Abstract
Purpose:
To evaluate the accuracy, reproducibility, and contouring time of RV mass in end-systole (ES) and end-diastole (ED). Magnetic resonance imaging (MRI) has been shown to be accurate and reproducible for the evaluation of right ventricular (RV) volume and function. RV mass, assessed in end-diastolic (ED) phase, is one of the least reproducible variables. The choice of end-systolic (ES) phase could offer an alternative to improve reproducibility, since the selection of the basal slice and the visualization of the usually thin RV wall are easier in this phase.
Materials and Methods:
To evaluate accuracy, 11 sheep were imaged in vivo and their RV free walls were weighed after removing epicardial fat. To evaluate reproducibility, 30 normal subjects and 30 subjects with pulmonary arterial hypertension (PAH) were imaged and interobserver and intraobserver variabilities were assessed in the ES and the ED. Segmentation time was recorded after visual selection of ES and ED phases.
Results:
ES RV mass measurement has less absolute variability (5.2% ± 3.2) compared to ED (10.6% ± 6.3) using weighed RV mass in sheep as the gold standard (P < 0.001). ES segmentation yielded higher intraobserver (intraclass correlation coefficients [ICC] = 0.94–0.99; coefficient of variability [CoV] = 6–7.3%) and interobserver (ICC = 0.85–0.98; CoV = 10.9–11.7%) reproducibility than ED segmentation. Segmentation time in humans was 25–28% faster in ES (P < 0.001).
Conclusion:
The MRI assessment of RV mass is more accurate, reproducible, and faster in the ES phase.
Many studies have demonstrated that the assessment of right ventricular (RV) mass has important diagnostic and prognostic values in right-sided cardiovascular diseases.1–5 The establishment of an accurate and highly reliable method for RV mass assessment is therefore crucial for research and clinical application.
Magnetic resonance imaging (MRI) has been shown to be accurate and reproducible for RV function evaluation.6–8 However, RV mass assessment, when performed in the end-diastolic (ED) phase, is one of the least reproducible MRI-derived variables.7–10 We hypothesized that the end-systolic (ES) phase could reduce intra-and interobserver variability and processing time, since the selection of basal slice and visualization of the usually thin RV wall are easier in this phase.
The aim of this study was to evaluate the accuracy of MRI-derived RV mass measurements in ED and ES using ovine hearts and compare the reproducibility and contouring time of ED and ES in healthy human subjects and patients with pulmonary arterial hypertension (PAH).
MATERIALS AND METHODS
Human Subjects
The control group was composed of healthy volunteers (n = 12) and clinical studies (n = 18) referred for suspected cardiac disease but were found to have completely normal biventricular size and function and no delayed enhancement. The PAH group was composed of 30 patients who were diagnosed as PAH with documented elevated mean pulmonary artery pressure (mPAP) and increased pulmonary vascular resistance with normal pulmonary capillary wedge pressure according to WHO Group 1 classification. Both groups gave written consent approved by the Institutional Review Board (IRB). The IRB also approved the retrospectively reviewing of data from clinical patients with a waiver of consent.
Animals
Eleven fresh normal adult sheep hearts were obtained from experiments approved by the Institutional Animal Care and Use Committee. MRIs were obtained in vivo before the sheep were sacrificed. The free wall of the RV was separated from the rest of the heart and epicardial fat and papillary muscles were removed before the weight was obtained (Fig. 1).
FIGURE 1:
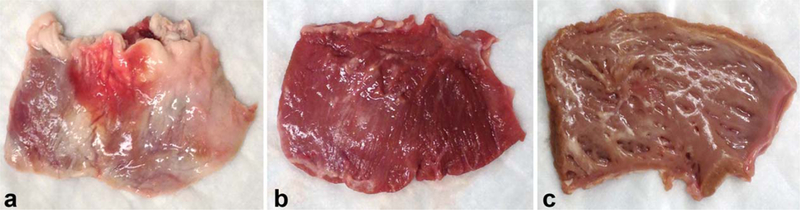
Dissection and processing of RV free wall before weight measurement. (A) RV free wall separated from the rest of the heart. (B) Epicardial surface after removal of the epicardial fat. (C) Endocardial surface after removal of attached papillary muscles.
