Abstract
Background
Due to the recent appearance of organisms that are resistant to several drugs (multidrug-resistant) like Enterobacteriaceae that produce extended-spectrum β-lactamase (ESBL, concerns have remarkably increased regarding the suitable treatment of infections. The present study was an investigation into ESBL molecular characteristics among clinical isolates of Klebsiella pneumoniae and Escherichia coli resulting in urinary tract infections (UTIs) and their pattern of antimicrobial resistance in order to come up with helpful information on the epidemiology of these infections and risk factors accompanied with them.
Methods
In order to conduct the study, 20 K. pneumoniae and 48 E. coli were isolated and retrieved from thalassemia center in Erbil, Iraq during July 2016 and September 2016. The collected strains were analyzed and the profile of their antimicrobial susceptibility was specified. In order to spot β-lactamase genes (i.e. blaTEM, blaSHV, and blaCTX-M), polymerase chain reaction was conducted.
Results
The findings obtained from multiplex PCR assay showed that out of the collected strains of ESBL-producing E. coli, had 81% blaTEM, 16.2% blaSHV, and 32.4% blaCTX-M genes. Similarly, 64.7% blaTEM, 35.2% blaSHV, and 41.1% blaCTX-M genes existed in the isolates of K. pneumoniae. It was found that antibiotic resistance pattern of E. coli and K. pneumoniae isolates to 20 antibiotics varied widely. It was also concluded that the majority of the K. pneumoniae and E. coli isolates were multi-drug resistant (MDR). Moreover, 75% and 87.5% of respectively K. pneumoniae and E. coli isolates showed the MDR phenotypes.
Conclusion
TEM prevalence was high among other types of ESBLs. Over all, the most active antimicrobial agents in vitro remained to be the carbapenems.
Keywords: Escherichia coli; Klebsiella pneumoniae; ESBL; blaTEM, blaSHV and blaCTX-M; Thalassemia
Introduction
It has been reported that bacteria that belong to the Enterobacteriaceae family are etiologic factors of numerous nosocomial infections all over the world.1 It is difficult to control diseases induced by bacilli Enterobacteriaceae given the limitation of therapeutic possibilities caused by constantly rising resistance of such organisms to antibiotics. In fact, Ojdana et al. (2014) introduced ESBLs as one of the most well-known resistance mechanisms in Gram-negative bacilli.2 ESBLs are a group of enzymes that lead to resistance increase in Aztreonam, Ceftazidime, Cefotaxime, related Oxyimino-β-lactams, cephalosporins, and penicillins, but Clavulanic acid inhibits them. TEM, SHV, and CTX-M are the 3 main types of ESBLs. CTX-M, which has become more prevalent than SHV and TEM, includes a rapidly expanding family which has spread among a wide range of clinically important bacteria and over wide geographic areas.3 Furthermore, strains that produce ESBL often demonstrate resistance to antibiotics belonging to other classes (i.e. aminoglycosides, quinolones, and sulfonamides), which makes strategies of treatment more complex.4
In addition, Enterobacteriaceae family members such as Klebsiella pneumoniae and Escherichia coli often produce ESBLs; however, other genera of the Enterobacteriaceae family have recently been reported to contain some other enzymes. A higher level of resistance in such organisms was first observed in patients with prolonged hospital stays in intensive care units in Europe. However, isolates were identified in Africa, Asia, the Middle East, and South and North Americas, and ESBL GNB soon became a global problem and concern.5
Common ESBL genes coding for isolates of K. pneumoniae and E. coli were determined as CTX-M (cefotaximase that preferentially hydrolyzes cefotaxime), TEM (found and isolated in the early 80s from Teminora who was a Greek patient), and SHV (for variable of sulphydryl which was first observed in a single Klebsiella ozaenae strain retrieved in Germany). These genes which are mediated by transposons, plasmids, or chromosomes are all sporadically described all over the world.6
Because there is an increase in the rates of bacterial resistance every year, leading to rising global concern, it is highly significant to understand susceptibility patterns as hospital stays may prolong and mortality rates increase due to inappropriate empirical antimicrobial therapy, which can be controlled given appropriate therapy.7 Acquiring additional PBPs insensitive to β-lactam or changing the normal PBPs are known as the commonest cause of resistance in cocci such as MRSA and pneumococci which are gram positive. However, a mixture of endogenous acquired β-lactamases with natural efflux and up-regulated impermeability is the main reason for resistance in the gram-negative bugs.8 It should be noted that there are well-prepared documents on the fact that routine disc-diffusion tests fail to detect ESBL production. Moreover, the significance and detection method of ESBLs are not fully recognized by many clinical laboratories; therefore, there may be a lack of enough resources in laboratories to reduce the spread of these mechanisms of resistance.9
A wide variety of ESBLs including SHV, TEM, OXA, CTX, AmpC, and so forth exist; however, most of them are derivatives of SHV, TEM, and CTX-M enzymes which are most often found in K. pneumoniae and E. coli. In this regard, the current study was aimed at determining the prevalence of the ESBL phenotype and examines the existence of blaSHV, blaCTX-M, and blaTEM genes in isolates.
