Abstract
Because of a soaring number of opioid-related deaths during the past decade, opioid use disorder has become a prominent issue in both the scientific literature and lay press. Although most of the focus within the emergency medicine community has been on opioid prescribing—specifically, on reducing the incidence of opioid prescribing and examining alternative pain treatment—interest is heightening in identifying and managing patients with opioid use disorder in an effective and evidence-based manner. In this clinical review article, we examine current strategies for identifying patients with opioid use disorder, the treatment of patients with acute opioid withdrawal syndrome, approaches to medication-assisted therapy, and the transition of patients with opioid use disorder from the emergency department to outpatient services.
INTRODUCTION
Opioid misuse is a major public health emergency in the United States, affecting communities large and small, urban and rural, affluent and poor.1,2 The opioid epidemic is unique in its vast reach, and a rapid increase in opioid-related deaths has led to declarations of a national crisis, with urgent calls to focus on evidence-based strategies to curb the epidemic and increase federal funding for treatment programs and opioid abuse-related research.3
According to the 2015 National Survey on Drug Use and Health, an estimated 3.8 million individuals, composing 1.4% of the US population aged 12 years and older, were current misusers of pain relievers.4 An additional 329,000 people aged 12 years and older use heroin. During the same year, more than 2.1 million individuals initiated the inappropriate use of prescription pain medications, and nearly 135,000 became new heroin users.5 There were 63,632 drug overdose deaths in 2016, representing a 21.4% increase from 2015.6 Furthermore, 66.4% of drug overdose deaths involved an opioid (illicit, prescription, or both), an increase of 27.7% from 2015.7 Since 2000, there has been a 200% increase in the rate of opioid overdose deaths, with heroin and synthetic opioids other than methadone considered the primary drivers.8
Within the US health care system, emergency departments (EDs) are often at the forefront of the opioid epidemic, treating individuals with opioid overdose, complications from opioid use, or long-term opioid addiction.7 To date, much of the focus within the emergency medicine community has been on opioid prescribing patterns, addressing concerns that physician prescribing may be an important driver of opioid abuse, dependence, and overdose.9 Since 2012, the American College of Emergency Physicians (ACEP) has promoted an opioid prescribing policy that encourages the use of nonopioid analgesics to treat pain when appropriate.10,11 However, ED prescribing reflects less than 5% of total opioid prescribing in terms of the total quantity of opioids in morphine equivalents.12
There is significantly less emphasis on establishing best practices for transitioning patients with opioid use disorders from the ED to appropriate longitudinal services and development of evidence-based treatment strategies. Furthermore, the Center for Behavioral Health Statistics and Quality recognizes that many patients with substance use disorders, including opioid use disorder, are not receiving treatment and many of these patients are not seeking treatment in traditional inpatient treatment centers.4 Expanding the availability of medication-assisted therapy and facilitating entry into appropriate outpatient treatment centers is a critical step in addressing this treatment gap.
This article examines the current body of evidence underpinning the identification of patients at risk for opioid use disorder, ED-based symptomatic treatment of acute opioid withdrawal, medication-assisted treatment of opioid use disorder on discharge from the ED, and transition to outpatient services.
Screening for Opioid Use Disorder in the ED
Emergency physicians require screening tools to identify patients with opioid use disorder, as well as those at risk for opioid-related harms, including overdose and misuse. Screening tools must be accurate, reliable, and easy to administer in the ED environment. They also must be brief and integrate seamlessly into existing ED work flows to promote widespread uptake and use.13 Competing clinical care priorities, limited time, and staff turnover present significant challenges to screening in a busy ED environment. Although many opioid screening tools have been validated, not all have been examined in the ED environment, and therefore generalizability should be examined before their use in the ED.
Table 1 provides an overview of commonly used screening tools for opioid abuse, misuse, and dependence. The Opioid Risk Tool is a self-report screening tool developed to assess the likelihood of opioid misuse among patients with chronic pain. It was initially tested and validated in patients presenting to a pain management clinic before initiation of prescription opioid therapy.14 The Current Opioid Misuse Measure and the Addiction Behaviors Checklist were both developed and validated in a pain clinic setting to clarify aberrant drug-related behavior in a population of patients already receiving long-term opioid therapy.15,16
Table 1.
Opioid use disorder screening tools.
| Tool | Author | Population | Methods | Screening Tool Characteristics |
|---|---|---|---|---|
| Opioid Risk Tool (ORT) | Webster (2005)14 | Newly enrolled adult patients at a pain clinic. Administered before beginning of opioid therapy for pain management. | Brief self-report, 10 questions (yes, no). |
Assesses personal and family history of substance abuse, H/O sexual abuse, and psychological disease. |
| Revised Screener and Opioid Assessment for Patients With Pain (SOAPP-R) | Butler (2008)17 Reyes-Gibby (2016)18 Weiner (2015)19 |
Adult patients with chronic noncancer pain treated at pain clinics. Assessed for feasibility in the ED. | Self-report, 24 questions. Likert 5-point scale (“never” to “very often”). |
Short (95% completed in <5 min), easy to score, assessed in the ED setting. Sensitivity 0.81, specificity 0.68 (using a cutoff score of 18). |
| Current Opioid Misuse Measure (COMM) | Butler (2008)15 |
Adult noncancer chronic pain patients. Assesses risk for aberrant drug-taking behavior before the initiation of opioid therapy—chronic pain patients. | 17 items, patient selfassessment. Likert 5-point scale. |
Sensitivity 0.77, specificity 0.68 (using a cutoff score of 9). |
| Addiction Behaviors Checklist (ABC) |
Wu (2006)16 | Adult patients with chronic pain already prescribed opioids or sedative analgesics. | 20 questions (yes, no). | Assesses addictive behaviors exhibited “since the last visit” and “within the current visit.” Longitudinal assessment. Sensitivity 0.88, specificity 0.86 (using a cutoff score of 3). |
| Alcohol Smoking and Substance Involvement Screening Test (ASSIST V 3.0) | WHO (2002)20 | Adults with no history of substance use, history of use, and history of dependence. | Interviewer-administered pencil-and-paper questionnaire and screens. | Addresses multiple addictive substances, including opioids. Sensitivity and specificity developed for use/abuse and abuse/dependence. Sensitivity 0.75, specificity 0.65 (for abuse/dependence). |
| NIDA-Modified Alcohol, Smoking and Substance Involvement Screening Test (NIDA-m-ASSiST) |
NIDA21 Blow (2017)22 Bogenschutz (2014)23 Macias-Konstantopoulos (2014)24 |
Intended for adults in the primary care setting. Used effectively in the ED. | Patient interview or online self-assessment. | Patients are asked about street opioids, such as heroin, and misuse of prescription opioids separately. Has not been validated. |
NIDA, National Institute on Drug Abuse.