MR Protocol
Human MRI exams were performed on a 1.5T Siemens scanner (Avanto, Siemens Health Systems, Germany). Cine short-axis slices were acquired using a steady-state free precession (SSFP) sequence (flip angle = 55–75°, temporal resolution = 25–35 msec, TE = 1.0–1.5 msec, 25–30 phases per cardiac cycle, slice thickness of 8 mm with a skip of 2 mm). Average breath-hold was 12 ± 3 seconds.
Animal MRIs were performed on a 3T Siemens scanner (Tim Trio, Siemens Health Systems) using an SSFP sequence (flip angle = 45°, temporal resolution = 25–30 msec, TE = 1.5 msec, 60 images per cardiac cycle, slice thickness of 4 mm).
Segmentation
The RV contours were analyzed in both ES and ED phases for human and animals using QMASS (Medis, Leide, The Netherlands), according to the MRI postprocessing guidelines.11 Cross-reference between long-and short-axis planes were used to facilitate selection of basal slice.7 Papillary muscles were included in the ventricular volume. All the visible RV myocardium was included in the epicardial contour. Interventricular septum was not included in the RV mass. In most of the normal human subjects, the RV wall was traced just outside the black artifact of the usually thin RV in end-diastole (Fig. 2).12 The RV outflow tract (RVOT) was included in the RV volume. To decrease observer variability, if the RVOT volume was smaller than the adjacent right atrium, the RV mass was not segmented in this slice.
FIGURE 2:
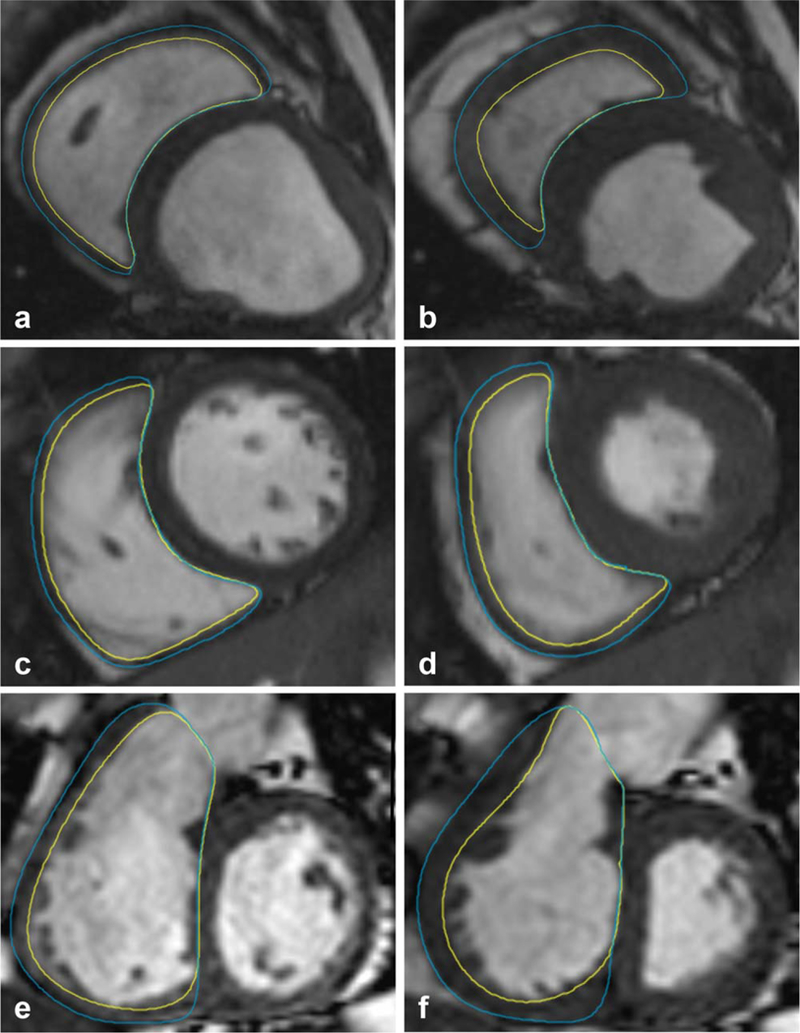
RV wall visualization in end-diastole and end-systole. RV segmentation in the end-diastolic (ED) phase in sheep (A), normal human subject (C), and a patient with pulmonary arterial hypertension (E). End-systolic (ES) phases are shown in (B,D,F), respectively. The epicardial contour was traced outside the black artifact of the thin RV wall. The RV wall is thicker and the edges of the myocardium are better visualized in ES compared to ED in all situations.