Materials and Methods
Isolates of bacteria
In total, 68 consecutive non-duplicate of K. pneumoniae and E. coli isolates (n = 20 and 48, respectively) were retrieved from specimens of urine at a Thalassemia center in Erbil, Iraq. The samples were obtained from both outpatients and inpatients between July 2016 and September 2016. Standard microbiological techniques were used for isolation.10 Conventional microbiological procedures were employed to identify the isolates. Besides, the VITEK 2 compact system was utilized to re-identify them (BioMerieux, France).
Antimicrobial susceptibility testing
According to the guidelines of the Clinical and Laboratory Standards Institute (CLSI), the isolates were screened by the disc diffusion method (Kirby-Bauer disc diffusion method) on Mueller-Hinton agar (MHA) plates in order to test their antimicrobial susceptibility.11 The utilized antimicrobials included Amoxicillin+Clavulanic acid (20+10μg), Amikacin (10 μg), Azithromycin (15μg), Cefixime (5μg), Cefotaxime (30μg), Chloramphenicol (30μg), Ceftazidime (30μg), Ciprofloxacin (10μg), Doxycycline (30μg), Imipenem (10μg), Gentamicin (10μg), Kanamycin (30μg), Nalidixic acid (30μg), Meropenem (10μg), Nitrofurantoin (100μg), Norfloxacin(10μg), Ofloxacin (5μg), Streptomycin (25μg), Piperacillin (100μg), and Tobramycin (10μg).
Testing for production of ESBL (MDDST)
Using a disc of Amoxicillin-Clavulanate (20/10 μg) with four cephalosporins of Ceftriaxone, 3GC-Cefotaxime, 4GC-Cefepime, and Cefpodoxime, the Modified Double Disc Synergy Test (MDDST) was employed to test all strains in terms of their production of Extended Spectrum Beta-Lactamase (ESBL). A lawn culture belonging to the organisms was created on a Mueller-Hinton agar plate following the recommendations by CLSI.11 A disc that contained Amoxicillin-Clavulanate (20/10 μg) was put in the middle of the plate. The 3GC and 4GC discs were placed respectively 15mm and 20mm center-to-center apart from the center of the amoxicillin-clavulanate disc.12 Any increase or distortion in the zone toward the Amoxicillin-Clavulanate disc was regarded positive for the production of ESBL. According to CLSI guidelines, the combined disc test was used to confirm ESBL production.
Detection of ESBL genotypes by multiplex PCR amplification
Using the method utilized by Monstein et al. (2007) with slight modifications, multiplex PCR was employed to examine the positive isolates in the initial screening test for ESBL production for the existence of blaSHV, blaCTX-M, and blaTEM genes.13 Freshly cultured isolates bacteria were used to prepare template deoxyribonucleic acid (DNA) was prepared using PrestoTM Mini gDNA bacterial kit. All reactions of PCR were conducted by utilizing 2 μl DNA template (density of 10 ng/μl), the Master Mix consisting of 3 mM MgCl2, 0.2% Tween® 20, 20 mM Tris-HCl pH 8.5, (NH4)2S04, 0.4 mM of each dNTP, 0.4 μM of each primer, and 0.2 units/μl Ampliqon Taq DNA polymerase. The conditions of polymerase chain reaction amplification were set up as follow: primary denaturation step for 10 minutes at 95°C; 30 denaturation cycles for 30 seconds at 94°C, annealing 30 seconds at 60°C for, extension for 2 minutes at 72°C, and a final extension step for 10 minutes at 72°C. Using agarose gel electrophoresis, size separation PCR amplicons were utilized to detect respective genes (Table1).
