Abstract
Peripheral sensory stimulation has been used as a method to stimulate the sensorimotor cortex, with applications in neuro-rehabilitation. To improve delivery modality and usability, a new stimulation method has been developed in which imperceptible random-frequency vibration is applied to the wrist concurrently during hand activity. The objective of this study was to investigate effects of this new sensory stimulation on the sensorimotor cortex. Healthy adults were studied. In a transcranial magnetic stimulation (TMS) study, resting motor threshold, short-interval intracortical inhibition, and intracortical facilitation for the Abductor Pollicis Brevis muscle were compared between vibration on vs. off while subjects were at rest. In an electroencephalogram (EEG) study, alpha and beta power during rest and event-related desynchronization (ERD) for hand grip were compared between vibration on vs. off. Results showed that vibration decreased EEG power and decreased TMS short-interval intracortical inhibition (i.e., disinhibition) compared with no vibration at rest. Grip-related ERD was also greater during vibration, compared to no vibration. In conclusion, subthreshold random-frequency wrist vibration affected release of intracortical inhibition and both resting and grip-related sensorimotor cortical activity. Such effects may have implications in rehabilitation.
Keywords: physical stimulation, subliminal stimulation, hand, brain mapping, sensorimotor cortex, cortical excitability
1. Introduction
Afferent input drives changes in the motor cortex.1-3 Based on this framework, peripheral sensory stimulation has been used as a method to influence the motor cortex.4-6 For example, corticomotor neuronal excitability as measured using transcranial magnetic stimulation (TMS) has been shown to increase during muscle vibration, compared to no vibration.7, 8 After 30 min of electrical stimulation of the hand, functional magnetic resonance imaging (fMRI) blood-oxygen-level-dependent (BOLD) signals during finger movement in the primary motor (M1) and somatosensory area (S1) increased, compared to that before the stimulation.9 After 2 hours of transcutaneous electrical nerve stimulation, corticomotoneuronal excitability increases via GABAergic mechanism.4, 5 Therefore, electrical or vibratory peripheral sensory stimulation has been used as a means to induce neuroplastic changes to complement neurorehabilitation for movement impairment.4, 10-15 Unfortunately, most modalities of peripheral sensory stimulation involves wearing of a glove or similar device or suprathreshold stimulation. Gloves hamper dexterous finger movement and cause a sense of discomfort.16, 17 Suprathreshold electrical stimulation causes tingling sensation9, 10 and increasing vibration amplitude may lead to an illusion of movement or tonic vibration reflex.18 Thus most modalities of peripheral sensory stimulation are administered for a limited duration (ranging from 20 minutes to 2 hours a day) while a person is in a sedentary posture. Furthermore, the effect diminishes after the stimulation,5, 19 requiring repeated receipts of the stimulation. These constraints substantially lower patient adherence to a stimulation regimen.
To improve the delivery mode while leveraging the benefits of sensory stimulation, a new stimulation method has been proposed.20, 21 This new stimulation method involves application of vibratory stimulation to wrist skin, below a level perceptible to a person. The imperceptible nature of the stimulation, along with the location of the stimulation device being off from the fingers and hand, may enable natural sensorimotor processing for concurrent hand tasks. Furthermore, the stimulation can be delivered during activities of daily living continuously for a substantially longer dosage than existing stimulation methods. This stimulation may potentially be delivered via a watch-type wearable device to provide user-friendly stimulation to complement rehabilitation. Early studies using this specific stimulation are promising, with improved ability to detect light touch on fingertips 20, 22 and to grasp and manipulate objects21 in patients after stroke and healthy adults during stimulation. However, there is little understanding of its mechanism of action.
Early studies postulated a broad cortical mechanism given non-specificity of the stimulation site in which detection of light touch on fingertips improved during stimulation to volar wrist, dorsal wrist, dorsum of the hand, and thenar and hypothenar area.20, 22 Yet, a question remained whether this imperceptible stimulation even reaches the sensorimotor cortex. Therefore, in the present study, we examined whether this stimulation results in measurable changes in the sensorimotor cortex. Specifically, we hypothesized that this stimulation would increase cortical motor excitability as measured by using TMS and cortical sensorimotor activity as measured by electroencephalogram (EEG), compared with no stimulation.
2. Materials & Methods
2.1. Participants
Eighteen healthy right-handed adults (eight males and ten females) with a mean ± standard deviation (SD) age of 27 ± 5 years participated in the TMS study. Twenty healthy right-handed adults (twelve males and eight females) with a mean age of 25 ± 5 years participated in the EEG study. There was no overlap in subjects between the TMS and EEG study. All subjects verbally disclosed that they had no history of upper limb injury or musculoskeletal or neurologic disorders. All TMS subjects were screened for TMS safety. The two studies took place at two universities (one study in each university). Each study protocol was approved by the Institutional Review Board of the university where the study was performed. All subjects read and signed written informed consent forms approved by the Institutional Review Board prior to participating in the study.
2.2. Experimental Design and Statistical Analysis
2.2.1. Wrist stimulation
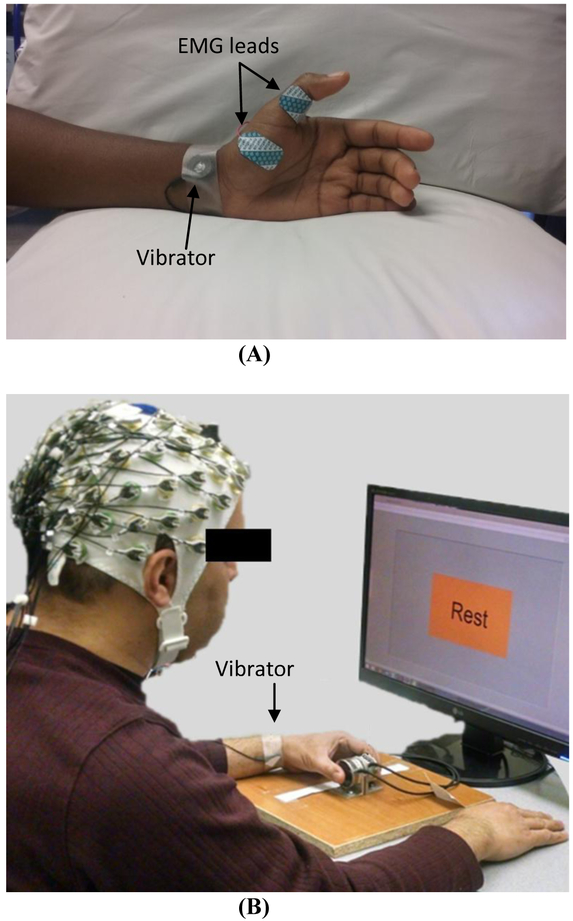
Vibration was applied using a C-3 Tactor (EAI Inc., Casselberry, FL, USA) attached to the left volar wrist using tape for both the TMS and EEG studies (Figure 1). The vibrator was driven by white-noise signals low-pass filtered at 500 Hz. White-noise signal was used based on literature in stochastic facilitation that collectively demonstrates, broadband white-noise stimulation enhances signal transmission23 and neural communication.24 A review25 and many applications exist.23, 26-28 At the beginning of each study, subjects’ sensory threshold for this wrist vibration was determined as the minimum vibration intensity (i.e., amplitude) that the subject was able to feel. Specifically, the sensory threshold was determined by increasing or decreasing the vibration intensity until the subject could or could not perceive the vibration, respectively, and taking the average of the vibration intensities at which the perception changed, using the ascending and descending limits method. The vibration amplitude corresponding to the sensory threshold was 4.5 ± 5.1 micron. Vibration intensity was subsequently set to 60% of the sensory threshold for the TMS and EEG evaluation, as this intensity has been shown to affect finger sensation,20, 22 finger sensory processing,29 and hand motor function.21
Figure 1.