Two observers (Ob1 = S.P.L.A., with 1 year of segmentation experience) and Ob2 = L.A.T., with 3 months of experience) practiced under supervision with five selected cases before the study began. Ob1 processed RV mass of 30 normal subjects, 30 PAH patients, and all animals. Segmentation time for the human studies was recorded after the visual selection of ES and ED phases. After an interval of at least 3 weeks, Ob1 and Ob2 independently reprocessed all animal studies and 15 randomly selected subjects from each human group to assess intraobserver and interobserver reproducibility.
To evaluate accuracy, the sheep MRI-derived RV mass was compared to the respective weighed RV free wall. The percent difference was computed as a ratio of the difference between the true weighed mass and MRI-derived mass (at ED or ES phase) over the weighed mass.
Statistical Analysis
The continuous variables are expressed as mean ± standard deviation (SD). Absolute variability (%) was calculated by dividing the absolute difference between two measurements by the respective mean. Coefficient of variability (CoV) was calculated by dividing the SD of the differences by the measurement mean. Intra-and interobserver absolute agreements were evaluated with two-way mixed intraclass correlation coefficients (ICC).
Two-sided paired t-tests were used to compare MRI-derived and weighed RV mass in the animal experiment. Two-sided unpaired t-tests were used to evaluate the variability, CoV, ICC, and processing time in ES vs. ED in human studies. The significance level was set at 0.05. All the analyses were performed with SPSS Statistics v. 23 (IBM, Chicago, IL).
RESULTS
The values for MRI-derived and weighed RV mass of each animal are presented in Table 1. There was no statistical difference between the weighed RV mass (35.5 ± 9.8 g) and the measurements done in end-systole (34.8 ± 9.5 g, P = 0.22) or end-diastole (34.2 ± 8.7 g, P = 0.23). The MRI-derived percent difference from the true weighted mass was significantly lower when the contours were drawn in ES (5.2% ± 3.2) as compared to 10.6% ± 6.3% in ED (P< 0.001).
TABLE 1.
