Abstract
Mice are a widely utilized in vivo model for translational salivary gland research but must be used with caution. Specifically, mouse salivary glands are similar in many ways to human salivary glands (i.e., in terms of their anatomy, histology and physiology) and are both readily available and relatively easy and affordable to maintain. However, there are some significant differences between the two organisms, and by extension, the salivary glands derived from them that must be taken into account for translational studies. The current review details pertinent similarities and differences between human and mouse salivary glands and offers practical guidelines for using both for research purposes.
Keywords: tissue engineering, primary cells, tissue culture, anatomy, histology, physiology
Introduction
Despite the many similarities between human and mouse salivary glands (SG) in terms of their anatomy, histology and physiology, significant differences are noted with regard to glandular size, life span in culture and cell organization that cause the respective specimens to behave differently, sometimes greatly affecting the degree to which results derived from the former can be considered to be representative of the latter. Although this issue would appear to be of significance for salivary research in light of the frequency with which such comparisons are made, in fact very little has been done to understand the above noted differences and predict their impact on research design as well as the translational potential for results thus derived. Consequently, the points compiled below are offered in the hope that they will help to bring about just such discussion and exploratory studies in the future.
Mice have been the experimental model of choice for the majority of SG studies due to the ease of acquisition and the well-established institutional protocols surrounding their use (Schapiro & Everitt, 2006) as well as the degree of genetic overlap between the species (Justice & Dhillon, 2016, Vandamme, 2015). However, emerging information regarding genetic differences (Cheng et al., 2014, Lin et al., 2014) seems to make translation of mouse studies to humans increasingly difficult, and although an interpretation of these genetic findings is beyond the scope of this paper and the authors’ expertise, such studies highlight the need for an overall awareness of important similarities and differences between the two species for research purposes. To that end, the following review is intended as a practical guide to help researchers compare the relative benefits of the two sources of SG and make informed choices regarding their use.
Comparisons
Anatomy
Both humans and mice have three pairs of major SG (i.e., parotid gland or PG, sublingual gland or SL and submandibular gland or SMG) as well as many minor SG (i.e., anterior and posterior lingual, labial, buccal, molar, incisive, and palatine glands) throughout the oral cavity (Treuting & Dintzis, 2011). SG produce and secrete specialized solutions onto epithelial surfaces through a complex branching system consisting of cells that produce salivary proteins and fluids (i.e., acinar cells) as well as those dedicated to modifying and transporting saliva (i.e., ductal cells) (Dobrosielski-Vergona, 1993). Another cell type, the less studied myoepithelial cells, wrap around the terminal ends of acini and proximal segments of ducts and appear to contract, thereby furthering saliva secretion into the lumens of the branching network (Garant, 2003).
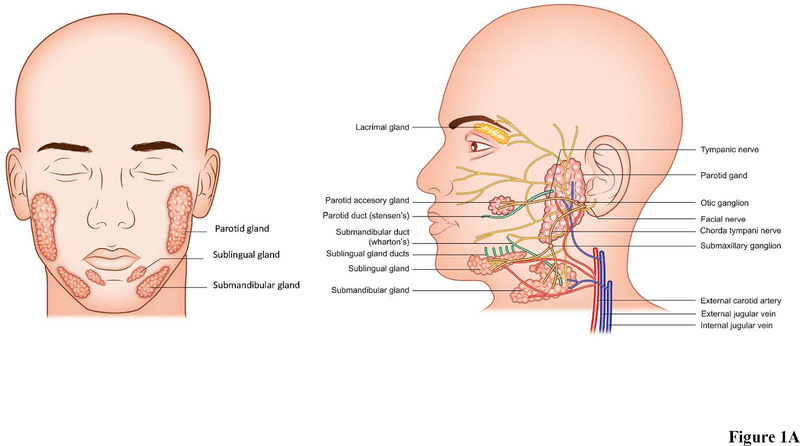
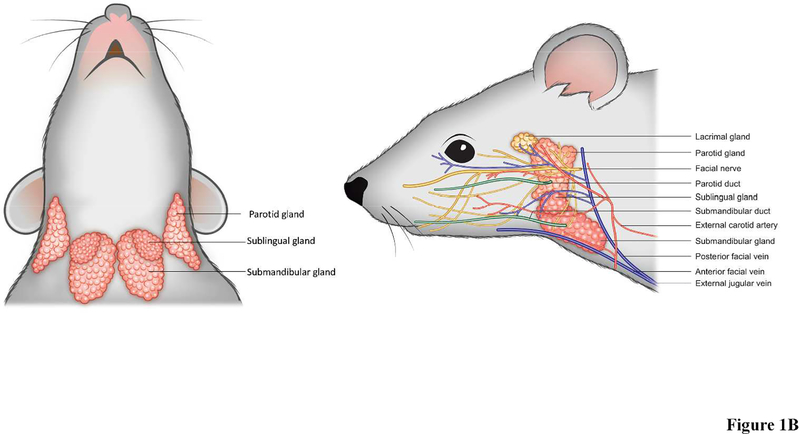
In humans, the SMG is located in level 1B of the neck (Markey et al., 2017, Hemmat et al., 2017), inferior to the mandible, superior to the hyoid, and posterior to the anterior belly of the digastric muscle (Fig. 1A), while in mice it is located in the ventral cervical subcutaneous region (Fig. 1B) (Treuting & Dintzis, 2011). The SMG is approximately half the size of the PG in humans, but it is the largest of the three major gland types in mice (Dobrosielski-Vergona, 1993, Treuting & Dintzis, 2011). In humans, the SMG is divided into two main parts: a large superficial portion located between the mandible and mylohyoid muscle and a smaller deep portion located in the sublingual facial space (Dobrosielski-Vergona, 1993, Ellis, 2012). Humans and mice both have a single duct that directs the saliva to the oral cavity (known as “Wharton’s Duct” in humans and unnamed in mice) (Dobrosielski-Vergona, 1993). However, significant anatomical differences are noted with regard to the SMG and SL glands, as they are separate structures in humans but fused in mice (Jonjic, 2001) (leading to the need for caution in performing dissections of the latter, given that they could easily be taken for a single gland).
Figure 1: Anatomy of (A) human and (B) mouse salivary glands.
Both humans and mice possess three pairs of major salivary glands (the parotid, sublingual, and submandibular glands), as well as hundreds of minor salivary glands (not illustrated due to space constraints). Each major gland empties its contents into a specific duct that terminates within the oral cavity. The major salivary glands also receive blood and neuronal signals from complex networks of vessels and nerves; however, only parasympathetic innervation is depicted (again, in the interest of space). Shown are nerves (yellow), arteries and veins (red and blue respectively), ducts (green). Both frontal and side view are shown.
The PG is the largest of the major SG in humans and is located bilaterally in the parotid space. The gland abuts the muscles of mastication anteriorly and traverses behind the ascending ramus of the mandible posteriorly (Dobrosielski-Vergona, 1993). In mice, the PG is the second largest major SG and is positioned between the SMG and the base of the ear (Treuting & Dintzis, 2011). Furthermore, there is a second portion of the PG which is only present in about 20% of the human population, called the accessory PG (Fig. 1A), which is detached from the main body of the gland and sits on the masseter muscle (Dobrosielski-Vergona, 1993). Although the mouse PG is comprised of only one portion, it extends diffusely over a large area and greatly resembles the nearby exorbital lacrimal gland in its fatty texture and whitish color (Fig. 1B) (Treuting & Dintzis, 2011). Secreted components from the PG travel through the parotid duct in both humans and mice (referred to as Stenson’s duct in humans) where they are carried to the oral cavity.
