Abstract
Sepsis involves a disordered host response to systemic infection leading to high morbidity and mortality. Despite intense research, targeted sepsis therapies beyond antibiotics have remained elusive. The cornerstone of sepsis research is the development of animal models to mimic human bacterial infections and test novel pharmacologic targets. Nonhuman primates (NHPs) have served as an attractive, but expensive, animal to model human bacterial infections due to their nearly identical cardiopulmonary anatomy and physiology, as well as host response to infection. Several NHP species have provided substantial insight into sepsis-mediated inflammation, endothelial dysfunction, acute lung injury, and multi-organ failure. The use of NHPs has usually focused on translating therapies from early preclinical models to human clinical trials. However, despite successful sepsis interventions in NHP models, there are still no FDA-approved sepsis therapies. This review highlights major NHP models of bacterial sepsis and their relevance to clinical medicine.
Subject terms: Bacterial infection, Translational research, Drug development, Experimental models of disease, Animal disease models
Treatment for bacterial sepsis remains limited beyond the use of antibiotics. Lingye Chen, Karen Welty-Wolf, and Bryan Kraft review nonhuman primate models of sepsis and highlight their advantages and limitations compared to other preclinical models.
Main
Sepsis is a common clinical syndrome arising from a dysregulated host response to systemic infection leading to life-threatening end-organ damage1. While the incidence of sepsis varies2, the substantial burden of critical illness reflects an aging and susceptible population with escalating comorbid conditions3,4. With the exception of early antibiotics and fluids5–7, targeted new therapies for sepsis have remained elusive8. However, through research, our understanding of sepsis has evolved from being seen solely as an exuberant host immune response9 to the intersection of pro- and anti-inflammatory, endothelial, coagulation, metabolic, endocrine, and neural pathways10,11. The foundation of such research has been the use of small animals in studies to unravel mechanisms of sepsis pathophysiology and develop novel therapeutics12,13.
This review briefly presents the common nonhuman primate (NHP) models of bacterial sepsis and summarizes the contributions to our understanding of sepsis pathophysiology and drug discovery, and how they might impact on future targeted research.
Finding an ideal animal model
An ideal animal model of sepsis must balance biological feasibility and practical considerations. The animal species should recapitulate the same hemodynamic changes to infection seen in humans, such as hypotension, low systemic vascular resistance (SVR), and a compensatory increase in cardiac output (CO) following fluid resuscitation14–16. Animals should also demonstrate similar signs and symptoms of infection, such as decreased activity or oral intake, change in body temperature, and change in white blood cell count12. The model should include provision of antibiotics17,18, a standard of care treatment for patients1 that may alter host cytokine responses19 and significantly improves survival20. The species should also activate similar molecular pathways, such as cytokine release in response to circulating pathogen-associated molecular patterns (PAMPs)11, and should lead to development of multiple organ dysfunction (MOD)21,22. Practically, the model must be economically reasonable, easy to support, and large enough for the collection of physiological data.
The earliest and most widely used animal species for sepsis studies has been rats and mice. Murines are attractive because they are inexpensive, easy to breed, versatile, and genetically modifiable8,12,13. The rat model of endotoxemic sepsis with fluid resuscitation relies on intravenous lipopolysaccharide (LPS) to mimic the physiological responses of Gram-negative sepsis in humans. Unlike human sepsis, which is a protracted response to live pathogens, LPS induces a rapid, transient inflammatory surge that causes mainly vascular injury13. The rat cecal-ligation and puncture (CLP) technique was developed to mimic human abdominal sepsis from live polymicrobial bacteria12,23,24. Though this model has been widely considered the gold standard for rodent studies, it is limited by variability in technique (e.g. number of punctures, size of puncture)23 and pathogen (e.g. polymicrobial)24. As an alternative rat model, the monomicrobial Staphylococcus aureus impregnated clot model of peritoneal sepsis was developed to simulate post-operative abdominal wound infections25. Another model using intravenous injection of live bacteria simulates the bacteremia seen in up to 69% of human septic shock24, but does so by artificially infusing a large inoculum of bacteria into the bloodstream rather than by slow release from an established nidus of infection12.
Rodent models suffer from important technical limitations. Their small size makes invasive hemodynamic monitoring difficult, and their small total blood volume (approximately 2 ml in mice and 20 ml in rats) limits serial phlebotomy23. Mice are also more resistant to LPS and bacterial pathogens than are humans, requiring 104- to 105-fold higher doses to achieve shock8,13,24. Once shock develops, the hemodynamic and cytokine profiles of small rodents differ greatly from those of humans8,24, though the inconsistent use of fluid resuscitation in these studies may have affected the results.
The sizable technical and biological challenges ultimately led to the use of larger mammals such as rabbits, sheep, dogs, and pigs, whose sizes permitted invasive and serial procedures12,23. Many large animals also exhibit sepsis physiology that is similar to humans. For instance, pigs are prone to sepsis-induced capillary leak and development of pulmonary edema, and sheep display similar hyperdynamic responses (i.e. elevated heart rate, cardiac output, and oxygen delivery with depressed systemic vascular resistance) to endotoxin as humans23. Nevertheless, notable physiological differences still exist between these large mammals and humans: Sheep and other ruminants have significantly more pulmonary intravascular macrophages compared to primates, which alters their susceptibility to sepsis-induced pneumonia and acute lung injury26,27. Pigs demonstrate a profound rise in pulmonary vascular resistance in response to bacterial sepsis which can be fatal and experiment-limiting23. And dog models of septic shock are confounded by significant intestinal mucosal congestion and hemorrhage23.
The nonhuman primate model of sepsis
To circumvent the limitations of rodent and large mammal models, researchers developed nonhuman primate (NHP) models of bacterial sepsis20,28,29. Baboons, macaques, and chimpanzees have historically been the most popular primates studied, though as of 2013 the National Institutes of Health has limited its funding support to monkeys, as great apes are widely agreed to be too sentient to ethically justify their use. Originating from a common phylogenetic ancestor, humans and monkeys have nearly identical anatomy and physiology. For example, the baboon and human tracheobronchial trees share a similar dichotomous branching pattern with a bronchiolar transition zone between the small airways and distal alveolar regions30. Moreover, the alveolar region of baboons and humans contain a prominent alveolar interstitial compartment31. These features are less prominent or less uniform in rodents, which may partly explain differing patterns of pneumonia-induced acute lung injury (ALI) compared with humans30–32.
Humans and baboons also display almost identical hemodynamic and cytokine responses to intravenous endotoxin and live bacteria29,33–35. Following infusion of Gram-negative bacteria (E. coli), baboons develop tachycardia, increased cardiac output, decreased SVR, and hypotension similar to human sepsis29.
Furthermore, the use of NHPs carries the same advantages seen in other large animals. For instance, the NHPs’ large size allows for invasive monitoring, tissue collection, and serial phlebotomy23. Their use also satisfies the U.S. Food and Drug Administration (FDA) drug development requirement that investigational new drug (IND) applications include data from at least one non-rodent species23. As such, the NHP models are considered quite suitable for pre-clinical drug development studies.
Endotoxemia model
Endotoxin plays a central role in the development of Gram-negative sepsis. Located on the outer cell membrane of Gram-negative bacteria, LPS is a pathogen-associated molecular pattern (PAMP) recognized by the innate immune pathogen recognition receptor, toll-like receptor 4 (TLR4), and activates pro-inflammatory and coagulation cascades36. Healthy human volunteers given intravenous LPS (2–4 ng/kg) developed tachycardia, decreased SVR, and depressed left ventricular function34. These hemodynamic changes are associated with the release of pro-inflammatory cytokines, tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), and activation of coagulation and fibrinolysis pathways37 (Fig. 1a, b), similar to the responses of patients in septic shock.
Fig. 1.
