Abstract
Background:
In the conservative management of retinoblastoma, detection of tumor activity beneath large, calcified tumors presents a challenging aspect of care as local consolidation is limited in this area. Routine imaging modalities, including magnetic resonance imaging, B-scan ultrasound, and optical coherence tomography, are also limited in providing appropriate surveillance for recurrent disease.
Materials and Methods:
Medical records were reviewed to evaluate patients’ demographic data, ophthalmic exams, imaging studies, and histopathologic reports.
Results:
Three patients (two females and one male) were diagnosed with retinoblastoma (two bilateral and one unilateral) and managed with intravenous chemotherapy and local consolidation. In all three cases, the initial tumors regressed to form large, predominantly calcified tumors. However, it was observed that there continued to be nodular recurrences on the surface of the calcium without visible clinical activity at the base of the calcified lesion. All three cases ultimately required enucleation for these active nodular recurrences and massive choroidal invasion was noted under the calcified tumor. Ophthalmic exams and imaging studies did not provide consistent indication of choroidal disease in these cases, and the extensive calcification prevented detection of active disease at the tumor base on fundoscopy.
Conclusions:
Active choroidal disease at the base of large, calcified tumors cannot be ruled out with ophthalmologic examination and noninvasive imaging; suspicion of disease activity at the base should remain high for patients presenting with multiple recurrent nodules over a calcified tumor.
Keywords: Calcification, clinical imaging, high-risk histopathology, retinoblastoma
Introduction
A collective goal in treating retinoblastoma is that no child should experience extraocular relapse after attempted globe salvage (e.g., conservative therapy) for disease limited to the intraocular space. A corollary to this is the need to perform secondary enucleation on eyes with persistent or recurrent disease (not amenable to other consolidative therapies) before the development of high-risk pathologic features. It can be considered a failure of timely enucleation when eyes demonstrate high-risk histopathologic features after attempted salvage. This can be a particularly difficult situation if a child has already been treated with six cycles of systemic chemotherapy (or multiple cycles of intra-arterial chemotherapy) and then requires additional cycles of systemic chemotherapy as adjuvant treatment for high-risk disease. Unfortunately, due to the regression pattern of intraocular retinoblastoma with some tumors displaying significant calcification (Type 1 (1)), a recurrence under the calcium can be hidden from the treating ocular oncologist on routine examination under anesthesia (EUA). In this setting, a high index of suspicion is paramount to prevent choroidal invasion, which has been associated with the development of metastatic disease (2). Herein, we describe a small case series of patients with persistent or recurrent disease under a calcified tumor requiring enucleation who were found to have choroidal invasion. Imaging studies (magnetic resonance imaging [MRI], B-scan ultrasound, and optical coherence tomography (OCT)) were evaluated for signs of choroidal disease. It is hypothesized that the persistent or recurrent disease was not clinically evident due to the over-lying calcium, thus leading to a delay in definitive treatment.
Materials and methods
This report is a retrospective case series of three patients diagnosed with unilateral or bilateral retinoblastoma. They were all diagnosed and treated at Children’s Hospital Los Angeles (CHLA), and patient consent was obtained for participation in the study. Their medical records were reviewed to evaluate their demographic data, ophthalmologic examination findings, imaging studies, and histopathologic reports.
The previously published CHLA chemoreduction protocol (3) includes systemic chemotherapy and local consolidation therapy (diode or argon laser therapy (532 or 810 nm laser) and cryotherapy (freeze–thaw cycle ×2) for larger lesions anterior to the equator). Systemic chemoreduction includes intravenous carboplatin 780 mg/m2 (13 mg/kg for children <36 months) × 2 days, etoposide 150 mg/m2 (5 mg/kg for <36 months) × 2 days, and vincristine 1.5 mg/m2 (0.05 mg/kg for <36 months) × 1 day, for six cycles every 28 days (i.e., CEV). Infants less than 6 months of age at diagnosis receive modified doses of 50% decreases in all agents for the first cycle; subsequent doses are adjusted based on clinical response and toxicity. Vincristine is excluded for patients less than 2 months of age. All patients were clinically evaluated at routine EUA during their treatment plans with documentation of fundus findings. They were all managed with intravenous chemotherapy and local consolidation (laser consolidation, cryotherapy, intravitreal chemotherapy). If available, imaging studies included MRI, B-scan ultrasound, and OCT. Following enucleation, histopathologic reports were evaluated for high-risk pathologic findings, including post-laminar optic nerve invasion, massive choroidal invasion (>3 mm), anterior segment invasion, and scleral invasion.
