Abstract
Background
Intrahepatic cholestasis of pregnancy is a pregnancy‐specific liver disorder, possibly associated with an increased risk of severe fetal adverse events. Total serum bile acids (TSBA) concentration, alone or in combination with serum aminotransferases, have been the most often used biomarkers for the diagnosis of intrahepatic cholestasis of pregnancy in clinical practice. Serum bile acid profile, composed of primary or secondary, conjugated or non‐conjugated bile acids, may provide more specific disease information.
Objectives
To assess and compare, independently or in combination, the diagnostic accuracy of total serum bile acids or serum bile acids profile, or both, for the diagnosis of intrahepatic cholestasis of pregnancy in pregnant women, presenting with pruritus. To define the optimal cut‐off values for components of serum bile acid profile; to investigate possible sources of heterogeneity.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Hepato‐Biliary Group Diagnostic Test Accuracy Studies Register, the Cochrane Library, MEDLINE Ovid, Embase Ovid, Science Citation Index Expanded, Conference Proceedings Citation Index – Science, BIOSIS, CINAHL, two Chinese databases (CKNI, VIP), Latin American and Caribbean Health Sciences Literature (LILACS), Scientific Electronic Library Online (SciELO), Evidence Search: Health and Social Care by the National Institute for Health and Care Excellence (NICE), the World Health Organization (WHO) Reproductive Health Library (RHL), and the Turning Research into Practice database (TRIP). The most recent date of search was 6 May 2019. We identified additional references by handsearching the references of articles, meta‐analyses, and evidence‐based guidelines retrieved from the computerised databases, on‐line trial registries, and grey literature through OpenSIGLE, National Technical Information Service (NTIS), ProQuest Dissertations & Thesis Database, and Index to Theses in Great Britain and Ireland.
Selection criteria
Prospective or retrospective diagnostic case‐control or cross‐sectional studies, irrespective of publication date, format, and language, which evaluated the diagnostic accuracy of total serum bile acids (TSBA) or components of serum bile acid profile for the diagnosis of intrahepatic cholestasis of pregnancy in pregnant women of any age or ethnicity, in any clinical setting, symptomatic for pruritus.
Data collection and analysis
We selected studies by reading titles, abstracts, or full texts, and assessing their fulfilment of our inclusion criteria. We emailed primary authors to request missing data or individual participant data. Having extracted data from each included study, we built the two‐by‐two tables for each primary study and for all the index tests considered. We estimated sensitivity and specificity with their 95% confidence intervals (CI). We presented data in coupled forest plots, showing sensitivities and specificities of each study, and we plotted the studies in the Receiver Operating Characteristic (ROC) space. We performed meta‐analyses adopting the hierarchical summary ROC model (HSROC) or the bivariate model to meta‐analyse the data. We made indirect comparisons of the considered index tests by adding the index tests as covariates to the bivariate or HSROC models. We performed heterogeneity analysis and sensitivity analysis on studies assessing TSBA accuracy. We used Review Manager 5 (RevMan 5) and SAS statistical software, release 9.4 (SAS Institute Inc., Cary, NC, USA), to perform all statistical analyses. We used QUADAS‐2 domains to assess the risk of bias of the included studies.
Main results
Our search yielded 5073 references, but at the end of our selection process, only 16 studies fulfilled the review inclusion criteria. Nine of these provided individual participant data. We analysed only data concerning TSBA, cholic acid (CA), glycocholic acid (GCA), chenodeoxycholic acid (CDCA), and CA/CDCA because the remaining planned index tests were assessed in few studies. Only one study had low risk of bias in all four QUADAS‐2 domains. The most biased domains were the patient sampling and the reference standard domains. When considering all studies with a cut‐off of 10 μmol/L, TSBA overall sensitivity ranged from 0.72 to 0.98 and specificity ranged from 0.81 to 0.97. After a sensitivity analysis excluding case‐control studies, TSBA sensitivity ranged from 0.48 to 0.66 and specificity from 0.52 to 0.99. After a sensitivity analysis excluding studies in which TSBA was part of the reference standard, TSBA sensitivity ranged from 0.49 to 0.65 and specificity from 0.53 to 0.99. We found the estimates of the overall accuracy for some serum bile acid components (CA, GCA, CDCA, and CA/CDCA) to be imprecise, with the CI for sensitivity and specificity very wide or impossible to calculate. Indirect comparisons between serum bile acid profile components and TSBA were not statistically significant. None of the heterogeneity analysis performed was statistically significant, except for the timing of assessment of TSBA (onset of symptoms, peak value among multiple assessments, delivery) but without clinically relevant results. We could not analyse the diagnostic accuracy of combinations of index tests because none of the included studies carried them out, and because of the small number of included studies.
Authors' conclusions
The overall high risk of bias, the existing concern regarding applicability of the results in clinical practice, and the great heterogeneity of the results in the included studies prevents us from making recommendations and reaching definitive conclusions at the present time. Thus, we do not find any compelling evidence to recommend or refute the routine use of any of these tests in clinical practice. So far, the diagnostic accuracy of TSBA for intrahepatic cholestasis of pregnancy might have been overestimated. There were too few studies to permit a precise estimate of the accuracy of serum bile acid profile components. Further primary clinical research is mandatory. We need both further phase II and phase III diagnostic studies.
Plain language summary
Diagnostic accuracy of total serum bile acids or individual bile acids for intrahepatic cholestasis of pregnancy in woman claiming pruritus
Review question To assess and compare the diagnostic accuracy of total serum bile acids (TSBA) and some components of serum bile acid profile for the diagnosis of intrahepatic cholestasis of pregnancy in woman with onset of pruritus during pregnancy.
Background 'Diagnostic accuracy' means how well a test correctly identifies or rules out disease and informs subsequent decisions about treatment. Intrahepatic cholestasis of pregnancy is a pregnancy‐specific liver disorder, in which bile (a digestive fluid) builds up in the liver, impairing the liver (intrahepatic) function. Intrahepatic cholestasis of pregnancy is possibly associated with an increased risk of premature delivery and fetal death, which seems to occur most often during the last weeks of pregnancy. This is why most clinicians choose to induce early delivery of the baby.
In clinical practice, presence of severe pruritus (itchiness) during late pregnancy and 'otherwise unexplained' abnormalities in serum liver tests, seems enough to support the diagnosis of intrahepatic cholestasis of pregnancy. However, excluding all other possible underlying diseases is not always easy; hence confirmation of the intrahepatic cholestasis of pregnancy diagnosis may be possible only after delivery, when spontaneous disappearance of pruritus and improvement of liver tests on blood exams usually occur.
Total serum bile acids (TSBA) are the most used biomarkers for intrahepatic cholestasis of pregnancy in clinical practice. Some components of the serum bile acid profile might provide more specific information than total serum bile acids when diagnosing the disease, defining its severity and monitoring its response to treatment.
Study characteristics This review considered all evidence provided by studies that assess the diagnostic accuracy of total serum bile acids (TSBA) and any component of serum bile acid profile for intrahepatic cholestasis of pregnancy in woman claiming onset of pruritus during pregnancy.
We assessed all available reports from a wide, systematic search of databases of medical literature, irrespective of design, publication status, language, and study design. We finally included 16 studies, most of them assessing the accuracy (sensitivity and specificity) of TSBA with a cut‐off of 10 μmol/L. Most studies had a case‐control design, and these studies could have overestimated the diagnostic accuracy.
Key results When considering the studies with a cut‐off of 10 μmol/L for TSBA serum concentration, TSBA overall sensitivity (the ability to correctly identify women with the disease) ranged from 72% to 98% and specificity (the ability to correctly identify women without the disease) ranged from 81% to 97%. However, after performing two different analyses excluding studies with probably less reliable results, the diagnostic accuracy seemed lower. We calculated the overall accuracy also of some components of serum bile acid profile, but the small number of studies and the high variability of the results led to very imprecise data.
Quality of the evidence Only one of the 16 included studies was performed and reported well (low risk of bias). The remaining 15 studies had problems with study design or reporting (high risk of bias). Only five studies seemed to show low concern regarding applicability of the results in clinical practice.
Conclusions The overall high risk of bias, the existing concern regarding applicability of the results in clinical practice, and the poor uniformity of our results in the included studies prevents us from making recommendations and reaching definitive conclusions at present. Thus, we do not find any compelling evidence to recommend or refute the routine use of any of these tests in clinical practice. So far, the diagnostic accuracy of TSBA for intrahepatic cholestasis of pregnancy might have been overestimated. There were too few studies to permit a precise estimate of the accuracy of serum bile acid profile components. Further primary clinical research is mandatory. We need both further phase II and phase III diagnostic studies.
Summary of findings
Summary of findings'. 'Summary of findings table.
| What is the diagnostic accuracy of total serum bile acids (TSBA), cholic acid (CA), glycocholic acid (GCA), chenodeoxycholic acid (CDCA), or CA/CDCA for intrahepatic cholestasis of pregnancy (ICP), at different cut‐off values? | |||||
| Patients/population | Pregnant women with onset of pruritus from the second trimester or later | ||||
| Prior testing | History, serum tests, liver ultrasound | ||||
| Settings | Obstetrics and Gynaecology departments | ||||
| Index test | TSBA, CA, GCA, CDCA, CA/CDCA | ||||
| Importance | Early diagnosis, treatment and follow‐up to reduce fetal adverse events | ||||
| Reference standard | Clinical evaluation comprising common liver function tests, with exclusion of other possible underlying liver or dermatological diseases, and follow‐up after delivery assessing spontaneous normalization of signs and symptoms. | ||||
| Studies | Cross‐sectional and case‐control studies. Each study can be present in more than one subgroup and for more than one index test | ||||
| Test/Subgroup | Summary accuracy (95% CI) | N° part. (studies) | Median prevalence of ICP in pregnant women with pruritus | Implications for an hypothetical population of 100 pregnant women with pruritus | Quality and Comments |
| TSBA, any cut‐off | Sensitivity 0.88 (0.73 to 0.95) Specificity 0.90 (0.84 to 0.95) | 1645 (13) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 36 (15 to 81) women with ICP would be missed, and 70 (35 to 112) without ICP would be falsely diagnosed. | The overall accuracy found may be not applicable to a real clinical context, as most studies were at high risk of bias for patient selection and reference standard. |
| TSBA cut‐off = 10 μmol/L | Sensitivity 0.91 (0.72 to 0.98) Specificity 0.93 (0.81 to 0.97) | 839 (11) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 27 (6 to 84) women with ICP would be missed, and 49 (21 to 133) without ICP would be falsely diagnosed. | The overall accuracy found may be not applicable to a real clinical context, as most studies were at high risk of bias for patient selection and reference standard. |
| CA cut‐off = 2 μmol/L | Sensitivity 0.99 (0.33 to 1.00) Specificity 0.61 (0.23to 0.89) | 312 (4) | ‐‐‐ | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, both for sensitivity and specificity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. |
| CA cut‐off =3 μmol/L | Sensitivity 0.94 (0.66 to 0.99) Specificity 0.82 (0.68 to 0.91) | 312 (4) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 18 (3 to 102) women with ICP would be missed, and 126 (63 to 224) without ICP would be falsely diagnosed. | The overall accuracy found may be not applicable to a real clinical context, as most studies were at high risk of bias for patient selection and reference standard. |
| GCA, all cut‐offs | Sensitivity 0.92 (0.65 to 0.99) Specificity 0.99 (0.06 to 1.00) | 630 (6) | ‐‐‐ | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, especially for specificity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. |
| GCA cut‐off = 0.7 μmol/L | Sensitivity 0.97 (0.38 to 1.00) Specificity 0.86 (0.02 to 1.00) | 333 (5) | ‐‐‐ | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, both for sensitivity and specificity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. |
| GCA cut‐off =1.5 μmol/L | Sensitivity 0.99 (0.08 to 1.00) Specificity 0.90 (0.75 to 0.97) | 417 (4) | ‐‐‐ | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, especially for sensitivity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. |
| GCA cut‐off = 2 μmol/L | Sensitivity 0.99 (0.07 to 1.00) Specificity 0.97 (0.82 to 1.00) | 125 (3) | ‐‐‐ | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, especially for sensitivity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. |
| CDCA cut‐off = 2 | Sensitivity 0.98 (0.62 to 1.00) Specificity 0.66 (0.19 to 0.94) | 312 (4) | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, especially for specificity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. | |
| CDCA cut‐off = 3 | Sensitivity 0.75 (CI not calc) Specificity 0.94 (0.88 to 0.97) | 312 (4) | ‐‐‐ | ‐‐‐ | The CI of sensitivity was not calculable and the CI of specificity was too wide. Hence, we cannot know the precision of the estimates obtained and their applicability in a real clinical scenario. |
| CA/CDCA cut‐off = 1.8 | Sensitivity 0.89 (0.54 to 0.98) Specificity 0.92 (0.85 to 0.96) | 312 (4) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 33 (6 to 138) women with ICP would be missed, and 56 (28 to 105) without ICP would be falsely diagnosed | The cut‐off used has been chosen among the best ones, comparing Youden indexes at multiple cut‐offs applied to all studies. This may have led to biased results. Moreover, sensitivity estimate has a wide CI. This makes hard to judge the applicability the index test in a real clinical setting. |
| Subgroup analysis for TSBA cut‐off = 10 μmol/L: timing (P = 0.027) | |||||
| Onset of symptoms | Sensitivity 0.87 (0.68 to 0.96) Specificity 0.87 (0.76 to 0.94) | 839 (11) | 30% (30 out of 100 pregnant women with pruritus having ICP) | 39 (12 to 96) women with ICP would be missed, and 91 (42 to 168) without ICP would be falsely diagnosed | Sensitivity and specificity seem to be quite good if TSBA are tested when symptoms of ICP arise. However, the overall accuracy found may be not applicable to a real clinical context, as most studies were at high risk of bias for patient selection and reference standard. |
| Peak value among multiple assessments | Sensitivity 0.7 (0.24 to 0.94) Specificity 1.00 (CI not calc) | 839 (11) | ‐‐‐ | ‐‐‐ | The CI of sensitivity was too wide and the CI of specificity was not calculable. Hence, we cannot know the precision of the estimates obtained and their applicability in a real clinical scenario. |
| Delivery | Sensitivity 1.00 (1.00 to 1.00) Specificity 0.87 (0.68 to 0.95) | 839 (11) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 0 women with ICP would be missed, and 91 (35 to 224) without ICP would be falsely diagnosed | Sensitivity seems to be higher when TSBA are tested at the time of delivery, while specificity seems to be the same as when symptoms of ICP arise. However, clinicians need to diagnose ICP as soon as possible during pregnancy to monitor and strictly follow up diseased woman, in order to find possible signs of fetal distress and plan the timing of delivery. Delivery time is too late to make a diagnosis. |
| Sensitivity analysis for TSBA cut‐off=10 μmol/L: exclusion of studies with TSBA as part of reference standard | |||||
| Sensitivity 0.57 (0.49 to 0.65) Specificity 0.98 (0.53 to 1.00) | 497 (5) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 129 (105 to 153) women with ICP would be missed, and 14 (0 to 329) without ICP would be falsely diagnosed | The overall accuracy of TSBA, especially sensitivity, seems to be lower when considering only studies without TSBA inclusion in the reference standard. The accuracy of the index test in a real clinical context may be similar to this. However CIs are wide, and estimates too imprecise to judge with certainty their applicability in a real clinical setting. | |
| Sensitivity analysis for TSBA cut‐off=10 μmol/L: exclusion of case‐control studies | |||||
| Sensitivity 0.57 (0.48 to 0.66) Specificity 0.92 (0.52 to 0.99) | 436 (3) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 129 (102 to 156) women with ICP would be missed, and 56 (7 to 336) without ICP would be falsely diagnosed | The overall accuracy of TSBA, especially sensitivity, seems to be lower when excluding case‐control studies. The accuracy of the index test in a real clinical context may be similar to this. However CIs are wide, and estimates too imprecise to judge with certainty their applicability in a real clinical setting. | |
CAUTION: The results on this table should not be interpreted in isolation from the results of the individual included studies contributing to each summary test accuracy measure. These are reported in the main body of the text of the review.
Background
Intrahepatic cholestasis of pregnancy, also known as obstetric cholestasis, is a pregnancy‐specific liver disorder, known to be possibly associated with an increased risk of severe fetal adverse events. Intrahepatic cholestasis of pregnancy was described as early as 1883 (Ahlfeld 1883), and since then many other publications have followed. However, our knowledge of the disease is still incomplete (Reyes 1997; Sinakos 2010).
The prevalence of intrahepatic cholestasis of pregnancy in pregnant women varies according to geographical location and population, as genetic and environmental factors play a role in its manifestation (Geenes 2009). Following studies from past decades, in countries of North America, Southern Europe, Asia, and Australia, the range of intrahepatic cholestasis of pregnancy was calculated to be between 0.01% and 0.1% (Reyes 1997), in some countries of South America between 1.5% and 4.0% (Reyes 1997), and in Scandinavian countries, the reported prevalence was 1.5% (Glantz 2004). Chile, Bolivia, Finland, Sweden, and Portugal are among the most affected countries in the world (Geenes 2009).
Most often the disease seems to affect women with a history of intrahepatic cholestasis during previous pregnancies (Reyes 1997) or a history of cholestasis associated with the use of oral contraceptives (Pathak 2010), with a family or personal history of biliary disease (Diken 2014), with hepatitis C viral infection (Paternoster 2002), twin pregnancies (Gonzalez 1989), or women with in vitro fertilisation pregnancies (Koivurova 2002). It is also suggested that the risk of acquiring intrahepatic cholestasis of pregnancy is higher in women over the age of 35 years (Heinonen 1999).
There are multiple factors involved in the aetiopathogenesis of intrahepatic cholestasis of pregnancy. Among the genetic factors suspected of playing a part in causing the disease are mutations in genes which encode biliary transport proteins (Dixon 2014); or mutations in bile acid receptors (such as farnesoid X receptor) (Jacquemin 1999). Likewise, among factors suspected of having a role in causing the disease are seasonal variations (with higher prevalence reported in winter) (Brites 1998b), low selenium intake, erucic acid, increased gut absorption of bacterial endotoxins, pollutants (pesticides), infections, or drugs (Geenes 2009; Diken 2014;Ozkan 2015). Hormonal factors such as oestrogens, progesterone, or their metabolites can also play a role in its development (Reyes 2008; Abu‐Hayyeh 2013). Seasonal variations and an increase in dietary selenium intake may have played a role in the decrease of the prevalence of the disease observed in Chile and in Scandinavian countries during the last few decades (Kauppila 1987; Reyes 2000a). Probably owing to these variations, the prevalence of intrahepatic cholestasis of pregnancy in Chile decreased from a range of 11.8% to 27.7% during the 1970s (the higher value observed for Araucanian ethnicity) (Reyes 1978) to the most recently reported range of 1.5% to 4.0% during the 1990s (Reyes 1997).
Some studies showed an association between intrahepatic cholestasis of pregnancy and metabolic abnormalities in affected pregnant women, such as glucose‐impaired tolerance, hyperinsulinaemia, or dyslipidaemia (Martineau 2015), with consequent increased fetal growth and possible sex‐specific increased susceptibility to an obese, diabetic phenotype of the offspring (Desai 2013; Papacleovoulou 2013).
In clinical practice, presence of pruritus during the last third of pregnancy and the 'otherwise unexplained' abnormalities in the most common liver tests, seems enough to support the suspected diagnosis of intrahepatic cholestasis of pregnancy (Green‐top Guideline No.43). However, owing to the nonspecific features of the disease, the mandatory exclusion of all other possible underlying diseases is not always easy and to ascertain the right diagnosis may not be possible until a certain time point after the delivery, when the spontaneous relief of pruritus and normalisation of liver test values occur (Beuers 2006).
The pathophysiology of intrahepatic cholestasis of pregnancy is still poorly understood. An increase in bile acid serum concentration is thought to play a primary role in the onset of the typical cholestatic pruritus (Pathak 2010); however, a correlation between the bile acid serum concentration and severity of pruritus has never been shown. Moreover, the increased passage of bile acids through the placental barrier appears to be toxic for the foetus during intrahepatic cholestasis of pregnancy (Perez 2005; Sheik Abdul Kadir 2010), and this is why obstetricians are concerned about possible fetal adverse events. In an attempt to reduce the feared risk of stillbirth, which seems to occur most often during the last weeks of pregnancy, most clinicians choose an early delivery of the baby because of the medical condition of the mother, usually at week 36 (Puljic 2015, Lo 2015). Whether the increased preterm birth rate associated with intrahepatic cholestasis of pregnancy is due to the disease itself or to its active management is still uncertain (Henderson 2014).
Therapies for intrahepatic cholestasis of pregnancy so far have been empiric, and all aimed at reducing maternal symptoms, improving results of liver tests, and reducing total bile acid concentration. Ursodeoxycholic acid (UDCA), S‐adenosylmethionine (SAMe), dexamethasone, or cholestyramine as well as vitamin K (aiming at preventing possible postpartum bleeding) are the most used interventions (Ozkan 2015).
A Cochrane Review on interventions for treating cholestasis in pregnancy concluded that there was no evidence to recommend early‐term delivery and that there was insufficient evidence to support the use of SAMe, guar gum, activated charcoal, dexamethasone, cholestyramine, Yinchenghao decoction, Danxiaoling Pill, and Yiganling, either alone, or in combination (Gurung 2013). However, the review found that UDCA seemed to improve the maternal symptom of pruritus (Gurung 2013), which is in agreement with the result of the meta‐analysis by Bacq 2012. In addition, Bacq 2012 strongly suggested that UDCA was also beneficial for the fetal outcome; however, the Cochrane Review could not reach this conclusion as the evidence was insufficient (Gurung 2013).
Total serum bile acids (TSBA), alone or in combination with serum aminotransferases, are the most often used biomarker for intrahepatic cholestasis of pregnancy in current clinical practice. They are believed to be 'the best biomarker' for the disease, both diagnostic and prognostic for possible fetal adverse events, and they have been considered appropriately used in clinical practice and 'well known' by scientific literature for years. Hence, when TSBA serum concentration is found high (usually over 10 μmol/L to 14 μmol/L) in a pregnant women claiming pruritus and suspected to have intrahepatic cholestasis of pregnancy, this is enough to start an empirical treatment with UDCA and to start monitoring the woman's and her foetus's well‐being. The attempt is to prevent the feared fetal adverse events, and decide the best timing for delivery (Diken 2014; Geenes 2014).
At first, based on these 'taken for granted' premises on TSBA, we thought of planning a systematic review to assess the accuracy of components of serum bile acid profile, especially primary bile acid concentrations (Sjovall 1966; Laatikainen 1977; Heikkinen 1983a) or total concentration of tauro‐conjugated forms (Tribe 2010). They were studied as biomarkers for intrahepatic cholestasis of pregnancy some decades ago, especially in Scandinavia and South America (Sjovall 1966; Almuna 1986; Almuna 1987b). Later studies showed interest in serum bile acid profile as more sensitive laboratory techniques to assess their serum concentration became available (Sinakos 2010; Tribe 2010; Tripodi 2015), and they were hypothesised to provide more specific information than TSBA when diagnosing the disease, defining its severity, and monitoring its response to treatment (Chen 2013). Hence we wanted to find if they could be useful to improve the current clinical pathway as add‐on or replacement for TSBA. However, while looking for studies to write the background, we realised that no authors had systematically reviewed TSBA accuracy before, and we started to suspect that their use as the best available test might not have been based on solid evidence. So we finally planned a protocol and then a systematic review on the diagnostic accuracy of both TSBA (the current test for intrahepatic cholestasis of pregnancy) together with serum bile acid profile, in order to assess the accuracy of these tests and their role in the diagnostic pathway.
We asked ourselves why and when TSBA have been introduced in clinical practice. Looking back to past studies on intrahepatic cholestasis of pregnancy, we found that intrahepatic cholestasis of pregnancy was also known as “recurrent jaundice of pregnancy”, “icterus gravidarum”, or “pruritus gravidarum” until the 1950s; and that, at first, its diagnostic criteria were mainly clinical, with commonest liver function tests performed to support clinically suspected, and exclude alternative possible, diagnoses. TSBA were introduced in intrahepatic cholestasis of pregnancy clinical pathway on the basis of some case series and case‐control studies conducted mainly in Scandinavian and South America during the 1970s and 1980s (Sjovall 1966; Laatikainen 1984; Laatikainen 1977; Heikkinen 1981; Glasinovic 1982; Shaw 1982; Heikkinen 1983a; Heikkinen 1983b; Heikkinen 1983c; Lisoni 1983). They showed that the TSBA mean serum concentration, as well as cholic acid (CA), glycocholic acid (GCA), or chenodeoxycholic acid (CDCA) mean serum concentrations in some studies, were higher in women with intrahepatic cholestasis of pregnancy, when compared to healthy pregnant women. Since then, in most clinical settings, having TSBA serum concentration over a certain cut‐off value became itself part of the definition of the disease. However the studies named before, despite being cited by most in support of TSBA use for the diagnosis of intrahepatic cholestasis of pregnancy, did not have an appropriate design to demonstrate TSBA diagnostic accuracy, and did not perform an appropriate statistical analysis (Colli 2014). They compared the mean serum concentrations between diseased and non‐diseased pregnant women, but they did not estimate bile acids' sensitivity and specificity in detecting the disease. The publications usually reported only mean values, plus or minus two standard deviations (SD), of serum bile acid concentrations in the two groups of pregnant women, and the difference found between them was statistically significant.