Likewise, the Revised Screener and Opioid Assessment for Patients With Pain (SOAPP-R) was also initially developed and validated in a cohort of individuals seeking outpatient care for chronic pain before receiving opioid therapy.17 The SOAPP-R is a 24-question assessment, longer than most other drug and alcohol screening tools used in ED setting. However, SOAPP-R correlates highly with opioid use disorder in the ED setting, and another study demonstrated the ability to administer SOAPP-R through tablet computers in the ED.18,19 The latter study found that most patients were able to complete the screening tool in 5 minutes or less.19
The World Health Organization designed and validated the Alcohol, Smoking and Substance Involvement Screening Test to detect substance use problems among primary care patients.20 The National Institute on Drug Abuse developed the modified Alcohol, Smoking and Substance Involvement Screening Test to address illicit opioid use, including heroin, and misuse of prescription opioids.21 Although evidence is limited, use in ED research settings demonstrates the tool’s ability to successfully identify patients with nonmedical prescription opioid use and other substance use problems.22−24
ACEP and others have suggested that statewide prescription drug monitoring programs may also serve as an important screening tool to identify patients at risk for opioid misuse.25 At the population level, states that implement a robust prescription drug monitoring program realize significant reductions in opioid-related overdose deaths.26 However, the programs do not capture data on patients who obtain opioids without a prescription, and there is no current evidence that these programs alone are capable of identifying individual patients with opioid use disorder. One recent study attempted to determine whether a combination of SOAPP-R and use of a prescription drug monitoring program could predict which ED patients being considered for discharge with an opioid prescription could be considered high risk for abuse potential.27 Although the SOAPP-R has a high negative predictive value, the sensitivity of this self-report tool for detecting high-risk behavior based on prescription drug monitoring program criteria was low, suggesting that self-report tools and the prescription drug monitoring programs provide important but different types of information and are best used in tandem. Another study from a single academic urban medical center showed that prescription drug monitoring programs were unable to detect many patients with self-reported opioid use disorder.28 Therefore, we do not recommend using the prescription drug monitoring program alone to assess for risk for opioid use disorder. However, in combination with self-reported data, the program may present complementary objective data worth considering in the screening process.
Finally, significant questions remain about whom to screen for opioid use disorder in the ED. At present, no formal guidelines exist. In accordance with the current body of evidence, we do not believe in the justification of universal screening, given a large number of limitations to the currently available self-report screening tools. However, judicious use of prescription drug monitoring programs when implemented in an effective and easy-to-use manner, along with a targeted screening of at-risk individuals (eg, reported history of opioid misuse, positive drug screen result) or of individuals who will be discharged with opioids, is recommended and in fact mandated in many states.24,29 This is an area that requires further investigation.
ED Management of Acute Opioid Withdrawal
Abrupt discontinuation of long-term prescription or illicit opioids can produce withdrawal symptoms as early as hours after the last use (eg, 3 to 5 hours after last fentanyl use, 6 hours after last heroin use). Initial symptoms of anxiety, agitation, and restlessness are distressing to patients, which may lead to increased irritability and aggression directed toward health care providers. As a result, providers may be less empathetic toward their patients, often unintentionally, further deviating from a therapeutic patient-provider relationship. Without treatment, acute opioid withdrawal is likely to progress, and the patient may experience excessive yawning, lacrimation, rhinorrhea, diffuse myalgias, abdominal cramping, nausea, vomiting, diarrhea, and insomnia. Physical examination findings can include mydriasis, tachycardia, hypertension, diaphoresis, and piloerection.30
Symptoms of acute opioid withdrawal are often poorly tolerated. Even when the severity of concurrent medical conditions necessitates inpatient admission, patients experiencing acute opioid withdrawal may choose to leave against medical advice if they believe there is no prospect of pain relief.31 Symptomatic management of acute opioid withdrawal can improve compliance with necessary treatment of concurrent medical or surgical conditions and therefore improve health outcomes.32,33 Managing patient symptoms, along with expectations, is key to caring for patients with acute opioid withdrawal.
Treatment of opioid withdrawal requires identification of symptoms and assessment of clinical status. Several validated tools currently exist, including the Clinical Institute Narcotic Assessment scale, the Short Opiate Withdrawal Scale, and the Clinical Opiate Withdrawal Scale.34−36 Many experts consider the latter, an 11-item clinician-administered scale assessing opioid withdrawal, to be the most useful evidence-based tool in the ED setting. Its brevity and simplicity allow easy, rapid recognition of potential opioid withdrawal syndromes and can assist clinicians in making treatment decisions.