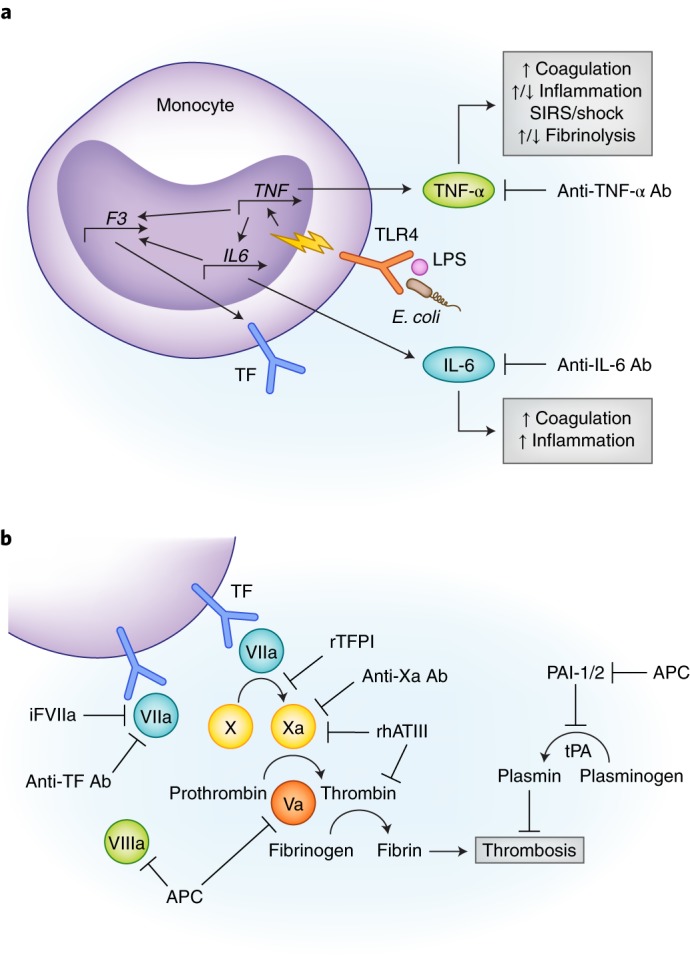
(a) Simplified rationale for performing studies in NHP sepsis models of antibody inhibition of TNF-α and IL-6. Lipopolysaccharide (LPS) or E. coli are shown binding to a TLR4 surface receptor of a monocyte which initiates downstream signal transduction (yellow lightning bolt) and transcriptional upregulation of TNF-α. TNF-α further activates other pro- and anti-inflammatory cytokines, including IL-6. Both cytokines promote hypercoagulability by upregulating tissue factor (TF) expression. TNF-α also simultaneously promotes and inhibits fibrinolysis via activation of tPA and PAI-1/2, respectively (see Fig. 1b). (b) Multiple therapeutics are shown inhibiting different components of the extrinsic coagulation pathway. These drugs were tested using LPS or E. coli NHP sepsis models. Abbreviations: Ab, antibody; APC, activated protein C; F3, factor 3; iFVIIa, site inactivated factor VIIa; IL6, interleukin 6; LPS, lipopolysaccharide; PAI-1/2, plasminogen activator inhibitor 1/2; rhATIII, recombinant human antithrombin III; SIRS, systemic inflammatory response syndrome; TF, tissue factor; TLR4, toll-like receptor 4; TNF, tumor necrosis factor.
NHPs are more resistant to endotoxin than humans. The baboon (Papio ursinus or cynocephalus) requires 0.1 mg/kg33 and the rhesus macaque (Macaca mulatta) 0.1 mcg/kg38 to achieve a hyperdynamic state. One exception appears in older studies of chimpanzees (Pan troglodytes) which, like humans, develop a mild and reversible inflammatory state at 4 ng/kg39,40. Nonetheless, NHP endotoxemia models are frequently employed to model Gram-negative sepsis without live pathogen inoculation, and they generally corroborate the hemodynamic and cytokine profiles seen in human sepsis33.
Pharmacologic inhibitors are commonly used to test mechanism of immune pathways, and monoclonal antibodies have been used in NHP sepsis experiments to inactivate a variety of pro-inflammatory and coagulation mediators38,40,41. Chimpanzees given monoclonal anti-TNF-α antibodies (15 mg/kg) immediately following LPS injection had decreased downstream production of IL-6 and CXCL840, and within the lungs, had increased fibrinolysis42 compared to LPS-only controls. Baboons and rhesus monkeys treated with anti-TNF-α antibodies had lower serum TNF-α levels, attenuated coagulation responses, and less severe organ dysfunction than control monkeys38,43. In the rhesus, treatment with anti-TNF-α antibodies significantly improved survival38. These findings suggest that TNF-α is not only a catalyst for other downstream inflammatory mediators, but also for sepsis-induced fibrinolysis and organ dysfunction (Fig. 1a).
Like TNF-α, IL-6 is also an early phase mediator (released within one hour) of LPS exposure33,37. In chimpanzees with mild endotoxemia, anti-IL-6 antibodies attenuate thrombin-antithrombin III (TAT) complex activity in both serum44 and lungs42, suggesting its role in the activation of sepsis-induced coagulation. Similarly, pretreatment with monoclonal antibody to tissue factor decreased TAT complex activity and coagulation response, but had no effect on cytokine release or fibrinolysis41 (Fig. 1a).
IL-6 also influences the development of acute lung injury (ALI). In endotoxemic chimpanzees given anti-IL-6 antibody, pro-coagulant proteins were significantly reduced in bronchoalveolar lavage fluid (BALF) compared with untreated animals42. These studies also found that pro-coagulant proteins were elevated in the BALF without evidence of vascular permeability, suggesting that coagulation in ALI is a local response42.
E. coli bacteremia model
Despite its ability to duplicate the initial hours of Gram-negative sepsis, the LPS model nonetheless cannot replace a live bacterial infection model. E. coli bacteremia in the baboon (Papio cynocephalus) is one of the earliest and most versatile models of NHP sepsis and has greatly expanded the understanding of sepsis-induced inflammation, coagulation, and organ injury45–47.
Disseminated intravascular coagulation
A frequent complication of sepsis, disseminated intravascular coagulation (DIC) is a syndrome of dysregulated microvascular coagulation that can lead to MOD45,47,48. In the baboon with E. coli bacteremia, the degree of coagulopathy varies directly with the dose of pathogen. The three most common dosing models, lethal dose 100 (LD100), LD50, and LD10, corresponding to a predicted mortality of 100%, 50%, and 10%, respectively48, have been used to elucidate mechanisms of sepsis-induced DIC and cellular injury, and to test novel sepsis therapies.
The LD100 model (1010 CFU/kg E. coli) produces a highly virulent phenotype wherein all animals die within 24 to 32 hours48,49. Administration of intravenous E. coli triggers the release of TNF-α, IL-6, and CXCL848, activation of endothelial cells, and microvascular migration of leukocytes50,51. Activated endothelial cells also express tissue factor (TF), a stimulus for intravascular and extravascular fibrin deposition45,47,48. Figures 1a,b demonstrate the TF-dependent coagulation pathways culminating in fibrin clot formation. Moreover, fibrinolysis is simultaneously triggered and inhibited by upregulation of tissue plasminogen activator (tPA), TAT complexes, and plasminogen activator inhibitor (PAI)45,46. These competing processes result in unchecked and widespread microvascular clot formation and MOD47.
NHP studies of coagulation cascade inhibitors further support DIC as a contributor to MOD (Fig. 1b, Table 1). Compared with control animals, baboons that received recombinant tissue factor-pathway inhibitor (TFPI) shortly after LD100 infusion had an attenuated coagulation response, significantly reduced organ injury, and 100% survival at 7 days52. Likewise, LD100E. coli animals given monoclonal antibodies against tissue factor53 or inactivated factor VIIa54 had attenuated coagulopathy and lethality. Moreover, treatment of E. coli-infected baboons with recombinant human antithrombin III (ATIII) reduced inflammation, coagulation, fibrinolysis, and mortality55.