Results
Antimicrobial susceptibility profile
In total, 68 consecutive non-duplicate of K. pneumoniae and E. coli isolates (n = 20 and 48, respectively) were retrieved, and their antimicrobial resistance profile against 20 different antimicrobial agents was tested. The current results revealed that K. pneumoniae and E. coli isolates vary widely to different antimicrobials. The resistance rates of isolates of K. pneumoniae and E. coli against the selected 20 antimicrobial agents obtained from urine samples. It was found that a majority of the K. pneumoniae and E. coli isolates were resistant to several drugs (multi-drug resistant: MDR) where a total of 87.5% and 75% of respectively E. coli and K. pneumoniae isolates indicated MDR phenotypes.
Furthermore, the results of the antimicrobial susceptibility test against E. coli revealed that E. coli showed 81.25% resistance to Amoxicillin+Clavulanic acid (Table 2), whereas susceptibility to doxycycline decreased to 39.5%. Similar patterns were observed for Piperacillin. Substantial decrease of 16.6–37.5% was observed in the susceptibility for all Cephalosporins. Imipenem, Meropenem, Amikacin, Gentamicin, Ciprofloxacin, Tobramycin and Ofloxacin with susceptibility rates of respectively 100%, 100%, 95.8%, 95.8%, 95.8%, 95.2%, and 93.75% were the most active agents against E. coli. Resistance to Azithromycin Norfloxacin and Streptomycin was comparatively less (10.5%, for them). On the other hand, E. coli showed a different sensitive rate to Chloramphenicol, Kanamycin, Nalidixic acid and Nitrofurantoin with 85.4%, 62.5%, 50% and 75%, respectively. Meanwhile, similar results were observed for K. pneumoniae which revealed that K. pneumoniae showed 65% resistant to Amoxicillin+Clavulanic acid, whereas susceptibility to Piperacillin dropped to 50% (Table 2).
Table 1.
List of primers used for Multiplex PCR amplification.
| Target gene | Primer | Sequence (5′-3′) | Amplicon size | References |
|---|---|---|---|---|
| blaTEM | Forward | TCG CCG CAT ACA CTA TTC TCA GAA TGA | 445-bp | [30] |
| Reverse | ACG CTC ACC GGC TCC AGA TTT AT | |||
| blaSHV | Forward | ATG CGT TATATT CGC CTG TG | 747-bp | [12] |
| Reverse | TGC TTT GTT ATT CGG GCC AA | |||
| blaCTX-M | Forward | ATG TGC AGY ACC AGT AAR GTK ATG GC | 593-bp | [30] |
| Reverse | TGG GTR AAR TAR GTS ACC AGA AYC AGC GG |
Table 2.
Antibiotic resistance pattern of K. pneumoniae and E. coli isolates.
| Name of Antibiotic | Symbol | E. coli | K. pneumoniae | ||
|---|---|---|---|---|---|
| Susceptible (%) | Resistance (%) | Susceptible (%) | Resistance (%) | ||
| Amikacin, | AK | 95.8 | 4.2 | 100 | 00 |
| Amoxicillin-Clavulanic acid | AMC | 18.75 | 81.25 | 35 | 65 |
| Azithromycin | AZM | 89.5 | 10.5 | 90 | 10 |
| Cefixime | CFM | 16.6 | 83.4 | 35 | 65 |
| Cefotaxime | CTX | 16.6 | 83.4 | 40 | 60 |
| Ceftazidime | CAZ | 37.5 | 62.5 | 30 | 70 |
| Chloramphenicol | C | 85.4 | 14.6 | 95 | 05 |
| Ciprofloxacin | CIP | 95.8 | 4.2 | 100 | 00 |
| Doxycycline | DOX | 39.5 | 60.5 | 95 | 05 |
| Gentamicin | CN | 95.8 | 4.2 | 100 | 00 |
| Imipenem | IPM | 100 | 00 | 100 | 00 |
| Kanamycin | KAN | 62.5 | 37.5 | 90 | 10 |
| Meropenem | MEM | 100 | 00 | 100 | 00 |
| Nalidixic acid | NA | 50 | 50 | 50 | 50 |
| Nitrofurantoin | F | 75 | 25 | 70 | 30 |
| Norfloxacin | NOR | 89.5 | 10.5 | 100 | 00 |
| Ofloxacin | OFL | 93.75 | 6.25 | 100 | 00 |
| Piperacillin | PIPER | 33.3 | 66.7 | 50 | 50 |
| Streptomycin | S | 89.5 | 10.5 | 95 | 05 |
| Tobramycin | TOB | 95.2 | 4.8 | 100 | 00 |
Similar patterns were noticed for Nalidixic acid. In addition, a substantial drop of 30–40% was observed in the susceptibility for all Cephalosporins. Nevertheless, K. pneumoniae showed a different sensitive rate to Chloramphenicol, Doxycycline, Streptomycin, Azithromycin, Kanamycin and Nitrofurantoin with 95%, 95%, 95%, 90%, 90% and 70% respectively.