Although acute opioid withdrawal is not typically life threatening, failure to address withdrawal and the circumstances that may ensue from untreated opioid withdrawal can result in morbidity and mortality.37 In particular, patients who have comorbid conditions, such as coronary artery disease, congestive heart failure, insulin-dependent diabetes mellitus, epilepsy, or liver failure, are at increased risk of death in cases of opioid withdrawal. This is an important distinction from the historical teaching that opioid withdrawal is not dangerous, often leading to neglect in addressing this potentially life-threatening situation. Whether persons engage in dangerous criminal activity to obtain opioids or choose to forgo necessary treatment for a serious medical condition, desperate actions they take to experience symptom relief from acute opioid withdrawal can increase the risk of life-threatening harm and death.
Below, we present options for targeted opioid withdrawal and management, as well as a variety of other medications to consider for symptomatic opioid withdrawal treatment for patients who do not require opioids for acute pain.
Buprenorphine
A partial μ-agonist with a long half-life (24 to 60 hours), buprenorphine has higher affinity yet lower intrinsic activation at the μ-type opioid receptor than many full agonists, including heroin, oxycodone, morphine, and methadone. Because buprenorphine will displace full opioid agonists without providing the same degree of receptor activation, a sufficient period after the last opioid use must transpire before administration of buprenorphine. Whether initiating induction or treating acute withdrawal, physicians should not administer buprenorphine until moderate symptoms of opioid withdrawal have developed. When administered before the onset of withdrawal, buprenorphine can precipitate moderate opioid withdrawal symptoms. The period of abstinence required both before induction and acute withdrawal treatment will vary in part as a function of the half-life of the opioid last used. Expect spontaneous withdrawal to occur within 6 to 12 hours in the case of short-acting opioids such as heroin and oxycodone, and within 24 to 72 hours for opioids with longer half-lives such as methadone.
Traditional teaching is that after an initial sublingual dose of 2 to 4 mg of buprenorphine, a 60- to 90-minute observation period is necessary to ensure that withdrawal symptoms improve.39 If symptoms persist, dose titration in 2- to 4-mg increments may be necessary to achieve clinical effectiveness. A maximum initial daily dose of 8 to 12 mg of buprenorphine is formally recommended.40 Nonopioid medications can be used to manage residual withdrawal symptoms. However, individual providers with significant experience managing opioid withdrawal have found that initiating treatment with higher doses of buprenorphine (eg, 8 mg), more rapid titration (eg, 8-mg increments), and a higher 24-hour maximum dose may be required in some patients with heavy routine opioid use. Simultaneously, given buprenorphine’s partial agonist mechanism of action, there is a “ceiling effect” whereby higher doses of the medication may not lead to additional receptor activation and desired effect.40,41 This results in a more favorable adverse effects profile compared with that of methadone and other opioid receptor agonists. Additional information on the requirements for prescribing opioids in the ED and the initiation of medication-assisted therapy is described below.
Buprenorphine is commonly paired with naloxone in the sublingual form to prevent abuse. Naloxone has a low bioavailability when taken orally, but if the tablets are dissolved and injected, the antagonist effects of naloxone will predominate, limiting abuse potential. However, the combined medication should not be provided to pregnant patients because fetal exposure to naloxone may precipitate withdrawal. Buprenorphine without naloxone is safe in pregnancy and, for compliant mothers, is associated with milder neonatal abstinence syndrome than in the neonates of mothers managed with methadone.42
Patients already enrolled in a methadone treatment program can continue to receive methadone treatment in the ED when being admitted to the hospital to prevent and treat acute methadone withdrawal. Methadone is a long-acting μ-agonist (full agonist) with a mean half-life of approximately 8 to 59 hours in adults. Methadone is also safe during pregnancy. The recommended starting dose is tailored to the patient’s opioid use history, concomitant substance abuse, previous experience with methadone, and other psychiatric and medical comorbidities.43 Adverse effects include QT-interval prolongation, and caution should be taken when combining methadone with other QT-interval-prolonging medications, such as ondansetron. Additionally, methadone is metabolized by the cytochrome P450 enzyme system of the liver and should be used with caution in patients with liver disease, concomitant use of cytochrome P450 inducers, or concomitant use of medications with the potential for hepatotoxicity.32
Clonidine
Albeit considered an off-label use of this α2-adrenergic agonist, research demonstrates the effective use of clonidine in controlling acute opioid withdrawal symptoms and lessening the likelihood of severe withdrawal.44 The recommended dose is 0.1 mg orally every 6 hours until symptoms resolve, up to a maximum starting dose of 0.4 mg during a 24-hour period. Transdermal clonidine patches may also be considered. Adverse effects include bradycardia and hypotension, and one should consider hemodynamic monitoring when administering intravenous clonidine.31 Clonidine alone may not be as effective as other monotherapies for the treatment of severe acute opioid withdrawal and therefore is often used in conjunction with μ-agonist therapy.45
Other Symptomatic Opioid Withdrawal Treatment
In addition to targeted opioid withdrawal treatment, symptom management can also bring comfort to patients presenting to the ED with acute withdrawal. In the case of excessive vomiting and diarrhea, standard ED resuscitation of a volume-depleted patient with crystalloid fluids is appropriate. Monitoring of electrolytes and appropriate repletion may be required in patients with evidence of moderate to severe dehydration. Table 2 presents commonly used medications for the symptomatic treatment of opioid withdrawal, as well as possible adverse effects.
Table 2.