Weighed RV Mass and MRI-Derived RV Mass in the Sheep
| Sheep number |
Weighted mass (g) |
End-systolic phase |
End-diastolic phase |
||||
|---|---|---|---|---|---|---|---|
| Ob1a | Ob1b | Ob2 | Ob1a | Ob1b | Ob2 | ||
| 1 | 34.4 | 32.5 | 32.3 | 30.3 | 30.8 | 32.6 | 28.7 |
| 2 | 49.1 | 50.2 | 51.1 | 54.6 | 46.7 | 43.3 | 59.2 |
| 3 | 38.9 | 40.5 | 36.4 | 37.0 | 35.6 | 33.0 | 39.7 |
| 4 | 26.2 | 25.9 | 27.1 | 24.0 | 26.1 | 24.5 | 22.9 |
| 5 | 57.6 | 52.8 | 58.4 | 50.5 | 50.6 | 52.7 | 49.1 |
| 6 | 32.8 | 32.6 | 33.7 | 31.7 | 32.2 | 34.8 | 37.4 |
| 7 | 32.4 | 30.5 | 29.0 | 30.3 | 28.9 | 30.1 | 29.2 |
| 8 | 28.8 | 29.7 | 30.0 | 29.4 | 31.2 | 35.2 | 37.2 |
| 9 | 34.4 | 31.7 | 32.4 | 32.1 | 27.6 | 33.5 | 30.8 |
| 10 | 25.5 | 26.3 | 26.9 | 26.4 | 23.8 | 22.2 | 29.3 |
| 11 | 30.8 | 29.0 | 30.9 | 31.9 | 29.7 | 28.3 | 33.5 |
| Difference from weighted mass (%) |
— | 5.2% ±3.2 | 10.6% ±6.3 | ||||
The measurements obtained in the human studies are summarized in Table 2. There was no significant difference between the RV mass obtained in ES and ED for both normal (P = 0.73) and PAH subjects (P = 0.12). The segmentation time was 25–28% faster when the contours were obtained in ES as compared to ED (P< 0.001).
TABLE 2.
MRI Derived Mass and Segmentation Time (Mean ± SD) in the 30 Normal Human Subjects
| Systole | Diastole | P-value | |
|---|---|---|---|
| Normal subjects (n = 30) | |||
| RV mass (g) | 30.6 ±6 | 31.2 ±6.6 | 0.71 |
| RV mass index (g/m2) |
16.5 ±3.0 | 16.8 ±3.2 | 0.41 |
| Segmentation time (min) |
4.6 ±0.8 | 6.4 ±1.2 | <0.001 |
| PAH cohort (n = 30) | |||
| RV mass (g) | 63.5 ±20.7 | 61.6 ±22.0 | 0.13 |
| RV mass index (g/m2) |
37.1 ±12.0 | 36.0 ±13.1 | 0.13 |
| Segmentation time (min) |
5 ±1.3 | 6.7 ±1.1 | <0.001 |
PAH, pulmonary arterial hypertension.
The reproducibility results of human and animal studies are shown in Table 3. The absolute intra-and interobserver variability and CoV were always lower when RV mass were obtained in systole. In healthy subjects, the ES segmentation almost halved interobserver (22.6% to 11.7%, P< 0.05) and intraobserver variability (13.7% to 7.3%, P= 0.001) when compared to ED segmentation. Similar results were found for the animals and the PAH subjects, all statistically significant (P < 0.05).
TABLE 3.
Intra-and Interobserver Variability of RV Mass in the Human and Animal Groups
| Intraobserver |
Interobserver |
||||||
|---|---|---|---|---|---|---|---|
| Variability | CoV | ICC | Variability | CoV | ICC | ||
| (%) | (%) | (%) | (%) | ||||
| Animal (11) | ES | 4.7 ± 3.7* | 6.8* | 0.99 | 5.7 ±3.1* | 6.9* | 0.99* |
| ED | 6.9 ± 2.6 | 8.8 | 0.97 | 12.2 ±10.6 | 14.2 | 0.91 | |
| Normal (15) | ES | 6.6 ± 5.1* | 7.3* | 0.94 | 12.2 ±7.8* | 11.7* | 0.85 |
| ED | 12.6 ± 4.1 | 13.7 | 0.88 | 18.6 ±9.7 | 22.6 | 0.67 | |
| PAH (15) | ES | 5.2 ± 4.1* | 6.0* | 0.99* | 7.9 ±4.2* | 10.9* | 0.98* |
| ED | 8.8 ± 5.0 | 10.4 | 0.97 | 14.7 ±11.8 | 18.0 | 0.96 | |
CoV, coefficient of variation; ICC, intraclass correlation coefficient; ES, end-systole; ED, end-diastole.
