Abstract
Objective:
To evaluate the effect of hospitalizations on patterns of sedentary/physical activity time in mobility-limited older adults randomized to either structured physical activity or health education.
Design:
Secondary analysis of an investigator-blinded, parallel-group, randomized trial conducted at 8 US centers between February 2010-December 2013.
Participants:
1,341 sedentary men and women aged 70-89 years at baseline who wore a hip-fitted accelerometer 7 consecutive days at baseline and 6, 12, and 24 months post-randomization.
Measurements:
Participants were randomized to either a physical activity (PA; n=669) intervention that included aerobic, resistance, and flexibility training or to a health education (HE; n=672) intervention that consisted of workshops on older adult health and light upper-extremity stretching. Accelerometer patterns were characterized as bouts of sedentary (<100 counts/min; ≥1, ≥10, ≥30, ≥60 lengths) and activity (100+ counts/min; ≥1, ≥2, ≥5, ≥10 lengths) time. Each participant was categorized as having 0, 1-3, or ≥4 cumulative hospital days prior to each accelerometer assessment.
Results:
Hospitalization increased sedentary time similarly in both intervention groups (8 and 16 minutes/day for 1-3 and ≥4 cumulative hospital days, respectively). Hospitalization was also associated with reduced physical activity time across all bouts <10 minutes (≥1: −7 and −16; ≥2: −5 and −11; ≥5: −3 and −4 minutes/day for 1-3 and ≥4 cumulative hospital days, respectively). There was no evidence of recovery to pre-hospitalization levels (time effect p-values > 0.41). When compared with HE, PA reduced sedentary time in bouts <30 minutes (−8 to −10 minutes/day) and increased total activity (+3 to +6 minutes/day). However, the hospital-related changes were similar between the intervention groups (interaction effect p-values > 0.26).
Conclusion:
Participating in a physical activity intervention prior to hospitalization had expected benefits, but participants remained susceptible to hospitalization’s detrimental effects on their daily activity levels. There was no evidence of improved activity recovery following a hospitalization.
Keywords: Hospital, accelerometer, sedentary behavior, exercise, clinical trial
INTRODUCTION
Although hospitalizations are intended to manage acute conditions and planned procedures, they are frequently associated with imposed bedrest and other iatrogenic conditions (e.g., malnutrition and sleep deprivation) that have long-term adverse effects on physical function (1,2). Furthermore, those hospitalized with pre-existing functional limitations are more susceptible to these adverse effects and more likely to develop outright disability (3). Disability onset ultimately leads to restricted community participation and reduced quality of life (4,5).
Older adults (≥70 years) are susceptible to reductions in activity levels that persist after hospitalization. For instance, hospitalized older adults report heightened levels of fatigue and apathy that are associated with lower daily activity (6–9). This is important because low physical activity is a major risk factor for disease (10), mortality (11), and disability (12) while sedentary behavior, particularly spent in prolonged and uninterrupted periods of time (i.e. bouts), is associated with poorer cardiometabolic profiles (13,14). However, little data exists to quantify hospitalization’s aftermath on activity levels and the patterns in which activity is accrued.
Characterizing the degree of change in sedentary and activity time following a hospitalization is an important step towards enhancing rehabilitation and physical activity programs surrounding a hospitalization. Though participating in structured physical activity provides mobility and health benefits (15,16), it’s capability to preserve activity levels following a hospitalization remains unclear.
The Lifestyles Intervention and Independence for Elders (LIFE) study provides a unique opportunity to examine the complex relationships between hospitalizations and the effects of a structured physical activity (PA) intervention. The primary aim was to evaluate the association between hospitalization and daily sedentary and activity time derived by accelerometry. We hypothesized that the occurrence and cumulative duration of hospitalization stays would increase sedentary time and decrease active time, particularly in longer bout lengths. The secondary aim was to assess the effect of a PA intervention on post-hospital sedentary and activity patterns. We hypothesized that a PA intervention, as compared to a health education (HE) intervention, would attenuate both increases in sedentary time and decreases in active time post-hospitalization.
METHODS
Trial Design and Participants
The study design, methods (17), recruitment (18), and primary results (15) of the LIFE study are outlined elsewhere. Briefly, the LIFE study was a Phase 3 randomized clinical trial conducted between February 2010-December 2013. The primary purpose of the LIFE study was to examine the ability of a structured, moderate-intensity physical activity intervention to reduce the risk of major mobility disability among 1,635 mobility-limited older adults (aged 70-89 years). Eligible participants were screened for functional limitation (Short Physical Performance Battery (SPPB) score < 10 (of 12), where 12 was the highest performance achievable) but were able to walk 400 meters within 15 minutes without sitting, leaning, or receiving any assistance.
At baseline, 1,341 participants had valid accelerometry and assessment data (see CONSORT diagram in Supplementary Figure S1). Study protocols were approved by the institutional review boards at all participating sites and all participants gave written informed consent. This paper represents a secondary analysis of existing data that uses data during the accelerometer collection periods in the LIFE study (see timeline in Figure S2).