Nonopioid pharmacologic treatment options for symptomatic treatment of opioid withdrawal.
| Symptom | Medication | Common/Dangerous Adverse Effects |
|---|---|---|
| Abdominal cramps | Dicyclomine | Anticholinergic, dizziness, nausea |
| Anxiety/restlessness | Diazepam, hydroxyzine | Oversedation (use caution when administering benzodiazepines in conjunction with μ-agonists) |
| Diarrhea | Loperamide | Torsades de pointes and cardiac arrest with higher doses |
| Dyspepsia | Famotidine | QT-interval prolongation |
| Myalgias | Methocarbamol | Dysphoria and suicidal thoughts with higher doses |
| Nausea/vomiting | Ondansetron | QT-interval prolongation |
| Pain | Acetaminophen, ibuprofen | Allergic reaction |
| Insomnia | Trazodone | Orthostatic hypotension |
TRANSITIONING PATIENTS FROM THE ED
Medication-Assisted Therapy
In 2015, the American Society of Addiction Medicine published the “National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use,” which was intended to provide information on evidence-based treatment of opioid use disorder.30 Medication-assisted therapy is a term that refers to any addiction treatment that includes the use of pharmacologic treatments. For opioids, such therapy uses pharmacologic properties of medications that act as agonists, partial agonists, or antagonists of the μ-type opioid receptor, including methadone, buprenorphine, and naltrexone. When methadone is used as medication-assisted therapy, follow-up care can be provided to patients only by an opioid treatment program that offers supervised dosing and is required to include elements of a psychosocial intervention.38,46 When buprenorphine is used as medication-assisted therapy, follow-up care can be obtained through opioid treatment programs, hospitals, health departments, and other qualified providers, as detailed below.
Medication-assisted therapy improves long-term outcomes for patients with opioid use disorder. Specifically, patients who receive opioid agonist therapy as part of treatment for opioid use disorder have a decreased chance of fatal overdose compared with those who receive psychological counseling alone.47 Furthermore, patients receiving maintenance buprenorphine for at least a year require fewer ED visits and hospitalizations compared with those who discontinue buprenorphine. Thus, early initiation and maintenance of medication-assisted therapy can significantly affect health care use and improve wellness for patients with opioid use disorder.48
Initiation of buprenorphine for patients with opioid use disorder in the ED is efficacious and safe. In a seminal study, D’Onofrio et al49 evaluated the efficacy of ED initiation of buprenorphine in patients with opioid use disorder compared with brief behavioral counseling alone or usual care. Patients who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)criteria for opioid dependence (replaced in DSM-5 by opioid use disorder) and had a urine specimen that tested positive for opioids were randomized to one of the treatment arms. Individuals randomized to the buprenorphine group were further assessed for withdrawal with the Clinical Opiate Withdrawal Scale. If a patient with opioid use disorder had symptoms consistent with moderate to severe opioid withdrawal, he or she was provided with a dose of buprenorphine in the ED, as well as a prescription for enough medication to last until a follow-up primary care appointment could take place within 72 hours of discharge. Patients not in active withdrawal were instructed to begin receiving buprenorphine once symptoms of moderate withdrawal developed and, likewise, were provided with a prescription and primary care follow-up in the hospital’s clinic that had established protocols within 72 hours. At 30 days after ED discharge, patients randomized to ED initiation of buprenorphine were more likely to be engaged in addiction treatment and had less illicit opioid use per week than those randomized to either brief behavioral counseling alone or usual care. These differences remained significant 2 months after the ED visit when primary care physicians continued buprenorphine in outpatient clinics.50 Furthermore, medication-assisted therapy initiated in the ED was found to be cost-effective compared with referral to community-based treatment, further supporting ED initiation of buprenorphine for patients with opioid use disorder when treatment can be continued in an outpatient office setting.51
Prescribing buprenorphine can present a number of challenges and requires careful consideration. First, the Drug Addiction Treatment Act of 2000 (DATA 2000) created a special licensing system for physicians to prescribe opioid-based medications to treat addiction.52 To obtain a Drug Enforcement Administration × license, or “DATA 2000 waiver,” a physician must complete an 8-hour training program either online or in person.53 Participants receive training on identifying appropriate candidate patients for buprenorphine treatment, how best to use medications in addiction treatment, and how to apply for the waiver to prescribe.53 The Drug Enforcement Administration also provides guidance to practitioners without a waiver in emergency situations. Specifically, physicians who have not completed the training and do not hold a DATA 2000 waiver can administer opioid medications (including buprenorphine) to a patient to relieve acute withdrawal symptoms while arranging for a referral to addiction treatment. This treatment can be administered only in the ED during 72 hours, and additional doses cannot be dispensed or prescribed without the appropriate waiver.54 Under the so-called 72-hour rule (21CFR, Part 1306.07[b]), either buprenorphine or methadone can be dispensed, not prescribed, as an induction or bridge medication from the ED up to 3 consecutive days while arrangements are made for referral to treatment. To our knowledge, there are no published trials evaluating the safety and efficacy of dispensing methadone to initiate medication-assisted therapy from the ED, and thus buprenorphine is most often used in this practice.
Patients under consideration for medication-assisted therapy should be evaluated to ensure that they meet criteria for opioid use disorder. Additionally, comorbidities including polysubstance use, current methadone use, and chronic pain requiring high daily doses of opioids are also important factors to consider before buprenorphine treatment is embarked on. Physicians with a DATA 2000 waiver can decide whether it is appropriate to initiate buprenorphine treatment at home or in the ED. Home induction of buprenorphine is an acceptable treatment strategy used by many physicians and can decrease the need for lengthy ED visits when a patient is not in withdrawal. Regardless of whether a patient receives medication-assisted therapy, supplying or prescribing naloxone rescue kits for all opioid use disorder patients, and counseling them on proper kit use, can be lifesaving, given their elevated risk of accidental overdose. This important harm reduction measure is well studied, effective, and supported by ACEP.55,56 Finally, any patient receiving ED initiation of buprenorphine should ideally be discharged with a streamlined plan for prompt follow-up in an outpatient clinic or addiction treatment facility to ensure continued medication-assisted therapy induction, stabilization, and long-term maintenance management of opioid use disorder. Treatment with buprenorphine in the ED does not preclude transitioning patients to methadone therapy as part of a comprehensive outpatient treatment plan.
