Abstract
The bioactive peptide bradykinin obtained from cleavage of precursor kininogens activates the kinin-B2 receptor functioning in induction of inflammation and vasodilatation. In addition, bradykinin participates in kidney and cardiovascular development and neuronal and muscle differentiation. Here we show that kinin-B2 receptors are expressed throughout differentiation of murine C2C12 myoblasts into myotubes. An autocrine loop between receptor activation and bradykinin secretion is suggested, since bradykinin secretion is significantly reduced in the presence of the kinin-B2 receptor antagonist HOE-140 during differentiation. Expression of skeletal muscle markers and regenerative capacity were decreased after pharmacological inhibition or genetic ablation of the B2 receptor, while its antagonism increased the number of myoblasts in culture. In summary, the present work reveals to date no functions described for the B2 receptor in muscle regeneration due to the control of proliferation and differentiation of muscle precursor cells.
Keywords: Mouse myoblast differentiation, Muscle repair, Kinin-B2 receptor, HOE-140
Introduction
The kinins bradykinin (BK) and kallidin are vasoactive peptides exerting their anti-hypertensive actions by activating metabotropic G protein-coupled kinin-B2 receptor (B2BKR). Kallikreins, which are serine proteases, cleave high and low molecular mass kininogens to originate BK and kallidin, respectively. Degradation of these peptides is mostly mediated by angiotensin-converting enzyme and carboxypeptidase activity. Along with other kinins, BK and kallidin elicit a wide range of physiological responses, being classically involved in the control of cardiovascular homeostasis, neurotransmission, control of nociceptive function, inflammation and various disease states including cardiovascular diseases, arthritis, pancreatitis, and asthma [1–3]. Although pro-inflammatory effects are attributed to BK, several studies have shown its involvement in mechanisms of cell survival [4, 5].
B2BKRs also participate in developmental processes, such as kidney and cardiovascular development [6, 7]. Moreover, expression of kallikreins in developing rat brains and kininogens and BK in neural progenitor cells obtained from embryonic rat brain [8, 9] as well as developmental stage regulated BK secretion and B2BKR expression during in vitro neuronal differentiation of P19 embryonic cells suggests roles for the kallikrein-kinin system during neuronal development [10]. Such findings are supported by the observation that in addition to an autocrine loop-mediated increase in BK secretion and receptor expression, the presence of the B2BKR-selective blocker HOE-140 interferes with early and later differentiation progress of these cells, including differentiation into neurons expressing functional cholinergic receptors [10]. Furthermore, it was shown using neural progenitor cells that BK-induced signaling is involved in neural fate determination and specification of neurotransmitter receptor expression [11].
Recently, BK-induced myogenic differentiation of murine C2C12 myoblasts was reported [12]; however, a possible role of BK and its receptors in protection against muscular degeneration and/or muscle regeneration has yet not been studied. Muscle injury may occur as a consequence of degenerative diseases, including muscular dystrophies, exposure to myotoxic agents, acute injuries, such as lacerations or bruising, ischemia, intense heat or cold exposure, and muscle contraction, especially eccentric contraction [13–16]. However, muscle tissue shows high regenerative activity due to the existence of satellite cells that are quiescent adult progenitor cells. Proper activation of satellite cells is critical to ensure the regenerative process [17–20], although proliferation and differentiation are antagonistic, mutually exclusive states in muscle cells [21]. Based on that, we focused on understanding B2BKR-mediated effects on regeneration and protection of degeneration of muscle tissue, since these are key issues in the muscle pathology field. For that, we have used a cardiotoxin-induced animal injury model to study tissue regeneration in B2BKR-knockout mice (B2BKR−/−) and the mouse myoblast C2C12 cell line as model for in vitro myogenic differentiation. This cell line represents the developmental stage of mononucleated myoblasts, which upon appropriate stimuli stop proliferation and initiate terminal differentiation characterized by expression of contractile muscle-specific proteins including myosin heavy chain (MHC) and formation of multi-nucleated syncytia [22].
Our data suggest that myoblast cells liberate BK, which in an autocrine loop activates B2BKRs for induction of myogenic differentiation. Consequently, after selective blockade of receptor activity in vitro, satellite cells have proliferation enhanced, and in vivo knockout animals for the B2BKR have the muscle regeneration decreased. Here, we provide evidence for the participation of B2BKR activity in the muscle regeneration due to the control of proliferation of muscle precursor cells.
Materials and Methods
Materials
Reagents were purchased in highest available quality from Sigma (St Louis, MO), unless otherwise stated. Mouse myoblast C2C12 cells were obtained from the American Tissue Cell Collection, Manassas, VA (ATCC CRL-1772).
Animals
Wild type and B2BKR−/− C57BL/6 mice (provided by the Center for Development of Experimental Models for Medicine and Biology – UNIFESP, São Paulo) were kept under optimal conditions, with food and water provided ad libitum. Muscle regeneration after lesion was induced in wild type and B2BKR−/− C57BL/6 mice. A total volume of 100 μl of either 40 μM cardiotoxin from Naja mossambica mossambica or vehicle (PBS) was injected in rectus femoral muscles. The procedure was performed using a 29G needle under anesthesia induced by intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). Five or ten days after the procedure, animals were killed by anesthesia hyper dose and rectus femoral muscle were collected for routine haematoxylin-eosin (H&E) histologic staining. For determining myogenin gene expression in the gastrocnemius skeletal muscle, C57BL/6 wild type mice (n = 4) were treated every day from birth to 21-days-old with an intraperitoneal injection of saline (NaCl 0.9%) or the selective B2BKR antagonist HOE-140 (1 mg/kg). Sixty-nine days after the end of treatment (i.e. 90-days-old) the animals were sacrificed by decapitation or a hyper dose of ketamine prior to extraction of gastrocnemius and rectus femoral skeletal muscles. All experiments using animals were performed according to guidelines of the local and NIH Ethics’ Committee.
Quantitative Description of Lesions
Transverse sections from medial portion of the rectus femoral tissues were analyzed under bright field microscopy. For each animal, ten representative images at 200× magnification were taken from lesion areas and subjected to blind analysis by two observers). Fibers were categorized as normal (polygonal shape with peripheral nuclei, intact sarcolemma and non-fragmented sarcoplasm), regenerative (small basophilic myotubes often with central nuclei) or degenerative (lighter staining than intact muscle and often with irregular shape and few nuclei) and quantified using the Fiji ImageJ “Cell Counter” plugin. Raw data from both observers were averaged for each animal (n = 6). Final data are expressed as mean values ± standard errors of mean (SEM) for each experimental condition.
