Abstract
Background
Deferoxamine (DFO) or Deferiprone (DFP) or Deferasirox (DFX) monotherapy and DFO and DFP combination therapy (DFO+DFP) were four commonly implemented now chelation regimens for the iron overloaded of β-thalassemia major. This systematic review aims to determine the cost-effectiveness of four chelation regimens and provide evidence for the rational use of chelation regimens for β-thalassemia major therapy in the clinic.
Methods
A systematic literature search in MEDLINE, EMBASE, the Cochrane Library, China Biology Medicine, China National Knowledge Infrastructure, VIP Data, and WanFang Data was conducted in April 2018. In addition, a manual search was performed. Two researchers, working independently, selected the papers, extracted the data, and assessed the methodological quality of the included documents. Each included paper was evaluated using a checklist developed by Drummond et al.
Results
The number of records was initially 968, and eight papers met the final eligibility criteria. All the included eight papers were cost-utility analyses, and their methodological quality was fair. In these eight papers, nineteen studies were present. Nine studies of DFX versus DFO had contradictory results. Out of the nineteen studies, three studies of DFX versus DFP established that using DFP was cost-effective. Three studies of DFP versus DFO proved that using DFP was cost-effective. One survey of DFO+DFP versus DFO found that using DFO was cost-effective. One study of DFO+DFP versus DFP found that using DFP was cost-effective. Moreover, there were two studies of DFO+DFP versus DFX, but we cannot be sure which one of two chelation regimens was cost-effective.
Conclusion
In brief, DFP is cost-effective, followed by DFO or DFX, when an iron chelator is to be used alone for β-thalassemia iron overload treatment. All studies that compared DFO+DFP with DFO (or DFP) monotherapy established that the DFO+DFP was not cost-effective. Existing studies about DFO+DFP versus DFX could not prove which one of two chelation regimens was cost-effective. However, due to the low number of DFO+DFP versus DFO (or DFP or DFX) monotherapy studies, more extensive, high-quality research is required for further analysis and confirmation of our findings. Moreover, the cost-effectiveness is not an absolute issue when in different countries (regions) the results are opposite for other countries (regions). As a result, the local/national context had a substantial influence on the results of the pharmacoeconomic evaluation.
Keywords: β-thalassemia major, Deferoxamine, Deferiprone, Deferasirox, Cost-effectiveness, Systematic Review
Introduction
β-thalassemia major is hemolytic anemia caused by inhibition of the synthesis of the β-globin peptide chain, caused by gene mutation.1 β-thalassemia major patients are homo- or double-heterozygous (beta0/beta0 or beta+/beta+ or beta0/beta+), whose parents are carriers of the β-thalassemia gene (beta0 or beta+).2,3 Approximately 1.5% of the world’s population carries the β-thalassemia gene, and the number of newborns diagnosed with homo- or double-heterozygous β-thalassemia every year exceeds 200,000.4,5 A survey on 600,000 people in China in the 1980s showed an average prevalence rate of β-thalassemia of 0.67%,6 reaching almost 2% in some instances.7 This disease causes from 50,000 to 100,000 deaths per year, or from 0.5% to 0.9% of all deaths of children under five years of age in low-income or middle-income countries.8
Patients with β-thalassemia major usually suffer from anemia throughout their lives. Moreover, they need a long-term regular blood transfusion to attenuate anemia and reduce mortality.1,9 Unfortunately, the human body does not have an iron excretory pathway, which leads to the accumulation of iron from the transfused blood, known as iron overloaded. The excessive iron is then deposited in the heart, liver, endocrine glands, and other organs, causing heart failure, liver damage, diabetes, hypogonadism, thyroid dysfunction, etc., eventually leading to death.9 Drug therapy with iron chelators is the only treatment used to clear the excessive iron from the body and reduce the end-organs damage and associated mortality. Three iron chelators have been licensed worldwide: Deferoxamine (DFO), Deferiprone (DFP), and Deferasirox (DFX).1,9
DFO appeared into the market in the 1970s and is the first-line drug for iron overload. The usual dose of DFO is 50 mg/Kg once a day. DFO is administered as a subcutaneous infusion for 8–12 h, 5–7 times per week, due to its short plasma half-life (20–30 min) and absence of oral activity.10–12 The life quality and compliance of patients are reduced by these lengthy infusions. Low compliance with DFO has a higher risk of iron overload-related complications and death.13 A literature review has shown that the rate of patients with good compliance had a 36% lower incidence of cardiac complications than patients with low compliance, and the RR for mortality of non-compliant group versus compliant group was 12.6.14 To overcome the disadvantages of DFO treatment, oral iron chelators, including DFP and DFX, were introduced.
DFP is a second-line drug, which means that when patients are unable to use DFO or are dissatisfied with the efficacy of DFO, they can use DFP. The usual dose of DFP is 75–100 mg/Kg once a day in three separate doses. DFP exists under the form of tablets and oral solution, and the latter can be used in one-year-old children. The common adverse reactions of DFP are gastrointestinal discomfort, arthralgia, joint effusion, vomiting, and zinc deficiency.15 Moreover, the severe adverse reactions of the treatment with DFP are neutropenia and agranulocytosis.16 To reduce the risk of agranulocytosis, patients who are using DFP need weekly blood cell count tests.
DFX is the latest oral iron chelator and is mainly used in patients aged two years and older. The usual dose of DFX is 20–40 mg/Kg once a day. The common adverse reactions of DFX are the following: gastrointestinal symptoms, rash, serum creatinine increased, and alanine aminotransferase increased.15,17,18 Moreover, the severe adverse reactions of DFX treatment are renal failure, liver failure, and related death. So it is necessary to make baseline and monthly monitoring of serum creatinine, serum transaminases, and bilirubin.19 Pediatric patients need to be focused on. The last study reported that DFX causes acute kidney injury (AKI) in a dose-dependent manner, and pediatric patients face a high risk of AKI and a cycle of repeated kidney injury due to a higher DFX exposure at a given eGRF.20 The US Food and Drug Administration recommended dose reduction or interruption when physicians often observed fever or dehydration adverse effects in children receiving DFX.19
The DFO, DFP, and DFX monotherapies can effectively reduce the liver iron concentration (LIC) and serum ferritin level (SFL) to avoid iron overload.21,22 A meta-analysis of 16 randomized controlled trials showed that three iron chelators monotherapies have the same effect for decreasing LIC, while DFX can reduce SFL more effectively.22 Heart disease related to myocardial iron deposition remains a major cause of morbidity and the primary cause of mortality in β-thalassemia major patients.23–25 Cardiac complications are reported to be the cause of the deaths in 71% of these patients.26,27 A multicentre prospective survey showed that DFO, DFP, and DFX monotherapies not only in removing iron from the liver, but also in improving myocardial siderosis and biventricular function.28 When the efficacy of DFO or DFP monotherapy is low, combination therapy of DFO and DFP (DFO+DFP) can be used. The Guidelines from the US, Italy, Australia, and China all recommended that β-thalassemia major patients with over iron-related cardiomyopathy should receive DFO+DFP.15,29–31
As β-thalassemia major patients need to use iron chelators for their whole life, the treatment cost is astronomic. It causes not only enormous economic pressure on the families of patients but also huge overall socio-economic losses to society as a whole. For example, Sheth et al. reported that the annual average treatment cost of adult transfusion-dependent β-thalassemia patients in the United States of America (USA) was United States dollar (USD) 128,062.32 Additionally, Esmaeilzadeh et al. found that the treatment of the approximately 18,000 β-thalassemia major patients in Iran led to an annual loss of nearly USD 150 million to Iran’s health system.33 Another study also showed that the annual average treatment cost of β-thalassemia major patients with blood transfusion and iron excretion in China was over the Chinese Yuan (CNY) 100,000, resulting in economic losses of more than CNY 1.4 billion per year.2 Therefore, the specific costs and the overall economic burden of the application of different chelation regimens in the treatment of β-thalassemia major areas are of substantial importance.