TSBA use in the clinical pathway of intrahepatic cholestasis of pregnancy may have been built up to much more than a merely diagnostic role when their primary role in the pathogenesis of fetal distress was hypothesised (Laatikainen 1984). This hypothesis lead to TSBA use also as prognostic marker for intrahepatic cholestasis of pregnancy, where prognosis meant fetal outcome. Due to the very short time between onset of the disease and delivery (which means fetal outcome, and so prognosis), diagnosis and prognosis were then considered together, as two faces of the same coin. One of the most important and most cited studies following this line was published by Glantz 2004. The study showed a positive correlation between TSBA serum concentration and fetal adverse events, especially when their concentration was above 40 μmol/L, with an increase of 1% of risk per additional μmol/L. Among the 505 participants with intrahepatic cholestasis, reported by Glantz and colleagues, there were three intrauterine deaths (IUDs) of foetuses observed, which meant 0.4% of the participants (two were twin pregnancies with an IUD of one twin in both cases, one of which was found to have a tight knot of the umbilical cord; only two out of three women had TSBA above 40 μmol/L). The incidence of IUD was surprisingly similar to the one observed for the healthy pregnant women group, in contrast with higher percentages reported in previously published studies. It was assumed that the low risk observed was attributable to the 'active' management adopted (i.e. induction or caesarean section a few weeks before term). Authors concluded that active management was to be performed only with pregnant women with a level of bile salts above 40 μmol/L. However, recent studies have questioned these conclusions. The review by Henderson 2014, in line with the ROCG Green‐top Guideline No.43, concluded that a correlation between IUD risk and intrahepatic cholestasis may not exist, and that there was no evidence to either support or refute active obstetric management. Moreover, a recently published prognostic systematic review by Ovadia 2019 concluded that there was an association between intrahepatic cholestasis and IUD only above 100 μmol/L of TSBA: active management should be set aside for these rare patients, while for TSBA below this serum concentration, pregnant women can probably be reassured that the risk of stillbirth is similar to that of pregnant women among the general population, provided that repeat bile acid testing is done until delivery.
Nowadays, debate on diagnosis and prognosis of intrahepatic cholestasis of pregnancy is still open, and clinicians ask for new evidence on the disease. In particular, their primary concern is to know if intrahepatic cholestasis of pregnancy determines an increased risk for fetal adverse events; and, if this is true, which monitoring methods and prognostic markers can reliably predict adverse outcomes in order to decide the best timing for delivery. However, as reliable prognosis cannot exist without reliable diagnosis, we focused our efforts on the 'first step' of diagnostic accuracy (Colli 2014): we planned a diagnostic test accuracy review instead of a prognostic accuracy review to try to summarise the best evidence available on this topic, and to provide 'state of the art' guidance over the diagnosis of intrahepatic cholestasis of pregnancy.
Target condition being diagnosed
Intrahepatic cholestasis of pregnancy is a gestation‐specific liver disorder, defined as onset of pruritus, most often from the third trimester of pregnancy, associated with abnormal liver test results or raised total serum bile acids (TSBA) or both, and spontaneous relief after delivery in the absence of other skin or liver diseases. Severe intrahepatic cholestasis of pregnancy (previously defined by TSBA greater than 40 µmol/L (Glantz 2004)) seems to be associated with an increased proportion of adverse fetal outcomes which include the following: fetal distress; preterm birth (spontaneous or iatrogenic); meconium staining of amniotic fluid; low birth weight; respiratory distress syndrome of the baby (Glantz 2004; Zecca 2006); sudden intrauterine death (IUD), possibly due to an acute anoxic event (Sepúlveda 1991); or impaired fetal cardiomyocyte function (Williamson 2001). However this last severe and feared complication has been recently associated with the disease only when TSBA are above 100 µmol/L (Ovadia 2019).
Pruritus in the absence of skin rash, with the exception of scratching excoriations, could be the only presenting symptom of the disease.
Despite the many available tests, an accurate and early diagnosis of intrahepatic cholestasis of pregnancy can be difficult: it shares some of its clinical features and laboratory findings with other skin diseases (e.g. stretch marks of pregnancy; eczema; pruritic urticarial papules and plaques of pregnancy; infectious, allergic, or immunological skin disorders, etc.) and liver diseases (e.g. viral and autoimmune hepatitis, tumours of hepatobiliary tract, bile stones of the biliary tree, etc.) (Diken 2014); conditions which may lead to icterus (e.g. severe hypoglycaemia, some types of encephalopathy, disseminated intravascular coagulation, etc.); obstetric‐specific benign diseases (e.g. pruritus gravidarum, defined as idiopathic onset of pruritus during pregnancy but with normal liver tests, or asymptomatic hypercholanaemia of pregnancy, defined as serum bile acids level above the upper normal limit without symptoms) (Castaño 2006); and also more serious diseases (e.g. pre‐eclampsia, haemolysis‐elevated liver enzymes‐low platelet count syndrome, or acute fatty liver disease) (Bacq 2011).
Even if most clinicians with the least suspicion of the disease initiate an empiric treatment with UDCA, prophylactic vitamin K, or antihistaminics (or also dexamethasone if pruritus is unbearable), the diagnosis can only be confirmed when the spontaneous relief of symptoms and signs after delivery occur within the usual 48 hours or at most eight weeks (Geenes 2009). In extremely rare occasions, women may have symptoms for longer periods of time (Olsson 1993; Aytaç 2006). If the symptoms or signs related to suspected intrahepatic cholestasis of pregnancy do not disappear within one month, clinicians should consider other differential diagnoses; and further investigations are mandatory (Bacq 2011).
Index test(s)
Total serum bile acids Total serum bile acids (TSBA) are present at very low concentration (below 5 µmol/L) in the systemic circulation in normal fasting status, depending mostly on absorption from the gut (in turn dependent upon bile canalicular secretion) and hepatic extraction (Walker 2002). The usefulness of TSBA measurements in serum for a variety of liver diseases has been debated (Cravetto 1985; Azer 1997), and almost their only accepted use in current clinical practice is for the diagnosis of intrahepatic cholestasis of pregnancy (see Background).
The most often used cut‐off value of TSBA concentration for the diagnosis of intrahepatic cholestasis of pregnancy is around 10 µmol/L to 14 µmol/L (Diken 2014). However, there is a certain variability in the cut‐off values provided in the literature because of the method of measurement, fasting status, population studied, or gestational age at diagnosis (Pathak 2010). In addition, an early finding of normal levels of bile salts during the course of the disease does not exclude the diagnosis of intrahepatic cholestasis of pregnancy, while isolated elevation of bile salts in asymptomatic pregnant women may occur. However, this finding is uncommon and stands most probably for asymptomatic hypercholanaemia of pregnancy (Tripodi 2015). This could be a reason why the high diagnostic accuracy attributed to total serum bile acids for intrahepatic cholestasis of pregnancy may be questionable (Brites 1998b; Diken 2014).
Serum bile acid profile The serum bile acid profile is composed of concentrations of individual primary bile acids (cholic acid (CA) and chenodeoxycholic acid (CDCA)), secondary bile acids (deoxycholic (DCA), lithocholic (LCA), ursodeoxycholic acid (UDCA)), and their individual or total glyco‐conjugated (G‐c) and tauro‐conjugated (T‐c) forms (Figure 1), including ratios of some of them (CA/CDCA, G‐c/T‐c), measured in µmol/L. As the measurement of the individual components of the serum bile acid profile for the diagnosis of intrahepatic cholestasis of pregnancy has never been introduced in clinical practice, universally accepted cut‐off values still do not exist.
1.
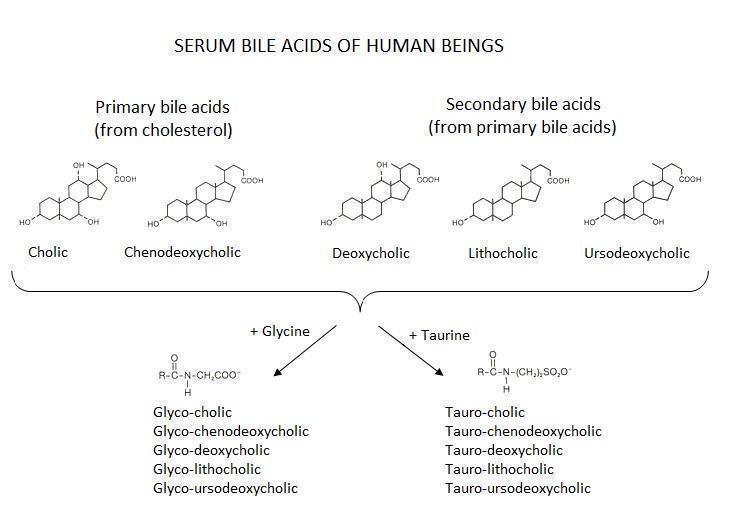
The current available laboratory methods for bile acid analysis (total or single components of serum bile acid profile) are enzyme assay, radioimmunoassay, enzyme immunoassay, and chromatographic methods such as thin‐layer chromatography, gas chromatography, high performance liquid chromatography, supercritical fluid chromatography, and capillary electrophoresis, coupled with mass spectrometry, fluorometry, UV detection, or electrochemical detection methods. This is why we expected to have heterogeneous results depending on the method used.
Clinical pathway
We describe the current clinical pathway for the diagnosis of intrahepatic cholestasis of pregnancy following the Green‐top Guideline No.43, published by Royal College of Obstetricians and Gynaecologists. Figure 2 presents a schematic overview of the current clinical pathway.
2.
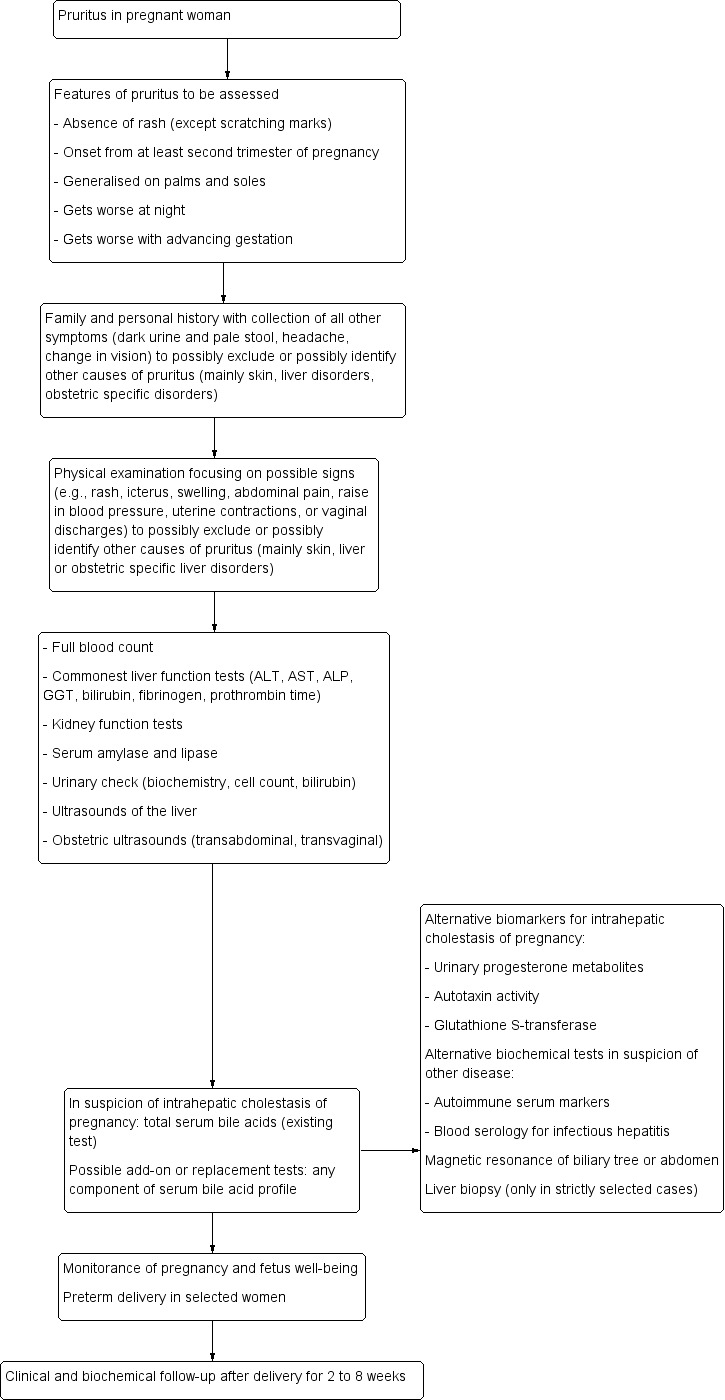
Clinical diagnostic pathway for the diagnosis of intrahepatic cholestasis of pregnancy
Clinical suspicion of intrahepatic cholestasis of pregnancy usually begins from the third — or at least the second — trimester of pregnancy with an onset of mild to severe pruritus, frequently generalised on the palms and soles, getting worse both at night and with advancing gestation (Kenyon 2001). In severe cases, it can also affect the ears, the eyelids, and even the oral cavity (Reyes 1997). Some studies describe instances of pruritus also from earlier stages of pregnancy (Brites 1998a; Keitel 2006; Hubschmann 2016).
After collecting all information concerning pruritus characteristics, it is mandatory to assess family and personal history, recording all other symptoms, and to carry out a physical examination to exclude or identify other plausible causes of pruritus. For example, in the presence of dark urine, pale stool, jaundice, steatorrhoea or right upper abdominal quadrant pain, alternative causes of cholestasis (e.g. bile stones of the biliary tree, tumours of hepatobiliary tract) or hepatitis of any aetiology should be suspected, as typical cholestatic symptoms in intrahepatic cholestasis of pregnancy are rare. Moreover, constitutional symptoms (insomnia, fatigue, anorexia, malaise, or abdominal pain) are not usually present (Hepburn 2008; Kondrakiene 2008;Mays 2010). Raised blood pressure, change in vision, headache, and epigastric abdominal pain may suggest pre‐eclampsia or haemolysis‒elevated liver enzymes‒low platelet count syndrome. Any kind of rash or swelling may suggest an infectious, allergic or immunologic skin disorder, while uterine contractions, or vaginal discharges should be carefully evaluated to exclude possible obstetric complications (Diken 2014). Last but not least, alcohol problems should be excluded. A laboratory test should follow, beginning with full blood count, liver function tests, urinary check, kidney function test, amylase, and lipase. Then depending on the suspected differential diagnoses, specific tests should be performed. In case of suspicion of immunological diseases (e.g. primary biliary cirrhosis, primary sclerosing cholangitis, or other autoimmune diseases), clinicians are advised to test nuclear, smooth muscle, mitochondrial, and liver–kidney microsomal autoantibodies, or other organ‐specific autoantibodies. In case of suspicion of liver infectious diseases, clinicians are advised to perform blood serology for the most common kind of hepatotropic viral agents such as hepatitis A, B, C, D, or E viruses, cytomegalovirus, and Epstein‐Barr virus. Also iron balance tests (ferritin, transferrin, saturation of transferrin) and copper metabolism biomarkers should be considered. Total serum bile acids are tested when intrahepatic cholestasis of pregnancy is suspected.
Among the imaging techniques, ultrasound examination of the abdomen focusing on the liver and biliary tree should be performed; and if it cannot rule out other cholestatic diseases, then magnetic resonance of the biliary tree or of the abdomen could be used to exclude possible causes of extrahepatic cholestasis such as choledochal stones, tumours of the biliary tree, or tumours of the pancreas (Boregowda 2013).
Liver biopsy is indicated only in jaundiced women without pruritus, beginning of symptoms before week 20 of gestation, and sustained abnormal laboratory findings beyond eight weeks after delivery (Boregowda 2013). Liver biopsy is not indicated for the diagnosis of intrahepatic cholestasis of pregnancy because of the risks associated with the procedure; but if performed, it may reveal pure cholestasis, with bile plugs sometimes visible in the hepatocytes and canaliculi (Bacq 2014).
An ideal method to predict fetal outcome does not exist, but obstetric examination with ultrasounds could help in ruling out high risk conditions of pregnancy and in assessing the well‐being of the foetus, while a 'non‐stress test' through cardiotocography (CTG) and biophysical profile (BPP) could also provide information about the well‐being of the baby at the time of the investigation (Diken 2014). If any signs of fetal distress are noticed, or if total serum bile acid shows an increment without benefit from UDCA therapy, the obstetrician may decide for an early delivery of the baby to prevent the feared fetal adverse events which seem to be related with intrahepatic cholestasis of pregnancy.
Prior test(s)
Liver biochemistry or liver function tests are commonly performed when intrahepatic cholestasis of pregnancy is suspected, but their normal upper limits in pregnant women are still discussed (Mullally 2002). Among the most common liver tests are serum aminotransferases (altered in up to 60% of women, but with lower values when compared to other aetiologies of liver disease such as viral hepatitis) (Diken 2014); gamma‐glutamyl transpeptidase (raised in less than one‐third of women with intrahepatic cholestasis of pregnancy) (Floreani 2006); alkaline phosphatases (not so reliable during pregnancy as its placental synthesis leads to physiologically increased values) (Bacq 1996); serum or urinary total, conjugated and unconjugated bilirubin (raised in about 25% of women, but with lower values when compared to other cholestatic diseases) (Reyes 1992); fibrinogen and prothrombin time. The prothrombin can be altered in case of severe liver dysfunction or vitamin K malabsorption due to cholestasis, leading to an increased risk of postpartum bleeding, but this is a very rare finding in intrahepatic cholestasis of pregnancy (Reyes 1992). Some women will have pruritus for days or weeks before the development of abnormal liver tests. In pregnant women with persistent unexplained pruritus, liver tests should be taken every week or two. If clinical evidence and liver tests show a pattern consistent with a viral or autoimmune aetiology (e.g. high elevation of serum aminotransferases), further testing is needed (Green‐top Guideline No.43).
Ultrasound examination of the liver and biliary tract could help to rule out other causes of liver disease or of cholestasis, especially extrahepatic cholestasis (e.g. stones or tumours of the biliary tree) (Boregowda 2013).
Role of index test(s)
The role of an index test, if related to an existent test within a diagnostic clinical pathway, can be one of replacement (substitution of the existent test), triage (addition before the existent test), or add‐on (addition after the existent test) (Bossuyt 2006).
Total serum bile acids (TSBA) are the existing test for the diagnosis of intrahepatic cholestasis of pregnancy. They are usually assessed after the most common liver tests described above when intrahepatic cholestasis of pregnancy is suspected. However, even if they are the existent test for intrahepatic cholestasis of pregnancy, a systematic review of their diagnostic accuracy has never been done, so we wanted first to assess their accuracy before looking for a test which may replace them or be added (add‐on).
Components of serum bile acid profile (cholic acid (CA), glycocholic acid (GCA), chenodeoxycholic acid (CDCA), cholic/chenodeoxycholic acid ratio (CA/CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), ursodeoxycholic acid (UDCA), total glyco‐conjugated bile acids (G‐c), total tauro‐conjugated bile acids (T‐c), total glyco‐conjugated bile acids/total taurine‐conjugated bile acid ratio (G‐c/T‐c)) could be considered as add‐on tests after TSBA. We calculated the overall diagnostic accuracy of some we wanted to consider as a replacement test of TSBA or add‐on tests after TSBA to improve the current clinical pathway.
Alternative test(s)
As alternative tests we can consider those that can be used to assess intrahepatic cholestasis of pregnancy, and all other serum and urinary biochemical tests or imaging techniques which can lead to exclusion of possible differential diagnosis (see Clinical pathway).
However, we also found some biomarker tests which were studied for their accuracy in diagnosing intrahepatic cholestasis of pregnancy, but these biomarker tests were mostly performed in a research setting. Among them are urinary progesterone metabolites, serum autotaxin activity, and glutathione S‐transferase. Urinary progesterone sulphated metabolites were found to be directly related to the pathogenesis of the disease and were studied for the diagnosis of intrahepatic cholestasis of pregnancy as well as for monitoring response to its treatment (Meng 1997; Reyes 2000b; Abu‐Hayyeh 2013). Serum autotaxin activity was shown to correlate with cholestasis‐associated pruritus and was considered able to distinguish intrahepatic cholestasis of pregnancy from other pruritic disorders of pregnancy or pregnancy‐related liver diseases (Kremer 2015). Glutathione S‐transferase is a detoxification liver enzyme with ubiquitous distribution in hepatic cells and its blood concentration was shown to rapidly increase in case of acute liver damage (Ozer 2008). Because of this, glutathione S‐transferase could be an earlier and more accurate indicator of hepatic dysfunction than liver aminotransferases or total bile acids alone (Dann 2004; Joutsiniemi 2008).
Rationale
Intrahepatic cholestasis of pregnancy is considered in clinical practice a high‐risk condition in pregnant women, mainly because of the increased risk of fetal adverse events. Currently, total serum bile acids are the most used diagnostic and prognostic marker for the disease, while serum bile acid profile components are less commonly used. A systematic review of diagnostic test accuracy of total serum bile acids and serum bile acid profile components has never been published. Thus, to assess the accuracy of total serum bile acids and serum bile acid profile components, independently or in combination, and to define which index test (or combination of index tests) could be better for use, may help to improve the current clinical pathway and clinicians' approach to the disease, leading to a direct benefit on the outcomes of both pregnant women and their babies. After that, a prognostic accuracy review to assess the reliability of our index tests as prognostic markers for the disease, could become feasible.
Objectives
To assess and compare, independently or in combination, the diagnostic accuracy of total serum bile acids or serum bile acids profile, or both, for the diagnosis of intrahepatic cholestasis of pregnancy in pregnant women, presenting with pruritus.
Secondary objectives
To define the optimal cut‐off values for components of serum bile acid profile; to investigate possible sources of heterogeneity (see below in Investigations of heterogeneity paragraph).
Methods
Criteria for considering studies for this review
Types of studies
We included studies that evaluated the diagnostic accuracy of the index tests for the diagnosis of intrahepatic cholestasis of pregnancy and used the proper reference standards (see below), irrespective of publication status, language, and prospective or retrospective design. We considered studies of cross‐sectional design including participants with clinical suspicion of intrahepatic cholestasis of pregnancy, as well as studies of case‐control design that compared people with known intrahepatic cholestasis of pregnancy to matched control (pregnant women without evidence of intrahepatic cholestasis of pregnancy participants).
Participants
Pregnant women of any age, recruited in any clinical setting. They should have been tested by at least one of the index tests and they should have undergone the reference standard (see below).
In cross‐sectional studies, participants should have been pregnant women with suspicion of having the target disease (e.g. presenting with new onset of pruritus during pregnancy).
In case‐control studies, the case group should have included pregnant women in which intrahepatic cholestasis of pregnancy had been confirmed through the reference standard (see below), while the control group should have comprised asymptomatic or symptomatic pregnant women in which the target condition had been ruled out with clinical evaluation and biochemical tests.
Index tests
The index tests we considered are total serum bile acids (TSBA) and the following components of serum bile acid profile.
Cholic acid (CA)
Glycocholic acid (GCA)
Chenodeoxycholic acid (CDCA)
Cholic/chenodeoxycholic acid ratio (CA/CDCA)
Deoxycholic acid (DCA)
Lithocholic acid (LCA)
Ursodeoxycholic acid (UDCA)
Total glyco‐conjugated bile acids (G‐c)
Total tauro‐conjugated bile acids (T‐c)
Total glyco‐conjugated bile acids/total taurine‐conjugated bile acid ratio (G‐c/T‐c)
The measurements of these index tests are performed in laboratories with different automated techniques (see Background). TSBA are commonly measured by almost all clinical laboratories, while techniques to measure components of serum bile acid profile are currently available only in clinical research settings.
Target conditions
Intrahepatic cholestasis of pregnancy defined as pruritus with onset during pregnancy associated with abnormal liver tests, both unexplained by other skin or liver diseases, and which resolves after delivery (Geenes 2009; Green‐top Guideline No.43).
Reference standards
The obstetric clinical evaluation in which the follow‐up after delivery is included. The clinical evaluation is the final judgment of the clinician who takes into account clinical assessment of the signs and symptoms suggestive for intrahepatic cholestasis of pregnancy and the presence of any otherwise unexplained, persistent abnormalities of AST, ALT, or bilirubin levels until delivery. The follow‐up after delivery is the assessment of spontaneous resolution of symptoms and normalisation of liver tests (i.e. liver test measurements below upper normal limits) within at least 48 hours or eight weeks at most.
For the final diagnosis, the obstetric evaluation could be enough when differential diagnoses can be easily ruled out. For difficult clinical cases, an evaluation and consensus with an hepatologist could be useful.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (maintained and searched internally by the CHBG Information Specialist via the Cochrane Register of Studies Web; 6 May 2019), the Cochrane Hepato‐Biliary Group Diagnostic Test Accuracy Studies Register (maintained and searched internally by the CHBG Information Specialist via the Cochrane Register of Studies Web; 6 May 2019), the Cochrane Library (2019, Issue 5), MEDLINE Ovid (1946 to 6 May 2019), Embase Ovid (1974 to 6 May 2019), Science Citation Index Expanded (Web of Science, 1900 to 6 May), Conference Proceedings Citation Index – Science (Web of Science; 1990 to 6 May 2019), BIOSIS (Web of Science; 1969 to 6 May 2019), and CINAHL (EBSCOhost; 1981 to 6 May 2019) (Royle 2003).
We searched Chinese literature through Chinese CKNI (1979 to May 2019) and VIP (1989 to May 2019) with the help of Maoling Wei from the Chinese Cochrane Centre.
As the highest prevalence of the disease is observed in Chile, on contacting some South American expert authors we were advised to search thoroughly two local databases: Literatura Latino‐Americana e do Caribe em Ciências da Saúde [Literature in the Health Sciences in Latin America and the Caribbean] (LILACS) and Scientific Electronic Library Online (SciELO) (both searched 6 May 2019).
We also searched through some field‐databases suggested by the Royal College of Obstetricians and Gynaecologists: the Evidence Search: Health and Social Care by NICE; the World Health Organization (WHO) Reproductive Health Library (RHL); and the Turning Research into Practice database (TRIP) (6 May 2019).
We applied no restrictions with regard to language or document type.
We give the search strategies with the time spans of the searches in Appendix 1.
Searching other resources
We identified additional references by handsearching the references of articles, meta‐analyses, and evidence‐based guidelines retrieved from the computerised databases, and the references suggested by the 'intrahepatic cholestasis of pregnancy support' web site (www.icpsupport.org/papers.shtml), in order to identify other potentially relevant studies for inclusion in our review.
We searched also for dissertations and theses through ProQuest Dissertations & Thesis Database and Index to Theses in Great Britain and Ireland, and grey literature through OpenSIGLE and National Technical Information Service (NTIS).
We searched on‐line trial registries such as ClinicalTrials.gov, European Medicines Agency (EMA) (www.ema.europa.eu/ema), WHO International Clinical Trial Registry Platform (www.who.int/ictrp), the Food and Drug Administration (FDA) (www.fda.gov), and pharmaceutical company sources.