Table 1.
Summary of experimental therapies and their associated nonhuman primate models
| Ref. | Species/Model | Pharmacological Target | Therapeutic | Outcome |
|---|---|---|---|---|
| 38 | Rhesus/i.v. LPS | TNF-α | Anti-TNF-α Ab | ↓ TNF-α, coagulation, MOF, death |
| 40,42 | Chimpanzee/i.v. LPS | TNF-α | Anti-TNF-α Ab | ↓ IL-6, CXCL840, & lung coagulation42 |
| 43 | Baboon/i.v. LPS | TNF-α | Anti-TNF-α Ab | ↓ TNF-α, coagulation, MOF |
| 95–98 | Baboon/i.v. E. coli | TNF-α | Anti-TNF-α Ab | ↓ coagulation, MOF, death |
| 42,44 | Chimpanzee/i.v. LPS | IL-6 | Anti-IL-6 Ab | ↓ coagulation in lung/BAL42, serum44 |
| 52 | Baboon/i.v. E. coli | Xa, FVIIa/TF/Xa complex | rTFPI | ↓ coagulation, MOF, death |
| 53 | Baboon/i.v. E. coli | TF | Anti-TF Ab | ↓ coagulation, SIRS, death |
| 18,54 | Baboon/i.v. E. coli | TF | Anti-FVIIa Ab | ↓ coagulation, inflammation, MOF, death |
| 70 | Baboon/i.v. E. coli | TF | Anti-FX Ab | ↓ coagulation, MOF |
| 55 | Baboon/i.v. E. coli | Thrombin pathway | rhATIII | ↓ DIC, inflammation, death |
| 58 | Baboon/i.v. E. coli | C3 convertase | compstatin | ↓ coagulation, MOF |
| 72 | Baboon/i.v. E. coli | C3 convertase | compstatin | ↓ post-ARDS fibrosis |
| 59 | Baboon/i.v. E. coli | C5 | C5 inhibitor | ↓ coagulation, MOF, death |
| 94 | Baboon/i.v. E. coli | E- and L-selectins | Anti-selectin Ab | Worsened UOP, acidosis, mortality |
| 48,49 | Baboon/i.v. E. coli | FVa, FVIIIa, PAI-1 | APC | ↓ coagulation/TNF-α48, MOF/death49 |
| 109–111 | Baboon/i.b. S. pneumoniae | HO-1, SPM | Inhaled CO gas | ↓ ALI severity109, ↑ SPM110,111 |
| 88 | Cynomolgus/i.b. B. anthracis | 30S ribosomal subunit | TP-271 antibiotic | ↓ infection-related mortality |
| 89, 90,113 | Cynomolgus/i.b. B. anthracis | Host immunity | vaccines | ↓ infection-related mortality |
Abbreviations: Ref, references; i.v., intravenous; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-alpha; Ab, antibody; MOF, multi-organ failure; IL, interleukin; BAL, bronchoalveolar lavage fluid; TF, tissue factor; rTFPA, recombinant tissue factor pathway inhibitor; TF, tissue factor; SIRS, systemic inflammatory response syndrome; rhATIII, recombinant human antithrombin III; DIC, disseminated intravascular coagulation; ARDS, acute respiratory distress syndrome; UOP, urine output; PAI-1, plasminogen activator inhibitor 1; APC, activated protein C; i.b., intra-bronchial; HO-1, heme oxygenase-1; SPM, specialized proresolving lipid mediators; CO, carbon monoxide; ALI, acute lung injury.
Multi-organ dysfunction
The LD100 model demonstrated that inflammation and coagulation interconnect through tissue factor, and that derangement of coagulation pathways culminates in DIC and lethal organ failure. However, due to its high lethality, the LD100 model was unable to define the cellular injury and repair that occurs in sub-lethal sepsis, prompting development of LD10 and LD50 models.
The baboon LD50 model (109 CFU/kg E. coli) produced hypotension and progressive MOD that was fatal in 50% of animals. The baboon LD10 model (107–108 CFU/kg E. coli) produced mild hypotension and non-progressive organ dysfunction with spontaneous resolution. Unlike the LD100 model where animals expired rapidly, these sub-lethal models survived long enough to demonstrate that sepsis is a multistage process driven by distinct mechanisms48. Although these experiments preceded the current standard of fluid resuscitation in early sepsis6, and ischemia-reperfusion is recognized and prevented clinically, these experiments show the natural course of under-resuscitated sepsis. The initial stage is a TNF-α- and tissue factor-dependent and PAMP-mediated intravascular innate immune activation leading to microvascular DIC and organ ischemia. The second stage is the extravascular (organ) sequelae of ischemia-reperfusion injury48, with accumulation of free radicals and further release of inflammatory mediators even after pathogen clearance56. Thus, whereas the LD100 animals expired from intravascular events (hemodynamic collapse and DIC) before reperfusion could develop, the LD50 animals expired from extravascular tissue injury48. This host-mediated, pathogen-independent injury is due to widespread complement activation48,56,57. Specifically, serum C5b9 levels were significantly elevated in LD50 non-survivors, comparable to that of LD100 animals, but not in LD50 survivors48. These findings provide a deeper understanding of the mechanisms of organ injury and rationale for modern clinical practice including early aggressive fluid resuscitation.
The role of complement activation in lethal MOD was demonstrated in greater detail by NHP complement inhibitor studies (Table 1). Administration of compstatin, a C3 convertase inhibitor, to LD50 baboons inhibited complement activation, protected against organ injury, and attenuated sepsis-mediated coagulopathy58. Concurrent administration of E. coli with a C5 inhibitor similarly decreased the inflammatory and coagulation responses, leading to organ protection and improved survival59.
Acute respiratory distress syndrome
Because of their similarities to human respiratory anatomy32 and physiology during Gram-negative sepsis60, baboon models are central to the study of sepsis-induced acute respiratory distress syndrome (ARDS), an ALI arising from systemic inflammatory responses61. ARDS is characterized by bilateral pulmonary infiltrates, reduced lung compliance, and impaired gas exchange, and in severe cases can lead to respiratory failure, MOD, and death62–64. The histopathologic hallmark is protein-rich alveolar edema and fibrin deposition due to breakdown of the alveolar-capillary barrier61,63. Compared with healthy controls, bronchoalveolar lavage fluid (BALF) from patients with ARDS demonstrates high factor X, extrinsic pathway inhibitor, and anti-plasmin activity, suggesting that a hypercoagulable environment develops within the lungs65.
The LD100 intravenous E. coli baboon model was further optimized to enhance ALI by the addition of a pre-inoculation bacterial priming step. Baboons pretreated with 109 CFU/kg heat-killed E. coli (65°C water bath for 30 minutes) prior to inoculation with 9 x 1010 CFU/kg live E. coli developed endotoxin tolerance with less shock and renal injury than unprimed animals66. However, the tolerance did not extend to the lungs, as priming worsened alveolar inflammation and fibroblast proliferation compared to unprimed animals66. These findings suggest that prior activation of the systemic inflammatory system leads to more severe lung injury in subsequent infections.
Using this primed LD100 E. coli model, the same tissue factor-dependent pathways involved in systemic endothelial activation were found to also mediate pulmonary microvascular injury (alveolar fibrin deposition) and contribute to ARDS18,67,68. Additionally, tissue factor is not only expressed on endothelial cells but also on pulmonary macrophages and alveolar epithelium, suggesting that these extravascular cells also regulate the procoagulant environment of the lung seen in ALI67,69. Further dysregulation comes from decreased expression of tissue factor pathway inhibitor (TFPI), a major checkpoint of the coagulation pathway68 (Fig. 1b). Baboons treated with site-inactivated factor VIIa18 or with factor X inhibitor70 to block tissue factor binding had reduced lung injury and improved renal function. These experiments support the causative relationship between coagulation and MOD.
