Abstract
Purpose
To evaluate the heritability of choroidal thickness and its relationship to age-related macular degeneration (AMD).
Design
Cohort Study
Subjects, Participants, and/or Controls
689 individuals from Amish families with early/intermediate AMD cases
Methods
Ocular Coherence Tomography was used to quantify choroidal thickness, and fundus photography to classify eyes into categories based on a modification of the Clinical Age-Related Maculopathy Staging (CARMS) system. Repeatability (R) and heritability (H2) of choroidal thickness, and both its phenotypic and genetic correlation (rA) with the AMD phenotype (CARMS category) were estimated using a generalized linear mixed model (GLMM) approach that accounted for pedigree-based relatedness, repeated measures (left and right eyes) and the main effects of age, sex, and refraction.
Main Outcome Measures
Heritability of choroidal thickness and its phenotypic and genetic correlation with the AMD phenotype (CARMS category)
Results
Phenotypic correlation between choroidal thickness and CARMS category was moderate (rs=−0.24, n=1,313 eyes) and significant (GLMM posterior mean=−4.27, 95% CI=−7.88 - −0.79, p=0.02) after controlling for relatedness, age, sex, and refraction. Eyes with advanced AMD had significantly thinner choroids than eyes without AMD (posterior mean=−73.8, 95% CI=−94.7- −54.6, p<0.001, n=1,178 eyes). Choroidal thickness measurements were highly repeatable within individuals (R=0.78, 95% credible interval, CI=0.68–0.89) and moderately heritable (H2=0.40, 95% CI 0.14–0.51), but did not show significant genetic correlation with CARMS category, although the effect size was moderate (rA=−0.18, 95% CI = −0.49-0.16). Choroidal thickness also varied with age, sex and refraction. CARMS category showed moderate heritability (H2=0.49, 95% CI=0.26–0.72).
Conclusions
This study is the first reported estimate of the heritability of choroidal thickness in humans, highlighting a heritable, quantitative trait that is measurable in all individuals regardless of AMD affection status, and moderately phenotypically correlated with AMD severity (CARMS category). Choroidal thickness may therefore capture variation not captured by the CARMS system. However, since the genetic correlation between choroidal thickness and AMD severity was not significant in our dataset, genes associated with the two traits may not overlap substantially. Future studies should test for genetic variation associated with choroidal thickness to determine whether genes underlying this trait overlap with or are distinct from those associated with AMD.
Introduction
Age-related macular degeneration (AMD) is a major cause of blindness in older adults 1. Both demographic and environmental factors, including advanced age, sex, smoking history and diet, contribute to the risk of developing AMD 2–4. Both intermediate and advanced AMD are also heritable (heritability, the proportion of phenotypic variation that is explained by genetic differences=0.44–0.71 5,6) with several common and rare genetic risk factors 4,7. Although identified genetic variants explain a relatively high proportion (40–60%) of the heritability of advanced disease, a substantial portion remains unexplained 6,7. AMD disease progression is also poorly understood and highly variable 8. In addition to unidentified rare variants or interaction effects, residual variation in disease risk, heritability, and progression may partly be a reflection of the currently used classification for AMD.
Despite the complexity of the AMD phenotype, eyes are usually classified into discrete categories using the Age-Related Eye Disease Study (AREDS) 9,10 or simplified Clinical Age-Related Maculopathy Staging (CARMS) classification systems 11 that are largely based on the presence and size of key hallmarks of AMD, such as drusen and/or retinal pigmentation. Furthermore, most studies of genetic association compare individuals with no or few signs of AMD (controls/CARMS categories 1 and 2) to those with late-stage disease (CARMS categories 4 and 5), while only a few studies have considered the genetics of early or intermediate AMD or specific AMD subtypes 6, 12, 13. Such broad-scale classification of disease stages may not adequately represent the biological basis of the disease and mask important sub-phenotypes that are more directly linked to the underlying disease process. Features found in AMD cases may also overlap with other retinal diseases that have a distinct genetic basis, confounding our ability to predict disease risk. We hypothesize that parsing the complex AMD phenotype into heritable finer-scale retinal traits that are easily measureable in all individuals, and each have a relatively simple genetic basis (“endophenotypes” 14), will increase our understanding of the biological basis of AMD, enable better prediction of disease risk and progression and aid the discovery of novel drug targets 15, 16. For example, an endophenotype approach was recently used to identify ocular traits and genes associated with glaucoma 15, 17 and myopia 18.