Interventions
Complete details about the LIFE interventions are provided elsewhere (19). Participants were randomized to either a PA or HE intervention. The PA intervention consisted of an individually-tailored plan to increase physical activity with a goal of achieving 150 minutes/week of moderate-intensity physical activity through the promotion of multiple ≥10 minute bouts. The PA intervention included aerobic, resistance, flexibility, and balance training—primarily consisting of a walking regiment which is easily administered and largely popular across the older adult population (20). The PA intervention entailed 2 center-based sessions accompanied by 3-4 home exercise sessions. Reducing sedentary behaviors, such as TV watching, was not a specific goal of the intervention.
Participants were placed on medical leave if they missed ≥4 consecutive sessions due to hospitalization, injury, or other health reasons or if their primary care physician ordered temporary suspension from physical activity. Participants on medical leave were periodically contacted for a status update and provided with support to restart the intervention when appropriate. Any prescribed rehabilitative therapy was completed prior to restarting the PA intervention.
The HE intervention consisted of workshops focused on older adult health while intentionally avoiding topics related to physical activity. Additionally, participants in the HE group were led through a 5-10 minute light-intensity upper-extremity stretching component during each session. HE participants were expected to meet weekly during the first 26 weeks of the intervention and at least once monthly thereafter. Additionally, they were encouraged to return to the intervention following a self-reported hospitalization, injury, or other health reason.
Hospitalization
Hospitalizations were ascertained by assessors masked to intervention assignment during scheduled clinic visits every 6 months. Every 6 months, hospital occurrence and length of stay were obtained via questionnaire, which initiated a review of the participant’s medical records. Self-reported hospitalization was verified through medical records along with extraction of admission and discharge dates (21).
A hospitalization variable was created for this analysis. It combined the occurrence and length of stay (LOS; in days) was created for the analysis. To do this, LOS was summed across the number of hospitalization events for each participant between accelerometer assessments—specifically, baseline to 6 months, 6 to 12 months, and 12 to 24 months. Then, a three-level categorical variable was created according to the LOS across time intervals. The median LOS was used to separate categories into the following: 0; 1-3 ; ≥4 cumulative days spent in the hospital.
Sedentary and Activity Pattern Outcomes
A hip-worn accelerometer (Actigraph™ GT3X) was used to collect daily activity patterns at baseline, pre-randomization and then 6, 12, and 24 months post-randomization. Data were first processed for valid wear time (22). Only participants with valid accelerometry—defined as ≥10 hours/day of data for ≥3 days—were included in the analytic sample (n=1,341; 196 missing at baseline and 98 with invalid accelerometer data). Three valid wear days was chosen to be sufficiently inclusive while being highly representative of activity patterns over 7 days (r = 0.94) (23).
Sedentary time is defined as the sum of minutes registering <100 counts/minute; whereas total physical activity time is the sum of all minutes registering at 100+ counts/minute (24). A bout is defined as a period when minutes in either a sedentary or active state occur consecutively without interruption. For sedentary time, we extracted time spent in ≥1 (total sedentary time), ≥10, ≥30, and ≥60 minute bout lengths. For active time, ≥1 (total activity time), ≥2, ≥5, and ≥10 minute bout lengths were used since significantly less time was spent being active, than sedentary (25,26).
Covariates
At baseline, age, sex, race/ethnicity, education, marital status, income, and smoking status were collected via self-report questionnaires. Body mass index (kg/m2) was calculated from height and weight measured by study staff. Global cognition was measured using the Modified Mini-Mental State Examination (3MSE), which was scored 0 to 100 where higher scores indicate better cognitive performance (27). Participants who reported seeking any medical attention for depression in the past 5 years were categorized as having depression. Hospitalization history was defined as reporting an in-patient stay 6 months prior to baseline. A comorbidity index was created by summing self-reported history of myocardial infarction, congestive heart failure, stroke, hypertension, cancer, diabetes, arthritis, and lung disease. Physical function was measured in two ways: usual pace walking speed during the 400m walk test and the SPPB, which consists of three physical tests scored from 0-4 (balance, walking speed, and chair rises). The SPPB score was calculated from the sum of the tests, and ranges from 0-12 (0-9 for the LIFE study), with higher scores indicate better performance (28).
Statistical Analysis
Baseline differences between those hospitalized (using first hospitalization) or not over 24 months were tested by either t-tests (means) or chi-square tests (frequency) within each intervention group. Accelerometer change values were derived by subtracting each accelerometer metric from its baseline value. To address each aim, a linear mixed effects (random and fixed) model was constructed with independent variables that included time-varying hospitalizations, intervention group, hospitalization by intervention interaction, time intervals, and additional covariates. Random effects included hospitalizations and time interval; other independent variables were considered fixed effects.