The smallest of the human major SG is the SL, located beneath the mucous membrane in the mouth in sublingual fossa (Fig. 1A) (Dobrosielski-Vergona, 1993). The mouse SL is similar to that of humans in that it is the smallest major SG and is comprised of only one lobe; it is positioned in the anterior neck of the mouse between the submandibular lymph nodes and SMG (Fig. 1B) (Treuting & Dintzis, 2011). Unlike the simple one-duct structure of the PG and SMG, the SL empties its contents into up to twenty small ducts, which are sometimes referred to as the Ducts of Ravinus (see green colored ducts Fig. 1A) (Dobrosielski-Vergona, 1993). In both mice and humans, secretions from the SL are deposited beneath the tongue onto the floor of the mouth.
Lastly, both mice and humans have numerous minor SG throughout the tissue of the oral cavity that produce saliva but do not contribute significant volumes when compared to the output of major SG. Human minor SG have been better characterized than those of mice due to use of the former in clinical diagnostic procedures (i.e. labial minor salivary glands biopsies are often collected during the diagnosis of Sjögren’s Syndrome), while minor SG in mice are difficult to dissect given their small size (Wicheta et al., 2017). In humans, the seven sub-types of minor SG are the anterior lingual, posterior lingual, labial, buccal, molar, incisive, and palatine glands. While the anterior lingual glands produce seromucous secretions, the posterior lingual glands are either entirely serous or entirely mucous, depending on their location on the tongue (Dobrosielski-Vergona, 1993). The labial glands are mucous in nature and found in the submucosal tissue of the lips, while the similarly structured buccal glands are located throughout the tissue in the walls of the cheek (Dobrosielski-Vergona, 1993). Saliva is deposited near the molars and the inferior part of the tongue by the molar and incisive glands, respectively (Dobrosielski-Vergona, 1993). Finally, the palatine glands are distributed in submucosal tissue of the soft and hard palate oral surfaces (Dobrosielski-Vergona, 1993). In mice, the minor SG are located in the oral submucosa and tongue but are not grossly visible, and in contrast to human glands, have both mucous and serous acini (Frith & Townsend, 1985).
Histology
Major SG in both humans and mice consist of protein and fluid secreting acinar structures draining into a sequential network of intercalated, striated and excretory ducts that transform the primary saliva into the final secretion product, which is then delivered into the oral cavity (Dobrosielski-Vergona, 1993, Treuting & Dintzis, 2011). Moreover, PG, SL, and SMG secrete fluids of comparable composition between both species. The PG is mainly comprised of serous acini that secrete a solution containing proline-rich proteins, secretory immunoglobulin A, immunoglobulin G, immunoglobulin M and amylase. Conversely, the SL is mostly comprised of mucous acini, as it secretes a viscous solution rich in mucins (Treuting & Dintzis, 2011, Korsrud & Brandtzaeg, 1980, Smith et al., 1987). Finally, the SMG is comprised of a mixed population of serous and mucous acini, with the greater portion being serous and serving as a source of the following: secretory immunoglobulin A, immunoglobulin G, immunoglobulin M, secretory immunoglobulin A in both human and mice (Kondo et al., 2015, Amano et al., 2012, Korsrud & Brandtzaeg, 1980, Smith et al., 1987). By contrast, minor SG are comprised of mostly mucous acini, which not only secrete mucins but also produce secretory immunoglobulin A (Crawford et al., 1975, Smith et al., 1987).
It is important to mention as well that mice display sexual dimorphism in their SMG, particularly in the granular convoluted and intercalated duct cells (GCT and GID, respectively). GCT of the mouse SMG are segments of the duct system situated between the intercalated ducts and the striated ducts that are composed of serous-like exocrine cells which are larger and more numerous in the glands of males than of females. GID cells are localized in the intercalated ducts of mouse SMG in females but not males (Jayasinghe et al., 1990, Kurabuchi, 2002, Chen et al., 1995, Gresik, 1994, Gresik et al., 1996). Human SMG, on the other hand, do not have granular convoluted tubules nor do they display such sexual dimorphism (Pinkstaff, 1998, Chen et al., 1995, Gresik, 1994, Gresik et al., 1996). Furthermore, researchers should be aware that factors beyond species and sex have been observed to affect histological features such as secretory granule presence and localization. For instance, the means of tissue fixation (e.g. immersion vs. perfusion and fixative solution used), the use of sialagogues prior to tissue collection, and whether or not the tissue source was fasting can impact downstream analyses (Watermann et al., 2016, de Lange & Vreugdenhil, 1979).
Physiology
Saliva secretion in both humans and mice is controlled by both parasympathetic and sympathetic innervation of the autonomic nervous system (Anderson & Garrett, 1998). Parasympathetic innervation in PG from both mice and humans starts with stimulation from the inferior salivatory nucleus via the auriculotemporal nerve, with axons running along the ninth cranial nerve then on through the jugular and otic ganglia (Garant, 2003). Furthermore, SMG and SL innervation originates for both species in the superior salivary nucleus, propagates along the chorda tympani branch of the facial nerve and SMG ganglion and finally innervates the gland via postganglionic fibers (Garant, 2003). As for minor SG in the upper jaw (i.e., soft and hard palates as well as in the upper lip), stimulation occurs through parasympathetic fibers that originate in the superior salivatory nucleus. These fibers then become part of a facial nerve that travels through the geniculate ganglion and later merges with the greater petrosal nerve (Dobrosielski-Vergona, 1993, Dumont, 1955). The greater petrosal nerve then synapses with post-ganglionic fibers in the pterygopalatine ganglion and reaches the minor SG via branches of the trigeminal nerve (Dobrosielski-Vergona, 1993, Dumont, 1955). At the same time, nerve fibers leave the geniculate ganglion as part of the chorda tympani nerve, synapse in the submandibular ganglion and depart as branches of the lingual nerve to innervate the incisive and anterior lingual glands (Dobrosielski-Vergona, 1993). Alternatively, parasympathetic innervation of the minor SG in the lower jaw (i.e., buccal, molar and those near the lower lip) begins in the inferior salivatory nucleus as the glossopharyngeal nerve, which synapses in the otic ganglion with post-ganglionic fibers that join the trigeminal nerve (Dobrosielski-Vergona, 1993, Garrett & Anderson, 1991, Garrett et al., 1991, Dumont, 1955). Total parasympathetic stimulation terminates in the activation of muscarinic cholinergic receptors with the neurotransmitter acetylcholine, thereby leading to fluid secretion with low protein content (Garrett et al., 1991, Holmberg & Hoffman, 2014).
As for sympathetic stimulation of the PG, SMG, SL and minor SG, this process starts in the first and second thoracic segments of the spinal cord. In this region, nerve fibers coalesce in the superior cervical sympathetic ganglion and then connect to post-ganglionic fibers following two possible routes, either traveling along the middle meningeal artery and through the otic ganglion to the PG or along the maxillary artery and through the submaxillary ganglion to the SMG and SL (Garant, 2003). Sympathetic fibers then travel to their respective glands where the neurotransmitter norepinephrine activates both α-adrenergic and β-adrenergic receptors, which in turn leads to protein secretion (Garrett et al., 1991, Garrett & Anderson, 1991).
Though the function of the autonomic nervous system is similar between mice and humans, there is one discernible difference that can be observed in cross-sections of peripheral nerve bundles, as detailed below. In both species, nerve axons are organized in bundles called fascicles, which in turn are bound into larger nerve trunks. There is connective tissue in addition to the myelin sheath that surrounds nerve tissue at each organizational level as follows: endoneurium surrounding axons, perineurium surrounding fascicles and epineurium surrounding the nerve trunk. Human peripheral nerves have a thicker myelin coating around axons as well as more connective tissue in the endoneurium, perineurium, and epineurium layers (Thomas, 1963). Furthermore, human axons are more spaced out due to dense connective tissue and the fiber diameter is also greater as compared to mouse axons (Treuting & Dintzis, 2011); by contrast, mice have a thinner myelin coating as well as less connective tissue surrounding the nerves. Though this size difference does not have any known impact on observation and comparisons of the innervations systems between the two species, it would appear prudent to keep such variations in mind should confounding results requiring in-depth interpretation arise.
