Abstract
Background:
Vulvodynia is a poorly characterized condition with multiple treatment options that have been described as largely ineffective in research settings.
Aim:
To describe treatment patterns in women enrolled in the National Vulvodynia Registry and determine if there is an association between selected treatments and patient-reported outcomes such as pain, sexual function, and psychological distress after 6 months of treatment.
Methods:
Participants completed questionnaires on general medical history and patient-reported outcomes using the short-form McGill Pain Questionnaire, the Female Sexual Function Index, the Short Form-12 quality-of-life questionnaire, the Coping Strategies Questionnaire, and the State-Trait Anxiety Inventory. The evaluation also included pain sensitivity assessment of the vaginal mucosa using a cotton-tipped applicator and the vaginal muscles using a single-digit. In this prospective cohort study, all measurements were collected at baseline and again at 6 months after treatment.
Outcomes:
Type of treatment, number of treatments, self-reported pain intensity, dyspareunia, and pain-related psychological distress measures are reported at baseline and 6 months.
Results:
Of 344 women enrolled, 282 received treatment; 78 different treatments were identified and categorized by type (eg, topical, oral, physical therapy) and number. The most commonly used treatments were topical (85%, n = 241), physical therapy (52%, n = 147), and oral medications (45%, n 128). Notably, 73% of participants received ≥ 2 treatments. There was no association between type or number of treatments and patient characteristics. At 6 months, women reported improvements in general pain (P = .001), pain during intercourse (P = .001), catastrophizing (P = .000), and anxiety (P .000). The Short Form-12 quality-of-life questionnaire showed improvements in physical limitations (P =.024), emotional limitations (P = .003), well-being (P = .025), and social (P = .010). function However, all domains of the Female Sexual Function Index indicated worsening in sexual function (P = .000) except for pain.
Clinical Translation:
Multi-modal treatments were most commonly used in clinical practice and improvements in patient-reported outcomes such as quality of life, distress, and pain were noted; however, participants who returned at 6 months continued to report poor sexual function.
Conclusions:
Strengths include a prospective and long-term study design that evaluated women in clinical settings. Limitations include a high rate of loss to follow-up for certain measures and inability to evaluate efficacy of individual treatments. In a setting where women were receiving highly specialized care, we found wide variation in the type and number of treatments used to treat vulvodynia. Despite this heterogeneity in treatment selection, women reported significant improvements in all study measures except sexual function.
Keywords: Vulvodynia, Painful Intercourse, Dyspareunia, National Vulvodynia Registry, Treatment, Outcomes
Condensation:
For women enrolled in the National Vulvodynia Registry, multi-modal treatment is common. At 6 months after initiating treatment, women with vulvodynia report improvements in pain, physical function, and distress but not in sexual function.
INTRODUCTION
Vulvodynia is a chronic pain disorder that affects nearly 14 million women in the United States.1 Approximately 18% of women have had pain consistent with vulvodynia at some point in their lives.1–3 Vulvodynia is defined as vulvar pain of unknown etiology lasting longer than 3 months.4 According to the International Society for the Study of Vulvovaginal Diseases (ISSVD) vulvodynia can be additionally described by location (generalized or localized to vaginal entrance or clitoris), whether the pain is provoked by contact or unprovoked, onset (primary from first genital contact or secondary if it occurred after a period of pain-free intercourse), and whether the pain is intermittent or persistent.4,5 Research shows that vulvodynia is consistently associated with poor quality of life, poor sexual function, and impaired physical function.2,6,7 In spite of this burden and the negative impact on women’s lives, less than 6% of women with vulvodynia receive an initial appropriate diagnosis and experience pain for many years.3
Research suggests that vulvodynia is a heterogeneous disorder and current diagnostic criteria may not adequately describe the full spectrum of disease.8 In 2003, vulvodynia was categorized by the ISSVD using diagnostic criteria based solely on location, timing, and onset of pain.9 More recent studies indicate that vulvodynia may co-exist with other disorders and should also be characterized based on associated pelvic floor muscle dysfunction, co-morbid pain disorders, and emotional distress.4,10–13 In the 2015 ISSVD criteria, the definition of vulvar pain was updated to include vaginal infections, neoplasms, or neurologic disorders; when pain with this type is identified it is defined as “vulvar pain caused by a specific disorder.”13 On the other hand, vulvodynia is defined as “vulvar pain of at least 3 months” duration, without clear identifiable cause, which may have potential associated factors, and thus “women may have both a specific disorder (eg, lichen sclerosus) and vulvodynia.”13 The challenge of defining vulvodynia and differentiating it from conditions that cause vulvar pain is further complicated by the fact that some inflammatory and neuropathic conditions associated with pain are not easily identified.5 Therefore, variation in disease presentation and the potential for multiple co-existing conditions make vulvodynia difficult to diagnose, and consequently difficult to treat.14,15
A wide range of vulvodynia treatments are available including topical anesthetics (lidocaine), anti-convulsants, tri-cyclic anti-depressants, surgical removal of the painful tissue with vestibulectomy, physical therapy, and cognitive behavioral therapy.5,16 In a 2005 systematic review of outcomes, most vulvodynia treatments were described as having insufficient evidence of efficacy.17 Yet, although not reported in the literature, we suspect that patients and providers often combine and improvise treatments, most with unknown efficacy and safety data.18 In 2016, Goldstein and colleagues16 concluded that there is still insufficient evidence to support the use of topical lidocaine, corticosteroids, or capsaicin for the treatment of localized vestibular pain. Additionally, they reported that the evidence does not support the use of botulinum toxin A, interferon, hormonal treatments, anti-depressants, or anti-convulsants. Stronger evidence was available for psychological interventions, pelvic floor physical therapy, and vestibulectomy (for localized vestibular pain).16 Interdisciplinary treatment was considered useful in the management of vulvodynia, although there was little evidence to support this approach.16 In spite of these recent recommendations, we suspect that the heterogeneity of this disease leads to significant variation in treatment selection. However, after an extensive PubMed search, using combinations of the MeSH terms “vulvodynia treatments” and “treatment patterns AND vulvodynia,” we were not able to identify any prospective studies that empirically describe treatments prescribed for vulvodynia by providers in the United States outside of a 2008 survey of members of the ISSVD.17 This survey showed that over 80% of respondents reported beliefs that anti-depressants, physical therapy, and psychological counseling were effective treatments for vulvodynia; however, the survey did not investigate actual treatments selected in clinical settings.19
Understanding how clinicians manage vulvodynia in tertiary settings will help determine: (1) the types of treatments pre-scribed; (2) the patient characteristics that guide physicians in selecting therapy; and (3) whether clinician practices are aligned with treatment recommendations.15,16 Although there is little published on these 3 factors, we speculate that they are important to study because they may contribute significantly to treatment outcomes. Therefore, our primary research goal was to perform an exploratory analysis of patients enrolled in the National Vulvodynia Registry (NVR) to better understand treatment patterns and factors that guide treatment selection. Our secondary goal was to determine if there was any association between selected treatments and patient-reported outcomes such as pain, sexual function, and psychological distress after 6 months of treatment.
