Abstract
Importance:
Although the most recent American Joint Committee on cancer staging guidelines for ocular surface squamous neoplasia place a heightened emphasis on biopsy and histopathologic analysis, the interpretation and clinical relevance of these staging criteria are not always clear. We address limitations of using histopathologic analysis to predict clinical outcomes and suggest less-invasive assessments.
Background:
To investigate the impact of histopathologic depth of invasion on outcomes for tumours with the common presentation of multiple structure involvement.
Design:
Retrospective chart review at tertiary institution.
Samples:
Of 41 eyes with ocular surface squamous neoplasia between 2012 and 2017, 27 tumours involving multiple ocular structures clinically were included.
Methods:
Biopsied tumours were determined to be invasive beyond the basement membrane or non-invasive; non-biopsied tumours were clinically identified with unknown depth of invasion. Outcomes were compared using Fisher’s exact or Student’s t tests.
Main Outcome Measures:
Proportion of tumours cured, recurred and/or persisting.
Results:
Twelve tumours (44%) received primary excisional biopsy, 10 (37%) received chemotherapy without biopsy and 5 (19%) received chemotherapy and biopsy. Clinical diagnosis was correct in all biopsied cases. While there were no significant differences in outcomes between invasive vs non-invasive tumours or treatments, there was a trend toward larger basal diameter in recurrent tumours regardless of treatment.
Conclusions and Relevance:
When ocular surface squamous neoplasia tumours with similar clinical involvement were compared, histopathologic depth of invasion was not predictive of clinical outcomes. Future staging criteria may consider the potential of largest basal dimension for more accurate prognostication.
Keywords: biopsy, chemotherapy, conjunctiva, neoplasia, staging
1 |. INTRODUCTION
The 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual,1 compared to the 7th edition,2 places a heightened emphasis on biopsy with histopathologic analysis for diagnosis and staging of ocular surface squamous neoplasia (OSSN) (Table 1). Stages T1 and T2, for example, are now both defined by histopathologic invasion of tumour cells beyond the epithelial basement membrane (a characteristic that is only discoverable through biopsy and histologic analysis), whereas stage Tis tumours are defined by a lack of basement membrane invasion. T3 is a particularly vast and common stage of OSSN,3 including tumours of any size that involve adjacent ocular structures (eg, cornea, tarsal conjunctiva, caruncle, eyelid lamellae, etc.); the majority of OSSN tumours fall into this category, demonstrating superficial extension onto the corneal surface or other adjacent structures.4–6 However, the interpretation of current AJCC criteria for stage T3 (tumours with multiple structure involvement) is ambiguous, as the presence or absence of invasion beyond the basement membrane—unlike for prior stages—is not clearly specified. Given this difficulty in applying AJCC criteria to OSSN tumours that involve multiple structures—and given that such tumours are often treated topically without excision in clinical practice—the necessity of biopsy for diagnosis, staging and treatment of these cases is questionable.
TABLE 1.
American Joint Committee on Cancer (AJCC) 7th and 8th edition staging criteria for ocular surface squamous neoplasiaa (8th edition updates to the 7th edition are bolded)
| Tumour (T) category | Tumour criteria (7th edition) | Tumour criteria (8th edition) |
|---|---|---|
| TX | Primary tumour cannot be assessed | Primary tumour cannot be assessed |
| TO | No evidence of primary tumour | No evidence of primary tumour |
| Tis | Carcinoma in situ; includes dysplasia, collectively referred to as conjunctival intraepithelial neoplasia | Carcinoma in situ; includes dysplasia, collectively referred to as conjunctival intraepithelial neoplasia |
| T1 | Tumour ≤5 mm in greatest dimension | Tumour (≤5 mm in greatest dimension) invades through the conjunctival basement membrane without invasion of adjacent structures (listed in T3) |
| T2 | Tumour >5 mm in greatest dimension, without invasion of adjacent structures (listed in T3) | Tumour (>5 mm in greatest dimension) invades through the conjunctival basement membrane without invasion of adjacent structures (listed in T3) |
| T3 | Tumour invades adjacent structures (excluding the orbit), including cornea, intraocular structures, forniceal conjunctiva, palpebral conjunctiva, tarsal conjunctiva, lacrimal punctum, canaliculi, plica, caruncle, anterior or posterior eyelid lamella, and/or eyelid margin | Tumour invades adjacent structures (excluding the orbit), including cornea, intraocular structures, forniceal conjunctiva, palpebral conjunctiva, tarsal conjunctiva, lacrimal punctum, canaliculi, plica, caruncle, anterior or posterior eyelid lamella, and/or eyelid margin |
| T4 | Tumour invades the orbit with or without further extension | Tumour invades the orbit with or without further extension |
Whether or not the absence of a biopsy for histopathologic analysis and staging leads to a difference in patient outcomes is not known. Although excisional biopsy with cryotherapy remains the gold standard for diagnosing and treating OSSN lesions,7 extensive tumours cannot be fully resected and thus clinical appearance and imaging features on anterior segment optical coherence tomography (ASOCT) are often used for clinical instead of pathologic diagnosis in these cases. The subsequent management is an area of active debate among ocular oncologists, with some preferring confirmatory incisional biopsy followed by definitive adjuvant therapy, while others diagnose clinically and then initiate treatment with primary topical chemotherapy.7,8 Although the latter approach involves treatment without confirmatory biopsy, many tumours in ocular oncology—including retinoblastoma and melanoma—are successfully treated based on clinical diagnosis without histopathologic confirmation.9,10 For OSSN, there have been no randomized controlled clinical trials to compare these options, but retrospective comparative studies demonstrate high cure rates with either primary surgery or chemotherapy alone.4,11,12
Although several retrospective case series have used 7th edition AJCC clinical staging criteria2 to classify OSSN lesions based on clinical presentation alone—without histopathologic examination5,6—no studies to our knowledge have yet examined the clinical relevance of the AJCC 8th edition for this disease, especially as it relates to tumours with multiple structure involvement. Thus, we examined the influence of histopathologic depth of invasion on tumour outcomes for OSSN with multiple structure involvement to better evaluate the clinical role of biopsy and staging for OSSN.