Aim 1 examined the time-varying hospitalization variable and its association with changes in accelerometer metrics. Aim 2 examined the interaction term to evaluate whether post-hospital changes in accelerometer metrics differed by intervention group. Non-significant interaction terms were removed from the final models and only the main effect of the hospitalization (Aim 1) and intervention (Aim 2) were interpreted. Models included wear time, baseline accelerometer value, age, sex, race/ethnicity, income, education, marital status, 3MSE score, self-reported depression, body mass index, smoked 100+ cigarettes ever, comorbidity index, previous hospitalizations in the 6 months before baseline, slow gait (< 0.8 m/s) (29), and clinical site. The hospitalization variable was included in the model as three categories with no hospitalization serving as the reference group. Additionally, the hospitalization variable was treated as continuous in the model to test for a trend across categories.
Hospitalizations occurred episodically and accelerometer collection occurred at relatively fixed intervals. Therefore, those experiencing a hospitalization closer in time to their scheduled visit may demonstrate larger changes. Pearson correlations were used to evaluate whether the days from the most recent hospitalization discharge date were related to the magnitude of accelerometer change.
Missing accelerometer data at follow-up assessments in our analytic sample (523/5,040 observations; 10%) were considered to be missing at random. Two-tailed alternative hypotheses and type 1 error rate of 0.05 were used for all analyses. Accelerometer data were processed using R (www.r-project.org) (22,30) and statistical analyses were performed in STATA v13 (STATA Corp.).
RESULTS
Sample Characteristics
Hospitalized PA participants were similar to those not hospitalized across demographics, behavioral factors, cognition, depression, medical conditions, physical function, and accelerometer wear metrics but had significantly more self-reported hospitalizations 6 months prior to baseline (11% vs. 5%, respectively, p=0.008) (Table 1). For HE, those hospitalized were older and had higher prevalence of women, non-Hispanic whites, reports a previous hospitalization 6 months prior to baseline, ≥2 comorbidities, low physical function, and low walking speed when compared to those not hospitalized (p-values<0.050).
Table 1.
Baseline participant characteristics by intervention group and hospitalization status over 24 months: the LIFE study
| Hosp. (n=187) | PA (n=669) No hosp. (n=482) | p-value | Hosp. (n=171) | HE (n=672) No hosp. (n=501) | p-value | |
|---|---|---|---|---|---|---|
| Age, mean(SD) | 79.0 (5.4) | 78.3 (5.2) | 0.14 | 79.8 (5.2) | 78.7 (5.2) | 0.02 |
| >= 80 years old, n(%) | 79 (42.3) | 192 (39.8) | 0.57 | 87 (50.9) | 206 (41.1) | 0.03 |
| Female, n(%) | 126 (67.4) | 305 (63.3) | 0.32 | 107 (62.6) | 354 (70.7) | 0.05 |
| Non-Hispanic white, n(%) | 139 (74.3) | 358 (74.3) | 0.68 | 144 (84.2) | 376 (75.1) | 0.04 |
| College or higher education, n(%) | 111 (59.4) | 306 (63.5) | 0.49 | 112 (65.5) | 318 (63.5) | 0.65 |
| Married, n(%) | 79 (42.3) | 174 (36.1) | 0.24 | 57 (33.3) | 184 (36.7) | 0.70 |
| Annual income < $25,000 | 54 (28.9) | 136 (28.2) | 0.95 | 45 (26.3) | 145 (28.9) | 0.51 |
| Body mass index, mean (SD) | 30.3 (5.8) | 30.1 (5.8) | 0.68 | 30.1 (6.1) | 30.6 (6.3) | 0.44 |
| Smoked 100+ cigarettes ever, n(%) | 102 (54.6) | 237 (49.2) | 0.41 | 84 (49.1) | 213 (42.5) | 0.32 |
| 3MSE score a, mean(SD) | 91.4 (5.3) | 91.9 (5.5) | 0.33 | 91.8 (5.4) | 91.6 (5.3) | 0.60 |
| Depression b, n(%) | 28 (15.0) | 69 (14.3) | 0.75 | 28 (16.4) | 57 (11.4) | 0.17 |
| Hospitalization history c, n(%) | 21 (11.2) | 26 (5.4) | 0.01 | 22 (12.9) | 30 (6.0) | <.01 |
| Comorbidities ≥ 2, n(%) | 57 (30.5) | 115 (23.9) | 0.18 | 63 (36.8) | 114 (22.8) | <.01 |
| SPPB score < 8 d, n(%) | 88 (47.1) | 198 (41.1) | 0.16 | 103 (60.2) | 206 (41.1) | <.01 |
| 400 meter walk < 0.8 m/sec, n(%) | 111 (59.4) | 287 (59.5) | 0.97 | 77 (45.0) | 299 (59.7) | <.01 |
| Wear days, mean(SD) | 8.0 (3.4) | 8.0 (3.2) | 0.89 | 8.1 (3.2) | 7.8 (3.1) | 0.27 |
| Wear minute/day, mean (SD) | 843.5 (120.2) | 837.4 (109.8) | 0.53 | 827.1 (85.8) | 837.7 (116.3) | 0.27 |
Note: PA – physical activity; HE – health education; hosp. – hospitalization. P-values were derived using either t-tests (continuous) or chi-squared tests (categorical) for each participant characteristic.