Cell Culture and Myogenic Differentiation of C2C12 Cells
C2C12 myoblasts were grown at low cellular density to avoid differentiation in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 100 μg/ml streptomycin, 100 U/mL penicillin and 1.5 g/L sodium bicarbonate. Cells at concentrations of 1.6 × 105 mL−1 or 0.8 × 105 mL−1 were plated into 35 or 22 mm cell culture dishes and kept in DMEM, supplemented with 10% FBS, for 24 h prior to differentiation induction. Differentiation into skeletal muscle was induced by changing the culture medium to DMEM containing 2% horse serum [23] and was complete following 7 days of culture in this medium.
Western-Blotting Assays
Undifferentiated C2C12 myoblasts and cultures from differentiating cells (days 0–7) kept in absence or presence of 1 μM HOE-140 were homogenized in ice-cold lysis buffer containing 50 mM Tris, pH 7.4, 150 mM NaCl and 0.5% Triton X-100 and protease inhibitors. Aliquots of obtained cell extracts (20 μg protein content) and molecular mass markers were denatured in SDS-sample buffer by boiling for 5 min and then separated by 12% SDS-PAGE. Electrophoretically separated proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane. Transference efficiency was verified by staining of the membrane with India ink. The membrane was then washed twice and blocked for 30 min by incubation in Tris-buffer saline with 0.03% Tween (TBS-T) containing 3% fat-free milk. Then the anti-MHC primary antibody at 1:1000 dilution was added and kept for 16 h at 4 °C. The membrane was washed, blocked again for 30 min with TBS-T containing 3% fat-free milk and then incubated for 2 h with the secondary antibody. India ink staining was used for comparison of protein content and for relative quantification of immunostaining by densitometric analysis with the ImageJ software.
Quantification of Bradykinin in Medium Supernatant from Differentiating C2C12 Myoblasts by High Performance Liquid Chromatography (HPLC)
BK secretion into the culture medium by C2C12 myoblasts on days 0 to 7 following induction to differentiation was analyzed as described previously [23]. Briefly, a cocktail of protease inhibitors (5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM p-hydroxymercuribenzoate, 1.5 mM ophenanthroline and 1 μM pepstatin) was immediately added after sample collection followed by freezing in liquid nitrogen. Two milliliters of each sample were pre-purified on Sep-Pak C18 cartridges (Waters, Milford, MA). Samples were eluted with 3 ml of 35% acetonitrile in 0.1% H3PO4. Following evaporation in a Speedvac concentrator (Boc Edwards, Wilmington, MA), the pre-purified extracts in a volume of 100 μl were separated on a reversed-phase Aquapore OD 300 column (250 × 4.6 mm, 5 μm, Brownlee, Applied Biosystems, Foster City, CA) connected to a HPLC system (Milton & Roy, Ivyland, PA). Following 5 min of isocratic chromatography with 5% acetonitrile in 0.1% H3PO4, peptides were eluted with a 0–90% linear gradient of 35% acetonitrile in 0.1% H3PO4, developed for 20 min at a flow rate of 1.5 mL/min. Absorbance was monitored at 214 nm. The elution position of the peptide was verified and its quantification by peak area was done by using synthetic BK as standard.
Quantification of Myoblasts and Myotubes in Culture
To count myoblasts and myotubes, C2C12 culture on day 7 following induction to differentiation in absence or presence of 1 μM HOE-140, a specific selective B2BKR antagonist, were fixed for 5 min in ice-cold isopropanol and then stained for 1 h with Alexa Fluor 488 Phalloidin (Cell Signaling, Danvers, MA) at a concentration of 300 nM, a high-affinity filamentous actin (F-actin) probe and 5 min for labeling nuclear DNA using DAPI (Sigma, St Louis, MO). By staining of the filaments and nuclei of the cells and imaging cytometry scanning and analysis of the cell cultures using TissueFAXS™ Cytometry platform (TissueGnostics GmbH, Vienna, Austria), we were able to distinguish myoblasts and fibers and quantify them using StrataQuest software (TissueGnostics GmbH). Such software provides cellular data in dot-plots to show multiple measurement parameters of single and multinucleated cells, multicellular structures and/or morphological features [24]. Briefly, the total cellular area was considered the area stained with Alexa 488 Phalloidin, then number of cells was determined using this cellular mask. Inside of each cell, number of nuclei was measured using the staining with DAPI. Accordingly, number of nuclei and size of cellular area, the cells were named as myoblasts, single-nucleus cells or nascent and mature myotubes, a newly formed multinucleated mouse muscle and a large mouse muscle cell in vitro that contains many nuclei, respectively [25].
Reverse Transcription and PCR
Total RNAs of undifferentiated C2C12 myoblasts and differentiating cells (days 1–7) were prepared by using the Trizol reagent (Invitrogen) and verified for their integrity by analyzing an aliquot on an ethidium-bromide stained agarose gel. Reverse transcription (RT)-PCR using 5 μg RNA as template was catalyzed by 200 U of RevertAid™ reverse transcriptase and Taq DNA polymerase (Fermentas, Campinas, Brazil) as described elsewhere [8]. Primer sequences for detection of RT-PCR of high-and low-molecular mass kininogens 1 and 2 [26] are detailed in Table 1. Following an initial denaturation step of 5 min at 95 °C, PCR reactions were cycled 35 times (95 °C for 45 s, 56 °C for 45 s and 72 °C for 105 s) plus a final extension step of 10 min at 72 °C.
Table 1.