Results
Case 1
A 13-month-old female with 13q and 16p deletions was diagnosed with bilateral retinoblastoma, Group C in the right eye and Group D in the left (TNM staging cT1b on the right, cT1c on the left). She was treated with 2-drug reduced-dose systemic chemotherapy, a regimen used for children less than 6 months of age (50% carboplatin and etoposide per the previously published protocol (4)) due to their smaller size. Since she had failure to thrive and growth retardation secondary to her multiple genetic anomalies, the reduced-dose regimen was used. Due to poor response of her intraocular disease, the dose was increased to 75% at cycle 3 and 100% at cycle 5. Response to treatment remained poor in both eyes, so systemic chemotherapy was discontinued after the fifth cycle. Control of vitreous seeding was achieved with intravitreal injection of melphalan in the right eye, but the left eye demonstrated a nodule of active disease at the apex of the main tumor with associated vitreous seeding. This nodule was treated with multiple cryotherapy, laser, and intravitreal melphalan injections. Intra-arterial infusion was attempted, but given her small size and anomalous vasculature, it was unsuccessful. Thus, the decision was made to proceed with enucleation. At that time, there was a larger recurrence on the calcified tumor with increased vascularity and seeding at the apex, but multiple new retinal tumors in the inferior quadrant had also developed from vitreous seeding. There did not appear to be disease at the base of the calcified tumor.
Imaging prior to her enucleation included MRI 19 weeks before enucleation as well as B-scan ultrasound 12 weeks prior. Neither study indicated interval progression of disease. Two weeks prior to enucleation, hemorrhage associated with the tumor recurrence was noted on EUA at the apex of the calcified mass, which remained evident on the day of enucleation (Figure 1).
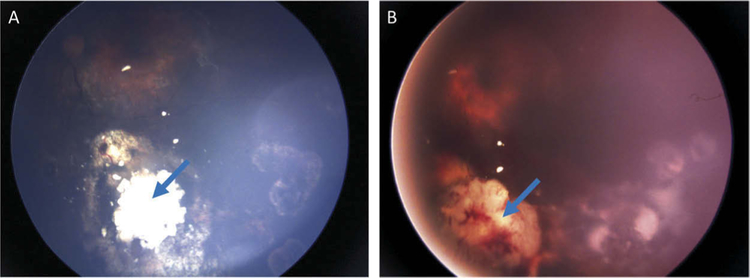
Figure 1.
Case 1: Fundus photos of the left eye. (A) Stable, calcified main tumor (blue arrow) 16 weeks prior to enucleation. (B) Hemorrhage (blue arrow) associated with recurrent tumor in the inferonasal region on the day of enucleation.
The microscopic histopathologic analysis indicated massive choroidal invasion of 6 mm in the largest dimension underlying the calcified tumor. Despite no peripapillary tumors, pre-laminar optic nerve invasion with well-differentiated cells was also noted. The patient was subsequently treated with six cycles of systemic chemotherapy for high-risk pathologic disease. The patient is currently alive without evidence of metastatic disease at 27 months of age (14 months of follow-up from diagnosis).
Case 2
A 13-day-old male with a family history of retinoblastoma and a positive RB1 mutation from amniotic fluid was diagnosed with bilateral retinoblastoma, Group A in the right eye, and Group B in the left (TNM cT1a, cT1b). He initially received three cycles of reduced-dose carboplatin and etopo-side given his age (4). At 5 months of age, he was noted to have a nodular recurrence in the left eye, and thus, was treated with another three cycles of intravenous carboplatin and etoposide in addition to three 30-μg intravitreal melphalan injections for vitreous seeding from the recurrence. There was good response with chemotherapy, cryotherapy, and laser consolidation.
Following treatment of the nodular recurrence and seeding in the left eye, he was actively monitored with EUAs and free of ocular disease for 16 months. A routine MRI screening was concerning for recurrent disease in the left eye described as a slightly increased volume of the posterior globe lesion with associated diffusion restriction and enhancement at the posterior aspect, suspicious for interval growth of known retinoblastoma (Figure 2). An urgent EUA was performed and there was a new large recurrence at the base and apex. Due to the significant tumor recurrence and poor visual potential, enucleation was performed.
