Abstract
Despite the enormous potential for the use of stereospecific cross-coupling reactions to rationally manipulate the three-dimensional structure of organic molecules, the factors that control the transfer of stereochemistry in these reactions remain poorly understood. Herein we report a mechanistic and synthetic investigation into the use of enantioenriched alkylboron nucleophiles in stereospecific Pd-catalyzed Suzuki cross-coupling reactions. By developing a suite of molecular descriptors of phosphine ligands, we could apply predictive statistical models to select or design distinct ligands that respectively promoted stereoinvertive and stereoretentive cross-coupling reactions. Stereodefined branched structures were thereby accessed through the predictable manipulation of absolute stereochemistry, and a general model for the mechanism of alkylboron transmetallation was proposed.
Palladium-catalyzed cross-coupling reactions have revolutionized the construction of C(sp2)–C(sp2) bonds. Among these cross-coupling processes, the Suzuki–Miyaura reaction has found particularly broad application due to its extensive reaction scope, as well as the stability, availability, and low toxicity of organoboron reagents (1). The 2010 Nobel Prize in chemistry was awarded, in part, to recognize the transformative impact of the Suzuki cross-coupling reaction on chemical synthesis. However, although C(sp2)–C(sp2) bond construction is now considered routine using the Suzuki reaction, extension of this process to the formation of C(sp3)–C(sp2) bonds using alkylboron nucleophiles remains a significant challenge. Of particular interest, a variant using secondary alkylboron nucleophiles with predictable and controllable stereospecificity would establish a powerful synthetic strategy to access molecular geometries with precise three-dimensional control, expanding the exceptional capabilities of the Suzuki reaction (Figure 1A).
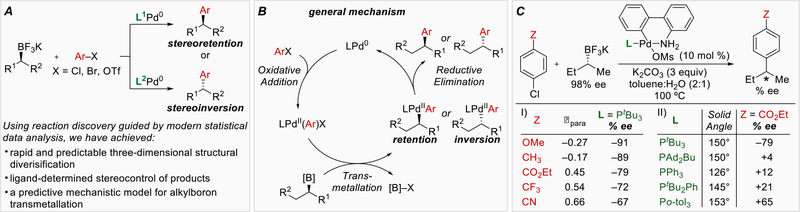
Figure 1:
A) Enantiodivergent Suzuki reactions of secondary alkylboron nucleophiles. B) General mechanism. C) Initial investigation of substrate and ligand influences on stereoselectivity. +% ee = net retention, −% ee = net inversion.
Many efforts have focused on the use of enantioenriched secondary alkylboron nucleophiles in Suzuki cross-coupling reactions (2–4). Significant limitations remain due to slow transmetallation of the highly covalent and sterically congested C(sp3)–B bond in these reagents, as well as the propensity of the resulting Pd-alkyl species to undergo β-hydride elimination/reinsertion sequences, which can result in isomerization of the alkyl group and racemization of the stereocenter. To circumvent prohibitively slow transmetallation, as well as competing β-hydride elimination/reinsertion pathways, most stereospecific Suzuki reactions have required the use of secondary alkylboron nucleophiles that are electronically activated via inclusion of a C(sp2) α-carbon, an a-heteroatom, and/or a strongly coordinating β-carbonyl group (5–17). In addition, alkylboron nucleophiles can undergo transmetallation via either stereoretentive or stereoinvertive pathways depending on the nature of the substrate, catalyst, and/or reaction conditions. In many cases, the factors controlling the dominant mechanism of transmetallation are not understood (Figure 1B). Thus, a predictive stereochemical model for transmetallation of alkylboron reagents remains elusive.
Recently, we reported a stereospecific Pd-catalyzed cross-coupling reaction using unactivated secondary alkylboron nucleophiles (18). With PtBu3 as a supporting ligand, enantioenriched arylation products were obtained with transmetallation proceeding primarily via a stereoinvertive mechanism. Whereas several enlightening mechanistic studies have recently been conducted on the transmetallation of arylboron nucleophiles (19–23), these studies have not addressed the transmetallation of alkylboron nucleophiles in C(sp3)–C(sp2) bond-forming processes (24–25). Thus, unactivated alkylboron nucleophiles constitute an attractive starting point from which to investigate the reaction parameters most influential to the mechanism of alkylboron transmetallation. This mechanistic work should simultaneously facilitate the development of new synthetic methods to rationally incorporate/manipulate stereocenters via cross-coupling strategies. To this end, we report a study using predictive statistical models (26–27) to relate phosphine ligand properties to stereochemical outcomes obtained from Pd-catalyzed Suzuki reactions of unactivated enantioenriched secondary alkylboron nucleophiles and aryl electrophiles. With statistical models that rely on a next generation set of molecular descriptors, we achieved a stereoretentive Pd-catalyzed cross-coupling reaction of such nucleophiles. Furthermore, we have identified an improved ligand for the stereoinvertive variant, thus enabling an entirely ligand-controlled enantiodivergent process from a single-enantiomer organoboron nucleophile (28). Our statistical models also provide compelling evidence that each transmetallation pathway is intimately tied to specific electronic properties of the supporting ligand, which serves as a predictive guide to the mechanism of alkylboron transmetallation to palladium.