Modified Mini-Mental State Examination; score ranges between 0-100 where higher scores indicate better performance
Sought medical advice for depression in past 5 years
Self-reported hospitalizations occurring 6 months prior to baseline
Short Physical Performance Battery; score ranges between 0-12 where higher scores indicate better performance; scoring < 8 indicates poor functioning.
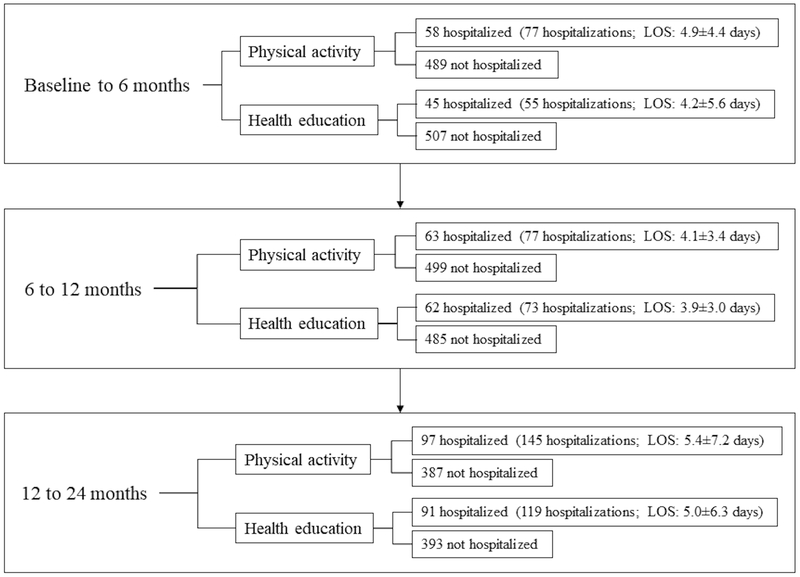
Participants hospitalized, total hospital events, and mean LOS appeared higher in the PA group than the HE group at each time interval (Figure 1), but these group differences were not statistically significant (p>0.114 for all hospitalization characteristics). Reasons for hospitalizations were largely heterogeneous (Table 2). When stratified by intervention group, no proportional differences by hospitalization reason were detected except for skin/subcutaneous tissue disorders, such as maculopapular rash (PA: 0% and HE: 2%; p=0.027).
Figure 1.
Participants hospitalized within each time interval according to intervention.
Note: LOS: length of stay (mean days +/− SD).
Table 2.
Reason for hospitalization during the first 24 months of follow-up by intervention group: the LIFE study
| Total inpatient hospitalizations* | Physical activity (n=299) | Health education (n=247) | p-value |
|---|---|---|---|
| n (%) | |||
| Blood and lymphatic systems disorders | 3 (1.0) | 3 (1.2) | 0.81 |
| Cardiac disorders | 53 (17.7) | 41 (16.6) | 0.73 |
| Ear and Labyrinth disorders | 2 (0.7) | 3 (1.2) | 0.51 |
| Endocrine disorders | 3 (1.0) | 0 (0.0) | 0.11 |
| Eye disorders | 0 (0.0) | 1 (0.4) | 0.27 |
| Gastrointestinal disorders | 34 (11.4) | 19 (7.7) | 0.15 |
| General disorders/administration site conditions | 8 (2.7) | 6 (2.4) | 0.86 |
| Hepatobiliary disorders | 4 (1.3) | 2 (0.8) | 0.56 |
| Immune system disorders | 3 (1.0) | 0 (0.0) | 0.11 |
| Infections and infestations | 28 (9.4) | 17 (6.9) | 0.29 |
| Injury, poisoning, and procedural complications | 22 (7.4) | 18 (7.3) | 0.98 |
| Investigations | 0 (0.0) | 1 (0.4) | 0.27 |
| Metabolism and nutrition disorders | 10 (3.3) | 6 (2.4) | 0.53 |
| Musculoskeletal and connective tissue disorders | 19 (6.4) | 25 (10.1) | 0.11 |
| Neoplasms: benign, malignant, and unspecified | 4 (1.3) | 2 (0.8) | 0.56 |
| Nervous system disorders | 29 (9.7) | 21 (8.5) | 0.63 |
| Psychiatric disorders | 1 (0.3) | 1 (0.4) | 0.89 |
| Renal and urinary disorders | 5 (1.7) | 8 (3.2) | 0.23 |
| Reproductive system and breast disorders | 2 (0.7) | 2 (0.8) | 0.85 |
| Respiratory, thoracic, and mediastinal disorder | 16 (5.4) | 16 (6.5) | 0.58 |
| Skin and subcutaneous tissue disorders | 0 (0.0) | 4 (1.6) | 0.03 |
| Surgical and medical procedures | 38 (12.7) | 38 (15.4) | 0.37 |
| Vascular disorders | 15 (5.0) | 13 (5.3) | 0.90 |
Based on Medical Dictionary for Regulatory Activities (MedDRA) system organ class, listed in alphabetical order
P-values were derived using chi-squared tests for each hospitalization class
Within the PA group, there were no differences in baseline time spent in sedentary and active behaviors between those who did or did not experience at least one hospitalization (Supplementary Table S1). For HE, sedentary time was generally higher and activity was lower among those hospitalized when compared to those not hospitalized (p<0.034 for all except ≥1 sedentary minute bouts).