Recent technological advances in Spectral Domain Ocular Coherence Tomography (SD-OCT) now allows detailed cross-sectional imaging of the retina’s ultrastructure, offering enhanced detection, measurement and analysis of retinal traits beyond that offered by traditional fundus photography 19. SDOCT may therefore aid the identification of AMD endophenotypes, or biomarkers that can be used to predict risk or progression to advanced stages 20, 21. Traits such as choroidal thickness 22, 23, drusen volume 20 and the presence of reticular pseudodrusen 20, 24 have previously been linked to AMD disease status and progression and may define AMD endophenotypes. For example, choroidal thickness was found to decrease with increasing AMD severity (AREDS category 1–4) 23. However, most studies have measured only the overall phenotypic correlation between retinal traits and AMD, but phenotypic correlation may result from genetic correction (overlapping genes) and/or environmental correlation. If environmental factors drive the correlation between retinal traits and AMD, rather than similar genes, then performing genetic association analyses on these fine-scale retinal traits may not be informative for AMD. The relationship between retinal features, AMD risk and progression, and genetics is therefore unclear and requires further investigation. Specifically, for a trait to be useful as an AMD endophenotype requires that the trait is shown to be heritable and genetically correlated, to some extent, with the AMD phenotype i.e. that there is some shared genetic basis between the quantitative trait and the disease 14, 15, 25. Such analyses can be performed by measuring the phenotypic similarity and relatedness between family members in a pedigree or twin study as this allows phenotypic variation to be separated into genetic variation, environmental variation and individual-level variation (repeatability).
To assess the use of choroidal thickness as an AMD endophenotype for future genetic studies, we therefore test whether the trait is heritable, (i.e. whether a significant proportion of the phenotypic variation is explained by genetic variation), and phenotypically and genetically correlated with the AMD phenotype (CARMS category) using families from the Amish Eye Study (AES); i.e. that the two traits are correlated and show overlapping genetic basis. The Amish are genetically and culturally isolated, and experience a relatively uniform environment, reducing genetic diversity and variance in disease risk due to environment. Additionally, their large extended families provide a powerful tool for heritability analyses. The frequency of smoking (a key environmental risk factor 2) is also low. The Amish therefore provides an excellent opportunity to examine the genetic architecture of complex disease.
Methods
Study population and data collection
Participants were recruited from Amish populations in Lancaster County, Pennsylvania, Holmes County, Ohio and Elkhart and LaGrange Counties, Indiana. Informed consent was obtained from all individuals. Institutional Review Board (IRB) approval was obtained, research is HIPAA-compliant and adhered to the tenets of the Declaration of Helsinki. Individuals and their siblings were recruited from families with at least 2 affected individual with early/intermediate AMD. Recruited families varied in size from nuclear families of up to 13 siblings to extended families of up to 30 individuals.
At each clinical center (Indiana, Ohio, Pennsylvania) participants underwent a health history and ophthalmologic exam that included color fundus photography and SD-OCT volume scans for both eyes where possible. For choroidal thickness assessments, SD-OCT imaging was performed with the Heidelberg Spectralis OCT device (Heidelberg Engineering Inc., Heidelberg, Germany) using a 20 x 20 degree field of view centered on the fovea with 97 B-scans each composed of 512 A-scans. Images were exported to the Doheny Image Reading Center and the choroidal thickness was measured at the foveal center using the caliper tool in the HEYEX software from the lower border of the RPE/Bruch’s membrane band to the choroidal-scleral junction in accordance with previous reports from the reading center 26. Eyes were classified by a modified CARMS classification (categories 0–5) at the Doheny Center from color fundus photographs (Table 1). The CARMS system grades eyes from 1–5 11, and considers eyes with no drusen and few small drusen as category 1. To achieve a more granular phenotype, for this analysis, eyes with no drusen were assigned to a new “Category 0” whereas only those with a few small drusen were included in Category 1. Category 2 included eyes with many small drusen or a few medium drusen, and thus includes eyes both without AMD and with early AMD (using the convention that medium drusen constitute the minimum criteria for AMD; 27). Category 3 includes eyes with intermediate AMD, and categories 4 and 5 includes eyes with advanced AMD as in the CARMS system (Table 1 11).