Initial investigations using electronically differentiated aryl chlorides with enantioenriched sBuBF3K revealed a trend correlating diminished stereofidelity with the use of more electron-deficient coupling partners (Figure 1C, I). This observation suggested that subtle electronic effects could influence the mechanism of transmetallation and the resulting stereochemical outcome. Additionally, when the phosphine ligand was varied in an initial screen with a common aryl chloride electrophile, a considerable change in the reaction outcome from stereoinvertion to stereoretention was found (Figure 1C, II). No obvious correlation was observed between these results and the steric properties (solid angle) of the ligand. Taken together, these outcomes were difficult to interpret and inspired the use of ligand parameterization tools to provide a platform for both predictive ligand performance and mechanistic interrogation.
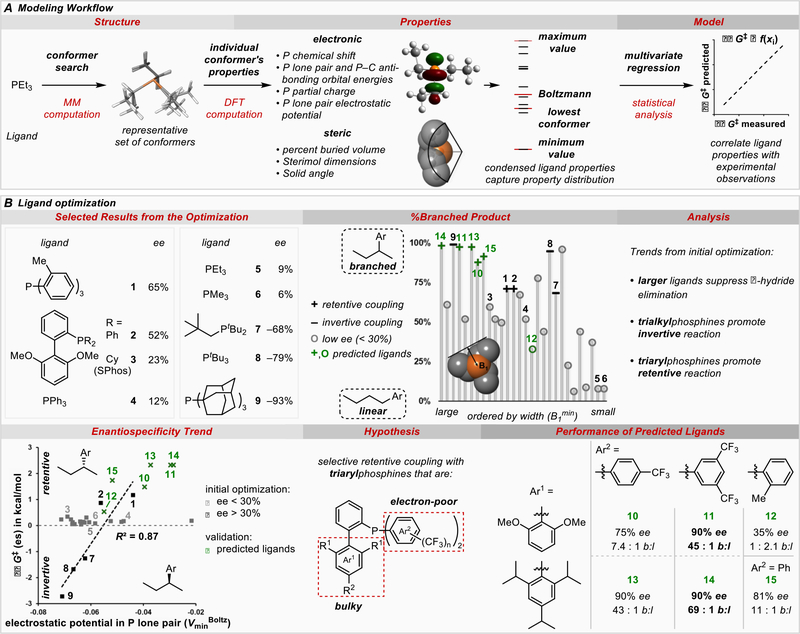
An expanded inventory of common phosphines with varied properties was evaluated in the Suzuki reaction of enantioenriched sBuBF3K and ethyl 4-chlorobenzoate. This dataset was then subjected to correlation analysis of phosphine structural features with the stereochemical outcomes as well as the ratios of branched:linear products in these reactions. We devised a workflow and universal parameter set to describe the catalyst properties from the phosphine itself (29–32). The workflow was initiated by performing a molecular mechanics (MM) conformational search to reveal representative low energy conformers. (Figure 2A). Next, geometry optimization of the conformers using DFT was followed by parameter collection. Subsequently, four descriptor subsets were defined to capture the conformational dynamics of the ligands by including the mathematical extreme descriptor values (minimum and maximum), the lowest energy conformer values, and the Boltzmann weighted averages. We viewed the unique treatment of representative conformers as a crucial means of describing ensemble properties such as chemical shift while also probing structural flexibility during catalysis.
Figure 2.
Phosphine parameterization. A) Workflow of parameter generation and statistical modeling. B) Application of phosphine parameterization to ligand optimization of the reaction shown in Figure 1C with Z = CO2Et. b:l = branched to linear ratio. +% ee = net retention, −% ee = net inversion.
