Abstract
Human papillomavirus (HPV) types 16 and 18 cause 70% of cervical cancer cases globally. The nonavalent HPV vaccine (9vHPV) was licensed in 2014 and protects against the next five most common cancer-causing HPV types (HPV 31/33/45/52/58) after HPV 16/18. Phase III clinical studies have demonstrated high vaccine efficacy (>90%) against cervical, vulvar, and vaginal precancers caused by these additional types, and have shown comparable immunogenicity to the shared genotypes to quadrivalent HPV vaccine (4vHPV). Vaccine efficacy and antibody responses for 9vHPV are found to persist for at least five years while longer-term observational studies are ongoing to monitor long-term vaccine effectiveness. The implementation of 9vHPV has the potential to prevent up to 93% of cervical cancer cases, as well as a significant proportion of other HPV-related anogenital cancers. This review article summarizes the current evidence for 9vHPV in terms of vaccine efficacy against HPV infection and related anogenital precancers, safety, and immunogenicity, as well as discussing the potential impact of this vaccine on the cervical cancer burden globally.
Keywords: nonavalent human papillomavirus vaccine, review, efficacy, immunogenicity, safety
Introduction
The human papillomavirus (HPV) vaccines are made using recombinant technology based on the self-assembly nature of the L1 capsid protein of the virus (viral-like particles, VLPs). As the VLPs do not contain the viral genome, they are not infectious. There are currently three licensed prophylactic HPV vaccines against HPV infections and cervical cancer precursors. The timeline of the Phase III clinical trials and licensure/registration of HPV vaccines is shown in Figure 1, and countries that have a national HPV immunization program are shown in Figure 2. The first-generation HPV vaccines, quadrivalent (4vHPV) and bivalent (2vHPV), were first licensed in 2006 and 2007, respectively. Both vaccines protect against HPV types 16 and 18 (major types causing cervical cancers), while 4vHPV also protects against HPV types 6 and 11 (major types causing genital warts). In 2014, a second-generation nonavalent HPV vaccine (9vHPV) was licensed by The United States (U.S.) Food and Drug Administration (FDA) for individuals aged 9–26 years, and subsequently extended to women up to age 45 years in 2018. This vaccine contains an additional five cancer-causing HPV types (HPV31, 33, 45, 52, and 58) in addition to the four types in 4vHPV, and has the potential to prevent up to 93% of cervical cancers.1 This review article summarizes the efficacy of 9vHPV in preventing HPV infection and related anogenital precancers as demonstrated in clinical trials to date, as well as the potential impact of this vaccine on the cervical cancer burden globally. We searched for studies and review articles that evaluated the safety, efficacy, and immunogenicity of 9vHPV using PubMed, Google Scholar, and scientific review/comment papers during August 2018–January 2019, and included them in this review article. Search terms used include “Gardasil9”, “nine-valent HPV vaccine”, “burden of cervical cancer”, “HPV vaccine licensure”, “HPV vaccination”, “HPV vaccines”, and “cost-effectiveness”.
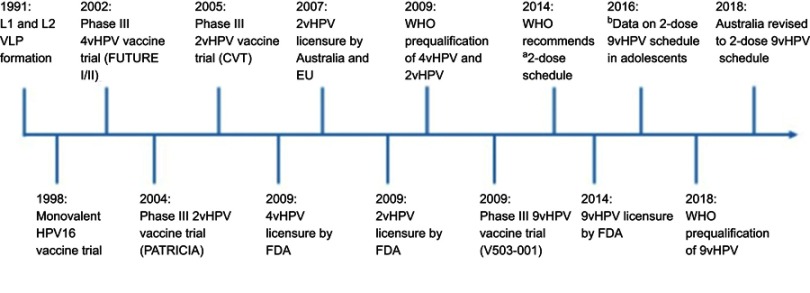
Figure 1.
Timeline of pivotal Phase III HPV vaccine trials and licensure/registration of the HPV vaccines.
Notes: aTwo-dose schedule separated by six months to adolescents aged <15 years. b Data from Iversen et al.57
Abbreviations: 4vHPV, quadrivalent HPV vaccine; 2vHPV, bivalent HPV vaccine; 9vHPV, nonavalent HPV vaccine; FDA, The U.S. Food and Drug Administration; HPV, human papillomavirus; EU, European Union; VLP, virus-like particle.
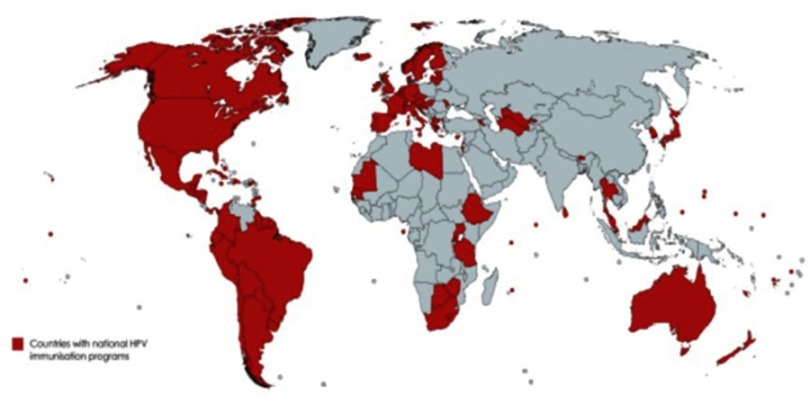
Figure 2.
Countries that have introduced a national human papillomavirus immunization program using any of the three licensed vaccines (91 countries, 47%).
Source: Data from WHO.85
Burden of HPV-associated diseases
HPV infection is one of the most common sexually transmitted infections worldwide.2 A meta-analysis of over one million women from 194 studies found an overall HPV prevalence of approximately 12% in asymptomatic women globally.3 The highest HPV prevalence is in the sub-Saharan African regions (24%), although these estimates vary by region, study design, as well as age of participants.3 Most individuals (approximately 90%) infected with HPV are asymptomatic and are able to clear the infection through host immune responses within two years.4 However, for the small proportion who have persistent infection, this can lead to a range of diseases from benign lesions to anogenital cancers as well as some oropharyngeal diseases. There are currently more than 100 HPV genotypes identified that can be classified into high- and low-risk types depending on the potential neoplastic disease they cause.
