Abstract
PURPOSE:
To review long-term outcomes of the University of Southern California Plaque Simulator (PS) software and Eye Physics (EP) plaques. We hypothesize that the PS/EP system delivers lower doses to critical ocular structures, resulting in lower rates of radiation toxicity and favorable visual outcomes compared to Collaborative Ocular Melanoma Study plaques, while maintaining adequate local tumor control.
METHODS AND MATERIALS:
Retrospective review of 133 patients treated for choroidal melanoma with 125I brachytherapy, using PS software and EP plaques, from 1990 through 2015. A dose of 85 Gy at a rate of 0.6 Gy/h was prescribed to the tumor apex (with a typical margin of 2 mm) over 7 days. Primary outcomes were local tumor recurrence, globe salvage, and metastasis. Secondary outcomes were changes in visual acuity and radiation complications.
RESULTS:
With median followup of 42 months, 5-year Kaplan–Meier estimated rates for tumor control, globe salvage, and metastatic-free survival were 98.3%, 96.4%, and 88.2%, respectively. Median doses to the macula and optic nerve were 39.9 Gy and 30.0 Gy, respectively. Forty-three percent of patients developed radiation retinopathy, and 20% developed optic neuropathy; 39% lost $ ≥6 Snellen lines of vision.
CONCLUSIONS:
The PS/EP system is designed to improve the accuracy and conformality of the radiation dose, creating a steep dose gradient outside the melanoma to decrease radiation to surrounding ocular structures. We report favorable rates of local tumor control, globe salvage, metastases, and radiation complications when compared to the Collaborative Ocular Melanoma Study and other studies. Overall, the PS/EP system results in excellent tumor control and appears to optimize long-term visual and radiation-related outcomes after brachytherapy. © 2018 American Brachytherapy Society. Published by Elsevier Inc. All rights reserved.
Keywords: Uveal melanoma, Choroidal melanoma, Brachytherapy, Eye physics, Plaque simulator, Radiation retinopathy
Introduction
The Collaborative Ocular Melanoma Study (COMS) was the first multicenter randomized clinical trial designed to compare the outcomes of brachytherapy with enucleation, the previous standard of care, for patients with medium-sized choroidal melanomas (measuring 2.5 to 10 mm apical height and 5- to 16-mm basal dimension). COMS was initiated in 1986 and included over 600 patients with medium-sized melanoma treated with 125I brachytherapy at a mean radiation dose of 85 Gy to the tumor apex. The COMS study established episcleral plaque radio-therapy as an attractive alternative to enucleation, with equal survival rates and the benefit of eye and potential vision preservation.
The Eye Physics (EP) plaques and Plaque Simulator (PS) were developed at the University of Southern California (USC) to treat choroidal melanomas. PS software fuses fundus photography, ocular ultrasonography, and CT or MRI for 3-dimensional tumor modeling. The goal was to use a 3-dimensional tumor-modeling program with the intended advantage over the COMS plaques of decreasing radiation-related morbidity by delivering conformal radiation to the tumor. EP plaques were designed with colli-mating slots for each 125I seed to eliminate wasted lateral radiation, thus reducing radiation doses to adjacent retina (1, 2). The slots are deeper near the center of the plaque and shallower near the periphery of the plaque, resulting in a more homogenous dose distribution (1). EP plaques are also available in a greater variety of shapes, sizes, and curvatures for customizable tumor coverage (1). An additional benefit was preoperative tumor localization, thus facilitating surgical plaque placement (1, 3).
In 2013, Berry et al. published results for a 20-year retrospective review of 82 patients at USC with mediumsized choroidal melanomas treated using 125I brachytherapy with the PS/EP system (4). The purpose of the study was to compare the outcomes of the PS/EP system with COMS outcomes. Median followup time was 46.8 months, and 97.6% of patients retained their eyes after treatment. The Kaplan–Meier estimated rate of local tumor control at 5 years was 97%. This compared favorably to the COMS Kaplan–Meier estimated enucleation rate of 12.5% and local tumor recurrence rate of 10.3% at 5 years. The rates of patient survival and metastatic disease were similar in both series. In terms of adverse ocular effects, the PS/EP system had lower rates of radiation retinopathy, optic neuropathy, and cataracts compared to COMS, although it was difficult to determine the significance of this potential advantage, given the small sample size. Finally, postoperative visual acuity was similar in both our series and the COMS. Overall, our 2013 study demonstrated that the PS/EP system is an effective and dependable method for treating medium-sized choroidal melanomas. Other studies using EP plaques have shown favorable outcomes as well. Tann et al. (5) recently reported favorable outcomes using PS for plaque localization and creation of custom EP plaques, although it was not used for dosimetry calculations. They reported 0% early local failure with a median follow-up of 22 months (5). Krohn et al. used PS for dosimetry and tumor localization in their study without EP plaques and also compared well with COMS outcomes (6).
The purpose of this report is to update the earlier 2013 study with additional followup of the initial 82 patients and to further expand upon the outcomes of new patients treated with plaque brachytherapy at USC from 2010 to 2015; additionally, small and large melanomas were included in this study in addition to medium-sized tumors. We hypothesized that there would be a continued clinical benefit of the PS/EP 3D modeling system as compared to COMS plaques in terms of tumor control, rates of radiation toxicity, and visual acuity outcomes.