The final step in the workflow involved the analysis of both the stereofidelity and the branched:linear product ratio. These two readouts presumably describe two stages of the reaction mechanism (Figure 1B): i) the competing stereoretentive and stereoinvertive transmetallation mechanisms that determine the final stereochemistry of the cross-coupling product and ii) the competitive β-hydride elimination/isomerization sequences that follow transmetallation. A correlation of the branched:linear ratio with the final enantiopurity of the product reveals that β-hydride elimination is responsible for both racemization and isomerization to the linear side product. Furthermore, a modest trend is observed relating the minimum width B1 of the phosphine ligand to the branched:linear ratio (Figure 2B). This is consistent with reports of large ligands facilitating reductive elimination over β-hydride elimination (33), and suggests the use of a parameterization approach to take into account the conformational flexibility of ligands.
Since the inherent selectivity of the transmetallation mechanism is masked by deleterious racemization as a consequence of β-hydride elimination, only ligands providing high selectivity were further investigated (>30% ee, Figure 2B). The molecular electrostatic potential minimum in the phosphorus lone pair region (Vmin) has been shown to correlate with the classical Tolman electronic parameter (34). Thus, Vmin serves as an easily computable measure for the overall ligand electronics. A correlation between enantioselectivity and Vmin was observed within the abridged dataset indicating that electronic properties of the ligand determine the mechanism of transmetallation. Specifically, electron-rich trialkylphosphines promoted stereoinvertive reactions, whereas the electron-poorer triarylphosphines provided modest selectivity for stereoretention. Use of the bulky, electron-rich ligand PAd3 (9), which was recently reported by Carrow (35), resulted in a particularly large preference for the stereoinvertive outcome. Based on these data, we hypothesized and virtually evaluated ligands for improved stereoretentive outcomes with the following features: i) large ligand bulk to prevent β-hydride elimination and racemization, and ii) electron-deficient aryl substituents at phosphorus to promote the stereoretentive mechanism and to accelerate reductive elimination (Figure 2B). Among the proposed ligands was a set of biaryl phosphines (11-15), as pioneered by Buchwald (36), featuring various electron-deficient aryl groups at phosphorus. Gratifyingly, ligands 11 and 14 promote the alkyl Suzuki cross-coupling reaction with significantly enhanced selectivity (up to 90% ee) and minimal alkyl isomerization. Thus, parameterization-driven optimization facilitated development of a stereoretentive Suzuki reaction involving unactivated alkylboron nucleophiles. When considered alongside the introduction of 9 to achieve stereoinvertive couplings, complete control of the absolute sense of enantioselectivity (retention or inversion) can be engendered by simply selecting the appropriate ligand.
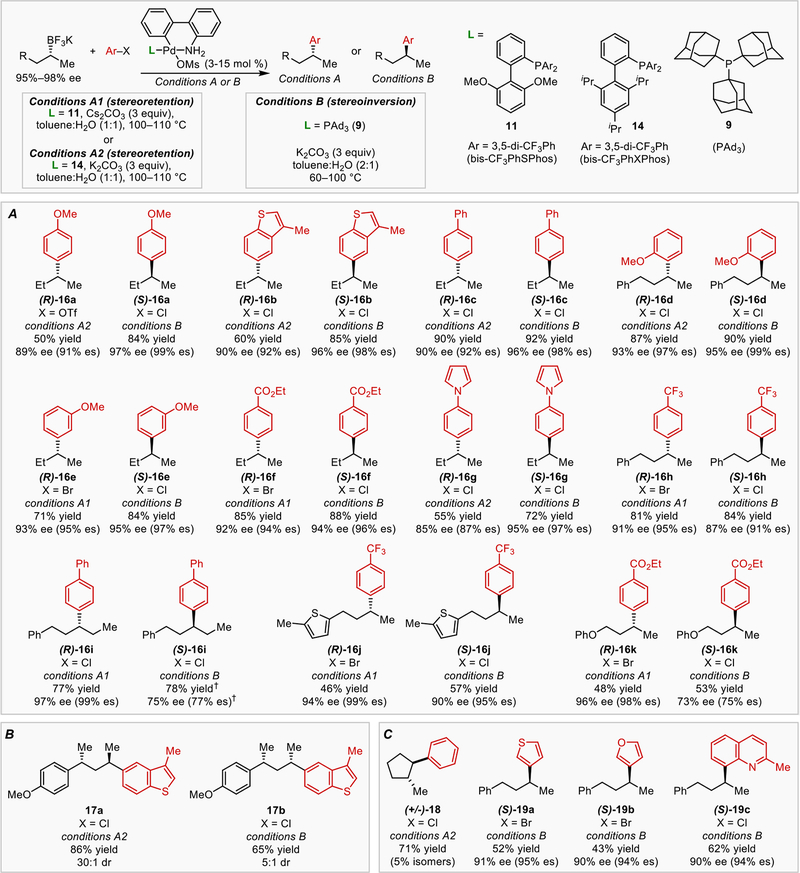
Our stereochemical investigations of secondary alkylboron transmetallation in the Suzuki reaction suggested that both enantiomers of a cross-coupling product could be selectively accessed through use of a single enantioenriched alkylboron reagent with the proper selection of the phosphine ligand. The scope of this process is depicted in Figure 3. Using enantioenriched, unactivated alkyltrifluoroborate nucleophiles, ligand-controlled stereoselectivity was broadly achieved in cross-coupling reactions with aryl electrophiles. Strongly π-accepting ligands bis- CF3PhSPhos (11) and bis-CF3PhXPhos (14), which emerged from our parameterization-guided optimization, preferentially promote the stereoretentive pathway, while strongly σ-donating ligand PAd3 (9) preferentially promotes the stereoinvertive pathway. Because electron-poor palladium