Low-risk HPV types 6 and 11 cause 90% of anogenital warts and benign/low-grade abnormalities in the genital areas.5,6 These HPV types are also responsible for causing recurrent respiratory papillomatosis in the respiratory tract.7 The high-risk HPV types are known to cause anogenital cancers, which includethe majority of cervical cancer cases and a significant proportion of vaginal (78%) and anal (88%) cancers, as well as some vulvar (25%) and penile (50%) cancers.8,9 In addition, high-risk types, in particular HPV16, are also responsible for an increasing number of oropharyngeal cancers (31%) largely in the base of the tongue and tonsillar areas.10,11 These diseases are more common in immunocompromised individuals such as those infected with HIV or post transplantation.12
Cervical cancer remains the fourth most common cancer in women worldwide, with an estimated 570,000 cases and 311,000 deaths annually.13 The majority of these cases occur in low-and lower-middle-income countries (LLMICs) due to multiple factors. These factors include the lack of comprehensive cervical cancer screening programs and treatment of lesions found on screening, no access to HPV vaccine due to the high vaccine cost, limited health services to deliver these vaccines, as well as the vaccine being seen as a low priority.14,15 Moreover, issues such as vaccine hesitancy, and social and cultural factors have also impeded the introduction of HPV vaccines.14 Globally, HPV16 and 18 are responsible for 70% of cervical cancer.9 Together with HPV types 31/33/45/52/58, these nine types are responsible for approximately 90% of cervical cancers worldwide.8,9 The prevalence of these five HPV types varies geographically, with higher prevalence of HPV52 and 58 in East Asia and North America, while HPV31, 33 and 45 are more common in European countries.3,16
First-generation HPV vaccines
The formulations and schedules of 4vHPV and 2vHPV are summarized in Table 1. Although both vaccines have aluminum-based adjuvant, 2vHPV also contains a monophosphoryl lipid A adjuvant component, which activates innate immunity through Toll-like receptor 4.17 This adjuvant system is also known as AS04 and is thought to account for the higher immunogenicity induced by 2vHPV when compared with 4vHPV, although the clinical significance remains unknown (there has been no breakthrough disease seen in those vaccinated and thus no defined immune correlate of protection).18,19 Both 4vHPV and 2vHPV have greater than 90% efficacy against cervical intraepithelial neoplasia grade 1–3, adenocarcinoma in situ, and invasive cervical carcinoma due to HPV16/18 in women aged 15–26 years.20–23 Although both vaccines might provide protection against other HPV-related cancers such as vulvar, vaginal, and anal,24 only 4vHPV is licensed against these cancers as well as against HPV6- and HPV11-related genital warts.
Table 1.
Characteristics of HPV VLP vaccines
| Manufacturer | Merck™ (Gardasil®) | GlaxoSmithKline™ (Cervarix®) | Merck™ (Gardasil® 9) |
|---|---|---|---|
| L1 VLP types | 6, 11, 16, and 18 | 16 and 18 | 6, 11, 16, 18, 31, 33, 45, 52, and 58 |
| Dose | 20/40/40/20 µg | 20/20 µg | 30/40/60/40/20/20/20/20/20 µg |
| Producer cells | Saccharomyces cerevisiae (baker’s yeast) expressing L1 | Trichoplusia ni (Hi 5) insect cell line infected with L1 recombinant baculovirus | Saccharomyces cerevisiae (baker’s yeast) expressing L1 |
| Adjuvant | 225 µg aluminium hydroxyphosphate sulfate | 500 µg aluminium hydroxide, 50 µg 3-O-deacylated-4ʹ-monophosphoryl lipid A | 500 µg aluminium hydroxyphosphate sulfate |
| Vaccination schedule | 0, 2, and 6 months | 0, 1, and 6 months | 0, 2, and 6 months |
Abbreviations: HPV, human papillomavirus; VLP, virus-like particle.
Neutralizing antibodies are thought to be the primary mediator of protection for HPV vaccines, although to date, the actual level required for protection (ie, correlate) has not been identified. Overall, seroconversion occurs in 99–100% of those vaccinated in randomized double-blind placebo-controlled trials.25 Antibody responses following vaccination peak at month seven (one month after the third dose) at titers between 10- and 100-fold higher than the levels found following natural infection.25 Such antibody responses are found to persist at a level several-fold higher than natural infection for more than 10 years.26 The high immunogenicity of the HPV vaccine is thought to be due to the dense and ordered structure of the VLPs, as well as the route of administration in comparison to the natural infection route.27
The high vaccine efficacy observed in vaccinated young adults (age 16–26 years) not infected or previously infected with the vaccine HPV types has prompted the use of HPV vaccine in young adolescents before the onset of sexual activity. Immunobridging studies evaluating either 4vHPV or 2vHPV in adolescent girls/boys and women following a three-dose schedule found at least one- to two-fold higher type-specific antibodies in the younger age group.28–30 Moreover, several studies then found that two doses given to adolescents six months apart had antibody responses that were non-inferior to women who were given the standard three-dose schedule, summarized by Toh et al.31 These studies have led the WHO to revise its recommendation in 2014 to include a two-dose HPV vaccine schedule for girls aged <15 years, provided the interval between each dose is at least six months.32
Both 2vHPV and 4vHPV also provide some degree of cross-protection to nonvaccine but phylogenetically related HPV types (ie, HPV31, 33, and 45), which is likely to be mediated by cross-neutralizing antibodies.33–38 In most cases, 2vHPV generated significantly higher neutralizing antibodies for HPV31 and 45 than 4vHPV, possibly due to its unique adjuvant properties, although the clinical significance of this is unknown.37,39,40 These cross-neutralizing antibodies are approximately 100-fold lower than vaccine-type antibodies,41 which raises the question of the duration of protection against these nonvaccine types.
Vaccine efficacy of 9vHPV
The appeal of 9vHPV is the potential to protect against the nine most common cancer-causing HPV types, as well as the majority of genital warts cases. Since 9vHPV was only licensed in the last five years and rollout in public health programs is only recent, population data are still several years away. Phase III studies have demonstrated that 9vHPV is safe and highly efficacious against HPV infection and anogenital precancer lesions in both men and women (Table 2). Of note, the pivotal trial that led to the licensure of 9vHPV was an international multicenter, double-blind randomized controlled trial of more than 10,000 women aged 16–26 years. The study reported greater than 96% vaccine efficacy against high-grade cervical, vulvar, or vaginal disease as well as six-month persistent infection caused by HPV31, 33, 45, 52, and 58 in women not previously infected with HPV following three doses of 9vHPV.42 This high efficacy (90–98%) of 9vHPV in preventing certain HPV-related precancers was sustained for up to six years.43 Due to ethical concerns, 9vHPV was compared with 4vHPV instead of a placebo control in this study. The incidence of disease related to HPV-6, 11, 16, and 18 was similar between both groups, with comparable antibody responses observed, suggesting that 9vHPV is just as effective as 4vHPV for these types. In addition, all participants who received 9vHPV seroconverted to the additional five HPV types (HPV31, 33, 45, 52 and 58) one month following the last dose, and the levels of these five additional HPV types were significantly higher than in 4vHPV recipients.
Table 2.
Studies reporting efficacy of 9vHPV against HPV infection and associated diseases
| Type of study | Primary endpoint | Number of participants and age | Study findings | References |
|---|---|---|---|---|
| Subgroup analyses of two Phase III RCTs | Efficacy, immunogenicity, and safety in Asian participants | Study 001: N=1717 (women, 16–26 years) Study 002: N=608 (boys and girls) |
Per-protocol 9vHPV efficacy against HPV31, 33, 45, 52, and 58:
|
44 |
| Subgroup analyses of two Phase III RCTs | Efficacy, immunogenicity, and safety in Latin American participants | Study 001: N=4744 (women, 16–26 years) Study 002: N=568 (boys and girls) |
Per-protocol 9vHPV efficacy against HPV31, 33, 45, 52, and 58 (Study 001):
|
45 |
| Phase III double-blind RCT |
Vaccine efficacy against incidence of high-grade CIN, AIS, ICC, VIN, VaIN, and vaginal/vulva cancer related to HPV31, 33, 45, 52, and 58 | N=14,215 (16–26 years) Follow up to 42 months for both groups, N=13,587; up to 60 months for 9vHPV group |
Per-protocol 9vHPV efficacy against HPV31, 33, 45, 52, and 58:
Per-protocol 9vHPV efficacy against HPV31, 33, 45, 52, and 58, 6 years post dose one:
Similar incidence of CIN, VIN, ValN, and persistent infection related to HPV6, 11, 16, and 18 between 4vHPV and 9vHPV |
42, 43 |
Abbreviations: 4vHPV, quadrivalent HPV vaccine; 9vHPV, nonavalent HPV vaccine; AIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; ICC, invasive cervical carcinoma; RCT, randomized controlled trial; VaIN, vaginal intraepithelial neoplasia; VIN, vulvar intraepithelial neoplasia.