Linkage to Treatment
Coordinated care for complex chronic conditions has repeatedly shown marked positive influence on disease trajectory.57,58 The treatment of opioid use disorder is no different, and a coordinated transfer of patient care to the outpatient setting has received increased attention.38,59,60 However, EDs face significant challenges in referring patients with opioid use disorder in a timely and coordinated fashion. Opioid use disorder treatment is often disconnected from the acute care health care system, and very few programs have competent referral mechanisms that can be accessed by an emergency physician. This disconnect places ED providers in a challenging position when attempting to care for patients with opioid use disorder. Although providers may be able to identify serious illness requiring specialty follow-up, EDs may not have the mechanisms or resources to ensure appropriate linkage to care.
In general, a comprehensive treatment strategy for patients with opioid use disorder involves 3 components: medication-assisted therapy, psychological interventions, and social support or case management. Although each has its own utility, a strategy combining all 3 is likely to be more successful in achieving lasting effects, especially in patients with severe injection opioid use disorder.61 As noted above, medication-assisted therapy has been shown to be safe when initiated in the ED, and consideration should be given to doing so. This is particularly true if appropriate follow-up services can be arranged. Psychological therapy may come in many forms, from individual psychosocial interventions to group or family therapy. Additionally, psychological therapy can be complemented by psychiatric care, depending on the presence and severity of other mental health conditions. Finally, social support and case management services help ensure that patients complete evaluation and treatment programs through improved navigation of social situations and overcoming of potential barriers to care, leading ultimately to a successful recovery. The concept of peer support or recovery coaching is one proposed support system, although others exist, and more evidence is generally needed in regard to the efficacy of these programs.62,63
Ideally, outpatient treatment should begin as soon as possible and preferably within 72 hours of ED evaluation. A rapid transition to outpatient care helps to ensure that patients receive the necessary services, including medication-assisted therapy.64 To facilitate this process, some institutions have developed a “bridge” clinic that assists with obtaining next-day evaluations for continuation of therapy and ensuring that patients receive an appropriate referral for opioid use disorder and associated comorbidity.65 However, it is recognized that robust linkages to outpatient medication-assisted therapy are not available at many institutions, and therefore providers and health care systems are encouraged to develop reasonable outpatient follow-up plans.
In addition to having appropriate and timely outpatient services available to ED patients with opioid use disorder, the way in which patients are referred may have a considerable effect on their long-term care. Traditionally, and in most communities today, there is little to no communication between EDs and treatment programs. These “cold handoffs” often result in delays in outpatient services, repeated assessments, gaps in medication-assisted therapy, and overall worse outcomes.66 One strategy for improving coordination of care between the ED and outpatient settings is a “warm handoff” between providers,67 which has been defined by the Agency for Healthcare Research and Quality as “a handoff that is conducted in person, between two members of the healthcare team, in front of the patient (and family if present).”68 Although it may not always be possible or realistic to expect such handoffs to occur in the ED environment, having a direct conversation with the outpatient team receiving the patient may help alleviate some of the barriers in care and provide a more streamlined transition process. In busy EDs, training and using allied health professionals (eg, social workers) as important conduits in the transition process may improve the rate at which communication with outpatient providers and clinics occurs. Although not all EDs will be adequately resourced to perform warm handoffs, moving toward an improved system of communication is likely to benefit patients.
During times when the outpatient treatment team may not be readily accessible (eg, nights, weekends), hotlines or referral coordinators can help bridge communication gaps. Where available, regional referral resources provide a mechanism by which patients can be linked with outpatient providers capable of delivering comprehensive opioid use disorder treatment, including medication-assisted therapy. Complicated assessment processes that delay appropriate access are challenging for patients to navigate and lead to poor follow-up compliance. Referral coordinators must facilitate the most rapid entry to comprehensive treatment possible and should be well trained in the overall management of opioid use disorder.
The transition of care from the ED to the outpatient setting represents a high-risk period for patients, and carefully coordinated care is essential to minimize the potential for acute opioid withdrawal and relapse. Outpatient services should be able to provide medication-assisted therapy, often after it has been initiated in the ED. Furthermore, clinics should be capable of providing or coordinating the appropriate counseling and social services, both of which are likely to lead to improved compliance and better health outcomes. Finally, a rapid referral system is essential because delays are likely to result in poor follow-up rates. Warm handoffs and regional referral systems are 2 mechanisms by which communication between the ED and outpatient providers can be improved and rapid entry into long-term treatment achieved.
CONCLUSION
In addition to providing high-quality acute care around the clock, EDs also function as a key entry point into the health care system for many patients. This is particularly true for vulnerable populations with poor access to care, including many individuals with opioid use disorder. Uniquely situated on the front lines of the opioid epidemic, EDs treat opioid overdoses, as well as the complications of opioid use disorder and long-term addiction daily. As the opioid epidemic continues, EDs will play an integral part in mitigating the human toll on many levels through screening and identification of patients at risk for opioid use disorder, managing acute opioid withdrawal, initiating medication-assisted therapy, and coordinating linkage to outpatient treatment. However, much work remains to be done to create, validate, disseminate, and implement effective evidence-based strategies to accomplish these challenging tasks within the unique care environment of the ED.
Future research will need to focus on more than opioid prescribing and alternative pain management strategies in the ED. Specifically, more work is required to identify which patients to screen, what tools to use, and what technology can be leveraged (eg, portable electronic devices, waiting room kiosks) to adequately assess opioid use disorder risk while minimizing the effect on ED patient turnaround times and ED provider workload. Additionally, ED initiation of buprenorphine is safe and efficacious, and EDs should consider how such a treatment program with aggressive linkages to an outpatient medication-assisted therapy program could be initiated in their setting. Ongoing, multicenter, randomized trials will assess for safety and generalizability to both academic and community ED settings and provide information on the best implementation strategy for this evidence-based treatment. Future clinical studies fielding the use of novel opioid use disorder treatment agents, including pharmacology and vaccines, need to include ED patient populations. Finally, the effect of a coordinated systems-based approach to treating opioid use disorder, spanning the ED to the outpatient setting, needs to be evaluated rigorously in large, pragmatic trials.