Methods
This is a retrospective review of all patients treated for choroidal melanomas with 125I episcleral plaque brachy-therapy at the USC between January 1, 1990 and December 31, 2015. The study was approved by the institutional review board at USC.
Patient eligibility
Eligible patients were diagnosed with primary choroidal melanoma without evidence of metastatic disease. Unlike the prior publication, which included only medium-sized tumors, this review included small, medium, and large size category tumors. Tumors predominantly involving the ciliary body or iris were excluded. All patients were educated on treatment options including observation, enucleation, and proton beam therapy. Patients who gave informed consent for brachytherapy were treated with 125I EP plaques with an average prescribed dose of 85 Gy to the tumor apex.
Data collection and patient followup
At diagnosis, an ocular oncologist obtained an ocular history from each patient and completed a detailed ophthalmic examination. The examination included measurement of best-corrected visual acuity, slit lamp examination, and fundoscopy. Gonioscopy was performed when there was clinical concern for glaucoma. Standard A-scan and B-scan echography, as well as color fundus photography, were performed at initial and followup examinations. Echograms were reviewed to measure the apical height of the tumor, basal diameters, and internal reflectivity of the tumor. Orbital imaging with computed tomographic scan or magnetic resonance imaging was also performed for plaque simulation planning.
Preoperative systemic evaluation was performed, which included CT or MRI imaging of the chest, abdomen, and pelvis. Postoperatively, serum liver function tests and liver imaging were done for surveillance, given the high risk of hepatic metastases in choroidal melanomas.
Following brachytherapy, patients were examined for immediate postoperative eye care. Afterward, patients were followed up with ophthalmology appointments at approximately 3- to 4-month intervals for the first 2 years followed by 4- to 6-month intervals for postoperative years, 3–5. At each scheduled followup, the patients were examined for any evidence of tumor expansion on clinical evaluation including ultrasound biomicroscopy, as well as for adverse radiation effects. Treatment failure was defined, as in COMS, as greater than 15% increase in tumor size, greater than 250 mm increase of tumor border (repeatable × two on ultrasound and including clinical signs of growth on fundus photography), extrascleral extension greater than 2 mm or evidence of orbital recurrence. Primary outcome measures were local tumor recurrence, enucleation, and metastases. Secondary outcome measures were radiation adverse effects (including radiation retinopathy, optic neuropathy, cataracts), diplopia, ptosis, and changes in visual acuity. Diagnoses of radiation retinopathy and optic neuropathy were defined by the development after initial treatment of at least one of the features listed in the COMS report 30 (7), including the presence of hemorrhages, exudates, or vascular abnormalities. Measurements from the tumor to the optic disc and fovea were performed as described previously by Kim et al. (8).
Plaque protocol
A dose of 85 Gy at a rate of 0.6 Gy/h was prescribed to the tumor apex (with a typical margin of 2 mm) over 7 days. The use of PS and EP plaques has been described previously (4, 9, 10). The suture coordinates for plaque placement were expressed as a retinal map meridian (clock hour) and as a caliper distance from the limbus. The surgical procedure used has also been described previously (3, 4).
Statistical analysis
Mean, median, range, and standard deviation were computed using Microsoft Excel functions. All other statistical analyses were performed using JMP Pro 13. Kaplan–Meier survival analyses were performed to evaluate primary outcomes. Fisher’s exact tests comparing incidence of radiation retinopathy and optic neuropathy were performed on groups with tumor distances 0, 0–1, 1–2, 2–3, and 3 + disc diameters from the fovea and optic nerve.
Results
From January 1, 1990 to December 31, 2015, 133 consecutive patients with choroidal melanoma were treated using PS software and EP plaques loaded with 125I seeds. Baseline patient demographic and tumor characteristics are described in Table 1. Median age at time of diagnoses was 63 years (range, 27–86 years). Mean tumor height at diagnosis was 4.60 mm (SD 1.82; range, 1.70–10.32). Mean largest basal dimension was 11.06 mm (SD 3.18; range, 5.90–28.86). Most tumors were located in the posterior pole, with the posterior border behind the equator in 117 patients (92.86%). Seven tumors (5.26%) involved the ciliary body. The median followup was 42 months (range, 0–171 months); 96 patients (72.18%) followed up for at least 24 months.
Table 1.
Baseline characteristics and primary and secondary outcomes in the Collaborative Ocular Melanoma Study (11, 12) versus University of Southern California Eye Physics Plaques
| COMS | USC | |
|---|---|---|
| Patient and tumor characteristics | ||
| Baseline demographics | ||
| Patients, number | 638 | 133 |
| Median followup, mo | 67 | 42 |
| Patients, % male | 50% | 57.9% |
| Median age at diagnosis | 61 | 63 |
| Patients, % white | 98% | 89.5% |
| Tumor characteristics | ||
| Median tumor height, mm | 4.2 | 4.1 |
| Median largest basal diameter, mm | 11.5 | 10.8 |
| Location of anterior border | ||
| Ciliary body | 11.1% | 5.6% |
| Equator to ora serrata | 34.5% | 35.7% |
| Posterior to equator | 54.5% | 58.7% |
| Location of posterior border | ||
| Equator to ora serrata | 2.6% | 7.1% |
| Posterior to equator | 97.4% | 92.9% |
| Treatment characteristics | ||
| Median dose to tumor apex, Gy | 85.0 | 85.0 |
| Median dose to fovea, Gy | 79.0 | 39.9 |
| Median dose to optic nerve, Gy | 52.1 | 30.0 |
| Median dose to lens, Gy | 15.6 | 12.3 |
COMS = Collaborative Ocular Melanoma Study; USC = University of Southern California.