Setup for TMS (A) and EEG (B) experiments.
2.2.2. TMS evaluation
Hand motor cortex excitability assessed by using TMS was compared between the vibration on and off conditions. Specifically, the resting motor threshold (RMT), short-interval intracortical inhibition (SICI), and intracortical facilitation (ICF) for the primary motor cortex area for the thumb muscle, Abductor Pollicis Brevis (APB), were compared between the vibration on and off conditions. The vibrator was placed on the subject throughout the session (approximately 50 min) and was turned on or off depending on the vibration condition. All motor cortex excitability measures were taken in one vibration condition, and then again in the other vibration condition, with the order of the vibration on and off conditions randomized across subjects. For the vibration ‘on’ condition, the subthreshold vibration was on continuously during the evaluation (approximately 25 min). The experimenter administering the TMS was blinded to the vibration condition. The vibration intensity was set to be subthreshold (i.e. imperceptible); thus, subjects should not have felt when the vibration was on. When asked after the testing was complete, one out of 18 subjects reported that s/he could feel when the vibration was on during testing, and two subjects reported feeling vibration briefly at some point during testing.
Subjects were comfortably seated in a TMS chair (Rogue Research Inc., Montreal, Quebec, Canada) and at rest. TMS was applied to the motor ‘hotspot’ in the subject’s right hemisphere, using a 70 mm figure-of-eight coil connected to Magstim Bistim2 2002 (Jali Medical, Waltham, MA, USA). The coil was held tangential to the scalp with the handle pointing backward and laterally at 45° from the midline, and moved in a systematic grid search over the right primary motor cortex at a slightly suprathreshold stimulus intensity to find the hotspot. The hotspot was determined as the location which consistently induced the largest motor evoked potential (MEP) amplitude in the left APB muscle. This stimulation location was kept consistent throughout the study with guidance from the Brainsight® system (Rogue Research Inc. Montreal, Quebec, Canada).
Surface electromyography (EMG) was recorded from the right APB muscle using disposable silver/silver chloride electrodes (Natus Medical Inc., Pleasanton, CA, USA) in a belly-tendon montage with the ground electrode on the back of the hand (Figure 1A). The EMG data were obtained using a CED 1902 Signal conditioner and 1401 interface (Cambridge Electronic Design, Cambridge, UK) and recorded using Spike2 software (Cambridge Electronic Design Ltd., Cambridge, England) at a sampling frequency of 5 kHz.
The RMT, SICI, and ICF were assessed at rest following the guidelines of the International Federation of Clinical Neurophysiology.30 Specifically, the RMT was determined as the percentage of the maximum stimulator output (%MSO) to elicit a 0.05 mV peak-to-peak MEP amplitude on the resting hand muscle with a 50% probability using the Parameter Estimation by Sequential Testing.31, 32 SICI and ICF were assessed using paired pulse protocols, involving a subthreshold conditioning stimulus followed by a suprathreshold test stimulus applied to the hotspot. The interstimulus interval (ISI) between the conditioning and test stimuli was 3 ms for SICI to inhibit the MEP response, while ISI was 15 ms for ICF to facilitate the MEP response. The reduction in the peak-to-peak MEP amplitude with the conditioning stimulus, compared to MEP amplitude with testing stimulus alone, indicated SICI. The increase in the peak-to-peak MEP amplitude with the conditioning stimulus compared to without the conditioning stimulus indicated ICF. The testing stimulus intensity was set as the percentage of the %MSO that evoked a peak-to-peak MEP amplitude of approximately 1 mV at rest consistently for three consecutive trials. The conditioning stimulation intensity was set to 5%MSO below RMT. The unconditioned and conditioned MEPs were obtained eight times each for SICI and also for ICF. The average peak-to-peak MEP amplitude values were used to compute SICI and ICF.
2.2.3. TMS analysis
A custom-made program in MATLAB (The MathWorks, Natick, MA) was used to extract the peak-to-peak MEP amplitudes. All extracted data were visually reviewed for accuracy. The generalized extreme Studentized deviate test33 was used to detect outliers in the distribution of data points for each cortical excitability measure that had multiple measurements (conditioned and unconditioned MEPs which had 8 data points each per subject). In the event of an outlier in the distribution of values for a single subject on a single parameter, this number was deleted. This occurred 4.8% for all data points. The MEPs were averaged across repetitions for each subject for each measure. The repetition-averaged data were assessed for normality before commencing subsequent analyses. Non-normality was not evidenced (Kolmogorov-Smirnov normality test p>0.05) for all measures (RMT, SICI, and ICF). The vibration on and off conditions for each measure were compared using a paired t-test. The significance level of 0.05/3, adjusted for multiple outcome variables (Bonferroni), was used. Additional paired t-tests were performed to compare testing stimulus intensities and unconditioned MEP amplitudes between the two vibration conditions. All statistical analysis was performed using SAS (SAS Institute Inc, Cary, NC, USA).
2.2.4. EEG acquisition
Cortical sensorimotor activity measured by EEG was compared between the two vibration conditions. Subjects were instructed to rest, then perform 2-sec long grips, and rest again per visual cues. The beginning of subsequent grips was separated by a random time interval between 7 and 8 seconds (s) (jittered by the computer). Visual cues prompted subjects when to start the grip and when to rest. Subjects performed 20 grips in one vibration condition followed by 20 grips in the other vibration condition in a single run of EEG collection. Subjects completed a total of 5 runs (100 grips in each vibration condition). The order of the vibration condition was randomized for each run and each subject. The vibrator was placed on the subject throughout the study (approximately 45 min) and was turned on or off depending on the vibration condition by the computer. During the trials for which the vibration condition was ‘on’, the vibration was continuously on throughout the half run duration including 20 grips and rests in between. The vibration intensity was set to be subthreshold. Subjects were informed that there would be vibration applied to the wrist, but they did not know when the vibration would be applied. After the EEG testing was complete, when asked if they felt the vibration at any time during the EEG recording, all subjects reported that they did not feel the vibration during the EEG recording. Therefore, none of the subjects knew for which trials the vibration was on.
