Abstract
Objective: Administration of drugs targeting anti-cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) is often associated with serious immune-related adverse events (irAEs). Here, we performed a comprehensive analysis of organ-specific irAEs and treatment-related hematologic abnormalities and musculoskeletal disorders resulting from anti-CTLA-4 treatment.
Materials and methods: PubMed, the Cochrane library, Web of Science, and ClinicalTrials.gov were searched for studies between January 1990 and March 2018 reporting AEs associated with anti-CTLA-4 therapies.
Results: A total of 11 clinical trials with 7,088 patients were included; of these, data were accessible for 10 on ClinicalTrials.gov. Compared with control therapies (placebo, chemotherapy, radiation therapy, or vaccine), anti-CTLA-4 therapies (ipilimumab and tremelimumab) were associated with an increased risk of serious irAEs, predominantly dermatologic (rash: odds ratio [OR] 3.39, P<0.01), gastrointestinal (diarrhea and colitis: OR 6.57 and 14.01, respectively; both P<0.001), endocrine (hypophysitis, hypothyroidism, adrenal insufficiency, and hypopituitarism: OR 4.22, 3.72, 3.77, and 4.73, respectively; all P<0.05), and hepatic (hepatitis, elevated alanine aminotransferase, and elevated aspartate aminotransferase: OR 4.44, 3.28, and 3.12, respectively; all P<0.05). The most common serious organ-specific irAEs were gastrointestinal (diarrhea 9.8% and colitis 5.3%). Although the incidence of selected events was higher in anti-CTLA-4-treated patients, no significant differences were found between anti-CTLA-4 and the control therapies in treatment-related hematologic abnormalities or severe musculoskeletal disorders.
Conclusion: Anti-CTLA-4 therapies are associated with an increased risk of serious organ-specific irAEs, most frequently involving the gastrointestinal system; however, no increased risk of hematologic abnormalities or severe musculoskeletal disorders was detected compared with other therapies. These results underscore the need for clinical awareness and prompt and effective management of multi-organ irAEs related to anti-CTLA-4 drugs.
Keywords: immune-related adverse events, anti-CTLA-4 drugs, ipilimumab, tremelimumab
Introduction
Recent years have seen increasing interest in immunotherapy-based immune checkpoint blockade for various advanced or metastatic solid tumors, such as prostate cancer,1 melanoma,2 lung cancer,3 and mesothelioma.4 Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is expressed exclusively on the surface of T cells and inhibits their activation and function by binding to the ligand B7.5,6 As such, CTLA-4 is a crucial negative regulator of the anti-tumor immune response and a key target of immune checkpoint therapy. Several anti-CTLA-4 monoclonal antibodies (mAbs) have been developed that block the inhibitory pathway, thereby restoring or enhancing T cell functions in the anti-tumor response.
Currently, the two most widely used anti-CTLA-4 drugs in clinical practice are the fully human mAbs ipilimumab and tremelimumab. Both mAbs have shown benefit for several cancers in a number of clinical trials.7,8 Nevertheless, despite the beneficial effects of immune checkpoint inhibitors in promoting the anti-tumor response, their mechanism of action in enhancing T cell activity9 also increases the risk of immune-related adverse events (irAEs), which often affect multiple organs.8 The most common anti-CTLA-4-associated organ-specific irAEs involve the skin, gastrointestinal tract, liver, and endocrine and nervous systems. In addition, treatment-related hematologic abnormalities, including thrombocytopenia, anemia, and neutropenia, are commonly seen in patients treated with anti-CTLA-4 mAbs and may be related to their immune activities.10 Musculoskeletal disorders, which can seriously impact the patient’s quality of life, have also been observed following treatment with immune checkpoint inhibitors. Their incidence has been investigated for drugs that disrupt the interaction between programmed cell death-1 and its ligand (PD-1–PDL-1);11 however, little is known about the incidence of musculoskeletal disorders in patients administered anti-CTLA-4 drugs.
Several meta-analyses have analyzed irAEs of cancer patients undergoing treatment with CTLA-4 inhibitors,12–14 but most of these studies have focused mainly on ipilimumab and examined only a limited number of irAEs. Moreover, a more complete review of the incidence of AEs is justified by the increasing clinical use of anti-CTLA-4 drugs and the potentially severe outcomes if irAEs are not recognized and managed in a timely manner. Here, we performed a systematic review and meta-analysis to evaluate irAEs related to anti-CTLA-4 drugs, with most data collected from ClinicalTrials.gov.
Materials and methods
Search strategy
We searched three databases (PubMed, Cochrane library and Web of Science) from January 1990 to march 2018 to identify all qualified trials. The following items: “anti-cytotoxic T-lymphocyte-associated protein-4”, “anti-CTLA-4”, “Ipilimumab”, and “tremelimumab” were adopted in Cochrane library and Web of Science with the restriction to language (English) and publication type (clinical trial). As for PubMed searching, the following search strategy was used “((((((clinical trial[Title/Abstract]) OR randomized controlled trial[Title/Abstract]) OR randomized trial[Title/Abstract]) OR clinical study[Title/Abstract]) OR trial[Title/Abstract])) AND ((((((. (anti-cytotoxic T lymphocyte-associated antigen 4[Title/Abstract]) OR anti CTLA4[Title/Abstract]) OR anti CTLA-4[Title/Abstract]) OR ipilimumab[Title/Abstract]) OR tremelimumab[Title/Abstract])))” EndNote, a bibliography managing software, was used to integrate our search results and find duplicates.
Study selection
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines,15 we applied the Population, Intervention, Comparator, Outcome, and Study design (PICOS) approach to identify eligible studies. Trials were selected if they were randomized and controlled in nature (S), compared anti-CTLA-4 drugs with control therapies (I, C) in cancer patients (P), and provided information on AEs (O). Non-randomized controlled trials, including case reports, commentaries, reviews, and quality of life studies, were excluded. Studies were also excluded if the intervention arm was a combination of mAbs, if the control arm was one or more mAb, or the enrolled population had previously been treated with mAbs. After duplicates were removed, two authors (H.X. and P.T.) independently screened the titles and abstracts, and the full text of the selected studies was further reviewed. Any disagreements were resolved by consulting a third reviewer (L.Y.).
Data extraction and outcomes
Data extraction was performed independently by two authors using a pre-designed extraction form. Any discrepancies were resolved by discussion. The following items were extracted from each study by two authors (H.X. and P.T.) independently: author, year, NCT number, trial phase, cancer type, sample size, intervention drugs and doses, control therapies, median follow-up duration in the experiment group, the primary outcome and the description of irAEs. Our primary outcome was the incidence of organ-specific irAEs including dermatologic (pruritus, rash,), gastrointestinal (diarrhea, colitis), endocrine (hypophysitis, hyperthyroidism, hypothyroidism, adrenal insufficiency, hypopituitarism), hepatic (hepatitis, elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels), and other (pneumonitis, pancreatitis, Guillain–Barre syndrome) adverse events. Our secondary outcome was the treatment-related hematologic abnormalities (anemia, neutropenia, thrombocytopenia) and musculoskeletal disorders (arthralgia, arthritis, back pain, bone pain, musculoskeletal pain, myalgia). ClinicalTrials.gov provided the main sources of adverse events data (search deadline: 20 March 2018). The information on adverse events from published literature was also extracted only when the information was not exhibited in ClinicalTrials.gov (the ClinicalTrials.gov had the priority over the published literature given its complete AEs information). Serious and other AEs were defined as grades ≥3 or grade 1–2 in published articles according to The Common Terminology of Clinical Adverse Events (CTCAE) categorization. We deemed an AE did not happen if an adverse event was not reported in both two sources.