A large number of pharmacoeconomic studies on chelators in β-thalassemia major patients have been performed worldwide but there was no systematic review to summarize the existing evidence. Meanwhile, some results are inconsistent. In the comparison of DFX versus DFO, Delea et al.34 thought DFX was cost-effective but Luangasanatip et al.35 thought the opposite.
Therefore, the authors of this paper systematically reviewed the existing publications in the pharmacoeconomic literature related to the four chelation regimens, DFO or DFP or DFX monotherapy and DFO+DFP. This study aimed to determine the cost-effectiveness of four chelation regimens and provide evidence for the rational use of chelation regimens for β-thalassemia major therapy in the clinic.
Methods
Search strategy
A systematic literature search in MEDLINE (PubMed),36 EMBASE (Ovid),37 Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane Library),38 Health Technology Assessment Database (HTAD, Cochrane Library),38 NHS Economic Evaluation Database (NHS EED, Cochrane Library),38 China Biology Medicine (CBM, SinoMed),39 China National Knowledge Infrastructure (CNKI),40 VIP Data,41 and Wanfang Data42 were conducted on April 2018, with no restrictions on the date. In addition, a manual search was performed to identify conferences and symposia. Chinese and English search terms were both used. Searches of these databases were performed using Mesh terms such as “thalassaemia”, “beta-thalassemia”, “iron overload”, “iron chelating agents”, “deferoxamine”, “deferiprone”, “deferasirox”, “pharmacoeconomics”, “cost-benefit analysis”, “cost-effectiveness analysis”, “cost-utility analysis” and “cost analysis”; other search terms included Cooley’s anaemia, Mediterranean anaemia, beta type thalassemia, beta type microcytemia, hemoglobin F disease, erythroblastic anemia, thalassemia major, transfusional hemosiderosis, iron chelate, iron chelating, iron chelation, desferrioxamine, desferrioxamine, Hdmpp, L1, pharmaceutical economics, marginal analysis, cost-benefit, cost comparison, cost-minimization analysis, cost measure, and their variations.
Inclusion criteria
Population: β-thalassemia major patients with chronic iron overload requiring blood transfusions;
Intervention: DFO monotherapy or DFP monotherapy or DFX monotherapy, or DFO+DFP;
Control: DFO monotherapy or DFP monotherapy DFX monotherapy, or DFO+DFP, or placebo;
Outcome: incremental cost-effectiveness ratios (ICER, ICER = incremental cost/incremental effectiveness);
Study type: the full economic evaluation studies, including cost-effectiveness analyses (CEA), cost-utility analyses (CUA), cost-benefit analyses (CBA) and cost-minimization analyses (CMA);
Exclusion criteria
Patients with other diseases;
Patients with bone marrow transplantation;
The language was not Chinese or English;
The published study was not with a full text, including abstract, poster, comment, etc.
Papers selection and data extraction
The study selection and data extraction were conducted independently by two researchers. The differences between the decisions of the reviewers were resolved by consensus. All double papers were excluded, and the remaining papers were assessed in a two-stage procedure. Firstly, the title and abstract were screened using the defined inclusion and exclusion criteria. If reviewers could not determine from the abstract and title whether a paper met the eligibility criteria, the paper was excluded. Secondly, the full texts of the remaining papers were assessed for inclusion. Further, we collected the following data from each included paper: (1) Basic information: the first author, year, country, study type, study method, etc.; (2) The basic characteristics of patients: age, treatment, etc.; (3) Economic parameters: perspective, discount, incremental costs (ΔCOST), incremental quality-adjusted life year (ΔQALY), ICER, payment threshold, etc.
Methodological quality assessment
Two researchers independently assessed the methodological quality of the included papers. Differences in the decisions between the reviewers were resolved by consensus. Each included paper was evaluated using a checklist developed by Drummond et al. (the Drummond checklist).43 The Drummond checklist consists of 10 questions. Each paper was scored for quality using a scale from 0 to 10 points. If “yes” was the answer to the question, question received a score of 1 point, whereas if the answer was “no” or “uncertain,” the score was 0 points. A rating of “A” (9–10 points), “B” (7–8 points), “C” (5–6 points), and “D” (0–4 points) was assigned.44
Results
Search results
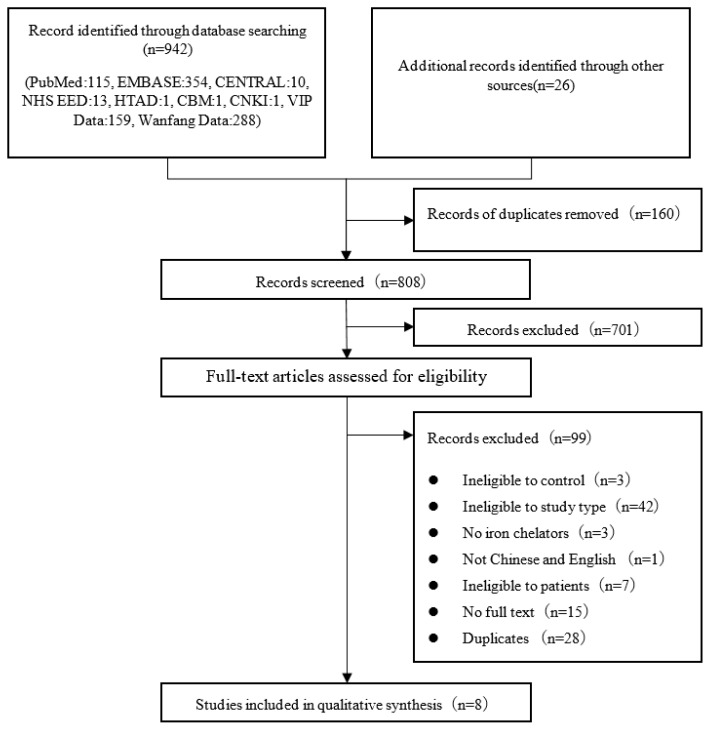
The overall search results and steps with elimination schema are provided in Figure 1. The initial number of records was 968, but after evaluation of the abstract and the full text, only eight papers met the final eligibility criteria.34,35,45–50
Figure 1.
Flow diagram of articles identified and evaluated.