There are basic anatomical features pertaining to irrigation that are similar between mice and humans. While these features can be readily identified and flow patterns thus traced in each of the glandular subgroups, little research has been conducted to determine how such blood flow contributes to SG function (Lee et al., 2005). With that in mind, information regarding the routes of blood flow specific to each species and within the various SG subtypes is detailed below to serve as a starting point for such functional studies as follows: Mouse PG are irrigated by the anterior auricular artery and blood then passes through the anterior auricular vein towards the external jugular vein (Fig. 1B) (Greene, 1955). Similarly, human PG are irrigated by the superficial temporal and maxillary arteries, which branch off of the external carotid artery, with blood then draining back into the external jugular vein via the temporal and maxillary veins (Fig. 1A) (Holsinger & Bui, 2007). As for SMG and SL, they are irrigated in mice by the lingual and maxillary branches of the external carotid artery (Greene, 1955), with blood returning to the heart through the anterior facial vein. Likewise, human SMG and SL receive blood from the lingual and facial arteries that branch off the external carotid artery (Holsinger & Bui, 2007), with the blood then draining through the small facial vein branches that coalesce to form the facial vein prior to joining the internal jugular vein (Holsinger & Bui, 2007).
Practice guidelines
Obtaining mouse and human tissue
Prior to beginning work, all institutions that perform research with laboratory animals are required to have an Institutional Animal Care and Use Committee (IACUC)-approved protocol (Silverman et al., 2017, Silverman, 2017, Hansen et al., 2017). Likewise, all institutions performing research of any type involving human subjects are required to have an Institutional Review Board (IRB)-approved protocol, the key component differentiating the latter from the former being the informed consent process, which attempts to assure subjects’ right to choose participation in a given study on the basis of the risks and benefits as presented by the researchers (Kane & Gallo, 2017, Goldenberg et al., 2015). Note that while IACUC and IRB are guidelines used within the United States, similar ethical committee guidelines are followed by much of the international community (Ghooi, 2014, Grady, 2015, Gettayacamin & Retnam, 2017, Newcomer & McGlone, 2015).
Mice and mouse specimens can be obtained with relative ease from commercial vendors or from other research institutions. Human specimens, though admittedly far more difficult to obtain, can potentially be found through online tissue repositories with several potential limitations as follows: a) availability is unpredictable, b) costs may be high, c) errors (e.g., sending the wrong tissue) are common in the authors’ experience and d) vendors may not be at liberty to disclose or may not even know vital information regarding the human tissue donor. Alternatively, local institutions such as hospitals may offer the possibility of obtaining fresh tissues from routine surgeries (e.g., head and neck cancer patients undergoing cervical lymphadenctomy) and, if they do, are more likely in the authors’ experience to disclose helpful information regarding the donor while not violating basic privacy standards. As a final note on obtaining human tissue, success has been gained by the authors only with a personal contact (i.e., you have to know somebody at the institution providing the tissue or know somebody who does) and the source must be in close enough proximity that the samples can be sent cold on ice rather than frozen on dry ice, which would render them useless for studies that require fresh specimens (e.g., when fresh isolated cells for tissue culture are needed).
Isolating and culturing cells
For our studies, human SMG specimens have been obtained with informed consent from patients undergoing neck dissection surgery at a cancer treatment facility within the University of Utah campus, while mouse SMG specimens have been obtained from female C57BL/6J mice. Following euthanasia procedures, mouse SMG can be easily isolated from the anterior region of the body, inferior to the mouth and superior to the fore legs (Baker, 2017). Male mice yield a large pair of glands that is tough in texture and light in color, while females at comparable ages yield a smaller pair that is more delicate and pink in color (per authors’ observations). As such, male SMG tissue may require additional mechanical digestion prior to filtration to achieve a level of dissociation similar to that found in females (again, per authors’ observations). Likewise, additional mechanical digestion is typically required for human SMG as well; however, the yield is significantly greater than of mouse SMG, with production from the former being anywhere from 5–10 times greater than that from the latter.
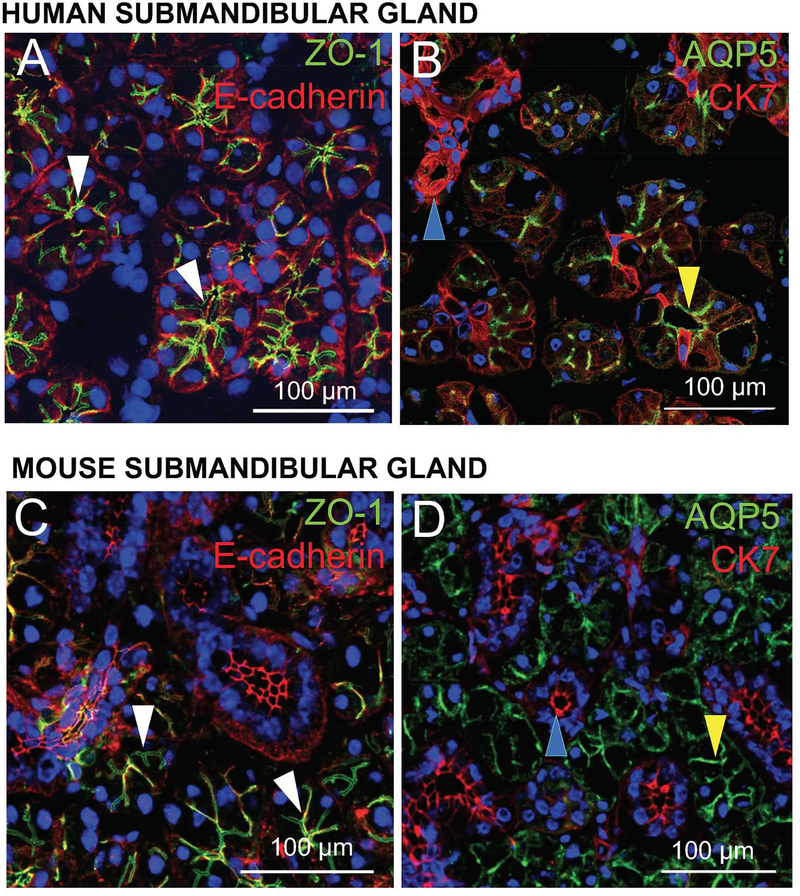
As for obtaining primary cells, they can be harvested from either human or mouse SMG, with the details of this process covered in Appendix A (Cell isolation and culturing). Several concerns should be born in mind during the isolation and culturing process. First, choice of substrate determines the type of cells that can be grown, such that plastic yields cell suspensions (Baker et al., 2006, Turner & Camden, 1990) while permeable supports (Tran et al., 2005) and scaffold proteins (Baker, 2017) lead to monolayers and sphere formations, respectively. Further details regarding the types of spheres that may be formed are found in Appendix B (Scaffold usage), with no additional information on cell suspensions and/or monolayers provided given their rudimentary structures. Next, larger sections of cells are more susceptible to breaking off and deterioration in general, given that they adhere to supporting surfaces to a lesser degree than smaller cell units and also present greater surface area and mass, both of which increase their chances of being swept up in the surrounding medium (i.e., during washing and fixation). As such, it is advisable to encapsulate cells within histogel and cut slices greater than 200 μm in order to protect against cell cluster loss during staining, as detailed in Appendix C (Histogel processing). Additionally, confocal microscopy must be performed to demonstrate that key structural components of the cell are present. For instance, tight junctions (e.g., ZO-1), adherens junctions (e.g., E-cadherin), acinar markers (e.g., aquaporin-5, AQP5) and ductal markers (e.g., cytokeratin 7, CK7) markers, see example of a human and mouse tissue sections in Fig. 4. Finally, measurement of agonist-induced intracellular calcium increases and cyclic AMP must be performed in live samples to demonstrate functionality.