Quality assessment
The Cochrane Risk of Bias Tool16 was applied to assess the quality of each included trial using the following six items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting and other sources of bias. Discrepancies were resolved by discussion.
Data synthesis and analysis
When possible, a meta-analysis was performed, and the pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated. A fixed effects model or random effects model (using the inverse variance method) was adopted for the meta-analysis according to the heterogeneity. Heterogeneity was assessed using Cochran Q and I2 statistics (if P<0.1, heterogeneity was considered present and the random effects model was used). A two-sided P-value of less than 0.05 was deemed statistically significant. If a study involved more than one intervention arm (eg, the trials conducted by Reck et al,17 Lynch et al,18 and Hodi et al8), we separately compared each intervention arm with the control arm. Subgroup analyses were conducted according to the control therapies. Publication bias was detected using the Egger’s test. All statistical analyses were performed using STATA 12.0 (Stata Corp, College Station, TX, USA).
Results
Basic characteristics of included trials
The process of study selection is shown in Figure S1. Table 1 summarizes the basic characteristics of the included trials. All 11 studies7,8,17–25 included were multicenter, randomized, controlled trials and included a total of 7,088 patients (intervention 3,985 vs control 3,103). Four trials were conducted in melanoma,7,8,24,25 two in metastatic non-small cell lung cancer,18,20 two in small cell lung cancer,17,22 two in metastatic castration-resistant prostate cancer,21,23 and one in mesothelioma.19 Ipilimumab and tremelimumab were used in nine and two trials, respectively. Doses of 10 mg/kg and 15 mg/kg of tremelimumab were administered in Maio et al19 and Ribas et al,24 respectively, and a 3 mg/kg dose of ipilimumab was administered in Hodi et al;8 the remaining 8 studies administered 10 mg/kg dose of ipilimumab or tremelimumab. The trials conducted by Rech et al17 and Lynch et al18 assessed two regimens of ipilimumab in the experimental group: ipilimumab + paclitaxel/carboplatin followed by placebo + paclitaxel/carboplatin; and placebo + paclitaxel/carboplatin followed by ipilimumab + paclitaxel/carboplatin. The trial conducted by Hodi et al8 included three arms: ipilimumab + gp100 (melanoma peptide vaccine), ipilimumab alone, and gp100 alone. The control arms consisted of a single chemotherapy drug in one trial, two chemotherapy drugs in four trials, radiotherapy in one trial, vaccine (gp100) in one trial, and placebo in three trials.
Figure S1.
Flow diagram for study selection.
Table 1.
Characteristics of included trials
| Trials | NCT | Phase | Cancer type | Size (Intervention/Control) | Intervention | Dose | Control | Follow-up duration (intervention) | Primary outcome | Description of irAEs |
|---|---|---|---|---|---|---|---|---|---|---|
| Maio 201719 | 01843374 | 2 | Mesothelioma | 569 (380/189) | Tremelimumab | 10 mg/kg | Placebo | NA | OS | No |
| Govindan 201720 | 01285609 | 3 | NSCLC | 948 (475/473) | Ipilimumab + Chemotherapy | 10 mg/kg | Chemotherapy + Placebo | 12.5 months | OS | Yes |
| Beer 201721 | 01057810 | 3 | mCRPC | 600 (399/199) | Ipilimumab | 10 mg/kg | Placebo | NA | OS | Yes |
| Reck 201622 | 01450761 | 3 | SCLC | 954 (562/561) | Ipilimumab + Chemotherapy | 10 mg/kg | Chemotherapy + Placebo | 10.5 months | OS | Yes |
| Eggermont 20167 | 00636168 | 3 | Melanoma | 945 (471/474) | Ipilimumab | 10 mg/kg | Placebo | 63.6 months | RFS | Yes |
| kwon 201423 | 00861614 | 3 | mCRPC | 799 (393/396) | Ipilimumab + Radiotherapy | 10 mg/kg | Radiotherapy + placebo | 9·9 months | OS | Yes |
| Ribas 201324 | 00257205 | 3 | Melanoma | 644 (325/319) | Tremelimumab | 15 mg/kg | Chemotherapy | 31 months | OS | No |
| Reck 201217 | 00527735 | 2 | SCLC | 86 (42/44) | Ipilimumab (Phased)# | 10 mg/kg | Chemotherapy + Placebo | NA | irPFS | Yes |
| 86 (42/44) | Ipilimumab# (Concurrent) | 10 mg/kg | Chemotherapy + Placebo | |||||||
| Lynch 201218 | 00527735 | 2 | NSCLC | 132 (67/65) | Ipilimumab# (Phased) | 10 mg/kg | Chemotherapy + Placebo | NA | irPFS | Yes |
| 136 (71/65) | Ipilimumab# (Concurrent) | 10 mg/kg | Chemotherapy + Placebo | |||||||
| Robert 201125 | 00324155 | 3 | Melanoma | 498 (247/251) | Ipilimumab + Chemotherapy | 10 mg/kg | Chemotherapy + Placebo | NA | OS | Yes |
| Hodi 20108 | 00094653 | 3 | Melanoma | 512 (380/132) | Ipilimumab + gp100 | 3 mg/kg | gp100 | 21 months | OS | Yes |
| 263 (131/132) | Ipilimumab | 3 mg/kg | gp100 | 27.8 months |
Notes: # concurrent, ipilimumab + paclitaxel/carboplatin followed by placebo + paclitaxel/carboplatin; phased, placebo + paclitaxel/carboplatin followed by ipilimumab + paclitaxel/carboplatin.
Abbreviations: NA, not available; OS, overall survival; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; mCRPC, metastatic castration resistant prostate cancer; irAEs, immune-related adverse events; irPFS, immune-related progression-free survival.
The median follow-up duration was 21 months (range 9.9–63.6 months), and the primary end point in all trials was survival. Data on AEs were available on ClinicalTrials.gov for 10 of the 11 studies (except Ribas et al24); only two trials did not describe irAEs. The risk of bias in the included trials is shown in Table 2. One of the trials was an open-label, randomized, comparative study and we considered it at high risk of bias for assessing random sequence generation and allocation concealment.
Table 2.
Risk of bias of included trials
| Study | Year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data* | Selective reporting* | Other sources of bias |
|---|---|---|---|---|---|---|---|---|
| Maio19 | 2017 | Low | Low | Low | Low | High | High | Low |
| Govindan20 | 2017 | Low | Low | Low | Low | High | High | Low |
| Beer21 | 2017 | Low | Low | Low | Low | High | High | Low |
| Reck22 | 2016 | Low | Low | Low | Low | High | High | Low |
| Eggermont7 | 2016 | Low | Low | Low | Low | High | High | Low |
| kwon23 | 2014 | Low | Low | Low | Low | High | High | Low |
| Ribas24 | 2013 | Low | Low | High | High | High | High | Low |
| Reck17 | 2012 | Low | Unclear | Low | Low | High | High | Low |
| Lynch18 | 2012 | Low | Unclear | Low | Low | High | High | Low |
| Robert25 | 2011 | Low | Unclear | Low | Low | High | High | Low |
| Hodi8 | 2010 | Low | Unclear | Low | Low | High | High | Low |
Note: *Applies to adverse events.