Data collection and analysis
We followed the available guidelines provided in the Cochrane Diagnostic Reviewer's Handbook (DTA Handbook 2010).
Selection of studies
Two authors (CM, TS) independently conducted the first selection of studies by reading titles or abstracts or both of the identified studies. The two authors independently reviewed the full texts for eligibility, assessing the fulfilment of the inclusion criteria. During this second selection stage, when the two authors found multiple publications of one study fulfilling the inclusion criteria, they grouped them together and they screened these publications for complementary data or checked them for discrepancies. When in doubt, the review authors emailed study authors to ensure that publications referred to the same study and to check the correctness of data.
We solved disagreements by discussion or by consulting a third author (CG, GC, or DN).
Data extraction and management
Two authors (CN, TS) independently completed data extraction from each included study. They solved disagreements by discussion or by consulting a third author (CG, GC, or DN).
They retrieved the following study data.
General information: title, journal, year, publication status, study design (cross‐sectional or case‐control, prospective or retrospective, single centre or multicentre), time span considered.
Total number of women screened for inclusion in the study, number of pregnant women included, and prevalence of the disease in the considered population.
Baseline characteristics: age, ethnicity, country, if pregnancies were twin or singleton, week of pregnancy in which the index tests were performed, disease severity if reported, and concurrent medications used.
If most common liver tests were performed, and their findings.
Index tests (total serum bile acids or any component of serum bile acid profile): technique used for the measurement, fasting or postprandial status of women while the test was performed, predefined cut‐off values for the diagnosis if reported.
Follow‐up after delivery: length of follow‐up, length of time needed for assessment of the spontaneous relief of symptoms and normalisation of liver tests.
Number of true positive, true negative, false positive, and false negative comparing index test results with reference standard.
Information related to the QUADAS‐2 items for evaluation of the risk of bias of the studies (Whiting 2011).
The two authors summarised data from each study in two‐by‐two tables (false positive (FP), false negative (FN), true positive (TP), true negative (TN)) and entered the data into Review Manager 5 (Review Manager 2014).
Missing data
When information on any of the FP, FN, TP, or TN diagnostic test values were missing, we attempted to contact the authors of the included studies in order to obtain missing information. We also contacted authors if other types of information needed for this review was missing, especially when the publication was in the form of an abstract or poster presentation. We used Excel and Review Manager 5 to add data required for statistical analyses (Review Manager 2014).
We contacted primary authors for missing data by e‐mail. In absence of a reply, we sent a second e‐mail a week after, and when possible we tried to contact the study authors by telephone. We acknowledged study authors for providing missing data, and we created references to unpublished studies when such study data were obtained through personal communication (see notes under each study in Characteristics of included studies).
We excluded the studies when we could not obtain the data needed for the two‐by‐two tables.
Assessment of methodological quality
Design flaws in test accuracy studies can produce biased results (Lijmer 1999; Whiting 2004; Rutjes 2006). In addition, evaluation of study results is quite often impossible due to incomplete reporting (Smidt 2005).
To limit the influence of different biases, two review authors independently assessed the risk of bias of the included studies using four QUADAS‐2 domains (Whiting 2011). A third review author (GC) checked the extracted information and the risk of bias assessments. We resolved disagreements by discussion or by consulting a fourth review author (CG or DN). We contacted study authors if information on methodology was lacking in order to assess correctly the risk of bias of the studies.
We used the domains in Appendix 2 to address aspects of study quality involving the participant selection, index test, reference standard, and flow and timing. We classified a study at low risk of bias only if classified at low risk of bias in all the four domains; otherwise, we considered the study at high risk of bias (Jüni 1999; Whiting 2005).
We used tabular and graphical displays to summarise QUADAS‐2 assessments.
Statistical analysis and data synthesis
We carried out the analyses following guidelines in Chapter 10 (Analysing and Presenting Results) of the Cochrane Diagnostic Reviewer's Handbook (DTA Handbook 2010). We used Review Manager 5 software for analyses and forest plots (Review Manager 2014).
We built two‐by‐two tables for each primary study and for all the index tests considered. We estimated sensitivity and specificity, with their 95% confidence intervals (CI). We presented data in coupled forest plots, showing sensitivities and specificities of each study, with their 95% CI. In forest plots, studies were ordered according to study authors’ names or, when heterogeneity analysis was performed, according to the source of heterogeneity.
We plotted the studies in summary Receiver Operating Characteristic (ROC) curves when studies with different cut‐offs were considered together, or we represented the studies as circles in the ROC space when the studies included in the analysis had the same cut‐off, reporting sensitivity against specificity. The size of circles in the ROC space are proportional to the number of participants included in the study. We represented the 95% confidence regions of mean specificity and sensitivity as areas surrounding the circles.
When individual participant data were available, or when studies used a common cut‐off, we attempted to meta‐analyse data at different cut‐offs and, when possible, to obtain multiple estimates of accuracy for the same index tests.
When the included primary studies reported accuracy data using different cut‐off values, we adopted the hierarchical summary ROC model (HSROC) to pool data and to estimate a summary ROC (SROC) curve. When a sufficient number of primary studies reported data using a common cut‐off value, we performed meta‐analyses using the bivariate model, and we provided the estimate of the summary operating point (mean sensitivity and mean specificity) at that cut‐off value.
For primary studies that reported accuracy results for more than one cut‐off point, we reported sensitivities and specificities for all of the cut‐off points, but we used a single cut‐off point for each study in HSROC or bivariate model analysis. We based our choice on the cut‐off value most commonly reported, or in case a study did not report data on the most commonly used cut‐off, we based our choice on the value corresponding to the maximum of the Youden's index (sensitivity + specificity − 1),
We made direct and indirect comparisons between the considered index tests by adding a covariate term for the index test to the bivariate or HSROC model, as appropriate. We assessed the significance of differences in test accuracy by using the log‐likelihood ratio test for comparison of models with and without the index test covariate term. We considered P values less than 0.05, two‐sided, as statistically significant.
We used SAS statistical software, release 9.4 (SAS Institute Inc., Cary, NC, USA) to perform all statistical analyses.
Investigations of heterogeneity
For the eleven studies assessing TSBA diagnostic accuracy with cut‐off equal to 10 μmol/L, we investigated the following sources of heterogeneity.
Laboratory technique used to measure the index test
Therapy (if study patients underwent therapy with UDCA or not)
Timing of measurement of index test (onset of symptoms, peak values among multiple assessments during pregnancy, immediately before delivery)
We investigated heterogeneity first by visual inspection of the paired forest plots of sensitivities and specificities for each index test. Then, we performed a formal analysis by adding covariates to the bivariate or HSROC model.
Sensitivity analyses
We performed the following sensitivity analysis.
Excluding all studies with case‐control design
Excluding studies in which the index test was part of the reference standard
Assessment of reporting bias
We decided not to assess publication bias.
'Summary of findings' table
To construct a 'Summary of findings' table for presenting the key findings of our review, we used the approach developed by the Cochrane GRADEing group (formerly, the Cochrane Applicability and Recommendations Methods Group) which is in conformity with QUADAS 2 (see Chapter 11, DTA Handbook 2013; Whiting 2011).
Results
Results of the search
We identified a total of 5073 references. We identified 3511 references through electronic searches of the Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 22) and the Cochrane Hepato‐Biliary Group Diagnostic Test Accuracy Studies Register (n = 0), the Cochrane Library (n = 115), MEDLINE Ovid (n = 770), Embase Ovid (n = 1409), Science Citation Index Expanded and Conference Proceedings Citation Index – Science (n = 592), BIOSIS Previews (n = 539), and CINAHL (EBSCO) (n = 64). We also identified 1043 references through the two Chinese databases CNKI and VIP, but we only considered nine were relevant; these were sent to us by the Chinese colleague who performed the search, and 242 references through electronic searches of the two South American databases LILACS and SciELO. In addition, we identified 277 references through electronic search of Evidence search: Health and Social Care, RHL, TRIP, and handsearching ProQuest Dissertations & Thesis Database, Index to Theses in Great Britain and Ireland, OpenSIGLE, NTIS, on‐line trials registers, conference proceedings and references taken from other publications and found to be potentially relevant. After exclusion of 1324 duplicates, 2715 publications were screened for inclusion. We excluded 2644 publications at a first selection, all by reading the title and abstract; and 259 publications by reading also the full text. Seventy‐one full‐text articles seemed to fulfil the inclusion criteria, and we finally excluded 55, most of them only after contacting study authors in order to bring together more information.
We included 16 relevant studies in this systematic review. Figure 3 presents a schematic overview of the study selection process.
3.
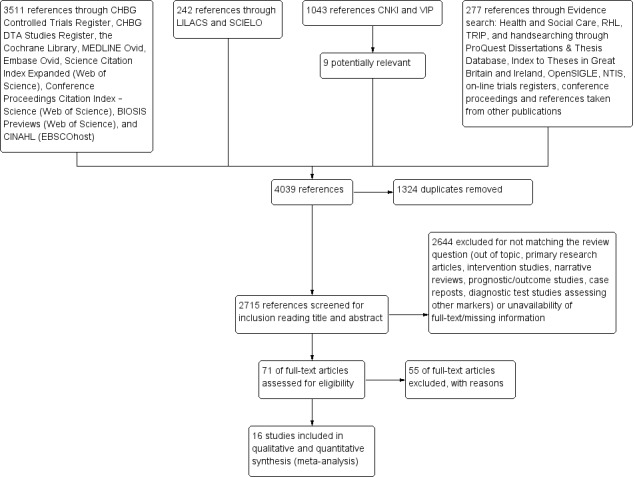
Study selection flow diagram.
References of included studies, ordered by study ID, are given in Included studies.
Additional Table 3 provides the main published or unpublished (given by email from study authors) characteristics of the included studies. For more detailed information on each study's characteristics, comprising the information obtained by email from authors, see Characteristics of included studies.
1. Characteristics of included studies ‐ Summary.
| Author | Year | Country | # | Study design | ICP definition | Index test(s) | Cut‐off (μmol/l)a | Laboratory technique | Timing |
| Almuna R | 1986 | Chile | 241 | case‐control | Only clinical/with FU | GCA | 0,7 | Immunoenzymatic assay | Onset |
| Almuna R | 1987 | Chile | 22 | case‐control | Clinical + lab/with FU | GCA | 0,7 | Immunoenzymatic assay | Delivery |
| Brites D * | 1998 | Portugal | 77 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 10 | Enzymatic fluorimetric | Onset |
| CA§ | not given | HPLC | Onset | ||||||
| CDCA§ | not given | HPLC | Onset | ||||||
| GCA | not given | HPLC | Onset | ||||||
| CA/CDCA§ | not given | ||||||||
| Brites D * | 1998 | Portugal | 14 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 10 | Enzymatic fluorimetric | Delivery |
| CA§ | not given | HPLC | Onset | ||||||
| CDCA§ | not given | HPLC | Onset | ||||||
| GCA | not given | HPLC | Onset | ||||||
| CA/CDCA§ | not given | ||||||||
| Gonzalez MC | 1989 | Chile | 62 | Cross‐sectional | Clinical + lab + TSBA/with FU | TSBA | 10 | Enzymatic assay | Onset |
| Guducu N* | 2013 | Turkey | 33 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 10 | Enzymatic assay | Peak |
| Huang W* | 2009 | USA | 193 | Cross‐sectional | Clinical + lab/with FU | TSBA | 10 | LC‐MS | Onset |
| CA | not given | LC‐MS | Onset | ||||||
| CDCA | not given | LC‐MS | Onset | ||||||
| CA/CDCA | 3,4 | ||||||||
| Jiang Y | 2012 | China | 700 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 11 | Enzymatic colorimetric | Onset |
| Kowalska‐Kanka A* | 2013 | Poland | 73 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 11 | Enzymatic colorimetric | Peak |
| Laatikanen T | 1984 | Finland | 177 | case‐control | Clinical + lab/with FU | TSBA | 10 | Enzymatic assay | Delivery |
| Lang T* | 2012 | UK | 66 | Cross‐sectional | Clinical + lab/with FU | TSBA | 14 | Enzymatic colorimetric | Onset |
| Lunzer M | 1986 | Australia | 297 | case‐control | Clinical + lab/with FU | GCA | 1,5 | Radioimmuno assay | Onset |
| Roger D* | 1994 | France | 34 | case‐control | Only clinical/with FU | TSBA | 6 | Enzymatic assay | Onset |
| GCA | 0,7 | Immunoenzymatic assay | Onset | ||||||
| Sjovall K* | 1966 | Sweden | 28 | case‐control | Only clinical/with FU | TSBA | not given | Gas‐liquid chromatography | Onset |
| CA | not given | Gas‐liquid chromatography | Onset | ||||||
| CDCA | not given | Gas‐liquid chromatography | Onset | ||||||
| CA/CDCA§ | not given | ||||||||
| Sun Y | 2011 | China | 105 | case‐control | Clinical + lab/with FU | TSBA | 20 | Enzymatic colorimetric | Onset |
| Tripodi V*b | 2006‐2015 | Argentina | 83 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 10 | Enzymatic assay | Onset |
ICP ‐ Intrahepatic cholestasis of pregnancy
*Studies with individual participant data (provided in the publications or received from authors by email) § The index test was calculated by review authors on the basis of individual participant data. See full‐text for explanations aCut‐offs given in this table are those provided in the publications bWe refer to all publications given under the study ID "Tripodi 2015" (see "References of included studies") “Clinical”: based on symptoms and physical examination “lab”: based on laboratory exams (e.g. liver tests, viral serology, autoimmunity biomarkers) “with FU”: comprising follow‐up after delivery “without FU”: follow‐up after delivery not performed
We retrieved individual participant data (marked with “*” in Additional Table 3) of nine included studies from their authors by email personal correspondence, while two studies already contained individual participant data in the publication. This allowed us to consider also the index tests performed by the study authors but not published, and to calculate the diagnostic accuracy of the index tests performed in these studies at cut‐offs different from the published ones; (for more detailed information on unpublished data, as well as on how diagnostic accuracy of unpublished index tests was calculated, see Characteristics of included studies). This is the reason why we finally had 13 studies assessing the diagnostic accuracy of TSBA, 4 studies of CA and CDCA, 4 studies of CA/CDCA, and 6 studies of GCA.
Some of the studies assessing the diagnostic accuracy of the components of serum bile acid profile analysed in our review assessed also the diagnostic accuracy of LCA, DCA, UDCA, G‐c, T‐c, and G‐c/T‐c. They reported that T‐c acids increased in the third trimester of pregnancy in women with intrahepatic cholestasis of pregnancy, and that G‐c/T‐c of less than 1.0 μmol/L had high diagnostic accuracy. Another finding was that LCA was higher in serum of women with intrahepatic cholestasis of pregnancy, while UDCA was lower (Brites 1998b; Brites 1998c; Tripodi 2015). However, as the number of studies assessing the diagnostic accuracy of LCA, DCA, UDCA, G‐c, T‐c, and G‐c/T‐c was three or fewer for each, we found it useless to meta‐analyse their data, even when taking into account the studies for which we had individual participant data, owing to statistical limitations and the high risk of random errors.
We could not analyse the accuracy of combinations of the index tests, defined in our protocol as TSBA plus any component of serum bile acid profile, as none of the included studies measured these combinations.
For our analysis of TSBA, irrespective of the cut‐off values used to assess the TSBA accuracy, we had data from 1645 participants; and with TSBA serum concentration of 10 μmol/L as diagnostic cut‐off value, we had data from 839 participants. Thanks to the availability of individual participant data for all studies assessing CA, GCA, CDCA, and CA/CDCA, the number of participants for each test was the same for every cut‐off considered: 312 participants for CA, 630 for GCA, 312 for CDCA, and 312 for CA/CDCA.
Methodological quality of included studies
Figure 4 shows the risk of bias for each study across each QUADAS‐2 domain and Figure 5 shows the overall risk of bias assessment for all included studies across each QUADAS‐2 domain.
4.
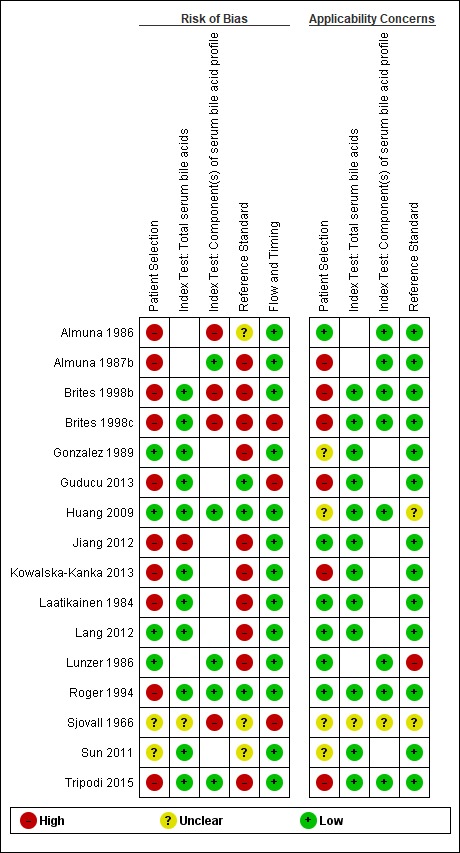
Risk of bias and applicability concerns summary: review authors' (CM and TS) judgements about each domain for each included study.
N.B. The empty cells stand for the test, not performed in the study.
5.
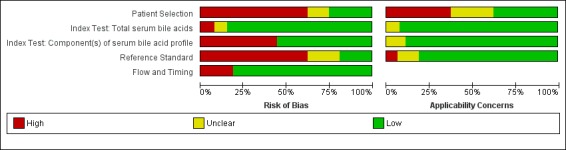
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
We assessed only one study at low risk of bias in all four QUADAS‐2 domains (Huang 2009). The most biased domains were the patient‐sampling domain and the reference standard domain. We judged the patient‐sampling domain at high risk of bias in most cases because of the case‐control design, as it excludes all 'difficult to diagnose' patients. We judged the reference standard domain at high risk of bias in most studies because of the inclusion of the index test TSBA in its definition. For applicability concerns, most studies had a high concern regarding patient selection, because the characteristics of selected participants (exclusion of 'difficult to diagnose' patients from cases group; absolutely asymptomatic and healthy pregnant women, often in a very early stage of pregnancy in control groups) excluded them as representative for clinical practice. Only five studies were representative for all domains (Laatikainen 1984; Almuna 1986;Roger 1994;Jiang 2012;Lang 2012).
For more detailed information on each study's quality assessment according to QUADAS‐2, see Characteristics of included studies.
Findings
In this review, we estimated the overall accuracy of TSBA and some components of serum bile acid profile (CA, GCA, CDCA and CA/CDCA) for the diagnosis of intrahepatic cholestasis of pregnancy. We did not perform the analysis of the remaining components of serum bile acid profile (i.e. LCA, DCA, UDCA, G‐c, T‐c, G‐c/T‐c) as there were too few data available.
Total serum bile acids (TSBA)
TSBA was the index test with the highest number of studies assessing its accuracy (Figure 6). The commonest cut‐off value used by studies was 10 μmol/L. Hence, after a first analysis considering all 13 studies (Figure 7), we analysed together only the 11 studies for which estimates of accuracy were available for a cut‐off of 10 μmol/L (Figure 8). Considering only studies using a cut‐off of 10 μmol/L, or those which it was possible to apply this cut‐off, we found the overall sensitivity and specificity estimated for TSBA to be respectively 0.91 (95% CI 0.72 to 0.98) and 0.93 (95% CI 0.81 to 0.97) (Figure 9).
6.
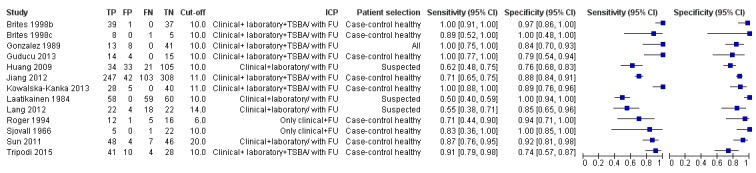
Forest plot of total serum bile acids (TSBA) (all studies) for the diagnosis of intrahepatic cholestasis of pregnancy
7.
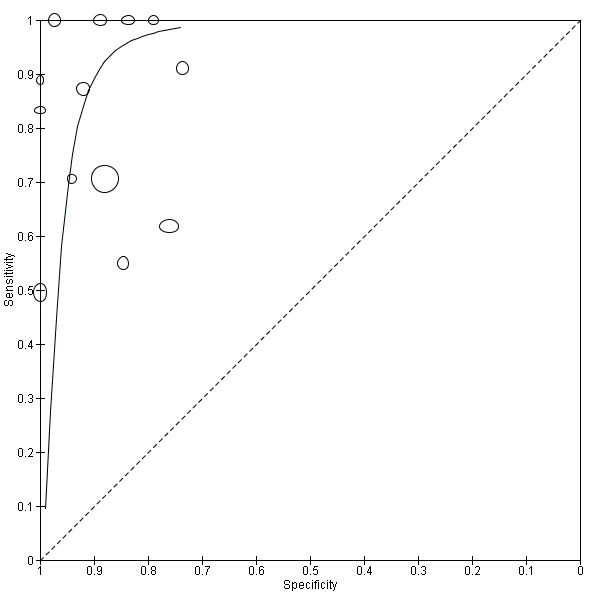
Summary receiver operating characteristic (ROC) plot of total serum bile acids (TSBA) (all studies) for the diagnosis of intrahepatic cholestasis of pregnancy. Statistical method used: HSROC (hierarchical summary ROC) model.
8.
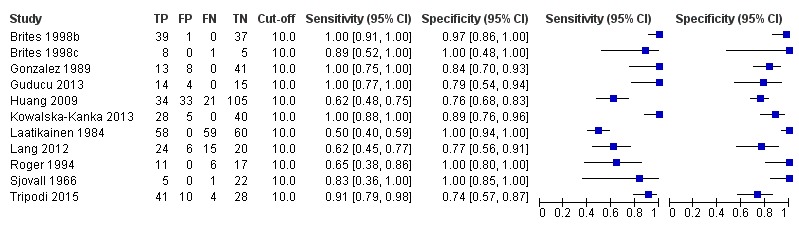
Forest plot of total serum bile acids (TSBA) with cut‐off = 10 µmol/L for the diagnosis of intrahepatic cholestasis of pregnancy
9.
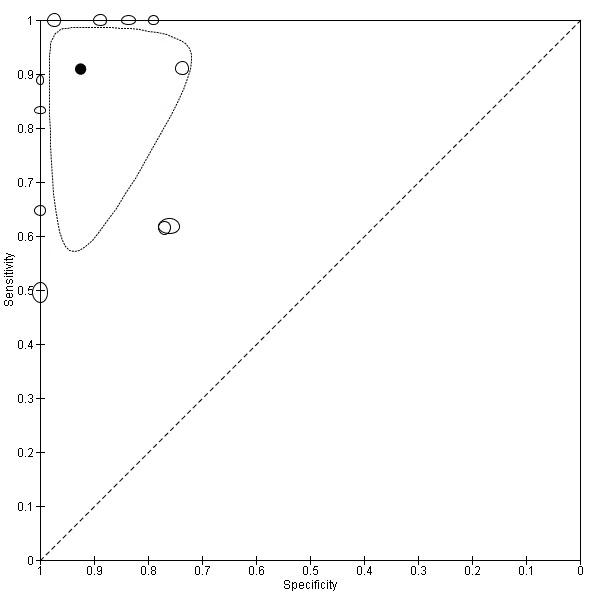
Summary receiver operating characteristic (ROC) plot of of total serum bile acids (TSBA) with cut‐off = 10 µmol/L for the diagnosis of intrahepatic cholestasis of pregnancy. Statistical method used: bivariate model.
Glycocholic acid (GCA)
At first we considered all six studies together when estimating GCA accuracy (Figure 10, Figure 11). Then we also estimated GCA accuracy at the three most reported cut‐offs: 0.7 μmol/l (5 studies), 1.5 μmol/L (4 studies), and 2 μmol/L (3 studies).
10.
Forest plot of glycocholic acid (GCA) (all studies) for the diagnosis of intrahepatic cholestasis of pregnancy
11.
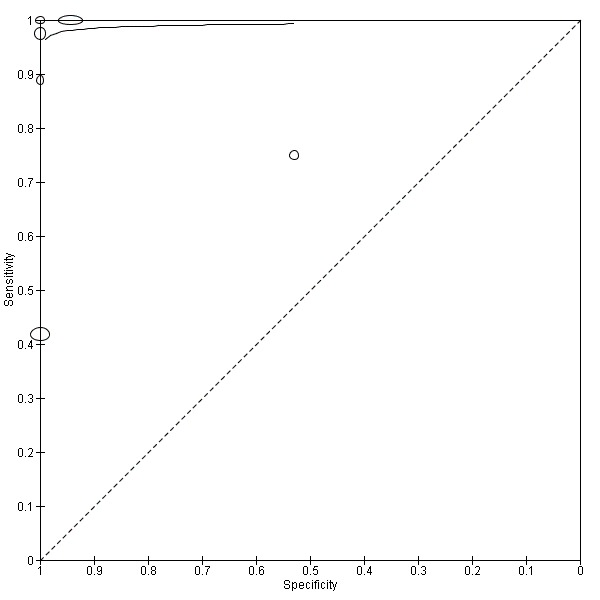
Summary receiver operating characteristic (ROC) plot of glycocholic acid (GCA) (all studies) for the diagnosis of intrahepatic cholestasis of pregnancy. Statistical method used: HSROC (hierarchical summary ROC) model.
The overall sensitivity was 0.92 (95% CI 0.65 to 0.99) and the overall specificity was 0.99 (95% CI 0.06 to 1.00), considering all studies. When considering a cut‐off of 0.7 μmol/L, they were 0.97 (95% CI 0.38 to 1.00) and 0.86 (95% CI 0.02 to 1.00) respectively; when the cut‐off was 1.5 μmol/L, they were 0.99 (95% CI 0.08 to 1.00) and 0.90 (95% CI 0.75 to 0.97) respectively; and when the cut‐off 2.0 μmol/L, they were 0.99 (95% CI 0.07 to 1.00) and 0.97 (95% CI 0.82 to 1.00) respectively.