catalysts commonly undergo slow oxidative addition with aryl chlorides, we also evaluated aryl bromide and triflate electrophiles in reactions involving 11 and 14. A particular highlight of this protocol is the uniformity of the conditions used for both the stereoinvertive and stereoretentive reactions: each operates in a toluene/water mixture as solvent, with a carbonate base, and no additional additives. Both reaction variants tolerated the use of electron-rich and electron-deficient aryl electrophiles, as well as an aryl electrophile bearing an ortho-substituent. High stereofidelity was achieved for all of these reactions, including those involving alkylboron nucleophiles bearing thiophenyl and phenoxide substituents. Use of an alkylboron nucleophile containing a larger substituent at the stereogenic center was also well-tolerated (16i). Diastereomeric products 17a and 17b could be generated from a single alkylboron diastereomer (37) using 14 and PAd3, respectively (Figure 3B). In these reactions, replacement of ligand 14 with PAd3 resulted in a change in diastereoselectivity from 30:1 to 1:5, a 3.6 kcal/mol free energy of activation difference dependent only on the ligand identity. No erosion of specificity was observed for electron-deficient aryl substrates in stereoinvertive Suzuki reactions using PAd3, in contrast to analogous reactions using PtBu3. Furyl and thiophenyl electrophiles are also compatible with our system (Figure 3C). As an additional mechanistic probe, trans-2- methylcyclopentyltrifluoroborate was subjected to the stereodivergent reaction conditions. Because trans-2-methylcyclopentyltrifluoroborate is sterically impeded from undergoing stereoinvertive transmetallation, only the stereoretentive process using 14 should be mechanistically viable. Indeed, we observed that use of ligand 14 smoothly generates 18 with stereoretention, while use of PAd3 results in low alkylboron conversion.
Figure 3.
Stereodivergent Pd-catalyzed cross-coupling reactions using enantioenriched alkylboron nucleophiles. % es = % ee (final product) / % ee (starting material). † 44% yield, 84% ee (86% es) when run at 60 °C.
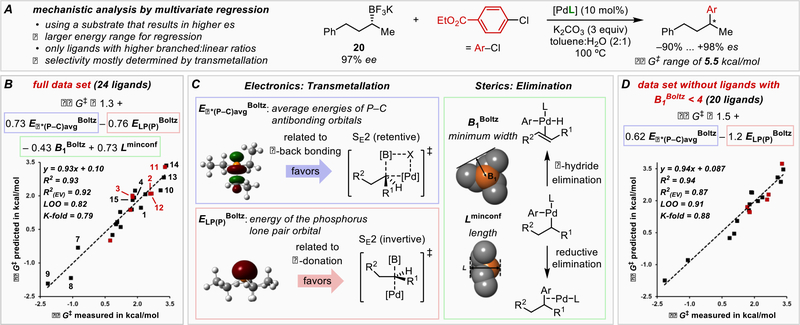
To further probe the origin of the ligand-dependent enantiodivergent process, we interrogated the mechanism of transmetallation using the parameterization strategy described above (Figure 4). To accomplish this, phenyl-substituted substrate 20 was selected due to enhanced performance and thus a greater output range. Additionally, 24 ligands were tested, excluding smaller ligands to reduce the complexity associated with β-hydride elimination. Multivariate linear regression revealed that most of the outputs can be expressed in two readily interpretable terms that discriminate the transmetallation pathways: the average energy of the P−C antibonding orbitals Eσ*(P−C), representative of π-back bonding, and the energy of the lone pair orbital of phosphorus ELP(P), a measure of the ligand’s σ-donation capability (Figure 4B). This outcome suggests that the stereoinvertive pathway is dependent on strong σ-donation from the ligand, which may stabilize a two-coordinate, cationic palladium complex. Conversely, the stereoretentive pathway is enhanced by π-back bonding, which may stabilize the coordination of a π-donor ligand X (presumably OH−) to Pd. Including two steric descriptors such as B1 and L improves the model fit by treating the competitive β-hydride elimination that occurs using smaller ligands and decreases the observed specificity. This becomes evident when the four smallest ligands in this dataset are removed from the analysis, which results in an excellent correlation using just the two electronic descriptors with the experimentally observed stereochemical outcomes (Figure 4D). Multivariate regression analysis thereby provides compelling evidence for the electronic factors favoring each transmetallation mechanism and thus a guideline for future developments in stereospecific cross-coupling reactions.