EDs will continue to care for patients with opioid overdoses, complications of opioid misuse, and chronic addiction. National calls to declare the opioid epidemic a public health emergency and rapidly increase treatment capacity across the United States must include and engage the emergency medicine community. A robust infrastructure to support, educate, and enable emergency physicians to manage opioid use disorder in an evidence-based fashion and rapidly transition care to outpatient services is a necessary step in turning the tide against an opioid epidemic affecting communities nationwide.
RECOMMENDATIONS
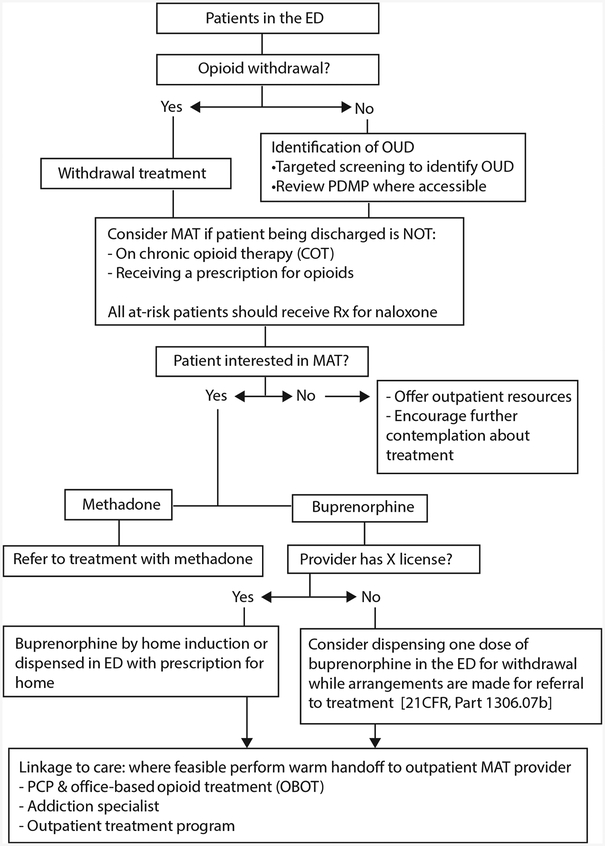
The Figure presents an overview of the ED screening, treatment, and referral cascade for patients with suspected opioid use disorder. In addition, we provide the following recommendations:
Figure.
ED screening, treatment, and referral for opioid use disorder. OUD, Opioid use disorder; MAT, medication-assisted therapy; PCP, primary care physician.
- Consider targeted screening of individuals at risk (eg, history of opioid misuse, positive drug screen result) for opioid use disorder. We do not recommend universal screening.
- All current opioid use disorder screening tools have some weakness. Of the tools currently available, we recommend using the SOAPP-R or National Institute on Drug Abuse-modified Alcohol, Smoking and Substance Involvement Screening Test given before testing and feasibility in the ED setting.
- ED providers should use the prescription drug monitoring program when prescribing opioids and consider it as an adjunct tool when screening for opioid use disorder.
- We recommend treating acute opioid withdrawal in symptomatic ED patients with opioid use disorder who are not receiving long-term opioid therapy for pain.
- Consider buprenorphine in patients with moderate withdrawal symptoms.
- Patients already receiving methadone or buprenorphine treatment in the outpatient setting should continue receiving these therapies after confirmation of current doses.
- Nonopioid medications should be used as needed for symptomatic treatment of acute withdrawal.
- All ED patients with acute opioid withdrawal should be considered for medication-assisted therapy and provided appropriate follow-up.
- We recommend that ED-initiated medication-assisted therapy be considered for all patients with opioid use disorder.
- Buprenorphine should be considered the medication of choice when medication-assisted therapy is initiated in the ED.
- All ED patients with identified opioid use disorder should receive a naloxone rescue kit, or a prescription for such a kit, and be counseled about proper kit use regardless of whether medication-assisted therapy is initiated.
- We recommend the development of systems of care that facilitate the transition of patients with opioid use disorder from the ED to the community setting.
- When possible, warm handoffs are the preferred method of transition.
- Outpatient settings should be able to continue or initiate medication-assisted therapy, provide psychological interventions, and offer social support or case management.
Acknowledgments
The authors acknowledge the ACEP Public Health and Injury Prevention Committee for their feedback and insight, and Margaret Montgomery, RN, for facilitating this working group and driving the project to completion. We would also like to thank Ly Huynh for her assistance with tables, figures, and references.
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. Dr. Whiteside’s institution has received grant money from the National Institute of Drug Abuse, City of Seattle, Department of Justice, and the Laura and John Arnold Foundation for investigator-initiated research.
REFERENCES
- 1.Bosman J Inside a killer drug epidemic: a look at America’s opioid crisis. January 6, 2017. Available at: https://www.nytimes.com/2017/01/06/us/opioid-crisis-epidemic.html?_r=1. Accessed May 5, 2018.
- 2.US drug overdose deaths: a global challenge. Lancet. 2016;387:404. [DOI] [PubMed] [Google Scholar]
- 3.Williams AR, Bisaga A. From AIDS to opioids: how to combat an epidemic. N Engl J Med. 2016;375:813–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2015 National Survey on Drug Use and Health. HHS Publication SMA 16–4984, NSDUH Series H-51. Available at: http://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2015/NSDUH-FFR1-2015/NSDUH-FFR1-2015.pdf. Accessed December 29, 2017.