Primer sequences for RT-PCR detection of kininogen gene expression
| Gene | GenBank™ Access. No. | Primer | Sequence |
|---|---|---|---|
| High molecular | AY462056 | forward | 5′-GCCAACTTCTCACAGAGCTGTAC-3′ |
| mass kininogen 1 | AY462057 | ||
| AY462058 | |||
| AY462059 | |||
| High molecular | AY462056 | forward | 5′-GCATTAGATAAGACTATTCC-3′ |
| mass kininogen 2 | AY462057 | reverse | 5′-CATTTGTGAAGCTACCTAGG-3′ |
| AY462058 | |||
| AY462059 | |||
| Low molecular | AY462056 | forward | 5′-GCCAACTTCTCACAGAGCTGTAC-3′ |
| mass kininogen 1 | AY462057 | reverse | 5′-TCCCTCTGGGTCATTGCTGG-3′ |
| AY462059 | |||
| Low molecular | AY462056 | forward | 5′-GCATTAGATAAGACTATTCC-3′ |
| mass kininogen 2 | AY462057 | reverse | 5′-TCCCTCTGGGTCATTGCTGG-3′ |
| AY462059 |
Primer sequences were identical with those used by Martins et al. 2005 [10]. High and low molecular mass kininogens are distinguished by the size of RT-PCR amplified fragments (HMWK 1: 603 and 854 bp; LMWK 2: 194 and 281 bp). Reverse primers for HMWK1 and HMWK2 were identical
Analysis of Gene Expression by Real Time PCR
Total RNAwas prepared from C2C12 myoblasts at days 0 and 7 of differentiation in the absence or presence of 1 μM HOE-140 from gastrocnemius skeletal muscles of 90 day–old mice treated from birth on for 21 days with HOE-140 (1 mg/kg), or from the gastrocnemius skeletal muscles of WTand B2BKR−/− mice at 15 and 30 days of life using the TRIzol Reagent (Invitrogen, USA) according to the manufacture’s recommendation. One microgram of RNA was reverse transcribed using Moloney Murine Leukemia Virus reverse transcriptase (Invitrogen). The cDNAwas synthesized from total RNAwith random hexamer primers. Standard curves were determined for each primer set and cDNA sample to verify the efficiency of the reaction. As the efficiency of all reactions was >95%, the 2−ΔΔCt parameter was employed for relative quantification of gene expression. The data shown were obtained from three independent samples and RT-PCR real-time reactions were prepared in triplicates for each analyzed gene. Relative quantification of gene expression data was done against acidic ribosomal protein 36B4 or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression levels. Primer sequences used for real time PCR are given in Table 2.
Table 2.
Primer sequences for real-time PCR of myogenic marker protein expression
| Gene | GenBank™ Access. No. | Primer | Sequence |
|---|---|---|---|
| MHC 1 | NM_030679.1 | forward | 5′-TCCCTAAAGGCAGGCTCTCT-3′ |
| reverse | 5′-ACTTCCGGAGGTAAGGAGCA-3′ | ||
| MHC 3 | NM_001099635.1 | forward | 5′-GACTCAGCCGACACCATGAG-3′ |
| reverse | 5′-AAGGGCTGGTTCTGAGCTTC −3′ | ||
| Myog | NM_031189.2 | forward | 5′-GTGAATGCAACTCCCACAGC −3′ |
| reverse | 5′-CGCGAGCAAATGATCTCCTG-3′ | ||
| Des | NM_010043.1 | forward | 5′-AACTTCCGAGAAACCAGCC-3′ |
| reverse | 5′-TCTCCATCCCGGGTCTCAAT-3′ | ||
| 36B4 | NM_007475.5 | forward | 5′-CGACCTGGAAGTCCAACTAC-3′ |
| reverse | 5′-ATCTGCTGCATCTGCTTG-3′ | ||
| GAPDH | NM_008084 | forward | 5′-TGCACCACCAACTGCTTA G-3′ |
| reverse | 5′-GGATGCAGGGATGATGTTC-3′ |
For quantification of relative gene expression of myosin-heavy chain (MHC) 1 and 3, myogenin (Myog) and desmin (Des), data were normalized with the expression of endogenous controls glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 36B4, whose expression levels did not change under the used experimental conditions
Immunofluorescence Studies
For immuno-fluorescence studies, C2C12 myoblasts were grown in 22 mm cell-culture dishes and kept in differentiation-inducing medium for 5 or 7 days in absence or presence of 1 μM HOE-140. Cell fixation was performed by incubation for 20 min with 4% para-formaldehyde in phosphate-buffered saline (PBS) at 4 °C. Cells were washed twice with PBS and then permeabilized for 30 min in PBS containing 0.1% Triton X-100. Following two further washing steps, PBS supplemented with 2% FBS was added for 45 min for blocking unspecific binding sites. Subsequently, incubation followed overnight with primary antibodies recognizing MHC (1:250). Following removal of antibody solution and two washing steps with the blocking solution, anti-IgG-Alexa Fluor 488 secondary antibodies were used for protein expression detection. Cell nuclei were visualized by counter-staining with 4′, 6′-diamidino-2′-phenylindole dihydrochloride (DAPI). Cover slips were mounted, and cell preparations were analyzed under an epifluorescence microscope Axiovert 200 (Zeiss, Jena, Germany) equipped with a Nikon 1200F digital camera (Nikon Inc., Melville, NY) using the Metamorph Program (Molecular Devices, Sunny Valley, CA).
Results
Repair and Degeneration of Muscle are Affected in Condition of B2BKR Deletion
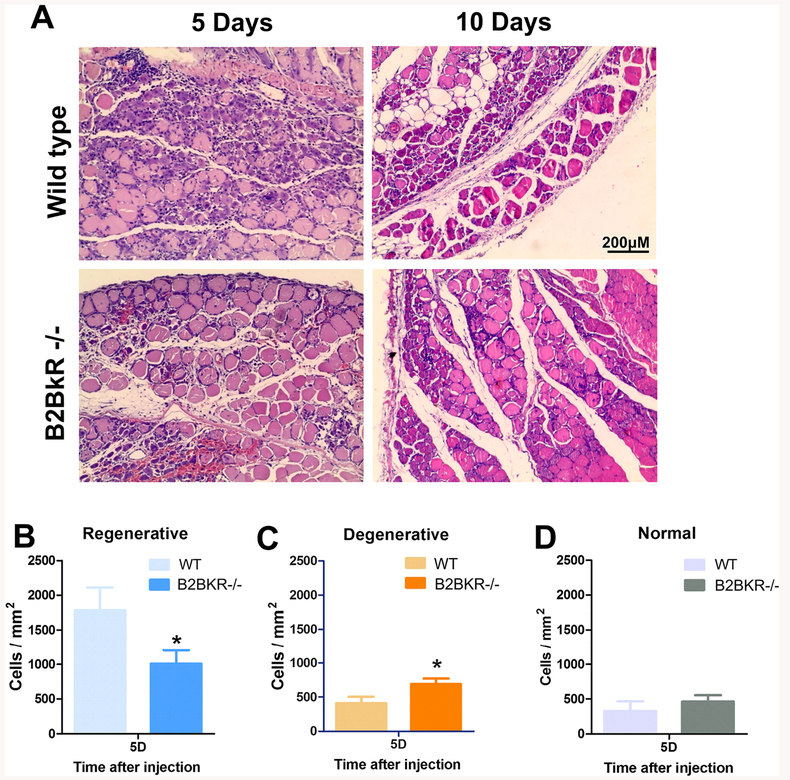
In order to investigate the role of B2BKR activity in muscle regeneration, we induced muscle injury injecting cardiotoxin into femoral muscles of B2BKR−/− and WT mice, Histological analyses on day 5 and day 10 showed more inflammatory cells infiltrated the necrotic area of the WTanimal than B2BKR−/− (Fig. 1a). Moreover, the quantification of regenerative, degenerative and normal fibers revealed that following 5 days of lesion, B2BKR−/− muscles showed significantly lower signs of nascent myotube formation (1008.3 ± 196.3 cells/mm2) than muscle from WT animals (1781.5 ± 329.2 cells/mm2) (Fig. 1b). This indicates that B2BKR−/− mice have less regenerative ability than animals expressing this receptor. In agreement with this data, the number of degenerative fibers, characterized by irregular shape and few nuclei was significantly higher in knockout (691.9 ± 79.2 cells/mm2) than WT mice (409.7 ± 93.3 cells/mm2) (Fig. 1c), while the amount of normal fibers did not change between the two groups (Fig. 1d).