METHODS
This is an analysis of data obtained from the NVR and methods were previously published.8 Briefly, the NVR was a prospective cohort study that enrolled women from 8 geographically different clinical sites in the United States from 2009e2014. The primary objective of the registry was to characterize women with vulvodynia as they progressed from evaluation to treatment by collecting information on self-reported and evoked pain severity, physical function, sexual function, and psychological distress. As part of the registry, women with vulvodynia were managed by gynecology providers considered experts in this condition at these 8 different sites. Although the participants were evaluated using a study protocol to facilitate collection of data, any medications or treatments were prescribed at the discretion of their provider and treatments were recorded over a period of 6 months.8 All treatments prescribed by the providers were recorded at the initial and subsequent visits (baseline, 4–8 weeks, 3 months, and 6 months).
The study protocol was institutional review board approved (no. 2273–7801, 8/3/2009) at all sites and women had to provide informed consent to participate in the registry. Prior to initiating the registry, the primary investigator of the NVR (G.L.) standardized the examination protocol, pain assessment technique, instrument calibration, and data collection with the help of the co-investigators. These methods and selection of the gynecologic experts are extensively described in our previous publication.8 All investigators were trained (via face-to-face, video, or telephone conference) for approximately 2 hours prior to the start of enrollment. Data collection was done by the investigators using paper data abstraction forms. The data were then faxed via a secure line to the NVR data repository at Florida Hospital (Orlando). Data entry was performed by a nosologist and missing or incorrect data were reconciled by contacting the local site investigator and review of medical records.
Approximately 900 women were screened and 344 women were consented and enrolled in the registry after screening positive on Harlow questionnaire, a validated questionnaire assessing the presence of vaginal pain lasting longer than 3 months.1,20 Women and girls were excluded if they were younger than 20 years because the resources needed to address the following issues were unavailable: (1) asking minors to provide consent; (2) answering sensitive questions related to sexual function; and (3) having a parent present during a pelvic examination if the patient is a minor. Women were also excluded if they were unable to provide consent, did not speak English, were unable to fill out the questionnaires, were pregnant, and/or had any other major diseases such as cancer or HIV. Lastly, women were excluded if there was evidence of acute vaginitis, dermatitis, or neoplasia on examination.8
After consent, participants completed a variety of self-reported questionnaires, including demographic information, and general and gynecological medical history. Next, they underwent a vaginal exam that included: (1) a cotton-tipped applicator neurosensory exam of the perineum, vulva, and vestibule; (2) a single-digit exam to assess for pelvic floor muscle pain; and (3) a speculum exam with vaginal swabs, wet preparation, potassium hydroxide, and pH. Amsel criteria (discharge, pH >5, amino odor when potassium hydroxide solution is added to the vaginal secretions, and presence of clue cells on wet preparation) was used to diagnose bacterial vaginosis.21 The vaginal examination was used to rule out causes for pain other than vulvodynia and to clinically confirm the diagnosis. Vaginal mucosa and pelvic muscle evoked pain sensitivity testing have been previously described.8,22 Using the cotton-tipped applicator at 5 sites on the vestibular mucosa (2, 5, 6, 7, and 10 o’clock), the examiner assessed the static pressure pain threshold (SPP), defined as the point at which the sensation of pressure first changed to pain and the patient reported a corresponding pain level using a 0–10 Numeric Rating Pain Scale. The pelvic floor muscles were examined by using a single digit to apply approximately 2 kg of pressure to the perineal body, the levatorani, and the bulboca-vernosus muscles, thus a total of 3 muscle sites were examined on both sides of the pelvis. The clinicians were trained to calibrate their index digit using a pressure algometer (Force Dial Algometer; Wagner Instruments, Greenwich, CT), and apply 2 kg of pressure at each site while reporting the patient’s level on the 0–10 Numeric Rating Pain Scale. This pain threshold was recorded as the SPP for the muscular sites.