2 |. METHODS
2.1 |. Study design
This retrospective case series was approved by the University of Southern California Institutional Review Board, and the methods were compliant with the Health Insurance Portability and Accountability Act and the Declaration of Helsinki. In order to investigate invasive and non-invasive tumours with the common presentation of multiple structure involvement, all patients at the USC Roski Eye Institute between January 2012 and June 2017 with OSSN tumours demonstrating involvement of bulbar conjunctiva and adjacent ocular surface structures (eg, cornea, fornices, tarsus, lacrimal punctum, canaliculi, plica, caruncle, eyelid lamellae, eyelid margins and/or intraocular tissue) were included in the review. Patients initially received either surgical or topical treatment for their ocular surface disease, followed by subsequent therapies for persistent or recurrent disease.
2.2 |. Clinical tumour characteristics
All OSSN patient records were reviewed for tumour characteristics such as size and involvement of adjacent ocular structures based on clinical descriptions, slit lamp photographs and RTVue (Optovue Inc., Fremont, California) or Spectralis (Heidelberg Engineering Inc., Franklin, Massachusetts) AS-OCT images. In order to achieve standardized size measurements, tumour size was determined from slit lamp photographs and recorded as a ratio of the largest basal tumour diameter to the corneal diameter. Clinical imaging was considered in order to investigate whether tumour features on AS-OCT correlate with histopathology, thus facilitating non-invasive pathologic diagnosis.
2.2.1 |. Tumour treatment and histopathologic analysis
Treatment (as recorded in patient charts) consisted of topical chemotherapy, excisional biopsy, or both depending on the clinical course of each case. Chemotherapeutic regimens included 5-fluorouracil (5-FU) drops (1%, 4 times daily given 2 weeks on — 2 weeks off × 2 courses), mitomycin C (MMC) drops (0.02%, 4 times daily given 2 weeks on—2 weeks off × 2 courses), interferon-a2b (IFN-a2b) drops (1 million IU/mL, 4 times daily given for 4–6 months continuously), and/or IFN-a2b subconjunctival injections (3 million IU in 0.5 mL with an average of 3–6 injections given weekly), with varying lengths of treatment depending on response of the tumour to chemotherapy. For lesions requiring surgical treatment, excisional biopsy was performed using a no-touch technique with double freeze-thaw cryotherapy along conjunctival margins.13 Absolute alcohol was applied to the corneal surface in cases of corneal epithelial involvement, and amniotic membrane grafts were used to achieve closure of wide excisions when necessary. The presence or absence of invasion beyond the basement membrane was determined for every biopsied lesion based on microscopic examination of formalin-fixed samples.
2.3 |. Tumour outcomes
Main outcomes following treatment were recorded for each tumour, including cure, recurrence and/or persistence. Tumours that demonstrated clinical resolution following treatment and remained completely clinically resolved for the duration of recorded follow up were considered cured. A recurrence was denoted for any tumour that demonstrated complete clinical resolution following treatment, with a subsequent clinical reappearance of the tumour on follow up. Tumours that remained clinically evident following initiation of treatment through to final recorded follow up were considered persistent.
2.4 |. Statistical analysis
Statistical analyses were performed using XLSTAT version 19.4 software (Addinsoft, New York, New York), and P-values less than 0.05 were considered statistically significant. Categorical variables were compared using the Fisher’s exact test, and quantitative variables were compared using the Student’s t test.