The lung pathology from patients at autopsy or from patients undergoing open lung biopsies show that ARDS is an acute inflammatory process followed by either resolution or fibrosis63, but less is known about long term ARDS sequelae61. Keshari et al. used the LD50E. coli model to characterize the evolution of ARDS-associated fibro-inflammatory changes for up to 27 months post-inoculation71. Lung histopathology within the first 24 to 48 hours after inoculation demonstrated an acute exudative process with increased alveolar-capillary permeability and intra-alveolar hemorrhage, fibrin, and edema. By day 7, a restorative phase had developed with proliferation of type 2 epithelial cells. In baboons surviving to six to 27 months, lung tissue universally showed fibroblast proliferation, collagen deposition, and significantly increased macrophage accumulation compared to controls, suggesting the long-term sequelae of ARDS is fibrosis and chronic inflammation71. Treatment of LD50 baboons with the complement inhibitor compstatin, however, decreased expression of fibrosis mediators, such as transforming growth factor-beta (TGF-β) and connective tissue growth factor (CTGF), myofibroblast accumulation, and collagen deposition72.
Organ-specific NHP models of sepsis
In addition to the pathophysiology of sepsis, NHPs are also used to model organ-specific disease. The most common source of sepsis in adults is pneumonia followed by abdominal and urinary tract infections21. While no NHP model for urinary tract infections has been reported, abdominal sepsis has been modeled in baboons via intraperitoneal implantation of E. coli-laden fibrin clot at a dose of 1011 CFU73. This model produced coagulopathy, MOD, and a 42% mortality rate. However, the interpretation was limited because the animals did not receive antibiotics or surgical source control, while both are standard-of-care treatments for patients with peritonitis.
Necrotizing fasciitis has also been modeled in baboons by intramuscular injection with Streptococcus pyogenes (group A Streptococcus)74. The animals develop a multistage, suppurative, soft tissue infection similar to that of humans and characterized by abscess formation, violaceous skin discoloration, and bullae formation. Histopathology showed early intense neutrophilic influx at the site of infection followed by lymphoplasmacytic infiltration. Non-survivors, however, had a paucity of neutrophilic infiltration and a peripheral blood leukopenia, suggesting death may be linked to secondary immune paralysis. Neither of these surgical sepsis models has gained widespread use.
Pneumonia
The most extensively studied organ system is the lungs. The use of NHP to model pneumonia dates back to the 1970s, most commonly using S. pneumoniae, the leading cause of bacterial community-acquired pneumonia (CAP) in adults75. An inoculation is performed under direct visualization by bronchoscopic instilment of live bacteria into the airway17,76,77. Multiple species of NHP are susceptible to S. pneumoniae infection. Squirrel monkeys endotracheally inoculated with either influenza A or low-dose Streptococcus pneumoniae (770 CFU) develop a self-limited illness with cough, fever, and tachypnea much like in humans76. However, the two pathogens together produce severe bronchopneumonia, leading to death in three of four animals76.
The rhesus macaque (Macaca mulatta) demonstrates a dose-dependent response to S. pneumoniae ranging from no clinical symptoms at 106 CFU to fever, leukocytosis, and respiratory distress at 108–109 CFU77. A more recent model of S. pneumoniae pneumonia was published in baboons (P. cynocephalus). Animals given 106 CFU experienced spontaneous bacterial clearance without signs or symptoms of pneumonia, whereas animals given 109 CFU consistently developed severe lobar pneumonia that closely mimics the human disease17. This novel model has since been externally validated19.
The inflammatory responses are also consistent across primate species. In baboons infected with high dose S. pneumoniae (109 CFU), peripheral blood cytokines and chemokines such as IL-6, CCL2, G-CSF, IL-1ra, and IL-10 are significantly elevated by 24 to 48 hours post-inoculation, but return to normal or near normal levels with time (~ 1 week) and antibiotic therapy17,19. Reyes et al. found that antibiotic treatment may reduce cytokine and chemokine concentrations in the lung tissue itself, although differences in experimental duration between the antibiotic-treated and untreated groups (9–14 days vs. 4–9 days, respectively), could also account for this observation19. In the BALF of baboons and squirrel monkeys with S. pneumoniae pneumonia, TNF-α, IL-1β, and IL-6 levels are significantly elevated by 24 to 48 hours17,77 and similarly decline during the resolution phase17. These plasma and BALF cytokine profiles corroborate observations in patients with pneumonia78. Lung histopathology at 168 hours also mirrors human disease and shows ALI with intra-alveolar fibrin, edema, neutrophilic infiltration, and hemorrhage, and in some cases, early organization with collagen deposition17.
Models for methicillin-resistant Staphylococcus aureus (MRSA) pneumonia have also been developed in the cynomolgus macaque (Macaca fasicularis)79 and rhesus macaque80 to study virulence factors. Historically considered a nosocomial pathogen, MRSA is now a recognized cause of severe, necrotizing CAP75,81, especially following influenza infection82,83. The MRSA cytotoxin Panton-Valentine leukocidin (PVL) has been implicated in necrotic S. aureus soft tissue infections and CAP84, however in macaques inoculated with wild-type MRSA strain USA300 versus PVL deletion-mutant strain, no difference in disease severity was seen79. Influenza A with MRSA co-infection also did not potentiate morbidity in this NHP model85, suggesting that other host factors must be present to predispose healthy individuals to highly morbid secondary bacterial pneumonia.
NHPs have also been used to develop models of Bacillus anthracis pneumonia86. Baboons were intravenously infused with B. anthracis at 105–109 CFU and then treated with i.v. levofloxacin daily starting at 2 hours post-inoculation. Infected animals displayed a dose-dependent increase in severity of ALI, DIC, systemic inflammation, and capillary leak (vascular permeability and pleural effusions) that closely mirrored the human disease. At the highest doses (≥108 CFU), mortality was 100%86. Similar findings were seen in baboons infused with B. anthracis-derived peptidoglycan, the predominant carbohydrate of the Gram-positive bacterial cell wall, indicating this molecule is a pathogen-associated molecular pattern (PAMP) likely responsible for the inflammation and hypercoagulability seen with clinical anthrax infection87. Other studies using rhesus or cynomolgus macaques administered aerosolized B. anthracis spores via nose- or head-only exposure chambers88–90. Animals developed a moribund, disseminated anthrax infection with sepsis and respiratory failure over several days88,89. The inhalational model mimics the likely mechanism of exposure to B. anthracis (airborne dispersion) in a possible terrorist attack and is therefore invaluable to researchers developing novel anthrax therapies for such a public health emergency90,91.
NHP models in sepsis therapy
The E. coli baboon bacteremia model has been tested for proof-of-mechanism studies to test inhibitors of the inflammatory and coagulation pathways8,92. Some of these inhibitors, such as antibodies to the cell surface glycoproteins E- and L-selectin, which mediate leukocyte migration93, worsened organ dysfunction and survival and never progressed beyond animal studies94. Other inhibitors did improve sepsis survival and/or organ dysfunction, and NHP models have been instrumental in translating these preclinical studies into clinical trials. Table 1 shows a summary of the experimental therapies and their associated NHP models.