The burden of cervical cancer and risk is different across populations around the world. Subgroup analyses from two Phase III double-blind randomized controlled trials were performed among Asian and Latin American participants to determine the population-specific vaccine efficacy.44,45 Similar high vaccine efficacy (>96%) against any grade of cervical, vulvar, and vaginal diseases, as well as greater than 93% against six-month persistent HPV infection, were found for both ethnic populations in individuals who were not infected with HPV. These findings were consistent with the vaccine efficacy globally, suggesting that 9vHPV is as efficacious in Asia and Latin America.
Reactogenicity and immunogenicity of 9vHPV
Antibody responses to 9vHPV types and the comparison of the shared HPV genotypes (HPV6, 11, 16, and 18) between 9vHPV and 4vHPV were investigated in clinical studies consisting of young women (aged 16–26 years) and young adolescent boys and girls (aged 9–15 years) (Table 3).46–49 Almost 100% of individuals who received 9vHPV seroconverted to all vaccine types one month following the third dose, and the antibody levels for HPV6, 11, 16, and 18 were similar between the two vaccine groups, suggesting that the addition of five new types to 9vHPV did not result in interference and hence did not alter immunogenicity to the 4vHPV types.42,50 Prior to 9vHPV licensure, three immunobridging Phase II studies evaluated the vaccine dose formulation to ensure the optimal formulation in terms of immunogenicity and safety of a multivalent HPV vaccine.51
Table 3.
Studies reporting immunogenicity and reactogenicity of 9vHPV
| Type of study | Immunogenicity endpoint | Study findings | References |
|---|---|---|---|
| Subgroup analyses of Asian and Latin American participants from two Phase III RCTs | Non-inferior GMTs for anti-4vHPV types in girls/boys aged 9–15 years and young women aged 16–26 years at month seven |
Immunogenicity: >98% of girls, boys, and young women seroconverted for each 9vHPV type GMTs for anti-HPV6/11/16/18 at month seven were generally similar between the qHPV vaccine and 9vHPV vaccine groups for Asian participants, while for Latin American participants, GMTs for HPV types 6 and 16 were similar in both vaccine groups; GMT for HPV11 was lower in the 9vHPV vaccine group than in the qHPV vaccine group and GMT for HPV18 was higher in the 9vHPV vaccine group than in the qHPV vaccine group Month seven GMTs in girls and boys were higher than those in young women for all 9vHPV types for both populations For Latin America participants, >90% of girls/boys remained seropositive at month 36 for all 9vHPV types; no follow-up data for Asian participants Reactogenicity: For both Phase III studies, injection-site AEs were 62.4–89.6% and 77.7–83.1% for 9vHPV and 4vHPV, respectively; most common were injection-site pain, swelling, and erythema, which were mild to moderate in intensity For Asian population, vaccine-related systemic AEs (9vHPV, 4.3–18.8%; 4vHPV, 6.3–13.9%) were lower than in the overall study population (9vHPV, 21.1–29.5%; 4vHPV, 27.3%), except for Thailand (9vHPV, 26.4–42.2%; 4vHPV, 40.3%) which were higher due to higher incidence of pyrexia; no participants experienced vaccine-related serious AEs For Latin American population, vaccine-related systemic AEs were 27.2–37.5% and 29.3% for 9vHPV and 4vHPV, respectively; most were headache and pyrexia; three participants experienced vaccine-related serious AEs, which were found to be due to previous medical history; they fully recovered following treatment |
44, 45 |
| Phase III double-blind-RCT | Immunogenicity in young women aged 16–26 years at month seven |
Immunogenicity: 99.6–100% seroconverted to all 9vHPV types at month seven aNon-inferior antibody responses for HPV6, 11, 16, and 18 when compared with 4vHPV at month seven (GMT ratio 0.8–1.19 depending on types) 77.5–100% remained seropositive at month 60 aNon-inferior antibody responses for HPV6, 11, 16, and 18 persist to 42 months (GMT ratio 0.80–1.26 type dependent) Reactogenicity: AEs related to injection site higher in 9vHPV than 4vHPV group (90.7% and 84.9%, respectively); most were injection-site pain Systemic AEs (headache, pyrexia, nausea, dizziness, and fatigue) are similar in both groups (55.8% and 54.9% in 9vHPV and 4vHPV, respectively). Discontinuation due to serious AE: <0.1% |
42, 43 |
| Phase III, open-label, safety and immunogenicity study | Immunogenicity, safety, and tolerability of 9vHPV to HPV types 6/11/16/18/31/33/45/52/58 in girls aged 9–15 years at month seven |
Immunogenicity: 100% of participants seroconverted to 9vHPV types at month seven, and maintained for two years GMTs in girls aged 9–15 years were comparable to girls of the same age group in the V503-002 study, and at least two-fold higher than those observed in Japanese women aged 6–26 years (from V503-001) Reactogenicity: Most common injection-site AE was pain at the site of injection (93%), followed by swelling (42%) and erythema (33%) Vaccine-related systemic AEs were 14.0%; most common was pyrexia (3%). No serious AEs, deaths, or discontinuation due to an AE |
46 |
| Prospective cohort study | Persistence of HPV antibody response and immune memory at five years post 9vHPV vaccination in girls aged 9–15 years |
Immunogenicity: Seropositivity rates to 9vHPV types at month 60 range from 77.5% to 100% Significant increase in GMT to all 9vHPV types at one week and one month post dose four, which were 1.25–4.10- and 1.65–4.88-fold higher, respectively, than levels observed at month seven Seropositivity rates were >99% and 100% at one week and one month post dose four, respectively Reactogenicity: A higher proportion of participants reported injection-site AEs after dose four (84.0%) than after doses one, two, or three (70.7%, 69.3%, 67.3%, respectively); most common were pain, swelling, erythema; mild to moderate in intensity Most common vaccine-related systemic AE after dose four was headache (6.7%) No participants experienced vaccine-related serious AEs following fourth dose of 9vHPV |
49 |
| Phase III, double-blind RCT | Non-inferior GMTs for anti-4vHPV types in the 9vHPV group compared with the 4vHPV group at month seven in men aged 16–26 years |
bGMTs to HPV 6/11/16/18 elicited by 9vHPV were non-inferior to those elicited by the qHPV vaccine Anti-9vHPV GMTs were higher in the younger age group (16–17 years) than the older age group (18–26 years) Anti-HPV 31/33/45/52/58 GMTs were greater by at least two-fold and up to 15-fold in 9vHPV group compared with 4vHPV group at month 7 100% seroconversion for HPV31, 33, 45, 52, and 58 in 9vHPV group Reactogenicity: One or more vaccine-related AEs and systemic AEs were similar between 9vHPV (81.5%, 23.0%, respectively) and 4vHPV (79.0%, 21.0%, respectively) No participants experienced vaccine-related serious AEs |
47 |
| Phase III double-blind RCT | Non-inferior GMTs for HPV16 and 18 antibody response between 9vHPV and 4vHPV in young girls aged 9–15 years at month seven |
Immunogenicity: aNon-inferior antibody GMTs to HPV 6/11/16/18 induced by 9vHPV when compared with 4vHPV post dose three Robust anti-HPV 31/33/45/52/58 GMTs post dose three were elicited by 9vHPV (one- to two-fold higher than 4vHPV) Higher antibody responses to 9vHPV vaccine types in younger girls (age 9–12 years) compared with older girls (age 13–15 years) Reactogenicity: Injection-site AEs are 93.3% and 90.3% for 9vHPV and 4vHPV, respectively; most were injection-site pain Vaccine-related systemic AEs were 20.7% and 24.3% for 9vHPV and qHPV vaccine, respectively; most common vaccine-related systemic AEs were headache, pyrexia, and nausea No participants experienced vaccine-related serious AEs |