Subjects were seated during the EEG testing. Subjects were instructed to perform precision pinch grip using the index finger and thumb of the left hand per visual cue. Visual cues were provided on a computer screen by a custom LabVIEW program (National Instruments, Austin, TX, USA) (Figure 1B). For grip, subjects were instructed to produce and maintain 4 N grip force which they practiced prior to testing using force sensors (2 Mini40, ATI Industrial Automation Inc, Apex, NC). This force level was deemed low enough not to induce excessive fatigue and also clearly distinguishable from the rest.
EEG signals were continuously recorded at 1 kHz using a 64 channel active electrode system (actiCAP, Brain Products GmbH, Gilching, Germany) and a Synamps2 amplifier system (Neuroscan, Charlotte, NC). The electrode position followed the international 10-20 system with an average reference and a ground at AFz. The EEG cap was placed on the subject’s head such that the Cz electrode was at the vertex. Each electrode site was hydrated using SuperVisc gel (Brain Products GmbH, Gilching, Germany). All electrodes’ impedance was checked to be below 20 kΩ. EEG signals were amplified, applied with a bandwidth filter at 0.10 to 200 Hz and a notch filter at 60 Hz, and recorded at 1 kHz using the Neuroscan software (SCAN 4.5). The timing of the visual cues to grip and rest was recorded together with the EEG data by the Neuroscan software via an external trigger input.
2.2.5. EEG analysis
The EEG data were analyzed using MATLAB and the EEGLAB toolbox. The data were band-pass filtered at 0.5 to 50 Hz. Independent component analysis was performed on the data to remove sources of artifacts using the ADJUST algorithm.34 Current source density estimates for surface potential were computed using a toolbox.35 Then, data were divided into epochs ranging from −2.5 to 4.5 s relative to the grip cue, with the time period before the grip cue (−2.5 to 0 s) as the baseline (resting) brain activity. Epochs with values beyond −450 to 450 μV were removed. This resulted in rejection of average 9% of the total trials for both vibration conditions, except for one subject for which over 90% of the trials were rejected. This one subject’s data was excluded from the analysis.
Baseline power and event-related spectral perturbation (ERSP) were obtained. The baseline power represents the resting brain activity. ERSP was obtained using time-frequency analysis, and depicts the dynamic changes in power of the EEG frequency spectrum from the baseline, specific for the grip activity. Specifically, reduction in power from the baseline with the grip task (event-related desynchronization, ERD) represents the brain activity for the grip, while ERD during grip termination represents the brain activity for grip termination.36-43 ERSP was obtained in time bins of 32 ms and frequency bins of 1 Hz. The power for the alpha (8-12 Hz) and beta band (13-30 Hz) was computed by averaging over each frequency band. These frequency bands are chosen as they have been shown to be relevant for sensorimotor processing, especially with sensory stimulation.44-47
For statistical analysis, two repeated measures ANOVAs were performed for baseline power and ERD, separately. For baseline power, the independent variables included vibration condition (on vs. off), frequency band (alpha and beta), and electrode (FC4, C4, and CP4, covering the sensorimotor areas including premotor, motor, and sensory areas). For ERD, the independent variables included vibration condition (on vs. off), frequency band (alpha and beta), electrode (FC4, C4, and CP4), and task (initiating a grip, and relaxing from a grip). Specifically, ERD for grip initiation was the event-related spectral perturbation averaged over the 1 s period immediately after the grip cue, and ERD for grip termination was the event-related spectral perturbation averaged over the 1 s period immediately after the rest cue. In addition, to examine any change in baseline power or ERD over the testing session, potentially due to desensitization to the continuous vibration, an additional independent variable of first vs. second half of the testing session was included for both ANOVAs. All second order interactions were also included in both ANOVAs. The significance level of 0.025 (adjusted for multiple tests) was used. ANOVAs were performed using SAS. Furthermore, a topographical distribution of the differences between the two vibration conditions was visually examined using t-maps.
3. Results
3.1. TMS
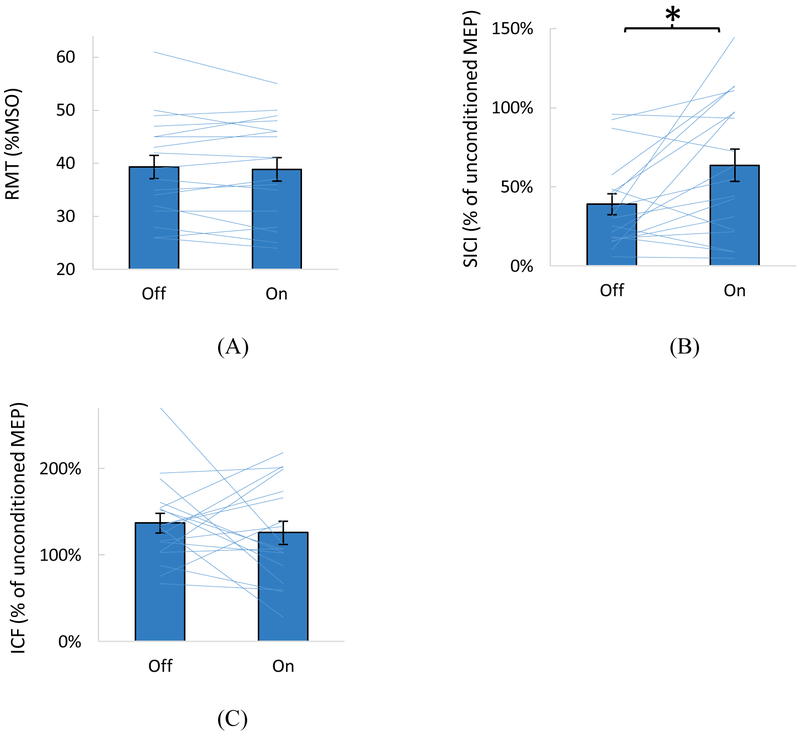
Motor cortex excitability was compared between the wrist vibration on vs. off conditions for each measure (Figure 2). The vibration resulted in a significant reduction in SICI (i.e., less inhibition) compared to vibration off (p=0.014): The conditioned MEP was, on average, 39% of the unconditioned MEP (61% inhibition) with the vibration off vs. 64% of the unconditioned MEP (36% inhibition) with the vibration on. The RMT and ICF were not significantly different between the two vibration conditions (p=0.521 and p=0.515, respectively).