Figure 4.
Mechanistic investigation by multidimensional regression modeling. A) Data from the arylation of 20 was used. [PdL] is the precatalyst as shown in Figure 3 with varying ligands. B) Regression model containing all 24 ligands of this data set. Eσ*(P−C)avg Boltz: Boltzmann-weighted average across the conformers of the average energies of the three P−C σ* antibonding orbitals in each phosphine. ELP(P) Boltz: Boltzmann-weighted average of the energy of the phosphorus lone pair orbital. Sterimol B1 Boltz is the least width and Sterimol Lminconf is the length of the lowest-energy conformer as seen from opposite the P substituents. Red points in the diagram: validation data (EV) not used in the model training. LOO = leave-one-out cross validation score. K-fold: average leave-three-out cross-validation score. C) Illustration and interpretation of the model terms. D) Regression model after removing the four smallest ligands in this data set to exclude the influence of competitive β-hydride elimination on the data.
Supplementary Material
Acknowledgements:
We acknowledge R. Kinthada for contributions to the diastereoselectivity studies. We thank G. Ralph for assistance with chiral HPLC analysis. Funding: we are grateful to the National Institutes of Health (grant SC1GM110010 to M.R.B.), the National Science Foundation (grant CHE-1665189 to M.R.B. and grant CHE-1361296 to M.S.S), and the Leopoldina Fellowship Programme of the German National Academy of Sciences Leopoldina (LPDS 2017–18, to T.G.). The support and resources from the Center for High Performance Computing at the University of Utah are gratefully acknowledged. Further computational resources were provided by the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the NSF (ACI-1548562) and provided through allocation TG-CHE180003.
Footnotes
Competing Interests:
The authors declare no competing interests.
Data and material availability:
All additional data and materials are contained within the supplement. This includes:
Materials and Methods
Supplementary Text
Figure S1
Tables S1–S6
References 38–81
NMR Spectra
References and Notes:
- 1.Lee JCH, Hall DG, “State-of-the-art in metal-catalyzed cross-coupling reactions of organoboron compounds with organic electrophiles” in Metal-Catalyzed Cross-Coupling Reactions and More, de Meijere A, Bräse S, Oestreich M, Eds. (Wiley-VCH, ed. 3, 1, 2014), pp. 65–132. [Google Scholar]
- 2.Wang C-Y, Derosa J, Biscoe MR, Configurationally stable, enantioenriched organometallic nucleophiles in stereospecific Pd-catalyzed cross-coupling reactions: an alternative approach to asymmetric synthesis. Chem. Sci 6, 5105–5113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandford C, Aggarwal VK, Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun 53, 5481–5494 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Rygus JPG, Crudden CM, Enantiospecific and iterative Suzuki-Miyaura cross- couplings. J. Am. Chem. Soc 139, 18124–18137 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Imao D, Glasspoole BW, Laberge VS, Crudden CM, Cross coupling reactions of chiral secondary organoboronic esters with retention of configuration. J. Am. Chem. Soc 131, 5024–5025 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Sandrock DL, Jean-Gérard L, Chen C.-y., Dreher SD, Molander GA, Stereospecific cross-coupling of secondary alkyl β-trifluoroboratoamides. J. Am. Chem. Soc 132, 17108–17110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohmura T, Awano T, Suginome M, Stereospecific Suzuki–Miyaura coupling of chiral α-(acylamino)benzylboronic esters with inversion of configuration. J. Am. Chem. Soc 132, 13191–13193 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Awano T, Ohmura T, Suginome M, Inversion or retention? Effects of acidic additives on the stereochemical course in enantiospecific Suzuki–Miyaura coupling of α-(acetylamino)benzylboronic esters. J. Am. Chem. Soc 133, 20738–20741 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Lee JCH, McDonald R, Hall DG, Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds. Nature Chem 3, 894–899 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Partridge BM, Chausset-Boissarie L, Burns M, Pulis AP, Aggarwal VK, Enantioselective synthesis and cross-coupling of tertiary propargylic boronic esters using lithiation–borylation of propargylic carbamates. Angew. Chem. Int. Ed 