Hospitalization Effect on Sedentary and Activity Patterns
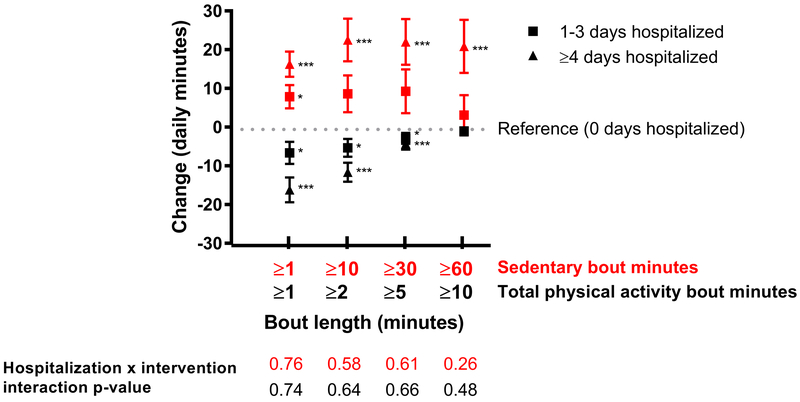
Across the entire sample, accruing either 1-3 or ≥4 cumulative hospital days was positively associated with total daily sedentary time (8 minutes for 1-3 days, p=0.01; 16 minutes for ≥4 days, p<0.001) when compared to no cumulative hospital days (trend p-value<0.001) (Figure 2). Spending ≥4 hospital days, but not 1-3 days, was associated with increases in ≥10 (22 minutes, p=0.002), ≥30 (22 minutes, p=0.002), and ≥60 (20.9 minutes, p=0.002) minute sedentary bouts.
Figure 2.
Effect of hospital duration on daily accelerometer pattern changes (daily minutes) in mobility-limited older adults (beta coefficient (SEM))^
^Reference value is no hospitalizations (0 days).
All models were adjusted for time (months), intervention status, wear time, baseline accelerometer value, age, sex, race/ethnicity, income, education, marital status, modified mini-mental status exam score (3MSE where 100 is highest cognition score), self-reported depression, body mass index (kg/m2), smoked 100+ cigarettes ever, comorbidity index, previous hospitalizations in the past 6 months, gait speed < 0.8 m/s, and clinical site.
*p<0.05 **p<0.01 ***p<0.001
Accruing 1-3 hospital days was negatively associated with total daily physical activity changes in ≥1, ≥2, and ≥5 minute bouts (−6 minutes, p=0.021; −5 minutes, p=0.021; and −3 minutes, p=0.035, respectively) when compared to those without a hospitalization. Those accruing ≥4 hospital days experienced declines in ≥1 (−16 minutes, p<0.001), ≥2 (−12 minutes, p<0.001), and ≥5 (−4 minutes, p=0.004) minute activity bouts compared to those without a hospitalization (trend across hospital days p-values<0.002). Neither 1-3 or ≥4 hospital days were associated with activity bouts lasting ≥10 minutes.
Intervention Effect on Post-Hospital Sedentary and Activity Patterns
Across all sedentary and activity outcomes, the hospitalization effect (both 1-3 and ≥4 cumulative hospital days vs. none) did not differ between intervention groups (interaction p-value>0.260 for all). Additionally, there were no interactions between hospitalization and time (2-way interaction p-values>0.412) or by intervention and time (3-way interaction p-values>0.451) for all outcomes. Lastly, most recent hospitalization discharge date was not related to changes in sedentary or physical activity bouts; hospitalizations occurring closer to accelerometer data collection periods were not correlated with changes in sedentary and activity outcomes (p>0.443 for all correlations).
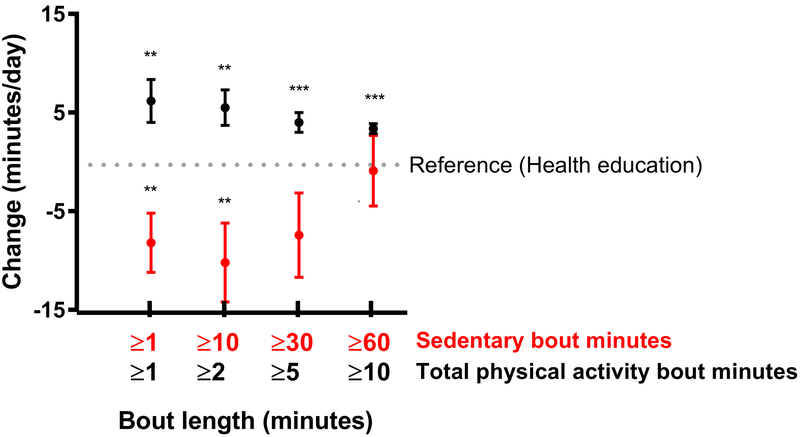
The intervention main effects demonstrated that the PA group decreased sedentary time for ≥1 minute bouts (−8 minutes, p=0.006) and ≥10 minute bouts (−10 minutes, p=0.010) when compared to the HE group, regardless of hospitalization (Figure 3). Also, the PA group increased their time spent being active across all bouts lengths (3-6 minutes, p<0.006 for all). These increases appeared to be lower with longer bout lengths.