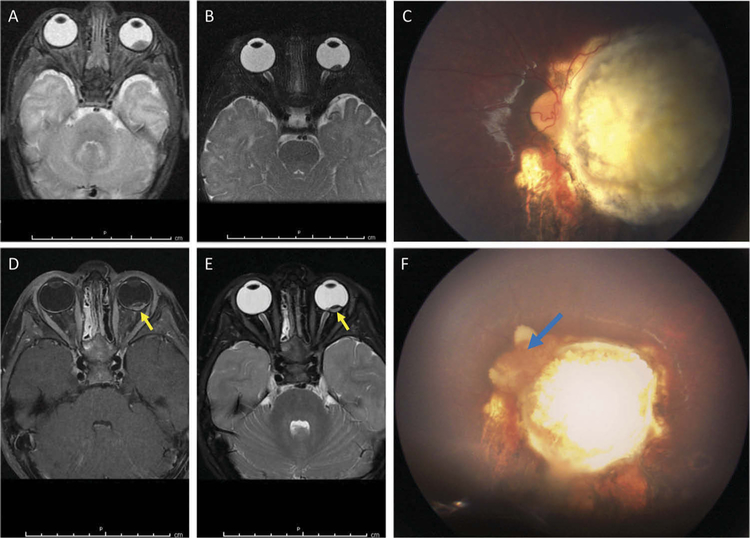
Figure 2.
Case 2: MRI and fundus photos prior to enucleation. (A) T2-weighted MRI image of the left intraocular tumor at diagnosis at 2 weeks of age. (B) T2-weighted image of the stable, calcified tumor at 8 months of age without abnormal enhancement. (C) At 35 months of age, there was a stable calcified tumor on funduscopic exam. (D, E) The T1-weighted (D) and T2-weighted (E) images 1 week prior to enucleation demonstrate increased enhancement (yellow arrows) at the posterior aspect. (F) Fundus image at the time of enucleation shows an active vascularized recurrence (blue arrow) at the nasal aspect of the calcified tumor, corresponding with the area of enhancement on MRI.
On microscopic histopathologic exam, massive choroidal invasion, measuring 5 mm × 0.2 mm, was noted. Moderate anaplasia of tumor cells and pre-laminar optic nerve invasion were also observed. The patient was subsequently treated with six cycles of systemic chemotherapy for high-risk pathologic disease. The patient is currently alive without evidence of metastatic disease at 49 months of age (48 months of follow-up from diagnosis).
Case 3
A 9-month-old female was diagnosed with unilateral Group D retinoblastoma in the right eye (TNM cT2b). She completed six cycles of intravenous carboplatin, etoposide, and vincristine with a good response, significant intra-tumoral calcification, and resolution of the subretinal fluid after the first cycle. However, 3 months after completion of the systemic chemotherapy, she was noted to have new reactive subretinal and vitreous seeding. She received four injections of 20-μg intravitreal melphalan, combined with continued laser consolidation, which controlled the vitreous and subretinal disease.
At 22 months of age, she was noted to have an active tumor recurrence at the inferonasal margin of the calcified tumor, treated with cryotherapy. One month later, she was found to have two new, nodular recurrences on top of the calcified tumor mass, which were too large for laser consolidation. At this point, the only feasible treatment options were intra-arterial chemotherapy and enucleation. Due to the poor visual potential of the eye, enucleation was performed.
Imaging prior to enucleation included B-scan ultrasound 15 weeks prior to enucleation which did not indicate interval progression of disease. An orbital MRI was also performed 3 weeks prior to enucleation; compared to the MRI at diagnosis, mild residual enhancement of the tumor was observed. There was no evidence of extraocular extension or involvement of the right optic nerve (Figure 3).
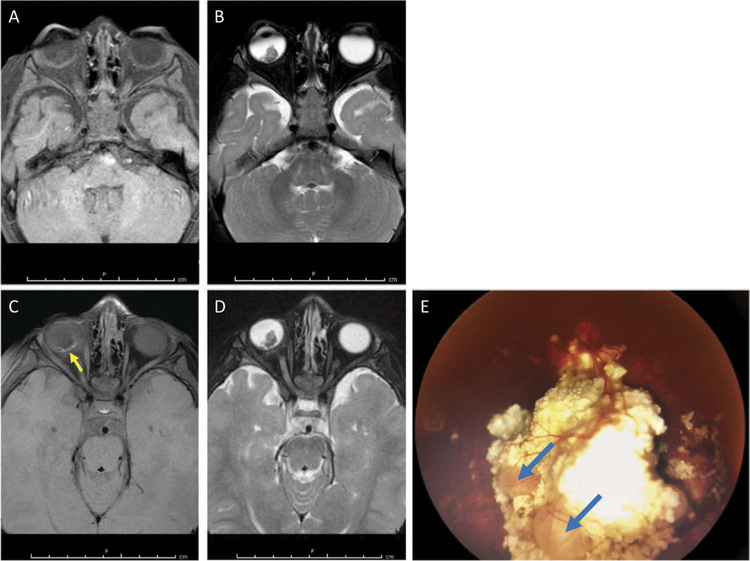
Figure 3.