Self-reported current pain intensity was measured using the validated short-form McGill Pain Questionnaire (MPQ).23 The short-form MPQ has 2 components; a 0- to 100-mm visual numeric pain intensity scale and the Pain Rating Index. The index is made up of 15 qualitative descriptors of pain measuring 11 sensory (pain severity) questions to create the MPQ-Sensory subscale and 4 affective (emotional experience of pain) qualities to create the MPQ-Affective subscale. Each descriptor is rated on a scale from 0–3 where 0 is no pain in the last 2 weeks, 1 is mild, 2 is moderate, and 3 is severe pain. Maximum scores range from 0–33 for the sensory scale, 0–12 for the affective scale, and 0–45 for the total score with higher numbers indicating higher levels of severity. At each visit, participants completed a Gracely Box Pain Scale (GPS),24,25 which was modified to address sensory and affective components of pain specifically related to intercourse. Sexual function was assessed with the Female Sexual Function Index (FSFI), a validated tool to evaluate multiple dimensions of self-reported sexual function such as desire, arousal, lubrication orgasm, satisfaction, and pain.26 The scores for these 6 domains are summed to obtain a total score where a score below 26.5 is classified as sexual dysfunction and lower scores are indicative of higher dysfunction.
Quality of life was evaluated using the Short Form-12 quality-of-life questionnaire (SF-12) that provides an assessment of mental and physical functioning as well as overall health-related quality of life. The SF-12 generates physical and mental health composite scores that range from 0–100, where 100 indicates the best level of health.27 Additional questionnaires used to measure levels of distress included: the Coping Strategies Questionnaire (CSQ),28 the Beck Depression Inventory (BDI),29 and the State-Trait Anxiety Inventory (STAI).30 The CSQ measures the degree to which catastrophization is used to cope with pain. The catastrophization subscale consists of 6 questions each scored from 0 (never) to 6 (always) with higher scores indicating worse levels of catastrophization. For this subscale the total score may range from 0–36.28 The BDI is a 21-item questionnaire used to evaluate depressive symptoms where scores from 0–9 indicate minimal, 10–18 mild, 19–29 moderate, and 30–63 severe depression.29 The STAI measures state and trait anxiety with higher scores indicating greater levels of anxiety.30
Post hoc, provider prescribed treatments were categorized as topical vulvovaginal therapies, oral medications, psychological therapies, physical therapy, dilators, injections, vaginal suppositories, surgery, and other. All prescribed treatments are described in Table 1. For the purposes of this analysis only baseline treatments and 6-month outcomes are reported.
Table 1.
Prescribed treatments
| Topical vaginal | |
| Hormone | Estradiol |
| Lidocaine | |
| Lidocaine + estradiol | |
| Estradiol + nifedipine | |
| Other | |
| Steroid | Testosterone |
| Methylprednisolone | |
| Betamethasone | |
| Hydrocortisone | |
| Other | |
| Anti-convulsant suppositories | Neurontin/gabapentin |
| Other | |
| Anti-fungal | Fluconazole |
| Ketoconazole | |
| Other | |
| Anti-biotic/anti-bacterial | Clindamycin |
| Other | |
| Oral medication | |
| Tricyclic anti-depressants | Amitriptyline/Elavil |
| Nortriptyline | |
| Desipramine/Norpramin | |
| Other | |
| SNRI | Duloxetine |
| Venlafaxine | |
| Cymbalta | |
| Pristiq | |
| Other | |
| SSRI | Celexa |
| Lexapro | |
| Paxil | |
| Sertraline | |
| Citalopram | |
| Effexor | |
| Other | |
| Anti-convulsants | Neurontin/gabapentin |
| Pregabalin | |
| Lamotrigine | |
| Topamax | |
| Keppra | |
| Lyrica | |
| Other | |
| Hormone | Estrogen |
| Testosterone | |
| OCP | |
| IUD | |
| Other | |
| Muscle relaxant | Flexeril |
| Baclofen | |
| Other | |
| Opioids | Percocet |
| Other | |
| Anti-biotic/anti-bacterial | Flagyl |
| Other | |
| Anti-inflammatory | Motrin |
| Piroxicam | |
| Elmiron | |
| Other | |
| Anti-fungal | Fluconazole/Diflucan |
| Other | |
| Anxiolytics | Klonopin |
| Valium/diazepam | |
| Other | |
| Alternative therapies | |
| Yoga/mindfulness | |
| Meditation | |
| Relationship therapy | |
| Sex therapy | |
| Dietary changes/supplements | |
| Acupuncture | |
| Other | |
| Physical therapy | |
| Physical therapy | Pelvic floor physical therapy |
| Biofeedback physical therapy | |
| Other | |
| Dilators | |
| Dilators | Vaginal dilators |
| Vibrators | |
| Other | |
| Injections | |
| Trigger point | Anesthetic |
| Saline | |
| Steroid | |
| Botox | |
| Interferon | |
| Other | |
| Blocks | Pudendal |
| Caudal | |
| Other | |
| Vaginal suppository insert | |
| Suppository | Neurontin/gabapentin |
| Valium | |
| Estring | |
| Other | |
| Surgery/procedures | |
| Vestibulectomy | With vaginal advancement |
| With modification | |
| Other | |
| Other | Vulvoscopy |
| Perineoplasty | |
| Other | |
| Other/experimental | |
| Neurostimulators | Spinal cord |
| Transcranial | |
| TENS | |
| Sacral neuromodulation | |
| Other | |
| Topical | Cromolyn |
| Nitroglycerin | |
| Capsaicin | |
| Other | |
| Experimental | Leukotriene receptor |
| Antagonist/montelukast | |
| Laser therapy (KTP-Nd:YAG) | |
| Photodynamic therapy | |
| Heparin | |
| Other | |
IUD = intrauterine device; Nd:YAG = neodymium:yttrium-aluminum-garnet; OCP = oral contraceptive pill; SNRI = selective serotonin–norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; TENS = transcutaneous electrical nerve stimulation.
Data were collected in a centralized database specifically created for the NVR. All data were de-identified prior to use in this analysis. Statistics were completed with software (SPSS Statistics for Windows, Version 22, IBM Corp, Armonk, NY). Frequency of treatment type and number of treatments pre-scribed were recorded at the initial evaluation. Evoked pain sensitivity was measured with the 0–10 Numeric Rating Pain Scale to SPP at the vestibule and pelvic floor muscles. A composite score was created based on the average of the SPPs reported for the 5 vestibule sites and a separate composite score was generated for 3 pelvic floor muscle sites.