Figure 4. Markers of human and mouse salivary glands.
Submandibular glands (SMG) were frozen, fixed and stained as described in Appendix D. Then, confocal microscopy was utilized to localize some of the structural markers in native tissue from human (A, B) and mouse (C, D) SMG. White arrowheads highlight the apical localization of the tight junction protein, zonula occludens-1 (A, C; ZO-1, green) in both human (A) and mouse (C) SMG. Aquaporin-5 (B, D; AQP5, green) and cytokeratin 7 (B, D; CK7, red) identify acinar (yellow arrowheads) and ductal epithelial cells (blue arrowheads), respectively in human (B) and mouse (D) SMG.
Maintaining cells
Recent studies have demonstrated the importance of acinar cell duplication for promoting salivary gland homeostasis (Aure et al., 2015b, Aure et al., 2015a). Specifically, acinar cells were shown to have a significant contribution to cell proliferation and SG maintenance, as evidenced by acinar cell replacement during homeostasis, growth and regeneration. These studies used an inducible CreERT2 expressed under the control of the Mist1 gene locus and demonstrated the following: a) genetic labeling, followed by a chase period, showed that acinar cell replacement is not driven by the differentiation of unlabeled stem cells, b) analysis using R26Brambow2.1 reporter revealed continued proliferation and clonal expansion of terminally differentiated acinar cells in salivary glands and c) induced injury also demonstrated the regenerative potential of pre-labeled acinar cells (Aure et al., 2015b). These findings suggest that SG maintenance relies to some extent on self-duplication, an assertion supported by a handful of earlier studies in salivary glands (Redman, 1995, Chang, 1974) and other organs (Dor et al., 2004). However, there is longstanding evidence that stem cells also contribute to salivary gland homeostasis (Pringle et al., 2016, Nanduri et al., 2011, Lombaert et al., 2008, Jeong et al., 2013, Feng et al., 2009). As such, the origin of differentiated epithelial cell types is still incompletely understood in the salivary gland and confirmatory studies are warranted to both better understand the relative contributions from the likely candidates mentioned above and determine if other cells may also be involved in this process.
Areas of growth
The following is a list of research programs that are currently being conducted primarily with mice and that ostensibly will be of clinical relevance for humans. As such, findings from each of these particular areas must be tested within human cells at a minimum to begin to make explicit the connections between the two species. Note that, consistent with the theme of this review, the following list of topics is not intended to be exhaustive but rather to highlight some obvious needs for exploration and provide some examples of how translational viability may be provisionally established.
Mouse salisphere transplantation
The term “salispheres” from salivary gland stem/progenitor cells is a neologism patterned after similar terms from other parts of the body (e.g., mammospheres and neurospheres) (Liao et al., 2007, Marshall et al., 2007). In a 2011 study, primary mouse SMG cells were cultured as salispheres in vitro for three days prior to transplantation into irradiated SMG. For these studies, mouse SMG were irradiated with 15 Gy and after 30 days either received a suspension of selected cells expressing stem cell markers or did not receive any transplanted cells. 120 days post-irradiation or 90 days after transplantation, the degree of functional regeneration was assessed by measuring salivary flow rate in mice receiving transplantations of various stem cell-rich sub-populations. Results indicate that mice receiving primarily c-kit positive and CD133 positive cells showed the most significant recovery in salivary flow rates (Nanduri et al., 2011), thereby offering preliminary support for the use of progenitor cell-based salispheres in SG regeneration.
Human salisphere transplantation
Results of a recent study suggest salisphere cell therapy may be beneficial for the treatment of xerostomia (Pringle et al., 2016). In it, primary human salispheres were cultured from healthy human SMG biopsies for three to five days and injected at various cell densities to post-irradiated mouse SMGs (30 days post-irradiation; 5 Gy dose) (Pringle et al., 2016). Results showed observable cell proliferation and differentiation of the human salisphere cells in the mouse SMG 30–60 days after transplantation or 60–90 days post-irradiation, with mice receiving the greatest number of transplanted cells demonstrating the highest degree of salivary flow rate recovery at end-stage analyses. Further studies are needed, however, to better understand signaling mechanisms between donor and recipient stem cells and to determine what other cell types might be involved in this process.
Bioengineered organ germ
In some cases, gland damage is so extensive that there is not a great enough reservoir of intact cells to harbor transplanted salispheres or single cells, thereby making whole organ transplantation desirable. For this reason, techniques to generate whole glands starting with isolated epithelial and mesenchymal cells from embryonic mice have been explored (Ogawa et al., 2013). While some studies have focused solely on the epithelium as it pertains to bioengineering a gland, this technique aims to maintain proper epithelium-mesenchyme signaling for organized gland morphogenesis, as would be observed in vivo. Interestingly, mice with SG defects receiving engraftments of bioengineered glands demonstrated pilocarpine- and citrate-induced saliva secretion (Ogawa et al., 2013); however, application of such findings to humans appears not to be an option in the near future, given difficulties in obtaining human embryonic cells in light of federal funding bans on such research. (Reisman & Adams, 2014).
Radioprotective nanoparticle delivery
Radiation exposure for head and neck cancer treatment causes apoptosis of salivary acinar, ductal, and progenitor cells, thereby leading to hyposalivation (Stephens et al., 1991, Avila et al., 2009). In order to overcome this issue, local delivery of siRNA-complexed nanoparticles against the pro-apoptotic PKCδ gene has been shown to reduce cell death following radiation treatment (Arany et al., 2013). This method was first used in primary SG cultures and later repeated using a mouse model with similar findings noted (Arany et al., 2013). Specifically, post-treatment functional studies involving a mouse model indicated enhanced saliva secretion rates three months following irradiation when PKCδ-targeted siRNA nanoparticles were employed (Arany et al., 2013).
Hedgehog pathway activation
Further attempts at protecting and restoring irradiated tissue involve activating the Hedgehog pathway, which signals during regeneration of the adult mouse SG and appears to promote the preservation of parasympathetic innervation (Hai et al., 2014). Specifically, it has been observed that irradiation inhibits the expression of two important markers of parasympathetic nerves, namely acetylcholinesterase (AChE) and glial cell line-derived neurotrophic factor family receptor a2, but that transient activation of the hedgehog pathway mitigated this effect (Hai et al., 2014). Additionally, both qRT-PCR and ELISA confirmed an upregulation of a brain-derived neurotrophic factor (Hai et al., 2014) and that genes associated with salivary stem and progenitor cells are also affected, making this stand out as a promising post-irradiation therapy (Hai et al., 2014, Hai et al., 2016). Finally, other pathways implicated in SG development and regeneration (e.g. FGF, Wnt, Hedgehog, Eda, Notch, Chrm1/HB-EGF) could potentially be exploited to promote cell survival and glandular secretion in post-irradiated glands (Liu & Wang, 2014).
Induction of aquaporin gene expression
Gene therapy using aquaporin-1 (AQP1) has been used for both pre-clinical and clinical studies to treat radiation-induced salivary gland hypofunction (Baum et al., 2006, Delporte & Baum, 1998, Delporte et al., 1996a, Delporte et al., 1996b, O’Connell et al., 1999). More recent work on this topic has shown that beneficial effects can continue years after parotid gland delivery (Alevizos et al., 2017), while other studies have demonstrated that that AQP1 gene therapy can also restore fluid movement in a murine model of Sjögren’s syndrome (Lai et al., 2016). Together, these results indicate the feasibility and benefits of using AQP1 gene delivery for treating various salivary gland conditions; however, new delivery methods are warranted to minimize any risk for immune response with repeated administrations (Lowenstein et al., 2007). To that end, a recent study demonstrated that ultrasound-assisted non-viral gene transfer could be a viable alternative means for delivering the AQP1 gene to the salivary gland (Wang et al., 2015). The potential reach of this technology is great due to the vast number of candidate genes that may be targeted as well as the possibility of applying it with patients while avoiding invasive surgical procedures. Finally, if used in combination, techniques such as hedgehog pathway activation (Liu & Wang, 2014, Hai et al., 2016) together with progenitor cell transplantation (Pringle et al., 2016, Nanduri et al., 2011) may yield even better outcomes than either technique alone, as the former preserves native structure while the latter supplements cell populations too vulnerable to survive irradiation.