Organ-specific irAEs
Table 3 summarizes the incidence of organ-specific irAEs related to anti-CTLA-4 drugs, and Table 4 shows the pooled ORs of irAEs compared with the control therapies.
Table 3.
Incidence of organ-specific immune-related adverse events related to anti-CTLA-4 drugs. Values are percentages (95% confidence intervals)
| Drugs | Ipilimumab (n=3280) | Tremelimumab (n=705) | Total (n=3985) | |||
|---|---|---|---|---|---|---|
| IrAEs* | All# | Serious† | All | Serious | All | Serious |
| Dermatologic | ||||||
| Pruritus | 25.0 (23.5–26.5) | 0.1 (0.0–0.3) | 28.8 (25.5–32.3) | 0.4 (0.1–1.2) | 25.6 (24.3–27.0) | 0.2 (0.1–0.4) |
| Rash | 26.6 (25.1–28.2) | 0.7 (0.4–1.1) | 26.2 (23.0–29.7) | 1.4 (0.7–2.6) | 26.5 (25.2–28.0) | 0.8 (0.6–1.2) |
| Gastrointestinal | ||||||
| Diarrhea | 46.2 (44.5–47.9) | 8.4 (7.4–9.4) | 55.3 (51.6–59.0) | 16.5 (13.8–19.4) | 47.8 (46.2–49.4) | 9.8 (8.9–10.8) |
| Colitis | 6.6 (5.8–7.5) | 5.3 (4.6–6.1) | 5.2 (3.7–9.2) | 5.2 (3.7–9.2) | 6.4 (5.6–7.2) | 5.3 (4.6–6.0) |
| Endocrine | ||||||
| Hypophysitis | 3.9 (3.3–4.6) | 2.0 (1.6–2.6) | 0.4 (0.1–1.2) | 0.4 (0.1–1.2) | 3.3 (2.8–3.9) | 1.7 (1.3–2.2) |
| Hypothyroidism | 2.5 (2.0–3.1) | 0.3 (0.2–0.6) | 2.7 (1.6–4.2) | 0.6 (0.2–1.4) | 2.5 (2.0–3.0) | 0.4 (0.2–0.6) |
| Hyperthyroidism | 0.3 (0.1–0.5) | 0.3 (0.1–0.5) | 0.0 (0.0–0.5) | 0.0 (0.0–0.5) | 0.2 (0.1–0.4) | 0.2 (0.1–0.4) |
| Adrenal insufficiency | 0.6 (0.3–0.9) | 0.6 (0.3–0.9) | 0.9 (0.3–1.8) | 0.7 (0.2–1.6) | 0.6 (0.4–0.9) | 0.6 (0.4–0.9) |
| Hypopituitarism | 0.8 (0.5–1.2) | 0.8 (0.5–1.2) | 0.1 (0.0–0.8) | 0.1 (0.0–0.8) | 0.7 (0.4–1.0) | 0.7 (0.4–1.0) |
| Hepatic | ||||||
| Hepatitis | 0.5 (0.3–0.8) | 0.5 (0.3–0.8) | 0.3 (0.0–1.0) | 0.3 (0.0–1.0) | 0.5 (0.3–0.7) | 0.5 (0.3–0.7) |
| ALT increased | 12.3 (11.2–13.4) | 2.6 (2.1–3.2) | 0.0 (0.0–0.5) | 0.0 (0.0–0.5) | 10.1 (9.2–11.1) | 2.1 (1.7–2.6) |
| AST increased | 11.0 (10.0–12.1) | 2.5 (2.0–3.1) | 0.0 (0.0–0.5) | 0.0 (0.0–0.5) | 9.1 (8.2–10.0) | 2.0 (1.6–2.5) |
| Other | ||||||
| Pneumonitis | 1.2 (0.8–1.6) | 0.8 (0.5–1.2) | 0.3 (0.0–1.0) | 0.3 (0.0–1.0) | 1.0 (0.7–1.4) | 0.7 (0.5–1.0) |
| Pancreatitis | 0.1 (0.0–0.3) | 0.1 (0.0–0.3) | 0.7 (0.2–1.6) | 0.7 (0.2–1.6) | 0.2 (0.1–1.4) | 0.2 (0.1–0.4) |
| Guillain-Barre syndrome | 0.2 (0.0–0.4) | 0.2 (0.0–0.4) | 0.1 (0.0–0.8) | 0.1 (0.0–0.8) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) |
Notes: *IrAEs, immune-related adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase. #Includes both serious and other adverse events if data were extracted from ClinicalTrials.gov; includes all Common Terminology of Clinical Adverse Events (CTCAE) grades if data were extracted from the publication. †Serious adverse events if data were extracted from ClinicalTrials.gov; CTCAE grades ≥3 if data were extracted from the publication.