Table 1.
Modified Clinical Age-Related Maculopathy Staging (CARMS) classification system11
| Category | Description |
|---|---|
| 0 | No drusen |
| 1 | <20 hard drusen |
| 2 | >20 hard drusen or some medium drusen |
| 3 | >20 medium drusen, or a single large drusen |
| 4 | Foveal geographic atrophy |
| 5 | Choroidal neovascularization |
Statistical analysis
To assess the use of choroidal thickness as an AMD endophenotype we quantified a) its overall phenotypic correlation with the AMD phenotype, b) its heritability, and b) its genetic correlation with the AMD phenotype to assess the extent to which genetic variation underlying the two traits overlapped. Our primary analyses treated the AMD phenotype as an ordinal trait, CARMS category (0–5), since this approach was more powerful than dichotomizing the phenotype as a binary trait (presence/absence of AMD). However, in a secondary analysis we re-analyzed data treating the AMD phenotype as a binary trait where possible
Broad sense heritability (H2, the proportion of phenotypic variance that is explained by genetic variance) of both choroidal thickness and CARMS category, and their correlation were quantified in a generalized linear mixed model (GLMM) framework. A GLMM approach enabled the use of repeated measures (both left and right eyes per subject) and hence an estimate of the proportion of phenotypic variance in each trait that was explained by individual identity (repeatability), the inclusion of covariates such as age and maximized the use of relatedness information from a pedigree 28.
Analyses were run using the R-package MCMCglmm that fits models in a Bayesian framework using Markov Chain Monte Carlo methods 29. Firstly, a univariate GLMM of choroidal thickness was used to test whether choroidal thickness varied with AMD severity (CARMS category) while controlling for age, sex, spherical equivalent refraction (sphere plus half the cylinder, as a covariate for choroidal thickness), relatedness, and repeated measures (left and right eyes). A bivariate GLMM with a 2 trait response variable was then fit with CARMS category (0–5) specified as an ordinal (threshold) trait with probit link, and choroidal thickness as a gaussian trait to quantify the heritability of each traits, and their genetic correlation, while controlling for age, sex, and spherical equivalent refraction. A pedigree (subject, mother, father) was used to estimate a genetic variance-covariance matrix, and a random effect of individual ID was fit to account for repeated measures per person (left and right eyes), allowing phenotypic variance to be partitioned into genetic, individual-level, and residual variance. Shared environmental effects between family members were not accounted for and may conflate our estimate of genetic variance, but since AMD is a late-onset disease, it was unclear whether accounting for shared sibship environment was relevant (see also 5). Eyes that were missing one of the two traits (CARMS category or choroidal thickness; n=61) were included in analyses as bivariate GLMMs can handle missing data in the response variable. In the bivariate model, random effect and residual variances were specified using the “us(trait)” structure thereby allowing the variance and covariance to vary between the two traits. Default priors were used for fixed effects. Priors on variance components for residual terms were inverse Wishart distributed with variance of 1 and low degree of belief (nu=0.002) with variance fixed at 1 for the ordinal trait 29. For random effects we used parameter expanded priors to facilitate mixing with a mean (mu) of 0 and variance (V) of 1000 for choroidal thickness and 100 for CARMS category 29. Variance estimates were similar when models were run with alternative priors. The model was run for 10,500,000 iterations with a burn-in interval of 500,000 and thin of 2000 to give an effective sample size of approximately 5000 and autocorrelation between consecutive samples <0.1. Model convergence and fixing was assessed by visual inspection of plots and using the ‘heidel.diag’ function in the Coda package 30. All analyses were conducted in R version 3.0.1 (http://www.cran.r-project.org). The heritability of choroidal thickness was quantitatively similar when run in a (restricted) maximum likelihood framework using the R-package “pedigreemm”.
Results
A total of 689 participants (417 females and 272 males) from Indiana (n=248), Pennsylvania (n=315) and Ohio (n=126) were recruited and examined between August 2013 and November 2015 (n=1,378 eyes; Table 2). Mean baseline age of participants was 66.4±10.9 (range 33–99 years of age). Considering the most severely affected eye per individual, approximately 42.1% of participants were CARMS category 0, 27.1% were category 1, 11.6% were category 2, 11.5% category 3, 5.4% had advanced AMD (categories 4 or 5) and 2.3% were not graded due to a fundus camera malfunction at one site (Table 3). Mean spherical equivalent refraction was 0.55D±1.62 for right eyes and 0.56D±1.65 for left eyes.