Of the 133 patients included, Kaplan–Meier (KM) estimate of globe salvage was 96.41% (95% CI, 92.94–99.88%) at 5 years. The KM estimate of local tumor control at 5 years was 98.29% (95% CI, 95.94–100%) (Fig. 1). Mean time to local tumor recurrence, seen in two patients (1.50%), was 11.50 months (SD 3.50, range 8–15). Two patients (1.50%) were enucleated for an indication other than tumor recurrence–one for a blind painful eye secondary to neovascular glaucoma and one secondary to massive tumor hemorrhage. Mean time to enucleation was 15.25 months (SD 5.72, range 8–24).
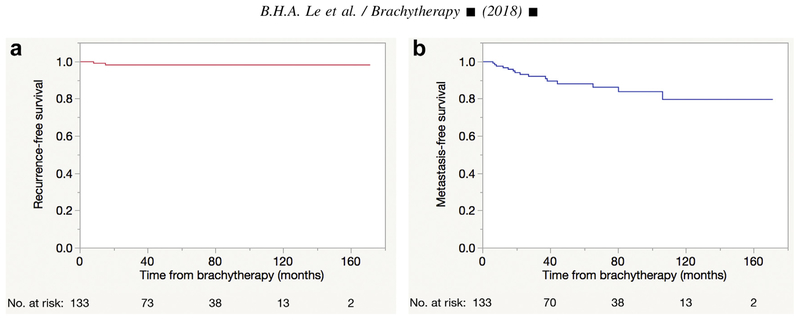
Fig. 1.
Kaplan–Meier curves demonstrating postbrachytherapy (a) local tumor control rate of 98.29% (95% CI, 95.94–100%) at 5 years and (b) globe salvage rate of 96.41% (95% CI, 92.94–99.88%) at 5 years.
Fifteen patients (11.3%) developed metastatic disease. The mean time to development of metastatic disease was 33.6 months (SD 29.4; range, 6–106 months). Metastases to the liver (n = 15) occurred in all cases. Other sites of metastasis were lungs (n = 4), bone (n = 3), abdominal cavity (n = 2), brain (n = 1), and breast (n = 1). The KM estimate of survival without metastatic disease at 5 years was 88.17% (95% CI, 81.68–94.66%) (Fig. 1).
Radiation dose and related adverse effects
Adverse effects of brachytherapy were radiation retinopathy in 57 patients (42.86%), optic neuropathy in 27 patients (20.30%), and cataracts in 62 patients (46.6%). Transient blepharoptosis was seen in 21 patients (15.79%) and transient diplopia in 24 patients (18.05%). None of the patients with blepharoptosis or diplopia required corrective surgery either due to poor vision in the affected eye or patient’s choice.
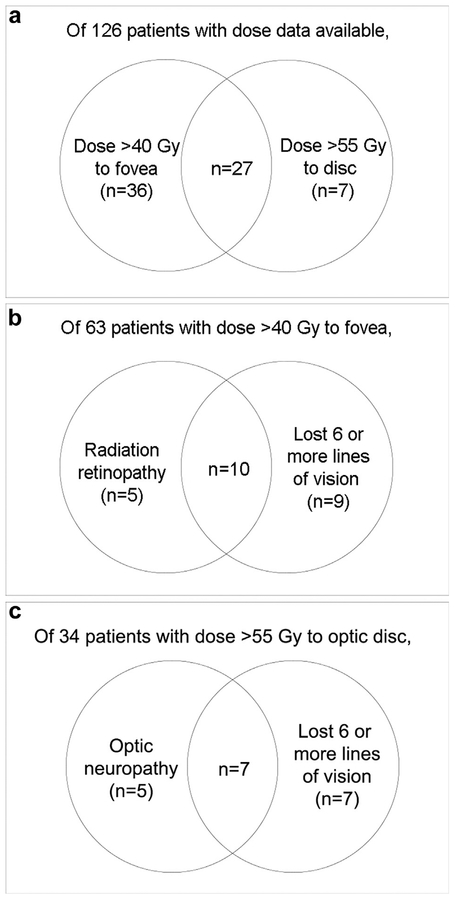
The median dose to the tumor apex was 85.0 Gy (SD 19.92; range, 70.59–188.20) (Table 1). The median doses to the optic nerve, lens, and fovea were 30.05 Gy (SD 32.74 Gy; range, 5.19–191.70), 12.29 Gy (SD 11.59 Gy; range, 3.80–73.65), and 39.92 Gy (SD 66.97 Gy; range, 4.22–312.40 Gy), respectively. Thirty-four patients (26.98%) received a dose greater than 55 Gy to the optic nerve; 12 of these 34 patients (35.29%) developed optic neuropathy (RR 2.32, p = 0.0237). Sixty-three patients (50.00%) received a dose greater than 40 Gy to the fovea; 34 of these 63 patients (53.97%) developed radiation retinopathy (RR 1.70, p = 0.0189), and 34 of these 63 patients (53.97%) lost six or more lines of vision (RR 3.09, p < 0.0001) (Fig. 2).