Figure 2.
Comparison between the vibration on and off conditions for the resting motor threshold (RMT) (A), short-interval intracortical inhibition (SICI) (B), and intracortical facilitation (ICF) (C). Means with SE are shown as bar graphs with error bars. Individual subjects’ data are shown as lines. (B) and (C) show conditioned MEP amplitudes expressed as % of the unconditioned MEP amplitudes. Increase in values in (B) indicates reduced inhibition. A significant difference between the vibration on and off conditions was found for SICI only (noted with * in B).
The mean RMT ± SD was 39 ± 9%MSO. The conditioning stimulus intensity mean ± SD was 34 ± 9%MSO, or 87 ± 4%RMT. The mean testing stimulus intensity was 74 ± 16%MSO. The testing stimulus intensity for the paired pulse protocol was not significantly different between the vibration on and off conditions (p=0.554). The unconditioned MEP amplitude was also not significantly different between the vibration on and off conditions (p=0.298, mean ± SE = 1.0 ± 0.1 mV for vibration off, 1.1 ± 0.2 mV for vibration on).
3.2. EEG
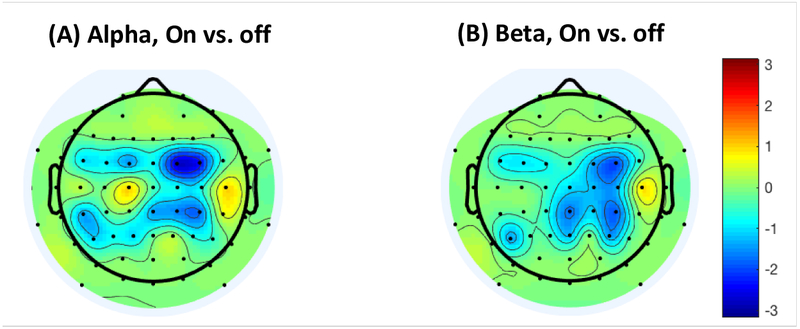
EEG power during rest and ERD for the grip was compared between the vibration on vs. off conditions. During rest, repeated measures ANOVA showed that EEG power significantly differed by the vibration condition (on vs. off), frequency band (alpha vs. beta), electrode (FC4/C4/CP4), and session (first vs. second half) (p<0.025). No interactions were found to be significant (p>0.5). Specifically, the resting power was lower while the vibration was on, compared to while it was off (p=0.011, mean ± SE = 12.8 ± 0.3 μV2/Hz for vibration on, 13.3 ± 0.3 μV2/Hz for vibration off). The t-map showing t-statistics comparing vibration on vs. off for each electrode is shown in Figure 3. The resting power was lower for the alpha than the beta frequency band (p=0.001, mean ± SE = 12.7 ± 0.3 μV2/Hz for alpha, 13.3 ± 0.3 μV2/Hz for beta). The resting power was lowest for the FC4 electrode (p=0.001, mean ± SE = 12.6 ± 0.3 μV2/Hz for FC4, 13.4 ± 0.4 μV2/Hz for C4, and 13.1 ± 0.3 μV2/Hz for CP4). The resting power increased over the testing session (p<0.001, mean ± SE = 12.6 ± 0.3 μV2/Hz for the first half, 13.5 ± 0.3 μV2/Hz for the second half).
Figure 3.
The t-statistics comparing power during baseline (rest) between the vibration ‘on’ and ‘off’ conditions, for the alpha (A) and beta band (B), with the blue color indicating reduced power with vibration compared to without.
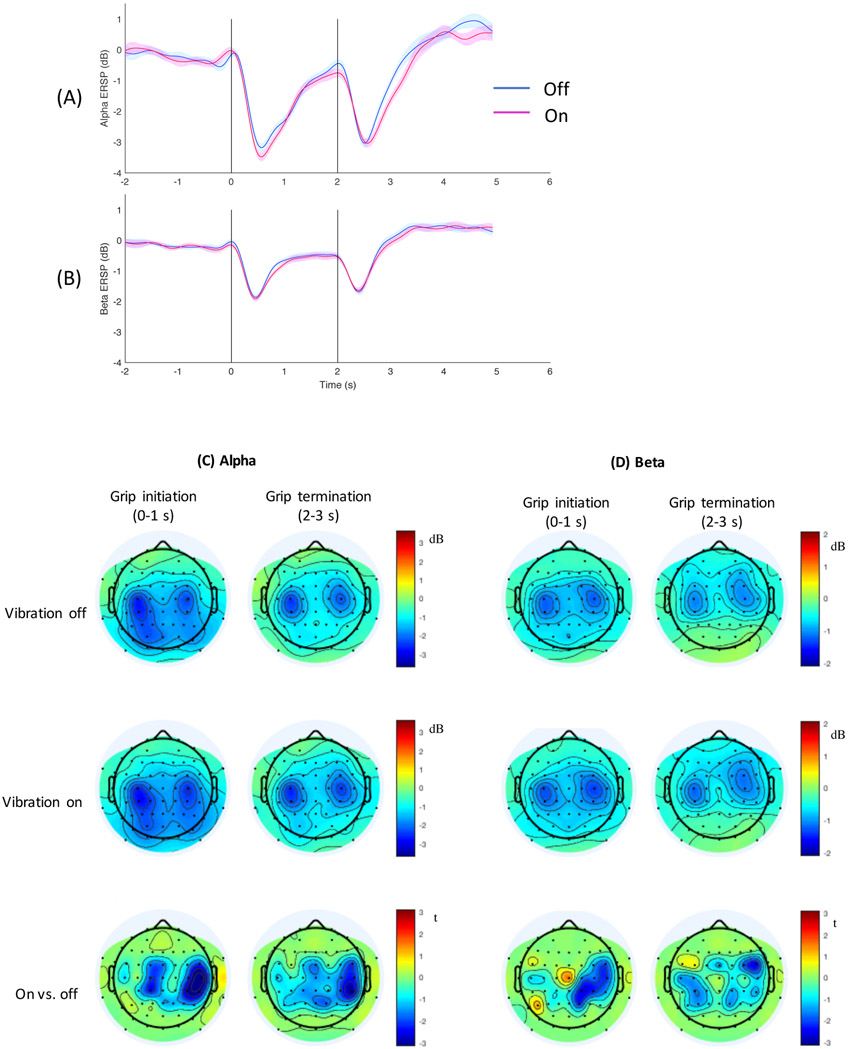
During the grip initiation and termination, ERSP time course is shown in Figure 4A-B and topography is shown in Figure 4C-D. ERD occurred shortly after the grip cue (at 0 s) and again shortly after the rest cue (at 2 s) for both alpha (Figure 4A) and beta band (Figure 4B), consistent with literature.41, 48, 49 ERD was topographically located in the bilateral sensorimotor cortical area for grip initiation and termination, for the alpha (Figure 4C) and beta band (Figure 4D), consistent with literature.36-43 The t-map showed the topography for difference in ERD between the vibration on vs. off conditions (Figure 4 bottom row).