Table 2.
Demographic parameters for 689 participants.
| Parameter | Pennsylvania | Indiana | Ohio | All |
|---|---|---|---|---|
|
| ||||
| Sex | 193 female, 122 male | 142 female, 106 male | 82 female, 44 male | 418 female, 272 male |
| Age, mean ±SD | 65.6 ±10.5 | 65.5 ±11.4 | 70.1 ±9.9 | 66.3±11.0 |
Table 3.
Number of participants and modified CARMS category (most advanced eyes).
| CARMS category | ||||||||
|---|---|---|---|---|---|---|---|---|
| Population | 0 | 1 | 2 | 3 | 4 | 5 | Not graded | all |
| Pennsylvania | 131 | 82 | 43 | 36 | 7 | 1 | 15 | 315 |
| Indiana | 114 | 75 | 25 | 21 | 8 | 4 | 1 | 248 |
| Ohio | 45 | 30 | 12 | 22 | 17 | 0 | 0 | 126 |
|
| ||||||||
| Total | 290 | 187 | 80 | 79 | 32 | 5 | 16 | 689 |
Mean choroidal thickness of 679 right eyes was 253.9 ±70.6μm and of 670 left eyes was 253.5 ±69.4μm. Choroidal thickness was highly correlated between left and right eyes (Pearson’s correlation coefficient, r=0.82, n=660). As expected, CARMS category was also highly correlated between eyes (Spearman’s correlation coefficient, rs=0.84, n=665); eyes therefore tended to be at the same disease stage. However, correlation coefficients suggested that there was sufficient variation to justify the inclusion of both eyes in future analyses. Choroidal thickness showed moderate negative phenotypic correlation with CARMS category (rs=−0.24, n=1,313 eyes); eyes more severely affected with AMD had thinner choroids. This correlation was significant in a univariate GLMM of choroidal thickness controlling for relatedness, repeated measures (left and right eyes), age, sex, and refraction (posterior mean=−4.27, 95% credible intervals, CI=−7.88 - −0.79, p=0.02). Secondary analyses defining AMD severity as a binary trait showed that choroidal thickness was marginally thinner in eyes with AMD (categories 3, 4, and 5, n=190 eyes; posterior mean=−10.7, 95% CI=−20.87-0.15, p=0.05) compared to eyes with no AMD (CARMS categories 0, 1, and 2, n=1,123 eyes). Interestingly, this difference in choroidal thickness between affected and unaffected individuals was substantially stronger and significant if category 3 (intermediate AMD) eyes were excluded (posterior mean=−73.8, 95% CI=−94.7- −54.6, p<0.001, n=1178 eyes); eyes with advanced AMD therefore had significantly thinner choroids than those without AMD. Although note that the sample size for this secondary analysis was small (n=37 individuals with at least one advanced AMD eye).
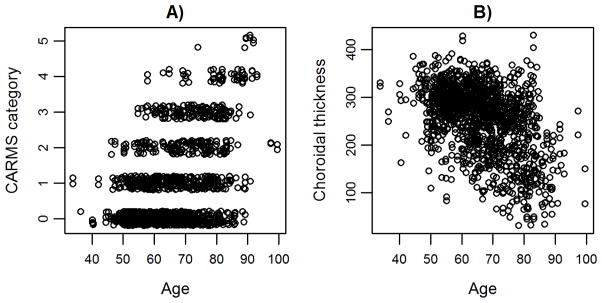
A bivariate GLMM estimated that the repeatability (proportion of phenotypic variation that was explained by an individual’s identity) of choroidal thickness was high (0.78, 95% CI=0.75–0.81, n=1,378 eyes) and that the heritability of choroidal thickness was moderate (0.40, 95% CI = 0.24–0.56; Table 4). CARMS category was also highly repeatable (0.93, 95% CI = 0.91–0.94; Table 4), and moderately heritable (H2 on the liability scale was 0.49 (95% CI =0.26–0.72), similar to that estimated by a twin study (H2=0.46 5). Choroidal thickness (r= −0.46; pMCMC<0.001; Figure 4) and CARMS category were both significantly negatively correlated with age (r=−0.38, pMCMC<0.001; Figure 4), but only choroidal thickness varied with sex; males had slightly thinner choroidal thickness (p=0.04; Table 5). Choroidal thickness was also positively correlated with refraction (Table 5). Genetic correlation between choroidal thickness and CARMS category was −0.18 but 95% credible intervals overlapped zero (−0.49-0.16), the correlation was therefore not statistically significant (Table 4). The bivariate model also showed that the overall phenotypic correlation between choroidal thickness and CARMS category was moderate, negative, and significant (−0.14, 95% CI=−0.22- −0.06). We did not test for the genetic correlation between choroidal thickness and the AMD phenotype as a binary trait due to the complexity of a bivariate model and the relatively small number of advanced AMD cases (62 eyes versus 1,137 eyes with no AMD) preventing model convergence despite long run time.