Cholic acid (CA)
Four included studies assessed the diagnostic accuracy of CA. Having obtained individual participant data of all four, we were able to calculate specificity and sensitivity for each study at multiple cut‐offs (2, 3, 4, and 5 μmol/l) (Figure 12). However, the only cut‐off values for which it was possible to estimate the overall accuracy and its 95% CI were 2 μmol/L and 3 μmol/l: CA with a cut‐off of 2 μmol/L had a sensitivity of 0.99 (95% CI 0.33 to 1.00) and a specificity of 0.61 (95% CI 0.23 to 0.89), while CA with a cut‐off of 3 μmol/L had a sensitivity of 0.94 (95% CI 0.66 to 0.99) and a specificity of 0.82 (95% CI 0.68 to 0.91). For a cut‐off of 4 μmol/L and 5 μmol/L, we were not able to estimate the overall sensitivity and specificity.
12.
Forest plots of cholic acid (CA) with different cut‐offs for the diagnosis of intrahepatic cholestasis of pregnancy: a) cut‐off 2 µmol/L; b) cut‐off = 3 µmol/L; c) cut‐off = 4 µmol/L; d) cut‐off = 5 µmol/L
Two studies took into account only the conjugated (tauro‐ and glyco‐) forms of CA, excluding free CA serum levels, because the laboratory techniques used cannot detect the free form as the concentration is too low (Sjovall 1966; Huang 2009). The other two studies for which we had individual participant data also measured free CA levels as separate assessments, but in order to make the study comparable to others we decided not to add them to the CA values (which then comprised only conjugated CA) (Brites 1998b; Brites 1998c).
Chenodeoxycholic acid (CDCA)
We estimated CDCA sensitivity and specificity at two different cut‐offs for each study (Figure 13). It was possible to do this because of the availability of individual participant data for all four studies. At a cut‐off of 2 μmol/l, the estimated overall sensitivity was 0.98 (95% CI 0.62 to 1.00) and the overall specificity was 0.66 (95% CI 0.19 to 0.94); at a cut‐off of 3 μmol/L we could not estimate the overall diagnostic accuracy. High heterogeneity among study results made it impossible to determine the precision of these estimates and their applicability in a real clinical setting.
13.
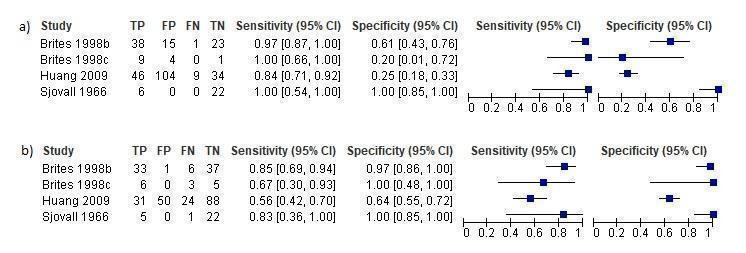
Forest plots of chenodeoxycholic acid (CDCA) at different cut‐offs for the diagnosis of intrahepatic cholestasis of pregnancy: a) cut‐off = 2 µmol/L; b) cut‐off = 3 µmol/L
Two studies took into account only the conjugated (tauro‐ and glyco‐) forms of CDCA, excluding free CDCA serum levels, because of the laboratory techniques used which cannot detect the free form as the concentration is too low (Sjovall 1966; Huang 2009). The other two studies for which we had individual participant data measured also free CDCA levels as separate assessments, but in order to make the study comparable to others we decided not to add them to CDCA values provided (which then comprised only conjugated CA) (Brites 1998b; Brites 1998c).
CA/CDCA
No study other than Huang 2009 provided a cut‐off for CA/CDCA. We found that for most of the studies, the cut‐off corresponding to the Youden index maximum was around 1.8. For this reason, we performed the meta‐analysis considering only the cut‐off of 1.8. The overall sensitivity was 0.89 (95% CI 0.54 to 0.98), and the overall specificity was 0.92 (95% CI 0.85 to 0.96) (Figure 14).
14.
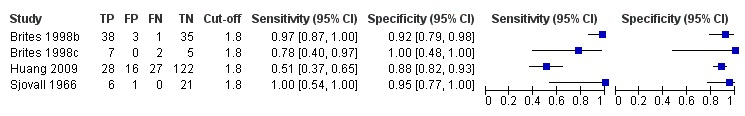
Forest plot of CA/CDCA with cut‐off = 1.8
Comparisons
We made indirect comparisons between TSBA for a cut‐off of 10 μmol/L and the following index tests for their most accurate cut‐off: CA with a cut‐off of 3 μmol/L, GCA with a cut‐off of 1.5 μmol/L, CA/CDCA with a cut‐off of 1.8 μmol/L. We made the indirect comparisons by adding the index tests as covariates to the bivariate or HSROC model. None of the indirect comparisons was found to be statistically significant (TSBA vs CA: P = 0.29; TSBA vs GCA: P = 0.096; TSBA vs CA/CDCA: P = 0.096). An indirect comparison between TSBA for a cut‐off of 10 μmol/L and CDCA for a cut‐off of 3 μmol/L was not statistically possible.
Direct comparisons were not statistically possible because of the small number of included studies.
Heterogeneity
Taking into account the number and characteristics of the included studies, heterogeneity analysis was possible only for the 11 studies assessing TSBA diagnostic accuracy with cut‐off equal to 10 μmol/L. Moreover, this analysis was possible only for some of the sources of heterogeneity planned at the protocol stage: laboratory technique used to measure the index test, therapy (if study patients underwent therapy with UDCA or not), and timing of measurement of index test (onset of symptoms, peak values among multiple assessments during pregnancy, immediately before delivery). Results are reported below.
Laboratory technique
We investigated laboratory technique by grouping studies according to five different techniques: enzymatic assay (5 studies), enzymatic colorimetric (2 studies), gas‐liquid chromatography (1 study), liquid chromatography‒mass spectrometry (LC‐MS) (1 study), enzymatic fluorometric (2 studies). However, the number of studies for each group was too small to calculate the confidence intervals of accuracy estimates and regions around summary ROC points (except for enzymatic assay). The differences found between techniques were not statistically significant (P = 0.42).
Therapy
We excluded three studies from this heterogeneity analysis because of lack of information regarding therapy (Roger 1994; Huang 2009; Lang 2012); and one because both kind of patients were included (Brites 1998b). We investigated therapy, grouping the seven remaining studies in two different groups: studies including patients who underwent UDCA therapy (5 studies) and studies including patients who did not (2 studies). The differences found between the two groups in TSBA diagnostic accuracy estimates were not statistically significant (P = 0.60).
Timing
We investigated timing, grouping studies in three groups (onset, peak, delivery) according to when the TSBA measurement took place. Sensitivity and specificity estimated for each group were the following: 0.87 (95% CI 0.68 to 0.96) and specificity 0.87 (95% CI 0.76 to 0.94) for onset; 0.70 (95% CI 0.24 to 0.94) and 1.00 (CI not calculable) for peak; 1.00 (95% CI 1.00 to 1.00) and 0.87 (95% CI 0.68 to 0.95) for delivery. The differences in diagnostic accuracy estimates were statistically significant (P = 0.027).
Sensitivity analysis
At the protocol stage, we had planned to perform a sensitivity analysis by excluding studies at high risk of bias (studies judged as high risk of bias or unclear risk of bias in at least one of the domains of QUADAS‐2) in order to explore the influence of the quality of the included studies. However, the overall low quality of included studies did not allow this, as we judged only one study at low risk of bias in all domains (Huang 2009). Hence, we performed only the remaining two sensitivity analyses planned in the protocol.
The first sensitivity analysis excluded studies in which TSBA were included in the reference standard for intrahepatic cholestasis of pregnancy, assuming that this could have been one of the most important sources of bias. The overall sensitivity and specificity were 0.57 (95% CI 0.49 to 0.65) and 0.98 (95% CI 0.53 to 1.00), respectively (Figure 15).
15.
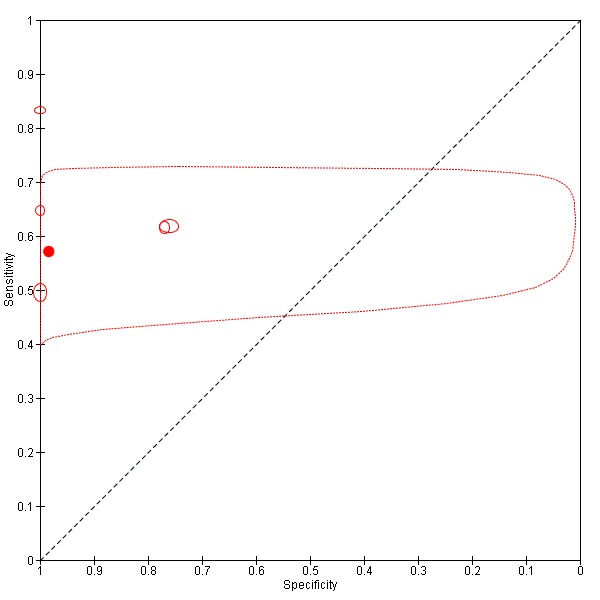
Summary ROC Plot of sensitivity analysis of TSBA cut‐off=10 μmol/L excluding studies in which TSBA assessment was part of the reference standard. Statistical method used: HSROC (hierarchical summary ROC) model.
The second sensitivity analysis excluded all studies with case‐control design, as all of them enrolled as controls asymptomatic pregnant women. The resultant overall sensitivity and specificity were 0.57 (95% CI 0.48 to 0.66) and 0.92 (95% CI 0.52 to 0.99), respectively (Figure 16).
16.
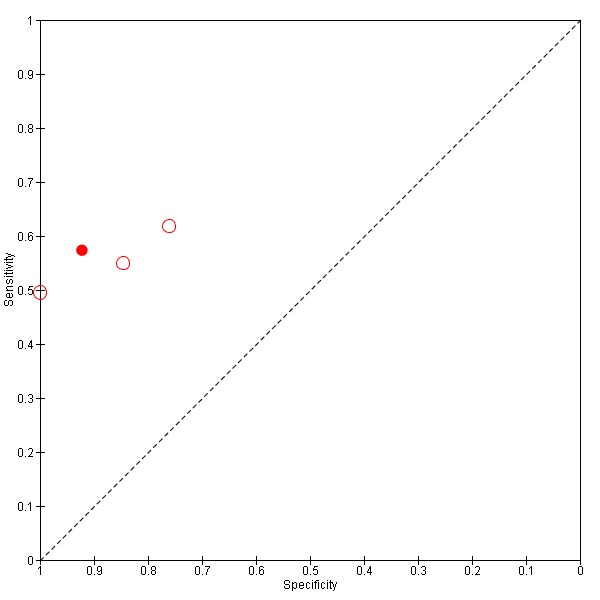
Summary ROC Plot of sensitivity analysis of TSBA cut‐off=10 μmol/L excluding studies with case‐control design (95% confidence region not estimable because of too few studies included in the analysis). Statistical method used: HSROC (hierarchical summary ROC) model.
'Summary of findings' table
Table 1 shows, whenever possible, all considered index tests summary accuracy estimates and consequences of their application in a hypothetical clinical context. In the Table 1, we give the prevalence of intrahepatic cholestasis of pregnancy of 30% among pregnant women claiming pruritus; that is, the median of prevalences provided by four included studies with a cross‐sectional design: Huang 2009 (55/193, prevalence 28%), Lang 2012 (40/66, prevalence 60%), Lunzer 1986 (15/69, prevalence 22%), Roger 1994 (17/49, prevalence 35%). Based on this, we calculated implications of the use of our index tests for the diagnosis of intrahepatic cholestasis of pregnancy on a hypothetical population of 100 pregnant women with pruritus. For some index tests, especially for GCA and CA, we found a small number of studies, and there was a great heterogeneity in accuracy estimates among them. Hence, we found that the overall accuracy was imprecise, and the confidence intervals (CI) for sensitivity and specificity estimates were very wide or impossible to calculate. To apply these results in a real clinical setting is not possible.
Discussion
Summary of main results
Our analysis of the eleven studies assessing TSBA diagnostic accuracy for a cut‐off of 10 μmol/L (the most used cut‐off among the studies we included) provided estimates of sensitivity ranging from 0.72 to 0.98, and specificity ranged from 0.81 to 0.97. Considering the respective pooled estimates of 0.91 and 0.93, in a population of 1000 pregnant women claiming for pruritus, with a prevalence of intrahepatic cholestasis of 30%, 27 (6 to 84) diseased women would be missed, and 49 women (21 to 133) without the disease would be falsely diagnosed. However, with a first sensitivity analysis excluding case‐control studies, TSBA overall sensitivity was lower, ranging from 0.48 to 0.66, and its overall specificity had a wider confidence interval, 0.52 to 0.99. This could mean that when patients were pre‐selected with a clear diagnosis of intrahepatic cholestasis of pregnancy and compared with healthy and asymptomatic pregnant women, as in most included studies, accuracy of TSBA was higher than when TSBA were performed on a population of symptomatic pregnant women in a real clinical setting. A second sensitivity analysis, with exclusion of studies which comprised TSBA in the reference standard, estimated a sensitivity range from 0.49 to 0.65 and specificity from 0.53 to 1.00. These results support the conclusion that TSBA accuracy may not be so high as believed, and that existent studies about diagnostic accuracy of TSBA are not well designed to assess it. What should have been done before introducing TSBA in clinical practice (and to know better the diagnostic accuracy of TSBA) was the completion of cross‐sectional studies enrolling consecutive pregnant women suspected of intrahepatic cholestasis of pregnancy (and followed up after delivery to confirm the right diagnosis). This would have allowed the index test to identify the disease among suspected population (Colli 2014). Only few of the studies included in this systematic review had a design similar to this (Gonzalez 1989; Huang 2009; Lang 2012).
Subgroup analysis considering possible sources of heterogeneity, such as a laboratory technique used to measure TSBA or a therapy for women with intrahepatic cholestasis of pregnancy during pregnancy, did not show statistically significant differences. A heterogeneity analysis considering timing of TSBA testing showed a statistically significant difference: assessing TSBA serum concentration at the time of delivery was more specific but less sensitive in diagnosing intrahepatic cholestasis of pregnancy, while taking the peak value among multiple assessments was more sensitive but less specific than assessing TSBA at the time of onset of symptoms (i.e. pruritus). However, this is not sufficiently clinically relevant, as obstetricians need an index test with high sensitivity at an earlier time, which can allow them to strictly follow up the pregnant woman and her foetus in order to find, as soon as possible, signs of potential fetal distress and to decide the best management. If diagnosis is made at the time of delivery, it is too late and almost useless, because it means that a fetal adverse event (fetal death) did not occur; if we make multiple assessments during pregnancy, the evaluation of the peak value could be only 'a posteriori'.
The data to allow a direct comparison between index tests were limited. Through indirect comparisons, none of the serum bile acid profile components were shown to be better than TSBA. However, there were too few and biased studies included and analysed to obtain a precise estimate of serum bile acid profile accuracy.
We could not analyse the accuracy of combinations of the index tests (that in our review protocol was defined as TSBA plus any component of serum bile acid profile) as none of the included studies did so.
Strengths and weaknesses of the review
To our knowledge, this is the first systematic review on the accuracy of diagnostic tests for intrahepatic cholestasis of pregnancy. The rigorous methodology adopted in all phases of the work is a strength of this review: our findings are based on a thorough searching of studies and strict inclusion criteria, as well as standardised and independent data extraction and analysis. Another strength of our review is our success in contacting many study authors to retrieve individual participant data and useful additional data: this allowed us to make more analyses than would have been possible based only on published data.
Major limitations are: the small number of included studies, especially for serum bile acid profile components; the overall low quality of included studies due to a design that was not properly tailored for diagnostic accuracy, and inclusion of the index tests in the reference standard; the relatively small sample sizes of study participants; and the unexplained heterogeneity among studies on serum bile acid profile, most probably due to other possible sources of heterogeneity, not planned at the protocol stage.
Applicability of findings to the review question
The overall low quality, high risk of bias, and great heterogeneity of the results of some index tests prevents us from reaching definitive conclusions and making recommendations. Thus, at present, we do not find any compelling evidence to recommend or refute the routine use of any of these tests in clinical practice.
Authors' conclusions
Implications for practice.
So far, TSBA diagnostic accuracy for intrahepatic cholestasis of pregnancy might have been overestimated: TSBA can add some more information, but they should not be used as the most reliable and unique marker for the diagnosis of intrahepatic cholestasis of pregnancy. Our review suggests to obstetricians who suspect intrahepatic cholestasis of pregnancy in a pregnant woman claiming for pruritus that they may not base their evaluation only on TSBA levels. To consider all possible differential diagnoses with the use of other laboratory and imaging tests, consulting a hepatologist if needed, may be of help.
Implications for research.
As evidence on intrahepatic cholestasis of pregnancy is lacking and current clinical practice questionable, we need continuous primary clinical research. New insights into the pathogenesis of intrahepatic cholestasis of pregnancy may help to find out more accurate biomarkers for the disease, both diagnostic and prognostic. We need well‐designed diagnostic test accuracy studies with a cross‐sectional prospective design on pregnant women suspected for intrahepatic cholestasis of pregnancy (i.e. with onset of pruritus during pregnancy), assessing total serum bile acids (TSBA) or components of serum bile acid profile concentrations, independently or in combination, and comprehensive evaluation of fetal outcomes, with a clear and unbiased definition of the reference standard for the disease (with an eight weeks' follow‐up after delivery assessing normalisation of liver tests). This will allow estimating the diagnostic and prognostic accuracy of these tests with high quality of evidence. Such studies ought to be reported according to the STARD statement.
Acknowledgements
We thank Sarah Louise Klingenberg (Denmark) for designing search strategies, Arturo Martí‐Carvajal (Venezuela) for helping to define the search strategies for South American databases, and Maoling Wei (China) for searching for Chinese literature. We also thank Dario Conte (Italy) for firing the interest of the first listed author, CM, in systematic reviews and for very useful suggestions on the review.
We thank Dora Brites, José Garcia‐Flores, Nilgun Kutay Guducu, William Huang, Titta Joutsiniemi, Ayse Kirbas, Aneta Kowalska‐Kanka, Tim Lang, Efser Öztaş, Denis Roger, and Valeria Tripodi (study authors), for individual participant data and further information on their published studies. We thank Yannick Bacq, Tomáš Binder, Libor Vitek, Min Ding, Ronald Oude Elferink, Annarosa Floreani, Anna Locatelli, Monika Oktaba, Ramesh Saeedi, Dorothy Shaw, Rachel Tribe, Alexandra Bouzouki, Humberto Reyes, Laura Bull, and Denis Gallot for providing us with additional information on their published studies.
We thank the following for helping with language translations: Maoling Wei, China (Chinese); Radan Bruha, Czech Republic (Czech).