Figure 3.
Change in daily sedentary and activity minutes across bout lengths among those in the Physical Activity group when compared to the Health Education group across 24 months
All models were adjusted for hospitalization status, time (months), wear time, baseline accelerometer value, age, sex, race/ethnicity, income, education, marital status, modified mini-mental status exam score (3MSE where 100 is highest cognition score), self-reported depression, body mass index (kg/m2), smoked 100+ cigarettes ever, comorbidity index, previous hospitalizations in the past 6 months, gait speed < 0.8 m/s, and clinical site.
*p<0.05 **p<0.01 ***p<0.001 when compared to the health education intervention.
DISCUSSION
In this study, accumulated hospitalization time was negatively associated with objectively-measured sedentary and physical activity time in mobility-limited older adults. A hospitalization increased time in short sedentary bouts while decreasing time in short physical activity bouts. These effects were magnified after accumulating ≥4 hospital days. These results support our primary hypothesis. Additionally, a physical activity intervention did not attenuate the hospitalization’s effect on changes in sedentary and activity time and when accumulated in bouts. This is contrary to our second hypothesis because the PA group was expected to have recovered more from their hospitalization than the HE group. As expected, the PA group had an overall higher activity and lower sedentary time when compared to the HE group, but those in the PA group remained susceptible to the aftermath of a hospitalization.
Hospitalization Effect on Sedentary and Activity Patterns
The literature characterizing sedentary and activity patterns in older adults who have experienced a hospitalization is sparse. Previous studies using activity monitors show that >80% of a hospital stay is spent in sedentary time (31,32). Older stroke survivors were observed to have higher total sedentary time after hospitalization when compared to matched counterparts with no existing medical problems—a behavior that remained 6 months post-hospitalization (33,34). Our results are similar, as they demonstrate that spending ≥4 cumulative days, but not ≤ 3 days, in the hospital was associated with higher sedentary time across all bout lengths. We also noted that the increased sedentary time did not diminish, suggesting a lack of recovery to pre-hospitalization levels. This lack of recovery also suggests that the sequela of hospitalization may contribute to the reprogramming of behavior to a higher sedentary time set-point and thus help explain higher sedentary levels that are often observed in older adults (35).
Hospitalizations were associated with declines in total activity time. This finding is supported by previous work demonstrating that short-duration bed rest, a common occurrence in hospitalizations, reduces muscle mass and function (36) that may accelerate with co-occurring illness in the hospital (37). Additionally, more accumulated time in the hospital led to greater activity declines, similar to previous observations (1,32,38). Therefore, reduced physical activity levels are likely due to a culmination of factors surrounding a hospitalization (1,2,39). Interestingly, while hospitalized participants had activity declines as a whole, they paradoxically retained their activity lasting ≥10 minutes. One explanation is that the time spent in these longer bout lengths was too low in this sample to be influenced by hospitalizations (< 5% time spent ≥10 minute activity bouts). Another explanation is that longer bout lengths may represent an activity pattern that is routine and more likely to be retained in life following a hospitalization event (e.g. retrieving mail) (33,40). The overall findings suggest activity levels are reduced following a hospitalization, but the impact may vary according to an individual’s lifestyle which reflects how activity is accumulated.
Intervention Effect on Post-Hospital Sedentary and Activity Patterns
Previously published data from the LIFE study demonstrated a slightly higher rate of hospitalizations among the PA group (15,21) and those who were hospitalized had significantly higher rates of major mobility disability (41). Adding to these findings, our results show that the PA intervention did not attenuate the association between hospitalization and time spent in sedentary or physical activity behaviors when compared with a HE intervention. However, overall activity was higher and sedentary time was lower in the PA group compared to the HE group. This suggests that the PA intervention shifted pre-hospitalization activity to a higher level, but did not enhance activity recovery following a hospitalization. Therefore, a hospitalization appears to have a powerful effect on reducing activity levels, despite the substantial efforts of the PA intervention to re-engage older adults in their physical activity program. These results have implications for developing new strategies to facilitate activity recovery following a hospitalization in mobility-limited older adults.
Strengths/Limitations
The strengths of this study include a large sample of mobility-limited older adults from 8 field centers across the US who were followed for at least 2 years. Hospitalizations were collected frequently and verified by a central committee blinded to intervention assignment. Time spent in sedentary and physically active behaviors was measured objectively using a hip-worn accelerometer and repeated 3 more times over 2 years. Free-living accelerometer patterns were examined by sedentary versus activity and by bout lengths to reveal associations with intervening hospitalizations. One limitation is that activity patterns were captured in snapshots over time (6, 12, and 24 months post-randomization) and thus the results are likely to underestimate the acute effects of a hospitalization. Another limitation was that hospitalization admission diagnosis was not evaluated in this analysis because there was substantial heterogeneity across categories, which limited a robust analytic strategy. Lastly, the accelerometer cut-point used in this analysis was developed in adults of all ages (24) and not explicitly calibrated for older adults who tend to expend more energy than younger adults for a given activity (42,43).