Properties of the included papers
Table 1 provides the characters of the included papers, and Table 2 displays the results of the included studies. Remarkably, one paper49 adopted two perspectives for the analysis, including the perspective of the public payer for health services in Poland (Polish National Health Fund, NHF) and the perspective of the patient NHF. However, the results for both perspectives were identical. The three health states in Ho et al.47 were β-thalassemia with complications, β-thalassemia without complications, and death. The complications included cardiac disease, diabetes mellitus, hypogonadism, hypoparathyroidism, and hypothyroidism.
Table 1.
The characters of included papers.
| Study ID | Country | Study Type | Perspective | Study Method | Time Horizon (year) | Health State | Cost | Sensitivity Analysis |
|---|---|---|---|---|---|---|---|---|
| Delea 200734 | US | CUA* | Health service | Markov model | 50 | β-thalassemia with cardiac complication, β-thalassemia without cardiac complication and death | direct medical cost: chelation, DFO administration, complication therapies(treatment of iron overload-related cardiac disease) | One-way & two-way & probabilistic |
| Luangasanatip 201135 | Thailand | CUA | Society | Markov model | lifetime | β-thalassemia with cardiac complication, β-thalassemia without cardiac complication and death |
direct medical cost: chelation, cost of a medical visit, DFO administration, adverse events management cost, complication therapies(treatment of iron overload-related cardiac disease) direct non-medical cost: transportation cost additional food cost, caregiver cost indirect cost: productivity |
One-way & probabilistic |
| Karnon 201245 | Australian | CUA | Health service | Markov model | 50 | β-thalassemia with cardiac and endocrine complications, β-thalassemia without cardiac and endocrine complications and death | direct medical cost: chelation, adverse events management cost, additional monitoring, complication therapies | One-way & Multi-way & probabilistic |
| Bentley 201346 | UK | CUA | Health service | Markov model | 5 | β-thalassemia with cardiac complication, β-thalassemia without cardiac complication and death | direct medical cost: chelation, DFO administration, monitoring, adverse events management cost | One-way & two-way & probabilistic |
| Ho 201347 | China Taiwan |
CUA | Health service | Markov model | 50 | β-thalassemia with complications, β-thalassemia without complications and death | direct medical cost: chelation, DFO administration, complication therapies | One-way |
| Keshtkaran 201348 | Iran | CUA | Society | Markov model | lifetime | β-thalassemia with cardiac complication, β-thalassemia without cardiac complication and death |
direct medical cost: chelation, DFO administration, adverse events management cost, complication therapies(treatment of iron overload-related cardiac disease) indirect cost: transfusion time |
One-way & probabilistic |
| Walczak 201349 | Poland | CUA | NHF# & patient and NHF | Simple decision model | 1 | - | direct medical cost: chelation, DFO administration, Cost of hospitalizations for monitoring and blood transfusions, Cost of blood units, Cost of additional monitoring | - |
| Pepe 201750 | Italy | CUA | Health service | Markov model | 5 | β-thalassemia with cardiac complication, β-thalassemia without cardiac complication and death | direct medical cost: chelation, DFO administration, monitoring | One-way & two-way & probabilistic |
CUA: cost-utility analyses
NHF: Polish National Health Fund.
Table 2.
The results of included studies.
| study ID | Age | Discount (%) | currency* | ΔCOST | ΔQALY | ICER (ΔCOST/ΔQALY) | Payment threshold | Conflicts of interest | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Δdirect medical care costs | Δdirect non-medical care cost | Δindirect cost | ΔCOSTt# | |||||||||||
|
|
|
|||||||||||||
| Cost | Outcome | Chelation | DFO Administration | Other | ||||||||||
| DFX VS DFO | ||||||||||||||
| Delea 200734 | 3 | 3 | 3 | USD | 313823 | −179331 | −8474 | 126018 | 4.500 | 28255 | 100000 | Novartis Parma | ||
| Luangasanatip 201135 | 6 | 3 | 3 | USD | 526996 | −3679 | −453 | 0 | 522863 | 5.770 | 90618 | 8707 | No conflicts | |
| Karnon 201245 | 6 | - | - | GBP | 102017 | −170533 | −3573 | −72089 | 4.850 | −14864 | - | Novartis Parma | ||
| Karnon 201245 | 2 | - | - | GBP | 91745 | −171190 | −3399 | −82846 | 5.020 | −16503 | - | Novartis Parma | ||
| Bentley 201346 | - | 3.5 | 3.5 | GBP | 80670 | −44429 | −1321 | 34921 | 0.813 | 42953 | 20000 | ApoPharma | ||
| Ho 201347 | 2 | 3 | 3 | USD | 46889 | −9078 | −1519 | 36291 | 2.300 | 15596 | 100000 | - | ||
| Keshtkaran 201348 % | - | 5 | 3 | USD | −2772 | −415.9 | 358 | −4525 | −96493 | 4.520 | −21348 | 39159 | No conflicts | |
| Walczak 201349 | 2–15 | PLN | 4376 | 336 | 4712 | 0.180 | 26180 | 105801 | - | |||||
| Pepe 201750 | - | 3 | 3 | EUR | 103113 | −12034 | 707 | 91787 | 0.826 | 111118 | 20000 | ApoPharma | ||
|
| ||||||||||||||
| DFX VS DFP | ||||||||||||||
| Luangasanatip 201135 | 6 | 3 | 3 | USD | 614067 | −39 | −48 | 0 | 613980 | 5.7700 | 106409 | 8707 | No conflicts | |
| Bentley 201346 | - | 3.5 | 3.5 | GBP | 80497 | 0 | −327 | 80172 | −0.099 | −809818 | 20000 | ApoPharma | ||
| pepe 201750 | - | 3 | 3 | EUR | 134021 | 0 | 218 | 134240 | −0.107 | −1254579 | 20000 | ApoPharma | ||
|
| ||||||||||||||
| DFP VS DFO | ||||||||||||||
| Luangasanatip 201135 | 6 | 3 | 3 | USD | −87071 | −3640 | −406 | 0 | −9117 | 0 | Dominant | 8707 | No conflicts | |
| Bentley 201346 | - | 3.5 | 3.5 | GBP | 173 | −44429 | −994 | −45251 | 0.912 | −49617 | 20000 | ApoPharma | ||
| Pepe 201750 | - | 3 | 3 | EUR | −30908 | −12034 | 489 | −42453 | 0.933 | −45502 | 20000 | ApoPharma | ||
|
| ||||||||||||||
| DFO+DFP VS DFO | ||||||||||||||
| Bentley 201346 | - | 3.5 | 3.5 | GBP | 21034 | −8226 | −1025 | 14205 | 0.240 | 59093 | 20000 | ApoPharma | ||
|
| ||||||||||||||
| DFO+DFP VS DFP | ||||||||||||||
| Bentley 201346 | - | 3.5 | 3.5 | GBP | 20861 | 36203 | 2391 | 59456 | −0.672 | −88476 | 20000 | ApoPharma | ||
|
| ||||||||||||||
| DFO+DFP VS DFX | ||||||||||||||
| Karnon 201245 (high-dose DFX) | 6 | - | - | GBP | −144599 | 127388 | 4902 | −12310 | −2.500 | 4925 | - | Novartis Parma | ||
| Bentley 201346 | - | 3.5 | 3.5 | GBP | −59636 | 36203 | 2718 | −20716 | −0.573 | 36154 | 20000 | ApoPharma | ||
USD: United States dollar, GBP: Great Britain Pound, EUR: European Dollar, PLN: Polish Zloty.