ESBL screening of E. coli and K. pneumoniae. Out of the 48 E. coli isolates, a total of 37 isolates (77%) showed positive results in initial screening test of ESBL production by MDDST and phenotypic confirmatory test of ESBL production. Meanwhile, out of the 20 K. pneumoniae isolates, a total of 17 isolates (85%) showed positive results in initial screening test of ESBL production and phenotypic confirmatory test of ESBL production.
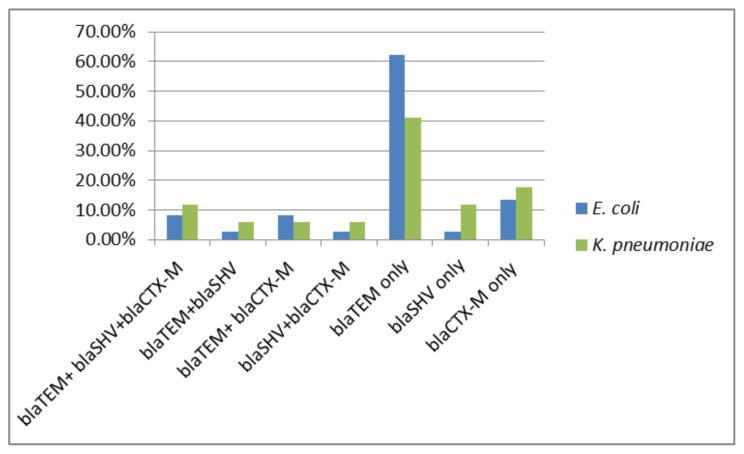
In PCR detection of ESBL genotypes, it was found that all of the ESBL screening positive K. pneumoniae and E. coli isolates had one or more ESBL genes that were tested in the present study. Overall, 85% (17/20) of K. pneumoniae and 77% (37/48) of E. coli isolates were positive for one or more ESBL genes. The multiplex PCR assay results indicated that 32.4% blaCTX-M genes, 16.2% blaSHV genes, and 81% blaTEM genes were detected in the E. coli isolates. Similarly, the isolates of K. pneumoniae contained 64.7% blaTEM, 35.2% blaSHV, and 41.1% blaCTX-M genes. The overall incidence of ESBL genotypes in K. pneumoniae and E. coli isolates is illustrated in Figure 1.
Figure 1.
The overall incidence of ESBL genotypes in screening positive K. pneumoniae and E. coli isolates.