Conclusion
In conclusion, these findings suggest that accumulating hospital days increases sedentary time and reduces time spent in activity among mobility-limited older adults. Additionally, a physical activity program begun prior to hospitalization did not enhance activity recovery following a hospitalization. However, while all participants experienced this lack in recovery due to a hospitalization, the pre-hospitalization shift towards higher activity levels and lower sedentary time due to a physical activity intervention remained significant. Overall, increasing physical activity levels prior to hospitalization events and development of post-hospital strategies to increase activity levels are paramount to help preserve mobility and thwart disability among mobility-limited older adults.
Supplementary Material
Supplementary Figure S1. Participant flow through study
a Data pre-processed for at least 10 hours of accelerometer data which were screened for data key-in errors, accelerometer malfunctions, participant operation errors, visual identification of unexplained patterns or phenomenon, and duplicate files.
b Data were screened for outliers and processed for valid wear/non-wear times based on an accelerometer wear time classification algorithm (22).
Supplementary Figure S2. Analysis timeline
Supplementary Table S1. Baseline sedentary and activity bouts by intervention group and hospitalization^: the LIFE study
^cumulative days spent in a hospital setting.
Note: hosp. – hospitalized. P-values were derived using either t-tests (continuous) or chi-squared tests (categorical) for each participant characteristic.
Supplementary Material S1. LIFE study acknowledgements
IMPACT STATEMENT.
We certify that this work is novel. We utilized repeated measures of accelerometry as an exploratory outcome to assess 1) the degree to which hospitalizations were associated with daily, objective activity and sedentary patterns and 2) the role, if any, a physical activity trial played in mitigate those negative hospitalization effects among mobility-limited older adults.
ACKNOWLEDGEMENTS
Please refer to Supplementary Material S1.
FUNDING
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #U01 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH. Dr. Wanigatunga is currently supported by T32AG000247 and P30AG021334 and previously supported for a majority of the work by T32AG020499 and a diversity supplement awarded by P30AG028740. Dr. Manini is supported by R01AG042525 and R01HL121023.
ROLE OF THE SPONSOR
The National Institutes of Health, the National Institute on Aging, National Heart, Lung and Blood Institute, and the Claude D. Pepper Older Americans Independence Center had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223. [DOI] [PubMed] [Google Scholar]
- 2.Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292(17):2115–2124. [DOI] [PubMed] [Google Scholar]
- 3.Gill TM. Disentangling the disabling process: Insights from the precipitating events project. Gerontologist. 2014;54(4):533–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterji S, Byles J, Cutler D, Seeman T, Verdes E. Health, functioning, and disability in older adults—present status and future implications. The Lancet. 2015;385(9967):563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manton KG, Vaupel JW. Survival after the age of 80 in the united states, sweden, france, england, and japan. N Engl J Med. 1995;333(18):1232–1235. [DOI] [PubMed] [Google Scholar]
- 6.Barnason S, Zimmerman L, Nieveen J, et al. Relationships between fatigue and early postoperative recovery outcomes over time in elderly patients undergoing coronary artery bypass graft surgery. Heart & Lung: The Journal of Acute and Critical Care. 2008;37(4):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zatzick D, Jurkovich GJ, Rivara FP, et al. A national US study of posttraumatic stress disorder, depression, and work and functional outcomes after hospitalization for traumatic injury. Ann Surg. 2008;248(3):429–437. [DOI] [PubMed] [Google Scholar]
- 8.Lenze EJ, Munin MC, Skidmore ER, et al. Onset of depression in elderly persons after hip fracture: Implications for prevention and early intervention of Late‐Life depression. J Am Geriatr Soc. 2007;55(1):81–86. [DOI] [PubMed] [Google Scholar]
- 9.Hardy SE, Studenski SA. Fatigue and function over 3 years among older adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63(12):1389–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee I, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. The lancet. 2012;380(9838):219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manini TM, Everhart JE, Patel KV, et al. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296(2):171–179. [DOI] [PubMed] [Google Scholar]
- 12.Mankowski RT, Anton SD, Axtell R, et al. Device‐Measured physical activity as a predictor of disability in Mobility-Limited older adults. J Am Geriatr Soc. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healy GN, Dunstan DW, Salmon J, et al. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care. 2007;30(6):1384–1389. [DOI] [PubMed] [Google Scholar]
- 14.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: Beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–666. [DOI] [PubMed] [Google Scholar]
- 15.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzetti E, Calvani R, Tosato M, et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging clinical and experimental research. 2017;29(1):35–42. [DOI] [PubMed] [Google Scholar]