ΔCOSTt=Δdirect medical care costs+Δdirect non-medical care cost+Δindirect cost.
Annual cost.
The cost of three main components was considered in the included papers: direct medical cost, direct non-medical cost, and indirect cost. The different papers have collected different cost data. The specific costs are shown in Table 1 and Table 2. As can be seen in Table 2, the costs affecting the economy of the four chelation regimens were predominantly constituted by the chelation cost, DFO administration cost, and indirect cost.
Methodological quality assessment
The results of the methodological quality assessment using the Drummond checklist are presented in Table 3. Out of the eight papers, six papers (75.00%)34,35,46–48,50 were considered as A, one paper (12.50%)45 as B, and one paper (12.50%)49 as C, without D. The papers scored well on questions 1, 2, 3, 5, 6, 8, 9, and worse on questions 4, 7, 10.
Table 3.
Results of the methodological quality assessment*
| Question# | Point | Rating | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| study ID | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | ||
| Delea 200734 | Y | Y | Y | Y | Y | Y | Y | Y | Y | UN | 9 | A |
| Luangasanatip 201135 | Y | Y | Y | Y | Y | Y | Y | Y | Y | UN | 9 | A |
| Karnon 201245 | Y | Y | Y | Y | Y | Y | N | Y | Y | UN | 8 | B |
| Bentley 201346 | Y | Y | Y | Y | Y | Y | Y | Y | Y | UN | 9 | A |
| Ho 201347 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10 | A |
| Keshtkaran 201348 | Y | Y | Y | Y | Y | Y | Y | Y | Y | UN | 9 | A |
| Walczak 201349 | Y | Y | Y | N | Y | Y | Y | Y | N | N | 7 | C |
| Pepe 201750 | Y | Y | Y | Y | Y | Y | Y | Y | Y | UN | 9 | A |
Y: yes, N: no, UN: uncertain.
Question: (1) Was a well-defined question posed in answerable form? (2) Was a comprehensive description of the competing alternatives given? (3) Was the effectiveness of the programs or services established? (4) Were all the important and relevant costs, and consequences for each alternative identified? (5) Were costs and consequences measured accurately in the appropriate physical units prior to valuation? (6) Were costs and consequences valued credibly? (7) Were costs and consequences adjusted for differential timing? (8)Was and incremental analysis of costs and consequences of alternatives performed? (9) Was uncertainty in the estimates of costs and consequences adequately characterized? (10) Did the presentation and discussion of study results include all issues of concern to users?
The reason for that the answer of Walczak et al.49 to question 4 was “NO” was as follows. Because β-thalassemia major patients with DFO treatment need lengthy infusions, the lengthy infusions cost much time, and which leads to much indirect cost, such as the productive loss, wages loss, etc. As a result, if a patient perspective for the analysis is adopted, the indirect cost will be essential and should be identified. However, Walczak et al.49 adopted a patient perspective and did not identify the indirect cost. The answer of Karnon et al.45 to question 7 was “NO” because the costs and consequences of this paper had no discount with a study time horizon of 50 years. The answer of Walczak et al.49 to question 10 was “NO” because the paper of Walczak et al. had no discussion. The answer of Delea et al.,34 Luangasanatip et al.,35 Karnon et al.,45 Bentley et al.,46 Keshtkaran et al.,48 Pepe et al.50 to question 10 were “UNCERTAIN” because the discussion of these papers lacked some issues of concern to users. The papers of Delea et al.,34 Luangasanatip et al.,35 and Keshtkaran et al.48 did not discuss the generalizability of their research results to other patients or settings. The paper of Pepe et al.50 did not compare its research results with those of others who have investigated the same question. The papers of Karnon et al.45 and Bentley et al.46 did not discuss the impact of foreign utility data on their research results.
Economic evaluation
DFX versus DFO. There were nine cost-utility analyses of DFX versus DFO. In three of nine studies,34,47,49 the ΔQALY and ΔCOST were positive numbers, which meant that using DFX achieved better utility and a higher cost than when using DFO. Due to the lower ICERs of these three studies34,47,49 than their payment threshold, the cost of using DFX was acceptable, and using DFX was cost-effective. In the three studies from two papers,45,48 the ΔQALY values were positive, whereas those of ΔCOST values were negative, which meant that using DFX led to a better utility and a lower cost compared the use of DFO. As a result, using DFX was cost-effective. In three other studies,35,46,50 the ΔQALY and ΔCOST were positive numbers, and their ΔICER values were higher than their payment threshold, indicating that the cost of using DFX was unacceptable and suing DFO was more cost-effective.
DFX versus DFP. There were three cost-utility analyses of DFX versus DFP. In the three included studies, ΔQALY, and ΔCOST values determined by Luangasanatip et al.35 were both positive numbers, so using DFX resulted in better utility and a higher cost than when using DFP. Unfortunately, the ΔICER of Luangasanatip et al.35 was USD 106,409 and higher than its payment threshold. As a result, the cost of using DFX was unacceptable, and using DFP was cost-effective. In two other studies,46,50 ΔQALY values were negative, whereas those of ΔCOST were positive, showing that using DFX led to a worse utility and a higher cost as compared with the cases of using DFP. As a result, the DFX was not cost effective.
DFP versus DFO. There were three cost-utility analyses of DFP versus DFO. These three studies revealed that DFP was cost-effective in comparison with the utilization of DFO. In the study of Luangasanatip et al.,35 ΔQALY value was zero and ΔCOST value was USD -9,117, which meant that using DFP led to the same utility and a lower cost compared the use of DFO, and using DFP was cost-effective. In two other studies,46,50 the ΔQALY values were positive, and ΔCOST values were negative, which meant that using DFP led to a better utility and a lower cost compared with using DFO. As a result, the DFP was not cost effective.
DFO + DFP versus DFO. Only one study35 assessed that comparison. In the study of Luangasanatip et al.,35 ΔQALY and ΔCOST were positive numbers, and the ΔICER was higher than the payment threshold. This outcome meant that the DFO+DFP led to a better utility and a higher cost compared with DFO monotherapy. Because the ΔICER was higher than its payment threshold, using DFO+DFP was unacceptable in Thailand, and using DFO monotherapy was established as cost-effective.
DFO + DFP versus DFP. In the only one study (Bentley et al.)46 included in that category, ΔQALY value was negative, whereas ΔCOST value positive. This result indicated that the DFO+DFP had a worse utility and a higher cost compared with DFP monotherapy. Hence, using DFP monotherapy was more cost-effective.
DFO + DFP versus DFX. In the two studies46,50 in this group, ΔQALY and ΔCOST were both negative numbers, which meant the DFO+DFP led to a worse utility and a lower cost in comparison with DFX monotherapy. Therefore, it was impossible to be sure which of two chelation regimens was cost-effective. More related research is required for further analysis.