Table 4.
Posterior means (and 95% credible intervals) for variance and covariance components estimated from a bivariate GLMM of choroidal thickness and modified Clinical Age-Related Maculopathy Staging (CARMS) category. Repeatability (R=Va+Vpe/(Va+Vpe+Ve)), heritability (H2= Va/(Va+Vpe+Ve) and genetic correlation between choroidal thickness and CARMS category (rA=Cov1,2/√(VA1*VA2) are shown where Va is genetic variance, Vpe is permanent environmental variance, Ve is the residual variance and Cov12 is the genetic covariance between traits.
| Trait | Genetic variance (VA) | Permanent individual variance (Vpe) | Residual variance (Ve) | Heritability (H2) | Genetic covariance (Cov1,2) | Genetic correlation (rA) |
|---|---|---|---|---|---|---|
| Choroidal thickness | 1540.85 (850.90–2273.01) | 1479.79 (889.49–2031.82) | 857.21 (769.19–951.88) | 0.40 (0.24–0.56) | −18.43 (−53.02-16.48) | −0.18 (−0.49 - 0.16) |
| CARMS category | 7.00 (3.01–11.12) | 6.08 (2.77–9.26) | 1 (fixed) | 0.49 (0.26 – 0.72) |
Table 5.
Main effect parameters (posterior mean, 95% credible interval and MCMCp-values) from a bivariate Generalized Linear Mixed Model (GLMM) investigating variation in choroidal thickness and modified Clinical Age-Related Maculopathy Staging (CARMS) category with respect to age, sex, and refraction.
| Trait | Posterior mean | 95% CI | MCMCp-value |
|---|---|---|---|
| Choroidal thickness intercept (females) | 476.1 | 446.2 – 504.4 | <0.001 |
| CARMS category intercept (females) | −11.3 | −13.6 - 9.1 | <0.001 |
| Choroidal thickness: age | −3.4 | −3.8 - 3.0 | <0.001 |
| CARMS category: age | 0.17 | 0.1 – 0.2 | <0.001 |
| Choroidal thickness: males | −8.6 | −17.1– −0.5 | 0.04 |
| CARMS category: males | 0 | −0.6 – 0.6 | 0.91 |
| Choroidal thickness: refraction | 6.0 | 3.5 – 8.3 | <0.001 |
Discussion
Decomposing disease phenotypes into heritable sub-components may be especially useful for unravelling the complex genetic basis of multifactorial diseases such as AMD. Endophenotypes may also potentially be useful as biomarkers of disease risk or progression thereby influencing clinical decision and allowing for intervention to alter disease progression 16. Genetic studies on heritable, novel AMD phenotypes may also provide additional biological pathways and therapeutic targets for early/intermediate AMD. One retinal trait that is quantifiable in all individuals using OCT imaging, regardless of AMD disease affection status, is choroidal thickness. The choroid performs many of the retina’s essential metabolic functions and thinning of choroid has previously been associated with age and AMD; older individuals 31–33 and those with AMD 22, 34–39 had thinner choroids, although some studies did not find a difference with AMD status 33, 40–42.