51, 11795–11799 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Molander GA, Wisniewski SR, Stereospecific cross-coupling of secondary organotrifluoroborates: Potassium 1-(benzyloxy)alkyltrifluoroborates. J. Am. Chem. Soc 134, 16856–16868 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthew SC, Glasspoole BW, Eisenberger P, Crudden CM, Synthesis of enantiomerically enriched triarylmethanes by enantiospecific Suzuki–Miyaura cross-coupling reactions. J. Am. Chem. Soc 136, 5828–5831 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Sun C, Potter B, Morken JP, A catalytic enantiotopic-group-selective Suzuki reaction for the construction of chiral organoboronates. J. Am. Chem. Soc 136, 6534–6537 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaisdell TP, Morken JP, Hydroxyl-directed cross-coupling: A scalable synthesis of debromohamigeran E and other targets of interest. J. Am. Chem. Soc 137, 8712–8715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamura S, Araki M, Suzuki T, Yamaguchi J, Itami K. Stereodivergent synthesis of arylcyclopropylamines by sequential C–H borylation and Suzuki–Miyaura coupling. Angew. Chem. Int. Ed 54, 846–851 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Lou Y, Cao P, Jia T, Zhang Y, Wang M, Liao J, Copper-catalyzed enantioselective 1,6-boration of para-quinone methides and efficient transformation of gem-diarylmethine boronates to triarylmethanes. Angew. Chem. Int. Ed 127, 12302–12306 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Hoang GL, Takacs JM, Enantioselective γ-borylation of unsaturated amides and stereoretentive Suzuki–Miyaura cross-coupling. Chem. Sci 8, 4511–4516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Zhao S, Joshi-Pangu A, Diane M, Biscoe MR, Stereospecific Pd-catalyzed cross-coupling reactions of secondary alkylboron nucleophiles and aryl chlorides. J. Am. Chem. Soc 136, 14027–14030 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amatore C, Jutand A, Le Duc G, Kinetic data for the transmetalation/reductive elimination in palladium-catalyzed Suzuki–Miyaura reactions: Unexpected triple role of hydroxide ions used as base. Chem. Eur. J 17, 2492–2503 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Carrow BP, Hartwig JF, Distinguishing between pathways for transmetalation in Suzuki–Miyaura reactions. J. Am. Chem. Soc 133, 2116–2119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas AA, Denmark SE, Pre-transmetalation intermediates in the Suzuki-Miyaura reaction revealed: The missing link. Science 352, 329–332 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Thomas AA, Wang H, Zahrt AF, Denmark SE, Structural, kinetic, and computational characterization of the elusive arylpalladium(II)boronate complexes in the Suzuki–Miyaura reaction. J. Am. Chem. Soc 139, 3805–3821 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas AA, Zahrt AF, Delaney CP, Denmark SE, Elucidating the role of the boronic esters in the Suzuki–Miyaura reaction: Structural, kinetic, and computational investigations. J. Am. Chem. Soc 140, 4401–4416 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridgway BH, Woerpel KA, Transmetalation of alkylboranes to palladium in the Suzuki coupling reaction proceeds with retention of stereochemistry. J. Org. Chem 63, 458–460 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Matos K, Soderquist JA, Alkylboranes in the Suzuki–Miyaura coupling: Stereochemical and mechanistic studies. J. Org. Chem 63, 461–470 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Santiago CB, Guo J-Y, Sigman MS, Predictive and mechanistic multivariate linear regression models for reaction development. Chem. Sci 9, 2398–2412 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigman MS, Harper KC, Bess EN, Milo A, The development of multidimensional analysis tools for asymmetric catalysis and beyond. Acc. Chem. Res 49, 1292–1301 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Krautwald S, Carreira EM, Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc 139, 5627–5639 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Tolman CA, Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis. Chem. Rev 77, 313–348 (1977). [Google Scholar]