Figure 4.
The time course of event-related spectral perturbation (ERSP) is shown for the alpha (A) and beta band (B) for the two vibration conditions, with shades showing the standard error for each vibration condition. ERSP is expressed as change from the baseline power in dB. The vertical lines represent the times the grip cue was presented to the subject at 0 s and the rest cue at 2 s. ERSP time course averaged for three electrodes (FC4/C4/CP4) is shown. Topography of event-related desynchronization (ERD) is shown for the alpha (C) and beta band (D). In C & D, the first column shows the topography for grip initiation (averaged over 1 s period immediately after the cue to grip), and the second column shows the topography for grip termination (averaged over 1 s period immediately after the cue to rest). The first row shows ERD topography for the vibration off condition, the second row for the vibration on condition, and the third row for t-statistics comparing the vibration on vs. off conditions with the blue color indicating greater ERD with vibration compared to without.
Repeated measures ANOVA showed that ERD significantly differed by the vibration condition (on vs. off), frequency band (alpha vs. beta), electrode (FC4/C4/CP4), task (grip initiation vs. termination), and interactions between frequency band and electrode, electrode and task, and frequency band and session (p<0.025). The main effect of session (first vs. second half) and other second-order interactions were not significant (p>0.3). Specifically, vibration increased ERD (p=0.009, mean ± SE = 1.6 ± 0.1 dB for vibration on, 1.4 ± 0.1 dB for vibration off, Figure 4). ERD was greater for alpha than beta (p<0.001, Figure 4). ERD differed by electrode (p<0.001) and electrode × frequency band (p=0.001), in which mean ERD was the largest for C4 followed in order by CP4 and FC4 for the alpha band, whereas for the beta band, mean ERD was the largest for C4 followed in order by FC4 and CP4 (Figure 4C-D). ERD differed by task (p=0.018) and task × electrode (p=0.009), in which mean ERD was larger for grip initiation than termination for C4 and CP4, and vice versa for FC4. As for the interaction between frequency band and session (p=0.010), mean ERD decreased in the second half compared to the first half of the testing session for alpha (mean ± SE = 2.1 ± 0.1 dB for the first half and 1.9 ± 0.1 dB for the second half), whereas the opposite trend was seen for beta (mean ± SE = 1.0 ± 0.1 dB for the first half and 1.1 ± 0.1 dB for the second half).
4. Discussion
The main finding of this study is that subthreshold random-frequency wrist vibration activated the sensorimotor cortex (as seen by reduced EEG power) and released intracortical inhibition of the primary motor cortex (as seen by reduced SICI, i.e., disinhibition) at rest. For grip activity, vibration resulted in additionally increased brain activity (as seen by greater EEG ERD). We postulate mechanisms of these vibration effects as follows.
Pathway from peripheral stimulation to M1:
At rest, subthreshold vibration, although imperceptible to subjects, may activate mechanoreceptors in the wrist skin and induce action potentials in the afferent neurons, because the minimum intensity of tactile stimuli on the palmar and wrist skin to activate sensory neurons (i.e., neuronal threshold) is lower than the perceptual threshold (i.e., the minimum intensity that is perceptible to a person).50 Subthreshold electrical cutaneous stimulation has been shown to induce evoked potentials in the somatosensory cortex.51 In regards to EEG power, suprathreshold electrical or mechanical stimulation decreases the EEG power over the sensorimotor cortex (compared to that without stimulation) at rest.45-47, 52 Specifically, beta power reduction indicates activation of the sensorimotor cortex for receipt and processing of the sensory stimulation.45-47, 52 The present study shows that subthreshold vibration also decreased the EEG power over the sensorimotor cortex at rest (Figure 3). To the authors’ best knowledge, this study is the first to show activation of the sensorimotor cortex in terms of resting EEG power reduction with subthreshold peripheral sensory stimulation.
Vibration affected not only beta (for sensory processing) but also alpha power. Alpha rhythm is generated by the thalamic oscillator function of the lateral thalamic nuclei, and sensory afference disrupts this oscillator function, thereby reducing alpha power.53 The lateral thalamic nuclei mainly project afferences to the sensory cortex, corroborating the observation that the alpha rhythm originates from postcentral sensory areas.40, 54 Functionally, reduction in alpha power represents activation of a broad synaptic network with distributed cortical representations that provide an ancillary support41 such as attention and release of inhibition.55, 56 Therefore, subthreshold vibration might have resulted in arousal and release of inhibition for the sensorimotor cortex.
Decrease in power with vibration was spread over the frontoparietal areas (Figure 3), suggesting that stimulation was received in the somatosensory/parietal area and further processed in the premotor area, as secondary processing of sensory input in the premotor area has been documented.57, 58 Further, the premotor cortex influences M1.59 Functional connections from the S1 to M1 have been demonstrated in the long-latency (~50 ms) cutaneomuscular reflex60-62 and the positive correlation between S1 and M1 excitability6 and intracortical microstimulation studies.1-3
Effect on M1:
Through these pathways, it has been shown that suprathreshold electrical cutaneous stimulation (with intensity 2-3 times the sensory threshold) reduces SICI in M1.63, 64 The present study showed that subthreshold vibratory cutaneous stimulation (with intensity 40% below the sensory threshold) also reduced SICI. Reduction in SICI was due to the sensory stimulation, not due to different stimulation parameters, as there were no significant differences in the unconditioned MEP sizes, test stimulus intensities, and conditioning stimulus intensities between the stimulation on and off conditions. While SICI decreased, ICF and RMT did not change. These findings in which effects were seen only on SICI but not on ICF and RMT are consistent with previous studies using cutaneous electrical stimulation.4, 7 SICI represents GABAergic inhibitory circuit activity, while ICF represents glutamatergic excitatory activity.30 Therefore, change only in SICI could be because somatosensory-induced M1 excitability changes are predominantly influenced by GABAergic function.5 The lack of the overall excitability change (RMT, MEP) could be due to the low stimulation intensity and limited effects of this stimulation on spinal mechanisms, as other studies have found effects on the overall excitability using suprathreshold stimulation,6, 63 contributed by spinal mechanisms.65, 66 In summary, subthreshold vibration appears to have affected M1 through a GABAergic mechanism.