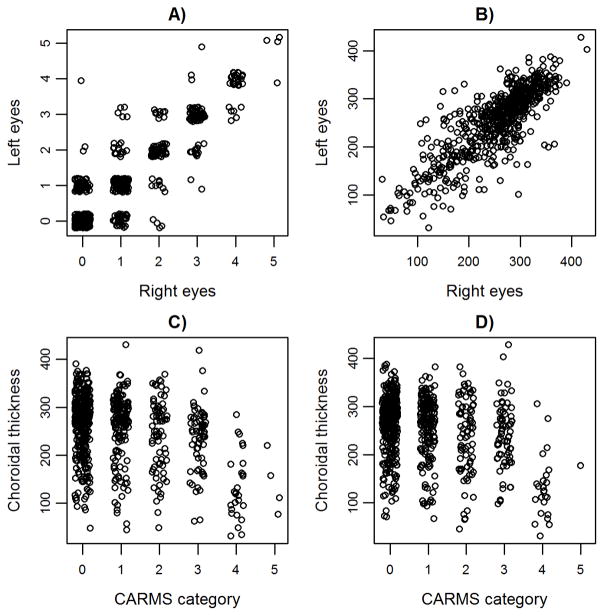
Here we show that, after controlling for age, sex and refraction, choroidal thickness is phenotypically negatively correlated with CARMS category and hence the severity of AMD. Eyes with AMD also had marginally thinner choroids than those without AMD. Phenotypic but not genetic correlation between choroidal thickness and AMD has been tested previously. Several studies found advanced AMD cases had thinner choroids than controls 34, 38, 39. Some studies also found a difference in choroidal thickness between early AMD and controls 35–37, while others did not 22, 23, 41 and some found the strength of the correlation depends on the AMD subtype e.g. 39, 43. We found a stronger difference between AMD cases and controls when eyes with intermediate AMD were excluded, albeit with small sample size of advanced cases, which suggested that the correlation between choroidal thickness and AMD severity is driven primarily by the considerable decrease in choroidal thickness in advanced AMD cases (Figure 2).
Figure 2.
Phenotypic correlation of A) CARMS category and B) subfoveal choroidal thickness between left and right eyes, and correlation between subfoveal choroidal thickness and CARMS category for C) right eyes, and D) left eyes. Points have been jittered for visualization.
Moreover, we show for the first time that choroidal thickness is significantly heritable, and therefore has a substantial genetic component. Choroidal thickness may therefore define an AMD endophenotype useful for genetic studies. However, for a trait to define an endophenotype, it should also show some (but not perfect) genetic correlation with the disease phenotype. Although the effect size of the genetic correlation between choroidal thickness and CARMS category was moderate (−0.18), suggesting that moderate negative phenotypic correlation observed between the two traits may reflect some overlap in genetic basis the genetic correlation was not significantly different from zero. Genetic correlation is the extent to which two traits share the same genes, whereas phenotypic correlation also encompasses environmental correlation. IIt is likely that the absence of significant genetic correlation results from relatively low power to detect correlation with the ordinal trait, CARMS category, since our study is primarily focused on families with early/intermediate AMD cases and therefore our sample consisted of many controls (ca. 70% of individuals were categories 0 or 1) and relatively few individuals with advanced AMD (5%). Any genetic correlation between choroidal thickness and AMD may be more likely to be detected across a sample with a larger proportion of advanced AMD cases, or a more variable sample of unrelated individuals using genome-wide trait analysis. Finally, CARMS category is only one measure of AMD severity/presence. Indeed, many studies dichotomize AMD scales into case-control status to study the genetics of advanced AMD risk. Our preliminary analyses on this relatively small sample size suggested that the genetic correlation between these two traits was stronger than for CARMS category. Therefore the absence of a significant genetic correlation in this dataset of early/intermediate cases and their unaffected relatives does not preclude the use of choroidal thickness as an AMD endophenotype.
As the correlation between choroidal thickness and AMD severity was, at most, moderate, choroidal thickness may capture novel genetic and phenotypic variation and therefore be especially informative for future studies of AMD. Numerous common and rare variants are associated with advanced AMD 7, although cumulatively they explain no more than 60% of the heritability of advanced disease, and less for early/intermediate AMD 6, 12. AMD endophenotypes may be associated with a subset of these known AMD variants, and/or with novel variants. Cohort studies focusing on families are especially useful for detecting genetic causes of disease, and the Amish Eye Study is such a resource. Finding the genetic causes for choroidal thickness has the potential to uncover novel biology for the progression of early to late AMD and ultimately may lead to better prognostic indicators and treatments to prevent AMD.
Figure 1.