Descriptive statistics were used to identify missing data and to describe baseline frequencies and percentages for categorical variables and means, SD, medians, and ranges for continuous variables. Paired t tests were used to compare self-reported pain intensity, dyspareunia, and pain-related psychological distress measures at baseline and 6 months. Pearson correlation coefficients were used to evaluate the relationship between the number of treatments and changes in self-reported psychological distress measures. A 2-sided P value ≤.05 and 80% power were used to represent significant differences. For measures in which multiple comparisons were conducted, including the SF-12 and pelvic pain sensitivity testing, we adjusted the P value using a Bonferroni correction based on the number of tests. For example, because 2 pain sensitivity assessments were done at the pelvic region, we divided our original alpha of 0.05 by 2, for an adjusted P value of .025 for these measures. Similar, for the SF-12 with 8 tests conducted, our adjusted P value was .006 (.05/8). Post hoc sample size calculations indicated an adequate sample size for hypothesis testing for all variables studied (MPQ, CSQ, STAI, BDI, GPS, pain evoked, and FSFI) except for the SF-12.
RESULTS
After screening for eligibility, 344 women were consented and enrolled in the NVR from 2009–2014. Of the 344 women, 282 had at least 1 prescribed treatment recorded after initial examination. Demographic characteristics for the study sample are presented in Table 2. No significant differences were found in age (P = .92) and pain duration (P .99) between those who were prescribed at least 1 treatment= at baseline (n 282) compared to those who were not included in the analysis=due to missing treatment data (n = 62).
Table 2.
Demographic characteristics of the study population
| Demographic, n = 282 | Value |
|---|---|
| Age, y, mean (SD) | 34.1 (12.2) |
| Median pain duration, mo (range) | 24.0 (0.33–360) |
| Education, n (%) | |
| GED | 2 (0.7) |
| Complete HS | 7 (2.5) |
| Some college | 42 (14.9) |
| Completed college | 87 (30.9) |
| Post-graduate | 55 (19.5) |
| Missing | 89 (31.6) |
| Marital status, n (%) | |
| Married | 105 (37.2) |
| Single | 50 (17.7) |
| Separated | 2 (0.7) |
| Divorced | 3 (1.1) |
| Stable relationship >2 y | 31 (11.0) |
| Stable relationship <2 y | 15 (5.3) |
| Other | 6 (2.1) |
| Missing | 69 (24.5) |
| Race, n (%) | |
| Caucasian | 187 (66.3) |
| African American | 3 (1.1) |
| Native American | 1 (0.4) |
| Asian | 2 (0.7) |
| Latino/Hispanic | 15 (5.3) |
| Other | 6 (2.1) |
| Missing | 68 (24.1) |
| Income, n (%) | |
| Less than $20,000 | 13 (4.6) |
| $20,000–50,000 | 48 (17.0) |
| $50,000–100,000 | 62 (22.0) |
| More than $100,000 | 65 (23.0) |
| Missing | 94 (33.3) |
| Employment, n (%) | |
| Unemployed | 17 (6.0) |
| Employed | 136 (48.2) |
| Self-employed | 17 (6.0) |
| Other | 29 (10.3) |
| Missing | 83 (29.4) |
GED = General Equivalency Diploma; HS = high school.
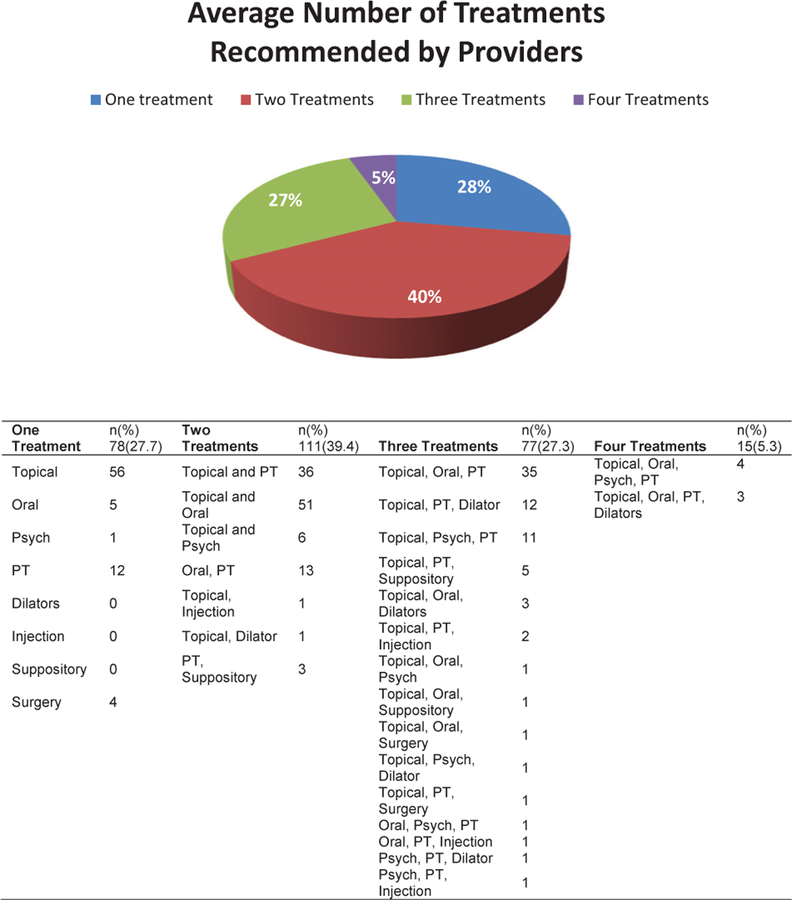
Descriptive baseline analysis for the cohort of 282 showed that at the first visit, providers recommended 78 different treatments. These treatments were categorized into 9 groups and 25 subgroups that are presented in Table 3; 241 patients were prescribed topical therapy (85%) and for 229 patients (95%) the topical contained lidocaine. Physical therapy was recommended for 147 patients (52%) and oral medications were prescribed for 128 patients (45%). The most frequent oral medications recommended were tricyclic anti-depressants. Alternative therapies, which included yoga, mindfulness, and sexual and couple therapy, was recommended in 33 patients (12%) and 4 (1%) received a recommendation for vestibulectomy. For 203 (72%) patients, providers recommended more than 1 treatment; number and combinations of treatments are described in Figure 1. Most often providers selected combinations of 2 (n 111, 39.4%) treatments that included topical and physical therapy, = or topical and oral therapy.