Discussion
As a global challenge, antimicrobial resistance in pathogenic bacteria is accompanied with high rates of mortality and morbidity. In addition, because of multidrug resistant patterns, infections have been reported to be difficult or even impossible to treat with conventional antimicrobials. Because many healthcare centers fail to diagnose causative microorganisms and their patterns of antimicrobial susceptibility timely in patients with bacteremia and other serious infections, antibiotics are broadly, liberally and mostly unnecessarily used.14
In the current study, high prevalence of MDR isolates of K. pneumoniae and E. coli was noticed in the clinical samples. The overall prevalence of MDR phenotypes in K. pneumoniae and E. coli isolates was respectively 75% and 87.5%. Among the MDR isolates of E. coli and K. pneumoniae, a majority of them were producers of ESBL. Similar to the results of the present study, also in a research by Bora et al. (2014) reported the same ratios.15
In the current study, the antimicrobial susceptibility patterns were determined in all isolates, and the results obtained from the test of antimicrobial susceptibility against E. coli and K. pneumonia revealed that isolated bacteria were different in their susceptibility to the tested antimicrobials. Liao et al. (2017)4 and Tabar et al. (2016) reported similar results.16 Carbapenems are often the final influential therapy that exists for infections resulting from MDR Enterobacteriaceae.17 According to other studies, 100% sensitivity was seen with Imipenem and Meropenem, which has been reported to be the most effective antibiotic including the isolates that produce ESBLs. This is an important result of the present study because many infections can be treated with Carbapenemes. This result can be relevant to the fact that these antibiotics are more expensive and thus used less in this region.
Paterson et al. (2001) stated that even if ESBL producers show an in vitro susceptibility, they are intrinsically resistant to all cephalosporins.18 In the present study, 9% and 13% of the producers of ESBL were found to have false susceptibilities respectively to cefotaxime and Ceftazidime. This can be attributed to the fact that different ESBL enzymes possess various optimal substrate profiles.19
In fact, ESBLs are reported to be a challenge among hospitalized patients all over the world. It has also been reported that ESBLs have different prevalence rates among clinical isolates in different parts of the world, and there is a rapid continuous change in their prevalence rate over time.20 Given the increased prevalence of ESBLs-producing Enterobacteriaceae, it is highly crucial to develop laboratory testing methods in order to accurately diagnose the existence of such enzymes in clinical isolates.21 Among all ESBL detection methods, modified double disc synergy tests were the most sensitive ones.22 A study carried out by23 presented similar findings and indicated positive MDDST in 40/40 isolates, while it was positive in 25/40 and 39/40 isolates respectively in double disk synergy test (DDST) and phenotypic confirmatory disc diffusion test (PCDDT).
By following the MDDST screening criteria for ESBL production, respectively 85% and 77% of K. pneumoniae and E. coli isolates were screened for detecting production of ESBL. Existence of one or more ESBL genes in all screened positive isolates revealed that K. pneumoniae and E. coli isolates that produce ESBL are highly frequent in the geographical region under investigation. In India, Kaur et al. (2013) observed that 63.4% E. coli and 60.3% K. pneumoniae isolates produced ESBL.24 Phenotypic tests for detection of ESBL can only confirm ESBL production but fail to recognize the subtypes of ESBL. As reported by Nüesch-Inderbinen et al. (1996), molecular methods have been proved to be sensitive, but they costly and conducting them requires a long time, expertise, and specialized equipment.25 Ultimate identification is only probable through methods of molecular detection. The results of a study conducted by (Navon-Venezia et al., 2003) revealed that it is necessary to periodically evaluate these phenotypic tests because introduction of new enzyme can change their performance.26 In their study of phenotypic and genotypic methods of ESBL detection, (Grover et al., 2006) stated that PCR is a reliable method for detecting ESBL.27 In the present study, multiplex PCR amplification assay was utilized to detect blaCTX-M, blaSHV, and blaTEM genes in the retrieved clinical isolates of K. pneumoniae and E. coli because one of the advantages of this assay rapid screening of large numbers of clinical isolates, moreover, if it is required, further molecular epidemiological studies can take advantage of the DNA that is isolated via this assay.13
Furthermore, it is essential to identify beta-lactamase in order to conduct a reliable epidemiological investigation into antimicrobial resistance. The current study was conducted to survey antimicrobial drug resistance, ESBL phenotypes, and blaSHV, blaTEM and blaCTX-M genes detection in K. pneumonia and E. coli isolates retrieved from urinary tract infections in Erbil, Iraq.