Microfluidic systems
Several groups are trying to build organ-on-chip systems which create microfluidic cell culture devices using microchip manufacturing methods. This system contains continuously perfused chambers inhabited by living cells arranged to simulate tissue- and organ-level physiology (Kong et al., 2016). By resembling tissue-tissue interfaces, physicochemical microenvironments and vascular irrigation of the body, these devices produce levels of tissue and organ functionality not possible with conventional 2D or 3D culture systems while also enabling high-resolution, real-time imaging and in vitro analysis of biochemical, genetic and metabolic activities of living cells in a functional tissue and organ context (Mosier et al., 2014). Given that organ-on-chip methods have already proven useful for the study of multiple organ systems (Alexander et al., 2017, Caballero et al., 2017, Esch et al., 2015, Escutia-Guadarrama et al., 2017, Kodzius et al., 2017, Mosig, 2017, van der Helm et al., 2016), there is little doubt that this technology shows great potential to advance the study of SG development, physiology and disease etiology. Moreover, it could be a game changer in the context of drug discovery and development by making possible nearly limitless trials, thereby leading to cheaper and better targeted studies of molecular mechanisms of action, prioritization of lead candidates, toxicity testing and biomarker identification (Liu et al., 2017).
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSC) can be directly generated from adult cells, thereby circumventing the controversy of using stem cells from embryos (Yu et al., 2007). Specifically, iPSC propagate indefinitely and also give rise to many other cell types in the body (e.g., neurons, pancreatic, and liver cells) (Stadtfeld & Hochedlinger, 2010). Moreover, they represent a single source that could be used to replace those cells lost to damage or disease. Finally, they could potentially be made in a patient-matched manner, meaning that each individual could have their own targeted pluripotent stem cell line (Lin et al., 2017). As a preliminary model, iPSC were obtained from parotid gland-derived mesenchymal stem cells (Yan et al., 2016); however, advancement of this process would require that safety be determined, a significant hurdle in light of frequent teratoma formation (Ono et al., 2015).
Conclusion
We would like to close this review with a thought experiment that we hope highlights the need for us to change the way we think and our research. What if we had infinite resources to conduct our current research programs – would we know more about how our findings relate to the real world human problems that we are working towards fixing? What if we had infinite money, access to human tissue and even clinical trials, and no oversight worries to constrain us? Would we be closer to hitting the mark of knowing how what we study in simulation with mice actually would play out in the real world with humans? We fear that we would not. Even with the obvious impediments above removed, there is still something very real getting in the way of such progress. Research is incentivized for us to work in isolation on discrete problems, thereby leading to the next publication and hopefully the next grant. One of the big questions, this review has attempted to raise a very big methodological issue, that of how our simulations using mice relate to the real world of human treatment. In order for us to get over the hump, we would need some basic opportunities to present themselves, including funding and access to human specimens as well as a reasonable accord with those who review and oversee our work. However, we would also need to see the relevance ourselves a pursuing such difficult questions as what our mouse-based research has to do with the problems that we are supposedly tracking. To do this, we would need to look beyond our immediate self-interest and work together to a greater degree than we are typically accustomed to doing. On the positive side, however, we really are establishing the relevance for and ensuring the survival of what we do if we are able to mobilize and work together to make sure that our work reaches and benefits those who ultimately support it, humans of the general public. Hopefully this review contributes in some small way to just such an endeavor.
Appendix
A –. Methods, cell isolation and culturing
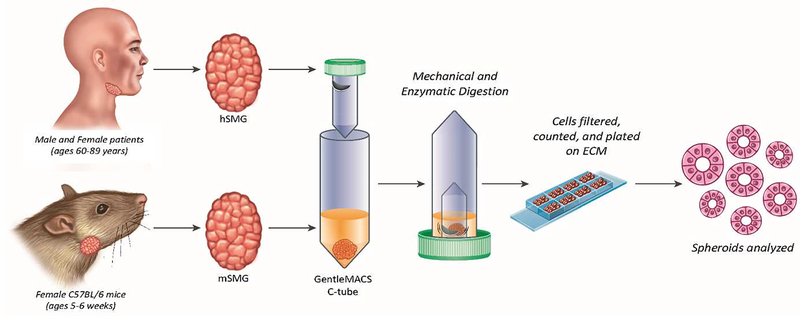
Human studies have been approved by the University of Utah Institutional Review Board on August 15th 2017 under the number IRB_00077943. Mouse studies have been approved by the University of Utah Institutional Animal Care and Use Committee on May 24th 2017 under the number IACUC_14–06012. Mouse and human submandibular glands were mechanically and enzymatically dissociated and homogenized (Fig. 2). The homogenate of each specie was further dispersed by filtration and centrifugation to obtain salivary acinar and ductal cells. Specifically, MACS Human enzyme kit solution [4.7 ml DMEM, 100 μl Enzyme R, 20 μl Enzyme A, 200 μl Enzyme H] (Miltenyi Biotec, Bergisch Gladbach, Germany) was thawed and mixed according to the manufacturer’s instruction. If Next, both mouse and human tissue samples were placed in weigh dishes, covered with MACS Human enzyme kit solution (Miltenyi Biotec) and minced into approximately one mm2 pieces using two sterile scalpel blades. The minced tissue and enzyme solution were then transferred to a GentleMACS C-Tube (Miltenyi Biotec), inverted and processed on a GentleMACS Tissue Dissociator (Miltenyi Biotec). The following pre-defined protocols were utilized on the GentleMACs tissue dissociator in succession: h_tumor_01, h_tumor_02 and h_tumor_03. Next, the tube was re-oriented and incubated at 37°C for twenty min in a shaking water bath. The tube was then inverted once again and processed using the GentleMACS Tissue Dissociator to further break down the tissue mechanically. At this point, the tube was re-oriented and incubated for another twenty min at 37°C in a shaking water bath. The tissue was ultimately incubated at 37°C and processed on the tissue dissociator three or more times, depending on the degree of tissue dispersion observed. In most instances, a defined protocol (h_tumor_01) was run a fourth or fifth time on the GentleMACs tissue dissociator in order to achieve a level of homogenization comparable to mouse SMG tissue. The tube was then centrifuged at 150 × g for eight min at 37°C, the solution removed and the pellet re-suspended in complete proliferation medium [DMEM/Ham’s F12 (1:1) containing 2.5% (v/v) fetal bovine serum (GIBCO BRL, Gaithersburg, MD) along with the following supplements: 2 nM triiodothyronine, 0.1 μM retinoic acid, 0.4 μg/ml hydrocortisone, 80 ng/ml epidermal growth factor, 5 ng/ml sodium selenite, 5 mM glutamine, 5 μg/ml insulin, 5 μg/ml transferrin]. At this time, cells were passed successively through 100 μm, 70 μm, and 40 μm sterile cell strainers and washed via centrifugation (150 × g for 5 min at 37°C), with the pellet re-suspended in complete proliferation medium (as described above). Suspended cells and cell clusters were then diluted 1:1 in Trypan Blue (Thermo Fisher Scientific, Waltham, MA) and counted using a hemocytometer (Hausser Scientific, Horsham, PA) and EVOS microscope (Life Technologies, Carlsbad, CA). Note that this same procedure can be performed without access to such specialized equipment as follows: a) Glands can be finely minced with blades in DMEM-F12 medium supplemented with enzymes (i.e., hyaluronidase and collagenase). B) Tissue is then dispersed in a plastic flask while shaking in water bath at 37°C with 95% O2-5% CO2 gas and cell aggregates are obtained by pipetting homogenates at 20 and 30 min and washing with buffer. C) Cells are then centrifuged at room temperature, re-suspended in supplemented medium and plated for tissue culture. For more details on this alternate procedure, see previous publications (Baker et al., 2006, Nelson et al., 2014).