Table 4.
Pooled odds ratio of adverse events for anti-CTLA-4 drugs compared with control therapies
| All# | Serious† | |||||
|---|---|---|---|---|---|---|
| IrAEs* | OR | 95%CI | P | OR | 95%CI | P |
| Dermatologic | ||||||
| Pruritus | 4.35 | 3.74–5.07 | <0.0001 | 3.64 | 0.89–14.91 | 0.072 |
| Rash | 4.03 | 3.22–5.04 | <0.0001 | 3.39 | 1.52–7.59 | 0.003 |
| Gastrointestinal | ||||||
| Diarrhea | 2.88 | 2.35–3.78 | <0.0001 | 6.57 | 4.09–10.58 | <0.0001 |
| Colitis | 14.62 | 8.61–24.83 | <0.0001 | 14.01 | 7.34–26.77 | <0.0001 |
| Endocrine | ||||||
| Hypophysitis | 5.30 | 1.71–16.46 | 0.004 | 4.22 | 1.72–10.34 | 0.002 |
| Hypothyroidism | 7.86 | 4.10–15.04 | <0.0001 | 3.72 | 1.18–11.75 | 0.025 |
| Hyperthyroidism | 3.78 | 0.94–15.17 | 0.061 | 3.78 | 0.94–15.17 | 0.061 |
| Adrenal insufficiency | 3.88 | 1.46–10.36 | 0.007 | 3.77 | 1.41–10.06 | 0.008 |
| Hypopituitarism | 4.73 | 1.73–12.95 | 0.003 | 4.73 | 1.73–12.95 | 0.003 |
| Hepatic | ||||||
| Hepatitis | 4.44 | 1.51–13.04 | 0.007 | 4.44 | 1.51–13.04 | 0.007 |
| ALT increased | 3.28 | 1.79–6.02 | <0.0001 | 11.37 | 4.45–29.10 | <0.0001 |
| AST increased | 3.12 | 1.92–5.09 | <0.0001 | 4.9 | 1.41–17.07 | 0.013 |
| Other | ||||||
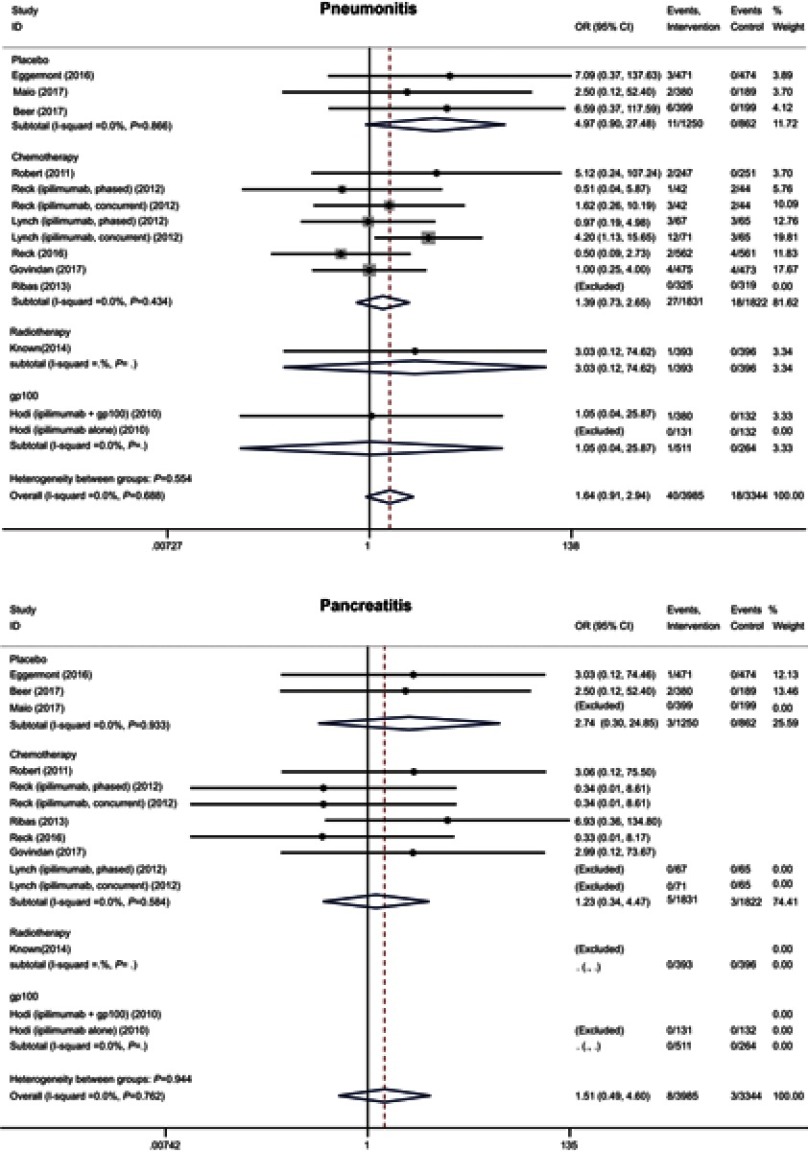
| Pneumonitis | 1.64 | 0.91–2.94 | 0.098 | 1.27 | 0.66–2.44 | 0.477 |
| Pancreatitis | 1.51 | 0.49–4.60 | 0.472 | 1.51 | 0.49–4.60 | 0.472 |
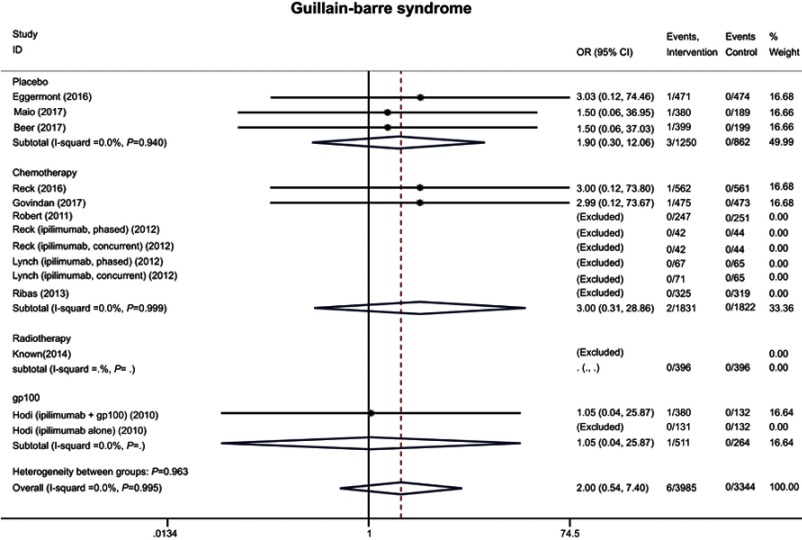
| Guillain-Barre syndrome | 2.00 | 0.54–7.40 | 0.299 | 2.00 | 0.54–7.40 | 0.299 |
| Treatment-related AEs | ||||||
| Hematologic | ||||||
| Anemia | 1.11 | 0.83–1.49 | 0.484 | 0.95 | 0.68–1.34 | 0.782 |
| Neutropenia | 1.05 | 0.66–1.68 | 0.826 | 0.72 | 0.37–1.42 | 0.349 |
| Thrombocytopenia | 0.84 | 0.47–1.52 | 0.569 | 0.57 | 0.34–0.96 | 0.035 |
| Musculoskeletal problems | ||||||
| Arthritis | 1.30 | 0.25–6.77 | 0.755 | 1.30 | 0.25–6.77 | 0.755 |
| Arthralgia | 1.02 | 0.86–1.20 | 0.851 | 0.84 | 0.27–2.61 | 0.769 |
| Back pain | 0.77 | 0.65–0.90 | 0.001 | 0.65 | 0.36–1.15 | 0.137 |
| Musculoskeletal pain | 0.72 | 0.58–0.90 | 0.003 | 0.7 | 0.24–2.01 | 0.503 |
| Bone pain | 0.76 | 0.58–0.99 | 0.044 | 0.73 | 0.30–1.78 | 0.488 |
| Myalgia | 1.33 | 1.00–1.77 | 0.05 | 0.71 | 0.07–6.83 | 0.765 |
Notes: *IrAEs, immune-related adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; OR, odds ratio; CI, confidence interval. #Includes both serious and other adverse events if data were extracted from ClinicalTrials.gov; includes all Common Terminology of Clinical Adverse Events (CTCAE) grades if data were extracted from the publication. †Serious adverse events if data were extracted from ClinicalTrials.gov; CTCAE grades ≥3 if data were extracted from the publication.