P < 0.05 comparing ES versus ED.
Values for ICC were high for almost all measurements, except for interobserver end-diastolic RV segmentation of normal subjects (ICC = 0.659). This value, however, increased for 0.846 when the contours were drawn in the ES phase. The intra-and interobserver ICCs, in both animal and human studies, were always higher when the segmentations were performed in systole and the statistical significance was achieved in interobserver ICC for animals and both inter-and intraobserver ICC for PAH cohort.
DISCUSSION
Our results showed that MRI RV mass assessment is accurate to measure true mass and is associated with high intra-and interobserver reproducibility. In addition, we demonstrated that both accuracy and reproducibility of these measurements are higher when the segmentation is performed in the end-systolic phase.
The accuracy of in vivo MRI to predict RV mass had previously been validated by McDonald et al 13 in dog studies and Beygui et al 14 in minipigs, both using ED segmentation. Neither study evaluated the differences between segmentation phases. Although both ED and ES segmented mass showed no significant difference compared to the weighed mass, we demonstrated that systolic measurements yielded smaller differences to true RV mass when compared to the diastolic measurements in the sheep.
In the human studies, the RV mass measurements yielded higher intra-and interrater reproducibility when performed in end-systole, which confirms previous findings reported by Blalock et al.9 In both the human and animal studies, we suspect the higher reproducibility is explained by the better RV wall visualization in ES when compared to the usually thin, and sometimes not well visualized, end-diastolic wall in ED. Additionally, the ES phase delineation process (selection of basal slice and endocardial tracing) are known to have higher ICC than ED phase.10
Beyond reproducibility improvement, we also demonstrated that the ES phase segmentation is less time-consuming than segmentation in ED, which makes it more desirable in clinical practice.
Although there was no previous consensus on the choice of ED vs. ES phase that RV mass should be measured, most studies on RV reproducibility were conducted by end-diastolic segmentation.1,2,7,9,10,12–15 In general, our ED results are similar to those previously reported; however, our intra-and interobserver CoV and ICC derived from ES measurements are higher than virtually all the ED values reported in the literature.
The higher accuracy and reproducibly of end-systolic RV mass assessment might have an impact in both research and clinical settings. For instance, a more precise method to obtain RV mass is desirable for monitoring the effects of drug therapy in longitudinal studies for patients with right sided cardiopulmonary diseases.15 Additionally, the higher accuracy of ES measurement of RV mass may be important for studies assessing demographical differences in the RV structure.
In the clinical context, RV mass indexed to body surface area (RVMI) has been shown to have diagnostic and prognostic significance. Katz et al 1 found that RVMI has a high positive correlation to mPAP. More recently, RV mass normalized to left ventricular mass, known as ventricular mass index (VMI), has shown to be a better predictor of mPAP2–4, suggesting MRI-derived VMI may be better than Doppler echocardiography in noninvasive assessment of pulmonary hemodynamics.3 Moreover, in a study on patients with chronic obstructive lung disease, RV mass was found to be more important than RV ejection fraction for earlier diagnosis of cor pulmonale.5
There were some limitations in our study. Our sample size is relatively small, although the sample sizes were in the 10–20 range for reproducibility in the normal hearts and 10–60 range in the abnormal RVs.1,2,7,9,10,15 We did not have patients with tachycardia, which could affect ES measurements. Despite some evidence in favor of a higher reproducibility of axial segmentation,16 we obtained our measurements from short-axis images according to the common practice.
In conclusion, MRI RV mass assessment is more accurate, reproducible, and faster when performed in the end-systolic phase. Future studies involving RV mass analysis may potentially benefit from this choice to reduce intra-and interobserver variability of measurements.
Acknowledgments
Contract grant sponsor: Cardiovascular Medical Research and Education Fund, Philadelphia, PA (to Y.H.); Contract grant sponsor: National Institutes of Health (NIH); Contract grant numbers: HL063954 and HL103723 (to R.C.G.).