Fig. 1. Muscle regeneration following cardiotoxin injection into WT and B2BKR−/−rectus femoral skeletal muscles.
H&E staining of muscle provided fiber status of fibers in cardiotoxin (100 μl of a 40 μM solution)-treated rectus femoral skeletal muscles of WT and B2BKR−/− mice. a Representative images of regenerating muscles of WT and B2BKR−/− mice at 5 and 10-days after lesion. Scale bars: 200 μm. Quantification of b regenerative, c degenerative and d normal fibers number per area (mm2) after 5 days. Data are expressed as mean ± SEM from 6 independent experiments. * p < 0.05 compared to wild-type (WT) mice
Bradykinin Secretion and Kininergic System Expression during C2C12 Cell Differentiation into Muscle Cells
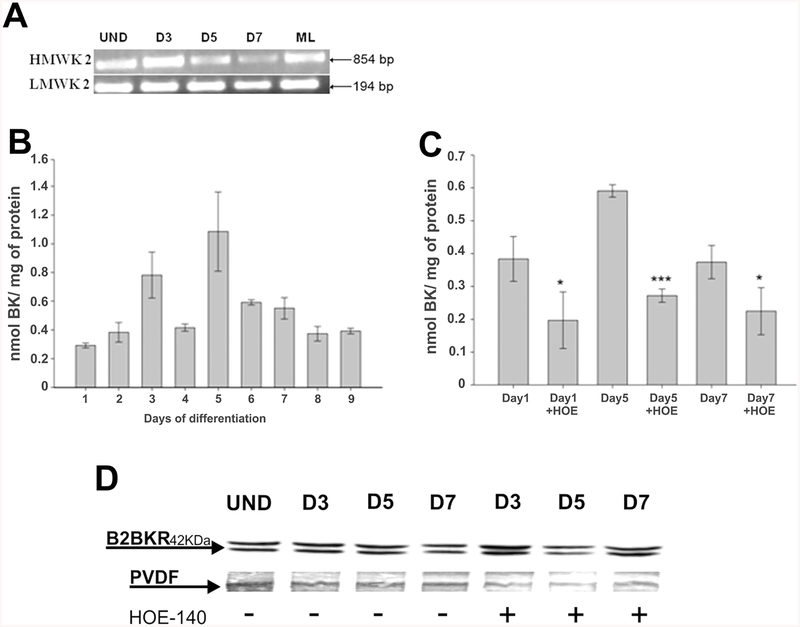
Murine C2C12 myoblasts were differentiated into skeletal muscle type cells by culturing them for 7 days in DMEM medium containing 2% horse serum. Undifferentiated cells and cells undergoing differentiation (day 1–7) expressed high and low molecular mass kininogens 2 as detected by RT-PCR (Fig. 2a), while expression of high and low mass kininogens 1 could not be identified (data not shown). Mice express two kininogen genes named 1 and 2, which generates the B2BKR agonists BK and kallidin [26]. Secretion of BK by differentiating C2C12 cells was quantified by HPLC analysis of collected cell culture supernatants. In culture supernatants of undifferentiated cells, BK secretion could be detected, while following induction to differentiation BK liberation increased and reached maximal values of 1.06 ± 0.27 nmol BK/mg of protein (mean ± S.E.M.) on day 4 (Fig. 2b). In the presence of the B2BKR antagonist HOE-140, that at 1 μM concentration completely blocks B2BKR activity [10], BK secretion into the culture medium was significantly decreased (Fig. 2c). B2BKR expression was also present during all days of differentiation in the presence and absence of HOE-140, including in undifferentiated C2C12 cells (Fig. 2d). Receptor activity during differentiation was confirmed by calcium imaging (data not shown).
Fig. 2. Kininogen mRNA transcription and bradykinin secretion into the culture medium during muscle differentiation of C2C12 cells.