3 |. RESULTS
3.1 |. Tumour treatment and histopathologic analysis
Out of 41 eyes of 40 patients managed for OSSN during the study period, 27 tumours in 20 males (74%) and 7 females (26%) demonstrated multiple ocular structure involvement, including cornea, plica, caruncle, forniceal conjunctiva, eyelid, eyelid margin, lacrimal punctum and/or tarsal conjunctiva (Figure 1). Table 2 delineates the initial treatments provided for the 27 cases, as well as treatments for recurrent tumours and tumour invasiveness if applicable. Sixty-three percent (n = 17) underwent either incisional or excisional biopsy sometime during management. Although all 17 biopsied tumours demonstrated clinical involvement of adjacent ocular structures, only 65% of the biopsied lesions (n = 11) had clear histopathologic invasion beyond the basement membrane whereas 35% (n = 6) demonstrated an intact basement membrane without identifiable invasion. No tumour in this series demonstrated full thickness stromal or scleral invasion.
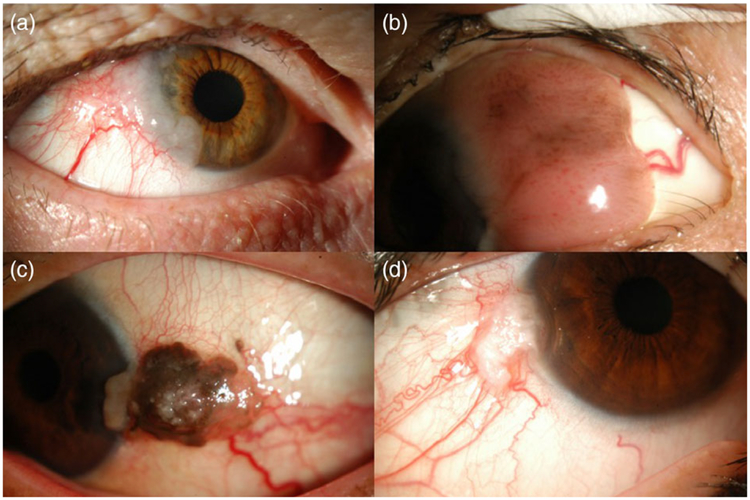
FIGURE 1.
Slit lamp photographs demonstrating a variety of clinical presentations of ocular surface squamous neoplasia. (a) Extensive gelatinous lesion on the temporal conjunctiva and extending onto the cornea from 7 to 11 o’clock, with large feeder vessels noted inferotemporally. (b) Large papillary mass on the temporal bulbar conjunctiva and extending onto the cornea. (c) Pigmented lesion involving the left temporal bulbar conjunctiva, with feeder vessels and extension onto the cornea. (d) Focal gelatinous white mass at the temporal limbus, with extension onto the cornea and with sentinel-type vessels
TABLE 2.
Management and histopathologic analysis of tumours involving multiple ocular surface structures
| Primary chemotherapy w/o biopsy | Primary chemotherapy with biopsy | Primary excisional biopsy | |
|---|---|---|---|
| Total, n | 10 | 5 | 12 |
| Cured, n (%)a | 10 (100%) | 3 (60%) | 11 (92%) |
| Recurred, n (%)a | 1 (10%) | 1 (20%) | 1 (8%) |
| Secondary excisional biopsy, n (%)b | 0 (0%) | 1 (100%) | 1 (100%) |
| Secondary chemotherapy, n (%)b | 1 (100%) | 0 (0%) | 0 (0%) |
| Time to recurrence (mo) | 5 | 18 | 8 |
| Persisting, n (%)a | 0 (0%) | 2 (40%) | 1 (8%) |
| Not invasive beyond basement membrane, n (%)a | n/a | 2 (40%) | 4 (33%) |
| Invasive beyond basement membrane, n (%)a | n/a | 3 (60%) | 8 (67%) |
Percentage of total tumours in column.
Percentage of recurred tumours in column.