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
The Cochrane Hepato‐Biliary Group Diagnostic Test Accuracy Reviews Editorial Team Contact editors: Agostino Colli, Italy Sign‐off Editor: Agostino Colli, Italy
The Cochrane Diagnostic Test Accuracy Reviews Editorial Team Contact Editor: Agostino Colli, Italy
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategies |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | 6 May 2019 | (((bile or cholic or CA or glycocholic or GCA or choliglycine or chenodeox*cholic or CDCA or deox*cholic or DCA or lithocholic or LCA or ursodeox*cholic or UDCA or glyco‐conjugated or tauro‐conjugated or glycine or taurine) and acid*) AND ((cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum) AND (pregnan* or obstetric* or gestation*) |
| Cochrane Hepato‐Biliary Group Diagnostic Test Accuracy Studies Register | 6 May 2019 | (((bile or cholic or CA or glycocholic or GCA or choliglycine or chenodeox*cholic or CDCA or deox*cholic or DCA or lithocholic or LCA or ursodeox*cholic or UDCA or glyco‐conjugated or tauro‐conjugated or glycine or taurine) and acid*) AND ((cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum) AND (pregnan* or obstetric* or gestation*) |
| Cochrane Library | 2019, Issue 5 | #1 MeSH descriptor: [Bile Acids and Salts] explode all trees #2 ((bile or cholic or CA or glycocholic or GCA or choliglycine or chenodeox*cholic or CDCA or deox*cholic or DCA or lithocholic or LCA or ursodeox*cholic or UDCA or glyco‐conjugated or tauro‐conjugated or glycine or taurine) and acid*) #3 #1 or #2 #4 MeSH descriptor: [Cholestasis, Intrahepatic] explode all trees #5 (cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum #6 #4 or #5 #7 MeSH descriptor: [Pregnancy] explode all trees #8 pregnan* or obstetric* or gestation* #9 #7 or #8 #10 #3 and #6 and #9 |
| MEDLINE Ovid | 1946 to 6 May 2019 | 1. exp "Bile Acids and Salts"/ 2. ((bile or cholic or CA or glycocholic or GCA or choliglycine or chenodeox*cholic or CDCA or deox*cholic or DCA or lithocholic or LCA or ursodeox*cholic or UDCA or glyco‐conjugated or tauro‐conjugated or glycine or taurine) and acid*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 3. 1 or 2 4. exp Cholestasis, Intrahepatic/ 5. ((cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 6. 4 or 5 7. exp Pregnancy/ 8. (pregnan* or obstetric* or gestation*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 9. 7 or 8 10. 3 and 6 and 9 |
| Embase Ovid | 1974 to 6 May 2019 | 1. exp bile acid/ 2. ((bile or cholic or CA or glycocholic or GCA or choliglycine or chenodeox*cholic or CDCA or deox*cholic or DCA or lithocholic or LCA or ursodeox*cholic or UDCA or glyco‐conjugated or tauro‐conjugated or glycine or taurine) and acid*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp intrahepatic cholestasis/ 5. ((cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. 4 or 5 7. exp pregnancy/ 8. (pregnan* or obstetric* or gestation*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 9. 7 or 8 10. 3 and 6 and 9 |
| Science Citation Index Expanded (Web of Science) | 1900 to 6 May 2019 | #4 #1 AND #2 AND #3 #3 TS=(pregnan* or obstetric* or gestation*) #2 TS=((cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum) #1 TS=((bile or cholic or CA or glycocholic or GCA or choliglycine or chenodeox*cholic or CDCA or deox*cholic or DCA or lithocholic or LCA or ursodeox*cholic or UDCA or glyco‐conjugated or tauro‐conjugated or glycine or taurine) and acid*) |
| Conference Proceedings Citation Index – Science (Web of Science) | 1900 to 6 May 2019 | #4 #1 AND #2 AND #3 #3 TS=(pregnan* or obstetric* or gestation*) #2 TS=((cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum) #1 TS=((bile or cholic or CA or glycocholic or GCA or choliglycine or chenodeox*cholic or CDCA or deox*cholic or DCA or lithocholic or LCA or ursodeox*cholic or UDCA or glyco‐conjugated or tauro‐conjugated or glycine or taurine) and acid*) |
| BIOSIS Previews (Web of Science) | 1969 to 6 May 2019 | #4 458 #1 AND #2 AND #3 #3 406,795 TS=(pregnan* or obstetric* or gestation*) #2 26,078 TS=((cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum) #1 464,139 TS=((bile or cholic or CA or glycocholic or GCA or choliglycine or chenodeox*cholic or CDCA or deox*cholic or DCA or lithocholic or LCA or ursodeox*cholic or UDCA or glyco‐conjugated or tauro‐conjugated or glycine or taurine) and acid*) |
| CINAHL (EBSCOhost) | 1981 to 6 May 2019 | S14 64 S6 AND S9 S9 167,584 S7 OR S8 S8 167,584 TX pregnan* or obstetric* or gestation* S7 99,820 MW Pregnancy S6 2,167 S4 OR S5 S5 2,104 TX (cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum S4 149 MW Intrahepatic Cholestasis S3 3,538 S1 OR S2 S2 3,538 TX (bile OR cholic glycocholic OR GCA OR choliglycine OR chenodeox?cholic OR CDCA OR deox?cholic OR DCA OR lithocholic OR LCA OR ursodeox?cholic OR UDCA OR glyco?conjugated OR tauro?conjugated OR glycine OR taurine) S1 412 MW Bile Acids and Salts |
| CNKI (Chinese database) |
1979 to May 2019 | ((bile acid OR bile) AND pregnancy) OR ICP |
| VIP (Chinese database) |
1989 to May 2019 | (bile AND pregnancy) AND diagnosis |
| LILACS (VHL) | 6 May 2019 | 1. (tw:((tw:(cholestasis )) AND (tw:(pregnancy OR obstetric)))) OR (tw:((tw:( colestasis)) AND (tw:(gravídica OR (intrahepática AND embarazo) OR obstétrica)))) OR (tw:((tw:(ictericia)) AND (tw:(embarazo OR gravídica)))) OR (tw:((tw:(colestase)) AND (tw:(gravidez OR gestacional OR obstétrica)))) OR (tw:( (tw:(icterícia)) AND (tw:(gravidez OR colestática)))) AND (instance:"regional") AND ( db:("LILACS")) 2. (tw:(acidos biliares)) AND (tw:(embarazo OR gravidez OR obstétrica OR gestational OR gravidica)) AND (instance:"regional") AND ( db:("LILACS")) |
| SCIELO | 6 May 2019 | 1. ((cholestasis) AND (pregnancy OR obstetric) ) OR ((colestasis) AND (embarazo OR obstétrica) ) OR ((ictericia) AND (embarazo OR gravídica) ) OR ( (icterícia) AND (gravidez OR colestática)) OR ((colestase) AND (gravidez OR gestacional) ) 2. (bile acids) AND (pregnancy OR obstetric) 3. (acidos biliares) AND (embarazo OR gravidez OR obstétrica OR gestational OR gravidica) |
| Evidence search: Health and Social Care, RHL, TRIP, OpenSIGLE, NTIS | 6 May 2019 | cholestasis AND (obstetric OR pregnancy) AND (bile acid) |
Appendix 2. QUADAS‐2
| Domain | Participant selection | Index test | Reference standard | Flow and timing |
| Description |
Describe methods of participant selection: describe inclusion criteria for participants (prior testing, presentation, intended use of index test, and setting): The studies that fulfil the inclusion criteria of this review should have included as participants pregnant women recruited in any clinical setting. They should have been evaluated for personal history of skin or liver diseases, presence of pruritus during their pregnancy, and they should have been assessed with any most common liver test (or tests), followed by any of the already mentioned index tests (total bile acids, cholic acid, glycocholic acid, chenodeoxycholic acid, cholic/chenodeoxycholic acid ratio, deoxycholic acid, lithocholic acid, ursodeoxycholic acid, total glyco‐conjugated bile acids, total tauro‐conjugated bile acids, total glyco‐conjugated bile acids/total taurine‐conjugated bile acids ratio) |
Describe the index test and how it was be conducted and interpreted: Total bile acids, cholic acid, glycocholic acid, chenodeoxycholic acid, cholic/chenodeoxycholic acid ratio, deoxycholic acid, lithocholic acid, ursodeoxycholic acid, total glyco‐conjugated bile acids, total tauro‐conjugated bile acids, total glyco‐conjugated bile acids/total taurine‐conjugated bile acids ratio, are non‐ invasive laboratory serum tests performed after the first clinical evaluation of the pregnant women for the diagnosis of intrahepatic cholestasis of pregnancy. The serum concentration of the index test(s) can be assessed through different techniques. Laboratory methods and diagnostic cut‐off values could vary between different studies. |
Describe the reference standard and how it was conducted and interpreted: Clinical evaluation including the follow‐up after delivery. The clinical evaluation is the final judgment of the clinician who takes into account the clinical assessment of suggestive signs and symptoms for intrahepatic cholestasis of pregnancy and the presence of any otherwise unexplained, persistent abnormalities of AST, ALT, or bilirubin levels until delivery. The follow‐up after delivery is the assessment of spontaneous relief of symptoms and normalisation of liver tests within eight weeks at most. |
Describe any people who did not receive the index test(s) or reference standard (or both) or who will be excluded from the 2 x 2 table (refer to flow diagram): describe the time interval and any interventions between index test(s) and reference standard: As mentioned in the protocol, we will exclude participants who lack data for the two‐by‐two table. To define a time interval between our index tests and our reference standard is not relevant, as the index tests should be performed when the suspicion of intrahepatic cholestasis of pregnancy arises and the reference standard comprises the follow‐up after delivery. |
| Signalling questions: yes/no/unclear |
Was a consecutive or random sample of participants enrolled? Yes: all consecutive participants or random sample of people with suspected intrahepatic cholestasis of pregnancy were enrolled in the study. No: selected participants were not included. Unclear: insufficient data were reported to permit a judgement. |
Were the index test results interpreted without knowledge of the results of the reference standard? We think this will not be a relevant question for our review as the index tests are objective laboratory tests and the answer should always be 'Yes'. |
Is the reference standard likely to classify the target condition correctly? Yes: if participants underwent a through clinical evaluation excluding all possible differential diagnosis and if they underwent an adequate follow‐up after delivery assessing the spontaneous relief of symptoms and normalisation of the previously found abnormal liver tests. No: clinical evaluation including the follow‐up after delivery was not able to rule out other possible differential diagnosis. Unclear: insufficient data were reported to permit a judgement. |
Was there an appropriate interval between index test(s) and reference standard? This is not a relevant question to our review (see above). |
|
Was a case‐controldesign avoided? Yes: case‐control design was avoided. No: case‐control design was not avoided. Unclear: insufficient information was reported to permit a judgement. |
If a threshold was used, was it pre‐specified? Yes. No. Unclear: it is not reported or not clearly described. |
Were the reference standard results interpreted without knowledge of the results of the index test? Yes: clinical evaluation including the follow‐up after delivery was performed without knowledge of the results of total serum bile acids or any component of serum bile acid profile. No: clinical evaluation including the follow‐up after delivery was performed with knowledge of the results of total serum bile acids or any component of serum bile acid profile. Unclear: insufficient data were reported to permit a judgement. _________________ Was the index test evaluation not part of the reference standard? Yes: the index test evaluation was not part of the reference standard. No: index test evaluation was part of the reference standard. Unclear: insufficient data were reported to permit a judgement. |
Did all participants receive the reference standard? Yes: all participants underwent the reference standard, i.e., clinical evaluation including the follow‐up after delivery. No: not all participants underwent the reference standard, i.e., clinical evaluation including the follow‐up after delivery. Unclear: insufficient data were reported to permit a judgement. |
|
|
Did the study avoid inappropriate exclusions? Yes: the study avoided inappropriate exclusions (e.g., women having a previously assessed value of the index test(s) below a defined cut‐off). No: the study excluded participants inappropriately. Unclear: insufficient data were reported to permit a judgement. |
Did all participants receive the same reference standard? Yes: all participants received the same reference standard, i.e., clinical evaluation including the follow‐up after delivery. No: not all participants received the same reference standard, i.e., clinical evaluation including the follow‐up after delivery. Unclear: insufficient data were reported to permit a judgement. |
|||
|
Were all participants included in the analysis? Yes: all participants meeting the selection criteria (selected participants) were included in the analysis, or data on all the selected participants were available so that a 2 x 2 table including all selected participants could be constructed. No: not all participants meeting the selection criteria were included in the analysis or the 2 x 2 table could not be constructed using data on all selected participants. Unclear: insufficient data were reported to permit a judgement. | ||||
| Risk of bias: high/low/unclear |
Could the selection of participants have introduced bias? High risk of bias: yes, if the selection of participants have introduced bias. Low risk of bias: no, if the selection of participants have not introduced bias. Unclear risk of bias: insufficient data on participants selection were reported to permit a judgement on the risk of bias. |
Could the conduct or interpretation of the index test have introduced bias? High risk of bias: if the answer to the signalling questions on the conduct or interpretation of the index test is 'no'. Low risk of bias: if the answer to the signalling questions on the conduct or interpretation of the index test is 'yes'. Unclear risk of bias: if the answers to the two signalling questions on the conduct or interpretation of the index test is either 'unclear' or any combination of 'unclear' with 'yes' or 'no'. |
Could the reference standard, its conduct, or its interpretation have introduced bias? High risk of bias: if the answer to the signalling questions on the reference standard, its conduct, or its interpretation is 'no'. Low risk of bias: if the answer to the signalling questions on the reference standard, its conduct, or its interpretation is 'yes'. Unclear risk of bias: if the answers to the three signalling questions on the reference standard, its conduct, or its interpretation is either 'unclear' or any combination of 'unclear' with 'yes' or 'no'. |
Could the participant flow have introduced bias? High risk of bias: if the answer to the signalling questions on flow and timing is 'no'. Low risk of bias: if the answer to the signalling questions on flow and timing is 'yes'. Unclear risk of bias: if the answers to the 4 signalling questions on flow and timing is either 'unclear' or any combination of 'unclear' with 'yes' or 'no'. |
| Concerns regarding applicability: high/low/unclear |
Are there concerns that the included participants do not match the review question? High concern: there is high concern that the included participants do not match the review question. Low concern: there is low concern that the included participants do not match the review question. Unclear concern: if it is unclear. |
Are there concerns that the index test, its conduct, or interpretation differ from the review question? High concern: there is high concern that the conduct or interpretation of total serum bile acids or any component of serum bile acid profile differs from the way likely to be used in clinical practice. Low concern: there is low concern that the conduct or interpretation of total serum bile acids or any component of serum bile acid profile differs from the way likely to be used in clinical practice. Unclear concern: if it is unclear. |
Are there concerns that the target condition as defined by the reference standard does not match the review question? High concern: all participants did not undergo clinical evaluation including the follow‐up after delivery. Low concern: all participants undergo clinical evaluation including the follow‐up after delivery. Unclear concern: If it is unclear. |
‐‐ |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 TSBA (all studies) | 13 | 1645 |
| 2 TSBA cut‐off=10 μmol/L | 11 | 839 |
| 4 CA cut‐off=2 μmol/L | 4 | 312 |
| 5 CA cut‐off=3 μmol/L | 4 | 312 |
| 6 CA cut‐off=4 μmol/L | 4 | 312 |
| 7 CA cut‐off=5 μmol/L | 4 | 312 |
| 8 CDCA cut‐off=2 μmol/L | 4 | 312 |
| 9 CDCA cut‐off=3 μmol/L | 4 | 312 |
| 10 GCA (all studies) | 6 | 630 |
| 11 GCA cut‐off=0.7 μmol/L | 5 | 333 |
| 12 GCA cut‐off=1.5 μmol/L | 4 | 417 |
| 13 GCA cut‐off=2 μmol/L | 3 | 120 |
| 14 CA/CDCA cut‐off=1.8 | 4 | 312 |
| 15 TSBA cut‐off=10 μmol/L sensitivity excl TSBA in reference standard | 5 | 497 |
| 16 TSBA cut‐off=10 μmol/L sensitivity excl case‐control | 3 | 436 |
1. Test.
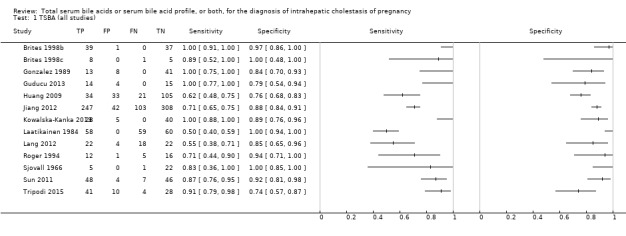
TSBA (all studies).
2. Test.
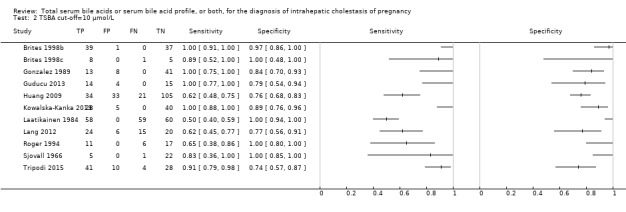
TSBA cut‐off=10 μmol/L.
4. Test.
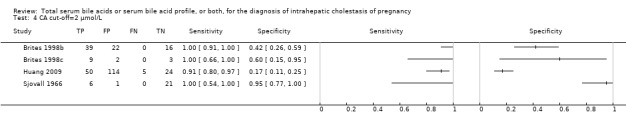
CA cut‐off=2 μmol/L.
5. Test.
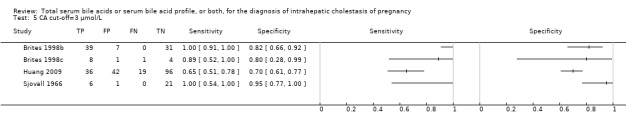
CA cut‐off=3 μmol/L.
6. Test.
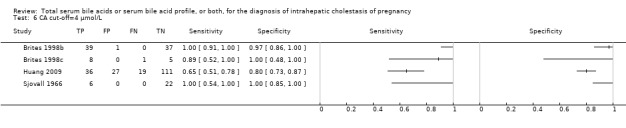
CA cut‐off=4 μmol/L.
7. Test.
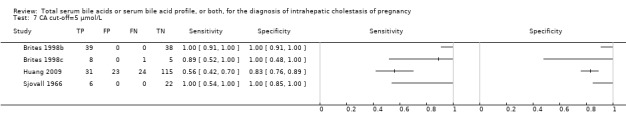
CA cut‐off=5 μmol/L.
8. Test.
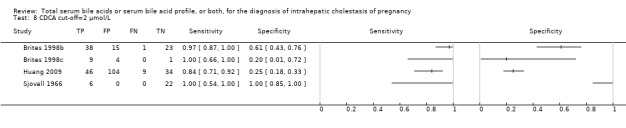
CDCA cut‐off=2 μmol/L.
9. Test.
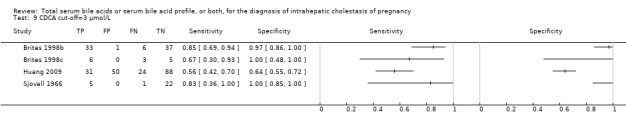
CDCA cut‐off=3 μmol/L.
10. Test.
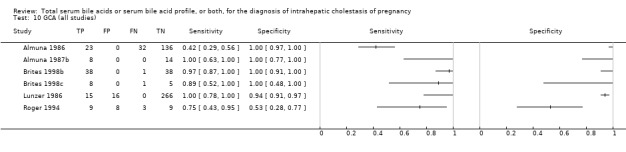
GCA (all studies).
11. Test.
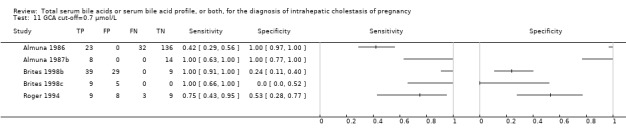
GCA cut‐off=0.7 μmol/L.
12. Test.
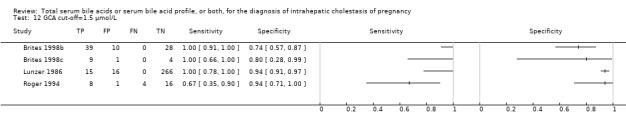
GCA cut‐off=1.5 μmol/L.
13. Test.

GCA cut‐off=2 μmol/L.
14. Test.
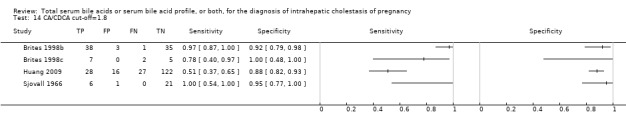
CA/CDCA cut‐off=1.8.
15. Test.
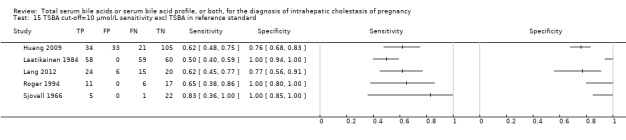
TSBA cut‐off=10 μmol/L sensitivity excl TSBA in reference standard.
16. Test.

TSBA cut‐off=10 μmol/L sensitivity excl case‐control.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almuna 1986.
| Study characteristics | |||
| Patient sampling | Case‐control, prospective, single centre study. Time span considered and total number of women screened for inclusion not provided. Cases: 55 pregnant women with pruritus (generalised or palm‐plantar, mostly at night) in the absence of other dermatological or allergic diseases, were enrolled as patients with ICP. Controls: 136 pregnant women without symptoms and without clinical and laboratory evidence of hepato‐biliary diseases, who did not take drugs for at least a week before the time of the assessment, were enrolled as controls. |
||
| Patient characteristics and setting | Setting: Hospital Militar de Santiago, Chile Characteristics of patients in case group: gestational age 28 to 39 weeks. Age of patients not provided. Characteristics of patients in control group: age 20 to 38 years old, gestational age 29 to 41 weeks |
||
| Index tests | Glycocholic acid, performed on serum samples of pregnant women at fasting (all participants) or postprandial (in all 55 patients and only 25 controls) status. Patients were tested when the suspicion of disease arose following pruritus. Laboratory technique: enzyme immunoassay ("ENDAB Cholyglicine, Enzime Immunoassay") Diagnostic cut‐off values: > 0.7 μg/L. Defined only after controls test results were found (their highest value was 0.66 μg/L). |
||
| Target condition and reference standard(s) | ICP and clinical diagnosis including follow‐up assessing spontaneous relief of pruritus after delivery, assessed by obstetricians. They were blind to the index test results. | ||
| Flow and timing | Glycocholic acid was performed at a fasting status in all participants after week 29 of pregnancy, and all of them were included in the analysis. At a postprandial status, it was performed in all 55 patients and only in 25/136 controls, and all of them were included in the analysis. All patients were followed‐up after delivery. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Component(s) of serum bile acid profile | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was the index test not part of the reference standard? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Almuna 1987b.
| Study characteristics | |||
| Patient sampling | Case‐control, prospective, single centre study Time span considered and total number of women screened for inclusion not provided. Cases: 22 pregnant women who underwent cesarean section after week 36 of pregnancy were enrolled at the time of delivery. 8 women were enrolled as patients with clinical diagnosis of ICP. They had pruritus, at least 3 previous determinations of fasting glycocholic acid > 0.7 µg/L, and absence of other possible maternal or fetal diseases. Controls: 14 women were enrolled, after exclusion of personal history of liver diseases, anaemia, diabetes, hypertension, urinary infections, Rh sensibilization, post‐term pregnancy, fetal distress or malformations. |
||
| Patient characteristics and setting | Setting: Military Hospital de Santiago, Chile Pregnant women enrolled at the time of cesarean delivery. They were pre‐selected with at least 3 previous determinations of fasting glycocholic acid > 0.7 µg/L |
||
| Index tests | Glycocholic acid (GCA) tested on serum fasting samples collected from pregnant women at the time of cesarean section (see notes) Laboratory technique: enzyme immunoassay ("ENDAB Cholyglicine, Enzime Immunoassay") Predefined cut‐off value: 0.7 µg/L Individual participant data regarding glycocholic acid assessment are given in the publication. |
||
| Target condition and reference standard(s) | ICP and clinical diagnosis including follow‐up after delivery assessing disappearance of pruritus. The index test was part of the reference standard, obstetricians who assessed the reference standard were not blind. | ||
| Flow and timing | Glycocholic acid performed at the time of cesarean section in all patients. Follow‐up after delivery assessing disappearance of pruritus in all patients | ||
| Comparative | |||
| Notes | The study analysed glycocholic acid concentration in maternal serum, cord blood and amniotic fluid tested on samples collected at the time of cesarean section. We considered for our analysis only maternal serum samples, according to our review question. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Component(s) of serum bile acid profile | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Brites 1998b.
| Study characteristics | |||
| Patient sampling | Case‐control, prospective, single centre study 39 pregnant women were enrolled as patients with ICP from the largest maternity ward and hospital in Lisbon (Portugal). They had: ‐ severe pruritus ‐ total serum bile acids > 10 µmol/l, with cholic acid representing > 40% ‐ at least one elevated among aminotransferases or bilirubin ‐ absence of any other known disease that could cause liver dysfunction (chronic liver disease, symptomatic cholelithiasis, viral hepatitis). The total number of woman screened for inclusion was not recorded. The prevalence of the disease at the time of the study was about 1.5%. 38 pregnant women were enrolled from "routine antenatal clinics" as controls. 20 healthy non‐pregnant women were also studied, but we didn't include them in our analysis following our inclusion criteria. Important data which could not be found in the publication were received by email from authors (see notes). |
||
| Patient characteristics and setting | Characteristics of patients in case group: 19 to 41 years old, at their 33.4 ± 4.4 week of pregnancy. They were pre‐selected for having total serum bile acids > 10 µmol/l, of which cholic acid representing > 40%. Characteristics of patients in control group: 18 to 44 years old, at their 30.0 ± 6.6 week of pregnancy. |
||
| Index tests | Total serum bile acids (TSBA), cholic acid (CA), glycocholic acid (GCA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), total glyco‐conjugated acids (G‐c), total tauro‐conjugated acids (T‐c), CA/CDCA, G‐c/T‐c, evaluated on serum fasting samples taken at the time of diagnosis (see notes) Laboratory techniques used:
NB: in the study, "unconjugated ursodeoxycholic acid was not individually quantified because it does not separate from chenodeoxycholic acid during thin‐layer chromatography". Cut‐off value for total serum bile acids: > 10 µmol/l, predefined. Cut‐off values (in µmol/l) for other index tests: GCA > 2, CA/CDCA > 1.5, G‐c/T‐c < 1.0. All defined only after data analysis. |
||
| Target condition and reference standard(s) | ICP and clinical diagnosis including the follow‐up after delivery showing disappearance of pruritus and normalisation of bile acids and previously found abnormal liver tests within 1 month. | ||
| Flow and timing | Index tests performed in all participants (in patients, at the time of diagnosis). All patients were followed up after delivery for 1 month. | ||
| Comparative | |||
| Notes | Following e‐mail correspondences with Dora Brites (see study references), we received detailed inclusion criteria and individual participant data, regarding laboratory findings needed for our analysis. In particular, individual data received were: serum concentrations of total bile acids (TSBA), glyco/tauro/free cholic acid (GCA,TCA, fCA), glyco/tauro/free chenodeoxycholic acid (GCDCA, TCDCA, fCDCA), glyco/tauro/free deoxycholic acid (GDCA, TDCA, fDCA), glyco/tauro ursodeoxycholic (GUDCA, TUDCA) and glyco/tauro/free lithocholic acid (GLCA, TLCA, fLCA). Using received data, we calculated:
We did not consider the free forms of CA and UDCA in the sums described above, in order to allow a comparison of the value of CA and CDCA provided by these studies with values provided in the other studies assessing their diagnostic accuracy; in fact, owing to the techniques used, they measured only the sum of conjugated forms of CA and CDCA, without their free form. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Component(s) of serum bile acid profile | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Brites 1998c.
| Study characteristics | |||
| Patient sampling | case‐control, prospective, multicentre study Pregnant women attending two centres from 1994 to 1997 were considered for inclusion (total number screened not provided). Cases: 16 women with ICP were enrolled as patients. They had:
Of these patients, 7 patients were treated with UDCA therapy (14 mg/Kg/day, from 21 days before the term to the time of delivery), while 9 patient were not treated. We received additional information from authors (see notes). Controls: 5 healthy pregnant women at full‐term gestation, without history of hepatic disease or preeclampsia, were voluntary enrolled as controls. |
||
| Patient characteristics and setting | Setting: Santa Maria Hospital, Garcia de Orta Hospital and Dr. Alfredo da Costa Maternity Ward (Portugal) Characteristics of patients in case group: 16 to 35 years old, 28 to 37 week of pregnancy at the time of diagnosis. They were pre‐selected for having total serum bile acids > 10 µmol/L, with cholic acid representing > 40%. |
||
| Index tests | Total serum bile acids and cholic acid (CA), glycocholic acid (GCA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), total glyco‐conjugated (G‐c) and total tauro‐conjugated (T‐c) acids, CA/CDCA, G‐c/T‐c (see notes) The index tests were assessed on serum fasting samples taken at the time of delivery. Laboratory techniques:
NB: in the study, "unconjugated ursodeoxycholic acid was not individually quantified due to difficult separation from chenodeoxycholic acid during thin‐layer chromatography". Diagnostic cut‐off value for TSBA: 10 µmol/l Cut‐off values not provided for any of the other index tests For total serum bile acid analysis, we included in our analysis all 21 selected participants. For serum bile acid profile components analysis, we obtained individual data (received from authors, see notes) for only the 9 non‐treated patients and the 5 controls. |
||
| Target condition and reference standard(s) | ICP and clinical evaluation including follow‐up after delivery (see notes) | ||
| Flow and timing | Index tests and reference standard performed in all participants at the time of delivery Follow‐up after delivery not performed in all patients (see notes) |
||
| Comparative | |||
| Notes | The primary aim of the study was to investigate bile acid concentration in colostrum of women with ICP, and to compare them with serum concentrations. We considered only data regarding concentration of the index tests performed on maternal serum. Following e‐mail correspondences with Dora Brites (see study references), we received: 1. detailed inclusion criteria for participants 2. individual data of the 9 non‐treated patients and 5 controls Among the individual data we received, were: serum concentrations of total bile acids (TSBA), glyco/tauro/free cholic acid (GCA,TCA, fCA), glyco/tauro/free chenodeoxycholic acid (GCDCA, TCDCA, fCDCA), glyco/tauro/free deoxycholic acid (GDCA, TDCA, fDCA), glyco/tauro ursodeoxycholic (GUDCA, TUDCA) and glyco/tauro/free lithocholic acid (GLCA, TLCA, fLCA). Using received data, we calculated:
We did not consider the free forms of CA and UDCA in the sums described above, in order to allow a comparison of the value of CA and CDCA provided by these studies with values provided in the other studies assessing their diagnostic accuracy: in fact, owing to the techniques used, they measured only the sum of conjugated forms of CA and CDCA, without their free form. 3. Information on follow‐up after delivery to assess the spontaneous relief of symptoms and normalization of liver tests: "some patients did not return to the hospital to see whether they have returned to normal. However, the majority were evaluated as accomplishing this item". |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Component(s) of serum bile acid profile | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Gonzalez 1989.
| Study characteristics | |||
| Patient sampling | Cross‐sectional, prospective, single centre study All 10,711 pregnant women admitted to the centre during a 12‐month period between the years 1983 and 1984, were serially evaluated and followed up during their pregnancy for the diagnosis of ICP. Among the women with twin pregnancies, only the 62 who underwent complete physical examination and clinical and laboratory follow‐up until one week post‐partum were consecutively enrolled in the study (see notes). The prevalence of the disease obtained in the study was about 20.9% among twin pregnancies and 4.7% among singleton pregnancies. |
||
| Patient characteristics and setting | Setting: Hospital del Salvador (Chile) Characteristics of patients: 66 had twin pregnancy, and 10,645 had singleton pregnancy. Singleton pregnancies were excluded. |
||
| Index tests | Total serum bile acids. The test was serially performed during a follow‐up of each pregnant woman until 1 week post‐partum. The values provided in the publication are the highest found during pregnancy (peak values). Laboratory technique: enzymatic method of Mashige et al (Mashige 1976) Predefined diagnostic cut‐off value: 10 µmol/L |
||
| Target condition and reference standard(s) | ICP and clinical diagnosis based on widespread skin pruritus, appearing in the second half of pregnancy and lasting until delivery, with total serum bile acids above 10 µmol/L, exclusion of other cholestatic diseases, follow‐up after delivery assessing spontaneous relief of symptoms and normalisation of previously found abnormal liver function tests. Reference standard assessed by obstetricians, who were not blind for the index test. | ||
| Flow and timing | Every participant was serially evaluated and followed up during pregnancy, with complete physical examination and clinical and laboratory tests until 1 week after delivery. The values provided for the index test are the peak values found during pregnancy. | ||
| Comparative | |||
| Notes | The study included all twin pregnancies recruited consecutively during a 1‐year period. Among the 10,645 singleton pregnancies admitted in the same period at the hospital, 492 were diagnosed with ICP, but authors included only 14 of them in their analysis, without explaining the criteria used to select them. We decide to exclude them from our analysis as their enrolment was not comparable to that used for twins. We do not know if the exclusion of singleton pregnancies and an analysis based only on twins may affect the applicability of the findings to clinical practice. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Guducu 2013.