a Detection of high and low molecular mass kininogen 2 (HMWK2 and LMWK2, respectively) mRNA transcription was performed by RT-PCR. b Quantification of BK in culture medium from differentiating C2C12 cells. c Quantification of BK in culture medium from differentiating C2C12 cells (day 1–7) in the absence or presence of 1 μM of the kinin-B2 antagonist HOE-140 (HOE). Cell culture supernatants from differentiating C2C12 cultures (days 0–7) were collected and analyzed for bradykinin content by reversed phase HPLC. Synthetic BK was used as a standard for elution and quantification. The results represent mean values ± S.D. of three independent experiments (* p < 0.05 and *** p < 0.001). d Detection of kinin-B2 receptor (B2BKR) expression in C2C12 cells during muscle differentiation by Western blot in the absence or presence of the antagonist HOE-140 (1 μM). Efficiency of protein transference from the polyacrylamide gel was confirmed by staining of the PVDF membrane and the gel with India ink. Density of bands was determinate using software Image J. ML mouse liver
Expression of Myogenic Protein Markers is Decreased when B2BKR is Inactivated
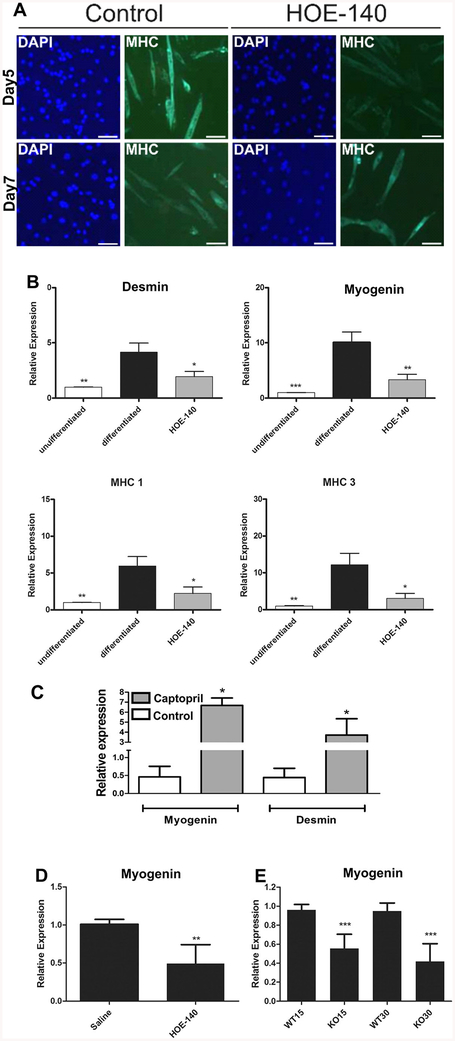
The secretion of BK and expression and functionality of B2BKRs along differentiation of C2C12 cells suggests an autocrine loop system between agonist secretion and receptor activation. Therefore, in order to confirm the participation of B2BKRs in myogenesis, undifferentiated C2C12 cells were induced to differentiation in the absence or presence of HOE-140 (1 μM). Morphological changes, cell fusion to form multinucleated myotubes and expression of MHC in differentiated cells on day 5 and 7 confirmed myogenic differentiation. (Fig. 3a). Gene expression of specific markers in undifferentiated or differentiated C2C12 myoblasts was further analyzed in conditions of absence or presence of HOE-140 during differentiation. Real-time PCR data revealed that desmin, myogenin, MHC-1 and MHC-3 expression was increased in differentiated cells by factors of 4 to 10 when compared to respective expression levels in undifferentiated myoblasts. Most importantly, even under conditions of HOE-140 treatment during differentiation of C2C12 myoblasts, the expression levels of these muscle markers were not different from those of undifferentiated myoblasts, (Fig. 3b). In order to verify BK-induced effects on muscle differentiation, the cells were treated with captopril. Captopril, is an angiotensin-converting enzyme inhibitor preventing BK breakdown, and therefore, potentiates BK effects [27]. In agreement with previously published data showing upregulation of MHC protein expression during C2C12 myogenic differentiation in the presence of captopril [28], we demonstrate here that chronic ACE inhibition resulted in increased desmin and myogenin expression (Fig. 3c). These results suggest that at least in part captopril-promoted myogenesis may result from BK accumulation in the extracellular medium. In vivo experiments showed that intraperitoneal injection of HOE-140 also interfered with myogenin expression levels in the gastrocnemius skeletal muscle of 90-day old mice (Fig. 3d). In agreement with B2BKR function in myogenesis, myogenin gene expression levels were reduced in the gastrocnemius skeletal muscle of B2BKR−/− mice at age of 15 and 30 days, when compared to WT animals (Fig. 3e).
Fig. 3. Myoblast differentiation depends on B2BKR activity in vitro and in vivo.
a Immunofluorescence studies of myosin heavy chain (MHC) expression in C2C12 cells on days 5 and 7 of differentiating in the absence or presence of HOE-140. Scale bars = 100 μm. b Relative gene expression analysis of desmin, myogenin, MHC 1 and MHC 3 in undifferentiated C2C12 cells, and cells induced to muscle differentiation for 7 days in the presence or absence of 1 μM HOE-140. c Relative gene expression levels of myogenin and desmin of C2C12 myoblasts induced to differentiation for 7 days in the absence or presence of 100 μM Captopril. d Myogenin gene expression in conditions of B2BKR inhibition in C57BL/6 90-days old mice injected with 1 mg/kg HOE-140 intraperitoneally for 21 days from birth. e Myogenin gene expression in B2BKR−/− mice on 15 (KO15) and 30-days of age (KO30). The results plotted represent mean values ± S.D. of three independent experiments (*p < 0.05, **p < 0.001 and ***p < 0.0001)
Effect of Inhibition of B2BKR in Cytoskeleton Rearrangement and Proliferation of Myoblasts in the Culture of C2C12 under Differentiation Induction
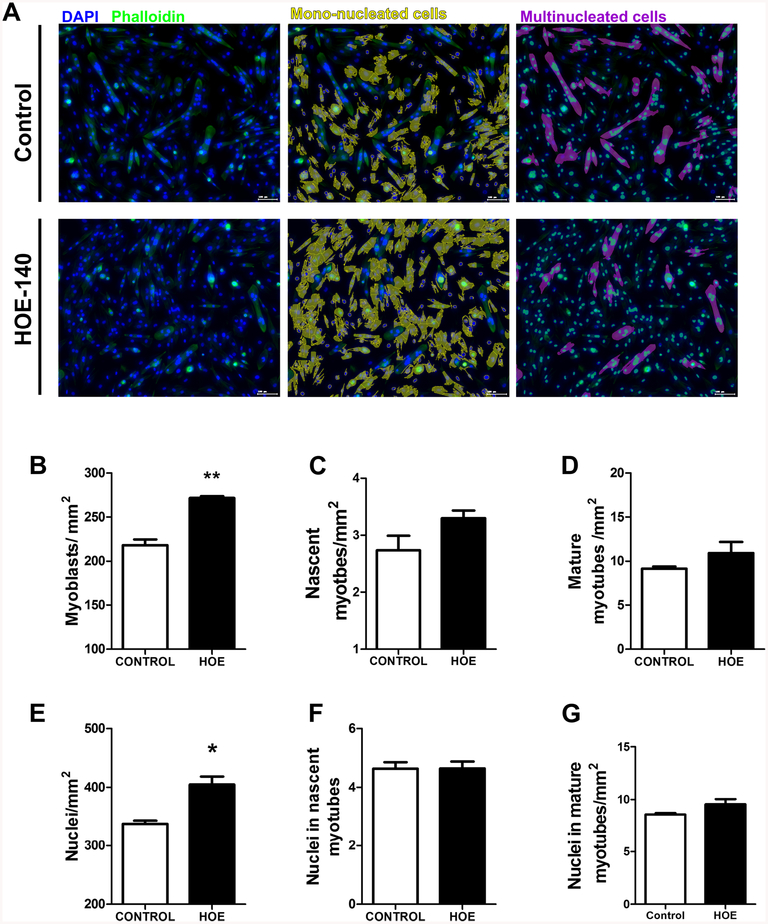
Proteomic analysis based on 2-D gel electrophoresis was performed using protein extract from cells on day 7 of myogenesis in the presence and absence of HOE-140. Expression of nestin and cardiac α-actin as well as β- and γ-actin was down-regulated in HOE-140 treated cells (Supplementary Material), suggesting that inhibition of B2BKR activity may interfere with the cytoskeleton arrangement for morphological changes during transition from myoblasts into differentiated muscle phenotypes. Next, Alexa phalloidin immunofluorescence staining for the actin cytoskeleton, revealed an important morphological change in the culture in the presence of HOE-140 when compared to control (Fig. 4a). For quantitative analysis of morphological changes in the treated and non-treated cultures, cellular areas were identified, followed by quantification of nuclei number using Strata Quest Software (TissueGnostics GmbH, Vienna, Austria). Indeed, such difference in overall culture occurred due to HOE-140-induced myoblast proliferation, reflected by higher number of mononucleated cells per area (271.8 ± 1.9) when compared to controls (218.2 ± 6.6), (Fig. 4b), but no significant alteration in number of nascent or mature myotube were observed (Fig. 4c and d). In agreement, in the control sample, there were less nuclei per area (337 ± 5.4) than treated sample (404.9 ± 13.8) (Fig. 4e); however, the number of nuclei per nascent or mature myotubes remained unchanged (Fig. 4f and g).