3.2 |. Clinical diagnosis and imaging
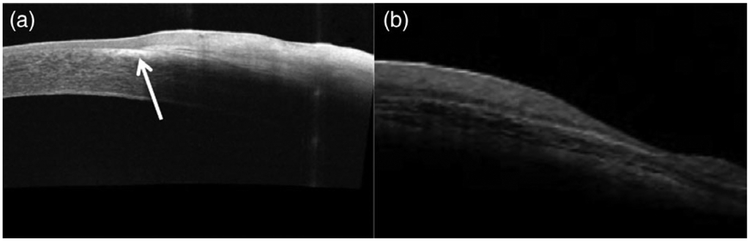
Regardless of histopathologic depth of invasion, no tumour with a clinical diagnosis of OSSN was subsequently found to have a different diagnosis on histopathologic evaluation. However, clinical diagnosis alone was unable to distinguish between invasive and non-invasive disease, as even eyes with very similar levels of ocular surface involvement and appearance were not clinically predictive of the presence or absence of basement membrane penetration (Figure 2). Similarly, clinical imaging with AS-OCT, while consistently useful for broad clinical diagnosis of OSSN, was unable to reliably distinguish between invasive and non-invasive lesions. Out of 12 tumours (six invasive, two non-invasive, four unknown depth of invasion) imaged with AS-OCT prior to treatment, all demonstrated thickening and hyper-reflectivity of the involved epithelial layer, as well as an abrupt transition between affected epithelium and normal epithelium as has been described classically for OSSN (Figure 3).14 However, although minor differences on ASOCT were occasionally appreciated between invasive and non-invasive tumours (Figure 4), these differences were neither consistent nor explicit, suggesting that clinical diagnosis and AS-OCT alone may not be reliably used to differentiate between histologic invasiveness or stages of OSSN (Figure 5).
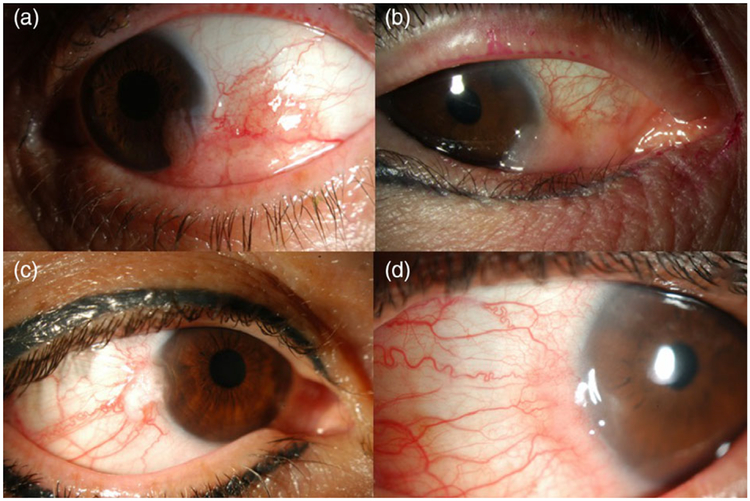
FIGURE 2.
Slit lamp photographs demonstrating that similar size and clinical involvement does not predict depth of invasion histopathologically. (a) Salmon-coloured inferotemporal vascularised mass with extension onto the cornea, subsequently found to be invasive ocular surface squamous neoplasia (OSSN) on biopsy. (b) Salmon-coloured inferonasal vascularised mass with extension onto the cornea, subsequently found to be non-invasive OSSN on biopsy. (c) Gelatinous nodular mass at the temporal limbus with extension onto the cornea and sentinel-type vessels, subsequently found to be invasive OSSN on biopsy. (d) Gelatinous nodular mass at the nasal limbus with extension onto the cornea and surrounding tortuous vessels, subsequently found to be noninvasive OSSN on biopsy
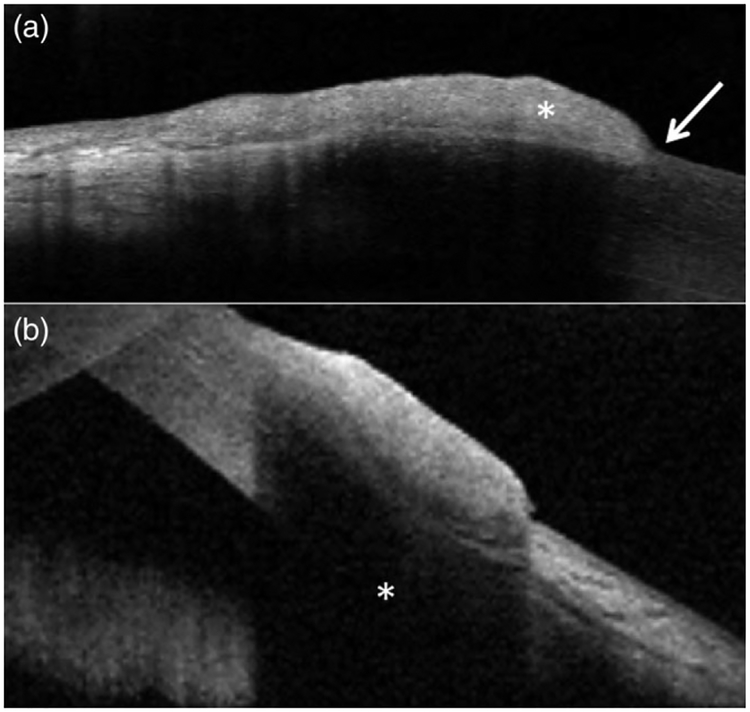
FIGURE 3.