| Study characteristics | |||
| Patient sampling | Case‐control, retrospective, single centre study Data of women who delivered at the centre between January 2008 and May 2013 were screened for inclusion (total number not provided). Cases: 20 women were enrolled as patients with ICP, for having pruritus with elevated total serum bile acids (> 10 µmol/L) or elevated liver aminotransferases, or both, in the absence of other possible liver diseases. Controls: 21 healthy pregnant women without pruritus were enrolled as controls. Exclusion criteria for participants were: diabetes mellitus, hypertensive diseases of pregnancy, diseases of thyroid gland, haematological abnormalities, use of drugs other than multivitamins or iron. |
||
| Patient characteristics and setting | Setting: Istanbul Bilim University Avrupa Florence Nightingale Hospital (Turkey) Characteristics of patients in case group: pre‐selected for having total serum bile acids > 10 µmol/L Women with diabetes mellitus, hypertensive diseases of pregnancy, diseases of thyroid gland, haematological abnormalities, use of drugs other than multivitamins of iron were excluded from the study. All participants were between 24 and 38 years old and after their 25th week of pregnancy. |
||
| Index tests | Total serum bile acids. Assessed on serum fasting samples. In patients, serum sample was taken at the time of onset of symptoms. Predefined diagnostic cut‐off value: 10 µmol/L (see notes) Laboratory technique: enzymatic assay (see notes) |
||
| Target condition and reference standard(s) | ICP and clinical evaluation including follow‐up after delivery assessing relief of symptoms and normalisation of liver tests in all patients. | ||
| Flow and timing | Index test performed after onset of pruritus (after week 25 of pregnancy). Follow‐up after delivery assessing relief of symptoms and normalisation of liver tests in all patients. Exclusion of 6 patients and 2 controls for lacking data on total serum bile acids, thus leading to a final inclusion in the analysis only 14 patients and 19 controls. |
||
| Comparative | |||
| Notes | Following e‐mail correspondences with Nilgun Kutay Guducu (see references), we received the information listed below.
|
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Huang 2009.
| Study characteristics | |||
| Patient sampling | Cross‐sectional, retrospective multicentric study All serum samples with complete bile acids and transaminases analysis from pregnant women attending clinics and hospitals of a corporation and sent to a unique laboratory between 1 January 2006 and 31 December 2006 were retrospectively considered. Authors assumed that bile acid testing was requested because of suspicion of ICP. Of 262 specimens, only 231 specimens from 208 pregnant women had complete data regarding bile acids and transaminases and were included (see notes). Having received individual participant data from authors, we decided to include only 1 specimen for each of the 208 women considered: in particular, whithin the database given by authors, we considered the first written specimen for each ID with multiple determinations, assuming that it was the first one collected as soon as the suspicion of diseases arose (see notes). Hence, the number of specimens finally included was 193. |
||
| Patient characteristics and setting | Setting: clinics and hospitals of Health and Hospital Corporation (New York). Serum samples sent to Quest Diagnostic Nichols Institute, San Juan Capistrano, CA, USA Serum samples were included if complete bile acids and transaminase data were available, assuming that they were performed because of the suspicion of ICP. In our opinion, the retrospective study design starting from laboratory test requests on serum samples, instead of starting from clinical suspicion of disease, does not allow a clear evaluation of the applicability of participant selection. |
||
| Index tests |
Total serum bile acids, CA/CDCA (published data). CA, CDCA, DCA (unpublished data, see notes) Diagnostic cut‐off values provided in the publication ("supplied by the laboratory"): TSBA > 19.2 µmol/l, CA/CDCA > 3.4. Serum samples were analysed at Quest Diagnostic Nichols institute, San Juan Capistrano, CA, USA. Through the web site of the laboratory (www.questdiagnostics.com/testcenter/BUOrderInfo.action?tc=8482N&labCode=SJC) we found:
|
||
| Target condition and reference standard(s) | ICP and clinical evaluation including a "general evaluation for resolution of symptoms at the post‐partum visit" (see notes) | ||
| Flow and timing | It is assumed that index tests were performed when the suspicion of ICP came out, but not provided any specific time point. A "general evaluation for resolution of symptoms at the post‐partum visit" took place (see notes). | ||
| Comparative | |||
| Notes | Following email correspondences with William Huang (see study references), we received the following information.
On the basis of individual participant data, we decided to redefine the index tests cut‐off values and the reference standard. The reference standard for the target condition as defined by authors was any elevation of total serum bile acids (> 19.2 µmol/L), or CA/CDCA (> 3.4) or liver biochemistry tests (ASP > 39 U/L, ALT > 35 U/L), assuming that they were performed in the suspicion of disease. This do not match with our review question. So, having received individual participant data from authors, we decided to redefine the reference standard as any elevation of serum aminotransferases (ASP>39 or ALT>35, or both) and clinical evaluation including "resolution of symptoms at the postpartum visit" (that took place for all patients, as supplied by authors), assuming (as authors did) that participants who underwent bile acid testing had suspicious symptoms for intrahepatic cholestasis. Moreover, we redefined the diagnostic cut‐off values as follows.
|
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Component(s) of serum bile acid profile | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was the index test not part of the reference standard? | Yes | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Jiang 2012.
| Study characteristics | |||
| Patient sampling | Case‐control, retrospective, single centre study Among 4930 total deliveries taking place at 1 hospital between January 2010 and August 2011, 350 pregnant women with ICP (incidence 7.1%) were enrolled as cases, and 350 pregnant women with uncomplicated pregnancies were enrolled as controls. Inclusion criteria for pregnant women diagnosed with ICP were:
|
||
| Patient characteristics and setting | Setting: First Affiliated Hospital, Chongqing (China) Characteristics of patients in case group: 18 to 45 years old Characteristics of patients in control group: 19 to 42 years old |
||
| Index tests | Total serum bile acid. Not mentioned if serum samples were taken at fasting or post‐prandial status of pregnant women. Laboratory technique: automatic biochemical Analyzer (7170A) which uses an enzymatic method. Diagnostic cut‐off value: not predefined, but derived from ROC curves as > 11 µmol/L. Provided sensitivity: 70.6% Provided specificity: 88.0% |
||
| Target condition and reference standard(s) | ICP and clinical evaluation comprising follow‐up after delivery assessing spontaneous relief of symptoms and normalisation of liver tests. Reference standard assessed by obstetricians, who were not blind for the index test as it was part of their evaluation. | ||
| Flow and timing | All patients were assessed with total bile acids at the time of onset of symptoms and were followed up after delivery to assess spontaneous relief of symptoms and signs. | ||
| Comparative | |||
| Notes | The translation of the article and the extraction of data was possible thanks to Ms Maoling Wei. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kowalska‐Kanka 2013.
| Study characteristics | |||
| Patient sampling | case‐control, retrospective, single centre study Among all pregnant women (total number not provided) attending 1 institute between March 2009 and September 2011, 33 were enrolled as patients for having pruritus, elevated aminotransferases, total total serum bile acids > 11 µmol/L, absence of other liver diseases. 40 women with uncomplicated pregnancy and fetal well‐being were selected as controls. The ethnicity of all women was Caucasian. Exclusion criteria for all participants were anaemia, kidney disease, bone diseases, acute or chronic bleedings in pregnancy, and pre‐eclampsia. |
||
| Patient characteristics and setting | Setting: Institute of Mother and Child, Warsaw (Poland) Characteristics of patients in case group: 21 to 42 years old, 8 twin pregnancies and 25 singleton; pre‐selected for having total serum bile acids > 11 µmol/L. Characteristics of patients in control group: 20 to 40 years old, 6 twin pregnancies and 34 singleton Exclusion criteria for all participants were anaemia, kidney disease, bone diseases, acute or chronic bleedings in pregnancy, and pre‐eclampsia (see notes). |
||
| Index tests | Total serum bile acids. Samples taken at a fasting status. Value provided for TSBA are peak values found among multiple assessments during pregnancy. Laboratory technique: enzymatic colorimetric assay (Randox bile acids kit and reagent) Predefined cut‐off value: 11 µmol/L |
||
| Target condition and reference standard(s) | ICP and clinical evaluation including an assessment with "laboratory tests of hepatic parameters (AST, ALT, bilirubin)" that "were performed two weeks after delivery" (information received by authors, see notes). Reference standard performed by the obstetrician, who was not blind for the index test as it was part of his evaluation. | ||
| Flow and timing | TSBA values provided are peak values among multiple assessments during pregnancy, found at week 30 to 38 of pregnancy in patients and at week 31 to 40 in controls (see notes). Assessment of liver tests' normalisation performed 2 weeks after delivery in all patients (see notes). | ||
| Comparative | |||
| Notes | The aim of the study was to assess the correlation between bile acids and erythropoietin in pregnant women with ICP. Thus, selected participant may not match properly our review question. Following email correspondences with Aneta Kowalska‐Kanka (see study references), we received the information listed below.
|
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Laatikainen 1984.
| Study characteristics | |||
| Patient sampling | Case‐control, retrospective, single centre study All 117 pregnant women referred because of pruritus and elevated serum aminotransferases to one hospital during 1980 to 1981 were included as cases (total number screened for inclusion not provided). 60 healthy pregnant women were included as controls. |
||
| Patient characteristics and setting | Setting: Helsinki University Central Hospital, Helsinki (Finland) Characteristics of patients in case group: mean age 30.2 years Characteristics of patients in control group not provided (only said that they were "healthy") |
||
| Index tests | Total serum bile acids. The values provided in the publication were performed 1 week before delivery. Not provided if serum samples were taken at a fasting or postprandial status. Technique: enzymatic method The range of normal values provided by the publication for controls is 0 to 5 µmol/L, but it is not clear if a concentration above 5 µmol/l was considered diagnostic. Thus, as data concerning this cut‐off value were insufficient to complete a two‐by‐two table, we redefined a diagnostic cut‐off value of 10 µmol/L (see notes). |
||
| Target condition and reference standard(s) | ICP and clinical evaluation including follow‐up after delivery assessing normalization of symptoms and laboratory tests. The reference standard was assessed by obstetricians, who were not blind for the index test. | ||
| Flow and timing | Total serum bile acid values provided in the publication were collected 1 week before delivery in all pregnant women diagnosed with ICP. A follow‐up after delivery assessing normalization of symptoms and laboratory tests was performed in all patients. | ||
| Comparative | |||
| Notes | The diagnostic cut‐off value for total serum bile acids provided in the publication is not clear, as authors only stated that the normal range found in the control group was between 0 and 5 µmol/L. For case group, it is provided only a table with the description of the 3 subgroups in which women were divided. The 3 subgroups were defined on total serum bile acids concentrations: group A (TSBA < 10 µmol/L), with 59 women; group B (TSBA from 10 to 40 µmol/L), with 37 women; group C (TSBA > 40 µmol/L) with 21 women. It is not clear if group A had a serum bile acid level between 5 and 10 or between 0 and 10 µmol/L. We therefore decided to consider as diagnostic a cut‐off value of 10 µmol/L, in order to have complete and unquestionable data to complete a two‐by‐two table. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Lang 2012.
| Study characteristics | |||
| Patient sampling | Cross‐sectional, retrospective, single centre study A retrospective audit of bile acids performed in all pregnant women suspected of ICP attending 1 centre between February 2009 and January 2010 was performed. 118 bile acid requests (associated with ALT) for 70 women were found, and linked to the patients' charts to assess the final diagnosis (see notes). Complete data to allow our analysis were available for 66 women (4 women lacked serum bile acid assessment result, see notes). |
||
| Patient characteristics and setting | Setting: Royal Jubilee Maternity Hospital, Belfast (UK) The treatments received by women diagnosed with ICP were: 53% vitamin K, 63% UDCA, 33% topical therapy, 65% anti‐histamines. The ethnicity of participants was mostly 'white', except for 3 women coming from India (1), Pakistan (1) and Nepal (1). |
||
| Index tests | Total serum bile acids. Assessed on serum fasting samples taken at the time of diagnosis Laboratory technique: Randox Total Bile Acid kit (enzymatic colorimetric method) Cut‐off value provided in published poster: 14 µmol/l (authors sent us individual patients data and their analysis, providing sensitivity and specificity calculated at each possible cut‐off value) |
||
| Target condition and reference standard(s) | ICP and RCOG Green‐top guideline n.43 diagnostic criteria (Green‐top Guideline No.43), assessed by obstetricians, who were not blind for the index test. | ||
| Flow and timing | Total bile acids were performed at the time of diagnosis (between week 28 to 40 of pregnancy, except for 2 patients before week 28) or they were assessed multiple times during pregnancy (see notes). The diagnosis was retrospectively confirmed viewing the women's charts and following the RCOG Green‐top guideline n.43 criteria (Green‐top Guideline No.43). All data from participants meeting the inclusion criteria were analysed. | ||
| Comparative | |||
| Notes |
|
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Lunzer 1986.
| Study characteristics | |||
| Patient sampling | Cross‐sectional, prospective, single centre study All pregnant women attending 1 hospital between March 1981 and December 1982 were asked to participate (total number not provided). 297 pregnant women were enrolled. They were followed up during pregnancy with at least 5 visits, which included clinical evaluation and serum testing for glycocholic acid (GCA) and liver biochemistry tests. The clinical evaluation assessed if cholestatic symptoms, intake of drugs or pruritus were present. There were no women with known hepato‐biliary, intestinal, pancreatic disease or with history of alcohol abuse. |
||
| Patient characteristics and setting | Setting: Royal North Hospital (Australia) Characteristics of patients: they were 16 to 42 years old, and their ethnicity was most often Mid‐European (267 women), Mediterranean or Asian. |
||
| Index tests | Glycocholic acid (GCA). Assessed on serum sample, taken at a post‐prandial status (2 h after a standard meal with 18 g of fat). Laboratory technique: radioimmunoassay (Abbott Diagnostics, North Chicago,III) (see notes) Diagnostic cut‐off values: GCA > 1.5 µmol/L (see notes) |
||
| Target condition and reference standard(s) | ICP and follow‐up during pregnancy with at least 5 visits, including clinical evaluation by the obstetrician and laboratory testing for glycocholic acid (GCA) and liver biochemistry tests. A decrease in serum glycocholic acid levels was assessed in all women 48 or 72 hours after delivery. NB: The assessment of raised GCA levels (the index test of the study) was included in the reference standard (see notes). The index test was part of the reference standard, assessed by obstetricians. |
||
| Flow and timing | Included pregnant women were assessed for pruritus and glycocholic acid after week 28 of pregnancy. They were followed up during pregnancy with multiple visits and until 48 or 72 hours after delivery to assess GCA levels reduction. | ||
| Comparative | |||
| Notes |
|
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Component(s) of serum bile acid profile | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Roger 1994.
| Study characteristics | |||
| Patient sampling | Case‐control, prospective, multicentric study Among the 3192 pregnant women who attended 2 hospitals between 1 November 1988 and 31 October 1989, 51 of them were claiming for pruritus during pregnancy or immediately post‐partum (< 7days) without previous other dermatological or systematic diseases. After the exclusion of 2 women, whose pruritus was unrelated to pregnancy, 49 women were enrolled in the study, and divided in different groups according to their final diagnosis. Case group was represented by 17 women who were diagnosed with “pruritus gravidarum”. These women were matched with 17 healthy pregnant controls taken by random sampling from excluded women with uncomplicated pregnancy. As the definition of "pruritus gravidarum" provided by the study matched our definition of ICP (see notes), we included the 17 woman with this diagnosis as patients. Their 17 matched controls were included as well. The prevalence of “pruritus gravidarum” as defined by article was 0.53%. |
||
| Patient characteristics and setting | Setting: Hôpital Trousseau and Hôpital Bretonneau and Beffroi, Tours (France) All participants were at their 26th to 40th week of pregnancy and there were 5 multiple and 12 singleton pregnancies in both groups. |
||
| Index tests | Total serum bile acids (TSBA), performed in all participants, and glycocholic acid (GCA), performed in all controls and 12 patients. Both index tests were assessed at the time of onset of symptoms. Laboratory technique:
Predefined diagnostic cut‐off values: TSBA > 6 µmol/L, GCA > 0.7 µg/L Further information not available in the publication and Individual participant data were received from authors (see notes). |
||
| Target condition and reference standard(s) | ICP (see notes) and clinical evaluation including laboratory serum testing and liver ultrasounds to exclude other differential diagnosis and follow‐up after delivery assessing spontaneous relief of pruritus (see notes). Reference standard assessed by obstetricians who were blind for the index test. | ||
| Flow and timing | Index test performed when the suspicion of disease arose in patients. All participants were at their 26th to 40th week of pregnancy. All participants underwent TSBA testing, while only 12 patients and all controls underwent GCA testing. A follow‐up after delivery assessed the spontaneous relief of symptoms in all patients (information received from authors, see notes) | ||
| Comparative | |||
| Notes | We received from Denis Roger (see study references) the full‐text paper of the study, where we took into account the following information: 1. Unpublished criteria for the diagnosis of "pruritus gravidarum":
2. The diagnostic criteria were assessed through a clinical evaluation including drug intake history, laboratory serum tests and liver ultrasounds. 3. A follow up after delivery assessed the spontaneous relief of symptoms in all patients. 4. The diagnostic cut‐off values for the index tests were: TSBA > 6 µmol/L, GCA > 0.7 µg/L. 5. TSBA were performed in all participants, while GCA only in 12 patients and all controls. 6. Individual participant data. The criteria for the diagnosis of "pruritus gravidarum" described above, match our protocol definition of ICP. Thus, we decided to include these women as patients diagnosed with ICP. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Component(s) of serum bile acid profile | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Was the index test not part of the reference standard? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Sjovall 1966.
| Study characteristics | |||
| Patient sampling | Case‐control, prospective, single centre study. 6 pregnant women with generalised pruritus in the last trimester of pregnancy, 1 of whom had jaundice, were enrolled as patients with "pruritus in pregnancy" (see notes). 22 pregnant women defined as "healthy" were enrolled as controls. |
||
| Patient characteristics and setting | Setting: Karolinska Institute ‐ Department of Obstetrics and Gynaecology, Stockholm, Sweden Characteristics of control group not specified in the publication (said only they were "healthy") |
||
| Index tests | Total serum bile acid (TSBA), cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA). All index tests were performed on serum fasting samples. Index tests were assessed once (at the time of onset of pruritus, week 30 to 38) in 4 patients, twice in 1 patient (week 38 and 39), 4 times (weeks 34, 38, 39, 40) in 1 patient, and once (week 14 to 39) in most controls. We considered for all patients and controls only the first assessment. Laboratory technique: gas‐liquid chromatography Predefined diagnostic cut‐off values: not provided for any index test |
||
| Target condition and reference standard(s) | ICP and clinical evaluation including follow‐up after delivery assessing spontaneous relief of pruritus. Not provided any information about other liver tests assessments during pregnancy (except for 3 patients) and if/how other possible liver or skin diseases which could explain pruritus were ruled out (see notes). | ||
| Flow and timing | Index tests were assessed once (at the time of onset of pruritus, week 30 to 38) in 4 patients, twice in 1 patient (week 38 and 39), 4 times (weeks 34, 38, 39, 40) in 1 patient, and once (week 14 to 39) in most controls. We considered for all patients and controls only the first assessment. A follow‐up after delivery assessed spontaneous relief of pruritus in all 6 patients (within 1 week after delivery), while the normalisation of liver tests was checked only in 2 patients. | ||
| Comparative | |||
| Notes | The publication is entitled "Serum bile acid levels in pregnancy with pruritus". No information was provided about liver tests assessments during pregnancy (except for 3 patients) and if/how other possible liver or skin diseases which could explain pruritus were ruled out. However, authors associated the study patients with women diagnosed with "idiopathic jaundice of pregnancy" or "recurrent jaundice of pregnancy, "endogenous hepatotoxaemia of pregnancy", or "hepatosis of pregnancy", which were all the old names for ICP between the 1930s and 1960s. In addition, after their findings, they write in the discussion that "serum bile acid pattern in pregnant women with pruritus is therefore in agreement with the results of other liver function tests and histologic findings which indicate that this condition is associated with cholestasis". Moreover, this study is one of the most cited among publications on ICP to support the use of bile acids for the diagnosis of the disease. Therefore, despite the scanty information provided by the publication on participants' characteristics and how they were tested, we decided to include this study in our review as it seems to match our review question. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Component(s) of serum bile acid profile | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Sun 2011.
| Study characteristics | |||
| Patient sampling | Case‐control, retrospective, single centre study The study enrolled pregnant women attending 1 hospital between October 2008 and May 2009. 55 pregnant women were enrolled as patients with ICP, while 50 "healthy" pregnant women were enrolled as controls. |
||
| Patient characteristics and setting | Setting: Henan Oil Field Hospital (China) Characteristics of patients in case group: 21 to 34 years old, in their 28th to 38th week of pregnancy Characteristics of patients in control group: 20 to 28 years old, in their 20th to 30th week of pregnancy The different gestational age between groups may not lead to comparability of obtained results. |
||
| Index tests | Total serum bile acids Predefined diagnostic cut‐off value: 20 µmol/l Laboratory technique: enzymatic colorimetric assay (automatic analyzer Aeroset 2000) Provided sensitivity: 87.5%. Provided specificity: 91.2 % |
||
| Target condition and reference standard(s) | ICP and diagnostic criteria provided by a Chinese obstetrics and gynaecology book (see notes), assessed by obstetricians who were not blind for the index test. | ||
| Flow and timing | Total serum bile acids were performed at the time of onset of symptoms in patients. Follow‐up after delivery assessing normalisation of signs and symptoms was part of the reference standard (see notes). | ||
| Comparative | |||
| Notes | ICP was diagnosed following criteria provided by the Chinese obstetrics and gynaecology book 曹泽毅. 中华妇产科学. 人民卫生出版社 [Cao Zeyi. Chinese Obstetrics and Gynecology. People's Health Publishing House] 1999; 501‐507. We were helped by Ms Maoling Wei to translate the paragraph concerning diagnostic criteria for ICP, which were:
|
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Tripodi 2015.