48 |
| Non-inferiority and immunogenicity substudies | (1) Non-inferiority of month seven GMTs in girls and boys versus young women (2) Immunogenicity of the lot consistency for 27 pairs of tests of equivalence for three manufacturing lots to nine HPV types at month seven in girls aged 9-15 years |
Immunogencity: (1) aNon-inferior antibody GMT responses in girls and boys compared with young women; month seven GMTs in girls and boys were higher than those in young women for all 9vHPV types; >90% of girls/boys remained seropositive at month 36 for all 9vHPV types (2) 99.6% of participants seroconverted by month seven to all nineHPV type GMT responses in groups who received the three vaccine lots were equivalent at month seven (lower bound of two-sided 95% CI of GMT ratio for each of three pairs of lots (lot 1 vs lot 2, lot 1 vs lot 3, and lot 2 vs lot 3) for all nine vaccine types was within the interval (0.5, 2.0)) Reactogenicity: Injection-site AEs and vaccine-related systemic AEs were lower among girls (81.9% and 20.9%, respectively) and boys (72.8% and 21.8%, respectively) than among young women (85.4% and 26.0%, respectively) Most AEs were mild to moderate in intensity (ie, pain, swelling, erythema, and pruritus) Discontinuation due to vaccine-related serious AE <0.2% |
49, 50 |
| Phase II studies, immunogenicity and safety of multivalent HPV VLP vaccine dose formulation | Immunogenicity of seven vaccine candidates in comparison to 4vHPV in three Phase II studies in girls aged 16–26 years cStudy 1: 8vHPV vs 4vHPV cStudy 2: 9vHPV vs 4vHPV Study 3: 5vHPV and 4vHPV vs 4vHPV |
Immunogenicity: Study 1: non-inferior antibody response between 8vHPV and 4vHPV, but lower GMTs for HPV types 6/11/16/18 in the 8vHPV recipients compared with the 4vHPV recipients Study 2: mid- and high-dose 9vHPV have similar and non-inferior antibody response for HPV types 6/11/16/18 as 4vHPV Study 3: non-inferior antibody response between 5vHPV and 4vHPV, but lower GMTs for HPV types 6/11/16/18 in the 5vHPV and 4vHPV recipients compared with the 4vHPV recipients Reactogenicity: Study 1: injection-site AEs were more common in girls who received 8vHPV (95.8–98.2%) or 9vHPV (92.4–92.8%) than 4vHPV (90.3–91.7%) Similar vaccine-related systemic AEs between vaccination groups for all three studies (Study 1: 50.6–55.0%; Study 2: 29.2–33.5%; Study 3: 23.8–25.6%) Serious AEs and discontinuations due to AE were rare (0–1.2% and 0–0.6%, respectively) No participants experienced serious vaccine-related AEs |
51 |
| Phase III, open-label, randomized, immunogenicity study | Safety and non-inferior antibody response to the 9vHPV types administered with Menactra and Adacel vaccines in girls and boys aged 11–15 years at month seven |
Immunogenicity: Similar and non-inferior anti-GMT between Groups A and B (fold difference range: 0.97–1.1) Seroconversion rates were 100% for all HPV types in both groups Non-inferiority antibody response for all four Neisseria meningitidis serogroups (Menactra) and diphtheria and tetanus (Adacel) Reactogenicity: Injection-site AEs were 46.7–80.9% and 46.5–80.4% for Group A and B, respectively; most were mild to moderate in intensity Vaccine-related systemic AEs were 16.1–43.1% and 15.0–42.4% for Group A and B, respectively Higher proportion in Group A reported swelling (14.4%) following 9vHPV compared with Group B (9.4%), although not statistically significant No participants experienced vaccine-related serious AEs |
63 |
| Phase III, open-label, randomized, immunogenicity and safety study | Safety and non-inferior antibody response to the 9vHPV types administered concomitantly (Group A) and nonconcomitantly (Group B) with Tdap-IPV 4 weeks postdose in girls and boys aged 11–15 years at month seven |
Immunogenicity: Anti-HPV GMTs against all HPV types were lower in Group A but non-inferior to Group B, with fold differences (ie, Group A/Group B) ranging from 0.89 to 0.97 Seroconversion rates were ≥99.8% for all HPV types in both groups Non-inferiority antibody response for diphtheria, tetanus, and all three poliovirus types Reactogenicity: Injection-site AEs were 60.7–93.9% and 60.2–90.1% for Group A and B, respectively; most were mild to moderate in intensity Vaccine-related systemic AEs were 19.2–48.6% and 18.0–48.6% for Group A and B, respectively Higher proportion of participants in Group A reported erythema (8.2%) and swelling (13.0%) at the 9vHPV injection site compared with Group B (5.7% and 8.2%); as well as for Tdap-IPV vaccination, erythema (26.9%) and swelling (39.4%) in Group A compared with Group B (21.7% and 31.3%, respectively) No participants experienced vaccine-related serious AEs |
62 |
| Phase III, randomized, double-blind, controlled, immunogenicity study | Non-inferior GMTs for anti-9vHPV types between young men and young women aged 16–26 years at month seven |
Immunogenicity: >99% in all three groups seroconverted to all 9vHPV types GMTs to all 9vHPV types were higher in HM than those in women or MSM at month seven aNon-inferior antibody GMTs between HM and young women (range 1.09–1.27) Reactogenicity: Proportion of participants who reported at least one injection-site or systemic AE were lower among young men (67.6% and 16.0% respectively) than among young women (84.1% and 23.4%); most common for injection-site AEs were pain, swelling, erythema, and pruritus; most were mild to moderate in intensity. Most common vaccine-related systemic AEs among young men were headache (7.3%) and pyrexia (2.4%) No participants experienced vaccine-related serious AEs |
53 |
| Double-blind, placebo-controlled, safety and immunogenicity study | Comparison of immunogenicity to HPV types 31/33/45/52/58 in girls and women aged 12–26 years who previously received three doses of 4vHPV following three doses of 9vHPV or placebo | >98% seroconverted for each 9vHPV type in the 9vHPV group Significant increase in GMTs for HPV types 31/33/45/52/58 following one dose, and increased further following doses two and three in the 9vHPV group Compared to HPV vaccination-naïve women (from another study): (1) month seven GMTs for HPV 6/11/16/18 were numerically higher in prior 4vHPV recipients (2) month seven GMTs for HPV 31/33/45/52/58 were generally numerically lower in prior 4vHPV recipients Reactogenicity: Injection-site AEs more common in 9vHPV vaccine group (91.1%) compared with saline placebo group (43.9%); most common were pain, swelling, erythema, pruritus, and hematoma; mild to moderate in intensity Most common vaccine-related systemic AEs in the 9vHPV group (30.6%) were headache (19.6%), pyrexia (5.1%), nausea (3.9%), and dizziness (3%) Discontinuations from study vaccination as a result of an AE were rare (0.5%) Incidence of serious vaccine-related AEs was low (0.5%) |
54 |
Notes: aNon-inferiority criterion: the lower bound of the two-sided 95% CI of the GMT ratio (9vHPV:4vHPV) was >0.67. bNon-inferiority criterion: the lower bound of the two-sided 95% CI for the GMT ratio (9vHPV: 4vHPV) was ⩾0.5. cThree-dose formulation: low, mid, and high.