Distribution of subfoveal choroidal thickness in microns for A) right eyes and B) left eyes.
Figure 3.
Correlation between A) CARMS category and age, and B) subfoveal choroidal thickness and age. Points are jittered for visualization.
Acknowledgments
Financial Support: Funding was provided by the NEI (5R01EY023164-02). RJS was supported by an NEI postdoctoral training fellowship (T32:EY023194). JNCB was supported by a PhRMA Informatics Fellowship. The funding organization had no role in the design or conduct of this research
Funding was provided by the NEI (5R01EY023164-02). RJS was supported by an NEI postdoctoral training fellowship (T32:EY023194). JNCB was supported by a PhRMA Informatics Fellowship.
Footnotes
Meeting Presentation: Presented as a poster at the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting in 2015, and the American Society for Human Genetics (ASHG) Annual Meeting in 2014 and 2015
Conflict of Interest: No conflicting relationship exists for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the united states. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Naj AC, Scott WK, Courtenay MD, et al. Genetic factors in nonsmokers with age-related macular degeneration revealed through genome-wide gene-environment interaction analysis. Ann Hum Genet. 2013;77:215–31. doi: 10.1111/ahg.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R, Peto T, Bird A, et al. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 4.Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-related eye disease study report number 3. Ophthalmology. 2000;107:2224–32. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seddon JM, Cote J, Page WF, et al. The US twin study of age-related macular degeneration: Relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123:321–7. doi: 10.1001/archopht.123.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Fritsche LG, Igl W, Bailey JNC, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016:48134–43. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45:433–9. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tikellis G, Robman L, Dimitrov P, et al. Characteristics of progression of early age-related macular degeneration: The cardiovascular health and age-related maculopathy study. Eye. 2007;21:169–76. doi: 10.1038/sj.eye.6702151. [DOI] [PubMed] [Google Scholar]
- 9.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS report no. 18. Arch Ophthalmol. 2005;123:1570–4. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Age-Related Eye Disease Study Research Group. The age-related eye disease study (AREDS): Design implications. AREDS report no. 1. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006;113:260–6. doi: 10.1016/j.ophtha.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Holliday EG, Smith AV, Cornes BK, et al. Insights into the genetic architecture of early stage age-related macular degeneration: A genome-wide association study meta-analysis. PloS one. 2013;8:e53830. doi: 10.1371/journal.pone.0053830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Ven Johannes PH, Smailhodzic D, Boon CJ, et al. Association analysis of genetic and environmental risk factors in the cuticular drusen subtype of age-related macular degeneration. Molecular vision. 2012:182271. [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 15.Charlesworth J, Kramer PL, Dyer T, et al. The path to open-angle glaucoma gene discovery: Endophenotypic status of intraocular pressure, cup-to-disc ratio, and central corneal thickness. Invest Ophthalmol Vis Sci. 2010;51:3509–14. doi: 10.1167/iovs.09-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorin MB, Weeks DE, Baron RV, et al. Endophenotypes for age-related macular degeneration: Extending our reach into the preclinical stages of disease. Journal of clinical medicine. 2014;3:1335–56. doi: 10.3390/jcm3041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman EE, Roy-Gagnon M, Descovich D, et al. The heritability of glaucoma-related traits corneal hysteresis, central corneal thickness, intraocular pressure, and choroidal blood flow pulsatility. PloS one. 2013;8:e55573. doi: 10.1371/journal.pone.0055573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JH, Chen H, Huang S, et al. Endophenotyping reveals differential phenotype-genotype correlations between myopia-associated polymorphisms and eye biometric parameters. 2012:18765–78. [PMC free article] [PubMed] [Google Scholar]
- 19.Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 20.de Sisternes L, Simon N, Tibshirani R, et al. Quantitative SD-OCT imaging biomarkers as indicators of age-related macular degeneration progression. Invest Ophthalmol Vis Sci. 2014;55:7093–103. doi: 10.1167/iovs.14-14918. [DOI] [PubMed] [Google Scholar]