The most globally common type of ESBL appeared to be CTX-M-type ESBLs with their higher incidence in most locations compared to SHV and TEM ESBLs.28 Among the three ESBL genotypes included in this study, the most prevalent one was found to be blaTEM (81%) and blaTEM (64.7%) respectively in ESBL-producing isolates of E. coli and K. pneumoniae. The less prevalent ESBL genotype was blaSHV, and the prevalence rate of blaSHV in ESBL-producing K. pneumoniae isolates (35.2%) was higher than E. coli isolates (16.2%). Also, the prevalence rate of blaCTX-M in ESBL-producing K. pneumoniae isolates (41.1%) was higher than E. coli isolates (32.4%). It was found that all of the ESBL-producing isolates of both organisms were positive for one or more ESBL genotypes. It was observed that blaTEM alone was more prevalent in E. coli (62.16%, 23/37), and in K. pneumoniae (41.17%, 7/17), while blaCTX-M and blaTEM together predominated in E. coli (8.1%), while blaSHV, blaTEM, and blaCTX-M together predominated in isolates of K. pneumoniae (11.76%). A study conducted by Manoharan et al. (2011) reported similar findings.29 In the present study; however, TEM ESBL was the prevalent genotype and CTX-M-type ESBL was not prevalent. The discrepancy is assumed to be because of regional variations, since the strains collected and evaluated in the current study were only from Erbil, Iraq.
Furthermore, in another study, Moghnieh et al. (2018) have reported that E. coli and Klebsiella spp resistance to third-generation cephalosporins is usual in whole countries, with outbreak reaching over 50% in Egypt and Syria30 and in our study, 30–40% was observed in the susceptibility for all Cephalosporins, which this prevalence is close to other Arabia countries, as well as in Moghnieh study, they reported that carbapenem resistance is emerging, albeit with a prevalence of less than 10%.30 In parallel, we have found that the most active antimicrobial agents in vitro remained to be the carbapenems. Khalaf and Al-Ouqaili et al. (2018) in Baghdad, during a period one year demonstrated that SHV gene was detected only in 12.5% E. coli, and 56.25% in K. pneumoniae.31 Approximately, we found close to findings above that 16.2% SHV genes in E. coli and 35.2% SHV genes existed in the isolates of K. pneumoniae. Of course, according the above findings several studies by Teawtrakul et al. (2015) Girmenia et al. (2016), Ricciardi et al. (2016) and Devrim et al. (2018) have shown that the rates and types of Klebsiella and Escherichia strains isolated are differed in other countries.32–35 These outcomes highlight require for structured national plans in the zone to target infection prevention and antimicrobial supervision.
Conclusions
Knowledge of the antimicrobial resistance patterns and resistance genes of bacterial pathogens in a geographical area is important for control and surveillance of antibiotic resistance. The results of the present study revealed that MDR was highly prevalent. In addition, the Carbapenems, Amikacin, and Ciprofloxacin were found to be the most to the least active antimicrobial agents in vitro. Based on the results obtained in the present study, TEM was highly prevalent among other types of ESBLs.
Acknowledgments
This work was supported by the Biology Department, Education College, Salahaddin University and Thalassemia Hospital in Erbil.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Gaynes R, Edwards JR National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 2.Ojdana D, Sacha P, Wieczorek P, Czaban S, Michalska A, Jaworowska J. The Occurrence of blaCTX-M, blaSHV, and blaTEM Genes in Extended-Spectrum β-Lactamase-Positive Strains of Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis in Poland. Intern J Antibi. 2014:7. doi: 10.1155/2014/935842. Article ID 935842. [DOI] [Google Scholar]
- 3.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao K, Chen Y, Wang M, Guo P, Yang Q, Ni Y, Yu Y, Hu B, Sun Z, Huang W. Molecular characteristics of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae causing intra-abdominal infections from 9 tertiary hospitals in China. Diagn Microbiol Infect Dis. 2017;87(1):45–48. doi: 10.1016/j.diagmicrobio.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Malloy AM, Campos JM. Extended-spectrum beta-lactamases: a brief clinical update. Pediatr Infect Dis J. 2011;30(12):1092–3. doi: 10.1097/INF.0b013e31823c0e9d. [DOI] [PubMed] [Google Scholar]