Anterior segment optical coherence tomography (AS-OCT) images of ocular surface squamous neoplasia (OSSN). (a) AS-OCT image of a lesion demonstrating characteristic OSSN features, including a thickened, hyper-reflective epithelial layer (asterisk) and an abrupt transition between abnormal and normal epithelium (arrow); subsequently found to be invasive OSSN on biopsy. (b) AS-OCT image of a nodular OSSN lesion demonstrating substantial sub-epithelial shadowing (asterisk) underlying thickened, reflective epithelium; subsequently found to be non-invasive OSSN on biopsy. The degree of invasion beneath the epithelium in (a) and (b) could not be determined from AS-OCT images alone
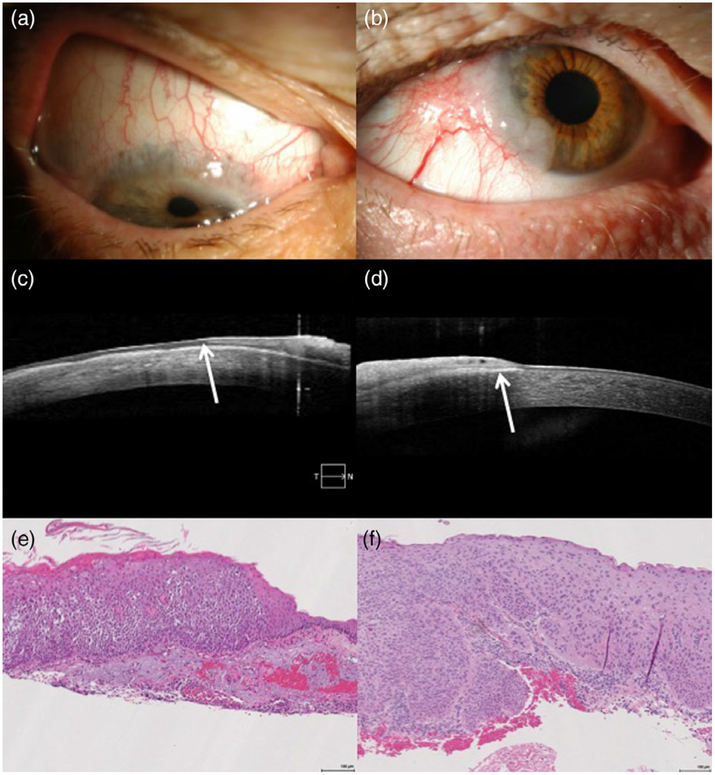
FIGURE 4.
Clinical, imaging and histopathologic appearances of non-invasive and invasive ocular surface squamous neoplasia (OSSN). (a) Slit lamp photograph of a superior gelatinous lesion with vascularity and extension onto the cornea. (b) Slit lamp photograph of a temporal gelatinous lesion with large inferotemporal vessels and extension onto the cornea. (c) Anterior segment optical coherence tomography (AS-OCT) image of the lesion from (a), demonstrating epithelial thickening and hyper-reflectivity consistent with OSSN as well as a lack of hyper-reflectivity at the epithelial base (arrow), possibly suggestive of more superficial epithelial disease. (d) AS-OCT image of the lesion from (b), demonstrating epithelial thickening and hyper-reflectivity as well as prominent sub-epithelial hyper-reflectivity (arrow), possibly suggestive of deeper invasion. (e) Histologic section of the lesion from (a) and (c), demonstrating moderate to focal severe epithelial dysplasia consistent with non-invasive OSSN. (f) Histologic section of the lesion from (b) and (d), demonstrating superficially invasive squamous cell carcinoma consistent with invasive OSSN
FIGURE 5.
Anterior segment optical coherence tomography (AS-OCT) images of non-invasive and invasive ocular surface squamous neoplasia (OSSN). (a) AS-OCT image demonstrating epithelial thickening and hyper-reflectivity, as well as sub-epithelial hyper-reflectivity (arrow) possibly suggestive of deeper invasion; however, the tumour was subsequently found to be non-invasive OSSN on biopsy. (b) AS-OCT image demonstrating epithelial thickening and only mild hyper-reflectivity, subsequently found to be invasive OSSN on biopsy. No definitive features were seen on AS-OCT that could predict invasive disease
3.2.1 |. Comparison of tumours with and without histopathologic invasion beyond the basement membrane
There were no significant differences in size, treatment, or outcomes when comparing invasive and non-invasive tumours (Table 3), although invasive tumours were smaller on average (mean = 0.855, SD = 0.349) than non-invasive tumours (mean = 1.172, SD = 0.463; P = 0.181). There was also no significant difference in proportion recurred and/or persisting, proportion cured, or time to cure when invasive and non-invasive tumours that received the same initial treatment were compared (ie, excised invasive tumours vs excised non-invasive tumours; medically treated invasive tumours vs medically treated non-invasive tumours; all P > 0.05).