Abbreviations: 4vHPV, quadrivalent HPV vaccine; 5vHPV, pentavalent HPV vaccine; 8vHPV, octavalent HPV vaccine; 9vHPV, nonavalent HPV vaccine; AE, adverse events; GMT, geometric mean titer; HPV, human papillomavirus; MSM, men who have sex with men; RCT, randomized controlled trial; Tdap-IPV, diphtheria, tetanus, pertussis, and poliomyelitis vaccines.
Around 77.5–100% of individuals who received three doses of 9vHPV remained seropositive to all 9vHPV after five years.43 When a fourth dose of 9vHPV was given to this group of individuals, antibody responses were 1.25–4.10- and 1.65–4.88-fold higher at one week and one month after the fourth dose, respectively, when compared to the levels at one month after the third dose, suggesting the induction of immunological memory to all nine HPV types following the three-dose primary series.52 Higher antibody responses to all 9vHPV types were observed in young adolescents when compared with young adults, consistent with the earlier findings for 4vHPV and 2vHPV.50 Between male and females, the immune responses were non-inferior at one month after the third dose, although the geometric mean titers to all nine HPV types were numerically lower in men who have sex with men and in women than in heterosexual men; geometric mean titers were lower among men who have sex with men compared to women, which may be due to the higher preexposure levels in these groups prior to vaccination.53
9vHPV is also immunogenic to all nine HPV types in young women previously vaccinated with three doses of 4vHPV.54 However, it is interesting to note that women who were naïve to any HPV vaccination and received three doses of 9vHPV had higher antibody responses to HPV31/33/45/52/58 when compared with women who previously received three doses of 4vHPV (who were seronegative to HPV31/33/45/52/58 after 12–36 months) and received three doses of 9vHPV. Several mechanisms have been proposed for this observation, including preferential activation of cross-reactive memory cells compared to naïve cells and/or preferential activation of memory response to HPV 16/18 L1 proteins, resulting in a blunting of primary responses to antigenically related HPV31/33/45/52/58 L1 proteins (the theory of original antigenic sin; the immune system activates the memory responses of the earlier infection/vaccination when the epitope varies slightly, inducing faster and stronger responses, rather than mounting another primary or secondary response to the new epitope).55 The clinical significance of this lower immune response to HPV31/33/45/52/58 following 9vHPV vaccination in women previously vaccinated with 4vHPV is unknown. Nevertheless, the antibody level to these types was still several-fold higher than natural infection and the study demonstrated that it was safe to give 9vHPV to individuals previously vaccinated with 4vHPV or 2vHPV. Therefore, individuals who wish to get additional protection to the five new types may still require the standard two- or three-dose 9vHPV schedule depending on their age and duration between doses.56
Similar to the findings of the 4vHPV and 2vHPV non-inferiority studies, two doses of 9vHPV given six or 12 months apart to adolescent boys and girls (age 9–14 years) generated non-inferior antibody responses (to all nine HPV types) when compared with young women aged 16–26 years who received three doses, one month after the last dose.57 This study was the basis of U.S. FDA approval for a two-dose 9vHPV schedule to girls and boys younger than age 15 years, including recommendations by the USA, European countries, and Australia.58–60
To date, more than 20,000 individuals have received three doses of 9vHPV in Phase II and III clinical trials (reactogenicity data summarized in Table 3). 9vHPV is shown to be safe and generally well tolerated in participants aged 9–26 years, with a similar adverse event profile to that of 4vHPV (which is used in many countries globally as part of national immunization programs).61 The most common adverse event from seven Phase III clinical trials was injection-site pain, swelling, and redness, which was more common for 9vHPV than 4vHPV with increasing number of doses.61 It is important to note that the adjuvant content in 9vHPV is more than double that of 4vHPV (0.5 vs 0.225 mg), and also has a higher VLP antigen content (refer to Table 1). Nevertheless, most adverse events were mild to moderate in intensity.61 Discontinuation due to adverse events and serious vaccine-related adverse events in the clinical trials was rare.61 There were three reports of vaccine-related serious adverse events, although the participants were later found to have preexisting medical conditions when receiving the vaccine. A 10-year-old boy who has a previous history of seasonal allergy and bronchial asthma experienced an asthma exacerbation one day after receiving the first 9vHPV dose.50 The boy was hospitalized, but fully recovered the following day. Another participant (age 21 years) from the same study experienced a severe headache on the day she received the third 9vHPV dose and was hospitalized the next day with neck stiffness, headache, and fever (39.0 °C). She was discharged from the hospital two days after receiving symptomatic treatment. It was noted that she was bitten by a spider five days before the third dose and was still undergoing treatment when she received the vaccine.50 In another study, one participant (age 18 years) was hospitalized for tonsillitis two days after receiving the first dose of 9vHPV vaccine, but fully recovered following incision and drainage of the tonsils as well as antibiotic treatment.54
Coadministration of 9vHPV and other adolescent vaccines (ie, Neisseria meningitidis serotypes A/C/Y/W-135, diphtheria/tetanus/acellular pertussis, or diphtheria/tetanus/acellular pertussis/inactivated poliomyelitis vaccine) to boys and girls aged 9–14 years was also found to be safe and immunogenic when compared with those who received the vaccines nonconcomitantly.62,63 Together, these data support the high safety profile of this vaccine, as well as the feasibility of concomitant administration of 9vHPV with common adolescent vaccines as a public health strategy.
Population effect and future directions
A long-term follow-up study to assess the effectiveness of 9vHPV for at least 14 years in participants in Scandinavian countries (ie, Denmark, Norway, and Sweden) from the Phase III 9vHPV efficacy study (V503-001) is currently ongoing.64 The incidence of cervical precancers and cancers due to the seven oncogenic types in the vaccine (HPV 16/18/31/33/45/52/58) will be compared to the estimated incidence rate in an unvaccinated cohort of similar age and risk level. This information will be critical for the longevity of protection offered by this vaccine.