| Study characteristics | |||
| Patient sampling | All 5 publications cited under the reference ID Tripodi 2015 (Included studies section) were based on the database we received by email from Valeria Tripodi (see notes). Case‐control, prospective, single centre study. Between January 2004 and June 2005, all pregnant women attending 1 hospital in their second half of pregnancy were screened for inclusion. Cases: 45 woman diagnosed with ICP for having pruritus, elevated aminotransferases, total serum bile acids > 11 µmol/l, absence of other known diseases (infectious, autoimmune, or skin diseases, alcohol intake, biliary obstruction). They all received UDCA treatment. Controls. 30 women among those clinically defined as healthy, were enrolled as controls. The calculated prevalence of ICP was 1.04%. |
||
| Patient characteristics and setting | Setting: Hospital JM Penna, Argentina (2596 yearly deliveries, thus meaning about 3894 estimated deliveries in the 18 months considered) Characteristics of patients in case group: 27.3 ± 1.0 years old, pre‐selected for having total serum bile acids > 11 µmol/L Characteristics of patients in control group: 26.1 ± 1.4 years old |
||
| Index tests | Total serum bile acid, lithocholic acid (LCA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), cholic acid (CA), ursodeoxycholic acid (UDCA), total tauro‐conjugated/total glyco‐conjugated bile acids (T‐c/G‐c). For TSBA, we received individual participant data (see notes). Laboratory technique: micellar electrokinetic chromatography with UV detection, on fasting serum sample Predefined diagnostic cut‐off values
|
||
| Target condition and reference standard(s) | ICP and clinical evaluation including assessment of normalisation of symptoms and liver tests after delivery. Reference standard assessed by obstetricians, who were not blind for the index test as it was part of their evaluation | ||
| Flow and timing | Assessment of total serum bile acids after onset of symptoms in all patients. Follow‐up assessing normalization of symptoms and liver biochemistry abnormalities after delivery in all patients. | ||
| Comparative | |||
| Notes | We received further information on the study and a complete database from which all participant data were taken by email from Valeria Tripodi (see Included studies). One of the publications defined the study design as 'cross‐sectional' but, following its design description and data received from authors, CM and TS agreed in defining it as 'case‐control'. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test Total serum bile acids | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Component(s) of serum bile acid profile | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Was the index test not part of the reference standard? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
ALT: alanine aminotransferase; AST: aspartate aminotransferase: CA: cholic acid; CDCA: chenodeoxycholic acid; CM: Cristina Manzotti (study author); DCA: deoxycholic acid; fCA: free (non‐conjugated) cholic acid; fCDCA: free (non‐conjugated) chenodeoxycholic acid; fDCA; free (non‐conjugated) deoxycholic acid; fLCA: free (non‐conjugated) lithocholic acid; G‐c: total glyco‐conjugated bile acids; GCA: glycocholic acid; GCDCA: glycochenodeoxycholic acid; GDCA: glycodeoxycholic acid; GLCA: glycolithocholic acid; GUDCA: glyco‐conjugated ursodeoxycholic acid; LCA: lithocholic acid; RCOG: Royal College of Obstetricians and Gynaecologists; T‐c Total tauro‐conjugated bile acids; TCA: taurocholic acid; TCDCA: taurochenodeoxycholic acid; TDCA: taurodeoxycholic acid; TLCA: tauro‐conjugated lithocholic acid; TS: Tea Stimac (study author); TSBA: total serum bile acids; TUDCA: tauro‐conjugated ursodeoxycholic acid; UDCA: ursodeoxycholic acid.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abu‐Hayyeh 2016 | Lacking data. Authors did not sent additional data when asked by email. |
| Ai 2004 | Lack of comparison with non‐diseased pregnant women. |
| Almuna 1987a | Lacking data (only mean value ± 2SD of the index test results for women included as controls). Authors' contacts not found. |
| Ambros‐Rudolph 2007 | Lack of comparison with non‐diseased pregnant women. |
| Anyikam 2013 | Lack of comparison with non‐diseased pregnant women. |
| Back 1974 | No information on inclusion criteria for patients. Bile acid assessments performed on pooled serum samples and not on individual serum samples. |
| Bacq 1995 | Lack of comparison with non‐diseased pregnant women. |
| Bacq 1997 | Lack of comparison with non‐diseased pregnant women. |
| Binder 2006 | Lacking data (only mean value ± SD of the index test results for both diseased and non‐diseased women). Further information received from T Binder (see study references), but data to complete a two‐by‐two table were not available. |
| Bouzouki 2013 | Lacking data. Authors did not send additional data when asked by email. |
| Ch'ng 2003 | Lacking data. Authors did not reply anymore during email correspondence. |
| Chen 2013 | Lacking data. Authors refused to send additional data when asked by email. |
| Chianale 1982 | Lacking data (only mean value ± SD of the index test results for both diseased and non‐diseased women). No reply from authors. |
| Cowles 2005 | Lacking data. No reply from authors contacted by email. |
| Dann 2004 | Not matching the review question. |
| Dann 2005 | Lacking data. Author's contact not found. |
| Favre 2010 | Not maching the review question. |
| Garcia‐Flores 2015 | Incomplete reference standard (lack of follow‐up after delivery). |
| Geenes 2012 | Lacking data. Authors did not sent additional data when asked by email. |
| Glasinovic 1982 | Lacking data (only mean value ± SD of the index test results for both diseased and non‐diseased women). No reply from authors. |
| Heikkinen 1981 | Lacking data (only mean value ± SD of the index test results for both diseased and non‐diseased women). Authors' contacts not found. |
| Heikkinen 1983a | Lacking data (only mean value ± SD of the index test results for both diseased and non‐diseased women). Authors' contacts not found. |
| Heikkinen 1983b | Lacking data (only mean value ± SD of the index test results for both diseased and non‐diseased women). Authors' contacts not found. |
| Heikkinen 1983c | Lacking data (only mean value ± SD of the index test results for both diseased and non‐diseased women). Author's contact not found. |
| Hong 2002 | Lack of comparison with non‐diseased pregnant women. |
| Hu 2015 | Lacking data. No reply from authors. |
| Huang 2007 | Not matching the review question. |
| Joutsiniemi 2008 | Index test (TSBA) not performed in control group: values provided in the publication are reference laboratory values for healthy women (information received from authors, see study references). |
| Jurate 2017 | Incomplete reference standard (lack of follow‐up after delivery). |
| Kenyon 2001 | Lack of comparison with non‐diseased pregnant women. |
| Khan 2013 | Lack of comparison with non‐diseased pregnant women. |
| Kirbas 2014 | Index test (TSBA) not performed in control group. |
| Kremer 2015 | Not matching the review question. |
| Laatikainen 1975 | Lacking data (only mean value ± SD of the index test results for women included as controls). Authors' contacts not found. |
| Laatikainen 1977 | Lacking data (only mean value ± SD of the index test results for women included as controls). Authors' contacts not found. |
| Laatikainen 1978 | Lacking data (only mean value ± SD of the index test results for women included as controls). Authors' contacts not found. |
| Lee 2006 | Incomplete reference standard (lack of follow‐up after delivery). |
| Li 2013 | Lack of comparison with non‐diseased pregnant women. |
| Lisoni 1983 | Lacking data (only mean value ± SD of the index test results for both diseased and non‐diseased women). No reply from authors. |
| Lo 2007 | Lack of comparison with non‐diseased pregnant women. |
| Lopez 1982 | Incomplete reference standard (lack of follow‐up after delivery). |
| Madazli 2015 | Lack of comparison with non‐diseased pregnant women. |
| Mahey 2009 | Lacking data. No reply from authors by email. |
| Meng 1997 | Lacking data. No reply from authors by email. |
| Nezer 2017 | Incomplete reference standard (lack of follow‐up after delivery). |
| Oktaba 2013 | Lack of comparison with non‐diseased pregnant women. |
| Qu 2015 | Index test (TSBA) not performed in control group. |
| Reyes 2006 | Lacking data. Authors stopped to reply during an e |
| Samuelson 1980 | Incomplete reference standard (lack of follow‐up after delivery). |
| Sargın Oruç 2014 | Lacking data. No reply from authors by email. |
| Sarria 1988 | Lack of comparison with non‐diseased pregnant women. |
| Shaw 1982 | Lack of comparison with non‐diseased pregnant women. |
| Subramaniam 2005 | Lacking data. Authors' contacts not found. |
| Tribe 2010 | Lacking data. |
| Yang 2014 | Lacking data. No reply from authors by email. |
Differences between protocol and review
At the protocol stage, we planned to analyse the accuracy of eleven index tests: total serum bile acids (TSBA), cholic acid (CA), glycocholic acid (GCA), chenodeoxycholic acid (CDCA), cholic/chenodeoxycholic acid ratio (CA/CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), ursodeoxycholic acid (UDCA), total glyco‐conjugated bile acids (G‐c), total tauro‐conjugated bile acids (T‐c), total glyco‐conjugated bile acids/total taurine‐conjugated bile acid ratio (G‐c/T‐c). However, we analysed only the diagnostic accuracy of TSBA, CA, CDCA, CA/CDCA, GCA, and we did not perform the analysis over the accuracy of LCA, DCA, UDCA, G‐c, T‐c, G‐c/T‐c, as there were too few data available.
We decided not to analyse the accuracy of combinations of the index tests (that in our review protocol was defined as TSBA plus any component of serum bile acid profile) as none of the included studies did so. However, we will examine the possibility for doing this analysis in the future, based on the individual participant data we have assembled.
We analysed sources of heterogeneity only for TSBA with a cut‐off of 10 μmol/L. Owing to the small number of studies, among the sources of heterogeneity planned at the protocol stage — country in which the study took place; participant selection: studies including only pregnant women with suspicion of intrahepatic cholestasis of pregnancy versus studies including all pregnant women; laboratory techniques used for the measurement of the index tests; participant treatment with UDCA versus no treatment; fasting or postprandial status of pregnant women at the time when the serum samples were taken; timing of assessment of the index test(s): the time when the symptoms arose, the peak values among multiple assessments during pregnancy, immediately before delivery; differences in study definitions of intrahepatic cholestasis of pregnancy — we analysed only laboratory technique used, timing of assessment of the index tests, and therapy (if included patient received UDCA or not).
We have deleted LRs and predictive values from the Methods section; we estimated only sensitivity and specificity.
At the protocol stage, we planned to perform the following sensitivity analyses.
Excluding studies at high risk of bias (studies judged as high risk of bias or unclear risk of bias in at least one of the domains of QUADAS‐2).
Excluding all studies with case‐control design.
Excluding only studies with case‐control design which enrolled as controls asymptomatic pregnant women (i.e. without symptoms suggestive for cholestasis).
Excluding studies in which the index test was part of the reference standards.
However, we could not perform a sensitivity analysis excluding the studies at high risk of bias, as only one study was judged at low risk of bias in all domains. Moreover, all case‐control studies enrolled asymptomatic pregnant women as controls; none of them included symptomatic pregnant women (i.e. with pruritus). Hence, we made only the other two planned sensitivity analyses (excluding case‐control studies; and excluding studies where TSBA were part of the reference standard).
In the protocol, we planned to test for publication bias. However, considering that these statistical methods are not widely used in diagnostic test accuracy reviews, and also due to the lack of validated methods, we decided not to assess publication bias.
Contributions of authors
CM ‒ formulated the research question, searched and selected the articles, extracted data, assessed quality, drafted the manuscript and reviewed the final version of the manuscript. GC ‒ provided statistical expert opinion, drafted part of the manuscript, involved in decision making, provided methodological and statistical analysis, and reviewed the final version of the manuscript. TS ‒ provided content expert opinion, searched and selected the articles, extracted data, assessed quality, and reviewed the final version of the manuscript. DN ‒ provided advice and suggestions, involved in decision making, corrected writing style and language, and reviewed the final version of the manuscript. CG ‒ provided methodological expert opinion, involved in decision making, and reviewed the final version of the manuscript.
All authors approved the final review for publication.
Sources of support
Internal sources
The Cochrane Hepato‐Biliary Group, Denmark.
-
CM's parents, Italy.
Supported CM while working in Copenhagen at the CHBG Editrial Team office
External sources
No sources of support supplied
Declarations of interest
CM: none known GC: none known TS: none known DN: none known CG: none known
New
References
References to studies included in this review
Almuna 1986 {published data only}
- Almuna R, Galindo E, Mella M, Alarcón M. Serum levels of cholylglycine in fasting and after meals fats in control group and in patients with intrahepatic cholestasis of pregnancy [Niveles séricos de colilglicina en ayunas y post‐ingesta grasa, en grupo control y en pacientes con colestasia intrahepática del embarazo]. Revista Chilena de Obstetetricia y Ginecologia 1986;51(4):325‐33. [Google Scholar]
Almuna 1987b {published data only}
- Almuna R, Galindo E, Sepulveda V. Bile salts (cholylglycine) in the amniotic fluid and umbilical cord blood in patients with intrahepatic cholestasis of pregnancy [Sales biliares (coliglicina) en liquido amniotico y en sangre de cordon umbilical en pacientes con colestasia intrahepatica del embarazo (CIE)]. Revista Chilena de Obstetricia y Ginecologia 1987;52(6):327‐32. [PubMed] [Google Scholar]
Brites 1998b {published and unpublished data}
- Brites D. Further information on published study [personal communication]. Email to: C Manzotti. November 2015.
- Brites D. Individual participant data (as supplied November 2015). Data on file.
- Brites D, Rodrigues CMP, Van‐Zeller H, Brito A, Silva R. Relevance of serum bile acid profile in the diagnosis of intrahepatic cholestasis of pregnancy in an high incidence area: Portugal. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1998;80(1):31‐8. [DOI] [PubMed] [Google Scholar]
Brites 1998c {published and unpublished data}
- Brites D. Further information on published study [personal communication]. Email to: C Manzotti. November 2015.
- Brites D. Individual participant data (as supplied 25 November 2015). Data on file.
- Brites D, Rodrigues CMP. Elevated levels of bile acids in colostrum of patients with cholestasis of pregnancy are decreased following ursodeoxycholic acid therapy. Journal of Hepatology 1998;29(5):743–51. [DOI] [PubMed] [Google Scholar]
Gonzalez 1989 {published data only}
- Gonzalez MC, Reyes H, Arrese M, Figueroa D, Lorca B, Andresen M, et al. Intrahepatic cholestasis of pregnancy in twin pregnancies. Journal of Hepatology 1989;9(1):84‐90. [DOI] [PubMed] [Google Scholar]
Guducu 2013 {published and unpublished data}
- Guducu N. Further information on published study [personal communication]. Email to: C Manzotti. November 2015.
- Guducu N. Individual participant data (as supplied 11 November 2015). Data on file.
- Guducu N, Kayan BO, Isci H, Aydinli K, Yigiter AB, Dunder I. Comparison of mean platelet volume and serum bile acid levels in intrahepatic cholestasis of pregnancy. Journal of Turkish Society of Obstetrics and Gynecology 2013;10(4):236‐41. [Google Scholar]
Huang 2009 {published and unpublished data}
- Huang W. Further information on published study [personal communication]. Email to: C Manzotti. October 2015.
- Huang W. Individual participant data (as supplied 27 October 2015). Data on file.
- Huang WM, Gowda M, Donnelly JG. Bile acid ratio in diagnosis of intrahepatic cholestasis of pregnancy. American Journal of Perinatology 2009;26(4):291‐4. [DOI] [PubMed] [Google Scholar]
Jiang 2012 {published data only}
- Jiang Y, Shao Y. Application of ROC curves in evaluating values of liver function indexes for diagnosing intrahepatic cholestasis of pregnancy [应用ROC 曲线评价肝功能指标在妊娠肝内胆汁淤积症诊断中的价值]. Journal of Modern Medicine and Health [现代医药卫生] 2012;28(10):1449‐53. [Google Scholar]
Kowalska‐Kanka 2013 {published and unpublished data}
- Kowalska‐Kanka A. Further information on published study [personal communication]. Email to: C Manzotti. November‐December 2015.
- Kowalska‐Kanka A. Individual participant data (as supplied 8 December 2015). Data on file.
- Kowalska‐Kanka A, Maciejewski T, Niemiec KT. The concentrations of bile acids and erythropoietin in pregnant women with intrahepatic cholestasis and the state of the fetus and newborn [Stężenia kwasów żółciowych i erytropoetynyu ciężarnych z cholestazą wewnątrzwątrobowąa stan płodu i noworodka]. Medycyna Wieku Rozowojowego 2013;17(3):232‐45. [PubMed] [Google Scholar]
Laatikainen 1984 {published data only}
- Laatikainen T, Tulenheimo A. Maternal serum bile acid levels and fetal distress in cholestasis of pregnancy. International Journal of Gynaecology and Obstetrics 1984;22(2):91‐4. [DOI] [PubMed] [Google Scholar]
Lang 2012 {published data only}
- Lang T. Further information on published poster [personal correspondence]. Emails to: C Manzotti. November‐December 2015.
- Lang T. Individual participant data (as supplied 1 December 2015). Data on file.
- Lang T, McKeown G, Hunter A. Biochemical investigation and clinical management of obstetric cholestasis (OC): how useful is bile acid quantification?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(S1):A50‐A51. [Google Scholar]
Lunzer 1986 {published data only}
- Lunzer M, Barnes P, Byth K, O'Halloran M. Serum bile acid concentrations during pregnancy and their relationship to obstetric cholestasis. Gastroenterology 1986;91(4):825‐9. [DOI] [PubMed] [Google Scholar]
Roger 1994 {published and unpublished data}
- Roger D. Further information on published study [personal communication]. Emails to: C Manzotti. November 2015.
- Roger D. Individual participant data (as supplied 5 November 2015). Data on file.
- Roger D, Vaillant L, Fignon A, Pierre F, Bacq Y, Brechot JF, et al. Specific pruritic diseases of pregnancy. A prospective study of 3192 pregnant women. Archives of Dermatology 1994;130(6):734‐9. [PubMed] [Google Scholar]
Sjovall 1966 {published data only}
- Sjövall K, Sjövall J. Serum bile acid levels in pregnancy with pruritus (bile acids and steroids 158). Clinical Chimica Acta 1966;13:207‐11. [DOI] [PubMed] [Google Scholar]
Sun 2011 {published data only}
- Sun Y. The clinical significance of biochemical parameters of intrahepatic cholestasis [生化指标检测对妊娠期肝内胆汁淤积症的临床意义]. China Practical Medical [中国实用医药] 2011;6(3):129‐30. [Google Scholar]
Tripodi 2015 {published and unpublished data}
- Castaño G, Lucangioli S, Sookoian S, Mesquida M, Lemberg A, Scala M, et al. Bile acid profiles by capillary electrophoresis in intrahepatic cholestasis of pregnancy. Clinical Science 2006;110(44):459‐65. [DOI] [PubMed] [Google Scholar]
- Lucangioli SE, Castano G, Contin MD, Tripodi VP. Lithocholic acid as a biomarker of intrahepatic cholestasis of pregnancy during ursodeoxycholic acid treatment. Annals of Clinical Biochemistry 2009;46(Pt 1):44‐9. [DOI] [PubMed] [Google Scholar]
- Martinefski M, Contin M, Lucangioli S, Carlo MB, Tripodi V. In search of an accurate evaluation of intrahepatic cholestasis of pregnancy. Scientifica (Cairo) 2012;2012:496489. [DOI: 10.6064/2012/496489] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi V. Further information on all the publications linked to the study [personal correspondence]. Emails to: C Manzotti. November ‐ December 2015.
- Tripodi V. Individual participant data (as supplied 4 and 16 Dicember 2015). Data on file.
- Tripodi V, Lucangioli S, Sookoian Silvia C, Mesquida M, Scala M, Masrian E, et al. New insight in the pathogenesis of intrahepatic cholestasis of pregnancy: bile acid profiles assessed by capillary electrophoresis is a useful tool in the differential diagnosis. Hepatology (Baltimore, Md.) 2004;40(4):296A. [Google Scholar]
References to studies excluded from this review
Abu‐Hayyeh 2016 {published data only}
- Abu‐Hayyeh S, Ovadia C, Lieu T, Jensen DD, Chambers J, Dixon PH, et al. Prognostic and mechanistic potential of progesterone sulphates in intrahepatic cholestasis of pregnancy and pruritus gravidarum. Hepatology (Baltimore, Md.) 2016;63(4):1287‐98. [DOI: 10.1002/hep.28265] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ai 2004 {published data only}
- Ai Y, Liu SY, Yao Q. Clinical characteristics of 1241 cases of intrahepatic cholestasis of pregnancy. Chinese Journal of Obstetrics & Gynecology [Chung‐Hua Fu Chan Ko Tsa Chih] 2004;39(4):217‐20. [PubMed] [Google Scholar]
- Wang XD, Yao Q, Peng B, Zhang L, Ai Y, Ying AY, et al. A clinical analysis of intrahepatic cholestasis of pregnancy in 1241 cases. Chinese Journal of Hepatology [Zhonghua Gan Zang Bing Za Zhi] 2007;15(4):291‐3. [PubMed] [Google Scholar]
Almuna 1987a {published data only}
- Almuna R, Mella M, Galindo E, Maturana J, Díaz L. Serum determination of ceruloplasmin and copper in pregnant women with intrahepatic cholestasis [Determinación de ceruloplasmina y cobre sérico en la colestasia intrahepatica del embarazo]. Revista Chilena de Obstetricia y Ginecologia 1987;52(2):142‐8. [Google Scholar]
Ambros‐Rudolph 2007 {published data only}
- Ambros‐Rudolph CM, Glatz M, Trauner M, Kerl H, Müllegger RR. The importance of serum bile acid level analysis and treatment with ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a case series from central Europe. Archives of Dermatology 2007;143(6):757‐62. [DOI] [PubMed] [Google Scholar]
Anyikam 2013 {published data only}
- Anyikam AL, Mays JK, Kaminsky SJ, Lee‐Hwang G, Nguyen ML. Recurrent intrahepatic cholestasis of pregnancy among a hispanic inner city population: an eight year retrospective cohort analysis. Reproductive Sciences (Thousand Oaks, Calif.) 2013;20(S3):324‐5. [Google Scholar]
Back 1974 {published data only}
- Back P, Sjovall J, Sjovall K. Mono hydroxy bile acids in plasma in intra hepatic cholestasis of pregnancy identification by computerized gas chromatography mass spectrometry. Medical Biology (Helsinki) 1974;52(1):31‐8. [PubMed] [Google Scholar]
- Back P, Sjovall K, Sjovall J. Identification of monohydroxy bile acids in intrahepatic cholestasis during pregnancy [Die Identifizierung von Monohydroxgallensauren bei intrahepatischer Schwangerschaftscholestase]. Verhandlungen der Deutschen Gesellschaft fur Innere Medizin 1972;78:1626‐30. [PubMed] [Google Scholar]
Bacq 1995 {published data only}
- Bacq Y, Myara A, Brechot MC, Hamon C, Studer E, Trivin F, et al. Serum conjugated bile‐acid profile during intrahepatic cholestasis of pregnancy. Journal of Hepatology 1995;22(1):66‐70. [DOI] [PubMed] [Google Scholar]
Bacq 1997 {published data only}
- Bacq Y, Sapey T, Brechot MC, Pierre F, Fignon A, Dubois F. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology (Baltimore, Md.) 1997;26(2):358‐64. [DOI] [PubMed] [Google Scholar]
Binder 2006 {published and unpublished data}
- Binder T. Further information on published study [personal correspondence]. Email to: C Manzotti. November 2015.
- Binder T, Salaj P, Zima T, Vitek L. Randomized prospective comparative study of ursodeoxycholic acid and S‐adenosyl‐L‐methionine in the treatment of intrahepatic cholestasis of pregnancy. Journal of Perinatal Medicine 2006;34(5):383‐91. [DOI] [PubMed] [Google Scholar]
- Binder T, Zima T, Vitek L. Biochemical parameters of the intrahepatic cholestasis of pregnancy [Czech]. Ceska Gynekologie 2007;72(2):90‐4. [PubMed] [Google Scholar]
Bouzouki 2013 {published data only}
- Bouzouki AB. Intrahepatic cholestasis in pregnancy (IHCP) ‐ A management assessment retrospective study. British Journal of Obstetrics and Gynaecology 2013;120:97‐8. [Google Scholar]
Ch'ng 2003 {published data only}
- Ch'ng CL, Morgan M, Kingham JG. Obstetric cholestasis in South Wales, UK. Journal of Hepatology 2002;36(S1):251. [Google Scholar]
- Ch'ng CL, Morgan M, Kingham JG. Prospective study of obstetric cholestasis in South Wales. Journal of Obstetrics & Gynaecology 2003;23:S44. [Google Scholar]
- Ch'ng CL, Morgan M, Kingham JG. Prospective study of obstetric cholestasis in South Wales, UK. Gastroenterology 2001;120(5):A76. [Google Scholar]
Chen 2013 {published data only}
- Chen J, Deng W, Wang J, Shao Y, Ou M, Ding M. Primary bile acids as potential biomarkers for the clinical grading of intrahepatic cholestasis of pregnancy. International Journal of Gynaecology and Obstetrics 2013;122(1):5‐8. [DOI] [PubMed] [Google Scholar]
- Ye L, Liu S, Wang M, Shao Y, Ding M. High‐performance liquid chromatography‐tandem mass spectrometry for the analysis of bile acid profiles in serum of women with intrahepatic cholestasis of pregnancy. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 2007;860(1):10‐7. [DOI] [PubMed] [Google Scholar]
Chianale 1982 {published data only}
- Chianale J, Glasinovic JC, Lopez J, Marinovic I, Vela P, Ahumada E, et al. Development of pruritus in cholestasis of pregnancy and its relation to the serum concentration of bile acids [Evolucion del prurito en la colestasia del embarazo y su relacion con la concentracion serica de sales biliares]. Revista Medica de Chile 1982;110(6):538‐41. [PubMed] [Google Scholar]
Cowles 2005 {published data only}
- Cowles K, Desilva V. The use of liver function tests to determine indication of bile acid analysis in patients with suspected obstetric cholestasis. Clinica Chimica Acta; International Journal of Clinical Chemistry 2005;355:S154. [Google Scholar]
Dann 2004 {published data only}
- Dann AT, Kenyon AP, Seed PT, Poston L, Shennan AH, Tribe RM. Glutathione S‐transferase and liver function in intrahepatic cholestasis of pregnancy and pruritus gravidarum. Hepatology (Baltimore, Md.) 2004;40(6):1406‐14. [DOI] [PubMed] [Google Scholar]
Dann 2005 {published data only}
- Dann A, Rafferty J, Kearney E. Is the evaluation of serum bile acids clinically warranted in pregnant women with pruritis?. Clinica Chimica Acta; International Journal of Clinical Chemistry 2005;355:S151‐2. [Google Scholar]
Favre 2010 {published data only}
- Favre N, Bourdel N, Sapin V, Abergel A, Gallot D. Importance of bile acids for intra‐hepatic cholestasis of pregnancy [Intérêt des acides biliaires dans la cholestase gravidique]. Gynecologie, Obstetrique & Fertilite 2010;38(4):293‐5. [DOI] [PubMed] [Google Scholar]
Garcia‐Flores 2015 {published and unpublished data}
- Garcia‐Flores J. Further information on published study [personal communication]. Email to: C Manzotti. November 2015.
- Garcia‐Flores J. Individual participant data (as supplied 6 November 2015). Data on file.
- Garcia‐Flores J, Canamares M, Cruceyra M, Garicano A, Espada M, Lopez A, et al. Clinical value of maternal bile Acid quantification in intrahepatic cholestasis of pregnancy as an adverse perinatal outcome predictor. Gynecologic and Obstetric Investigation 2015;79(4):222‐8. [DOI] [PubMed] [Google Scholar]
Geenes 2012 {published data only}
- Geenes V, Lawrance D, Lovgren‐Sandblom A, Bethin L, Chappell L, Thornton J, et al. Maternal and fetal bile acids in intrahepatic cholestasis of pregnancy. Reproductive Sciences 2012;19(3):180A. [Google Scholar]
Glasinovic 1982 {published data only}
- Glasinovic JC, Marinovic I, Vela P, Lopez J, Ahumada E, Valdivia MT, et al. Variations in serum bile acid levels in cholestasis of pregnancy. Revista Medica de Chile 1982;110(7):640‐3. [PubMed] [Google Scholar]
Heikkinen 1981 {published data only}
- Heikkinen J, Mäentausta O, Ylöstalo P, Jänne O. Changes in serum bile acid concentrations during normal pregnancy, in patients with intrahepatic cholestasis of pregnancy and in pregnant women with itching. British Journal of Obstetrics and Gynaecology 1981;88(3):240‐5. [PubMed] [Google Scholar]
Heikkinen 1983a {published data only}
- Heikkinen J. Serum bile acids in the early diagnosis of intrahepatic cholestasis of pregnancy. Obstetrics and Gynecology 1983;61(5):581‐87. [PubMed] [Google Scholar]
Heikkinen 1983b {published data only}
- Heikkinen J, Ylostalo P, Maentausta O, Janne O. Bile acids in maternal serum, umbilical cord serum and amniotic fluid of healthy women, women with pruritis and patients with intrahepatic cholestasis of pregnancy. Journal of Obstetrics and Gynaecology 1983;4(1):17‐20. [Google Scholar]
Heikkinen 1983c {published data only}
- Heikkinen J. Effect of a standard test meal on serum bile acid levels in healthy nonpregnant and pregnant women and in patients with intrahepatic cholestasis of pregnancy. Annals of Clinical Research 1983;15(5‐6):183‐8. [PubMed] [Google Scholar]
Hong 2002 {published data only}
- Hong C, Wu D, Li J. Clinical analysis of 34 cases of intrahepatic cholestasis of pregnancy [Chinese]. Medical Journal of Wuhan University [Wuhan Daxue Xuebao] 2002;23(3):270‐1, 281. [Google Scholar]
Hu 2015 {published data only}
- Hu YY, Liu JC, Xing AY. Oxidative stress markers in intrahepatic cholestasis of pregnancy: a prospective controlled study. European Review for Medical and Pharmacological Sciences 2015;19(17):3181‐6. [PubMed] [Google Scholar]
Huang 2007 {published and unpublished data}
- Huang W. Further information on published study [personal communication]. Email to: C Manzotti. November 2015.
- Huang W. Individual participant data (as supplied 29 November 2015). Data on file.