Fig. 4. Analysis of myoblasts, nascent and mature myotubes in C2C12 culture under condition of differentiation and B2BKR inhibition.
C2C12 myoblasts were induced to differentiation in DMEM supplemented with 2% horse serum in the absence or presence of HOE-140 a Fluorescence microscopy images are representative of cell nuclei staining with DAPI and actin filament staining with Alexa Fluor 488 phalloidin. Mono-nucleated cells (myoblasts) and Multi-nucleated cells (nascent and mature myotubes) were highlighted in both conditions. Scale bars = 100 μm. Quantification of b myoblasts, c nascent myotubes and d mature myotubes per area (mm2). Quantification of nuclei e per area, f in nascent myotubes and g in mature myotubes. Quantitative analyses were performed using Strata Quest software. Data represent mean values ± S.D. of three independent experiments(*p < 0.05 and **p < 0.001)
Discussion
Skeletal muscle plays important mechanical and metabolic activities, therefore, muscle-related diseases often come accompanied by a poor prognosis; however, muscle tissue has high regenerative activity [17]. Muscular dystrophies and degenerative muscle disorders are associated with ineffective or burnt-out regenerative potential of muscle tissue. Therefore, treatment of patients with muscular dystrophy or other muscle injury explores the regenerative potential of muscle stem cells [29].
BK and its receptor (B2BKR), members of the kallikrein-kinin system, have been originally attributed with functions in inflammation and induction of vasodilatation. However, recently it has been recognized that BK is also involved in regulation of proliferation [24, 30] and cellular differentiation of muscle from myoblasts [12]. Meanwhile, the role this system plays in muscle regeneration has yet not been described. The herein revealed data show the importance of BK-induced signaling through B2BKR activation for muscle repair functions in the adult animal. In the cardiotoxin-induced muscle injury model [31], B2BKR−/− mice following 5 days of rectus femoral muscle lesioning revealed significantly less regenerative fibers and more degenerative fibers than WT animals did.
We have demonstrated the expression of components of kallikrein-kinin system, such as high and low molecular mass kininogens and the B2BKR along differentiation of C2C12 myoblasts into myotubes. Autocrine receptor activation is suggested since following induction to differentiation, cells released BK into the culture medium. BK secretion along differentiation depended on B2BKR activity, as in the presence of the specific B2BKR antagonist HOE-140, secretion rates of the peptide were diminished on all studied days of differentiation. Using embryonic carcinoma cells as in vitro system for early neurogenesis, we have already shown that BK secretion and B2BKR activity formed an autocrine loop system. Moreover, B2BKR activity was essential for differentiation of embryonic cells into neurons expressing functional cholinergic receptors [10].
Myogenesis is the process for formation of muscle tissue, which consists of the proliferation of myoblasts, which subsequently fuse into multinucleated myotubes and form muscle fibers [29]. This process depends on specific genes whose expression rates are spatially and temporally regulated. B2BKR activity also contributed to the progress of myogenic differentiation, as the presence of HOE-140 during myoblast differentiation resulted in reduced muscle markers expression, such as myogenin, a member of the MyoD family, as well as desmin, a marker of muscle tissue in embryogenesis, and isoforms of MHC, such as MHC 1 and 3, expressed in adult and embryonic skeletal muscles, respectively [32–35].
In order to determine B2BKR activity in muscle differentiation, we have cultured C2C12 myotubes with captopril, an ACE inhibitor. BK is degraded by ACE into the biological inactive peptides BK-1–5 and 1–7. In this context, inhibition of ACE activity results in an increase of BK concentration in the extracellular medium and then augments B2BKR activation, which then would accelerate myogenesis. Indeed, captopril-treated C2C12 cells revealed higher myogenin and desmin expression levels than non-treated cells did. Such conclusion is in agreement with the inhibition of myogenic differentiation, observed in the presence of the B2BKR antagonist HOE-140. Potentiation of BK-induced receptor responses was described for the ACE inhibitor enalapril in isolated guinea pig ileum [36]. A direct effect of B2BKRs on ACE activity, however, cannot be ruled out, as recent findings suggest that ACE/B2BKR receptor heterodimerization alters pharmacological properties of the receptor. Angiotensin I-converting enzyme inhibitors potentiate bradykinin’s inotropic effects independently from blocking its inactivation [37]. Our work is in line with previous studies, which had already revealed the participation of this enzyme in myotube differentiation of C2C12 cells. Mori and Tokuyama (2007) reported that inhibition of ACE activity resulted in upregulation of MHC expression during C2C12 myoblast differentiation [28]. On the other hand, ACE overexpression negatively affected MHC expression. The authors concluded that a probable mechanism for the ACE activity-dependent progression of myoblast differentiation could be the activation of angiotensin AT 2 receptors. The hypothesis that inhibition of ACE-induced conversion of angiotensin I (Ang I) into Ang II blocks C2C12 myoblast differentiation is supported by experiments in which blockade of A2 receptor activity led to an increase in MHC expression during C2C12 cell differentiation. Inhibition of the AT2 receptor resulted in less acceleration of myogenesis when compared to rates obtained in the presence of captopril [28]. This observation leads to the conclusion that inhibition of Ang I into Ang II conversion is not the only mechanism for ACE-induced myotube differentiation and points at the relevant participation of B2BKR.