Case 3: MRI and fundus photos. (A, B) T1-weighted (A) and T2-weighted (B) images at diagnosis demonstrate an intraocular tumor which is hypointense to the vitreous on T2 imaging. (C, D) The T1-weighted (C) and T2-weighted (D) images 3 weeks prior to enucleation demonstrate mild residual enhancement on the T1-weighted image at the posterior aspect of the globe (yellow arrow). (E) Fundus photo at the time of enucleation demonstrates two nodular recurrences on the surface of the large calcified tumor (blue arrows).
On microscopic pathologic analysis, there was massive choroidal invasion of 5 mm in the greatest dimension. The cells were moderately differentiated, and no optic nerve invasion was observed. The patient was subsequently treated with six cycles of systemic chemotherapy for high-risk pathologic disease. The patient is currently alive without evidence of metastatic disease at 30 months of age (21 months of follow-up from diagnosis).
Discussion
In these three cases, massive choroidal invasion, a high-risk histopathologic feature associated with metastatic disease (2), was noted on histopathologic analysis following attempted conservative treatment. In all three cases, extensive calcification was the primary regression pattern of the main tumors, which prevented fundoscopic diagnosis of active choroidal disease at the base of the calcified tumor. Predominant calcification of the main tumor is a common regression pattern for retinoblastoma seen both on imaging (5–7) and histopathologic analysis (8,9). In general, a completely calcified tumor (Type 1 regression (1)) indicates an inactive tumor (1). However, calcification also prevents adequate views of the tumor base for detection of recurrences, both on fundoscopy and ultrasound evaluation. In two cases (#2 and #3), MRI was indicative of possible recurrent tumor activity and aided the clinical decision-making for prompt enucleation. In the first case, MRI did not indicate interval progression, but enucleation was performed due to worsening tumor activity at the apex of the calcium on fundus exam.
Previous studies have evaluated the use of various imaging modalities to assess retinoblastoma tumor activity. A case report of a patient with a tumor containing retinoblastoma and retinocytoma components suggested the differences on MRI correlated with histopathologic analysis to determine the differences between the two tumor types on imaging (10). Another study reported a sensitivity of 75% and specificity of 100% in detecting choroidal invasion at diagnosis prior to local and systemic treatment on MRI (7). Handheld OCT can detect small retinoblastoma tumors and monitor edge recurrences that are difficult to detect on indirect ophthalmoscopy (11–14). While OCT is a useful tool, extensive calcification is a limiting factor in using OCT to detect persistent or recurrent disease underlying calcified tumors, as the calcium causes shadowing artifact.
The common theme in these three cases is that despite a significantly calcified tumor, continued nodular recurrences were noted along the surface of the calcium without tumor activity noted at the base. Often initially, there was regression of these active lesions with local consolidation therapy. However, that too is limited with a recurrence on calcium; diode laser has less penetration given the lack of pigmentation, and cryotherapy may not be safe given either the size of ice-ball formation that would be required and/or the increased risk of retinal tear in the setting of calcium. In all cases, despite early regression, more significant recurrences were noted, in case 2 separated by 16 months, and in all the foci, active disease was underlying the main calcified tumor. Thus, more significant recurrences at the base of a calcified tumor should be considered in the setting of recurrent nodules overlying the calcified tumor mass.
In this case series, we report three cases of recurrent tumors lurking beneath large calcified tumors and associated with massive choroidal invasion. Although imaging in addition to funduscopic exam may elucidate the presence of a tumor recurrence, it remains difficult to assess disease activity at the base of calcified tumors. As such, multiple recurrent (or persistent) tumors at the surface of an otherwise extensively calcified regressed tumor should raise suspicion for tumor activity underneath the calcium. In this clinical setting, the treating ocular oncologist may consider therapy targeted not just at the recurrence but also at the base of the calcified lesion, such as further systemic or intra-arterial chemotherapy, or earlier secondary enucleation to prevent undetected tumor growth and development of high-risk pathologic features.