- 30.Jover J et al. , Expansion of the ligand knowledge base for monodentate P-donor ligands (LKB-P). Organometallics 29, 6245–6258 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niemeyer ZL, Milo A, Hickey DP, Sigman MS, Parameterization of phosphine ligands reveals mechanistic pathways and predicts reaction outcomes. Nature Chem 8, 610–617 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Wu K, Doyle AG, Parameterization of phosphine ligands demonstrates enhancement of nickel catalysis via remote steric effects. Nat. Chem 9, 779–784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jana R, Pathak TP, Sigman MS, Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev 111, 1417–1492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suresh CH, Koga N, Quantifying the electronic effect of substituted phosphine ligands via molecular electrostatic potential. Inorg. Chem 41, 1573–1578 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Ren P, Carrow BP, Tri(1-adamantyl)phosphine: Expanding the boundary of electron-releasing character available to organophosphorus compounds. J. Am. Chem. Soc 138, 6392–6395 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Surry DS, Buchwald SL, Dialkylbiaryl phosphines in Pd-catalyzed amination: a user’s guide. Chem. Sci 2, 27–50 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balieu S, Hallett GE, Burns M, Bootwicha T, Studley J, Aggarwal VK, Toward ideality: The synthesis of (+)-kalkitoxin and (+)-hydroxyphthioceranic acid by assembly-line synthesis. J. Am. Chem. Soc 137, 4398–4403 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Li H, Wang L, Zhang Y, Wang J, Transition-Metal-Free synthesis of pinacol alkylboronates from tosylhydrazones, Angew. Chem. Int. Ed 51, 2943–2946 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Bagutski V, Ros A, Aggarwal VK, Improved method for the conversion of pinacolboronic esters into trifluoroborate salts: facile synthesis of chiral secondary and tertiary trifluoroborates, Tetrahedron, 65, 9956–9960 (2009). [Google Scholar]
- 40.Molander GA, Cavalcanti LN, Oxidation of Organotrifluoroborates via Oxone, J. Org. Chem 76, 623–630 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dreher SD, Domer PG, Sandrock DL, Molander GA, Efficient cross-coupling of secondary alkyltrifluoroborates with aryl chloride-reaction discovery using parallel microscale experimentation. J. Am. Chem. Soc 130, 9257–9259 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey WF, Jiang X, Cyclization of (4-methoxy-5-hexenyl)lithium, J. Org. Chem 59, 6528–6533 (1994). [Google Scholar]
- 43.Gauthier RL, Elford TG, Aggarwal VK, Ate-Complexes of secondary boronic esters as chiral organometallic-type nucleophiles for asymmetric synthesis, J. Am. Chem. Soc 133, 16794–16797 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Bonet A, Odachowski M, Leonori D, Essafi S, Aggarwal VK, Enantiospecific sp2-sp3 coupling of secondary and tertiary boronic esters, Nat. Chem 6, 584–589 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Burns M, Essafi S, Bame JR, Bull SP, Webster MP, Balieu S, Dale JW, Butts CP, Harvey JN, Aggarwal VK, Assembly-line synthesis of organic molecules with tailored shapes, Nature, 513, 183–188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barder TE, Walker SD, Martinelli JR, Buchwald SL, Catalysts for Suzuki- Miyaura coupling process: scope and studies of the effect of ligand structure, J. Am. Chem. Soc 127, 4685–4696 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Hartmann N, Niemeyer M, Easy large-scale synthesis of sterically encumbered 2- iodobiphenyls and some derivatives thereof, Syn. Comm 21, 3839–3845 (2001). [Google Scholar]
- 48.Hicks JD, Hyde AM, Cuezva AM, Buchwald SL, Pd-catalyzed N-arylation of secondary acyclic amides: catalyst development, scope, and computational study. J. Am. Chem. Soc 131, 16720–16734 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruno NC, Tudge MT, Buchwald SL, Design and preparation of new palladium precatalysts for C-C and C-N cross-coupling reaction, Chem. Sci 4, 916–920 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruno NC, Niljianskul N, Buchwald SL, N-Substituted 2-aminobiphenylpalladium methanesulfonate precatalysts and their use in C-C and C-N cross-couplings, J. Org. Chem 79, 4161–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friis SD, Skrydstrup T, Buchwald SL, Mild Pd-catalyzed aminocarbonylation of (hetero)aryl bromides with a palladacyle precatalyst, Org. Lett 16, 4296–4299 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, Wang C, Huang R, Biscoe MR, Stereoretentive Pd-catalyzed Stille cross-coupling reaction of secondary alkyl azastannatranes and aryl halides, Nat. Chem 5, 607–612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green SA, Matos JL, Yagi A, Shenvi RA, Branch-selective hydroarylation: iodoarene-olefin cross-coupling, J. Am. Chem. Soc 138, 12779–12782 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Czaplik WM, Mayer M, Wangelin AJV, Direct cobalt-catalyzed cross-coupling between aryl and alkyl halides, Syn. Lett 18, 2931–2934 (2009). [Google Scholar]