One of the earliest inhibitor trials targeted TNF-α. Studies from the 1980s showed that LD100 baboons given monoclonal antibodies against TNF-α had preserved organ function and reduced mortality95,96. Subsequent studies showed the drug attenuated the coagulopathy97 and reduced mortality in recurrent LD10E. coli bacteremia98. These positive preclinical studies eventually led to the advancement of afelimomab, a murine monoclonal antibody against TNF-α, into phase III trials99,100. Similarly, recombinant TFPI reduced mortality in LD100 baboons52 and moved on to phase II101 and phase III trials102 in humans. However, neither afelimomab nor recombinant TFPI improved outcomes in patients and were ultimately abandoned.
One mediator-targeted sepsis therapy advanced as far as FDA-approval—activated protein C (APC). APC inhibits factors V and VIII and enhances fibrinolysis, providing negative feedback to the coagulation pathway103 (Fig. 1b). Levels of protein C, however, are depleted during septic shock104. Administration of APC to LD100 baboons prevented coagulopathy, hepatic injury, and lethality49. Conversely, administration of an antibody to protein C in LD10 (107–108 CFU/kg E. coli) baboons turned sub-lethal sepsis into lethal septic shock49. In contrast to the other coagulation inhibitors, APC attenuated the early stage mediators of the inflammatory and coagulation pathways, including TNF-α and fibrinogen48. These positive studies eventually led to the phase III clinical trial (PROWESS) of recombinant human APC (drotrecogin alfa) for the treatment of sepsis105, which showed a significant reduction in mortality in the treatment group. Drotrecogin alfa was approved by the FDA as a first-in-class drug for the treatment of sepsis. Subsequent phase IV trials however failed to demonstrate benefit106,107, and actually showed increased risk of major bleeding, eventually prompting the removal of drotrecogin alpha from the market.
Despite decades of research, there are currently no inflammation- or coagulation-targeted therapies approved for the treatment of sepsis. One reason for the discrepant findings between the animal and human studies is a lack of concordance between experimental design and clinical practice. For example, in baboons the TNF-α inhibitor was given prior to97 or immediately after96 E. coli infusion. Similarly, the recombinant TFPI was more efficacious if administered 30 minutes (rather than 4 hours) after E. coli infusion52. These animal studies do not reflect the real-world initiation of sepsis treatment, which typically occurs a day or more after onset of infection. The negative human trials also demonstrate the complexity and redundancy of molecular pathways wherein blockade of one mediator is counteracted by activation of another.
Another promising potential therapy for sepsis-induced ARDS that has been tested in baboons with S. pneumoniae pneumonia (108–109 CFU) is low-dose inhaled carbon monoxide (CO) gas. In mice, low-dose inhaled CO exerts anti-inflammatory and anti-apoptotic effects, reducing severity of sepsis-induced organ injury108. In S. pneumoniae-infected baboons, the pharmacokinetics and delivery of inhaled CO were described and preliminary evidence reported for reduction of ALI severity in CO-treated animals compared with historical controls109. This was linked to CO-mediated induction of mitochondrial biogenesis in alveolar type 2 cells and generation of specialized proresolving lipid mediators110,111. The pharmacokinetics data generated from this NHP study was used to support an FDA IND application, and a multicenter phase I clinical trial112.
The macaque anthrax model has also been used to develop novel anthrax therapies. Cynomolgus macaques inoculated with nebulized B. anthracis experienced significantly improved survival after receiving the novel tetracycline class antibiotic TP-27188. Survival of inhalational anthrax in this primate species was also significantly improved by administering a post-exposure prophylaxis vaccine, AV790989. The combination of both post-exposure treatments was tested in rhesus macaques where antibiotics (ciprofloxacin x 14 days) and vaccine therapy (anthrax vaccine adsorbed, AVA) significantly improved post-exposure survival compared to antibiotics alone, likely due to delayed germination of anthrax spores occurring after completion of antibiotics leading to anthrax infection90. Additionally, a pre-exposure vaccine study in rhesus macaques using a conjugated anti-capsule vaccine showed robust immune response and improved survival of anthrax infection compared to either component alone113.
Limitations of NHP models
Given the nearly identical pathophysiology amongst primates, it would appear that NHP can readily replace other animal models of sepsis in biomedical research. The reality, however, is that the use of NHPs in research is still subject to limitations23. As highly intelligent animals, NHPs require much more stimulation and environmental enrichment than rodents, and must be housed in spacious containment facilities subject to rigorous monitoring regulations23. Additionally, the cost of one NHP experiment, when taking into account housing, personnel, and equipment costs is equivalent to that of hundreds of mice23. Significant resources and expertise are especially essential for NHP experiments involving intravenous infusions, phlebotomy, or vital sign measurements; these procedures generally require sedation, which when administered to septic animals can worsen cardiopulmonary instability (similar to patients). While such sepsis experiments have been reported for chimpanzees40, baboons70, and rhesus macaques114, they have required provision of ICU-level support (e.g., mechanical ventilation, vasopressors, fluids). An alternative strategy involves inserting and tethering indwelling intravascular catheters or vital sign recording devices to a harness worn by the animal19,115. Such devices can continuously administer fluids or medications, and measure telemetry, heart rate, blood pressure, and body temperature19,115. The implantation procedure itself is still a surgery that requires technical expertise, equipment, sedation, and a recovery period, but obviates the need for and inherent risks of sedation while the animal is septic. There can be device complications, however, such as catheter-associated infections116, which could confound sepsis experiments. Cost aside, NHPs require greater skill and expertise to handle than small mammals. They are several times stronger than humans per pound and can carry communicable diseases, such as tuberculosis and herpes B virus23.
While NHPs share much of the same physiology and cell biology, the clinical course of sepsis in experimental models can nonetheless be different from that of patients. Because animals receive direct inoculation of pathogen, sepsis tends to be quicker onset with either rapid death or resolution. In the clinical arena, however, sepsis can be indolent and develop slowly as the nidus of infection grows. Furthermore, the outcomes of certain studies may be confounded when standard of care treatments are withheld, such as antibiotics or surgery8. And the timing of therapies administered to animals (either before or immediately after inoculation) does not reflect clinical practice when patients present several days after the onset of symptoms.
NHP models also suffer from the same limitations in experimental design as all other animal models: experiments are tightly controlled and frequently performed on a population of young, typically male animals with no comorbid diseases8,24. Baboons are generally three to eight years of age at the time of experimentation71,109, which is equivalent to an adolescent human. These conditions differ greatly from clinical sepsis, which is a notoriously heterogeneous disease affecting older adults of both sexes with multiple comorbidities. Thus the homogeneity that characterizes high quality experimental design becomes one of the biggest hindrances to integrating the findings into the clinical area.
Finally, there are ethical considerations that must be considered when discussing NHP sepsis research. The 2006 Weatherall Report commissioned by medical groups in the United Kingdom concluded that the use of NHPs in medical research is justified, provided that 1) there are no alternative means (e.g. lower animals or in vitro work) to answer the biological question; 2) the NHP model is representative of the human disease; 3) there are adequate regulations in place to ensure the welfare of NHPs; and 4) the work significantly advances human health117. The report further qualifies that each use of NHPs for medical research should be adjudicated on a case-by-case basis117. This ethical justification is predicated on the utilitarian principle that suffering by a small number of NHPs may be acceptable if the research leads to substantial benefits to many humans118. Applications of this principle include using NHP studies to prevent a harmful drug from going to clinical trial, or conversely, redirecting resources towards drugs that show significant benefits in NHPs119. Risks associated with this calculus can be attenuated by minimizing the number of animals used, and be ensuring adequate attention to animal welfare118. The former can be addressed by changes to experimental design, such as use of “rolling controls” where the control group changes by one or two animals every experiment, or “before-and-after” studies where an animal serves as its own control. Strategies to address the latter including providing the animals with adequate food, water, space, enrichment, and social interactions117. The latter can also be addressed by using humane endpoints, or predetermined changes in animal physiology, rather than experimental endpoints, such as mortality. Furthermore, distinctions between different NHP species are likely relevant. For instance, the use of use great apes (chimpanzees, bonobos, gorillas, orangutans) in medical research was banned in the European Union in 2011 and phased out in the U.S. in 2013119. These ethical and policy issues are far from settled, and the justification for using monkeys or lesser apes (siamangs and gibbons) in medical research going forward must be continually reassessed. At the very least, the authors propose that their use should be limited to therapeutic studies (rather than mechanistic ones), which are more likely to directly impact human health.