- 6.Akpaka PE, Legall B, Padman J. Molecular detection and epidemiology of extended-spectrum beta-lactamase genes prevalent in clinical isolates of Klebsiella pneumoniae and E coli from Trinidad and Tobago. West Indian Med J. 2010;59(6):591–6. [PubMed] [Google Scholar]
- 7.Fraser A, Paul M, Almanasreh N, Tacconelli E, Frank U, Cauda R, Borok S, Cohen M, Andreassen S, Nielsen AD, Leibovici L TREAT Study Group. Benefit of appropriate empirical antibiotic treatment: thirty-day mortality and duration of hospital stay. Am J Med. 2006;119(11):970–6. doi: 10.1016/j.amjmed.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Livermore DM, Paterson DL. Pocket Guide to Extended-spectrum [beta]-lactamases in Resistance. Current Medicine Group; London, UK: 2006. [Google Scholar]
- 9.Sharma J, Sharma M, Ray P. Detection of TEM & SHV genes in Escherichia coli & Klebsiella pneumoniae isolates in a tertiary care hospital from India. Indian J Med Res. 2010;132:332–6. [PubMed] [Google Scholar]
- 10.Collee JG, Miles RS, WB . Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, SA, editors. Mackie & McCartney practical medical microbiology. Churchill Livingstone; Edinburgh: 1996. [Google Scholar]
- 11.Wayne P. Performance standards for antimicrobial susceptibility testing. 2011. Clinical and laboratory standards institute. [PubMed] [Google Scholar]
- 12.Paterson DL, Hujer KM, Hujer AM, Yeiser B, Bonomo MD, Rice LB, Bonomo RA International Klebsiella Study Group. Extended-spectrum β-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: Dominance and widespread prevalence of SHV-and CTX-M-type β-lactamases. Antimicrob Agents Chemother. 2003;47(11):3554–60. doi: 10.1128/AAC.47.11.3554-3560.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monstein HJ, Ostholm-Balkhed A, Nilsson MV, Nilsson M, Dornbusch K, Nilsson LE. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS. 2007;115(12):1400–8. doi: 10.1111/j.1600-0463.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 14.Akova M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence. 2016;7:252–266. doi: 10.1080/21505594.2016.1159366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bora A, Hazarika NK, Shukla SK, Prasad KN, Sarma JB, Ahmed G. Prevalence of blaTEM, blaSHV and blaCTX-M genes in clinical isolates of Escherichia coli and Klebsiella pneumoniae from Northeast India. Indian J Pathol Microbiol. 2014;57(2):249–54. doi: 10.4103/0377-4929.134698. [DOI] [PubMed] [Google Scholar]
- 16.Tabar MM, Mirkalantari S, Amoli RI. Detection of ctx-M gene in ESBL-producing E. coli strains isolated from urinary tract infection in Semnan, Iran. Electron Physician. 2016;8(7):2686–90. doi: 10.19082/2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitout JD. The latest threat in the war on antimicrobial resistance. The Lancet Infectious Diseases. 2010;10:578–579. doi: 10.1016/S1473-3099(10)70168-7. [DOI] [PubMed] [Google Scholar]
- 18.Paterson DL, Ko WC, Von Gottberg A, Casellas JM, Mulazimoglu L, Klugman KP, Bonomo RA, Rice LB, McCormack JG, Yu VL. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum β-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol. 2001;39(6):2206–12. doi: 10.1128/JCM.39.6.2206-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong-Beringer A. Therapeutic challenges associated with extended-spectrum, beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Pharmacotherapy. 2001;21(5):583–92. doi: 10.1592/phco.21.6.583.34537. [DOI] [PubMed] [Google Scholar]
- 20.Babypadmini S, Appalaraju B. Extended spectrum-lactamases in urinary isolates of Escherichia coli and Klebsiella pneumoniae-prevalence and susceptibility pattern in a tertiary care hospital. Indian J Med Microbiol. 2004;22(3):172–4. [PubMed] [Google Scholar]