- 21.Liakopoulos S, Ongchin S, Bansal A, et al. Quantitative optical coherence tomography findings in various subtypes of neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:5048–54. doi: 10.1167/iovs.08-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung SE, Kang SW, Lee JH, et al. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118:840–5. doi: 10.1016/j.ophtha.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Lee JY, Lee DH, Lee JY, et al. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7812–8. doi: 10.1167/iovs.13-12284. [DOI] [PubMed] [Google Scholar]
- 24.Hogg RE. Reticular pseudodrusen in age-related macular degeneration. Optom Vis Sci. 2014;91:854–9. doi: 10.1097/OPX.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 25.Glahn DC, Curran JE, Winkler AM, et al. High dimensional endophenotype ranking in the search for major depression risk genes. Biol Psychiatry. 2012;71:6–14. doi: 10.1016/j.biopsych.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang Y, Heussen FM, Hariri A, et al. Optical coherence tomography–based observation of the natural history of drusenoid lesion in eyes with dry age-related macular degeneration. Ophthalmology. 2013;120:2656–65. doi: 10.1016/j.ophtha.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferris FL, Wilkinson C, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenesa A, Haley CS. The heritability of human disease: Estimation, uses and abuses. Nature Reviews Genetics. 2013;14:139–49. doi: 10.1038/nrg3377. [DOI] [PubMed] [Google Scholar]
- 29.Hadfield JD. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. Journal of Statistical Software. 2010:331–22. [Google Scholar]
- 30.Plummer M, Best N, Cowles K, et al. CODA: Convergence diagnosis and output analysis for MCMC. R news. 2006;6:7–11. [Google Scholar]
- 31.Wei WB, Xu L, Jonas JB, et al. Subfoveal choroidal thickness: The beijing eye study. Ophthalmology. 2013;120:175–80. doi: 10.1016/j.ophtha.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 32.Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147:801–10. doi: 10.1016/j.ajo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Kim SW, Oh J, Kwon SS, et al. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011;31:1904–11. doi: 10.1097/IAE.0b013e31821801c5. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Lai C, Huang EJ, et al. Axial length and subfoveal choroidal thickness in individuals with age-related macular degeneration. Taiwan Journal of Ophthalmology. 2015;5:169–76. doi: 10.1016/j.tjo.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Switzer DW, Jr, Mendonca LS, Saito M, et al. Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina. 2012;32:1265–71. doi: 10.1097/IAE.0b013e31824453ac. [DOI] [PubMed] [Google Scholar]
- 36.Sigler EJ, Randolph JC. Comparison of macular choroidal thickness among patients older than age 65 with early atrophic age-related macular degeneration and normals. Invest Ophthalmol Vis Sci. 2013;54:6307–13. doi: 10.1167/iovs.13-12653. [DOI] [PubMed] [Google Scholar]
- 37.Capuano V, Souied EH, Miere A, et al. Choroidal maps in non-exudative age-related macular degeneration. Br J Ophthalmol. 2015 doi: 10.1136/bjophthalmol-2015-307169. [DOI] [PubMed] [Google Scholar]
- 38.Adhi M, Lau M, Liang MC, et al. Analysis of the thickness and vascular layers of the choroid in eyes with geographic atrophy using spectral-domain optical coherence tomography. Retina. 2014;34:306–12. doi: 10.1097/IAE.0b013e3182993e09. [DOI] [PubMed] [Google Scholar]
- 39.Lindner M, Bezatis A, Czauderna J, et al. Choroidal thickness in geographic atrophy secondary to age-related macular Degeneration Choroidal thickness in geographic atrophy. Invest Ophthalmol Vis Sci. 2015;56:875–82. doi: 10.1167/iovs.14-14933. [DOI] [PubMed] [Google Scholar]
- 40.McCourt EA, Cadena BC, Barnett CJ, et al. Measurement of subfoveal choroidal thickness using spectral domain optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2010;41(Suppl):S28–33. doi: 10.3928/15428877-20101031-14. [DOI] [PubMed] [Google Scholar]
- 41.Wood A, Binns A, Margrain T, et al. Retinal and choroidal thickness in early age-related macular degeneration. Am J Ophthalmol. 2011;152:1030, 1038. e2. doi: 10.1016/j.ajo.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Jonas JB, Forster TM, Steinmetz P, et al. Choroidal thickness in age-related macular degeneration. Retina. 2014;34:1149–55. doi: 10.1097/IAE.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 43.Koizumi H, Yamagishi T, Yamazaki T, et al. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2011;249:1123–8. doi: 10.1007/s00417-011-1620-1. [DOI] [PubMed] [Google Scholar]