Conclusions and Future Directions
NHP models mimic human sepsis more faithfully than any other species because of anatomic, molecular, and physiological similarities. They have helped to deeply shape our current understanding of sepsis pathophysiology. These models have evolved from discovery of biochemical pathways to drug development. The E. coli bacteremia model has translated dozens of therapies targeting the inflammatory and coagulation pathways from rodent experiments to human clinical trials. However, despite their initial promise in preclinical NHP studies, no pharmacologic therapy has yet demonstrated benefit in humans. Their lack of success may not be due to failure of the animal model itself, but rather to the need to identify crucial targets and pathways in sepsis. Owing to their high cost and intelligence, the use of NHPs in sepsis research today is reserved exclusively for preclinical drug and therapy development, rather than for mechanistic studies in order that novel sepsis treatments can either move to clinical testing or be abandoned before the expense of a clinical trial.
Acknowledgements
The authors thank Dr. Claude A. Piantadosi for his critical review of this manuscript.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA : the journal of the American Medical Association. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Critical care medicine. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. Journal of the American Geriatrics Society. 2012;60:1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA : the journal of the American Medical Association. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrer R, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Critical care medicine. 2014;42:1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 6.Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England journal of medicine. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 7.The ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. The New England journal of medicine370, 1683–1693 (2014). [DOI] [PMC free article] [PubMed]
- 8.Fink MP. Animal models of sepsis. Virulence. 2014;5:143–153. doi: 10.4161/viru.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy MM, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive care medicine. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 10.Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40:463–475. doi: 10.1016/j.immuni.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Wiersinga WJ, Leopold SJ, Cranendonk DR, van der Poll T. Host innate immune responses to sepsis. Virulence. 2014;5:36–44. doi: 10.4161/viru.25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 13.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat. Rev. Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 14.Groeneveld AB, Bronsveld W, Thijs LG. Hemodynamic determinants of mortality in human septic shock. Surgery. 1986;99:140–153. [PubMed] [Google Scholar]
- 15.Parker MM, Shelhamer JH, Natanson C, Alling DW, Parrillo JE. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Critical care medicine. 1987;15:923–929. doi: 10.1097/00003246-198710000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Abraham E, Shoemaker WC, Bland RD, Cobo JC. Sequential cardiorespiratory patterns in septic shock. Critical care medicine. 1983;11:799–803. doi: 10.1097/00003246-198310000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Kraft BD, et al. Development of a novel preclinical model of pneumococcal pneumonia in nonhuman primates. American journal of respiratory cell and molecular biology. 2014;50:995–1004. doi: 10.1165/rcmb.2013-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carraway MS, et al. Blockade of tissue factor: treatment for organ injury in established sepsis. American journal of respiratory and critical care medicine. 2003;167:1200–1209. doi: 10.1164/rccm.200204-287OC. [DOI] [PubMed] [Google Scholar]
- 19.Reyes LF, et al. A Non-Human Primate Model of Severe Pneumococcal Pneumonia. PloS one. 2016;11:e0166092. doi: 10.1371/journal.pone.0166092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinshaw LB, et al. Survival of primates in LD100 septic shock following steroid/antibiotic therapy. J Surg Res. 1980;28:151–170. doi: 10.1016/0022-4804(80)90158-4. [DOI] [PubMed] [Google Scholar]
- 21.Angus DC, van der Poll T. Severe sepsis and septic shock. The New England journal of medicine. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 22.Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364:545–548. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 23.Zanotti-Cavazzoni, S. L. & Goldfarb, R. D. Animal models of sepsis. Critical care clinics 25, 703–719, vii-viii, (2009). [DOI] [PubMed]
- 24.Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, Sannomiya P. Experimental models of sepsis and their clinical relevance. Shock. 2008;30(Suppl 1):53–59. doi: 10.1097/SHK.0b013e318181a343. [DOI] [PubMed] [Google Scholar]
- 25.Haden DW, et al. Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. American journal of respiratory and critical care medicine. 2007;176:768–777. doi: 10.1164/rccm.200701-161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redl H, Bahrami S. Large animal models: baboons for trauma, shock, and sepsis studies. Shock. 2005;24(Suppl 1):88–93. doi: 10.1097/01.shk.0000191339.46777.63. [DOI] [PubMed] [Google Scholar]
- 27.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. American journal of physiology. Lung cellular and molecular physiology. 2008;295:L379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinshaw LB, et al. Survival of primates in lethal septic shock following delayed treatment with steroid. Circulatory shock. 1981;8:291–300. [PubMed] [Google Scholar]
- 29.Hinshaw LB, Brackett DJ, Archer LT, Beller BK, Wilson MF. Detection of the ‘hyperdynamic state’ of sepsis in the baboon during lethal E. coli infusion. The Journal of trauma. 1983;23:361–365. doi: 10.1097/00005373-198305000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Miller FJ, Mercer RR, Crapo JD. Lower Respiratory Tract Structure of Laboratory Animals and Humans: Dosimetry Implications. Aerosol Science and Technology. 1993;18:257–271. [Google Scholar]
- 31.Crapo JD, et al. Morphometric characteristics of cells in the alveolar region of mammalian lungs. The American review of respiratory disease. 1983;128:S42–46. doi: 10.1164/arrd.1983.128.2P2.S42. [DOI] [PubMed] [Google Scholar]
- 32.Plopper CG, Hyde DM. The non-human primate as a model for studying COPD and asthma. Pulm Pharmacol Ther. 2008;21:755–766. doi: 10.1016/j.pupt.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Haudek SB, et al. Lipopolysaccharide dose response in baboons. Shock. 2003;20:431–436. doi: 10.1097/01.shk.0000090843.66556.74. [DOI] [PubMed] [Google Scholar]
- 34.Suffredini AF, et al. The cardiovascular response of normal humans to the administration of endotoxin. The New England journal of medicine. 1989;321:280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 35.Hinshaw LB, et al. Effectiveness of steroid/antibiotic treatment in primates administered LD100 Escherichia coli. Ann Surg. 1981;194:51–56. doi: 10.1097/00000658-198107000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 37.van Deventer SJ, et al. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990;76:2520–2526. [PubMed] [Google Scholar]
- 38.Fiedler VB, et al. Monoclonal antibody to tumor necrosis factor--alpha prevents lethal endotoxin sepsis in adult rhesus monkeys. The Journal of laboratory and clinical medicine. 1992;120:574–588. [PubMed] [Google Scholar]
- 39.van Leenen D, et al. Pentoxifylline attenuates neutrophil activation in experimental endotoxemia in chimpanzees. Journal of immunology. 1993;151:2318–2325. [PubMed] [Google Scholar]
- 40.van der Poll T, et al. Differential effects of anti-tumor necrosis factor monoclonal antibodies on systemic inflammatory responses in experimental endotoxemia in chimpanzees. Blood. 1994;83:446–451. [PubMed] [Google Scholar]
- 41.Levi M, et al. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. The Journal of clinical investigation. 1994;93:114–120. doi: 10.1172/JCI116934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levi M, et al. Differential effects of anti-cytokine treatment on bronchoalveolar hemostasis in endotoxemic chimpanzees. American journal of respiratory and critical care medicine. 1998;158:92–98. doi: 10.1164/ajrccm.158.1.9709007. [DOI] [PubMed] [Google Scholar]