- 17.Fielding RA, Rejeski WJ, Blair S, et al. The lifestyle interventions and independence for elders study: Design and methods. J Gerontol A Biol Sci Med Sci. 2011;66(11):1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle interventions and independence for elders study: Recruitment and baseline characteristics. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2013;68(12):1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rejeski WJ, Axtell R, Fielding R, et al. Promoting physical activity for elders with compromised function: The lifestyle interventions and independence for elders (LIFE) study physical activity intervention. Clin Interv Aging. 2013;8:1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King AC, Rejeski WJ, Buchner DM. Physical activity interventions targeting older adultsa: A critical review and recommendations. Am J Prev Med. 1998;15(4):316–333. [DOI] [PubMed] [Google Scholar]
- 21.Marsh AP, Applegate WB, Guralnik JM, et al. Hospitalizations during a physical activity intervention in older adults at risk of mobility disability: Analyses from the lifestyle interventions and independence for elders randomized clinical trial. J Am Geriatr Soc. 2016;64(5):933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kocherginsky M, Huisingh-Scheetz M, Dale W, Lauderdale DS, Waite L. Measuring physical activity with hip accelerometry among US older adults: How many days are enough? PloS one. 2017;12(1):e0170082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the united states measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181. [DOI] [PubMed] [Google Scholar]
- 25.Wanigatunga AA, Ambrosius WT, Rejeski WJ, et al. Association between structured physical activity and sedentary time in older adults. JAMA. 2017;318(3):297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wanigatunga AA, Tudor-Locke C, Axtell RS, et al. Effects of a long-term physical activity program on activity patterns in older adults. Medicine & Science in Sports & Exercise. 2017;49(11):2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987. [PubMed] [Google Scholar]
- 28.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. [DOI] [PubMed] [Google Scholar]
- 29.Van Kan GA, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an international academy on nutrition and aging (IANA) task force. J Nutr Health Aging. 2009;13(10):881–889. [DOI] [PubMed] [Google Scholar]
- 30.Van Domelen DR, Van Domelen, Maintainer Dane R, Rcpp I, Rcpp L. Package ‘accelerometry’. . 2015.
- 31.Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665. [DOI] [PubMed] [Google Scholar]
- 32.Mattlage AE, Redlin SA, Rippee MA, Abraham MG, Rymer MM, Billinger SA. Use of accelerometers to examine sedentary time on an acute stroke unit. J Neurol Phys Ther. 2015;39(3):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore SA, Hallsworth K, Plötz T, Ford GA, Rochester L, Trenell MI. Physical activity, sedentary behaviour and metabolic control following stroke: A cross-sectional and longitudinal study. PLoS One. 2013;8(1):e55263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tieges Z, Mead G, Allerhand M, et al. Sedentary behavior in the first year after stroke: A longitudinal cohort study with objective measures. Arch Phys Med Rehabil. 2015;96(1):15–23. [DOI] [PubMed] [Google Scholar]
- 35.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the united states, 2003-2004. Am J Epidemiol. 2008;167(7):875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297(16):1769–1774. [DOI] [PubMed] [Google Scholar]
- 37.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: An undiagnosed condition in older adults. current consensus definition: Prevalence, etiology, and consequences. international working group on sarcopenia. Journal of the American Medical Directors Association. 2011;12(4):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graf C Functional decline in hospitalized older adults: It’s often a consequence of hospitalization, but it doesn’t have to be. AJN The American Journal of Nursing. 2006;106(1):58–67. [DOI] [PubMed] [Google Scholar]
- 39.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roos MA, Rudolph KS, Reisman DS. The structure of walking activity in people after stroke compared with older adults without disability: A cross-sectional study. Phys Ther. 2012;92(9):1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill TM, Beavers DP, Guralnik JM, et al. The effect of intervening hospitalizations on the benefit of structured physical activity in promoting independent mobility among community-living older persons: Secondary analysis of a randomized controlled trial. BMC medicine. 2017;15(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koster A, Shiroma EJ, Caserotti P, et al. Comparison of sedentary estimates between activPAL and hip- and wrist-worn ActiGraph. Med Sci Sports Exerc. 2016;48(8):1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corbett DB, Valiani V, Knaggs JD, Manini TM. Evaluating walking intensity with hip-worn accelerometers in elders. Med Sci Sports Exerc. 2016;48(11):2216–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Participant flow through study
a Data pre-processed for at least 10 hours of accelerometer data which were screened for data key-in errors, accelerometer malfunctions, participant operation errors, visual identification of unexplained patterns or phenomenon, and duplicate files.
b Data were screened for outliers and processed for valid wear/non-wear times based on an accelerometer wear time classification algorithm (22).
Supplementary Figure S2. Analysis timeline
Supplementary Table S1. Baseline sedentary and activity bouts by intervention group and hospitalization^: the LIFE study
^cumulative days spent in a hospital setting.
Note: hosp. – hospitalized. P-values were derived using either t-tests (continuous) or chi-squared tests (categorical) for each participant characteristic.
Supplementary Material S1. LIFE study acknowledgements