TABLE 3.
Tumour treatments, size, and outcomes relative to histopathologic analysis
| Variable | Total cases, n (%) | Unknown depth of invasion, n (%) |
Invasive beyond basement membrane, n (%) | Not invasive beyond basement membrane, n (%) | P-valuea |
|---|---|---|---|---|---|
| Initial management | |||||
| Excisional biopsy | 12 (44%) | 0 (0%) | 8 (73%) | 4 (67%) | 1 |
| Chemotherapy | 15 (56%) | 10 (100%) | 3 (27%) | 2 (33%) | |
| Tumour sizeb | |||||
| Less than average (<0.988) | 16 (59%) | 5 (50%) | 9 (82%) | 2 (33%) | 0.109 |
| Greater than average (>0.988) | 11 (41%) | 5 (50%) | 2(18%) | 4 (67%) | |
| Recurred and/or persisting | |||||
| Yes | 6 (22%) | 1 (10%) | 3 (27%) | 2 (33%) | 1 |
| No | 21 (78%) | 9 (90%) | 8 (73%) | 4 (67%) | |
| Cured | |||||
| Yes | 24 (89%) | 10 (100%) | 9 (82%) | 5 (83%) | 1 |
| No | 3 (11%) | 0 (0%) | 2(18%) | 1 (17%) | |
P-values for comparisons of invasive vs non-invasive tumours (Fisher’s exact test).
Tumour size calculated as a ratio of the largest basal tumour diameter to corneal diameter.
3.3 |. Comparison of treatment modalities
Regardless of tumour depth of invasion, a comparison of primary treatment modalities (ie, excisional biopsy vs chemotherapy) demonstrated that patients treated initially with excision were significantly younger (mean = 63.2 years, SD = 10.5 years) than patients treated with chemotherapy (mean = 77.3 years, SD = 11.4 years; P = 0.0026), although there may be an effect of treatment choice on this variable, with patients with more comorbidities choosing to defer surgery. While not significantly different, tumours treated with excision were smaller on average (mean = 0.879, SD = 0.366) than those treated with chemotherapy (mean = 1.075, SD = 0.463; P = 0.232), which is reflective of the surgeon’s decision regarding the ability to fully resect the tumour. Notably, although time to cure was significantly greater for tumours treated with chemotherapy (mean = 6.5 months, SD = 5.2 months) than excised tumours (mean =1.5 months, SD = 1.9 months; P = 0.0056), there were no significant differences in proportion of tumours recurred and/or persisting (P = 0.662) or proportion cured (P =1) when comparing chemotherapy andexcision.
3.4 |. Recurrent and persistent tumours
In total, 22% (n = 6; three invasive, two non-invasive and one unknown depth of invasion) of the 27 tumours recurred and/or persisted over the study period. Cryotherapy +/−absolute alcohol was used intraoperatively for all excised tumours, so positive conjunctival margins were monitored closely and additional therapy was not given based on pathology results alone. Any signs of recurrent or persistent disease, however, were promptly treated with topical chemotherapy or excision. The tumours that recurred and/or persisted were larger on average (mean = 1.279, SD = 0.449) than those that did not (mean = 0.905, SD = 0.392; P = 0.105). The excised tumours that recurred and/or persisted (n = 2; one invasive, one non-invasive) both demonstrated positive surgical margins on histopathologic examination, whereas tumours with negative surgical margins all resolved without recurrence or adjuvant therapy. Tumours treated specifically with chemotherapy that recurred and/or persisted (n = 4; two invasive, one non-invasive, one unknown depth of invasion) and required additional treatment tended to have a larger size (mean = 1.417, SD = 0.461) than tumours that responded successfully to initial chemotherapeutic treatment (mean = 0.903, SD = 0.375), although this difference was not statistically significant (P = 0.069).
4 |. DISCUSSION
Previous studies have investigated the utility of the 7th edition AJCC staging system in predicting clinical outcomes of patients with OSSN. Although Nanji et al found no increased risk of recurrence with respect to AJCC classification,4 others demonstrated significant reduction in recurrence-free survival with higher T categories. Chauhan et al and Yousef and Finger, for example, showed higher T category (T3 or T4; involvement of adjacent structures or the orbit, respectively) to be an important predictor of tumour recurrence.15,16 Galor et al similarly demonstrated an increased risk of recurrence for more extensive lesions (stages T2 and T3; >5 mm in greatest dimension and involving adjacent structures, respectively) than for smaller lesions (stage T1; ≤5 mm in greatest dimension).17 As a result, some institutions have recommended routine tumour staging with histopathologic confirmation as a necessary adjunct in the management of every OSSN patient.15,16
However, although these studies emphasized the predictive value of clinical staging (based on clinical appearance of the tumours, as in the 7th edition of the AJCC clinical staging criteria2), it should be clearly noted that not all patients in these earlier studies underwent excisional (or incisional) biopsy and thus many were staged based on clinical features only—a method that is less compatible with more recent AJCC 8th edition staging. Additionally, in many of these previous studies stage Tis was not even utilized in classifying tumours even though multiple cases demonstrated biopsyproven carcinoma in situ.4,15,17 This disparity raises questions as to how prior findings translate to the more recent AJCC staging definitions—especially given the ambiguity in interpreting current stage T3 criteria—and whether prior recommendations of routine staging should still hold true today.