Figure 2: Human and mouse submandibular gland cell isolation process.
Submandibular glands (SMG) can be obtained from humans or mice, as described in Obtaining mouse and human tissue. Our lab utilizes a GentleMACS Tissue Dissociator (Miltenyi Biotec) and its associated enzyme cocktail for cell isolation, with cells later plated on ECM scaffolds to form 3D salivary spheroids (Baker, 2017).
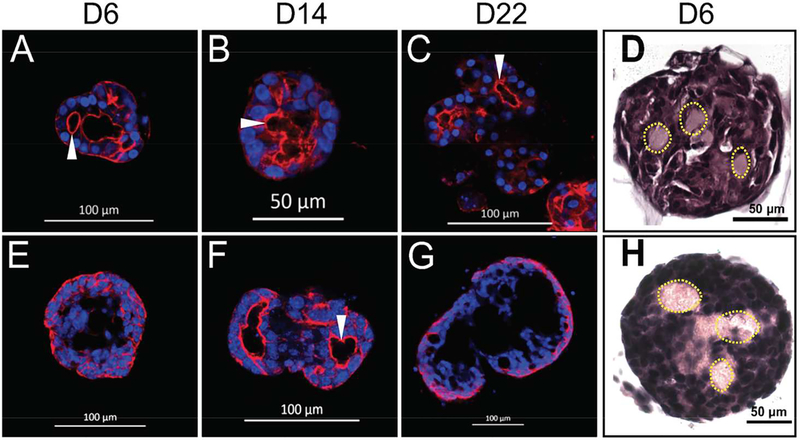
Once obtained, cells can be plated on various substrates that will dictate the type of salivary epithelial structures to be formed in vitro. For this study, 100 μl of highly purified Laminin-1 gel (Trevigen, Catalog Number: 3446-005-01) were plated on eight-well chambers mounted on #1 German borosilicate cover glass (Nalge Nunc International Corporation, Naperville, IL), while L1 gel was plated at a concentration of six mg/ml and allowed to solidify at 37°C for one hour. SMG cell clusters were then dissociated and seeded at approximately 8,500 cell clusters per well. Then, SMG cells were grown in an incubator at 37°C with 95% air and 5% CO2 in a supplemented DMEM/Ham’s F12 medium. Finally, TO-PRO™−3 iodide and phalloidin were used to stain nuclei blue and F-actin red, respectively (Fig. 3A, 3B, 3C, 3E, 3F, 3G).
Figure 3: Culturing human and mouse submandibular gland cells.
Human (A-D) and mouse (E-H) SMG tissue was obtained and dissociated (as detailed in Fig. 2). Suspended cells and cell clusters were then plated on Laminin-111 (L1) gel in 8-well chambered coverglass slides and grown for 6 (A, E), 14 (B, F), and 22 (C, G) days. TO-PRO™−3 iodide and phalloidin were used to stain nuclei blue and F-actin red, respectively (A, B, C, E, F, G). Human (D) and mouse (H) SMG cells were then cultured on Laminin-111 rich gel for six days and captured in histogel for fixing, sectioning, and staining with H&E. Yellow dotted lines are indicative of lumen formation (as evidenced by a lack of nuclei) and white arrowheads point to apically localized F-actin (A, B, C, F).
B –. Scaffold usage
SMG cells can grow on various scaffolds to form 3D structures. These matrices may consist of decellularized tissue, Matrigel, polymers and hydrogels (Maria et al., 2011, McCall et al., 2013), all of which can be further optimized with peptides and growth factors (McCall et al., 2013, Nam et al., 2016). Regarding decellularization, rat SMG have been successfully decellularized for use as a scaffold to reseed primary cells (Gao et al., 2014); however, this technique has not yet been performed with human cells. Regarding, Matrigel, they can support the growth of many cell types due to their rich composition (Kleinman & Martin, 2005); however, they are derived from Engelbreth-Holm-Swarm tumors, which have been observed to increase tumorigenesis in vivo (Noël et al., 1993). Regarding polymers and hydrogels, chemically defined and biologically inert compounds have shown great potential for supporting primary SG cell growth while minimizing the concerns for tumorigenesis upon implantation. For example, enzymatically degradable poly (ethylene glycol) hydrogels support an acinar cell phenotype in primary mouse SMG cells, as demonstrated by maintained expression of the markers AQP5 and Nkcc1 (Shubin et al., 2017). Additionally, hydrogels offer more flexibility by providing the option to plate cells on top of or to encapsulate them within a gel (Shubin et al., 2017, Pradhan-Bhatt et al., 2013). Moreover, repeatable encapsulation can be difficult to achieve given quick sedimentation rates of cell clusters. Finally, this issue has at times been addressed by utilizing photopolymerizable components that can be solidified quickly, thus trapping cells in suspension (Shubin et al., 2017).
C –. Histogel processing
Histogel (i.e., an aqueous hydroxyethyl agarose based gel useful in processing histological and cytological specimens; Thermo Fisher Scientific) was warmed to 60° C in a water bath to achieve a liquid consistency. Media from cell culture plates were then removed and 150–200 μl of warmed histogel added to each well of grown cell clusters. Using the pipette tip, histogel was gently mixed and cell clusters and ECM scaffold transferred into a small plastic mold on ice. The contents of multiple wells were then pooled to increase cell cluster density in each mold, and once the mixture was cooled, it was transferred to a labeled tissue cassette and fixed overnight (at 4° C in 10% buffered formalin). The following day, cassettes were moved to 70% ethanol, embedded in paraffin, sectioned and stained with H&E to visualize cell cluster organization (Fig. 3D and 3H). Confocal microscopy was then performed on SMG cell clusters fixed in 10% buffered formalin (Fisher Scientific, Waltham, MA) for fifteen min at room temperature, at which time they were incubated with 0.1% Triton X-100 (Fisher Scientific) for twenty min and washed with 1× PBS three times for five min. Cell clusters were then incubated in an AlexaFluor 568 Phalloidin filamentous actin stain (1:400 dilution, Thermo Fisher Scientific) for one hour, followed by a TO-PRO™−3 iodide nuclear stain (1: 1,000 dilution, Thermo Fisher Scientific) for fifteen min. Finally, cell clusters were washed three times for five min with 1× PBS prior to imaging with a Carl Zeiss LSM 700 confocal microscope (Zeiss, Oberkochen, Germany).
D –. Detection of salivary glands structural markers using confocal microscopy.
Frozen tissue sections of mouse and human SMG were fixed with 10% buffered formalin (Fisher Scientific) for 10 mins at room temperature, permeabilized with 0.1% Triton X-100 (Fisher Scientific) for 12 min, and washed three times with 1 × PBS for 5 min. Sections were then incubated in 5% goat serum (Sigma Aldrich, St. Louis, MO) for 1 h at room temperature. Then, the following primary antibodies were used (overnight incubation at 4°C in 5% goat serum) to localize various structural features in the SMG: rabbit anti-zonula occludens-1 (ZO-1; 1:200 dilution; Invitrogen, Carlsbad, CA), mouse anti-E-cadherin (1:250 dilution; BD Biosciences, San Jose, CA), rabbit anti-Aquaporin-5 (AQP-5; 1:250 dilution; Abcam, Cambridge, MA), and mouse anti-Cytokeratin-7 (CK-7; 1:250 dilution; Abcam). The following day tissue sections were washed 3 times for 5 min with 1× PBS, and incubated in corresponding fluorescently labeled secondary antibodies (1:500 dilution in 5% goat serum) for 1 h. Finally, tissue sections were washed again 3 times for 5 min with 1× PBS, incubated in TO-PROtm-3 iodide nuclear stain (1:1,000 dilution; Thermo Fisher Scientific) for 12 min at room temperature, washed 3 times for 5 min with 1× PBS, and mounted with coverglass and ProLongTM Gold Antifade mountant (Thermo Fisher Scientific) and imaging was done with a Carl Zeiss LSM 700 confocal microscope (Zeiss), see Fig. 4.