- 43.Emerson TE, Jr., Lindsey DC, Jesmok GJ, Duerr ML, Fournel MA. Efficacy of monoclonal antibody against tumor necrosis factor alpha in an endotoxemic baboon model. Circulatory shock. 1992;38:75–84. [PubMed] [Google Scholar]
- 44.van der Poll T, et al. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. The Journal of experimental medicine. 1994;179:1253–1259. doi: 10.1084/jem.179.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor FB., Jr. Staging of the pathophysiologic responses of the primate microvasculature to Escherichia coli and endotoxin: examination of the elements of the compensated response and their links to the corresponding uncompensated lethal variants. Critical care medicine. 2001;29:S78–89. doi: 10.1097/00003246-200107001-00026. [DOI] [PubMed] [Google Scholar]
- 46.de Boer JP, et al. Activation patterns of coagulation and fibrinolysis in baboons following infusion with lethal or sublethal dose of Escherichia coli. Circulatory shock. 1993;39:59–67. [PubMed] [Google Scholar]
- 47.Drake TA, Cheng J, Chang A, Taylor FB., Jr. Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. The American journal of pathology. 1993;142:1458–1470. [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor FB, Jr., Kinasewitz GT, Lupu F. Pathophysiology, staging and therapy of severe sepsis in baboon models. J Cell Mol Med. 2012;16:672–682. doi: 10.1111/j.1582-4934.2011.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor FB, Jr., et al. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. The Journal of clinical investigation. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kneidinger R, Bahrami S, Redl H, Schlag G, Robinson M. Comparison of endothelial activation during endotoxic and posttraumatic conditions by serum analysis of soluble E-selectin in nonhuman primates. The Journal of laboratory and clinical medicine. 1996;128:515–519. doi: 10.1016/s0022-2143(96)90049-9. [DOI] [PubMed] [Google Scholar]
- 51.Redl H, et al. Expression of endothelial leukocyte adhesion molecule-1 in septic but not traumatic/hypovolemic shock in the baboon. The American journal of pathology. 1991;139:461–466. [PMC free article] [PubMed] [Google Scholar]
- 52.Creasey AA, et al. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. The Journal of clinical investigation. 1993;91:2850–2860. doi: 10.1172/JCI116529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor FB, Jr., et al. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circulatory shock. 1991;33:127–134. [PubMed] [Google Scholar]
- 54.Taylor FB, et al. Active site inhibited factor VIIa (DEGR VIIa) attenuates the coagulant and interleukin-6 and -8, but not tumor necrosis factor, responses of the baboon to LD100 Escherichia coli. Blood. 1998;91:1609–1615. [PubMed] [Google Scholar]
- 55.Minnema MC, et al. Recombinant human antithrombin III improves survival and attenuates inflammatory responses in baboons lethally challenged with Escherichia coli. Blood. 2000;95:1117–1123. [PubMed] [Google Scholar]
- 56.de Boer JP, et al. Activation of the complement system in baboons challenged with live Escherichia coli: correlation with mortality and evidence for a biphasic activation pattern. Infection and immunity. 1993;61:4293–4301. doi: 10.1128/iai.61.10.4293-4301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bengtsson A, et al. Anti-TNF treatment of baboons with sepsis reduces TNF-alpha, IL-6 and IL-8, but not the degree of complement activation. Scand J Immunol. 1998;48:509–514. doi: 10.1046/j.1365-3083.1998.00433.x. [DOI] [PubMed] [Google Scholar]
- 58.Silasi-Mansat R, et al. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116:1002–1010. doi: 10.1182/blood-2010-02-269746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keshari, R. S. et al. Inhibition of complement C5 protects against organ failure and reduces mortality in a baboon model of Escherichia coli sepsis. Proceedings of the National Academy of Sciences of the United States of America (2017). [DOI] [PMC free article] [PubMed]
- 60.Huang YC, et al. VA/Q abnormalities during gram negative sepsis. Respiration physiology. 1996;105:109–121. doi: 10.1016/0034-5687(96)00039-4. [DOI] [PubMed] [Google Scholar]
- 61.Capelozzi VL. What have anatomic and pathologic studies taught us about acute lung injury and acute respiratory distress syndrome? Current opinion in critical care. 2008;14:56–63. doi: 10.1097/MCC.0b013e3282f449de. [DOI] [PubMed] [Google Scholar]
- 62.Levitt JE, Matthay MA. Clinical review: Early treatment of acute lung injury--paradigm shift toward prevention and treatment prior to respiratory failure. Critical care. 2012;16:223. doi: 10.1186/cc11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ware LB, Matthay MA. The acute respiratory distress syndrome. The New England journal of medicine. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 64.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. The Journal of clinical investigation. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Idell S, et al. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. The Journal of clinical investigation. 1989;84:695–705. doi: 10.1172/JCI114217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welty-Wolf KE, et al. Bacterial priming increases lung injury in gram-negative sepsis. American journal of respiratory and critical care medicine. 1998;158:610–619. doi: 10.1164/ajrccm.158.2.9704064. [DOI] [PubMed] [Google Scholar]
- 67.Welty-Wolf KE, et al. Tissue factor in experimental acute lung injury. Seminars in hematology. 2001;38:35–38. doi: 10.1053/shem.2001.29505. [DOI] [PubMed] [Google Scholar]
- 68.Tang H, et al. Sepsis-induced coagulation in the baboon lung is associated with decreased tissue factor pathway inhibitor. The American journal of pathology. 2007;171:1066–1077. doi: 10.2353/ajpath.2007.070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Welty-Wolf KE, Carraway MS, Ortel TL, Piantadosi CA. Coagulation and inflammation in acute lung injury. Thrombosis and haemostasis. 2002;88:17–25. [PubMed] [Google Scholar]
- 70.Welty-Wolf KE, et al. Blockade of tissue factor-factor X binding attenuates sepsis-induced respiratory and renal failure. American journal of physiology. Lung cellular and molecular physiology. 2006;290:L21–31. doi: 10.1152/ajplung.00155.2005. [DOI] [PubMed] [Google Scholar]
- 71.Keshari RS, et al. Acute lung injury and fibrosis in a baboon model of Escherichia coli sepsis. American journal of respiratory cell and molecular biology. 2014;50:439–450. doi: 10.1165/rcmb.2013-0219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silasi-Mansat R, et al. Complement inhibition decreases early fibrogenic events in the lung of septic baboons. J Cell Mol Med. 2015;19:2549–2563. doi: 10.1111/jcmm.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kinasewitz GT, Chang AC, Peer GT, Hinshaw LB, Taylor FB., Jr. Peritonitis in the baboon: a primate model which stimulates human sepsis. Shock. 2000;13:100–109. doi: 10.1097/00024382-200013020-00003. [DOI] [PubMed] [Google Scholar]
- 74.Taylor FB, Jr., et al. Staging of the baboon response to group A streptococci administered intramuscularly: a descriptive study of the clinical symptoms and clinical chemical response patterns. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1999;29:167–177. doi: 10.1086/520147. [DOI] [PubMed] [Google Scholar]
- 75.Jain S, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. The New England journal of medicine. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berendt RF, Long GG, Walker JS. Influenza alone and in sequence with pneumonia due to Streptococcus pneumoniae in the squirrel monkey. The Journal of infectious diseases. 1975;132:689–693. doi: 10.1093/infdis/132.6.689. [DOI] [PubMed] [Google Scholar]
- 77.Philipp MT, et al. Experimental infection of rhesus macaques with Streptococcus pneumoniae: a possible model for vaccine assessment. Journal of medical primatology. 2006;35:113–122. doi: 10.1111/j.1600-0684.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 78.Dehoux MS, et al. Compartmentalized cytokine production within the human lung in unilateral pneumonia. American journal of respiratory and critical care medicine. 1994;150:710–716. doi: 10.1164/ajrccm.150.3.8087341. [DOI] [PubMed] [Google Scholar]