The B2BKR antagonist also affected myogenesis in vivo. C57BL/6 90-days old WT mice treated from birth to 21 days old with HOE-140 by intraperitoneal injection revealed diminished myogenin expression levels, when compared to control animals. These results let to suggest that chronic inhibition of the B2BKR does not only cause a temporal delay of myogenic differentiation, but also maintains its effect in the adult mature muscle tissue. In agreement, lower gene expression levels were observed in B2BKR−/− mice than in WT mice, providing evidence that the B2BKR functions in promoting myogenesis in vitro and in vivo. In fact, with blocking effects of B2BKR on the progress of muscle differentiation, reduction of cardiac α-actin as well as β- and γ-actin gene expression levels was observed, which are known to be enhanced following induction to myogenesis [38]. In addition, blockade of B2BKR induced myoblast proliferation. Since proliferation and differentiation are antagonistic, mutually exclusive states in muscle cells [18, 20, 21], this data is one more evidence of B2BKR activity in muscle differentiation.
In summary, our results define a novel role for B2BKRs in muscle regeneration. Since neuronal differentiation of stem cell models also depended on B2BKR activity, a general role for BK in cell differentiation may be postulated. ACE inhibitors which leads to an increase of the half-time of BK may also be attributed with novel therapeutic applications in diseases related to muscle injury, including myocyte hypertrophy and muscle dystrophy.
Supplementary Material
Funding
This work was supported by grants awarded by Fundação de Amparo à Pesquisa do Estado de São Paulo (São Paulo Research Foundation, FAPESP, Project No. 2012/50880–4) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil, to H.U. and C.L. (2015/19128–2), by a NIH grant, to A.H.M (8G12MD007600) and by the Universidad Central del Caribe Biomedical Proteomics Facility Grant G12MD007583 awarded to NMB from the NIH National Institute on Minority Health and Health Disparities (NIMHD) RCMI Program.. J.M.A.’s Ph.D. thesis research was funded by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil. This study was financed by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior -Brazil (CAPES) – Finance Code 001.
Footnotes
Conflict of Interest All authors declare that they do not have conflict of interests of any type to publish the manuscript.
Summary statement A novel function of bradykinin is described for muscle differentiation and repair.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12015-018-9850-9) contains supplementary material, which is available to authorized users.
References
- 1.Tschöpe C, & Westermann D (2008). Development of diabetic cardiomyopathy and the kallikrein-kinin system–new insights from B1 and B2 receptor signaling. Biological Chemistry, 389(6), 707–711. [DOI] [PubMed] [Google Scholar]
- 2.Cruden NLM, & Newby DE (2008). Therapeutic potential of icatib ant (HOE-140, JE-049). Expert Opinion on Pharmacotherapy, 9(13), 2383–2390. [DOI] [PubMed] [Google Scholar]
- 3.Rodi D, Buzzi A, Barbieri M, et al. (2013). Bradykinin B2receptors increase hippocampal excitability and susceptibility to seizures in mice. Neuroscience, 17(/248), 392–402. [DOI] [PubMed] [Google Scholar]
- 4.Torres-Rivera W, Pérez D, Park KY, et al. (2013). Kinin-B2 receptor exerted neuroprotection after diisopropylfluorophosphate-induced neuronal damage. Neuroscience, 5(/247), 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martins AH, Alves JM, Perez D, et al. (2012). Kinin-B2 receptor mediated neuroprotection after NMDA excitotoxicity is reversed in the presence of kinin-B1 receptor agonists. PLoS One, 7(2), 3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Dahr SS, Dipp S, Meleg-Smith S, et al. (2000). Fetal ontogeny and role of metanephric bradykinin B2receptors. Pediatric Nephrology, 14(4), 288–296. [DOI] [PubMed] [Google Scholar]
- 7.Madeddu P, Emanueli C, Gaspa L, et al. (1999). Role of the bradykinin B2 receptor in the maturation of blood pressure pheno-type: Lesson from transgenic and knockout mice. Immunopharmacology, 15(/44), 9–13. [DOI] [PubMed] [Google Scholar]
- 8.Martins AH, Alves JM, Trujillo CA, et al. (2008). Kinin-B2 receptor expression and activity during differentiation of embryonic rat neurospheres. Cytometry. Part A, 73(/4), 361–368. [DOI] [PubMed] [Google Scholar]
- 9.Trujillo CA, Schwindt TT, Martins AH, et al. (2009). Novel perspectives of neural stem cell differentiation: From neurotransmitters to therapeutics. Cytometry. Part A, 75(/1), 38–53. [DOI] [PubMed] [Google Scholar]
- 10.Martins AHB, Resende RR, Majumder P, Faria M, Casarini DE, Tárnok A, Colli W, Pesquero JB, & Ulrich H (2005). Neuronal differentiation of P19 embryonal carcinoma cells modulates kinin B2 receptor gene expression and function. The Journal of Biological Chemistry, 280(/20), 19576–19586. [DOI] [PubMed] [Google Scholar]
- 11.Trujillo CA, Negraes PD, Schwindt TT, Lameu C, Carromeu C, Muotri AR, Pesquero JB, Cerqueira DM, Pillat MM, de Souza HDN, Turaça LT, Abreu JG, & Ulrich H (2012). Kinin-B2 receptor activity determines the differentiation fate of neural stem cells. Journal of Biological Chemistry, 287(53), 44046–44061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruno G, Cencetti F, Bernacchioni C, Donati C, Blankenbach KV, Thomas D, Meyer zu Heringdorf D, & Bruni P (2018). Bradykinin mediates myogenic differentiation in murine myoblasts through the involvement of SK1/Spns2/S1P2axis. Cellular Signalling, 45, 110–121. [DOI] [PubMed] [Google Scholar]
- 13.Campion DR (1984). The muscle satellite cell: a review. International Review of Cytology, 87, 225–251. [DOI] [PubMed] [Google Scholar]