References
- Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Billings ME, Goldsmith CM, Tandon M, Helmerhorst EJ, Catalan MA, Danielides SJ, Perez P, Nikolov NP, Chiorini JA, Melvin JE, Oppenheim FG, Illei GG and Baum BJ (2017). Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene Ther 24: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander F Jr., Eggert S and Wiest J (2017). A novel lab-on-a-chip platform for spheroid metabolism monitoring. Cytotechnology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano O, Mizobe K, Bando Y and Sakiyama K (2012). Anatomy and histology of rodent and human major salivary glands: -overview of the Japan salivary gland society-sponsored workshop. Acta Histochem Cytochem 45: 241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L and Garrett J (1998). Neural regulation of blood flow in the rat submandibular gland. European journal of morphology 36: 213–218. [PubMed] [Google Scholar]
- Arany S, Benoit DS, Dewhurst S and Ovitt CE (2013). Nanoparticle-mediated gene silencing confers radioprotection to salivary glands in vivo. Mol Ther 21: 1182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aure MH, Arany S and Ovitt CE (2015a). Salivary Glands: Stem Cells, Self-duplication, or Both? J Dent Res 94: 1502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aure MH, Konieczny SF and Ovitt CE (2015b). Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell 33: 231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila JL, Grundmann O, Burd R and Limesand KH (2009). Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int J Radiat Oncol Biol Phys 73: 523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker OJ (2017). Current Cell Models for Bioengineering Salivary Glands In: Cha S, ed. Salivary Gland Development and Regeneration: Advances in Research and Clinical Approaches to Functional Restoration. Springer International Publishing: Cham, pp. 133–144. [Google Scholar]
- Baker OJ, Camden JM, Ratchford AM, Seye CI, Erb L and Weisman GA (2006). Differential coupling of the P2Y 1 receptor to Gα 14 and Gα q/11 proteins during the development of the rat salivary gland. Archives oforal biology 51: 359–370. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Zheng C, Cotrim AP, Goldsmith CM, Atkinson JC, Brahim JS, Chiorini JA, Voutetakis A, Leakan RA, Van Waes C, Mitchell JB, Delporte C, Wang S, Kaminsky SM and Illei GG (2006). Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim Biophys Acta 1758: 1071–7. [DOI] [PubMed] [Google Scholar]
- Caballero D, Kaushik S, Correlo VM, Oliveira JM, Reis RL and Kundu SC (2017). Organ-on-chip models of cancer metastasis for future personalized medicine: From chip to the patient. Biomaterials 149: 98–115. [DOI] [PubMed] [Google Scholar]
- Chang WW (1974). Cell population changes during acinus formation in the postnatal rat submandibular gland. Anat Rec 178: 187–201. [DOI] [PubMed] [Google Scholar]
- Chen S, Gao F, Kotani A and Nagata T (1995). Age-related changes of male mouse submandibular gland: a morphometric and radioautographic study. Cell Mol Biol (Noisy-le-grand) 41: 117–24. [PubMed] [Google Scholar]
- Cheng Y, Ma Z, Kim BH, Wu W, Cayting P, Boyle AP, Sundaram V, Xing X, Dogan N, Li J, Euskirchen G, Lin S, Lin Y, Visel A, Kawli T, Yang X, Patacsil D, Keller CA, Giardine B, mouse EC, Kundaje A, Wang T, Pennacchio LA, Weng Z, Hardison RC and Snyder MP (2014). Principles of regulatory information conservation between mouse and human. Nature 515: 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JM, Taubman MA and Smith DJ (1975). Minor salivary glands as a major source of secretory immunoglobin A in the human oral cavity. Science 190: 1206–9. [DOI] [PubMed] [Google Scholar]
- de Lange GI and Vreugdenhil AP (1979). Preservation of ultrastructure after extended perfusion of mouse salivary glands with autonomic stimulating drugs. Stain Technol 54: 309–12. [DOI] [PubMed] [Google Scholar]
- Delporte C and Baum BJ (1998). Preclinical and biological studies using recombinant adenoviruses encoding aquaporins 1 and 5. Eur J Morphol 36 Suppl: 118–22. [PubMed] [Google Scholar]
- Delporte C, Chen ZJ and Baum BJ (1996a). Aquaporin 1 expression during the cell cycle in A5 cells. Biochem Biophys Res Commun 228: 223–8. [DOI] [PubMed] [Google Scholar]
- Delporte C, O’Connell BC, He X, Ambudkar IS, Agre P and Baum BJ (1996b). Adenovirus-mediated expression of aquaporin-5 in epithelial cells J Biol Chem 271: 22070–5. Dobrosielski-Vergona K (1993). Biology of the salivary glands, CRC. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI and Melton DA (2004). Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–6. [DOI] [PubMed] [Google Scholar]
- Dumont L (1955). [Parasympathetic innervation of the salivary glands]. C R Hebd Seances Acad Sci 240: 240–2. [PubMed] [Google Scholar]
- Ellis H (2012). Anatomy of the salivary glands. Surgery (Oxford) 30: 569–572. [Google Scholar]
- Esch EW, Bahinski A and Huh D (2015). Organs-on-chips at the frontiers of drug discovery. Nat Rev DrugDiscov 14: 248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escutia-Guadarrama L, Vazquez-Victorio G, Martinez-Pastor D, Nieto-Rivera B, Sosa-Garrocho M, Macias-Silva M and Hautefeuille M (2017). Fabrication of low-cost micropatterned polydimethyl-siloxane scaffolds to organise cells in a variety of two-dimensioanl biomimetic arrangements for lab-on-chip culture platforms. J Tissue Eng 8: 2041731417741505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, van der Zwaag M, Stokman MA, van Os R and Coppes RP (2009). Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiotherapy and Oncology 92: 466–471. [DOI] [PubMed] [Google Scholar]
- Frith CH and Townsend JW (1985). Histology and Ultrastructure, Salivary Glands, Mouse., Springer, Berlin, Heidelberg. [Google Scholar]
- Gao Z, Wu T, Xu J, Liu G, Xie Y, Zhang C, Wang J and Wang S (2014). Generation of bioartificial salivary gland using whole-organ decellularized bioscaffold. Cells Tissues Organs 200: 171–180. [DOI] [PubMed] [Google Scholar]
- Garant PR (2003). Oral cells and tissues.