Fig. 2.
Ven diagram demonstrating (a) overlap between patients who received >40Gy to the fovea and >55Gy to the disc. (b) Of patients with dose >40Gy to the fovea, the overlap of those who developed radiation retinopathy and lost >6 lines of vision. (c) Of patients with dose >55Gy to the optic disc, the overlap of those that developed radiation optic neuropathy and lost >6 lines of vision.
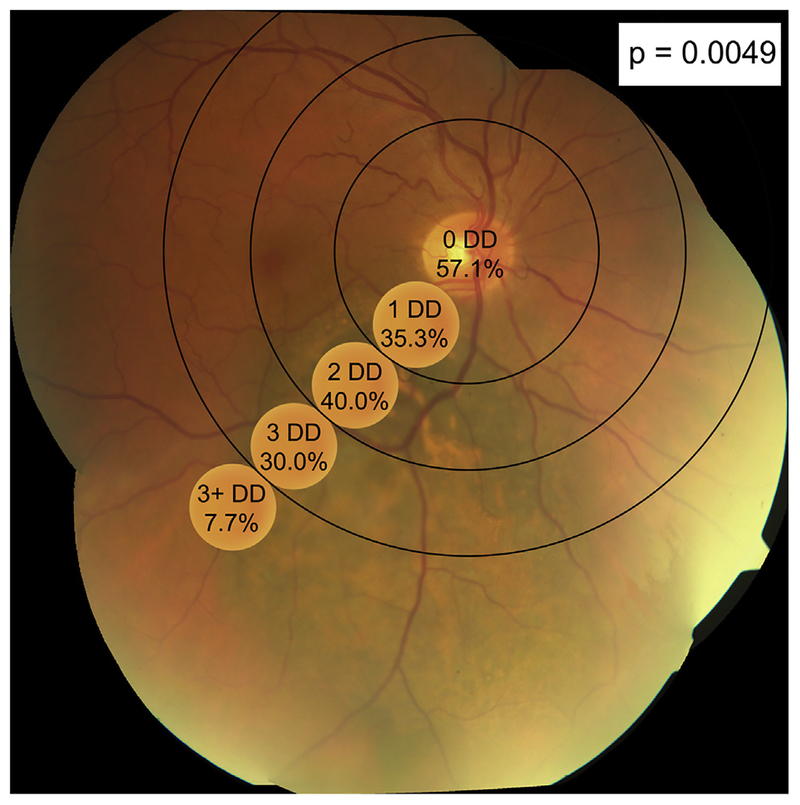
The average distance from the tumor edge to the fovea and optic nerve was 3.61 mm (SD 3.46 mm; range, 0–15.69 mm) to the fovea and 4.05 mm (SD 3.09 mm; range, 0–20.76 mm) to the optic nerve. When tumors were grouped by disc diameters from the optic disc, decreased distances from the optic disc significantly correlated with the development of optic neuropathy (p = 0.0049) (Fig. 3). There was no significant correlation between tumor distances to the fovea and the development of radiation retinopathy (p = 0.1408).
Fig. 3.
Percentage of patients with radiation optic neuropathy with tumors within 1, 1–2, 2–3, and 3 + disc diameters (DDs) of the optic nerve. There was a significant correlation between tumors located closer to the optic nerve and the development of optic neuropathy (p = 0.0049).
Vision
Snellen visual acuity at diagnosis was better than or equal to 20/40 in 86 patients (67.19%) and worse than or equal to 20/200 in 8 patients (18.75%). At final followup, visual acuity was better than or equal to 20/40 in 44 patients (33.08%), and worse than or equal to 20/200 in 59 patients (44.36%). After brachytherapy, 56 patients (44.09%) had visual acuities within two Snellen lines of preplaque visual acuity, and 50 patients (39.37%) lost six or more lines of vision.
Discussion
Primary survival outcomes
In a 25-year review of 133 patients treated for choroidal melanoma with the PS/EP system, we report long-term outcomes that compare favorably to the COMS outcomes (11) as well as outcomes reported by other centers (Table 2). The KM estimate of local tumor control at 5 years was 98.29% (95% CI, 95.94–100%) in our series compared to 89.7% in the COMS and 93% (95% CI, 92–94%) reported by the Ophthalmic Oncology Task Force (13). Other studies report local tumor recurrence rates of 0–11.6% (14–17). Our KM estimate of globe salvage at 5 years was 96.41% compared to other studies reporting 6–12.5% enucleation rates (11, 17, 18). The KM estimates of survival without metastatic disease were similar in our series (88.17%) compared to the COMS (90%) (11). The literature reports metastatic rates ranging 10–13% (13, 16, 17).
Table 2.