Sensitivity analyses
Sensitivity analysis was not conducted in only one49 out of eight papers. The details of the sensitivity analysis of the other seven papers can be found in Table 1. The sensitivity analysis of seven papers34,35,45–48,50 showed that the influential parameters were drug cost, DFO administration cost, drug compliance, the dose of the drug, the starting age of chelation therapy, the discount rate and the utility values associated with the route of administration or complication.
Discussion
Four chelation regimens for β-thalassemia major therapy were assessed in the study, including DFO monotherapy, DFP monotherapy, DFX monotherapy, and DFO+DFP. The focus was placed on the economic parameters of the four chelation regimens in an attempt to find the best cost-effective option, which would serve as useful guidance in clinical practice. Nine cost-utility analysis of DFX versus DFO had contradictory results. Three cost-utility analysis of DFX versus DFP established that using DFP was cost-effective. Three cost-utility analysis of DFP versus DFO established that using DFP was cost-effective. One study of DFO+DFP versus DFO found that using DFO was cost-effective. One study of DFO+DFP versus DFP found that using DFP was cost-effective. Remarkably, there was only one study of DFO+DFP versus DFO (or DFP). The number of studies was too small to ensure the accuracy of the results. As a result, more extensive, high-quality research is required for further analysis and confirmation of these findings. There were two studies of DFO+DFP versus DFX, and their ΔQALY and ΔCOST values were negative numbers. Thus, it is impossible to be sure which one of two chelation regimens was cost-effective. Cost-effectiveness was relatively sensitive to the influential parameters were drug cost, DFO administration cost, drug compliance, the dose of the drug, the starting age of chelation therapy, the discount rate and the utility values associated with the route of administration or complication.
With the development of the times, the combined iron chelation therapy used in β-thalassemia major patients is gradually increasing. The Guidelines from the US, Italy, Australia, and China all recommended that β-thalassemia major patients with over iron-related cardiomyopathy should receive DFO+DFP.29–31,15 Unfortunately, this study showed that there are a little number of pharmacoeconomic studies on combined iron chelation therapy. So more research on its utility and pharmacoeconomic would be necessary.
As can be observed in Table 2, the cost the iron chelation varies substantially in different countries (regions). In the studies of DFX versus DFO, the ΔCOSTt values from the US, Thailand, Australia, Taiwan (China) and Italy were positive numbers, whereas the ΔCOSTt from Iran was a negative number. The main reason is that same drugs are priced differently in various countries (regions). The studies of Delea et al.34 and Karnon et al.45 showed that Exjade® (DFX) was priced at USD 89.49 per gram in the United States and GBP 33.60 per gram in Australia, as well as Desferal® (DFO), was priced at USD 35.77 per gram in the United States, and GBP 9.34 per gram in Australia. According to the rate of calculation of GBP 1 = USD 1.3085,51 the price of Exjade® in the US was 2.04-fold higher than that in Australia, and the price of Desferal® in the US was 2.93-fold higher than that in Australia. The study of Luangasanatip et al.35 also showed that the difference in drug costs when changing from deferoxamine to deferasirox resulted in an increase of over 400% in Thailand, whereas the cost of the drug increased only 100% in both US and UK settings.34,52 The price of the three iron chelators in the considered countries has been showed in Table 4. The more details about that same drugs are priced differently in various countries (regions) could be seen in Table 4.
Table 4.
The price of the three iron chelators in the considered countries.
| Study ID | Country | the price of iron chelators (per gram) (USD*) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| DFO | DFP | DFX | |||||
|
| |||||||
| Brand | Price | Brand | Price | Brand | Price | ||
| Delea 200720 | US | Desferal® | USD 35.77 | Exjade® | USD 89.49 | ||
| Luangasanatip 201121 | Thailand | Desferal® | USD 10.77 | Kelfer® GPO-L-ONE® |
USD 2.09 USD 0.20 |
Exjade® | USD 58.56 |
| Karnon 201224 | Australian | generic Desferal® |
USD 8.52 USD 9.34 |
Ferriprox® | USD 3.04 | Exjade® | USD 33.60 |
| Bentley 201325 | UK | generic | GBP 8.50 (USD 11.12) | Ferriprox® | GBP 3.20 (USD 4.19) | Exjade® | GBP 33.60 (USD 43.97) |
| Ho 201326 | China Taiwan |
USD 20.00 | USD 60.70 | ||||
| Keshtkaran 201327 | Iran | - | - | - | - | - | - |
| Walczak 201328 | Poland | - | - | Exjade® | PLN 6.94 (USD 1.89) | ||
| Pepe 201729 | Italy | Desferal® (injection, 500mg/10ml) | EUR 10.90 (USD 12.69) | Ferriprox® (tablets) | EUR 2.90 (USD 3.38) | Exjade® | EUR 52.00 (USD 60.56) |
| Desferal®(injection, 2g/ | EUR 27.20 (USD 31.68) | Ferriprox (oral solution)® | EUR 3.60 (USD 4.19) | ||||
GBP 1 = USD 1.3085, EUR 1 = USD 1.1646, PLN 1 = USD 0.2727
It can also be seen in Table 2 that the difference in the DFO administration cost varies considerably in different countries (regions). The DFO administration cost was USD 179,331 in the US34 and USD9078 in Taiwan (China).47 The price of DFO administration cost in the US was 19.75-fold higher than that in Taiwan (China). The reason for the considerable difference in the DFO administration costs in different countries (regions) may be that the prices of infusion equipment, such as infusion pumps, alcohol, and disposables are different in various countries (regions). The Annual cost of a pump, for example, was GBP 230 in Australia45 but USD 85.1 in Iran.48 The pump prices in Australia are, therefore, 3.54 times higher than those in Iran, calculated at a rate of GBP 1 = USD 1.3085.51 Table 2 also shows that the difference in payment threshold varies significantly in different countries (regions). The main reason for this difference is the economic level of the countries (regions). Ordinarily, more developed national economies have higher payment thresholds, and the ΔICER of a drug is more likely to be below the payment threshold. Because the economy of Thailand is relatively backward, its GDP per capita in 2011 was USD 5,539.49,53 and its payment threshold was only USD 8,707. Hence, the study of Luangasanatip et al.35 indicated that the ΔICER of DFX VS DFO was USD 90,618, and using DFX was not cost-effective in Thailand nevertheless, if Thailand had the same payment threshold as the US,34 as high as USD 100,000, the results of Luangasanatip et al.35 would be the opposite.
Based on the above-discussed findings, we can summarize that the specific region considered has a substantial influence on the economy of drugs. Therefore, when referring to the results of this study for clinical decision-making, clinicians need to consider the impact of local economies, price, and other factors. Meanwhile, health care experts from different countries (regions) can actively conduct relevant economic research and obtain valuable localized evidence.
To ensure the quality of the research, we excluded incomplete papers, such as abstracts of the meeting. As a result, there may be fewer data available for final research. Moreover, it may bias the results. The risk of a language bias seems minor based on the one non-English and non-Chinese paper excluded.