Stimulation of the wrist affected intracortical inhibition for the thumb. Spillover effects of sensory stimulation on TMS measures for muscles that are not directly related to the stimulation site were seen in literature: cutaneous nerve stimulation of the index finger decreased SICI for not only the first dorsal interosseous (FDI) but also the APB and abductor digiti minimi (ADM), and cutaneous nerve stimulation of the little finger decreased SICI not only for ADM but also for FDI and APB using ISI of 3 ms (used in the present study).64 This spillover effect may in part be explained by the vibration affecting the alpha rhythm that tends to be topographically diffused41 and provides ancillary support such as attention and release of inhibition,55, 56 and may parallel GABAergic intracortical inhibition.55 Similar observations were made in literature in which imperceptible random-frequency peripheral stimulation influenced broad brain areas24, 67, 68 and led to behavioral changes beyond the site stimulated.29, 69 This diffusivity supports the previous reports on subthreshold wrist vibration’s effects on fingertip sensation20, 22 and hand motor function.21
Effect of vibration on grip-related ERD:
The subthreshold vibration not only changed the resting EEG power, but also increased the extent of brain activity for grip initiation and termination. This grip-specific change with vibration was additional to the change at rest; i.e., EEG power was reduced with vibration at rest, and yet more reduction in power was observed during grip with vibration compared to without. This amplified ERD for grip with sensory stimulation may have been enabled by decrease in cortical inhibitory circuit activity (as seen by decreased SICI in the present study) along with reduction in top-down inhibition70, 71 (as seen by decreased baseline alpha power) at rest. Greater alpha ERD with vibration may reflect increase in general excitability and supporting network activity41, 55, 56 for the grip task. Greater beta ERD with vibration may reflect increase in either motor planning/coordination activity or movement-induced afferent input.36, 37, 42, 43 On the flip side, a lack of afferent input due to pure somatosensory stroke has been shown to result in reduced ERD.38 Together, the present study results and literature suggest that somatosensory afference contributes to release of inhibition for movement and sensorimotor activity.
Change over time:
The potential of changing effects of the vibration over time was examined only in the EEG study. The EEG testing lasted for approximately 45 minutes. Baseline power increased from the first half to the second half of the EEG session, which may be associated with reduced attention for subjects over the testing duration. However, the effects of the stimulation on the EEG resting power and ERD were consistent over time, as it did not significantly alter from the first half to the second half of the testing session (with the interaction between vibration condition and session of p=0.481 for resting power and p=0.731 for ERD). Potential sensory habituation may have been minimized possibly by the random characteristics of the vibration frequency or breaks in between.
Implication:
The result of this study may be relevant as this vibration is considered for use during rehabilitation and motor learning. The GABAergic circuits play a role in maintaining the boundaries of the cortical motor map and a decrease in local GABAergic activity is essential in motor cortical plasticity and reorganization after injury.72, 73 For instance, less intracortical inhibition in the acute phase of stroke was associated with greater improvement in motor function many months after stroke.74, 75 Similarly, motor skill learning has been shown to be accompanied by reduction of SICI, indicating its role in use-dependent plasticity.76, 77 In addition, a decrease in alpha power during motor learning was attributed to increased attention to the task with maximal readiness and information processing capacity.78 Thus, a reduction of SICI and greater ERD for grip with imperceptible wrist vibration may potentially prime the sensorimotor cortex to facilitate hand motor activity, skill learning and reorganization. However, practical utility for rehabilitation or motor learning must be further investigated.
Conclusions:
The present study showed that subthreshold random-frequency vibration applied to the wrist led to release of inhibition for the APB muscle in M1, suppression of resting alpha and beta rhythms, and increased grip-related ERD activity, indicating increased excitability/activity of the sensorimotor cortex. These effects may explain improvements in hand motor function using the stimulation in earlier studies. The effects are similar to those of existing peripheral sensory stimulation. The specific modality using subthreshold wrist vibration can be mobile and cannot be detected and thus may have advantages in motor learning or rehabilitation settings.
Acknowledgments:
This work was supported by the COBRE for Stroke Recovery through an Institutional Development Award (IDeA) from the NIH/NIGMS under grant number P20GM109040, NIH/NHLBI R25HL092611, American Heart Association grant, and NIH/NIGMS U54-GM104941.
Footnotes
Declaration of interest: Seo is an inventor of a patent regarding the vibratory stimulation method studied in this manuscript. Other authors have no conflict of interest to report.
References
- 1.Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. II. Intracortical connections between neurons receiving somatosensory cortical input and motor output neurons of the cortex. J Comp Neurol. 1994. July 08;345(2):172–84. [DOI] [PubMed] [Google Scholar]
- 2.Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. I. Responses of morphologically identified motor cortical cells to stimulation of the somatosensory cortex. J Comp Neurol. 1994. July 08;345(2):161–71. [DOI] [PubMed] [Google Scholar]
- 3.Matyas F, Sreenivasan V, Marbach F, et al. Motor control by sensory cortex. Science. 2010. November 26;330(6008):1240–3. [DOI] [PubMed] [Google Scholar]
- 4.Celnik P, Hummel F, Harris-Love M, Wolk R, Cohen LG. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil. 2007. November;88(11):1369–76. [DOI] [PubMed] [Google Scholar]
- 5.Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002. April 15;540(Pt 2):623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schabrun SM, Ridding MC, Galea MP, Hodges PW, Chipchase LS. Primary sensory and motor cortex excitability are co-modulated in response to peripheral electrical nerve stimulation. PLoS One. 2012;7(12):e51298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003. September 1;551(Pt 2):649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenkranz K, Pesenti A, Paulus W, Tergau F. Focal reduction of intracortical inhibition in the motor cortex by selective proprioceptive stimulation. Exp Brain Res. 2003. March;149(1):9–16. [DOI] [PubMed] [Google Scholar]