Primary and secondary outcomes of University of Southern California eye physics plaques compared to results reported by other centers
| Outcomes | Other studies | USC |
|---|---|---|
| Primary outcomes | ||
| Tumor recurrence rate | 0–11.6% (11, 13–17) | 1.71% (KM estimate of cumulative incidence of local tumor recurrence at 5 years) |
| Enucleation rate | 6–12.5% (11, 17, 18) | 3.59% (KM estimate of cumulative incidence of enucleation at 5 years) |
| Metastases rate | 10–13% (11, 13, 16, 17) | 11.83% (KM estimate of cumulative incidence of metastases at 5 years) |
| Secondary outcomes | ||
| Preoperative visual acuity | ||
| ≥20/40–20/50 | 64.0–70.0% (12, 19) | 67.19% |
| ≤20/200 | 7.1–9.7% (12, 19) | 18.75% |
| Postoperative visual acuity | ||
| ≥20/40–20/50 | 34–37.5% (12, 20) | 33.08% |
| ≤20/200 | 22–95% (12, 19, 21) | 44.36% |
| Change in visual acuity | ||
| Loss of <2 lines of visual acuity from baseline | 26–62% (21) | 44.09% |
| Loss of ≥6 lines of visual acuity from baseline | 49% (12) | 39.37% |
| Radiation toxicity rates | ||
| Radiation retinopathy | 10–63% (7, 18, 21) | 42.9% |
| Optic neuropathy | 0–46% (7,18,21) | 20.3% |
KM = Kaplan–Meier; USC = University of Southern California.
Dose-dependent complications of radiation
There is widespread consensus that higher radiation doses to critical ocular structures are significantly correlated with higher rates of radiation-related complications (21)t308, as well as with poorer visual outcomes (17, 22–24). Radiation retinopathy is the most common complication (21, 25), with risk factors including dose to the structure as well as patient features such as age, male gender, diabetes, and tumor characteristics such as increased size, posterior location, or location close to the macula or nerve (21, 26, 27). Increased radiation dose to the fovea is associated with higher risks of radiation maculopathy and worse visual outcomes (27, 28). Optic neuropathy is another dose-dependent complication of radiation (22, 29, 30), with significantly increased risk seen at doses above 55 Gy to the optic nerve (30). Significantly increased risk of cataracts is associated with doses above 20 Gy to the lens (24, 31). Finger noted that plaque location, which is a determinant to dose to a given structure, is also related to the incidence of cataract and retinopathy after brachytherapy (32).
Our study similarly demonstrated an association between radiation doses and adverse ocular effects, with increased RRs of radiation retinopathy and optic neuropathy associated with higher doses to the fovea and optic nerve, respectively. A radiation dose above 40 Gy to the fovea was also associated with a greater RR of severe (>6 Snellen lines) vision loss. In our study, proximity to the optic disc was also significantly correlated with the development of optic neuropathy, but proximity to the fovea was not a significant predictor of radiation retinopathy.
Reduced doses and radiation toxicity using EP/PS and visual outcomes
Previously, Marwaha et al. used PS software with 3D planning to generate treatment plans and dose distributions for both COMS and EP917 plaques (33). Their results suggested a theoretical benefit of the EP917 plaque compared to COMS, as it significantly decreased radiation doses to the optic disc, opposite retina, macula, and sclera. Only the dose to the lens was not significantly different between the two plaques.
In our study, we similarly report radiation doses to critical ocular structures that are lower than those of COMS (Table 3). While maintaining a median dose to the tumor apex of 85 Gy, our median dose to the fovea (39.9 Gy) was 49% lower than COMS (79.0 Gy). Our median dose to the optic nerve (30.0 Gy) was 42% lower than COMS (52.1 Gy). Our median dose to the lens (12.3 Gy) was 21% lower than COMS (15.6 Gy). However, these calculations do not account for tumor location.
Table 3.
Doses to critical ocular structures in the Collaborative Ocular Melanoma Study (12) versus University of Southern California Eye Physics Plaques
| Details on radiation doses | COMS | USC |
|---|---|---|
| Dose to tumor apex, % | ||
| 85.0 | 24.5% | 51.1% |
| 85.1–98.0 | 25.4% | 23.3% |
| 98.1–120.0 | 24.5% | 10.5% |
| >120.0 | 24.5% | 9.8% |
| NA | 1.2% | 5.3% |
| Dose to fovea, % | ||
| 40.0 | 26.5% | 47.4% |
| 40.0–79.9 | 23.9% | 20.3% |
| 80.0–149.9 | 23.4% | 13.5% |
| ≥150.0 | 25.5% | 13.5% |
| NA | 0.6% | 5.3% |
| Dose to optic nerve, % | ||
| 30.0 | 21.7% | 47.4% |
| 30.0–49.9 | 25.8% | 20.3% |
| 50.0–74.9 | 26.0% | 13.5% |
| ≥75.0 | 25.5% | 13.5% |
| NA | 1.0% | 5.3% |
| Dose to lens, % | ||
| <12.0 | 23.6% | 45.1% |
| 12.0–15.9 | 27.0% | 14.3% |
| 16.0–23.9 | 26.8% | 14.3% |
| ≥24.0 | 21.8% | 21.1% |
| NA | 0.8% | 5.3% |
COMS = Collaborative Ocular Melanoma Study; USC = University of Southern California.