Table 3.
Frequency of treatments recommended by providers*
| Treatment type,* n (%) | Total N = 282 |
|---|---|
| Topical | 241 (85.5) |
| Topical 5% lidocaine (± estradiol) | 229 |
| Topical steroid | 52 |
| Topical anti-convulsants (eg, gabapentin, amitriptyline) | 49 |
| Topical anti-fungal | 4 |
| Topical anti-bacterial | 5 |
| Other topical | 5 |
| Oral | 128 (45.4) |
| Tricyclic anti-depressant | 54 |
| SNRI | 4 |
| SSRI | 8 |
| Anti-convulsant | 22 |
| Hormone | 19 |
| Muscle relaxant | 24 |
| Opioids | 1 |
| Anti-biotic | 3 |
| Anti-inflammatory | 4 |
| Anti-fungal | 8 |
| Other | 1 |
| Alternative therapies | 34 (12.1) |
| Yoga, mindfulness, mediation | 29 |
| Relationship, sex therapy | 8 |
| Injections | 13 (4.61) |
| Trigger point injections | 3 |
| Anesthetic blocks | 10 |
| Physical therapy | 147 (52.1) |
| Vaginal dilators | 26 (9.2) |
| Vaginal inserts | 11 (3.9) |
| Vestibulectomy | 4 (1.4) |
| Other experimental | 7 (2.5) |
SNRI = selective serotonin–norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor.
Participants may have received treatments in more than 1 category, therefore totals do not add up to 100%.
Figure 1.
Number and combination of treatments prescribed by providers at the first evaluation. PT = physical therapy. Figure 1 is available online at www.jsm.jsexmed.org .
Baseline and 6-month comparisons (and corresponding effect size) in patient-reported levels of pain, distress, quality of life, and sexual function are described in Tables 4–6, respectively. This analysis was limited to participants who were prescribed a treatment and had data at both time points; this cohort included: 275 (98%) patients who completed the STAI, 277 (98%) who competed the BDI, 193 (68%) who completed the FSFI, 82 (29%) who completed the MPQ, and 72 (25%) who completed the SF-12 and the CSQ. Only 37 (13%) reported their pain with intercourse on the GPS-Intercourse related pain scale at baseline and at 6 months, and 21 (7%) had all data (questionnaires and physical exam) at 6 months.
Table 4.
Changes in pain levels and distress baseline to 6 months
| Baseline | 6 mo | |||||
|---|---|---|---|---|---|---|
| Mean score | SD | Mean score | SD | P value* | Effect size (Cohen d) | |
| Measures of patient-reported pain sensitivity | ||||||
| MPQ-Sensory (n = 82) | 10.2 | 6.8 | 7.6 | 5.8 | .001 | 0.41 |
| GPS-Intercourse pain (n = 37) | 62.6 | 23.1 | 35.2 | 26.9 | .000 | 1.09 |
| MPQ-Numeric pain scale (n = 82) | 50.3 | 28.2 | 30.1 | 25.4 | .001 | 0.75 |
| Measures of pain evoked during clinical examination | ||||||
| Vestibule SPP rating* | 3.1 | 1.9 | 2.3 | 1.9 | .046 | 0.42 |
| Vaginal muscle SPP rating* | 2.6 | 2.4 | 1.5 | 1.4 | .007 | 0.56 |
| Measures of distress | ||||||
| MPQ-Affective (n = 82) | 3.0 | 3.6 | 1.9 | 2.6 | .005 | 0.35 |
| CSQ-Catastrophizing (n = 72) | 13.7 | 7.4 | 9.5 | 8.1 | .000 | 0.54 |
| STAI-State (n = 275) | 26.0 | 20.9 | 10.7 | 17.2 | .000 | 0.80 |
| STAI-Trait (n = 274) | 27.7 | 22.2 | 12.4 | 19.7 | .001 | 0.73 |
| BDI (n = 277) | 5.2 | 7.5 | 1.6 | 4.2 | .000 | 0.59 |
BDI = Beck Depression Inventory; CSQ = Coping Strategies Questionnaire; GPS = Gracely Box Pain Scale; MPQ = McGill Pain Questionnaire; SPP = static pressure threshold; STAI = State-Trait Anxiety Inventory.
Cohen d is an effect size to standardize the difference between 2 means, d = 0.2 is considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size. For example, if 2 means do not differ by 0.2 SD or more, the difference is trivial even if statistically different.
SPP composite scores were based on pain ratings using the 0–10 Numerical Pain Rating scale.
Alpha level is 0.05 for all measures except for pain evoked during clinical examination, which was adjusted to 0.025 after Bonferroni correction.
Table 6.