- 9.Golaszewski SM, Siedentopf CM, Koppelstaetter F, et al. Modulatory effects on human sensorimotor cortex by whole-hand afferent electrical stimulation. Neurology. 2004. June 22;62(12):2262–9. [DOI] [PubMed] [Google Scholar]
- 10.Meesen RL, Cuypers K, Rothwell JC, Swinnen SP, Levin O. The effect of long-term TENS on persistent neuroplastic changes in the human cerebral cortex. Hum Brain Mapp. 2011. June;32(6):872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conforto AB, Ferreiro KN, Tomasi C, et al. Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair. 2010. Mar-Apr;24(3):263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotze M, Ladda AM, Roschka S, Platz T, Dinse HR. Priming Hand Motor Training with Repetitive Stimulation of the Fingertips; Performance Gain and Functional Imaging of Training Effects. Brain Stimul. 2017. Jan-Feb;10(1):139–46. [DOI] [PubMed] [Google Scholar]
- 13.Gomes-Osman J, Field-Fote EC. Cortical vs. afferent stimulation as an adjunct to functional task practice training: a randomized, comparative pilot study in people with cervical spinal cord injury. Clin Rehabil. 2015. August;29(8):771–82. [DOI] [PubMed] [Google Scholar]
- 14.Cordo P, Wolf S, Lou JS, et al. Treatment of severe hand impairment following stroke by combining assisted movement, muscle vibration, and biofeedback. J Neurol Phys Ther. 2013. December;37(4):194–203. [DOI] [PubMed] [Google Scholar]
- 15.Marconi B, Filippi GM, Koch G, et al. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil Neural Repair. 2011. January;25(1):48–60. [DOI] [PubMed] [Google Scholar]
- 16.Yu A, Yick KL, Ng SP, Yip J. Case study on the effects of fit and material of sports gloves on hand performance. Appl Ergon. 2019. February;75:17–26. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita H Effect of gloves on prehensile forces during lifting and holding tasks. Ergonomics. 1999. October;42(10):1372–85. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari F, Clemente F, Cipriani C. The preload force affects the perception threshold of muscle vibration-induced movement illusions. Exp Brain Res. 2018. October 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith L, Brouwer B. Effectiveness of muscle vibration in modulating corticospinal excitability. J Rehabil Res Dev. 2005. Nov-Dec;42(6):787–94. [DOI] [PubMed] [Google Scholar]
- 20.Enders LR, Hur P, Johnson MJ, Seo NJ. Remote vibrotactile noise improves light touch sensation in stroke survivors' fingertips via stochastic resonance. J Neuroeng Rehabil. 2013. October 11;10(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo NJ, Kosmopoulos ML, Enders LR, Hur P. Effect of remote sensory noise on hand function post stroke. Front Hum Neurosci. 2014;8:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakshminarayanan K, Lauer AW, Ramakrishnan V, Webster JG, Seo NJ. Application of vibration to wrist and hand skin affects fingertip tactile sensation. Physiol Rep. 2015. July 14;3(7):e12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins JJ, Imhoff TT, Grigg P. Noise-enhanced tactile sensation. Nature. 1996. October 31;383(6603):770. [DOI] [PubMed] [Google Scholar]
- 24.Ward LM, MacLean SE, Kirschner A. Stochastic resonance modulates neural synchronization within and between cortical sources. PLoS One. 2010. December 16;5(12):e14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonnell MD, Ward LM. The benefits of noise in neural systems: bridging theory and experiment. Nat Rev Neurosci. 2011. June 20;12(7):415–26. [DOI] [PubMed] [Google Scholar]
- 26.Wells C, Ward LM, Chua R, Inglis JT. Touch noise increases vibrotactile sensitivity in old and young. Psychol Sci. 2005. April;16(4):313–20. [DOI] [PubMed] [Google Scholar]
- 27.Collins JJ, Imhoff TT, Grigg P. Noise-mediated enhancements and decrements in human tactile sensation. Physical Review E. 1997;56(1):923. [Google Scholar]
- 28.Kurita Y, Shinohara M, Ueda J. Wearable Sensorimotor Enhancer for Fingertip Based on Stochastic Resonance Effect. IEEE Transactions on Human-Machine Systems. 2013;43(3):333–7. [Google Scholar]
- 29.Seo NJ, Lakshminarayanan K, Bonilha L, Lauer AW, Schmit BD. Effect of imperceptible vibratory noise applied to wrist skin on fingertip touch evoked potentials - an EEG study. Physiol Rep. 2015. November;3(11):e12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015. June;126(6):1071–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awiszus F TMS and threshold hunting. Suppl Clin Neurophysiol. 2003;56:13–23. [DOI] [PubMed] [Google Scholar]
- 32.Ah Sen CB, Fassett HJ, El-Sayes J, Turco CV, Hameer MM, Nelson AJ. Active and resting motor threshold are efficiently obtained with adaptive threshold hunting. PLoS One. 2017;12(10):e0186007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosner B Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25(2):165–72. [Google Scholar]
- 34.Mognon A, Jovicich J, Bruzzone L, Buiatti M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 2011. February;48(2):229–40. [DOI] [PubMed] [Google Scholar]
- 35.Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol. 2006. February;117(2):348–68. [DOI] [PubMed] [Google Scholar]
- 36.Alegre M, Labarga A, Gurtubay IG, Iriarte J, Malanda A, Artieda J. Movement-related changes in cortical oscillatory activity in ballistic, sustained and negative movements. Exp Brain Res. 2003. January;148(1):17–25. [DOI] [PubMed] [Google Scholar]
- 37.Nakayashiki K, Saeki M, Takata Y, Hayashi Y, Kondo T. Modulation of event-related desynchronization during kinematic and kinetic hand movements. J Neuroeng Rehabil. 2014. May 30;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platz T, Kim IH, Pintschovius H, et al. Multimodal EEG analysis in man suggests impairment-specific changes in movement-related electric brain activity after stroke. Brain. 2000. December;123 Pt 12:2475–90. [DOI] [PubMed] [Google Scholar]
- 39.Toma K, Nagamine T, Yazawa S, et al. Desynchronization and synchronization of central 20-Hz rhythms associated with voluntary muscle relaxation: a magnetoencephalographic study. Exp Brain Res. 2000. October;134(4):417–25. [DOI] [PubMed] [Google Scholar]
- 40.Ohara S, Ikeda A, Kunieda T, et al. Movement-related change of electrocorticographic activity in human supplementary motor area proper. Brain. 2000. June;123 (Pt 6):1203–15. [DOI] [PubMed] [Google Scholar]
- 41.Crone NE, Miglioretti DL, Gordon B, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998. December;121 (Pt 12):2271–99. [DOI] [PubMed] [Google Scholar]
- 42.Formaggio E, Storti SF, Boscolo Galazzo I, et al. Time-frequency modulation of ERD and EEG coherence in robot-assisted hand performance. Brain Topogr. 2015. March;28(2):352–63. [DOI] [PubMed] [Google Scholar]