- 79.Olsen RJ, et al. Lack of a major role of Staphylococcus aureus Panton-Valentine leukocidin in lower respiratory tract infection in nonhuman primates. The American journal of pathology. 2010;176:1346–1354. doi: 10.2353/ajpath.2010.090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chertow DS, et al. Influenza A and methicillin-resistant Staphylococcus aureus co-infection in rhesus macaques - A model of severe pneumonia. Antiviral Res. 2016;129:120–129. doi: 10.1016/j.antiviral.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klevens RM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA : the journal of the American Medical Association. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 82.Centers for Disease Control & Prevention. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-August 2009. MMWR. Morbidity and mortality weekly report58, 1071–1074 (2009). [PubMed]
- 83.Kallen AJ, et al. Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Annals of emergency medicine. 2009;53:358–365. doi: 10.1016/j.annemergmed.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 84.Lina G, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi SD, et al. Seasonal H3N2 influenza A virus fails to enhance Staphylococcus aureus co-infection in a non-human primate respiratory tract infection model. Virulence. 2013;4:707–715. doi: 10.4161/viru.26572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stearns-Kurosawa DJ, Lupu F, Taylor FB, Jr., Kinasewitz G, Kurosawa S. Sepsis and pathophysiology of anthrax in a nonhuman primate model. The American journal of pathology. 2006;169:433–444. doi: 10.2353/ajpath.2006.051330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Popescu NI, et al. Peptidoglycan induces disseminated intravascular coagulation in baboons through activation of both coagulation pathways. Blood. 2018;132:849–860. doi: 10.1182/blood-2017-10-813618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grossman, T. H. et al. The Fluorocycline TP-271 Is Efficacious in Models of Aerosolized Bacillus anthracis Infection in BALB/c Mice and Cynomolgus Macaques. Antimicrobial agents and chemotherapy61, 10.1128/AAC.01103-17 (2017). [DOI] [PMC free article] [PubMed]
- 89.Savransky V, et al. Correlation between anthrax lethal toxin neutralizing antibody levels and survival in guinea pigs and nonhuman primates vaccinated with the AV7909 anthrax vaccine candidate. Vaccine. 2017;35:4952–4959. doi: 10.1016/j.vaccine.2017.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vietri NJ, et al. Short-course postexposure antibiotic prophylaxis combined with vaccination protects against experimental inhalational anthrax. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7813–7816. doi: 10.1073/pnas.0602748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Inglesby TV, et al. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA : the journal of the American Medical Association. 2002;287:2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 92.Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2:391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 93.Bevilacqua MP, Nelson RM. Selectins. The Journal of clinical investigation. 1993;91:379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carraway MS, et al. Antibody to E- and L-selectin does not prevent lung injury or mortality in septic baboons. American journal of respiratory and critical care medicine. 1998;157:938–949. doi: 10.1164/ajrccm.157.3.9707129. [DOI] [PubMed] [Google Scholar]
- 95.Tracey KJ, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 96.Hinshaw LB, et al. Survival of primates in LD100 septic shock following therapy with antibody to tumor necrosis factor (TNF alpha) Circulatory shock. 1990;30:279–292. [PubMed] [Google Scholar]
- 97.van der Poll T, et al. Pretreatment with a 55-kDa tumor necrosis factor receptor-immunoglobulin fusion protein attenuates activation of coagulation, but not of fibrinolysis, during lethal bacteremia in baboons. The Journal of infectious diseases. 1997;176:296–299. doi: 10.1086/514034. [DOI] [PubMed] [Google Scholar]
- 98.Schlag, G., Redl, H., Davies, J. & Haller, I. Anti-tumor necrosis factor antibody treatment of recurrent bacteremia in a baboon model. Shock 2, 10–18 ; discussion 19-22. (1994). [DOI] [PubMed]
- 99.Reinhart K, et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Critical care medicine. 2001;29:765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 100.Panacek EA, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Critical care medicine. 2004;32:2173–2182. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 101.Abraham E, et al. Assessment of the safety of recombinant tissue factor pathway inhibitor in patients with severe sepsis: a multicenter, randomized, placebo-controlled, single-blind, dose escalation study. Critical care medicine. 2001;29:2081–2089. doi: 10.1097/00003246-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Abraham E, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2003;290:238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 103.Marlar RA, Kleiss AJ, Griffin JH. Mechanism of action of human activated protein C, a thrombin-dependent anticoagulant enzyme. Blood. 1982;59:1067–1072. [PubMed] [Google Scholar]
- 104.Taylor FB, Kinasewitz G. Activated protein C in sepsis. J Thromb Haemost. 2004;2:708–717. doi: 10.1111/j.1538-7836.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- 105.Bernard GR, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. The New England journal of medicine. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 106.Ranieri VM, et al. Drotrecogin alfa (activated) in adults with septic shock. The New England journal of medicine. 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 107.Abraham E, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. The New England journal of medicine. 2005;353:1332–1341. doi: 10.1056/NEJMoa050935. [DOI] [PubMed] [Google Scholar]
- 108.Otterbein LE, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature medicine. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 109.Fredenburgh LE, et al. Effects of inhaled CO administration on acute lung injury in baboons with pneumococcal pneumonia. American journal of physiology. Lung cellular and molecular physiology. 2015;309:L834–846. doi: 10.1152/ajplung.00240.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shinohara M, et al. Cell-cell interactions and bronchoconstrictor eicosanoid reduction with inhaled carbon monoxide and resolvin D1. American journal of physiology. Lung cellular and molecular physiology. 2014;307:L746–757. doi: 10.1152/ajplung.00166.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dalli J, et al. The Regulation of Proresolving Lipid Mediator Profiles in Baboon Pneumonia by Inhaled Carbon Monoxide. American journal of respiratory cell and molecular biology. 2015;53:314–325. doi: 10.1165/rcmb.2014-0299OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fredenburgh LE, et al. A phase I trial of low-dose inhaled carbon monoxide in sepsis-induced ARDS. J. CI Insight. 2018;3:pii: 124039. doi: 10.1172/jci.insight.124039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chabot DJ, et al. Efficacy of a capsule conjugate vaccine against inhalational anthrax in rabbits and monkeys. Vaccine. 2012;30:846–852. doi: 10.1016/j.vaccine.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 114.Poliquin PG, et al. Delivering Prolonged Intensive Care to a Non-human Primate: A High Fidelity Animal Model of Critical Illness. Sci Rep. 2017;7:1204. doi: 10.1038/s41598-017-01107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shade RE, Bishop VS, Haywood JR, Hamm CK. Cardiovascular and neuroendocrine responses to baroreceptor denervation in baboons. The American journal of physiology. 1990;258:R930–938. doi: 10.1152/ajpregu.1990.258.4.R930. [DOI] [PubMed] [Google Scholar]
- 116.Friday KE, Lipkin EW. Long-term parenteral nutrition in unrestrained nonhuman primates: an experimental model. The American journal of clinical nutrition. 1990;51:470–476. doi: 10.1093/ajcn/51.3.470. [DOI] [PubMed] [Google Scholar]
- 117.Weatherall D. The use of non-human primates in research. London: Academy of Medical Sciences; 2006. [Google Scholar]
- 118.Arnason G. The ethical justification for the use of non-human primates in research: the Weatherall report revisited. Journal of medical ethics. 2018;44:328–331. doi: 10.1136/medethics-2016-103827. [DOI] [PubMed] [Google Scholar]
- 119.Barnhill A, Joffe S, Miller FG. The Ethics of Infection Challenges in Primates. Hastings Cent Rep. 2016;46:20–26. doi: 10.1002/hast.580. [DOI] [PubMed] [Google Scholar]