- Huang WM, Seubert DE, Donnelly JG, Liu ML, Javitt NB. Intrahepatic cholestasis of pregnancy: detection with urinary bile acid assays. Journal of Perinatal Medicine 2007;35(6):486‐91. [DOI] [PubMed] [Google Scholar]
Joutsiniemi 2008 {published and unpublished data}
- Joutsiniemi T. Further information on published study [personal correspondence]. Emails to: C Manzotti. December 2015.
- Joutsiniemi T. Individual participant data (as supplied 9 December 2015). Data on file.
- Joutsiniemi T, Leino R, Timonen S, Pulkki K, Ekblad U. Hepatocellular enzyme glutathione‐S‐transferase alpha and intrahepatic cholestasis of pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2008;87(12):1280‐4. [DOI] [PubMed] [Google Scholar]
Jurate 2017 {published data only}
- Jurate K, Rimantas Z, Jolanta S, Vladas G, Kupcinkas L. Sensitivity and specificity of biochemical tests for diagnosis of intrahepatic cholestasis of pregnancy. Annals of Hepatology 2017;16:569‐73. [DOI] [PubMed] [Google Scholar]
Kenyon 2001 {published data only}
- Kenyon AP, Piercy CN, Girling J, Williamson C, Tribe RM, Shennan AH. Pruritus may precede abnormal liver function tests in pregnant women with obstetric cholestasis: a longitudinal analysis. British Journal of Obstetrics and Gynaecology 2001;108(11):1190‐2. [DOI] [PubMed] [Google Scholar]
Khan 2013 {published data only}
- Khan N, Krishnamoorthy P. Audit of obstetric cholestasis in a district general hospital, UK. British Journal of Obstetrics and Gynaecology 2013;120:142. [Google Scholar]
Kirbas 2014 {published and unpublished data}
- Kirbas A. Further information on published study [personal correspondence]. Emails to: C Manzotti. November‐December 2015.
- Kirbas A. Individual participant data (as supplied 2 December 2015). Data on file.
- Kirbas A, Biberoglu E, Daglar K, Iskender C, Erkaya S, Dede H, et al. Neutrophil‐to‐lymphocyte ratio as a diagnostic marker of intrahepatic cholestasis of pregnancy. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014;180(1):12‐5. [DOI] [PubMed] [Google Scholar]
Kremer 2015 {published data only}
- Kremer AE, Bolier R, Dixon PH, Geenes V, Chambers J, Tolenaars D, et al. Autotaxin activity has a high accuracy to diagnose intrahepatic cholestasis of pregnancy. Journal of Hepatology 2015;62(4):897‐904. [DOI] [PubMed] [Google Scholar]
- Ovadia C, Abu‐Hayyeh S, Dixon PH, Chambers J, Lovgren‐Sandblom A, Bolier R, et al. Biochemical markers for intrahepatic cholestasis of pregnancy and pruritus gravidarum in women with gestational pruritus. Journal of Hepatology 2014;60:S194. [Google Scholar]
Laatikainen 1975 {published data only}
- Laatikainen T, Hesso A. Determination of serum bile acids by glass capillary gas‐liquid chromatography. Clinica Chimica Acta; International Journal of Clinical Chemistry 1975;64(1):63‐8. [DOI] [PubMed] [Google Scholar]
Laatikainen 1977 {published data only}
- Laatikainen T, Ikonen E. Serum bile acids in cholestasis of pregnancy. Obstetrics and Gynecology 1977;50(3):313‐8. [PubMed] [Google Scholar]
Laatikainen 1978 {published data only}
- Laatikainen T. Postprandial serum bile acids in cholestasis of pregnancy. Annals of Clinical Research 1978;10(6):307‐12. [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee RH, Goodwin TM, Greenspoon J, Incerpi M. The prevalence of intrahepatic cholestasis of pregnancy in a primarily Latina Los Angeles population. Journal of Perinatology 2006;26(9):527‐32. [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li L, Zhao XY, Ou XJ, Jia JD. Clinical analysis of intrahepatic cholestasis of pregnancy [Chinese]. Chinese Journal of Hepatology 2013;21(4):295‐8. [DOI] [PubMed] [Google Scholar]
Lisoni 1983 {published data only}
- Lisoni M, Oyarzun E, Ahumada E, Glasinovic JC, Marinovic I. Intrahepatic cholestasis of pregnancy, serum bile acids, and electronic monitoring of the fetus [Colestasia intrahepatica del embarazo, acidos biliares sericos y monitorizacion electronica del feto]. Revista Chilena De Obstetricia Y Ginecologia 1983;48(5):366‐71. [PubMed] [Google Scholar]
Lo 2007 {published data only}
- Lo TK, Lau WL, Lam HS, Leung WC, Chin RK. Obstetric cholestasis in Hong Kong ‐ local experience with eight consecutive cases. Hong Kong Medical Journal 2007;13(5):387‐91. [PubMed] [Google Scholar]
Lopez 1982 {published data only}
- Lopez J, Glasinovic JC, Marinovic I, Vela P, Ahumada E, Valdivia MT, et al. Clinical and laboratory characterization in 100 cases of pregnancy cholestasis [Caracterizacion clinica y de laboratorio en cien casos de colestasia gravidica]. Revista Chilena de Obstetricia y Ginecología 1982;47(4):215‐21. [PubMed] [Google Scholar]
Madazli 2015 {published data only}
- Madazli R, Yuksel MA, Oncul M, Tuten A, Guralp O, Aydin B. Pregnancy outcomes and prognostic factors in patients with intrahepatic cholestasis of pregnancy. Journal of Obstetrics and Gynaecology 2015;35(4):358‐61. [DOI] [PubMed] [Google Scholar]
Mahey 2009 {published data only}
- Mahey R, Agarwal N, Kriplani A, Saraya A, Garg P. Role of serum bile acids in diagnosis of intrahepatic cholestasis of pregnancy and effect of ursodeoxycholic acid therapy on bile acids and perinatal outcome. International Journal of Gynaecology and Obstetrics 2009;107(S2):S444‐5. [Google Scholar]
Meng 1997 {published data only}
- Meng LJ, Reyes H, Palma J, Hernandez I, Ribalta J, Sjovall J. Profiles of bile acids and progesterone metabolites in the urine and serum of women with intrahepatic cholestasis of pregnancy. Journal of Hepatology 1997;27(2):346‐57. [DOI] [PubMed] [Google Scholar]
Nezer 2017 {published data only}
- Nezer M, Cohen Y, Drukker L, Farkash R, Samueloff A, Sela H Y. Intrahepatic cholestasis of pregnancy: Dose bile acid levels really matter?. American Journal of Obstetrics and Gynecology 2017;1(Suppl 1):S445‐6. [Google Scholar]
Oktaba 2013 {published data only}
- Oktaba M, Iruloh C. The diagnosis and management of obstetric cholestasis‐is random bile acid levels sufficient to establish diagnosis?. British Journal of Obstetrics and Gynaecology 2013;120:145. [Google Scholar]
Qu 2015 {published data only}
- Qu W, Yu T. The diagnostic value of neutrophil‐to‐lymphocyte ratio and serum bile acid in pregnant women with intrahepatic cholestasis of pregnancy [中性粒细胞一淋巴细胞比率和胆酸水平在妊娠期肝内胆汁淤积症中的诊断价值]. Chinese Journal of Clinical Healthcare [中国临床保健杂志] 2015;18(4):376‐9. [Google Scholar]
Reyes 2006 {published data only}
- Reyes H, Zapata R, Hernandez I, Gotteland M, Sandoval L, Jiron MI, et al. Is a leaky gut involved in the pathogenesis of intrahepatic cholestasis of pregnancy?. Hepatology (Baltimore, Md.) 2006;43(4):715‐22. [DOI] [PubMed] [Google Scholar]
Samuelson 1980 {published data only}
- Samuelson K, Thomassen PA. Radioimmunoassay of serum bile acids in normal pregnancy and in recurrent cholestasis of pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1980;59(5):417‐20. [DOI] [PubMed] [Google Scholar]
Sargın Oruç 2014 {published data only}
- Sargın Oruç A, Seçkin B, Özcan N, Özyer S, Uzunlar Ö, Danışman N. Role of postprandial bile acids in prediction of perinatal outcome in intrahepatic cholestasis of pregnancy. Journal of Obstetrics and Gynaecology Research 2014;40(7):1883‐9. [DOI] [PubMed] [Google Scholar]
Sarria 1988 {published data only}
- Sarria CJ, Sosa A, Gustavo A. Cholestasis intrahepatic in pregnancy: review of 13 years [Colestasis intrahepática del embarazo: revisión de 13 años]. Revista de Obstetricia y Ginecologia Venezolana 1988;48(3):144‐7. [Google Scholar]
Shaw 1982 {published and unpublished data}
- Shaw D. Further information on published study [personal correspondence]. Emails to: C Manzotti. November 2015.
- Shaw D, Frohlich J, Wittmann BA, Willms M. A prospective study of 18 patients with cholestasis of pregnancy. American Journal of Obstetrics and Gynecology 1982;142(6 Pt 1):621‐5. [DOI] [PubMed] [Google Scholar]
Subramaniam 2005 {published data only}
- Subramaniam S, Shyama S. Role of serum bile acids in diagnosis of intrahepatic cholestasis of pregnancy. Clinical Chemistry 2005;51(S6):A39‐40. [Google Scholar]
Tribe 2010 {published data only}
- Tribe RM, Dann AT, Kenyon AP, Seed P, Shennan AH, Mallet A. Longitudinal profiles of 15 serum bile acids in patients with intrahepatic cholestasis of pregnancy. American Journal of Gastroenterology 2010;105(3):585–95. [DOI] [PubMed] [Google Scholar]
Yang 2014 {published data only}
- Yang Q, Feng Q. Analysis of total bile acid diagnostic value in cholestasis of pregnancy [总胆汁酸检测对妊娠期胆汁淤积的诊断价值分析]. Journal of Modern Medicine and Health [现代医药卫生] 2014;30(14):2150‐2. [Google Scholar]
Additional references
Abu‐Hayyeh 2013
- Abu‐Hayyeh S, Papacleovoulou G, Lövgren‐Sandblom A, Tahir M, Oduwole O, Jamaludin NA, et al. Intrahepatic cholestasis of pregnancy levels of sulphated progesterone metabolites inhibit FXR resulting in a pro‐cholestatic phenotype. Hepatology (Baltimore, Md.) 2013;57:716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahlfeld 1883
- Ahlfeld F. Berichte und Arbeiten aus der Geburtshülflich‐Gynaekologischen Klinik zu Giessen 1881‐1882. Vol. 1, Grunow, 1883. [Google Scholar]
Aytaç 2006
- Aytaç S, Kargili A, Türkay C. A prolonged gestational intrahepatic cholestasis: a case report. Turkish Journal of Gastroenterology 2006;17(3):206–8. [PubMed] [Google Scholar]
Azer 1997
- Azer SA, Klaassen CD, Stacey NH. Biochemical assay of serum bile acids: methods and applications. British Journal of Biomedical Science 1997;54(2):118‐32. [PubMed] [Google Scholar]
Bacq 1996
- Bacq Y, Zarka O, Bréchot JF, Mariotte N, Vol S, Tichet J, et al. Liver function tests in normal pregnancy: a prospective study of 103 pregnant women and 103 matched controls. Hepatology (Baltimore, Md.) 1996;23(5):1030‐4. [DOI] [PubMed] [Google Scholar]
Bacq 2011
- Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clinics and Research in Hepatology and Gastroenterology 2011;35:182‐93. [DOI] [PubMed] [Google Scholar]
Bacq 2012
- Bacq Y, Sentilhes L, Reyes HB, Glantz A, Kondrackiene J, Binder T, et al. Efficacy of ursodeoxycholic acid in treating intrahepatic cholestasis of pregnancy: a meta‐analysis. Gastroenterology 2012;143(6):1492‐501. [DOI] [PubMed] [Google Scholar]
Bacq 2014
- Bacq Y, Sentilhes L. Intrahepatic cholestasis of pregnancy: diagnosis and management. Clinical Liver Disease 2014;4(3):58‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beuers 2006
- Beuers U, Pusl T. Intrahepatic cholestasis of pregnancy ‐ A heterogeneous group of pregnancy related disorders?. Hepatology (Baltimore, Md.) 2006;43(4):647‐9. [DOI] [PubMed] [Google Scholar]
Boregowda 2013
- Boregowda G, Shehata HA. Gastrointestinal and liver disease in pregnancy. Best Practice & Research. Clinical Obstetrics & Gynaecology 2013;27:835‐53. [DOI] [PubMed] [Google Scholar]
Bossuyt 2006
- Bossuyt PM, Irwig L, Craig J, Glasziou P. Comparative accuracy: assessing new tests against existing diagnostic pathways. BMJ (Clinical Research Ed.) 2006;332(7549):1089‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brites 1998a
- Brites D, Rodrigues CM, Cardoso Mda C, Graça LM. Unusual case of severe cholestasis of pregnancy with early onset, improved by ursodeoxycholic acid administration. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1998;76(2):165‐8. [DOI] [PubMed] [Google Scholar]
Colli 2014
- Colli A, Fraquelli M, Casazza G, Conte D, Nikolova D, Duca P, at al. The architecture of diagnostic research: from bench to bedside‐research guidelines using liver stiffness as an example. Hepatology (Baltimore, Md.) 2014;60(1):408‐18. [DOI] [PubMed] [Google Scholar]
Cravetto 1985
- Cravetto C, Molino G, Biondi AM, Cavanna A, Avagnina P, Frediani S. Evaluation of the diagnostic value of serum bile acid in the detection and functional assessment of liver disease. Annals of Clinical Biochemestry 1985;22(Pt 6):596‐605. [DOI] [PubMed] [Google Scholar]
Desai 2013
- Desai M, Ross MG. Reproductive endocrinology: maternal cholestasis and offspring metabolic abnormalities. Nature Reviews. Endocrinology 2013;9(10):567‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diken 2014
- Diken Z, Usta IM, Nassar AH. A clinical approach to intrahepatic cholestasis of pregnancy. American Journal of Perinatology 2014;31(1):1‐8. [DOI] [PubMed] [Google Scholar]
Dixon 2014
- Dixon PH, Wadsworth CA, Chambers J, Donnelly J, Cooley S, Buckley R, et al. A comprehensive analysis of common genetic variation around six candidate loci for intrahepatic cholestasis of pregnancy. American Journal of Gastroenterology 2014;109(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
DTA Handbook 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Version 1.0 [updated September 2010]. The Cochrane Collaboration, 2010. [Google Scholar]
DTA Handbook 2013
- Bossuyt P, Davenport C, Deeks J, Hyde C, Leeflang M, Scholten R. Chapter 11: Interpreting results and drawing conclusions. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 0.9. The Cochrane Collaboration, 2013. [Google Scholar]
Floreani 2006
- Floreani A, Carderi I, Paternoster D, Soardo G, Azzaroli F, Esposito W, et al. Intrahepatic cholestasis of pregnancy: three novel MDR3 gene mutations. Alimentary Pharmacology & Therapeutics 2006;23(11):1649‐53. [DOI] [PubMed] [Google Scholar]
Geenes 2009
- Geenes V, Williamson C. Intraepatic cholestasis of pregnancy. World Journal of Gastroenterology 2009;15(17):2049‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geenes 2014
- Geenes V, Chappell LC, Seed PT, Steer PJ, Knight M, Williamson C. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: a prospective population‐based case‐control study. Hepatology (Baltimore, Md.) 2014;59(4):1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glantz 2004
- Glantz A. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology (Baltimore, Md.) 2004;40(2):467‐74. [DOI] [PubMed] [Google Scholar]
Green‐top Guideline No.43
- Royal College of Obstetricians and Gynaecologists. Green‐top Guideline No. 43. www.rcog.org.uk/globalassets/documents/guidelines/gtg_43.pdf 2011 (accessed 18 July 2018).
Gurung 2013
- Gurung V, Middleton P, Milan SJ, Hague W, Thornton JG. Interventions for treating cholestasis in pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD000493.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heinonen 1999
- Heinonen S, Kirkinen P. Pregnancy outcome with intrahepatic cholestasis. Obstetrics and Gynecology 1999;94(2):189‐93. [DOI] [PubMed] [Google Scholar]
Henderson 2014
- Henderson CE, Shah RR, Gottimukkala S, Ferreira KK, Hamaoui A, Mercado R. Primum non nocere: how active management became modus operandi for intrahepatic cholestasis of pregnancy. American Journal of Obstetrics and Gynecology 2014;211(3):189‐96. [DOI] [PubMed] [Google Scholar]
Hepburn 2008
- Hepburn IS, Schade RR. Pregnancy‐associated liver disorders. Digestive Diseases and Sciences 2008;53(9):2334‐58. [DOI] [PubMed] [Google Scholar]
Hubschmann 2016
- Hubschmann AG, Orzechowski KM, Berghella V. Severe first trimester recurrent intrahepatic cholestasis of pregnancy: a case report and literature review. American Journal of Perinatology Reports 2016;6(1):e38‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacquemin 1999
- Jacquemin E, Cresteil D, Manouvrier S, Boute O, Hadchouel M. Heterozygous non‐sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet 1999;353(9148):210–1. [DOI] [PubMed] [Google Scholar]
Jüni 1999
- Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. Journal of the American Medical Association 1999;282(11):1054‐60. [DOI] [PubMed] [Google Scholar]
Kauppila 1987
- Kauppila A, Korpela H, Mäkilä UM, Yrjánheikki E. Low serum selenium concentration and glutathione peroxidase activity in intrahepatic cholestasis of pregnancy. British Medical Journal (Clinical Research Ed.) 1987;294(6565):150–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keitel 2006
- Keitel V, Vogt C, Haussinger D, Kubitz R. Combined mutations of canalicular transporter proteins cause severe intrahepatic cholestasis of pregnancy. Gastroenterology 2006;131(2):624‐9. [DOI] [PubMed] [Google Scholar]
Koivurova 2002
- Koivurova S, Hartikainen AL, Karinen L, Gissler M, Hemminki E, Martikainen H, et al. The course of pregnancy and delivery and the use of maternal healthcare services after standard IVF in Northern Finland 1990‐1995. Human Reproduction 2002;17(11):2897‐903. [DOI] [PubMed] [Google Scholar]
Kondrakiene 2008
- Kondrackiene J, Kupcinskas L. Intrahepatic cholestasis of pregnancy – Current achievements and unsolved problems. World Journal of Gastroenterology 2008;14(38):5781‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lijmer 1999
- Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, Meulen JH, et al. Empirical evidence of design‐related bias in studies of diagnostic tests. Journal of the American Medical Association 1999;282(11):1061‐6. [DOI] [PubMed] [Google Scholar]
Lo 2015
- Lo JO, Shaffer BL, Allen AJ, Little SE, Cheng YW, Caughey AB. Intrahepatic cholestasis of pregnancy and timing of delivery. Journal of Maternal‐fetal & Neonatal Medicine 2015;28(18):2254–58. [DOI] [PubMed] [Google Scholar]
Martineau 2015
- Martineau MG, Raker C, Dixon PH, Chambers J, Machirori M, King NM, et al. The metabolic profile of intrahepatic cholestasis of pregnancy is associated with impaired glucose tolerance, dyslipidaemia, and increased fetal growth. Diabetes Care 2015;38(2):243‐8. [DOI] [PubMed] [Google Scholar]
Mashige 1976
- Mashige F, Imai K, Osuga T. A simple and sensitive assay of total serum bile acids. Clinica Chimica Acta 1976;70:79‐86. [DOI] [PubMed] [Google Scholar]
Mays 2010
- Mays JK. The active management of intrahepatic cholestasis of pregnancy. Current Opinion in Obstetrics & Gynecology 2010;22(2):100‐3. [DOI] [PubMed] [Google Scholar]
Mullally 2002
- Mullally BA, Hansen WF. Intrahepatic cholestasis of pregnancy: review of the literature. Obstetrical & Gynecological Survey 2002;57(1):47‐52. [DOI] [PubMed] [Google Scholar]
Olsson 1993
- Olsson R, Tysk C, Aldenborg F, Holm B. Prolonged postpartum course of intrahepatic cholestasis of pregnancy. Gastroenterology 1993;105(1):267‐71. [DOI] [PubMed] [Google Scholar]
Ovadia 2019
- Ovadia C, Seed PT, Sklavounos A, Geenes V, Ilio C, Chambers J, Kohari K, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta‐analyses. Lancet 2019;393(10174):899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozer 2008
- Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008;245(3):194‐205. [DOI] [PubMed] [Google Scholar]
Ozkan 2015
- Ozkan S, Ceylan Y, Ozkan OV, Yildirim S. Review of a challenging clinical issue: intrahepatic cholestasis of pregnancy. World Journal of Gastroenterology 2015;21(23):7134‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papacleovoulou 2013
- Papacleovoulou G, Abu‐Hayyeh S, Nikolopoulou E, Briz O, Owen BM, Nikolova V. Maternal cholestasis during pregnancy programs metabolic disease in offspring. Journal of Clinical Investigations 2013;123(7):3172‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paternoster 2002
- Paternoster DM, Fabris F, Palù G, Santarossa C, Bracciante R, Snijders D, et al. Intra‐hepatic cholestasis of pregnancy in hepatitis C virus infection. Acta Obstetricia et Gynecologica Scandinavica 2002;81(2):99‐103. [PubMed] [Google Scholar]
Pathak 2010
- Pathak B, Sheibani L, Lee RH. Cholestasis of pregnancy. Obstetrics and Gynecology Clinics of North America 2010;37(2):269‐82. [DOI] [PubMed] [Google Scholar]
Perez 2005
- Perez MJ, Macias RI, Duran C, Monte MJ, Gonzalez‐Buitrago JM, Marin JJ. Oxidative stress and apoptosis in fetal rat liver induced by maternal cholestasis. Protective effect of ursodeoxycholic acid. Journal of Hepatology 2005;43(2):324‐32. [DOI] [PubMed] [Google Scholar]
Puljic 2015
- Puljic A, Kim E, Page J, Esakoff T, Shaffer B, LaCoursiere DY. The risk of infant and fetal death by each additional week of expectant management intrahepatic cholestasis of pregnancy by gestational age. American Journal of Obstetrics and Gynecology 2015;212(5):667.e 1‐5. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes 1978
- Reyes H, Gonzalez MC, Ribalta J, Aburto H, Matus C, Schramm G, et al. Prevalence of intrahepatic cholestasis of pregnancy in Chile. Annals of Internal Medicine 1978;88(4):487‐93. [DOI] [PubMed] [Google Scholar]
Reyes 1992
- Reyes H. The spectrum of liver and gastrointestinal disease seen in cholestasis of pregnancy. Gastroenterology Clinics of North America 1992;21(4):905‐21. [PubMed] [Google Scholar]
Reyes 1997
- Reyes H. Review: intrahepatic cholestasis. A puzzling disorder of pregnancy. Journal of Gastroenterology and Hepatology 1997;12(3):211‐6. [DOI] [PubMed] [Google Scholar]
Reyes 2000a
- Reyes H, Báez ME, González MC, Hernández I, Palma J, Ribalta J. Selenium, zinc and copper plasma levels in intrahepatic cholestasis of pregnancy, in normal pregnancies and in healthy individuals, in Chile. Journal of Hepatology 2000;325(4):42‐9. [DOI] [PubMed] [Google Scholar]
Reyes 2000b
- Reyes H, Sjövall J. Bile acids and progesterone metabolites in intrahepatic cholestasis of pregnancy. Annals of Medicine 2000;32(2):94‐106. [DOI] [PubMed] [Google Scholar]
Reyes 2008
- Reyes H. Sex hormones and bile acids in intrahepatic cholestasis of pregnancy. Hepatology (Baltimore, Md.) 2008;47(2):376‐9. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Rutjes 2006
- Rutjes AW, Reitsma JB, Nisio M, Smidt N, Rijn JC, Bossuyt PM. Evidence of bias and variation in diagnostic accuracy studies. CMAJ : Canadian Medical Association Journal 2006;174(4):469‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sepúlveda 1991
- Sepúlveda WH, González C, Cruz MA, Rudolph MI. Vasoconstrictive effect of bile acids on isolated human placental chorionic veins. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1991;42(3):211‐5. [DOI] [PubMed] [Google Scholar]
Sheik Abdul Kadir 2010
- Sheik Abdul Kadir SH, Miragoli M, Abu‐Hayyeh S, Moshkov AV, Xie Q, Keirel V, et al. Bile‐acid induced arrhyhtmia is mediated by muscarinic M2 receptors in neonatal rat cardiomyocytes. Public Library of Science 2010;5(3):e9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinakos 2010
- Sinakos E, Lindor KD. Bile acid profiles in intrahepatic cholestasis of pregnancy: is this the solution to the enigma of intrahepatic cholestasis of pregnancy?. American Journal of Gastroenterology 2010;105(3):596‐8. [DOI] [PubMed] [Google Scholar]
Smidt 2005
- Smidt N, Rutjes AW, Windt DA, Ostelo RW, Reitsma JB, Bossuyt PM, et al. Quality of reporting of diagnostic accuracy studies. Radiology 2005;235:347‐53. [DOI] [PubMed] [Google Scholar]
Walker 2002
- Walker IAL, Nelson‐Piercy C, Williamson C. Role of bile acid measurement in pregnancy. Annals of Clinical Biochemistry 2002;39(2):105‐13. [DOI] [PubMed] [Google Scholar]
Whiting 2004
- Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Annals of Internal Medicine 2004;140(3):189‐202. [DOI] [PubMed] [Google Scholar]
Whiting 2005
- Whiting P, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Medical Research Methodology 2005;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155:529‐36. [DOI] [PubMed] [Google Scholar]
Williamson 2001
- Williamson C, Gorelik J, Eaton BM, Lab M, Swiet M, Korchev Y. The bile acid taurocholate impairs rat cardiomyocyte function: a proposed mechanism for intra‐uterine fetal death in obstetric cholestasis. Clinical Science (London) 2001;100(4):363‐9. [PubMed] [Google Scholar]
Zecca 2006
- Zecca E, Luca D, Marras M, Caruso A, Bernardini T, Romagnoli C. Intrahepatic cholestasis of pregnancy and neonatal respiratory distress syndrome. Pediatrics 2006;117:1669‐72. [DOI] [PubMed] [Google Scholar]