In this review, we had taken into consideration the utilization of chelating agents in thalassemia only, where we found more data available to perform an economic evaluation. However, the importance of these drugs and their cost is of rising concern considering that the iron chelation is more and more utilized in iron overload of Sickle Cell Diseases54,55,56 and Myelodysplastic Syndromes.57 The conclusions driven by us for the thalassemia patients could be useful to have an idea about the most rewarding utilization among the chelating agents also for these two diseases; however, specific studies are advisable.
Conclusions
In brief, DFP is the best choice, followed by DFO or DFX, when an iron chelator is to be used alone for β-thalassemia major therapy. All studies that compared DFO+DFP with DFO (or DFP) monotherapy established that the DFO+DFP was not cost-effective. Moreover, existing studies about DFO+DFP versus DFX could not prove which one of two chelation regimens was cost-effective. However, due to the low number of DFO+DFP versus DFO (or DFP or DFX) monotherapy studies, more extensive, high-quality research is required for further analysis and confirmation of our findings.
Moreover, the evaluation of cost-effectiveness, even being a global problem, cannot have a unique solution when in the different countries (regions) the results are opposite. The specific legislation of regions, where clinicians operate, had a substantial influence on the economy of drugs. Health care experts can contribute significantly to fully elucidating these aspects by conducting localized economic research, which will facilitate the choice of the best approach in each specific location.
Acknowledgment
The authors have no conflicts of interest that are directly relevant to the content of this study.
Appendix. Pubmed-Search Strategy
#1 “Thalassemia”[Mesh]
#2 thalassaemia*[Title/Abstract]
#3 “thalassaemia major”[Title/Abstract]
#4 “cooley’s anaemia”[Title/Abstract]
#5 “mediterranean anaemia”[Title/Abstract]
#6 #1 or #2 or #3 or #4 or #5
#7 “beta-Thalassemia”[Mesh]
#8 “beta thalassemia”[Title/Abstract]
#9 “beta thalassemias”[Title/Abstract]
#10 “beta type Thalassemia”[Title/Abstract]
#11 “beta type Thalassemias”[Title/Abstract]
#12 (((Microcytemia[Title/Abstract]) OR Microcytemias[Title/Abstract])) AND “beta Type”[Title/Abstract]
#13 (disease*[Title/Abstract]) AND “Hemoglobin F”[Title/Abstract]
#14 (“Erythroblastic Anemias”[Title/Abstract]) OR “Erythroblastic Anemia”[Title/Abstract]
#15 “Thalassemia Major”[Title/Abstract]
#16 “beta-Thalassemia Major”[Title/Abstract]
#17 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 “Iron Overload”[Mesh]
#19 “Iron overload”[Title/Abstract]
#20 “transfusional hemosiderosis”[Title/Abstract]
#21 #18 or #19 or #20
#22 #6 or #17 or #21
#23 “Iron Chelating Agents”[Mesh]
#24 (“Iron chelating agents”[Title/Abstract]) OR “Iron chelating agent”[Title/Abstract]
#25 (“ iron chelate”[Title/Abstract]) OR “ iron chelates”[Title/Abstract]
#26 “iron chelating”[Title/Abstract]
#27 “iron chelation”[Title/Abstract]
#28 #23 or #24 or #25 or #26 or #27
#29 “Deferoxamine”[Mesh]
#30 Deferoxamine[Title/Abstract]
#31 Desferroxamine[Title/Abstract]
#32 Desferioximine[Title/Abstract]
#33 Desferrioxamine[Title/Abstract]
#34 DFO[Title/Abstract]
#35 Desferal[Title/Abstract]
#36 #29 or #30 or #31 or #32 or #33 or #34 or #35
#37 “Deferoxamine”[Mesh]
#38 Deferiprone[Title/Abstract]
#39 Hdmpp[Title/Abstract]
#40 Hdpp[Title/Abstract]
#41 L1[Title/Abstract]
#42 Ferriprox[Title/Abstract]
#43 CP20[Title/Abstract]
#44 #37 or #38 or #39 or #40 or #41 or #42 or #43
#45 “Deferoxamine”[Mesh]
#46 Deferasirox[Title/Abstract]
#47 DFX[Title/Abstract]
#48 Exjade[Title/Abstract]
#49 #45 or #46 or #47 or #48
#50 #28 or #36 or #44 or #49
#51 “Economics, Pharmaceutical”[Mesh]
#52 Pharmacoeconomics[Title/Abstract]
#53 “Pharmaceutical Economics”[Title/Abstract]
#54 (“economic evaluation”[Title/Abstract]) OR “economic evaluations”[Title/Abstract]
#55 “Cost-Benefit Analysis”[Mesh]
#56 (“cost-benefit analysis”[Title/Abstract]) OR “cost-benefit analyses”[Title/Abstract]
#57 (“marginal analysis”[Title/Abstract]) OR “marginal analyses”[Title/Abstract]
#58 (“cost benefit”[Title/Abstract]) OR “cost benefits”[Title/Abstract]
#59 (“cost-effectiveness analysis”[Title/Abstract]) OR “cost-effectiveness analyses”[Title/Abstract]
#60 (“cost-utility analysis”[Title/Abstract]) OR “cost-utility analyses”[Title/Abstract]
#61 “Costs and Cost Analysis”[Mesh]
#62 (“cost analyses”[Title/Abstract]) OR “cost analysis”[Title/Abstract]
#63 (“cost comparison”[Title/Abstract]) OR “cost comparisons”[Title/Abstract]
#64 (“cost-minimization analysis”[Title/Abstract]) OR “cost-minimization analyses”[Title/Abstract]
#65 (“cost measure”[Title/Abstract]) OR “cost measures”[Title/Abstract]
#66 ((cost[Title/Abstract]) OR costs[Title/Abstract]) OR pricing[Title/Abstract]
#67 #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66
#68 #22 and #50 and #67
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Yang Y, Dai BT. The Iron Overload and Iron-Cheleting Therapying of β-thalassemia J. Journal of Pediatric Pharmacy. 2011;17(3):58–61. [Google Scholar]
- 2.Beijing AngelMom Charity Foundation, China Siyuan Foundation for Poverty Alleviation, China Philanthropy Research Institute of Beijing Normal University. Blue Paper of Thalassemia in China [M] China Social Publishing House; 2016. [Google Scholar]
- 3.Pistoia L, Meloni A, Salvadori S, et al. Cardiac involvement by CMR in different genotypic groups of major thalassemia patients. J Blood Cells Mol Dis. 2019;(77):1–7. doi: 10.1016/j.bcmd.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Angastiniotis M, Modell B. Global epidemiology of hemoglobin disorders[J] Ann N Y Acad Sci. 1998;850:25l–259. doi: 10.1111/j.1749-6632.1998.tb10482.x. [DOI] [PubMed] [Google Scholar]
- 5.Vichinsky EP. Changing patterns of thalassemia worldwide J. Ann NY Acad Sci. 2005;1054:18–24. doi: 10.1196/annals.1345.003. [DOI] [PubMed] [Google Scholar]
- 6.National Co-ordination Group of Haemoglobinopathy Research. A survey on hemoglobinopathy among 600,000 residents of 20 provinces, cities, and autonomous regions of China. J Zhonghua Yi Xue Za Zhi. 1983;63(6):382–5. [PubMed] [Google Scholar]
- 7.Hematology SGO, Association CM. Guidelines for the diagnosis and treatment of beta-thalassemia major J. Zhonghua Er Ke Za Zhi. 2010;48(3):186–189. [PubMed] [Google Scholar]
- 8.Rehm J, Chisholm D, Room R, et al. Disease control priorities in developing countries[M] Oxford University Press; 2006. [Google Scholar]
- 9.Ee TA, von Riedemann S, Tricta F. Cost-utility of chelators in transfusion-dependent β-thalassemia major patients: a review of the pharmacoeconomic literature J. Expert Review of Pharmacoeconomics & Outcomes Research. 2014;14(5):651–60. doi: 10.1586/14737167.2014.927314. [DOI] [PubMed] [Google Scholar]