- 5.Lipari RN, Williams MR, Copello EAP, et al. Risk and protective factors and estimates of substance use initiation: results from the 2015 National Survey on Drug Use and Health. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-PreventionandInit-2015/NSDUH-PreventionandInit-2015.htm. Accessed June 15, 2017. [PubMed]
- 6.Hedegaard H, Warner M, Miniño AM. Drug Overdose Deaths in the United States, 1999–2016. Hyattsville, MD: US Dept of Health & Human Services, CDC, National Center for Health Statistics; 2017; NCHS Data Brief, No. 294. Available at: https://www.cdc.gov/nchs/data/databriefs/db294.pdf. Accessed May 5, 2018. [Google Scholar]
- 7.Vivolo-Kantor AM, Seth P, Gladden RM, et al. Vital signs: trends in emergency department visits for suspected opioid overdoses—United States, July 2016-September 2017. MMWR Morb Mortal Wkly Rep. 2018;67:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudd RA, Aleshire N, Zibell JE, et al. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378–1382. [DOI] [PubMed] [Google Scholar]
- 9.Barnett ML, Olenksi AR, Jena AB. Opioid prescribing by emergency physicians and risk of long-term use. N Engl J Med. 2017;376:1896. [DOI] [PubMed] [Google Scholar]
- 10.Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60:499–525. [DOI] [PubMed] [Google Scholar]
- 11.American College of Emergency Physicians. Policy statement: optimizing the treatment of acute pain in the emergency department. Available at: https://www.acep.org/clinical—practice-management/optimizing-the-treatment-of-acute-pain-in-the-emergency-department. Accessed October 1, 2017.
- 12.Axeen S, Seabury SA, Menchine M. Emergency department contribution to the prescription opioid epidemic. Ann Emerg Med. 2018;71:659–667.e3. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JA, Woychek A, Vaughan D, et al. Screening for at-risk alcohol use and drug use in an emergency department: integration of screening questions into electronic triage forms achieves high screening rates. Ann Emerg Med. 2013;62:262–266. [DOI] [PubMed] [Google Scholar]
- 14.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432–442. [DOI] [PubMed] [Google Scholar]
- 15.Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu SM, Compton P, Bolus R, et al. The Addiction Behaviors Checklist: validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manag. 2006;32:342–351. [DOI] [PubMed] [Google Scholar]
- 17.Butler SF, Fernandez K, Benoit C, et al. Validation of the Revised Screener and Opioid Assessment for Patients With Pain (SOAPP-R). J Pain. 2008;9:360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes-Gibby CC, Anderson KO, Todd KH. Risk for opioid misuse among emergency department cancer patients. Acad Emerg Med. 2016;23:151–158. [DOI] [PubMed] [Google Scholar]
- 19.Weiner SG, Horton LC, Green TC, et al. Feasibility of tablet computer screening for opioid abuse in the emergency department. West J Emerg Med. 2015;16:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO Assist Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183–1194. [DOI] [PubMed] [Google Scholar]
- 21.National Institute on Drug Abuse. Screening for drug use in general medical settings resource guide. Available at: https://www.integration.samhsa.gov/clinical-practice/sbirt/NIDA_screening_for_drug_use.pdf. Accessed June 15, 2017.
- 22.Blow FC, Walton MA, Bohnert ASB, et al. A randomized controlled trial of brief interventions to reduce drug use among adults in the low-income urban emergency department: the Healthier You study. Addiction. 2017;112:1395–1405. [DOI] [PubMed] [Google Scholar]
- 23.Bogenschutz MP, Donovan DM, Mandler RN, et al. Brief intervention for patients with problematic drug use presenting in emergency departments: a randomized clinical trial. JAMA Intern Med. 2014;174:1736–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macias-Konstantopoulos WL, Dreifuss JA, McDermott KA, et al. Identifying patients with problematic drug use in the emergency department: results of a multi-site study. Ann Emerg Med. 2014;64:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American College of Emergency Physicians. Policy statement: electronic prescription drug monitoring programs. Available at: https://www.acep.org/Clinical—Practice-Management/Electronic-Prescription-Drug-Monitoring-Programs/. Accessed October 1, 2017.
- 26.Patrick SW, Fry CE, Jones TF, et al. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff (Millwood). 2016;35:1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner SG, Horton LC, Green TC, et al. A comparison of an opioid abuse screening tool and prescription drug monitoring data in the emergency department. Drug Alcohol Depend. 2016;159: 152–157. [DOI] [PubMed] [Google Scholar]
- 28.Hawk K, D’Onofrio G, Fiellin DA, et al. Past-year prescription drug monitoring program opioid prescriptions and self-reported opioid use in an emergency department population with opioid use disorder. Acad Emerg Med. 2017; 10.1111/acem.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broida RI, Gronowski T, Kalnow AF, et al. State emergency department opioid guidelines: current status. West J Emerg Med. 2017;18:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kampman K, Jarvis M. American Society of Addiction Medicine (ASMA) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noska A, Mohan A, Wakeman S, et al. Managing opioid use disorder during and after acute hospitalization: a case-based review clarifying methadone regulation for acute care settings. J Addict Behav Ther Rehabil. 2015;4:1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saitz R Discharges against medical advice. CMAJ. 2002;167:L647–L648. [PMC free article] [PubMed] [Google Scholar]
- 33.Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35:56–59. [DOI] [PubMed] [Google Scholar]
- 34.Tompkins DA, Bigelow GE, Harrison JA, et al. Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend. 2009;105:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253–259. [DOI] [PubMed] [Google Scholar]
- 36.Vernon MK, Reinders S, Mannix S, et al. Psychometric evaluation of the 10-item Short Opiate Withdrawal Scale-Gossop (SOWS-Gossop) in patients undergoing opioid detoxification. Addict Behav. 2016;60:109–116. [DOI] [PubMed] [Google Scholar]
- 37.Darke S, Larney S, Farrell M. Yes, people can die from opiate withdrawal. Addiction. 2017;112:199–200. [DOI] [PubMed] [Google Scholar]
- 38.Center for Substance Abuse Treatment. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Rockville, MD: Substance Abuse & Mental Health Services Administration; 2004; Treatment Improvement Protocol (TIP) Series, No. 40. [PubMed] [Google Scholar]
- 39.Waller R Medication assisted treatment (MAT) guidelines for opioid use disorders. Available at: http://www.michigan.gov/documents/mdhhs/MAT_Guidelines_for_Opioid_Use_Disorders_524339_7.pdf. Accessed May 5, 2018.