- 14.Camillo AC, De Carvalho Rocha R, & Chopard RP (2004). Structural and microvascular study of soleous muscle of Wistar rats after section of the sciatic nerve. Arquivos de Neuro-Psiquiatria, 62, 835–838. [DOI] [PubMed] [Google Scholar]
- 15.Cooper RN, Tajbakhsh S, Mouly V, et al. (1999). In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. Journal of Cell Science, 112(/1), 2895–2901. [DOI] [PubMed] [Google Scholar]
- 16.Morgan JE, & Partridge TA (2003). Muscle satellite cells. The International Journal of Biochemistry & Cell Biology, 35, 1151–1156. [DOI] [PubMed] [Google Scholar]
- 17.Bernacchioni C, Cencetti F, Ouro A, Bruno M, Gomez-Muñoz A, Donati C, & Bruni P (2018). Lysophosphatidic acid signaling axis mediates ceramide 1-phosphate-induced proliferation of C2C12 myoblasts. International Journal of Molecular Sciences, 19(1), 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Čamernik K, Barlič A, Drobnič M, Marc J, Jeras M, & Zupan J (2018). Mesenchymal stem cells in the musculoskeletal system: from animal models to human tissue regeneration? Stem Cell Reviews and Reports, 14, 346–369. [DOI] [PubMed] [Google Scholar]
- 19.Siemionow M, Cwykiel J, Heydemann A, Garcia J, Marchese E, Siemionow K, & Szilagyi E (2018). Dystrophin expressing chimeric (DEC) human cells provide a potential therapy for Duchenne muscular dystrophy. Stem Cell Reviews and Reports, 14, 370–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siemionow M, Cwykiel J, Heydemann A, Garcia-Martinez J, Siemionow K, & Szilagyi E (2018). Creation of dystrophin expressing chimeric cells of myoblast origin as a novel stem cell based therapy for Duchenne muscular dystrophy. Stem Cell Reviews and Reports, 14, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blau HM, Pavlath GK, Hardeman EC, et al. (1985). Plasticity of the differentiated state. Science, 230, 758–766. [DOI] [PubMed] [Google Scholar]
- 22.Filigheddu N, Gnocchi VF, Coscia M, Cappelli M, Porporato PE, Taulli R, Traini S, Baldanzi G, Chianale F, Cutrupi S, Arnoletti E, Ghè C, Fubini A, Surico N, Sinigaglia F, Ponzetto C, Muccioli G, Crepaldi T, & Graziani A (2007). Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Molecular Biology of the Cell, 18, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burattini S, Ferri R, Battistelli M, et al. (2004). C2C12 murine myoblasts as a model of skeletal muscle development: Morpho-functional characterization. European Journal of Histochemistry, 48(/3), 223–233. [PubMed] [Google Scholar]
- 24.Ulrich H, Ratajczak MZ, Schneider G, et al. (2018). Kinin and purine signaling contributes to neuroblastoma metastasis. Frontiers in Pharmacology, 9, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abmayr SM, & Pavlath GK (2012). Myoblast fusion: lessons from flies and mice. Development, 139, 641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardoso CC, Garrett T, Cayla C, Meneton P, Pesquero JB, & Bader M (2004). Structure and expression of two kininogen genes in mice. Biological Chemistry, 385(3–4), 295–301. [DOI] [PubMed] [Google Scholar]
- 27.Morais KL, Ianzer D, Miranda JR, Melo RL, Guerreiro JR, Santos RA, Ulrich H, Lameu C (2013). Proline rich-oligopeptides: Diverse mechanisms for antihypertensive action. Peptides, 48, 124–133. [DOI] [PubMed] [Google Scholar]
- 28.Mori S, & Tokuyama K (2007). ACE activity affects myogenic differentiation via mTOR signaling. Biochemical and Biophysical Research Communications, 363(3), 597–602. [DOI] [PubMed] [Google Scholar]
- 29.Yusuf F, & Brand-Saberi B (2012). Myogenesis and muscle regeneration. Histochemistry and Cell Biology, 138(2), 187–199. [DOI] [PubMed] [Google Scholar]
- 30.Pillat MM, Lameu C, Trujillo CA, Glaser T, Cappellari AR, Negraes PD, Battastini AMO, Schwindt TT, Muotri AR, & Ulrich H (2016). Bradykinin promotes neuron-generating division of neural progenitor cells through ERK activation. Journal of Cell Science, 129(/18), 3437–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garry GA, Antony ML, & Garry DJ (2016). Cardiotoxin induced injury and skeletal muscle regeneration. Methods in Molecular Biology (Clifton, N.J.), 1460, 61–71. [DOI] [PubMed] [Google Scholar]
- 32.Zhu LN, Ren Y, Chen JQ, & Wang YZ (2013). Effects of myogenin on muscle fiber types and key metabolic enzymes in gene transfer mice and C2C12 myoblasts. Gene, 532(2), 246–252. [DOI] [PubMed] [Google Scholar]
- 33.Paulin D, & Li Z (2004). Desmin: A major intermediate filament protein essential for the structural integrity and function of muscle. Experimental Cell Research, 301(1), 1–7. [DOI] [PubMed] [Google Scholar]
- 34.Allen DL, Harrison BC, Sartorius C, Byrnes WC, & Leinwand LA (2001). Mutation of the IIB myosin heavy chain gene results in muscle fiber loss and compensatory hypertrophy. American Journal of Physiology. Cell Physiology, 280(3), C637–C645. [DOI] [PubMed] [Google Scholar]
- 35.Lyons GE, Ontell M, Cox R, Sassoon D, & Buckingham M (1990). The expression of myosin genes in developing skeletal muscle in the mouse embryo. Journal of Cell Biology, 111(4), 1465–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaffel R, Rodrigues MS, & Assreuy J (1991). Potentiation of bradykinin effects and inhibition of kininase activity in isolated smooth muscle. Canadian Journal of Physiology and Pharmacology, 69(7), 904–908. [DOI] [PubMed] [Google Scholar]
- 37.Minshall RD, Erdös EG, & Vogel SM (1997). Angiotensin I-converting enzyme inhibitors potentiate bradykinin’s inotropic effects independently of blocking its inactivation. American Journal of Cardiology, 3(80), 132A–136A. [DOI] [PubMed] [Google Scholar]
- 38.Bains W, Ponte P, Blau H, & Kedes L (1984). Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Molecular and Cellular Biology, 4(8), 1449–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.