Funding
This work was supported by Retinoblastoma International, Inc., The Institute for Families, Inc., Children’s Hospital Los Angeles, and an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Shields CL, Mashayekhi A, Cater J, Shelil A, Meadows AT, Shields JA. Chemoreduction for retinoblastoma. Analysis of tumor control and risks for recurrence in 457 tumors. Am J Ophthalmol. 2004;138(3):329–37. doi: 10.1016/j.ajo.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 2.Messmer EP, Heinrich T, Hopping W, De Sutter E, Havers W, Sauerwein W. Risk factors for metastases in patients with retinoblastoma. Ophthalmology. 1991;98(2):136–41. [DOI] [PubMed] [Google Scholar]
- 3.Berry JL, Kogachi K, Aziz HA, McGovern K, Zolfaghari E, Murphree AL, Jubran R, Kim JW. Risk of metastasis and orbital recurrence in advanced retinoblastoma eyes treated with systemic chemoreduction versus primary enucleation. Pediatr Blood Cancer. 2017;64(4). doi: 10.1002/pbc.26270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry JL, Jubran R, Lee TC, Murphree AL, Lee D, Kim JW. Low-dose chemoreduction for infants diagnosed with retinoblastoma before 6 months of age. Ocul Oncol Pathol. 2015;1(2):103–10. doi: 10.1159/000370215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tateishi U, Hasegawa T, Miyakawa K, Sumi M, Moriyama N. CT and MRI features of recurrent tumors and second primary neoplasms in pediatric patients with retinoblastoma. AJR Am J Roentgenol. 2003;181(3):879–84. doi: 10.2214/ajr.181.3.1810879. [DOI] [PubMed] [Google Scholar]
- 6.Char DH, Hedges TR 3rd, Norman D. Retinoblastoma. CT diagnosis. Ophthalmology. 1984;91(11):1347–50. [DOI] [PubMed] [Google Scholar]
- 7.Lemke AJ, Kazi I, Mergner U, Foerster PI, Heimann H, Bechrakis N, Schuler A, von Pilsach MI, Foerster M, Felix R, et al. Retinoblastoma – MR appearance using a surface coil in comparison with histopathological results. Eur Radiol. 2007;17(1):49–60. doi: 10.1007/s00330-006-0197-2. [DOI] [PubMed] [Google Scholar]
- 8.Sang DN, Albert DM. Retinoblastoma: clinical and histopathologic features. Hum Pathol. 1982;13(2):133–47. [DOI] [PubMed] [Google Scholar]
- 9.Lin CC, Tso MO. An electron microscopic study of calcification of retinoblastoma. Am J Ophthalmol. 1983;96(6):765–74. [DOI] [PubMed] [Google Scholar]
- 10.Benhamou E, Borges J, Tso MO. Magnetic resonance imaging in retinoblastoma and retinocytoma: a case report. J Pediatr Ophthalmol Strabismus. 1989;26(6):276–80. [DOI] [PubMed] [Google Scholar]
- 11.Rootman DB, Gonzalez E, Mallipatna A, Vandenhoven C, Hampton L, Dimaras H, Chan HS, Gallie BL, Heon E. Hand-held high-resolution spectral domain optical coherence tomography in retinoblastoma: clinical and morphologic considerations. Br J Ophthalmol. 2013;97(1):59–65. doi: 10.1136/bjophthalmol-2012-302133. [DOI] [PubMed] [Google Scholar]
- 12.Seider MI, Grewal DS, Mruthyunjaya P. Portable optical coherence tomography detection or confirmation of ophthalmoscopically invisible or indeterminate active retinoblastoma. Ophthalmic Surg Lasers Imaging Retina. 2016;47(10):965–68. doi: 10.3928/23258160-20161004-12. [DOI] [PubMed] [Google Scholar]
- 13.Park K, Sioufi K, Shields CL. Clinically invisible retinoblastoma recurrence in an infant. Retin Cases Brief Rep. 2017. doi: 10.1097/ICB.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Montpetit ME, Samara WA, Magrath GN, Shields CL. Detection of minimally visible recurrent retinoblastoma by hand-held spectral-domain optical coherence tomography. J Pediatr Ophthalmol Strabismus. 2017;54:e6–e8. doi: 10.3928/01913913-20170201-03. [DOI] [PubMed] [Google Scholar]