9vHPV has been licensed in a number of regions including Australia (Therapeutic Goods Association), the USA (FDA), Canada (Health Canada), the European Union (European Medicines Agency), and a number of countries in Asia such as China and Korea. To our knowledge, Australia, Canada, and the USA are using 9vHPV in their national immunization programs, and while both Australia and Canada have school-based programs, HPV vaccination is offered through primary care providers in clinic-based settings in the USA.65–67 Australia recently revised their HPV vaccination schedule in February 2018 from three doses of 4vHPV to two doses of 9vHPV in adolescent boys and girls aged 12–13 years. Australia was one of the first countries to introduce a government-funded school-based HPV vaccine program (4vHPV in 2007) with demonstrated high vaccine coverage (around 80% for three doses) in women aged <18 years and is associated with a significant decrease of 0.38% in high-grade cervical abnormalities within three years of vaccine introduction.68 The prevalence of high-risk vaccine HPV type declined from 22% in the prevaccine era to 1.5% among girls aged 18–24 years, nine years after vaccine introduction.69,70 It was postulated that the replacement of 4vHPV with 9vHPV in Australia can potentially protect against an additional 15% and 11% of cervical cancer and anal cancers, respectively.71 The impact of 9vHPV and the HPV prevalence in Australia can be evaluated by “a built in approach” which utilizes the genotyping from screening using HPV nucleic acid testing, which was recently introduced to replace Pap screening (largely due to the effects of HPV vaccination on Pap abnormalities and the introduction of a more sensitive and objective test).72 With the use of 9vHPV coupled with high vaccine coverage in a gender-neutral vaccination program, and robust HPV screening, Australia is likely to be the first country to eliminate cervical cancer (defined as four new cases per 100,000 women each year) by 2028.73 The incidence of cervical cancer is expected to further decrease to less than one case per 100,000 women by 2066.73 The national HPV vaccine schedule in Quebec, Canada is unique, consisting of one dose of 9vHPV followed by a dose of 2vHPV six months later to nine-year-old girls and boys; mixed HPV vaccine schedule. The potential cost-savings from this mixed schedule based on the lower cost for 2vHPV are approximately $3 million per vaccinated birth cohort compared with a two-dose program with 9vHPV. These savings could be used for catch-up vaccination in older age groups, representing an attractive vaccination strategy, provided it is safe and equivalent protection can be achieved.74 The HPV vaccines are safe, and have similar safety profiles between the mixed vaccine schedule and the standard schedule as demonstrated in several cohort studies.54,75–77 In addition, higher immunogenicity to HPV16 and 18 (possibly due to the unique adjuvant in 2vHPV) and lower immunogenicity to the HPV types that are not in 2vHPV are observed in the mixed schedule when compared with the standard 9vHPV schedule.75 This finding is not unexpected although the clinical significance is unknown. Therefore, long-term follow up including the prevalence of genital warts and other HPV-related diseases as well as circulating HPV types following such schedules are extremely important, and studies are in place to monitor these outcomes.74
Several studies have found the use of 9vHPV to be highly cost effective in high-income countries when the vaccine is given to both boys and girls, despite the higher costs associated with the use of 9vHPV compared with 2vHPV or 4vHPV.78–81 Although these results are largely dependent on the vaccine cost, they were generally robust across varying assumptions based on the burden of disease, vaccine coverage, and duration of protection including cross-protection of 2vHPV or 4vHPV, as well as health care costs within high-income countries. It is important to note that the majority of the HPV-associated cancers, particularly cervical cancer, occurs in LLMICs, and the use of HPV vaccine is inversely proportionate to countries with the highest disease burden; only 1.7% of women in LLMICs are estimated to have received one dose of any HPV vaccine.82 The use of 9vHPV in LLMICs in the foreseeable future is unlikely due to its high cost compared to either 2vHPV or 4vHPV. However, ongoing studies are underway to examine the efficacy and immunogenicity of a single-dose HPV vaccine (9vHPV and 2vHPV) in Costa Rica and Tanzania, respectively,83,84 which may be a “game-changer” for LLMICs. The continued investigation of novel strategies such as single-dose schedules, or mixed HPV vaccine schedules, will be important in the continued use and implementation of HPV vaccine in high burden settings.
Conclusion
9vHPV is safe and offers the potential to prevent 90% of cervical cancers and a significant proportion of HPV-related vulvar, vaginal, and anal cancers. In high-income countries that have high vaccine coverage and a robust HPV screening program, the transition from 2vHPV or 4vHPV to 9vHPV as a national immunization program will likely further decrease the incidence of HPV-associated diseases and the costs associated with cancer treatment due to the additional cancer-causing types in the vaccine. As for LLMICs, any HPV vaccine use in a national program (demonstrated to have the highest coverage) is the most feasible option with maximum impact on cervical cancer morbidity and mortality. Financial and logistical support in these countries are crucial, which will be alleviated with a reduced-dose schedule, particularly a single-dose schedule if proven effective.
Disclosure
FMR and PVL are recipients of National Health and Medical Research Council Fellowships. SMG has received grants through her institution from Merck, GlaxoSmithKline, CSL, and the Commonwealth Department of Health; and has delivered lectures and received speaking fees from MSD for work performed in her personal time. SMG is also a member of Global Advisory Board HPV. The authors report no other conflicts of interest in this work.
References
- 1.Brotherton JML, Tabrizi SN, Phillips S, et al. Looking beyond human papillomavirus (HPV) genotype 16 and 18: defining HPV genotype distribution in cervical cancers in Australia prior to vaccination. Int J Cancer. 2017;141(8):1576–1584. doi: 10.1002/ijc.30871 [DOI] [PubMed] [Google Scholar]
- 2.Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41(11):660–664. doi: 10.1097/OLQ.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789–1799. doi: 10.1086/657321 [DOI] [PubMed] [Google Scholar]
- 4.Molano M, Van den Brule A, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158(5):486–494. doi: 10.1093/aje/kwg171 [DOI] [PubMed] [Google Scholar]
- 5.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–814. doi: 10.1086/597071 [DOI] [PubMed] [Google Scholar]
- 6.Patel H, Wagner M, Singhal P, Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013;13:39. doi: 10.1186/1471-2334-13-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson DA, Derkay CS. Epidemiology of recurrent respiratory papillomatosis. APMIS. 2010;118(6–7):450–454. doi: 10.1111/j.1600-0463.2010.02619.x [DOI] [PubMed] [Google Scholar]
- 8.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi: 10.1002/ijc.30716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056. doi: 10.1016/S1470-2045(10)70230-8 [DOI] [PubMed] [Google Scholar]
- 10.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497 [DOI] [PubMed] [Google Scholar]
- 11.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789. doi: 10.1016/S1470-2045(10)70017-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92(18):1500–1510. doi: 10.1093/jnci/92.18.1500 [DOI] [PubMed] [Google Scholar]
- 13.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 14.Toh ZQ, Licciardi PV, Russell FM, Garland SM, Batmunkh T, Mulholland EK. Cervical cancer prevention through HPV vaccination in low- and middle-income countries in Asia. Asian Pac J Cancer Prev. 2017;18(9):2339–2343. doi: 10.22034/APJCP.2017.18.9.2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard N, Gallagher KE, Mounier-Jack S, et al. What works for human papillomavirus vaccine introduction in low and middle-income countries? Papillomavirus Res. 2017;4:22–25. doi: 10.1016/j.pvr.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruni L, Barrionuevo-Rosas L, Albero GAM, et al. Human papillomavirus and related diseases in Asia. Summary report. ICO Information Centre on HPV and Cancer (HPV Information Centre); 2018. Accessed April17, 2015.