The PS/EP system has thus been shown to reduce calculated doses to critical ocular structures, but the clinical benefit in terms of patient outcomes must also be evaluated. As it is evident that the risks of many ocular complications are radiation dose dependent, we hypothesized that minimizing radiation doses to critical ocular structures using the EP/PS system would produce clinically significant decreases in rates of radiation toxicity and visual decline after brachytherapy compared to COMS.
At final followup, 43% of our patients exhibited radiation retinopathy, 20% optic neuropathy, and 47% cataracts. These rates compare favorably to rates reported in the literature (7, 18, 21) (Table 2). Visual outcomes in our study were also favorable (12, 19–21) (Table 2). In the COMS, 49% of patients lost six or more lines of vision at 3 years (12), whereas 35.7% of our patients lost six or more lines of vision at the final followup. However, we did not perform a direct statistical comparison.
Dosimetric considerations
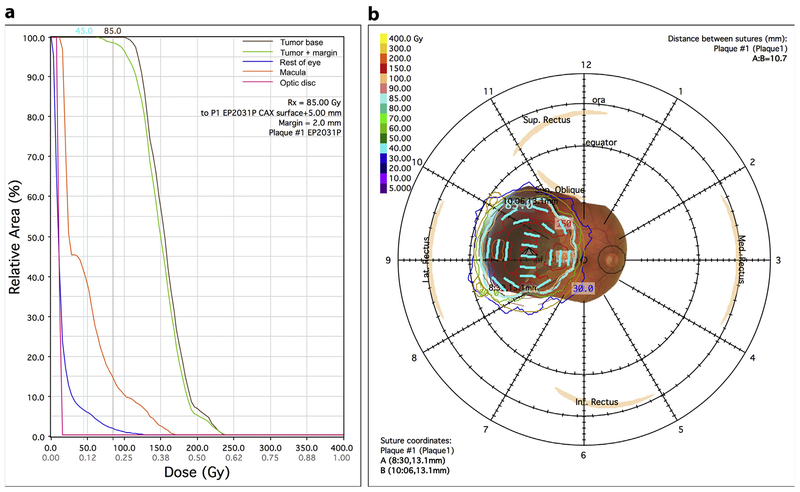
Compared to COMS plaques, PS improves conformation of the radiation dose to tumors, by incorporating not just tumor B-scan measurements but also all other available imaging modalities including MRI or CT in dose calculations. Astrahan dosimetrically compared rival plaques using retinal dose–area histograms and found that conformally loaded plaques delivered the lowest macular dose, as well as the least area outside the margin with doses above 40 Gy (34) (Fig. 4). In addition, PS software is useful in dosimetry planning because it incorporates correction fac tors such as source attenuation and scatter (35, 36).
Fig. 4.
(a) Sample retina dose–area histogram from Plaque Simulator (PS) demonstrating sharp drop-off of relative doses to the macula and optic disc. In this case, <45% of the macular area and <10% of the optic disc area received >45 Gy. (b) Sample diagram from PS showing tumor fundus photography overlaid with dose distributions.
The PS/EP system has been dosimetrically verified. Knutsen et al. (37)t330 experimentally verified dose distributions in three different plaques, including the EP917 plaque. Calculated PS central axis dose (CAX) in their study provided excellent agreement with their measured CAX, with deviations up to 4.3%. PS also performs well in comparison to Monte Carlo dosimetry, with CAX differences ranging 5.7–10.5% for the EP917 plaque calculated by Zimmermann et al. (38).
It should be noted that physical dose is an inherently accurate measurement. Calculated dose for brachytherapy, however, is subject to many different variables including assumptions made in the model used to calculate the dose. Source anisotropy, attenuation by the silastic carrier, and interactions with the gold shell are only some of the many factors that impact the accuracy of the calculation. It is well known that COMS disregards these factors in dose calculations (39). The American Brachytherapy Society Ophthalmic Oncology Task Force published consensus guidelines in 2014 that list the range of acceptable prescription doses between 70 and 100 Gy (40). The current COMS recommendation is 85 Gy but fails to take into account many variables affecting that calculation; thus, the physical dose is somewhat less than the calculated dose for COMS plaques. The intent of the PS/EP system was to accurately prescribe 85 Gy; however, given differences in the models for calculations 85 Gy for PS/EP is likely higher than the same prescribed dose for COMS plaques by 10–15%. Therefore, it is possible that prescribed doses lower than 85 Gy could be used with the PS/EP system to still achieve outstanding local control with even less ocular toxicity.
Limitations o f our study
COMS is the only large national multicenter clinical trial of brachytherapy for choroidal melanoma, making it the best standard of comparison. However, this analysis is limited by the lack of direct statistical comparison with COMS data. Compared to COMS, our study is also smaller, single-center, and the data were collected retrospectively. Because of advances in brachytherapy since the COMS trials ended, we additionally included tumors around or directly involving the optic nerve, select small and large sized tumors, and choroidal tumors involving the ciliary body and iris. Comparison of radiation doses is complicated by the use of different formalisms as well as methods of calculation.