Dermatologic AEs
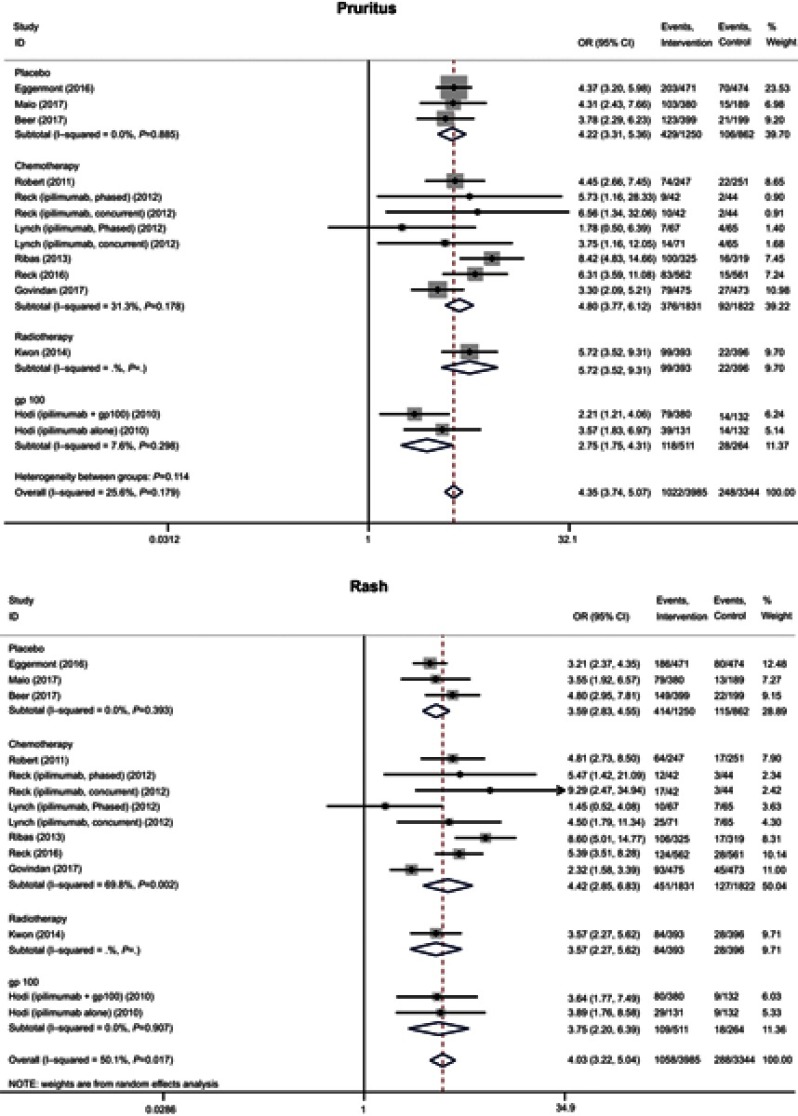
Pruritus and rash affected 1022 (25.6%) and 1058 (26.5%) patients, respectively, and were reported in all 11 trials at all grades. The incidence of all grades of pruritus (OR 4.35, 95% CI 3.74–5.07) and rash (OR 4.03, 95% CI 3.22–5.04) was significantly higher for the anti-CTLA-4 treatment groups than the corresponding control groups (Figure 1), as was the incidence of severe rash (OR 3.39, P=0.003). There was no significant difference between the experimental and control groups with respect to the incidence of severe pruritus (OR 3.64, P=0.072) (Table 4).
Figure 1.
Forest plot of the overall risk of pruritus and rash related to anti-CTLA-4 drugs.
Gastrointestinal AEs
Diarrhea and colitis were reported in 11 and 9 studies, and affected a total of 1905 (47.8%) and 254 (6.4%) patients, respectively (all grades). Compared with the control group, the anti-CTLA-4-treated patients had higher overall risks of diarrhea (OR 2.88, 95% CI 2.35–3.78) and colitis (OR 14.62, 95% CI 8.61–24.83) (Figure 2). The same trends were observed for serious diarrhea (OR 6.57, 95% CI 4.09–10.58) and serious colitis (OR 14.01, 95% CI 7.34–26.77) (Table 4).
Figure 2.

Forest plot of the overall risk of colitis and diarrhea related to anti-CTLA-4 drugs.
Endocrine AEs
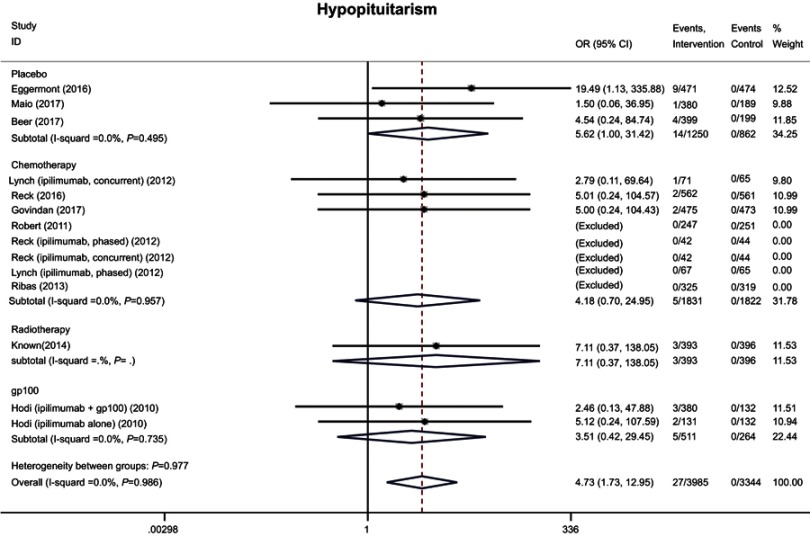
Hypophysitis, hyperthyroidism, hypothyroidism, adrenal insufficiency, and hypopituitarism of any grade were observed in 131 (3.3%), 100 (2.5%), 9 (0.2%), 25 (0.6%), and 27 (0.7%), respectively, of patients treated with anti-CTLA-4 drugs. Meta-analysis showed that patients in the intervention arms had higher risks of hypophysitis (OR 5.30, 95% CI 1.71–16.46), hypothyroidism (OR 7.86, 95% CI 4.10–15.04), adrenal insufficiency (OR 3.88, 95% CI 1.46–10.36), and hypopituitarism (OR 4.73, 95% CI 1.73–12.95), but not hyperthyroidism (OR 3.78, 95% CI 0.94–15.17), compared with the control therapies (Figures 3, 4, and S2). The same trends were observed when the rates of severe AEs were analyzed (Table 4).
Figure 3.

Forest plot of the overall risk of adrenal insufficiency and hypophysitis related to anti-CTLA-4 drugs.
Figure 4.

Forest plot of the overall risk of hyperthyroidism and hypothyroidism related to anti-CTLA-4 drugs.
Figure S2.
Forest plot of the overall risk of hypopituitarism related to anti-CTLA-4 drugs.