- 43.Gross J, Pollok B, Dirks M, Timmermann L, Butz M, Schnitzler A. Task-dependent oscillations during unimanual and bimanual movements in the human primary motor cortex and SMA studied with magnetoencephalography. Neuroimage. 2005. May 15;26(1):91–8. [DOI] [PubMed] [Google Scholar]
- 44.Melgari JM, Zappasodi F, Porcaro C, et al. Movement-induced uncoupling of primary sensory and motor areas in focal task-specific hand dystonia. Neuroscience. 2013. October 10;250:434–45. [DOI] [PubMed] [Google Scholar]
- 45.Tecchio F, Zappasodi F, Porcaro C, et al. High-gamma band activity of primary hand cortical areas: a sensorimotor feedback efficiency index. Neuroimage. 2008. March 1;40(1):256–64. [DOI] [PubMed] [Google Scholar]
- 46.Pfurtscheller G, Krausz G, Neuper C. Mechanical stimulation of the fingertip can induce bursts of beta oscillations in sensorimotor areas. J Clin Neurophysiol. 2001. November;18(6):559–64. [DOI] [PubMed] [Google Scholar]
- 47.Salenius S, Schnitzler A, Salmelin R, Jousmaki V, Hari R. Modulation of human cortical rolandic rhythms during natural sensorimotor tasks. Neuroimage. 1997. April;5(3):221–8. [DOI] [PubMed] [Google Scholar]
- 48.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999. November;110(11):1842–57. [DOI] [PubMed] [Google Scholar]
- 49.Gourab K, Schmit BD. Changes in movement-related beta-band EEG signals in human spinal cord injury. Clin Neurophysiol. 2010. December;121(12):2017–23. [DOI] [PubMed] [Google Scholar]
- 50.Vallbo AB, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol. 1984;3(1):3–14. [PubMed] [Google Scholar]
- 51.Nierhaus T, Forschack N, Piper SK, et al. Imperceptible somatosensory stimulation alters sensorimotor background rhythm and connectivity. J Neurosci. 2015. April 15;35(15):5917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Moraes Silva J, Lima FP, de Paula Junior AR, et al. Assessing vibratory stimulation-induced cortical activity during a motor task--A randomized clinical study. Neurosci Lett. 2015. November 3;608:64–70. [DOI] [PubMed] [Google Scholar]
- 53.Schreckenberger M, Lange-Asschenfeldt C, Lochmann M, et al. The thalamus as the generator and modulator of EEG alpha rhythm: a combined PET/EEG study with lorazepam challenge in humans. Neuroimage. 2004. June;22(2):637–44. [DOI] [PubMed] [Google Scholar]
- 54.Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms' strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum Brain Mapp. 2009. April;30(4):1168–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson KL, Ding M. Attentional modulation of the somatosensory mu rhythm. Neuroscience. 2011. April 28;180:165–80. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Ding M. Detection of a weak somatosensory stimulus: role of the prestimulus mu rhythm and its top-down modulation. J Cogn Neurosci. 2010. February;22(2):307–22. [DOI] [PubMed] [Google Scholar]
- 58.Auksztulewicz R, Blankenburg F. Subjective rating of weak tactile stimuli is parametrically encoded in event-related potentials. J Neurosci. 2013. July 17;33(29):11878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001. December;14(6):1444–53. [DOI] [PubMed] [Google Scholar]
- 60.Jenner JR, Stephens JA. Cutaneous reflex responses and their central nervous pathways studied in man. J Physiol. 1982. December;333:405–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen R, Ashby P. Reflex responses in upper limb muscles to cutaneous stimuli. Can J Neurol Sci. 1993. November;20(4):271–8. [DOI] [PubMed] [Google Scholar]
- 62.Corden DM, Lippold OC, Buchanan K, Norrington C. Long-latency component of the stretch reflex in human muscle is not mediated by intramuscular stretch receptors. J Neurophysiol. 2000. July;84(1):184–8. [DOI] [PubMed] [Google Scholar]
- 63.Ridding MC, Rothwell JC. Afferent input and cortical organisation: a study with magnetic stimulation. Exp Brain Res. 1999. June;126(4):536–44. [DOI] [PubMed] [Google Scholar]
- 64.Ridding MC, Pearce SL, Flavel SC. Modulation of intracortical excitability in human hand motor areas. The effect of cutaneous stimulation and its topographical arrangement. Exp Brain Res. 2005. June;163(3):335–43. [DOI] [PubMed] [Google Scholar]
- 65.Claus D, Mills KR, Murray NM. Facilitation of muscle responses to magnetic brain stimulation by mechanical stimuli in man. Exp Brain Res. 1988;71(2):273–8. [DOI] [PubMed] [Google Scholar]
- 66.Claus D, Mills KR, Murray NM. The influence of vibration on the excitability of alpha motoneurones. Electroencephalogr Clin Neurophysiol. 1988. May;69(5):431–6. [DOI] [PubMed] [Google Scholar]
- 67.Kitajo K, Doesburg S, Yamanaka K, Nozaki D, Ward L, Yamamoto Y. Noise-induced large-scale phase synchronization of human-brain activity associated with behavioural stochastic resonance. EPL (Europhysics Letters). 2007;80(4):40009. [Google Scholar]
- 68.Mendez-Balbuena I, Huidobro N, Silva M, et al. Effect of mechanical tactile noise on amplitude of visual evoked potentials: multisensory stochastic resonance. J Neurophysiol. 2015. October;114(4):2132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lugo E, Doti R, Faubert J. Ubiquitous crossmodal Stochastic Resonance in humans: auditory noise facilitates tactile, visual and proprioceptive sensations. PLoS One. 2008. August 06;3(8):e2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007. January;53(1):63–88. [DOI] [PubMed] [Google Scholar]
- 71.Hummel F, Andres F, Altenmuller E, Dichgans J, Gerloff C. Inhibitory control of acquired motor programmes in the human brain. Brain. 2002. February;125(Pt 2):404–20. [DOI] [PubMed] [Google Scholar]
- 72.Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991. February 22;251(4996):944–7. [DOI] [PubMed] [Google Scholar]
- 73.Bachtiar V, Stagg CJ. The role of inhibition in human motor cortical plasticity. Neuroscience. 2014. October 10;278:93–104. [DOI] [PubMed] [Google Scholar]
- 74.Huynh W, Vucic S, Krishnan AV, Lin CS-Y, Kiernan MC. 8. Evolution and recovery of motor function post-stroke. Clinical Neurophysiology. 2014;125(4):e3–e4. [Google Scholar]
- 75.Liuzzi G, Horniss V, Lechner P, et al. Development of movement-related intracortical inhibition in acute to chronic subcortical stroke. Neurology. 2014. January 21;82(3):198–205. [DOI] [PubMed] [Google Scholar]
- 76.Coxon JP, Peat NM, Byblow WD. Primary motor cortex disinhibition during motor skill learning. J Neurophysiol. 2014. July 01;112(1):156–64. [DOI] [PubMed] [Google Scholar]
- 77.Berghuis KM, Veldman MP, Solnik S, Koch G, Zijdewind I, Hortobagyi T. Neuronal mechanisms of motor learning and motor memory consolidation in healthy old adults. Age. 2015. June;37(3):9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhuang P, Toro C, Grafman J, Manganotti P, Leocani L, Hallett M. Event-related desynchronization (ERD) in the alpha frequency during development of implicit and explicit learning. Electroencephalogr Clin Neurophysiol. 1997. April;102(4):374–81. [DOI] [PubMed] [Google Scholar]