While in a small cohort, our study demonstrated that noninvasive tumours were clinically larger and more extensively involved on average than invasive tumours. In addition, we found that while AS-OCT is a reliable tool for the broad clinical diagnosis of OSSN, it was unable to consistently differentiate between invasive and non-invasive tumours. These findings emphasize that neither AS-OCT imaging18 nor clinical appearance alone can be used to reliably identify invasion, which is consistent with the AJCC’s current heightened emphasis on biopsy with histopathologic analysis for OSSN staging (particularly for stages Tis, T1 and T2, for which depth of histopathologic invasion is explicitly defined).
The critical question, of course, is whether or not this matters ultimately to patient care and outcomes. While generally considered safe, biopsy is not without its risks. Conjunctival surgery increases the potential for symblepharon formation and limbal stem cell deficiency, which can threaten or reduce vision.4 Biopsy involves a surgery, often with general anaesthesia, with its own subset of risks, and there is an increased cost to the health-care system with surgical intervention (however, it may be covered by insurance while topical chemotherapy may not be, thus decreasing direct patient costs).19 Alternatively, we and others have demonstrated that the diagnosis of OSSN may be accurately and non-invasively determined using clinical appearance and AS-OCT,14 followed by topical or subconjunctival chemotherapy for successful tumour resolution. Because the ultimate goal of management is to maximize treatment efficacy without causing undue harm, it is important to consider the role of confirmatory biopsy in tumour staging and whether an invasive procedure is truly necessary to appropriately manage patients with OSSN.
In our study, we found no significant difference in tumour outcomes when comparing invasive and noninvasive lesions, even when primary treatment modality was accounted for. This is consistent with larger retrospective studies that found no significant difference in recurrence of invasive vs non-invasive OSSN tumours.20,21 Additionally, a recent multicentre retrospective study demonstrated that tumour stage did not reliably predict initial treatment decisions, as both excision and chemotherapy were successfully utilized as treatment modalities for invasive and noninvasive lesions, despite their histopathologic differences.22 Thus, although routine staging and histopathologic analysis of OSSN tumours has been previously recommended in order to guide treatment planning,15,16 our findings suggest that a biopsy of each tumour in order to determine specific histopathologic characteristics may not be necessary in order to make effective management decisions and achieve reasonable treatment response and cure.
Rather than tumour depth of invasion, clinical features such as tumour size seemed more influential in determining both initial treatment modality and post-treatment outcomes. For example, our study demonstrated a trend toward smaller tumours receiving excisional biopsy and more extensive lesions receiving chemotherapy. Previous studies have similarly emphasized the use of medical treatment for large OSSN tumours, as complete resection can be very difficult for extensive lesions with diffuse involvement of ocular structures.23,24 While we, like others,4,11 found no significant difference in outcomes between excisional biopsy vs chemotherapy, tumour extent (regardless of depth of invasion or treatment) seemed to play a role—as recurrent or persistent tumours were larger on average than tumours that did not recur. This trend toward larger size in recurrent tumours agrees with prior studies that have shown a significant relationship between increased recurrence and larger baseline tumour diameter.20,21
Although the recent AJCC 8th edition staging criteria more strongly emphasize biopsy with histopathologic analysis for the evaluation of OSSN, we found that tumour outcomes were similar regardless of depth of invasion or treatment, and that initial tumour size—rather than histopathologic analysis—may be more useful for understanding potential risk of recurrence and the need for more aggressive management and more cautious follow-up. As our study is limited by its non-randomized, retrospective nature and relatively small sample size, future investigations with larger patient populations and randomized trials would be useful to more extensively evaluate the clinical relevance of the AJCC 8th edition in the diagnosis and management of patients with OSSN.
ACKNOWLEDGEMENTS
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. This work was supported by an unrestricted departmental grant from Research to Prevent Blindness that had no role in study design; the collection, analysis and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Funding information
Research to Prevent Blindness
Footnotes
CONFLICTS OF INTEREST
None declared.