- 55.Wilsilv A, Nguyen Y, Fillion E, Hydrogenolysis of unstrained carbon-carbon σ bonds: stereoselective entry into benzylic tertiary centers, J. Am. Chem. Soc 131, 15606–15607 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Baldwin JE, Bonacorsi S Jr, Stereochemistry of the thermal isomerizations of (1R, 2R)-1-((E)-Styryl)-2-methylcyclopropane to 3-phenyl-4-methylcyclopentenes, J. Am, Chem. Soc 115, 10621–10627 (1993). [Google Scholar]
- 57.Schrödinger Release 2018–1: MacroModel (Schrödinger, LLC, New York, NY, 2018). [Google Scholar]
- 58.Ermanis K, Parkes KEB, Agback T, Goodman JM, Expanding DP4: application to drug compounds and automation. Org. Biomol. Chem 14, 3943–3949 (2016). [DOI] [PubMed] [Google Scholar]
- 59. https://github.com/KristapsE/PyDP4/
- 60.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, and Fox DJ, Gaussian 16, Revision A.03 (2016). Gaussian, Inc, Wallingford CT [Google Scholar]
- 61.Adamo C, Barone V, Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys 110, 6158–6170 (1999). [Google Scholar]
- 62.Hehre WJ, Ditchfield R, Pople JA, Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys 56, 2257–2261 (1972). [Google Scholar]
- 63.Hariharan PC, Pople JA, The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 28, 213–222 (1973). [Google Scholar]
- 64.Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR, Efficient diffuse function-augmented basis sets for anion calculations. III. The 3–21+G basis set for first-row elements, Li-F. J. Comput. Chem 4, 294–301 (1983). [Google Scholar]
- 65.Krishnan R, Binkley JS, Seeger R, Pople JA, Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys 72, 650–654 (1980). [Google Scholar]
- 66.Cheeseman JR, A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys 104, 5497–5509 (1996). [Google Scholar]
- 67.Zhao Y, Truhlar DG, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Account 120, 215–241 (2008). [Google Scholar]
- 68.Weigend F, Ahlrichs R, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys 7, 3297 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Glendening ED, Reed AE, Carpenter JE, Weinhold F, NBO Version 3.1 (1990). [Google Scholar]
- 70.Latypov SK, Polyancev FM, Yakhvarov DG, Sinyashin OG, Quantum chemical calculations of 31 P NMR chemical shifts: scopes and limitations. Phys. Chem. Chem. Phys 17, 6976–6987 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Clavier H, Nolan SP, Percent buried volume for phosphine and N-heterocyclic carbene ligands: steric properties in organometallic chemistry. Chem. Commun 46, 841 (2010). [DOI] [PubMed] [Google Scholar]
- 72.Falivene L et al. , SambVca 2. A web tool for analyzing catalytic pockets with topographic steric maps. Organometallics 35, 2286–2293 (2016). [Google Scholar]
- 73. https://www.molnac.unisa.it/OMtools/sambvca2.0/index.html.
- 74.Verloop A, in Drug Design Ed. Ariens EJ (Academic Press, Vol. III, 1976). [Google Scholar]
- 75.Piou T et al. , correlating reactivity and selectivity to cyclopentadienyl ligand properties in Rh(III)-catalyzed C–H activation reactions: An experimental and computational study. J. Am. Chem. Soc 139, 1296–1310 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. https://github.com/bobbypaton/Sterimol.
- 77.Guzei IA, Wendt M, An improved method for the computation of ligand steric effects based on solid angles. Dalton Trans, 3991 (2006). [DOI] [PubMed] [Google Scholar]
- 78. http://xray.chem.wisc.edu/Resources.html.
- 79.MATLAB R2017a (Mathworks, Inc, Natick, MA, 2017). [Google Scholar]
- 80.Guo J-Y, Minko Y, Santiago CB, Sigman MS, Developing comprehensive computational parameter sets to describe the performance of pyridine-oxazoline and related ligands. ACS Catal 7, 4144–4151 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.