Female Sexual Function Index changes from baseline to 6 months
| Baseline | 6 mo | |||||
|---|---|---|---|---|---|---|
| Mean score | SD | Mean score | SD | P value* | Effect size (Cohen d) | |
| FSFI-Total (n = 193) | 12.4 | 8.4 | 6.4 | 10.1 | .000 | 0.65 |
| Desire | 1.6 | 0.9 | 0.7 | 1.1 | .000 | 0.90 |
| Arousal | 2.4 | 1.9 | 1.2 | 1.9 | .000 | 0.63 |
| Lubrication | 2.5 | 2.2 | 1.2 | 1.9 | .000 | 0.63 |
| Orgasm | 2.6 | 2.3 | 1.2 | 2.1 | .000 | 0.64 |
| Satisfaction | 2.3 | 1.7 | 1.2 | 1.9 | .000 | 0.61 |
| Pain | 1.0 | 1.3 | 0.8 | 1.6 | .174 | 0.14 |
FSFI = Female Sexual Function Index.
Cohen d is an effect size to standardize the difference between 2 means, d = 0.2 is considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size. For example, if 2 means do not differ by 0.2 SD or more, the difference is trivial even if statistically different.
FSFI lower levels indicate higher levels of sexual dysfunction.
Alpha level is 0.008 after Bonferroni correction.
In Table 4 we report significant reduction in the mean MPQ-Sensory score, the mean GPS-Intercourse-related pain rating, the mean MPQ-Numeric pain scale, and in the mean vestibular and muscular evoked pain ratings. Quality-of-life changes are reported in Table 5. At 6 months, participants reported improvement in physical limitations, emotional limitations, well-being, and social function. We found no significant changes in physical function, energy, pain, and health-related quality-of-life measures. For the FSFI shown in Table 6, where lower scores indicate higher levels of sexual dysfunction, we observed that the total FSFI mean score worsened, which reflected lower score in all domains (desire, arousal, lubrication, orgasm, satisfaction) except for pain, which was not statistically significant at 6 months.
Table 5.
Changes in Short Form-12 quality-of-life questionnaire measures from baseline to 6 months
| Baseline | 6 mo | |||||
|---|---|---|---|---|---|---|
| SF-12 Measure (n = 72) | Mean score | SD | Mean score | SD | P value* | Effect size (Cohen d) |
| Physical function | 83.6 | 26.2 | 86.1 | 24.7 | .300 | −0.1 |
| Physical limitations | 66.6 | 44.2 | 77.5 | 39.7 | .024 | −0.26 |
| Emotional limitations | 55.6 | 44.3 | 73.2 | 39.4 | .003 | −0.42 |
| Well-being | 46.8 | 18.2 | 51.3 | 17.1 | .025 | −0.25 |
| Energy | 52.2 | 23.3 | 52.5 | 20.8 | .914 | |
| Social function | 73.6 | 28.2 | 80.8 | 24.4 | .010 | −0.27 |
| Pain | 76.7 | 25.9 | 80.2 | 26.8 | .199 | −0.16 |
| Health | 69.0 | 22.0 | 66.3 | 23.0 | .132 | 0.11 |
SF-12 = Short Form-12 quality-of-life questionnaire.
Cohen d is an effect size to standardize the difference between 2 means, d = 0.2 is considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size. For example, if 2 means do not differ by 0.2 SD or more, the difference is trivial even if statistically different.
Alpha level is 0.006 after Bonferroni correction.
In our correlation matrix analyzing the relationship between baseline and 6-month changes in psychometric or physical exam outcomes and the number of treatments prescribed we did not find any significant associations.
DISCUSSION
In this group of women who were treated by gynecologists in specialty clinics, we confirm that there is marked variation in the types of treatments selected for vulvodynia; for this small cohort providers recommended 78 different treatments. In 73% of patients, 2 or more treatments were recommended simultaneously, again emphasizing that in clinical settings, unlike in research settings, health care providers are often mixing multiple therapies. Although topical treatments combined with physical therapy and oral pharmacotherapy emerged as the most common combination of treatments, even within each treatment category there was significant variation. Several explanations may account for this extreme heterogeneity in therapy selection. Treatment variation may reflect the heterogeneity of the disease itself, in other words, the patients who are being treated in these specialized clinical settings present with various complex symptoms, physical findings, and co-morbidities that necessitate a variety of multi-modal therapies. This explanation is not supported by our findings since we were not able to find any association between the number of treatments prescribed at baseline and patient characteristics such as the severity of pain, location of pain (vestibular vs muscular or generalized vs localized), demographics, or duration of pain. Alternatively, variation in treatment selection may represent discrepancy in provider knowledge about treatment guidelines, or a gap between real-world clinical practice and published recommendations that support the use of physical therapy, cognitive behavioral therapy, and vestibulectomy.16 For example, in our previous publication from the NVR, we demonstrated that 90% of this cohort reported pain on musculoskeletal examination, 41% had a history of anxiety, and 40% reported depression,8 yet this analysis shows that only 52% of the women were referred for physical therapy and 12% were referred for psychologic therapy including sexual and relationship therapy. Lastly, since participants reported being in pain for a median 2 years before entering the registry, it is possible that their experience with previous treatments could influence treatment choice and thus contribute to number and type of treatments prescribed. Unfortunately, the NVR was not specifically designed to identify clinical decision-making pathways, and our patient sample turned out to be too small to adequately examine each treatment individually and confirm why treatments were prescribed. However, our study does provide evidence that further research on treatment selection is warranted, because clinical practice may differ significantly from treatment recommendations supported by evidence-based, scientific publications.
In this study, participants were mostly Caucasian, educated, and employed. Prior research suggests that non-white and Latino women may have a higher risk for the development of vulvodynia1,31,32 yet Latino ethnicity was represented in less than 6% of our sample. This finding may be due to the exclusion of women who did not speak English or due to a potential disparity between women who are reported to have vulvodynia in the community and those who access specialty care. Participants had vulvodynia for a median 2 years before being evaluated by registry specialists, implying that even for women who can access care, they are not able to access it in a timely manner.