- 21.Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14(4):933–51. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modi D, Patel D, Patel S, Jain M, Bhatt S, Vegad M. Comparison of various methods for the detection of extended spectrum beta lactamase in Klebsiella pneumoniae isolated from neonatal Intensive Care Unit, Ahmedabad. Natl J Med Res. 2012;2(3):348–353. [Google Scholar]
- 23.Khan MK, Thukral SS, Gaind R. Evaluation of a modified double-disc synergy test for detection of extended spectrum β-lactamases in AMPC β-lactamase-producing Proteus mirabilis. Indian J Med Microbiol. 2008;26(1):58–61. doi: 10.4103/0255-0857.38860. [DOI] [PubMed] [Google Scholar]
- 24.Kaur J, Chopra S, Sheevani, Mahajan G. Modified double disc synergy test to detect ESBL production in urinary isolates of Escherichia coli and Klebsiella pneumoniae. J Clin Diagn Res. 2013;7(2):229–33. doi: 10.7860/JCDR/2013/4619.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nüesch-Inderbinen MT, Hächler H, Kayser FH. Detection of genes coding for extended-spectrum SHV beta-lactamases in clinical isolates by a molecular genetic method, and comparison with the E test. Eur J Clin Microbiol Infect Dis. 1996;15(5):398–402. doi: 10.1007/BF01690097. [DOI] [PubMed] [Google Scholar]
- 26.Navon-Venezia S, Hammer-Munz O, Schwartz D, Turner D, Kuzmenko B, Carmeli Y. Occurrence and phenotypic characteristics of extended-spectrum β-lactamases among members of the family Enterobacteriaceae at the Tel-Aviv Medical Center (Israel) and evaluation of diagnostic tests. J Clin Microbiol. 2003;41(1):155–8. doi: 10.1128/JCM.41.1.155-158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grover SS, Sharma M, Chattopadhya D, Kapoor H, Pasha ST, Singh G. Phenotypic and genotypic detection of ESBL mediated cephalosporin resistance in Klebsiella pneumoniae: emergence of high resistance against cefepime, the fourth generation cephalosporin. J Infect. 2006;53(4):279–88. doi: 10.1016/j.jinf.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen JH, McElmeel ML, Fulcher LC, Zimmer BL. Detection of CTX-M-type extended-spectrum beta-lactamase (ESBLs) by testing with MicroScan overnight and ESBL confirmation panels. J Clin Microbiol. 2010;48(1):120–3. doi: 10.1128/JCM.01507-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manoharan A, Premalatha K, Chatterjee S, Mathai D SARI Study Group. Correlation of TEM, SHV and CTX-M extended-spectrum beta lactamases among Enterobacteriaceae with their in vitro antimicrobial susceptibility. Indian J Med Microbiol. 2011;29(2):161–4. doi: 10.4103/0255-0857.81799. [DOI] [PubMed] [Google Scholar]
- 30.Moghnieh RA, Kanafani ZA, Tabaja HZ, Sharara SL, Awad LS, Kanj SS. Epidemiology of common resistant bacterial pathogens in the countries of the Arab League. Lancet Infect Dis. 2018;18(12):e379–e394. doi: 10.1016/S1473-3099(18)30414-6. [DOI] [PubMed] [Google Scholar]
- 31.Khalaf EA, Al-Ouqaili MTS. Molecular detection and sequencing of SHV gene encoding for extended-spectrum β-lactamases produced by multidrug resistance some of the Gram-negative bacteria. Intern J Green Pharm. 2018;12(4):S918. doi: 10.22377/ijgp.v12i04.2274. [DOI] [Google Scholar]
- 32.Teawtrakul N, Jetsrisuparb A, Sirijerachai C, Chansung K, Wanitpongpun C. Severe bacterial infections in patients with non-transfusion-dependent thalassemia: prevalence and clinical risk factors. Int J Infect Dis. 2015;39:53–6. doi: 10.1016/j.ijid.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Ricciardi W, Giubbini G, Laurenti P. Surveillance and Control of Antibiotic Resistance in the Mediterranean Region. Mediterr J Hematol Infect Dis. 2016;8(1):e2016036. doi: 10.4084/mjhid.2016.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devrim F, Serdaroğlu E, Çağlar İ, Oruç Y, Demiray N, Bayram N, Ağın H, Çalkavur S, Sorguç Y, Dinçel N, Ayhan Y, Yılmaz E, Devrim I. The Emerging Resistance in Nosocomial Urinary Tract Infections: From the Pediatrics Perspective. Mediterr J Hematol Infect Dis. 2018 Sep 1;10(1):e2018055. doi: 10.4084/mjhid.2018.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Girmenia C, Serrao A, Canichella M. Epidemiology of Carbapenem Resistant Klebsiella pneumoniae Infections in Mediterranean Countries. Mediterr J Hematol Infect Dis. 2016;8(1):e2016032. doi: 10.4084/mjhid.2016.032. [DOI] [PMC free article] [PubMed] [Google Scholar]