- 40.Dematteis M, Auriacombe M, D’Agnone O, et al. Recommendations for buprenorphine and methadone therapy in opioid use disorder: a European consensus. Expert Opin Pharmacother. 2017;18:1987–1999. [DOI] [PubMed] [Google Scholar]
- 41.Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. [DOI] [PubMed] [Google Scholar]
- 42.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baxter LE Sr, Campbell A, DeShields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377–386. [DOI] [PubMed] [Google Scholar]
- 44.Gowing L, Farrell M, Ali R, et al. Apha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2014;(3):CD002024. [DOI] [PubMed] [Google Scholar]
- 45.Gowing L, Ali R, White JM. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;(3):CD002025. [DOI] [PubMed] [Google Scholar]
- 46.Substance Abuse and Mental Health Services Administration. Medication and counseling treatment. Available at: https://www.samhsa.gov/medication-assisted-treatment/treatment#otps. Accessed May 5, 2018. [Google Scholar]
- 47.Pierce M, Bird SM, Hickman M, et al. Impact of treatment for opioid dependence on fatal drug-related poisoning: a national cohort study in England. Addiction. 2016;111:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo-Ciganic WH, Gellad WF, Gordon AJ, et al. Association between trajectories of buprenorphine treatment and emergency department and in-patient utilization. Addiction. 2016;111:892–902. [DOI] [PubMed] [Google Scholar]
- 49.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313:1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Onofrio G, Chawarski MC, O’Connor PG, et al. Emergency department-initiated buprenorphine for opioid dependence with continuation in primary care: outcomes during and after intervention. J Gen Intern Med. 2017;32:660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Busch SH, Fiellin DA, Chawarski MC, et al. Cost-effectiveness of emergency department-initiated treatment for opioid dependence. Addiction. 2017;112:2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drug Addiction Treatment Act of 2000, HR 2634,106th Congress, 2nd Sess (Wa 2000). [Google Scholar]
- 53.American Society of Addiction Medicine. Treatment of opioid use disorder course: includes waiver qualifying requirements. Available at: https://www.asam.org/education/live-online-cme/buprenorphine-course. Accessed December 19, 2017.
- 54.Drug Enforcement Administration. Administering or dispensing of narcotic drugs. 21 CFR §1306.07 (2016) Code of Federal Regulations. Available at: https://www.gpo.gov/fdsys/pkg/CFR-2016-title21-vol9/pdf/CFR-2016-title21-vol9-sec1306-07.pdf. Accessed December 19, 2017.
- 55.Bird SM, McAuley A, Perry S, et al. Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006–10) versus after (2011–13) comparison. Addiction. 2016;111:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.American College of Emergency Physicians. Naloxone prescriptions by emergency physicians. Available at: https://www.acep.org/Clinical—Practice-Management/Naloxone-Prescriptions-by-Emergency-Physicians/#sm.0001j6kleiafzdt5wgw1sc7thww4i. Accessed May 5, 2018.
- 57.Brennan-Ing M, Seidel L, Rodgers L, et al. The impact of comprehensive case management on HIV client outcomes. PLoS One. 2016;11:e0148865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whiteside LK, Darnell D, Jackson K, et al. Collaborative care from the emergency department for injured patients with prescription drug misuse: an open feasibility study. J Subst Abuse Treat. 2017;82:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss RD, Griffin ML, Potter JS, et al. Who benefits from additional drug counseling among prescription opioid-dependent patients receiving buprenorphine-naloxone and standard medical management? Drug Alcohol Depend. 2014;140: 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reif S, Braude L, Lyman DR, et al. Peer recovery support for individuals with substance use disorders: assessing the evidence. Psychiatr Serv. 2014;65:853–861. [DOI] [PubMed] [Google Scholar]
- 63.Samuels E Emergency department naloxone distribution: a Rhode Island department of health, recovery community, and emergency department partnership to reduce opioid overdose deaths. R I Med J (2013). 2014;97:38–39. [PubMed] [Google Scholar]
- 64.Substance Abuse and Mental Health Services Administration. Federal guidelines for opioid treatment programs. Available at: https://store.samhsa.gov/shin/content//PEP15-FEDGUIDEOTP/PEP15-FEDGUIDEOTP.pdf. Accessed December 21, 2017.
- 65.Chou R, Korthuis PT, Weimer M, et al. Medication-Assisted Treatment Models of Care for Opioid Use Disorder in Primary Care Settings. Rockville, MD: Agency for Healthcare Research & Quality; 2016. Technical Briefs, No. 28. Findings. Available at: https://www.ncbi.nlm.nih.gov/books/NBK402343/. Accessed December 29, 2017. [PubMed] [Google Scholar]
- 66.Herring AA. Emergency department medication-assisted treatment of opioid addiction. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/PDF%20E/PDF%20EDMATOpioidProtocols.pdf. Accessed October 1, 2017. [Google Scholar]
- 67.Sammer J Warm handoffs serve as the first step toward accountable care. Behav Healthc. 2015;35(24):26–27. [PubMed] [Google Scholar]
- 68.Agency for Healthcare Research and Quality. Warm handoff intervention: patient and family engagement in primary care. Available at: https://www.ahrq.gov/professionals/quality-patient-safety/patient-family-engagement/pfeprimarycare/interventions/warmhandoff.html. Accessed December 19, 2017.