Hepatic AEs
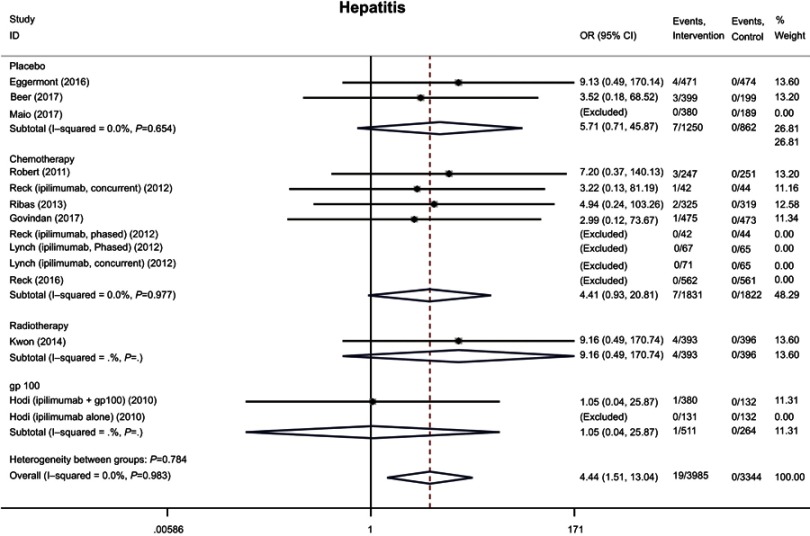
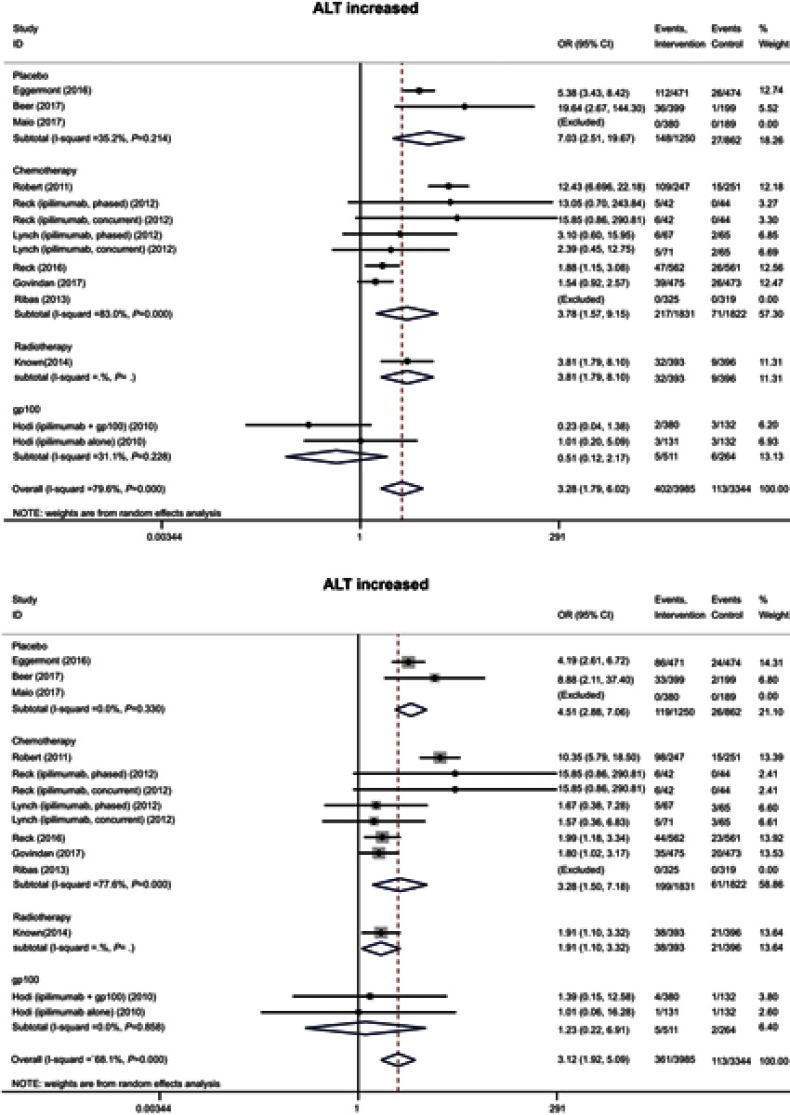
A total of 8, 9, and 9 studies evaluated the incidence of hepatitis, alanine aminotransferase (ALT) levels, and aspartate aminotransferase (AST) levels, respectively, and were found to affect 19 (0.5%), 402 (10.1%), and 361 (9.1%) patients, respectively, in the anti-CTLA-4 intervention groups. Meta-analysis showed that the risks of all three hepatic AEs were higher for the anti-CTLA-4 compared with control groups (hepatitis: OR 4.44, 95% CI 1.51–13.04; ALT: OR 3.28, 95% CI 1.79–6.02; AST: OR 3.12, 95% CI 1.92–5.09) (Figures 5 and S3). The same trend held when severe hepatic AEs were analyzed (Table 4).
Figure 5.
Forest plot of the overall risk of hepatitis related to anti-CTLA-4 drugs.
Figure S3.
Forest plot of the overall risk of ALT and AST elevation related to anti-CTLA-4 drugs.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Other irAEs
We also analyzed the incidence of pneumonitis, pancreatitis and Guillain–Barre syndrome in our study. Most of them were severe and the rates were low (<1%) in the intervention arms (18 [0.7%], 8 [0.2%], and 6 [0.2%] for severe pneumonitis, pancreatitis, and Guillain–Barre syndrome). No significant differences were observed between the two arms (pneumonitis: OR 1.64 95% CI 0.91–2.94; pancreatitis: OR 1.51 95% CI 0.49–4.60; Guillain–Barre syndrome: OR 2.00 95% CI 0.54–7.04) (Figure 6 and S4).
Figure 6.
Forest plot of the overall risk of pneumonitis and pancreatitis related to anti-CTLA-4 drugs.
Figure S4.
Forest plot of the overall risk of Guillain–Barre syndrome related to anti-CTLA-4 drugs.
Treatment-related AEs
In addition to the organ-specific irAEs, we also examined the incidence of treatment-related AEs including hematologic abnormalities and musculoskeletal disorders (seen in Table 5). The pooled odds ratios are exhibited in Table 4.
Table 5.
Incidence of treatment-related adverse events related to anti-CTLA-4 drugs. Values are percentages (95% confidence intervals)
| Drugs | Ipilimumab (n=3280) | Tremelimumab (n=705) | Total (n=3985) | |||
|---|---|---|---|---|---|---|
| Treatment-related AEs* | All# | Serious† | All | Serious | All | Serious |
| Hematologic | ||||||
| Anemia | 20.1 (18.7–21.5) | 2.3 (1.8–2.8) | 8.4 (6.4–10.7) | 0.7 (0.2–1.6) | 18.0 (16.8–19.2) | 2.0 (1.6–2.5) |
| Neutropenia | 13.6 (12.4–14.8) | 1.3 (1.0–1.8) | 0.7 (0.2–1.6) | 0.6 (0.2–1.4) | 11.3 (10.3–12.3) | 1.2 (0.9–1.6) |
| Thrombocytopenia | 6.1 (5.3–6.9) | 0.7 (0.5–1.1) | 1.0 (0.4–2.0) | 0.4 (0.1–1.2) | 5.2 (4.5–5.9) | 0.7 (0.4–1.0) |
| Musculoskeletal problems | ||||||
| Arthritis | 0.1 (0.0–0.3) | 0.1 (0.0–0.3) | 0.0 (0.0–0.5) | 0.0 (0.0–0.5) | 0.1 (0.0–0.2) | 0.1 (0.0–0.2) |
| Arthralgia | 11.4 (10.3–12.5) | 0.2 (0.1–0.4) | 0.0 (0.0–0.5) | 0.0 (0.0–0.5) | 9.4 (8.5–10.3) | 0.2 (0.1–0.3) |
| Back pain | 10.4 (9.4–11.5) | 0.6 (0.4–1.0) | 3.0 (1.9–4.5) | 0.0 (0.0–0.5) | 9.1 (8.2–10.0) | 1.5 (1.2–2.0) |
| Musculoskeletal pain | 5.5 (4.8–6.4) | 0.2 (0.1–0.4) | 0.1 (0.0–0.8) | 0.1 (0.0–0.8) | 4.6 (4.0–5.3) | 0.2 (0.1–0.4) |
| Bone pain | 3.4 (2.8–4.0) | 0.2 (0.1–0.5) | 0.0 (0.0–0.5) | 0.0 (0.0–0.5) | 2.8 (2.3–3.3) | 0.2 (0.1–0.4) |
| Myalgia | 4.7 (4.0–5.5) | 0.0 (0.0–0.1) | 0.1 (0.0–0.8) | 0.1 (0.0–0.8) | 3.9 (3.3–4.5) | 0.0 (0.0–0.1) |
Notes: *AEs, adverse events. #Includes both serious and other adverse events if data were extracted from ClinicalTrials.gov; includes all Common Terminology of Clinical Adverse Events (CTCAE) grades if data were extracted from the publication. †Serious adverse events if data were extracted from ClinicalTrials.gov; CTCAE grades ≥3 if data were extracted from the publication.