REFERENCES
- 1.Katz J, Whang J, Boxt LM, Barst RJ. Estimation of right ventricular mass in normal subjects and in patients with primary pulmonary hypertension by nuclear magnetic resonance imaging. J Am Coll Cardiol 1993;21:1475–1481. [DOI] [PubMed] [Google Scholar]
- 2.Vogel-Claussen J, Shehata ML, Lossnitzer D, et al. Increased right ventricular Septomarginal trabeculation mass is a novel marker for pulmonary hypertension: comparison with ventricular mass index and right ventricular mass. Invest Radiol 2011;46:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saba TS, Foster J, Cockburn M, Cowan M, Peacock AJ. Ventricular mass index using magnetic resonance imaging accurately estimates pulmonary artery pressure. Eur Respir J 2002;20:1519–1524. [DOI] [PubMed] [Google Scholar]
- 4.Hagger D, Condliffe R, Woodhouse N, et al. Ventricular mass index correlates with pulmonary artery pressure and predicts survival in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology (Oxford) 2009;48:1137–1142. [DOI] [PubMed] [Google Scholar]
- 5.Pattynama PM, Willems LN, Smit AH, van der Wall EE, de Roos A. Early diagnosis of cor pulmonale with MR imaging of the right ventricle. Radiology 1992;182:375–379. [DOI] [PubMed] [Google Scholar]
- 6.McLure LE, Peacock AJ. Imaging of the heart in pulmonary hypertension. Int J Clin Pract Suppl 2007:15–26. [DOI] [PubMed] [Google Scholar]
- 7.Mooij CF, de Wit CJ, Graham DA, Powell AJ, Geva T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J Magn Reson Imaging 2008;28:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J 2004;147: 218–223. [DOI] [PubMed] [Google Scholar]
- 9.Blalock SE, Banka P, Geva T, Powell AJ, Zhou J, Prakash A. Interstudy variability in cardiac magnetic resonance imaging measurements of ventricular volume, mass, and ejection fraction in repaired tetralogy of Fallot: a prospective observational study. J Magn Reson Imaging 2013;38:829–835. [DOI] [PubMed] [Google Scholar]
- 10.Caudron J, Fares J, Lefebvre V, Vivier PH, Petitjean C, Dacher JN. Cardiac MRI assessment of right ventricular function in acquired heart disease: factors of variability. Acad Radiol 2012;19:991–1002. [DOI] [PubMed] [Google Scholar]
- 11.Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakken NH, Velthuis BK, Vonken EJ, Mali WP, Cramer MJ. Cardiac MRI: standardized right and left ventricular quantification by briefly coaching inexperienced personnel. Open Magn Reson J 2008;1:104–111. [Google Scholar]
- 13.McDonald KM, Parrish T, Wennberg P, et al. Rapid, accurate and simultaneous noninvasive assessment of right and left ventricular mass with nuclear magnetic resonance imaging using the snapshot gradient method. J Am Coll Cardiol 1992;19:1601–1607. [DOI] [PubMed] [Google Scholar]
- 14.Beygui F, Furber A, Delepine S, et al. Routine breath-hold gradient echo MRI-derived right ventricular mass, volumes and function: accuracy, reproducibility and coherence study. Int J Cardiovasc Imaging 2004;20:509–516. [DOI] [PubMed] [Google Scholar]
- 15.Doherty NE 3rd, Fujita N, Caputo GR, Higgins CB. Measurement of right ventricular mass in normal and dilated cardiomyopathic ventricles using cine magnetic resonance imaging. Am J Cardiol 1992;69: 1223–1228. [DOI] [PubMed] [Google Scholar]
- 16.Alfakih K, Plein S, Bloomer T, Jones T, Ridgway J, Sivananthan M. Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging. J Magn Reson Imaging 2003;18:25–32. [DOI] [PubMed] [Google Scholar]