- 17.Garcon N, Wettendorff M, Van Mechelen M. Role of AS04 in human papillomavirus vaccine: mode of action and clinical profile. Expert Opin Biol Ther. 2011;11(5):667–677. doi: 10.1517/14712598.2011.573624 [DOI] [PubMed] [Google Scholar]
- 18.Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5(10):705–719. doi: 10.4161/hv.5.10.9518 [DOI] [PubMed] [Google Scholar]
- 19.Einstein MH, Levin MJ, Chatterjee A, et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: follow-up through Month 48 in a Phase III randomized study. Hum Vaccin Immunother. 2014;10(12):3455–3465. doi: 10.4161/hv.36117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741 [DOI] [PubMed] [Google Scholar]
- 21.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760 [DOI] [PubMed] [Google Scholar]
- 22.Dillner J, Kjaer SK, Future I/II Study Group, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 341;2010:c3493. doi: 10.1136/bmj.c3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):89–99. doi: 10.1016/S1470-2045(11)70286-8 [DOI] [PubMed] [Google Scholar]
- 24.Mensah FA, Mehta MR, Lewis JS Jr., Lockhart AC. The human papillomavirus vaccine: current perspective and future role in prevention and treatment of anal intraepithelial neoplasia and anal cancer. Oncologist. 2016;21(4):453–460. doi: 10.1634/theoncologist.2015-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper DM, DeMars LR. HPV vaccines - A review of the first decade. Gynecol Oncol. 2017;146(1):196–204. doi: 10.1016/j.ygyno.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 26.Schwarz TF, Galaj A, Spaczynski M, et al. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15-55 years of age. Cancer Med. 2017;6(11):2723–2731. doi: 10.1002/cam4.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiller J, Lowy D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 2018;36(32Pt A):4768–4773. doi: 10.1016/j.vaccine.2017.12.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118(5):2135–2145. doi: 10.1542/peds.2006-0461 [DOI] [PubMed] [Google Scholar]
- 29.Pedersen C, Petaja T, Strauss G, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007;40(6):564–571. doi: 10.1016/j.jadohealth.2007.02.015 [DOI] [PubMed] [Google Scholar]
- 30.Petaja T, Keranen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10-18 years. J Adolesc Health. 2009;44(1):33–40. doi: 10.1016/j.jadohealth.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 31.Toh ZQ, Licciardi PV, Fong J, et al. Reduced dose human papillomavirus vaccination: an update of the current state-of-the-art. Vaccine. 2015;33(39):5042–5050. doi: 10.1016/j.vaccine.2015.07.102 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Meeting of the Strategic Advisory Group Of Experts on immunization, April 2014 – conclusions and recommendations. Relevé Épidémiologique Hebdomadaire. 2014;89(21):221–236. [PubMed] [Google Scholar]
- 33.Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199(7):926–935. doi: 10.1086/597307 [DOI] [PubMed] [Google Scholar]
- 34.Wheeler CM, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J Infect Dis. 2009;199(7):936–944. doi: 10.1086/597309 [DOI] [PubMed] [Google Scholar]
- 35.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4 [DOI] [PubMed] [Google Scholar]
- 36.Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis. 2017;17(12):1293–1302. doi: 10.1016/S1473-3099(17)30468-1 [DOI] [PubMed] [Google Scholar]
- 37.Barzon L, Squarzon L, Masiero S, et al. Neutralizing and cross-neutralizing antibody titres induced by bivalent and quadrivalent human papillomavirus vaccines in the target population of organized vaccination programmes. Vaccine. 2014;32(41):5357–5362. doi: 10.1016/j.vaccine.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 38.Garland SM, Cornall AM, Brotherton JML, et al. Final analysis of a study assessing genital human papillomavirus genoprevalence in young Australian women, following eight years of a national vaccination program. Vaccine. 2018;36(23):3221–3230. doi: 10.1016/j.vaccine.2018.04.080 [DOI] [PubMed] [Google Scholar]
- 39.Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum Vaccin. 2011;7(12):1359–1373. doi: 10.4161/hv.7.12.18282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godi A, Bissett SL, Miller E, Beddows S. Relationship between humoral immune responses against HPV16, HPV18, HPV31 and HPV45 in 12-15 year old girls receiving Cervarix(R) or Gardasil(R) vaccine. PLoS One. 2015;10(10):e0140926. doi: 10.1371/journal.pone.0140926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kemp TJ, Hildesheim A, Safaeian M, et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011;29(11):2011–2014. doi: 10.1016/j.vaccine.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044 [DOI] [PubMed] [Google Scholar]
- 43.Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143–2159. doi: 10.1016/S0140-6736(17)31821-4 [DOI] [PubMed] [Google Scholar]
- 44.Garland SM, Pitisuttithum P, Ngan HYS, et al. Efficacy, immunogenicity, and safety of a 9-valent human papillomavirus vaccine: subgroup analysis of participants from Asian countries. J Infect Dis. 2018;218(1):95–108. doi: 10.1093/infdis/jiy133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz-Sternberg AM, Moreira ED Jr., Restrepo JA, et al. Efficacy, immunogenicity, and safety of a 9-valent human papillomavirus vaccine in Latin American girls, boys, and young women. Papillomavirus Res. 2018;5:63–74. doi: 10.1016/j.pvr.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwata S, Murata S, Rong Han S, Wakana A, Sawata M, Tanaka Y. Safety and Immunogenicity of a 9-valent human papillomavirus vaccine administered to 9- to 15-year-old Japanese girls. Jpn J Infect Dis. 2017;70(4):368–373. doi: 10.7883/yoken.JJID.2016.299 [DOI] [PubMed] [Google Scholar]
- 47.Van Damme P, Meijer C, Kieninger D, et al. A phase III clinical study to compare the immunogenicity and safety of the 9-valent and quadrivalent HPV vaccines in men. Vaccine. 2016;34(35):4205–4212. doi: 10.1016/j.vaccine.2016.06.056 [DOI] [PubMed] [Google Scholar]
- 48.Vesikari T, Brodszki N, van Damme P, et al. A randomized, double-blind, phase III study of the immunogenicity and safety of a 9-valent human papillomavirus L1 virus-like particle vaccine (V503) versus gardasil(R) in 9-15-year-old girls. Pediatr Infect Dis J. 2015;34(9):992–998. doi: 10.1097/INF.0000000000000773 [DOI] [PubMed] [Google Scholar]
- 49.Luxembourg A, Ed M Jr., Samakoses R, et al. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 2015;11(6):1306–1312. doi: 10.1080/21645515.2015.1009819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Damme P, Olsson SE, Block S, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136(1):e28–39. doi: 10.1542/peds.2014-3745 [DOI] [PubMed] [Google Scholar]
- 51.Luxembourg A, Brown D, Bouchard C, et al. Phase II studies to select the formulation of a multivalent HPV L1 virus-like particle (VLP) vaccine. Hum Vaccin Immunother. 2015;11(6):1313–1322. doi: 10.1080/21645515.2015.1012010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guevara A, Cabello R, Woelber L, et al. Antibody persistence and evidence of immune memory at 5years following administration of the 9-valent HPV vaccine. Vaccine. 2017;35(37):5050–5057. doi: 10.1016/j.vaccine.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 53.Castellsague X, Giuliano AR, Goldstone S, et al. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine. 2015;33(48):6892–6901. doi: 10.1016/j.vaccine.2015.06.088 [DOI] [PubMed] [Google Scholar]
- 54.Garland SM, Cheung TH, McNeill S, et al. Safety and immunogenicity of a 9-valent HPV vaccine in females 12-26 years of age who previously received the quadrivalent HPV vaccine. Vaccine. 2015;33(48):6855–6864. doi: 10.1016/j.vaccine.2015.08.059 [DOI] [PubMed] [Google Scholar]
- 55.Vatti A, Monsalve DM, Pacheco Y, Chang C, Anaya JM, Gershwin ME. Original antigenic sin: a comprehensive review. J Autoimmun. 2017;83:12–21. doi: 10.1016/j.jaut.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 56.Van Damme P, Bonanni P, Bosch FX, et al. Use of the nonavalent HPV vaccine in individuals previously fully or partially vaccinated with bivalent or quadrivalent HPV vaccines. Vaccine. 2016;34(6):757–761. doi: 10.1016/j.vaccine.2015.12.063 [DOI] [PubMed] [Google Scholar]
- 57.Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411–2421. doi: 10.1001/jama.2016.17615 [DOI] [PubMed] [Google Scholar]
- 58.Meites E, Kempe A, Markowitz L. se of a 2-dose schedule for Human Papillomavirus Vaccination — updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–1408. doi: 10.15585/mmwr.mm6549a5 [DOI] [PubMed] [Google Scholar]
- 59.European Medicines Agency Gardasil 9. Summary of product characteristics; 2018. Accessed October29, 2018.