On baseline patient-reported questionnaires, women reported moderate-severe levels of general pain (the mean MPQ-Numeric pain scale was 50.3 ± 28.3 mm with a maximum possible score of 100 mm), and intercourse-related pain (the GPS-Intercourse-related pain scale mean was 62.6 mm ± 23.1 mm with a maximum possible score of 100 mm). Not surprisingly, they also described poor sexual function (mean FSFI total score was 12.4 ± 8.4 with scores below 26.5 representing sexual dysfunction) across all domains including desire, arousal, lubrication, orgasm, and satisfaction. However, on clinical examination, average pain reported during vestibular SPP (VAS 3.1, SD 1.9) and muscular SPP (VAS 2.6, SD 2.6) assessments were low, indicating that there is little correspondence between higher levels of pain during intercourse and what providers can replicate with the cotton-tipped applicator test or the single-digit test used during physical examination. The clinical implication of this finding, which is confirmed by other researchers,33 is that self-reported questionnaires may more accurately depict the patient’s pain experience. Despite serving as the gold standard to diagnose vulvodynia, the cotton swab test may underestimate the degree of pain (and distress) generally experienced by these women, leading to erroneous exclusion of patients with low vaginal examination pain scores from clinical care or research.
At baseline, participants demonstrated little impairment of social function; however, they reported physical and emotional limitations, impairment of overall energy, impairment of overall sense of well-being, and sexual dysfunction. 6 Months after initiating treatment, women demonstrated significant improvements, with moderate to large effect sizes, in measures of distress such as catastrophizing and anxiety as well as severity of pain (evoked during clinical exam and on self-reported questionnaires). Similarly, although effect sizes were small, we observed improvements in physical limitations, emotional limitations, and social function. However, the total FSFI score, which measures sexual function, worsened by approximately 50% in every sub-scale (desire, arousal, lubrication, orgasm, and satisfaction), indicating that overall sexual function deteriorated over this period despite improvements in pain.
The most obvious explanation for this finding is that women commonly presented with poor sexual function and yet they were rarely referred for sexual counseling or equivalent psychological therapy. Alternatively, we may be finding improvements in pain without corresponding improvements in sexual function because a healthy sexual response depends on other factors such as libido, arousal, and relationship status.33 Another potential explanation for persistent poor sexual function may be that as daily and general body pain improves, women choose not to resume intercourse because dyspareunia persists despite use of the therapies prescribed. Therefore, women learn to fear and avoid intercourse and this may lead to an overall decrease in their sexual function over time. Unfortunately, we were unable to determine if those women who reported not having intercourse did so because they were avoiding it or because they did not have a partner. This is a previously described limitation of the FSFI34 that will need to be explicitly addressed in future work. Finally, the role of patient education and expectations cannot be underestimated. We suspect that as patients find supportive care, they also learn to accept living with chronic pain, which may decrease distress (eg, anxiety) and improve their overall quality of life but not necessarily improve sexual function.
Our study has several limitations including that the sample of women seen at NVR specialty centers may not necessarily represent women with vulvodynia in the general population. Because we found a much higher than expected number of treatments, we were not able to evaluate the efficacy for individual treatments or combinations of treatments. We were surprised by the extreme variation of treatment selection within this group of expert providers, and we speculate about a discrepancy in provider knowledge, however, it is important to note that our study did not include a formal assessment of provider knowledge about or compliance with published vulvodynia treatment guidelines.15,16 We are also unable to comment on patients’ compliance with treatment(s) because we did not have protocols to assess patient compliance. Additionally, we were unable to determine if those lost to follow-up did not return because they improved, or because they worsened and sought care elsewhere. Although our effect sizes were statistically significant and reassuring, it is important to emphasize some of our findings are based on small sample sizes. For example, many participants had missing vaginal exam data at 6 months. We believe that as patients were improving, providers may not have thought it was necessary to repeat the potentially invasive vaginal examination, despite the study protocol.
CONCLUSION
In summary, the women in our cohort tended to receive multi-modal treatments that did not necessarily correspond to current vulvodynia treatment guidelines, emphasizing the need for additional provider and patient education. Patient-reported outcomes were more pronounced than improvements in the physical exam leading us to strongly recommend the incorporation of validated patient-reported outcome questionnaires into clinical practice and research. We are reassured by the fact that patients reported significant improvements in quality of life, distress, and pain, regardless of the number of treatments pre-scribed at the initial visit. However, this leads us to question whether, as previously reported in the medical literature, vulvo-dynia may sometimes be a condition with periods of remission35 or episodic flares that only periodically require medical attention. We were surprised to find worsening sexual function over time despite improvements in pain, indicating that: (1) providers need to continually screen women for sexual dysfunction in addition to pain and distress; and (2) sexual therapy must be incorporated into treatment regimens when necessary. Lastly, our registry participants demonstrated long-term improvements in multiple measures when using multi-modal therapy. Yet, future research will still need to compare single vs multi-modal therapy, and identify which components of multi-modal therapy are most effective. We also hope that the information we provide in this study will be especially useful to investigators who will conduct future pragmatic trials where the emphasis is on conducting research in environments that replicate clinical settings.