REFERENCES
- 1.Amin MB, Edge S, Greene F, et al. , eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017. [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 3.Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39:429–450. [DOI] [PubMed] [Google Scholar]
- 4.Nanji AA, Moon CS, Galor A, Sein J, Oellers P, Karp CL. Surgical versus medical treatment of ocular surface squamous neoplasia: a comparison of recurrences and complications. Ophthalmology. 2014;121:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields CL, Kaliki S, Kim HJ, et al. Interferon for ocular surface squamous neoplasia in 81 cases: outcomes based on the American Joint Committee on cancer classification. Cornea. 2013;32:248–256. [DOI] [PubMed] [Google Scholar]
- 6.Shah SU, Kaliki S, Kim HJ, Lally SE, Shields JA, Shields CL. Topical inter-feron alfa-2b for management of ocular surface squamous neoplasia in 23 cases: outcomes based on American Joint Committee on cancer classification. Arch Ophthalmol. 2012;130:159–164. [DOI] [PubMed] [Google Scholar]
- 7.Othman IS. Ocular surface tumors. Oman J Ophthalmol. 2009;2:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayed-Ahmed IO, Palioura S, Galor A, Karp CL. Diagnosis and medical management of ocular surface squamous neoplasia. Expert Rev Ophthalmol. 2017;12:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkinson H Retinoblastoma: diagnosis and management—the UK perspective. Arch Dis Child. 2015;100:1070–1075. [DOI] [PubMed] [Google Scholar]
- 10.Murphy SF, Mahl CF, Bloom SM. Choroidal malignant melanoma. Optom Clin. 1993;3:63–77. [PubMed] [Google Scholar]
- 11.Sturges A, Butt AL, Lai JE, Chodosh J. Topical interferon or surgical excision for the management of primary ocular surface squamous neoplasia. Ophthalmology. 2008;115:1297–1302.e1. [DOI] [PubMed] [Google Scholar]
- 12.Antonietta Blasi M, Maceroni M, Grazia Sammarco M, Pagliara M. Mitomycin C or interferon as adjuvant therapy to surgery for ocular surface squamous neoplasia: comparative study. Eur J Ophthalmol. 2018;28:204–209. 10.5301/ejo.5001035. [DOI] [PubMed] [Google Scholar]
- 13.Shields JA, Shields CL, De Potter P. Surgical management of conjunctival tumors. The 1994 Lynn B. McMahan lecture. Arch Ophthalmol. 1997;115: 808–815. [DOI] [PubMed] [Google Scholar]
- 14.Shousha MA, Karp CL, Perez VL, et al. Diagnosis and management of conjunctival and corneal intraepithelial neoplasia using ultra high-resolution optical coherence tomography. Ophthalmology. 2011;118:1531–1537. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan S, Sen S, Sharma A, et al. American Joint Committee on cancer staging and clinicopathological high-risk predictors of ocular surface squamous neoplasia: a study from a tertiary eye center in India. Arch Pathol Lab Med. 2014;138:1488–1494. [DOI] [PubMed] [Google Scholar]
- 16.Yousef YA, Finger PT. Squamous carcinoma and dysplasia of the conjunctiva and cornea: an analysis of 101 cases. Ophthalmology. 2012;119: 233–240. [DOI] [PubMed] [Google Scholar]
- 17.Galor A, Karp CL, Oellers P, et al. Predictors of ocular surface squamous neoplasia recurrence after excisional surgery. Ophthalmology. 2012;119: 1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong SS, Vora GK, Gupta PK. Anterior segment imaging in ocular surface squamous neoplasia. J Ophthalmol. 2016;2016:5435092 10.1155/2016/5435092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon CS, Nanji AA, Galor A, McCollister KE, Karp CL. Surgical versus medical treatment of ocular surface squamous neoplasia: a cost comparison. Ophthalmology. 2016;123:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maudgil A, Patel T, Rundle P, Rennie IG, Mudhar HS. Ocular surface squamous neoplasia: analysis of 78 cases from a UK ocular oncology centre. Br J Ophthalmol. 2013;97:1520–1524. [DOI] [PubMed] [Google Scholar]
- 21.Gichuhi S, Macharia E, Kabiru J, et al. Topical fluorouracil after surgery for ocular surface squamous neoplasia in Kenya: a randomised, double-blind, placebo-controlled trial. Lancet Glob Health. 2016;4:e378–e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellerive C, Berry JL, Polski A, Singh AD. Conjunctival squamous neoplasia: staging and initial treatment. Cornea. 2018;37:1287–1291. 10.1097/ICO.0000000000001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudkin AK, Dempster L, Muecke JS. Management of diffuse ocular surface squamous neoplasia: efficacy and complications of topical chemotherapy. Clin Experiment Ophthalmol. 2015;43:20–25. [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, Shields CL, Shah SU, Kaliki S, Lally SE. Giant ocular surface squamous neoplasia managed with interferon alpha-2b as immunotherapy or immunoreduction. Ophthalmology. 2012;119:938–944. [DOI] [PubMed] [Google Scholar]