- 10.Neufeld EJ. Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions. Blood. 2006;107(9):3436–41. doi: 10.1182/blood-2006-02-002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neufeld EJ. Update on iron chelators in thalassemia. J Hematology American Society of Hematology Education Program. 2010;2010(6):451. doi: 10.1182/asheducation-2010.1.451. [DOI] [PubMed] [Google Scholar]
- 12.Hematology SGO, Association CM. Guidelines for the diagnosis and treatment of beta-thalassemia major J. Zhonghua Er Ke Za Zhi. 2010;48(3):186–189. [PubMed] [Google Scholar]
- 13.Hatzipantelis ES, Karasmanis K, Perifanis V, et al. Combined Chelation Therapy with Deferoxamine and Deferiprone in β-Thalassemia Major: Compliance and Opinions of Young Thalassemic Patients J. Hemoglobin. 2014;38(2):111–114. doi: 10.3109/03630269.2013.867407. [DOI] [PubMed] [Google Scholar]
- 14.Delea TE, Edelsberg J, Sofrygin O, et al. Consequences and costs of noncompliance with iron chelation therapy in patients with transfusion-dependent thalassemia: a literature review. J Transfusion. 2010;47(10):1919–1929. doi: 10.1111/j.1537-2995.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 15.Subspecialty Groups of Hematology, Society of Pediatrics, Chinese Medical Association; Editorial Board of Chinese Journal of Pediatrics. Guidelines for the diagnosis and treatment of beta-thalassemia major. J Zhonghua Er Ke Za Zhi. 2018;56(10):724–729. doi: 10.3760/cma.j.issn.0578-1310.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Borgna-Pignatti C, Marsella M. Iron chelation in thalassemia major J. Clinical Therapeutics. 2015;37(12):2866–2877. doi: 10.1016/j.clinthera.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Bollig C, Schell LK, Rücker G, et al. Deferasirox for managing iron overload in people with thalassaemia. Cochrane Database of Systematic Reviews. 2017;(8) doi: 10.1002/14651858.CD007476.pub3. Art. No.: CD007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. Label for Exjade revised:12/2018. https://www.accessdata.fda.gov/drugsatfdadocs/label/2018/021882s028s029lbl.pdf. Last retrieved on 7 May, 2019.
- 19.US Food and Drug Administration. FDA briefing document, Pediatric Advisory Committee Meeting, March 6, 2017: update on safety issues associated with Exjade (deferasirox) use in young children who have fever. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM543659.pdf. Last retrieved on 7 May, 2019.
- 20.Bird ST, Swain RS, Tian F, et al. Effects of deferasirox dose and decreasing serum ferritin concentrations on kidney function in paediatric patients: an analysis of clinical laboratory data from pooled clinical studies. J Lancet Child Adolesc Health. 2019;(3):15–22. doi: 10.1016/S2352-4642(18)30335-3. [DOI] [PubMed] [Google Scholar]
- 21.Kwiatkowski JL. Real-world use of iron chelators. J Hematology. 2011;2011(1):451–8. doi: 10.1182/asheducation-2011.1.451. [DOI] [PubMed] [Google Scholar]
- 22.Xia S, Zhang W, Huang L, et al. Comparative Efficacy and Safety of Deferoxamine, Deferiprone and Deferasirox on Severe Thalassemia: A Meta-Analysis of 16 Randomized Controlled Trials. J PLOS ONE. 2013:8. doi: 10.1371/journal.pone.0082662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijarnpreecha K, Kumfu S, Chattipakorn SC, et al. Cardiomyopathy Associated with Iron Overload: How Does Iron Enter Myocytes and What are the Implications for Pharmacological Therapy? J. Hemoglobin. 2015;39(1):9. doi: 10.3109/03630269.2014.987869. [DOI] [PubMed] [Google Scholar]
- 24.Kremastinos DT, Farmakis D, Aessopos A, et al. Beta-Thalassemia Cardiomyopathy: History, Present Considerations, and Future Perspectives J. Circulation Heart Failure. 2010;3(3):451–458. doi: 10.1161/CIRCHEARTFAILURE.109.913863. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter JP, Roughton M, Pennell DJ. International survey of T2* cardiovascular magnetic resonance in β-thalassemia major J. Haematologica. 2013;98(9):1368–1374. doi: 10.3324/haematol.2013.083634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galanello R, Origa R. Beta-thalassemia. J Orphanet Journal of Rare Diseases. 2010;5(1):11. doi: 10.1186/1750-1172-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo V, Rago A, Papa AA, et al. Electrocardiographic Presentation, Cardiac Arrhythmias, and Their Management in β-Thalassemia Major Patients J. Annals of Noninvasive Electrocardiology. 2016;21(4):335–342. doi: 10.1111/anec.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepe A, Meloni A, Pistoia L, et al. MRI multicentre prospective survey in thalassaemia major patients treated with deferasirox versus deferiprone and desferrioxamine. Br J Haematol. 183(5):783–795. doi: 10.1111/bjh.15595. [DOI] [PubMed] [Google Scholar]
- 29.Pennell DJ, Udelson JE, Arai AE, et al. Cardiovascular function and treatment in β-thalassemia major: a consensus statement from the American Heart Association J. Circulation. 2013;128(13):E203–E203. doi: 10.1161/CIR.0b013e31829b2be6. [DOI] [PubMed] [Google Scholar]
- 30.Angelucci E, Barosi G, Camaschella C, et al. Italian Society of Hematology practice guidelines for the management of iron overload in thalassemia major and related disorders J. Haematologica. 2008;93(5):741–752. doi: 10.3324/haematol.12413. [DOI] [PubMed] [Google Scholar]
- 31.Ho PJ, Tay L, Lindeman R, et al. Australian guidelines for the assessment of iron overload and iron chelation in transfusion-dependent thalassaemia major, sickle cell disease and other congenital anaemias J. Internal Medicine Journal. 2011;41(7):516–524. doi: 10.1111/j.1445-5994.2011.02527.x. [DOI] [PubMed] [Google Scholar]
- 32.Sheth S, Weiss M, Parisi M, Ni Q. Clinical and Economic Burden of Transfusion-Dependent β-Thalassemia in Adult Patients in the United States. [Accessed August 31, 2018];Blood. 2017 130(Suppl 1):2095. [Google Scholar]
- 33.Esmaeilzadeh F, Azarkeivan A, Emamgholipour S, et al. Economic Burden of Thalassemia Major in Iran, 2015. J Journal of Research in Health Sciences. 2017;16(3):111. [PMC free article] [PubMed] [Google Scholar]
- 34.Delea TE, Sofrygin O, Thomas SK, et al. Cost effectiveness of once-daily oral chelation therapy with deferasirox versus infusional deferoxamine in transfusion-dependent thalassaemia patients: US healthcare system perspective J. PharmacoEconomics. 2007;25(4):329–342. doi: 10.2165/00019053-200725040-00005. [DOI] [PubMed] [Google Scholar]
- 35.Luangasanatip N, Chaiyakunapruk N, Upakdee N, et al. Iron-chelating therapies in a transfusion-dependent thalassaemia population in Thailand: a cost-effectiveness study J. Clinical Drug Investigation. 2011;31(7):493–505. doi: 10.2165/11587120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.MEDLINE(Pubmed)[DB/OL] https://www.ncbi.nlm.nih.gov/pubmed. Last retrieved on 24 April, 2018.