Funding:
The National Vulvodynia Association and the Patty Brisben Foundation for Women’s Sexual Health funded this research. Methodology for the National Vulvodynia Registry was developed with the help of Dr Denniz Zolnoun and funded by NIH, K23 HD053631–01. Dr Alappattu is supported by the Rehabilitation Research Career Development Program (National and Rehabilitation Research and National Institute of Neurological Disorders and Stroke, K12 HD055929). Dr Lamvu was supported by Florida Hospital and the Orlando Veteran Affairs Medical Center. These data were presented in 2016 at the International Pelvic Pain Society in Chicago, and the International Association for the Study of Pain World Congress on Pain in Japan.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
REFERENCES
- 1.Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc 2003; 58:82–88. [PubMed] [Google Scholar]
- 2.Arnold LD, Bachmann GA, Rosen R, et al. Assessment of vulvodynia symptoms in a sample of US women: a prevalence survey with a nested case control study. Am J Obstet Gynecol 2007;196:128.e121–128.e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reed BD, Harlow SD, Sen A, et al. Prevalence and demographic characteristics of vulvodynia in a population-based sample. Am J Obstet Gynecol 2012;206:170.e171–170.e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. J Low Genit Tract Dis 2016;20:126–130. [DOI] [PubMed] [Google Scholar]
- 5.Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Low Genit Tract Dis 2005;9:40–51. [DOI] [PubMed] [Google Scholar]
- 6.Arnold LD, Bachmann GA, Rosen R, et al. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol 2006;107:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamvu G, Barron K. Vulvodynia: a prevalent yet under-diagnosed chronic pain syndrome. Pain Week J 2015;3:7. [Google Scholar]
- 8.Lamvu G, Nguyen RH, Burrows LJ, et al. The evidence-based vulvodynia assessment project. A national registry for the study of vulvodynia. J Reprod Med 2015;60:223–235. [PubMed] [Google Scholar]
- 9.Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: a historical perspective. J Reprod Med 2004;49:772–777. [PubMed] [Google Scholar]
- 10.Nguyen RH, Veasley C, Smolenski D. Latent class analysis of comorbidity patterns among women with generalized and localized vulvodynia: preliminary findings. J Pain Res 2013; 6:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brotto LA, Sadownik LA, Thomson S, et al. A comparison of demographic and psychosexual characteristics of women with primary versus secondary provoked vestibulodynia. Clin J Pain 2014;30:428–435. [DOI] [PubMed] [Google Scholar]
- 12.Reed BD, Plegue MA, Williams DA, et al. Presence of spontaneous pain and comorbid pain conditions identifies vulvodynia subgroups. J Low Genit Tract Dis 2016;20:57–63. [DOI] [PubMed] [Google Scholar]
- 13.Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. Obstet Gynecol 2016;127:745–751. [DOI] [PubMed] [Google Scholar]
- 14.ACOG Committee on Gynecologic Practice. ACOG committee opinion: number 345, October 2006: vulvodynia. Obstet Gynecol 2006;108:1049–1052. [DOI] [PubMed] [Google Scholar]
- 15.Stockdale CK, Lawson HW. 2013 Vulvodynia guideline update. J Low Genit Tract Dis 2014;18:93–100. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein AT, Pukall CF, Brown C, et al. Vulvodynia: assessment and treatment. J Sex Med 2016;13:572–590. [DOI] [PubMed] [Google Scholar]
- 17.Andrews JC. Vulvodynia interventions—systematic review and evidence grading. Obstet Gynecol Surv 2005;66:299–315. [DOI] [PubMed] [Google Scholar]
- 18.Veasley C, Clare D, Clauw DJ, et al. Impact of chronic over-lapping pain conditions on public health and the urgent need for safe and effective treatment. The Chronic Pain Research Alliance. Available at: www.chronicpainresearch.org: The Chronic Pain Research Alliance/TMJ Association LTD 2015: 1–46. [Google Scholar]
- 19.Reed BD, Haefner HK, Edwards L. A survey on diagnosis and treatment of vulvodynia among vulvodynia researchers and members of the International Society for the Study of Vulvo-vaginal Disease. J Reprod Med 2008;53:921–929. [PubMed] [Google Scholar]
- 20.Harlow BL, Vazquez G, MacLehose RF, et al. Self-reported vulvar pain characteristics and their association with clinically confirmed vestibulodynia. J Womens Health (Larchmt) 2009;18:1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14–22. [DOI] [PubMed] [Google Scholar]
- 22.Zolnoun D, Bair E, Essick G, et al. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. J Pain 2012;13:910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grafton KV, Foster NE, Wright CC. Test-retest reliability of the Short-Form McGill Pain Questionnaire: assessment of intra-class correlation coefficients and limits of agreement in patients with osteoarthritis. Clin J Pain 2005;21:73–82. [DOI] [PubMed] [Google Scholar]
- 24.Gracely RH. Evaluation of multi-dimensional pain scales. Pain 1992;48:297–300. [DOI] [PubMed] [Google Scholar]
- 25.Gracely RH, McGrath P, Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain 1978;5:19–29. [DOI] [PubMed] [Google Scholar]
- 26.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191–208. [DOI] [PubMed] [Google Scholar]
- 27.Busija L, Pausenberger E, Haines TP, et al. Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL). Arthritis Care Res (Hoboken) 2011; 63(Suppl 11):S383–S412. [DOI] [PubMed] [Google Scholar]
- 28.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother 2009;9:745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 30.Spielberger C, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 31.Reed BD, Payne CM, Harlow SD, et al. Urogenital symptoms and pain history as precursors of vulvodynia: a longitudinal study. J Womens Health (Larchmt) 2012;21:1139–1143. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen RH. With the identification of a high-risk group for the development of vulvodynia comes an eye on prevention. J Womens Health (Larchmt) 2012;21:1130–1131. [DOI] [PubMed] [Google Scholar]
- 33.Dargie EE, Chamberlain SM, Pukall CF. Provoked vestibulo-dynia: diagnosis, self-reported pain, and presentation during gynecological examinations. J Obstet Gynaecol Can 2017; 39:145–151. [DOI] [PubMed] [Google Scholar]
- 34.Meyer-Bahlburg HF, Dolezal C. The female sexual function index: a methodological critique and suggestions for improvement. J Sex Marital Ther 2007;33:217–224. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen RH, Mathur C, Wynings EM, et al. Remission of vulvar pain among women with primary vulvodynia. J Low Genit Tract Dis 2015;19:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]