Conclusion
The PS/EP system is a clinically effective treatment planning system for plaque brachytherapy of choroidal melanoma and the only existing ophthalmic-specific program for plaque planning. Its dosimetry has been experimentally verified, and various studies have shown that PS calculates dosimetry reliably. We demonstrate in this study that use of the PS/EP system is associated with favorable long-term clinical outcomes, in terms of local tumor control, globe preservation, radiation complications, and visual acuity. We also report lower radiation doses to critical ocular structures compared to the COMS although statistical comparison is hampered by the lack of patient-specific COMS data. This retrospective study provides additional clinical data documenting the effectiveness of the PS/EP system for treating uveal melanomas and its potential for minimizing rates of local tumor recurrence and ocular side effects.
Financial disclosure:
Financial support was provided by an unrestricted departmental grant from Research to Prevent Blindness. Dr. Astrahan holds an ownership position in Eye Physics LLC, which was incorporated in 2007 to continue development of the Plaque Simulator software and Eye Physics plaques following Dr. Astrahan’s emeritus retirement from the University of Southern California (USC) in 2010. During 1990–2010, no outside funding for development or material support for any of his contributions was received by USC. No compensation was received for any patient in this study. From 1995 to 2015, USC and Dr. Astrahan shared a royalty derived from licensed distribution of the Plaque Simulator software to other institutions. The authors have no other disclosures to report.
References
- [1].Eye physics. Available at: http://www.eyephysics.com/. Accessed December 20, 2017.
- [2].Astrahan MA, Luxton G, Pu Q, et al. Conformal episcleral plaque therapy. Int J Radiat Oncol Biol Phys 1997;39:505–519. [DOI] [PubMed] [Google Scholar]
- [3].Berry JL, Kim JW, Jennelle R, et al. Use of the toric surgical marker to aid in intraoperative plaque placement for the USC eye physics plaques to treat uveal melanoma: a new surgical technique. Ophthalmic Surg Lasers Imaging Retina 2015;46:866–870. [DOI] [PubMed] [Google Scholar]
- [4].Berry JL, Dandapani SV, Stevanovic M, et al. Outcomes of choroidal melanomas treated with eye physics: a 20-year review. JAMA Ophthalmol 2013;131:1435–1442. [DOI] [PubMed] [Google Scholar]
- [5].Tann AW, Teh BS, Scarboro SB, et al. Early outcomes of uveal melanoma treated with intraoperative ultrasound guided brachytherapy using custom built plaques. Pract Radiat Oncol 2017;7:e275–e282. [DOI] [PubMed] [Google Scholar]
- [6].Krohn J, Monge OR, Skorpen TN, et al. Posterior uveal melanoma treated with I-125 brachytherapy or primary enucleation. Eye Lond 2008;22:1398–1403. [DOI] [PubMed] [Google Scholar]
- [7].Boldt HC, Melia BM, Liu JC, et al. Collaborative ocular melanoma study group. I-125 brachytherapy for choroidal melanoma photographic and angiographic abnormalities: the collaborative ocular melanoma study: COMS report no. 30. Ophthalmology 2009;116:106–115.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim JW, Hassan AA, McGovern K, et al. Treatment outcomes of focal laser consolidation during chemoreduction for group b retinoblastoma - sciencedirect. Ophthalmol Retina 2017;1:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Astrahan MA, Luxton G, Jozsef G, et al. Optimization of 125I ophthalmic plaque brachytherapy. M ed Phys 1990;17:1053–1057. [DOI] [PubMed] [Google Scholar]
- [10].Astrahan MA, Luxton G, Jozsef G, et al. An interactive treatment planning system for ophthalmic plaque radiotherapy. Int J Radiat Oncol Biol Phys 1990;18:679–687. [DOI] [PubMed] [Google Scholar]
- [11].Jampol LM, Moy CS, Murray TG, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology 2002;109:2197–2206. [DOI] [PubMed] [Google Scholar]
- [12].Melia BM, Abramson DH, Albert DM, et al. Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16. Ophthalmology 2001;108:348–366. [DOI] [PubMed] [Google Scholar]
- [13].The Ophthalmic Oncology Task Force. Local recurrence significantly increases the risk of metastatic uveal melanoma. Ophthalmology 2016;123:86–91. [DOI] [PubMed] [Google Scholar]
- [14].Sanchez-Tabernero S, Garcia-Alvarez C, Munoz-Moreno MF, et al. Pattern of local recurrence after I-125 episcleral brachytherapy for uveal melanoma in a Spanish referral ocular oncology unit. Am J Ophthalmol 2017;180:39–45. [DOI] [PubMed] [Google Scholar]
- [15].Echegaray JJ, Bechrakis NE, Singh N, et al. Iodine-125 brachytherapy for uveal melanoma: a systematic review of radiation dose. Ocul Oncol Pathol 2017;3:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McCannel TA, Chang MY, Burgess BL. Multi-year follow-up of fineneedle aspiration biopsy in choroidal melanoma. Ophthalmology 2012;119:606–610. [DOI] [PubMed] [Google Scholar]