- 37.EMBASE (Ovid)[DB/OL] http://ovidsp.tx.ovid.com/sp-3.33.0b/ovidweb.cgi. Last retrieved on 24 April, 2018.
- 38.Cochrane Central Register of Controlled Trials (CENTRAL) [DB/OL], Health Technology Assessment Database(HTAD) [DB/OL], NHS Economic Evaluation Database (NHS EED) [DB/OL] in Cochrane Libray. https://www.cochranelibrary.com. Last retrieved on 24 April, 2018.
- 39.China Biology Medicine(CBM, SinoMed) [DB/OL] http://www.sinomed.ac.cn/zh/. Last retrieved on 24 April, 2018.
- 40.China National Knowledge Infrastructure(CNKI) [DB/OL] http://www.cnki.net/. Last retrieved on 24 April, 2018.
- 41.VIP [DB/OL] http://qikan.cqvip.com/. Last retrieved on 24 April, 2018.
- 42.Wanfang Data [DB/OL] http://www.wanfangdata.com.cn/index.html. Last retrieved on 24 April, 2018.
- 43.Drummond M, O’Brien B, Stoddart G, Torrance G. Methods for economic evaluation of healthcare programmes. 4nd ed. Oxford: Oxford Medical Publications; 2015. [Google Scholar]
- 44.Gc V, Wilson EC, Suhrcke M, et al. Are brief interventions to increase physical activity cost-effective? A systematic review J. British Journal of Sports Medicine. 2016;50(7):408–417. doi: 10.1136/bjsports-2015-094655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karnon J, Tolley K, Vieira J, et al. Lifetime cost-utility analyses of deferasirox in beta-thalassaemia patients with chronic iron overload: a UK perspective. J Clinical Drug Investigation. 2012;32(12):805–15. doi: 10.1007/s40261-012-0008-2. [DOI] [PubMed] [Google Scholar]
- 46.Bentley A, Gillard S, Spino M, et al. Cost-Utility Analysis of Deferiprone for the Treatment of β-Thalassaemia Patients with Chronic Iron Overload: A UK Perspective J. PharmacoEconomics. 2013;31(12):1185–6. doi: 10.1007/s40273-013-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho WL, Chung KP, Yang SS, et al. A pharmaco-economic evaluation of deferasirox for treating patients with iron overload caused by transfusion-dependent thalassemia in Taiwan. J Journal of the Formosan Medical Association. 2013;112(4):221. doi: 10.1016/j.jfma.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 48.Keshtkaran A, Javanbakht M, Salavati S, et al. Cost-utility analysis of oral deferasirox versus infusional deferoxamine in transfusion-dependent β-thalassemia patients. J Transfusion. 2013;53(8):1722–1729. doi: 10.1111/trf.12024. [DOI] [PubMed] [Google Scholar]
- 49.Walczak J, Sołtys E, Obrzut G, et al. PSY44. Cost-Utility Analysis of Deferasirox for the Treatment of Iron Overload Due to Frequent Blood Transfusions in the Children and Adolescents. J Value in Health. 2013;16(7):A379. doi: 10.1016/j.jval.2013.08.327. [DOI] [Google Scholar]
- 50.Pepe A, Rossi G, Bentley A, et al. Cost-Utility Analysis of Three Iron Chelators Used in Monotherapy for the Treatment of Chronic Iron Overload in β-Thalassaemia Major Patients: An Italian Perspective J. Clinical Drug Investigation. 2017:1–12. doi: 10.1007/s40261-017-0496-1. [DOI] [PubMed] [Google Scholar]
- 51.The Monetary Policy Department of The People’s Bank of China, On September 28, 2018, China Foreign Exchange Exchange Trading Center was authorized to announce the announcement of the mid-price of CNY exchange rate[EB/OL] http://www.pbc.gov.cn/zhengcehuobisi/125207/125217/125925/3636555/index.html. Last retrieved on 2 May, 2019.
- 52.Kamon J, Tolley K, Oyee J, et al. Cost utility analysis of deferasirox (Exjade*) versus deferoxamine (Desferal) for patients requiring iron chelation therapy in the United Kingdom. Curr Med Res Opin. 2008;24(6):1609–21. doi: 10.1185/03007990802077442. [DOI] [PubMed] [Google Scholar]
- 53.the World Bank, GDP per capita[EB/OL] http://data.worldbank.org.cn/indicator/NY.GDP.PCAP.CD. Last retrieved on 2 May, 2019.
- 54.Coates TD, Wood JC. How we manage iron overload in sickle cell patients. Br J Haematol. 2017 Jun;177(5):703–716. doi: 10.1111/bjh.14575. Epub 2017 Mar 14. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yassin M, Soliman A, De Sanctis V, Nashwan A, Abusamaan S, Moustafa A, Kohla S, Soliman D. Liver Iron Content (LIC) in Adults with Sickle Cell Disease (SCD): Correlation with Serum Ferritin and Liver Enzymes Concentrations in Trasfusion Dependent (TD-SCD) and Non-Transfusion Dependent (NT-SCD) Patients. Mediterr J Hematol Infect Dis. 2017 Jun 20;9(1):e2017037. doi: 10.4084/MJHID.2017.037. doi: 10.4084/MJHID.2017.037. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delicou S, Maragkos K, Tambaki M, Kountouras D, Koskinas J. Transient Elastography (TE) is a Useful Tool for Assessing the Response of Liver Iron Chelation in Sickle Cell Disease Patients. Mediterr J Hematol Infect Dis. 2018 Sep 1;10(1):e2018049. doi: 10.4084/MJHID.2018.049. doi: 10.4084/MJHID.2018.049. eCollection 2018. PubMed PMID: 30210742; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angelucci E, Urru SA, Pilo F, Piperno A. Myelodysplastic Syndromes and Iron Chelation Therapy. Mediterr J Hematol Infect Dis. 2017 Mar 1;9(1):e2017021. doi: 10.4084/mjhid.2017.021. eCollection 2017. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]