- 60.National Centre For Immunisation Research and Surveillance. Human papillomavirus (HPV) vaccines for Australians: information for immunisation providers; 2018. Accessed October29, 2018.
- 61.Moreira ED Jr., Block SL, Ferris D, et al. Safety profile of the 9-Valent HPV vaccine: a combined analysis of 7 phase iii clinical trials. Pediatrics. 2016;138(2):e20154387. doi: 10.1542/peds.2015-4387 [DOI] [PubMed] [Google Scholar]
- 62.Kosalaraksa P, Mehlsen J, Vesikari T, et al. An open-label, randomized study of a 9-valent human papillomavirus vaccine given concomitantly with diphtheria, tetanus, pertussis and poliomyelitis vaccines to healthy adolescents 11-15 years of age. Pediatr Infect Dis J. 2015;34(6):627–634. doi: 10.1097/INF.0000000000000694 [DOI] [PubMed] [Google Scholar]
- 63.Schilling A, Parra MM, Gutierrez M, et al. Coadministration of a 9-valent human papillomavirus vaccine with meningococcal and Tdap vaccines. Pediatrics. 2015;136(3):E563-E572. doi: 10.1542/peds.2015-0573 [DOI] [PubMed] [Google Scholar]
- 64.Luxembourg A, Kjaer SK, Nygard M, et al. Design of a long-term follow-up effectiveness, immunogenicity and safety study of women who received the 9-valent human papillomavirus vaccine. Contemp Clin Trials. 2017;52:54–61. doi: 10.1016/j.cct.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 65.Markowitz LE, Gee J, Chesson H, Stokley S. Ten years of human papillomavirus vaccination in the United States. Acad Pediatr. 2018;18(2S):S3–S10. doi: 10.1016/j.acap.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ward K, Quinn H, Menzies R, McIntyre P. A history of adolescent school based vaccination in Australia. Commun Dis Intell Q Rep. 2013;37(2):E168–E174. [DOI] [PubMed] [Google Scholar]
- 67.McClure CA, MacSwain MA, Morrison H, Sanford CJ. Human papillomavirus vaccine uptake in boys and girls in a school-based vaccine delivery program in Prince Edward Island, Canada. Vaccine. 2015;33(15):1786–1790. doi: 10.1016/j.vaccine.2015.02.047 [DOI] [PubMed] [Google Scholar]
- 68.Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377(9783):2085–2092. doi: 10.1016/S0140-6736(11)60551-5 [DOI] [PubMed] [Google Scholar]
- 69.Machalek DA, Garland SM, Brotherton JML, et al. Very low prevalence of vaccine human papillomavirus (HPV) types among 18 to 35 year old Australian women, nine years following implementation of vaccination. J Infect Dis. 2018. doi: 10.1093/infdis/jiy075 [DOI] [PubMed] [Google Scholar]
- 70.Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206(11):1645–1651. doi: 10.1093/infdis/jis590 [DOI] [PubMed] [Google Scholar]
- 71.Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Euro Surveill. 2018;23:41. doi: 10.2807/1560-7917.ES.2018.23.41.1700737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brotherton JM, Hawkes D, Sultana F, et al. Age-specific HPV prevalence among 116,052 women in Australia’s renewed cervical screening program: a new tool for monitoring vaccine impact. Vaccine. 2019;37(3):412–416. doi: 10.1016/j.vaccine.2018.11.075 [DOI] [PubMed] [Google Scholar]
- 73.Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2018;4(1):E19–E27. doi: 10.1016/S2468-2667(18)30183-X [DOI] [PubMed] [Google Scholar]
- 74.Gilca V, Sauvageau C, Trudeau G. Advisory report on the Human Papillomavirus (HPV) Vaccination Schedule. National Institute of Public Health of Quebec; 2018. Available from: https://www.inspq.qc.ca/sites/default/files/publications/2458_papillomavirus_vaccination_schedule.pdf [Google Scholar]
- 75.Gilca V, Sauvageau C, Panicker G, De Serres G, Ouakki M, Unger ER. Immunogenicity and safety of a mixed vaccination schedule with one dose of nonavalent and one dose of bivalent HPV vaccine versus two doses of nonavalent vaccine - A randomized clinical trial. Vaccine. 2018;36(46):7017–7024. doi: 10.1016/j.vaccine.2018.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toh ZQ, Russell FM, Reyburn R, et al. Sustained antibody responses 6 years following 1, 2, or 3 doses of quadrivalent human papillomavirus (HPV) vaccine in adolescent Fijian girls, and subsequent responses to a single dose of bivalent HPV Vaccine: a prospective cohort study. Clin Infect Dis. 2017;64(7):852–859. doi: 10.1093/cid/ciw865 [DOI] [PubMed] [Google Scholar]
- 77.Gilca V, Sauvageau C, Boulianne N, et al. The effect of a booster dose of quadrivalent or bivalent HPV vaccine when administered to girls previously vaccinated with two doses of quadrivalent HPV vaccine. Hum Vaccin Immunother. 2015;11(3):732–738. doi: 10.1080/21645515.2015.1011570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mennini FS, Bonanni P, Bianic F, et al. Cost-effectiveness analysis of the nine-valent HPV vaccine in Italy. Cost Eff Resour Alloc. 2017;15:11. doi: 10.1186/s12962-017-0073-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Largeron N, Petry KU, Jacob J, Bianic F, Anger D, Uhart M. An estimate of the public health impact and cost-effectiveness of universal vaccination with a 9-valent HPV vaccine in Germany. Expert Rev Pharmacoecon Outcomes Res. 2017;17(1):85–98. doi: 10.1080/14737167.2016.1208087 [DOI] [PubMed] [Google Scholar]
- 80.Durham DP, Ndeffo-Mbah ML, Skrip LA, Jones FK, Bauch CT, Galvani AP. National- and state-level impact and cost-effectiveness of nonavalent HPV vaccination in the United States. Proc Natl Acad Sci U S A. 2016;113(18):5107–5112. doi: 10.1073/pnas.1515528113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petrosky E, Bocchini JA Jr., Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 82.Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–e463. doi: 10.1016/S2214-109X(16)30099-7 [DOI] [PubMed] [Google Scholar]
- 83.ClinicalTrials.gov. Scientific evaluation of one or two doses of the bivalent or nonavalent prophylactic HPV vaccines; 2018. Accessed October7th, 2018.
- 84.ClinicalTrials.gov. A dose reduction immunobridging and safety study of two HPV vaccines in tanzanian girls (DoRIS); 2018. Accessed November7th, 2018.
- 85.The World Health Organization. Vaccine in national immunization programme update; 2018. [Updated October 2018]. Accessed December27th, 2018.