- [17].Perez BA, Mettu P, Vajzovic L, et al. Uveal melanoma treated with iodine-125 episcleral plaque: an analysis of dose on disease control and visual outcomes. Int J Radiat Oncol Biol Phys 2014;89:127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Günüz K, Shields CL, Shields JA, et al. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol 1960;1999:609. [DOI] [PubMed] [Google Scholar]
- [19].Aziz HA, Singh N, Bena J, et al. Vision loss following episcleral brachytherapy for uveal melanoma: development of a vision prognostication tool. JAMA Ophthalmol 2016;134:615–620. [DOI] [PubMed] [Google Scholar]
- [20].Quivey JM, Char DH, Phillips TL, et al. High intensity 125-iodine (125I) plaque treatment of uveal melanoma. Int J Radiat Oncol Biol Phys 1993;26:613. [DOI] [PubMed] [Google Scholar]
- [21].Wen JC, Oliver SC, McCannel TA. Ocular complications following I- 125 brachytherapy for choroidal melanoma. Eye (Lond) 2009;23: 1254–1268. [DOI] [PubMed] [Google Scholar]
- [22].Puusaari I, Heikkonen J, Kivela T. Effect of radiation dose on ocular complications after iodine brachytherapy for large uveal melanoma: empirical data and simulation of collimating plaques. Invest Ophthalmol Vis Sci 2004;45:3425. [DOI] [PubMed] [Google Scholar]
- [23].Jones R, Gore E, Mieler W, et al. Posttreatment visual acuity in patients treated with episcleral plaque therapy for choroidal melanomas: dose and dose rate effects. Int J Radiat Oncol Biol Phys 2002;52:989–995. [DOI] [PubMed] [Google Scholar]
- [24].Stack R, Elder M, Abdelaal A, et al. New Zealand experience ofI125 brachytherapy for choroidal melanoma. Clin Exp Ophthalm ol 2005; 33:490–494. [DOI] [PubMed] [Google Scholar]
- [25].Nag S, Quivey JM, Earle JD, et al. The American brachytherapy society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys 2003;56:544–555. [DOI] [PubMed] [Google Scholar]
- [26].Gragoudas ES, Li W, Lane AM, et al. Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology 1999;106:1571 discussion 1577–8. [DOI] [PubMed] [Google Scholar]
- [27].Finger PT, Chin KJ, Yu GP. Risk factors for radiation maculopathy after ophthalmic plaque radiation for choroidal melanoma. Am J Ophthalmol 2010;149:608. [DOI] [PubMed] [Google Scholar]
- [28].Newman H, Chin KJ, Finger PT. Subfoveal choroidal melanoma: pre-treatment characteristics and response to plaque radiation therapy. Arch Ophthalmol 2011;129:892. [DOI] [PubMed] [Google Scholar]
- [29].Lommatzsch PK, Alberti W, Lommatzsch R, et al. Radiation effects on the optic nerve observed after brachytherapy of choroidal melanomas with 106Ru/106Rh plaques. Graefes Arch Clin Exp Ophthalmol 1994;232:482. [DOI] [PubMed] [Google Scholar]
- [30].Mayo C, Martel MK, Marks LB, et al. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys 2010;76: S28–S35. [DOI] [PubMed] [Google Scholar]
- [31].Finger PT, Chin KJ, Yu GP, et al. Risk factors for cataract after palladium-103 ophthalmic plaque radiation therapy. Int J Radiat Oncol Biol Phys 2011;80:800–806. [DOI] [PubMed] [Google Scholar]
- [32].Finger PT. Tumour location affects the incidence of cataract and retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol 2000;84:1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marwaha G, Wilkinson A, Bena J, et al. Dosimetric benefit of a new ophthalmic radiation plaque. Int J Radiat Oncol Biol Phys 2012;84: 1226–1230. [DOI] [PubMed] [Google Scholar]
- [34].Astrahan MA. The retina dose-area histogram: a metric for quantitatively comparing rival eye plaque treatment options. J Contemp Brachytherapy 2013;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Astrahan MA. Improved treatment planning for COMS eye plaques. Int J Radiat Oncol Biol Phys 2005;61:1227–1242. [DOI] [PubMed] [Google Scholar]
- [36].Chiu-Tsao ST, Astrahan MA, Finger PT, et al. Dosimetry of (125)I and (103)Pd COMS eye plaques for intraocular tumors: report of Task Group 129 by the AAPM and ABS. M ed Phys 2012;39:6161–6184. [DOI] [PubMed] [Google Scholar]
- [37].Knutsen S, Hafslund R, Monge OR, et al. Dosimetric verification of a dedicated 3D treatment planning system for episcleral plaque therapy. Int J Radiat Oncol Biol Phys 2001;51:1159–1166. [DOI] [PubMed] [Google Scholar]
- [38].Zimmermann LW, Amoush A, Wilkinson DA. Episcleral eye plaque dosimetry comparison for the eye physics EP917 using plaque simulator and Monte Carlo simulation. J Appl Clin M ed Phys 2015;16:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kline R, Earle J. Implications of TG-43 for dose prescription and calculation for I-125 eye plaques. M ed Phys 1996;23:1054. [Google Scholar]
- [40].The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy 2014;13:1–14. [DOI] [PubMed] [Google Scholar]