Hematologic abnormalities
Anemia, neutropenia, and thrombocytopenia were assessed in nine studies. A total of 717 (18.0%), 451 (11.3%), and 206 (5.2%) patients occurred these three AEs when receiving anti-CTLA-4 drugs, respectively. Our meta-analyses demonstrated no significant difference existed between the two arms with regard to anemia (OR 1.11 95% CI 0.83–1.49), neutropenia (OR 1.05 95% CI 0.66–1.68), and thrombocytopenia (OR 0.84 95% CI 0.47–1.52). When looking at severe hematologic AEs, patients treated with anti-CTLA-4 drugs were less likely to suffer thrombocytopenia (OR 0.57 95% CI 0.34–0.96) (Table 4).
Musculoskeletal disorders
Six musculoskeletal disorders including arthritis, arthralgia, back pain, musculoskeletal pain, bone pain, and myalgia were analyzed in our study. The numbers and rates were 3 (0.1%), 374 (9.4%), 363 (9.1%), 183 (4.6%), 110 (2.8%), and 115 (3.5%) for each musculoskeletal problem related to anti-CTLA-4 drugs, respectively (Table 5). Meta-analysis showed that patients were less likely to experience back pain, musculoskeletal pain, and bone pain (OR 0.77, 0.72, 0.76; all P<0.05) and more likely to experience myalgia (OR 1.33, P=0.05) compared to the control group. There was no significant difference regarding the severe musculoskeletal disorders (arthritis: OR 1.30 95% CI 0.25–6.77; arthralgia: OR 0.84 95% CI 0.27–2.61; back pain: OR 0.65 95% CI 0.36–1.15; musculoskeletal pain: OR 0.70 95% CI 0.24–2.01; bone pain: OR 0.73 95% CI 0.30–1.78; myalgia: OR 0.71 95% CI 0.07–6.83) (Table 4).
Discussion
The aim of this study was to provide further understanding of the incidence of irAEs related to anti-CTLA-4 drugs with the ultimate goal of assisting clinicians in treating patients with this drug class. We analyzed 11 randomized controlled trials that included a total of nearly 4000 patients treated with ipilimumab or tremelimumab and provided precise data of the common and important AEs involving multiple organs. To our knowledge, this is the most comprehensive meta-analysis of the incidence and risk of organ-specific irAEs following anti-CTLA-4 therapy in cancer patients, and additionally, we believe this is the first meta-analysis to comprehensively evaluate the incidence of anti-CTLA-4 treatment-related hematologic abnormalities and musculoskeletal disorders compared with control treatments.
Among the strengths of our study is the inclusion of AE data mainly collected from ClinicalTrials.gov (10 of 11 trials). Several meta-analyses have previously investigated anti-CTLA-4-related AEs,12–14 but most of them focused mainly on ipilimumab and evaluated a limited number of irAEs. Compared with these studies, we included ipilimumab and tremelimumab and analyzed more trials and more irAEs, which is a strength but could also be a source of inconsistency between the studies. Unlike the study conducted by Velasco et al,13 we found an increased risk of all-grade AST elevation and high-grade hypothyroidism with CTLA-4 inhibitors compared with control therapies, although our findings were largely the same with regard to rash and colitis. Collection of the data directly from ClinicalTrials.gov enabled us to evaluate useful information on AEs with relatively low clinical awareness, such as hematologic and musculoskeletal disorders.
Our findings are of considerable clinical importance for multidisciplinary cooperation. As the use of immune checkpoint inhibitors in cancer treatment increases, AEs related to this drug class will require the participation of doctors from departments other than oncology26 to ensure adequate management of multi-organ AEs. In our study, the rates of all-grade pruritus, rash, diarrhea, and ALT elevation exceeded 10%, and serious diarrhea and colitis were also common (9.8% and 5.3%, respectively). Similarly, all-grade hematologic AEs were high (ranging from 18% to 5.2%), but serious events were less frequent (0.7–2%), consistent with previous reports.10,27,28 The incidence of musculoskeletal disorders associated with anti-PD-1–PD-L1 therapy has recently been investigated in a meta-analysis,11 and inflammatory arthritis is a well-known AE associated with immune checkpoint inhibitors.29,30 Optimal management of these potentially serious AEs requires multidisciplinary cooperation to best serve cancer patients treated with checkpoint inhibitors.
Many of the increased risks of irAEs identified here were also noted in the recent meta-analysis of irAEs associated with anti-PD-1 drugs,11 including all-grade rash (OR 4.03 here vs 2.34 in the study by Baxi et al11), colitis (OR 14.62 vs 2.88), hypothyroidism (7.86 vs 6.92), and hypophysitis (5.30 vs 3.38), although the risks were higher with anti-CTLA-4 mAbs, especially colitis. However, we detected an increased risk of diarrhea and hepatitis with anti-CTLA-4 mAbs, which was not observed with anti-PD-1/PD-L1,11 and conversely, an increased risk of pneumonitis (OR 3.82) was associated with anti-PD-1–PD-L1 drugs,11 but not with anti-CTLA-4 drugs (this study), compared with the control therapies.
Questions remain about whether some of these discrepancies may be due to inaccurate and/or inconsistent identification of AEs associated with immune checkpoint inhibitors. To avoid this, we must have a better understanding of the characteristics, timing, and outcomes of these AEs. The full spectrum of irAEs associated with immune checkpoint drugs is not yet clear, because some may emerge early and result in a high mortality rate (eg, myocarditis31) and others may appear too late to be recognized within the restricted period of follow-up for most studies. It is crucial that clinical trials collect and report as much information as possible on AEs resulting from administration of immune checkpoint inhibitors, and their reporting should also follow the most up-to-date edition of the Common Terminology of Clinical Adverse Events.
In our meta-analysis, we detected some inconsistencies in reporting terms for the irAEs across trials. Although collection of data from ClinicalTrials.gov enabled us to access more information on AEs (reported according to the Common Terminology of Clinical Adverse Events guidelines) compared with the published literature, recognition of AEs is highly subjective and relies on personal experience, which may contribute to cross-trial inconsistencies. For instance, some well-described irAEs (colitis, hepatitis, and hypothyroidism) may be correctly recognized and reported, whereas less common AEs (eg, Guillain–Barre syndrome and musculoskeletal disorders) may not. Increased attention should be paid to this topic to ensure that reporting of AEs in these clinical trials becomes more standardized and accurate. Overall, successful management of irAEs related to immune checkpoint inhibitors requires early diagnosis, high suspicion, excellent patient–provider communication, and rapid and aggressive use of corticosteroids and other immunosuppressants.26
Some limitations of our study should also be noted. First, some AEs may not have been recognized and/or reported, leading to underestimation of their rate and overestimation of drug safety.32 Second, pooling of the irAEs may have been affected by inconsistencies in the definition of irAEs.8,23,25 Third, our study included only two randomized controlled trials of tremelimumab, with the majority focusing on ipilimumab; thus, the incidence of AEs might be related mainly to ipilimumab rather than anti-CTLA-4 drugs as a class. Fourth, some AEs were relatively rare and only reported in a few trials, potentially contributing to publication bias. Fifth, because the control therapies differed (placebo, chemotherapy, radiation therapy, vaccine), we performed subgroup analyses to try to eliminate confounding bias. Sixth, a previous study showed that the rate of AEs was associated with the ipilimumab dose.33 Our dataset included one randomized controlled trial with 3 mg/kg ipilimumab, one with 15 mg/kg tremelimumab, and the remainder with 10 mg/kg mAb, which precluded analysis of dose dependency. Finally, the relatively short duration of follow-up may have underestimated the rates of some events. Going forward, standard criteria for irAEs and longer follow-up times might be helpful in understanding irAEs related to anti-CTLA-4 therapy.
Conclusion
In this meta-analysis, we found that anti-CTLA-4 mAbs carried an increased risk of serious organ-specific irAEs compared with control therapies, most frequently involving the gastrointestinal system. However, the rates of hematologic abnormalities and severe musculoskeletal disorders were the same as with control therapies. Our results underscore the need for clinical awareness of irAEs related to the treatment of cancer patients with anti-CTLA-4 drugs.
Acknowledgments
This program was supported by the National Natural Science Foundation of China (Grant No.81300627, No.81702536), Programs from Science and Technology Department of Sichuan Province (Grant No. 2018JY0089 and No. 2018HH0153), the Prostate Cancer Foundation Young Investigator Award 2013, Chengdu Technological Innovation Research and Development Project (Chengdu Science and Technology Bureau, 2018-YFYF-00131-SN), a grant from 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYGD18011), and Young Investigator Award of Sichuan University 2017 (Grant No. 2017SCU04A17). The funders had no role in patient selection, data extraction, statistical analysis or interpretation, writing of this article, or the decision to publish. The authors would like to thank Anne M. O’Rourke, PhD, from Liwen Bianji, Edanz Group China, for editing the English text of a draft of this manuscript.
Abbreviations
IrAEs, immune-related adverse events; Anti-CTLA-4, anti-cytotoxic T-lymphocyte-associated protein-4; ALT, alanine aminotransferase; AST, aspartate aminotransferase; OR, odds ratio; CI, confidence interval; CTCAE, Common Terminology of Clinical Adverse Events; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Supplementary materials
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hossain MK, Nahar K, Donkor O, Apostolopoulos V. Immune-based therapies for metastatic prostate cancer: an update. Immunotherapy. 2018;10(4):283–298. doi: 10.2217/imt-2017-0123 [DOI] [PubMed] [Google Scholar]
- 2.Ugurel S, Röhmel J, Ascierto P, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies-update 2017. Eur J Cancer. 2017;83:247–257. doi: 10.1016/j.ejca.2017.06.028 [DOI] [PubMed] [Google Scholar]
- 3.Xia B, Herbst RS. Immune checkpoint therapy for non-small-cell lung cancer: an update. Immunotherapy. 2016;8(3):279–298. doi: 10.2217/imt.15.123 [DOI] [PubMed] [Google Scholar]
- 4.Calabro L, Ceresoli GL, D‘Incecco A, Scherpereel A, Aerts J, Maio M. Immune checkpoint therapy of mesothelioma: pre-clinical bases and clinical evidences. Cytokine Growth Factor Rev. 2017;36:25–31. doi: 10.1016/j.cytogfr.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 5.O‘Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110(12):2614–2627. doi: 10.1002/cncr.23086 [DOI] [PubMed] [Google Scholar]
- 6.Kaehler KC, Piel S, Livingstone E, Schilling B, Hauschild A, Schadendorf D. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol. 2010;37(5):485–498. doi: 10.1053/j.seminoncol.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 7.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. doi: 10.1056/NEJMoa1611299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O‘Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiuan E, Beckermann KE, Ozgun A, et al. Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer. 2017;5:8. doi: 10.1186/s40425-017-0210-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793. doi: 10.1136/bmj.k793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komaki Y, Komaki F, Yamada A, Micic D, Ido A, Sakuraba A. Meta‐analysis of the risk of immune‐related adverse events with anticytotoxic T‐lymphocyte‐associated antigen 4 and antiprogrammed death 1 therapies. Clin Pharmacol Ther. 2017;103(2):318–331. [DOI] [PubMed] [Google Scholar]
- 13.De Velasco G, Je Y, Bossé D, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5(4):312. doi: 10.1158/2326-6066.CIR-16-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13(1):211. doi: 10.1186/s12916-015-0455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj Br Med J. 2011;343(7829):889–893. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83. doi: 10.1093/annonc/mds213 [DOI] [PubMed] [Google Scholar]
- 18.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–2054. doi: 10.1200/JCO.2011.38.4032 [DOI] [PubMed] [Google Scholar]
- 19.Maio M, Scherpereel A, Calabro L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18(9):1261–1273. doi: 10.1016/S1470-2045(17)30446-1 [DOI] [PubMed] [Google Scholar]
- 20.Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–3457. doi: 10.1200/JCO.2016.71.7629 [DOI] [PubMed] [Google Scholar]
- 21.Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–47. doi: 10.1200/JCO.2016.69.1584 [DOI] [PubMed] [Google Scholar]
- 22.Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–3748. doi: 10.1200/JCO.2016.67.6601 [DOI] [PubMed] [Google Scholar]
- 23.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 26.Weber J, Yang J, Atkins M, Disis M. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol. 2015;33(18):2092–2099. doi: 10.1200/JCO.2014.60.0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ban-Hoefen M, Burack R, Sievert L, Sahasrabudhe D. Ipilimumab-induced neutropenia in melanoma. J Investig Med High Impact Case Rep. 2016;4(3):2324709616661835. doi: 10.1177/2324709616661835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyers DE, Hill WF, Suo A, Jimenez-Zepeda V, Cheng T, Nixon NA. Aplastic anemia secondary to nivolumab and ipilimumab in a patient with metastatic melanoma: a case report. Exp Hematol Oncol. 2018;7:6. doi: 10.1186/s40164-018-0098-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Annals of the Rheumatic Diseases. 2016;76(1):43. doi: 10.1136/annrheumdis-2016-209595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leipe J, Christ LA, Arnoldi AP, et al. Characteristics and treatment of new-onset arthritis after checkpoint inhibitor therapy. RMD Open 2018;4(2): e000714. doi: 10.1136/rmdopen-2018-000714 [DOI] [PMC free article] [PubMed]
- 31.Moslehi J, Salem J, Sosman J, Lebrun-Vignes B, Johnson D. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet (London, England). 2018;391(10124):933. doi: 10.1016/S0140-6736(18)30302-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saini P, Loke YK, Gamble C, Altman DG, Williamson PR, Kirkham JJ. Selective reporting bias of harm outcomes within studies: findings from a cohort of systematic reviews. Bmj. 2014;349(nov21 3):g6501. doi: 10.1136/bmj.g6501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng Y, Roy A, Masson E, Chen TT, Humphrey R, Weber JS. Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2013;19(14):3977–3986. doi: 10.1158/1078-0432.CCR-12-3243 [DOI] [PubMed] [Google Scholar]