- Garrett J, Suleiman A, Anderson L and Proctor G (1991). Secretory responses in granular ducts and acini of submandibular glands in vivo to parasympathetic or sympathetic nerve stimulation in rats. Cell and tissue research 264: 117–126. [DOI] [PubMed] [Google Scholar]
- Garrett JR and Anderson LC (1991). Rat sublingual salivary glands: secretory changes on parasympathetic or sympathetic nerve stimulation and a reappraisal of the adrenergic innervation of striated ducts. Arch Oral Biol 36: 675–83. [DOI] [PubMed] [Google Scholar]
- Gettayacamin M and Retnam L (2017). AAALAC International Standards and Accreditation Process. Toxicol Res 33: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghooi RB (2014). Institutional review boards: Challenges and opportunities. Perspect Clin Res 5: 60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg AJ, Maschke KJ, Joffe S, Botkin JR, Rothwell E, Murray TH, Anderson R, Deming N, Rosenthal BF and Rivera SM (2015). IRB practices and policies regarding the secondary research use of biospecimens. BMC Med Ethics 16: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C (2015). Institutional Review Boards: Purpose and Challenges. Chest 148: 1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene EC (1955). Anatomy of the Rat. Anatomy of the rat. [Google Scholar]
- Gresik EW (1994). The granular convoluted tubule (GCT) cell of rodent submandibular glands. Microsc Res Tech 27: 1–24. [DOI] [PubMed] [Google Scholar]
- Gresik EW, Hosoi K, Kurihara K, Maruyama S and Ueha T (1996). The rodent granular convoluted tubule cell--an update. Eur J Morphol 34: 221–4. [DOI] [PubMed] [Google Scholar]
- Hai B, Qin L, Yang Z, Zhao Q, Shangguan L, Ti X, Zhao Y, Kim S, Rangaraj D and Liu F (2014). Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation. Clin Cancer Res 20: 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai B, Zhao Q, Qin L, Rangaraj D, Gutti VR and Liu F (2016). Rescue Effects and Underlying Mechanisms of Intragland Shh Gene Delivery on Irradiation-Induced Hyposalivation. Hum Gene Ther 27: 390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen BC, Gografe S, Pritt S, Jen KC, McWhirter CA, Barman SM, Comuzzie A, Greene M, McNulty JA, Michele DE, Moaddab N, Nelson RJ, Norris K, Uray KD, Banks R, Westlund KN, Yates BJ, Silverman J, Hansen KD and Redman B (2017). Ensuring due process in the IACUC and animal welfare setting: considerations in developing noncompliance policies and procedures for institutional animal care and use committees and institutional officials. FASEB J 31: 4216–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmat SM, Wang SJ and Ryan WR (2017). Neck Dissection Technique Commonality and Variance: A Survey on Neck Dissection Technique Preferences among Head and Neck Oncologic Surgeons in the American Head and Neck Society. Int Arch Otorhinolaryngol 21: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg KV and Hoffman MP (2014). Anatomy, biogenesis and regeneration of salivary glands. Monogr Oral Sci 24: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger FC and Bui DT (2007). Anatomy, function, and evaluation of the salivary glands Salivary gland disorders. Springer, pp. 1–16. [Google Scholar]
- Jayasinghe NR, Cope GH and Jacob S (1990). Morphometric studies on the development and sexual dimorphism of the submandibular gland of the mouse. J Anat 172: 115–27. [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Baek H, Kim Y-J, Choi Y, Lee H, Lee E, Kim ES, Hah JH, Kwon T-K and Choi IJ (2013). Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Experimental & molecular medicine 45: e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjic S (2001). Surgical removal of mouse salivary glands. Curr Protoc Immunol Chapter 1: Unit 1 11. [DOI] [PubMed] [Google Scholar]
- Justice MJ and Dhillon P (2016). Using the mouse to model human disease: increasing validity and reproducibility. Dis Model Mech 9: 101–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane EI 3rd and Gallo JJ (2017). Perspectives of IRB chairs on the informed consent process. AJOB Empir Bioeth 8: 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK and Martin GR (2005). Matrigel: basement membrane matrix with biological activity Seminars in cancer biology. Elsevier, pp. 378–386. [DOI] [PubMed] [Google Scholar]
- Kodzius R, Schulze F, Gao X and Schneider MR (2017). Organ-on-Chip Technology: Current State and Future Developments. Genes (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Nakamoto T, Jaramillo Y, Choi S, Catalan MA and Melvin JE (2015). Functional differences in the acinar cells of the murine major salivary glands. J Dent Res 94: 715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Luo Y, Jin D, An F, Zhang W, Liu L, Li J, Fang S, Li X, Yang X, Lin B and Liu T (2016). A novel microfluidic model can mimic organ-specific metastasis of circulating tumor cells. Oncotarget 7: 78421–78432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsrud FR and Brandtzaeg P (1980). Quantitative immunohistochemistry of immunoglobulin-and J-chain-producing cells in human parotid and submandibular salivary glands. Immunology 39: 129–40. [PMC free article] [PubMed] [Google Scholar]
- Kurabuchi S (2002). Morphologic changes in the granular convoluted tubule cells of the mouse submandibular gland following hypophysectomy and hormonal replacement. Odontology 90: 27–34. [DOI] [PubMed] [Google Scholar]
- Lai Z, Yin H, Cabrera-Perez J, Guimaro MC, Afione S, Michael DG, Glenton P, Patel A, Swaim WD, Zheng C, Nguyen CQ, Nyberg F and Chiorini JA (2016). Aquaporin gene therapy corrects Sjogren’s syndrome phenotype in mice. Proc Natl Acad Sci U S A 113: 5694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Matsuoka T and Aiyama S (2005). Changes in the distribution and fine structure of the intralobular blood vessels of the submandibular gland in the postnatally developing mouse. Anat Rec A Discov Mol Cell Evol Biol 287: 1272–80. [DOI] [PubMed] [Google Scholar]
- Liao MJ, Zhang CC, Zhou B, Zimonjic DB, Mani SA, Kaba M, Gifford A, Reinhardt F, Popescu NC, Guo W, Eaton EN, Lodish HF and Weinberg RA (2007). Enrichment of a population of mammary gland cells that form mammospheres and have in vivo repopulating activity. Cancer Res 67: 8131–8. [DOI] [PubMed] [Google Scholar]
- Lin H, Li Q and Lei Y (2017). An Integrated Miniature Bioprocessing for Personalized Human Induced Pluripotent Stem Cell Expansion and Differentiation into Neural Stem Cells. Sci Rep 7: 40191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Lin Y, Nery JR, Urich MA, Breschi A, Davis CA, Dobin A, Zaleski C, Beer MA, Chapman WC, Gingeras TR, Ecker JR and Snyder MP (2014). Comparison of the transcriptional landscapes between human and mouse tissues. Proc Natl Acad Sci U S A 111: 17224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F and Wang S (2014). Molecular cues for development and regeneration of salivary glands. Histol Histopathol 29: 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gill E and Shery Huang YY (2017). Microfluidic on-chip biomimicry for 3D cell culture: a fit-for-purpose investigation from the end user standpoint. Future Sci OA 3: FSO173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G and Coppes RP (2008). Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 3: e2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K and Castro MG (2007). Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther 7: 347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria OM, Zeitouni A, Gologan O and Tran SD (2011). Matrigel improves functional properties of primary human salivary gland cells. Tissue Engineering Part A 17: 1229–1238. [DOI] [PubMed] [Google Scholar]
- Markey JD, Morrel WG, Wang SJ and Ryan WR (2017). The effect of submandibular gland preservation during level 1B neck dissection on postoperative xerostomia. Auris Nasus Larynx. [DOI] [PubMed] [Google Scholar]
- Marshall GP 2nd, Reynolds BA and Laywell ED (2007). Using the neurosphere assay to quantify neural stem cells in vivo. Curr Pharm Biotechnol 8: 141–5. [DOI] [PubMed] [Google Scholar]
- McCall AD, Nelson JW, Leigh NJ, Duffey ME, Lei P, Andreadis ST and Baker OJ (2013). Growth factors polymerized within fibrin hydrogel promote amylase production in parotid cells. Tissue Engineering Part A 19: 2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier AP, Peters SB, Larsen M and Cady NC (2014). Microfluidic platform for the elastic characterization of mouse submandibular glands by atomic force microscopy. Biosensors (Basel) 4: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig AS (2017). Organ-on-chip models: new opportunities for biomedical research. Future Sci OA 3: FSO130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam K, Jones JP, Lei P, Andreadis ST and Baker OJ (2016). Laminin-111 Peptides Conjugated to Fibrin Hydrogels Promote Formation of Lumen Containing Parotid Gland Cell Clusters. Biomacromolecules 17: 2293–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van Os RP and Coppes RP (2011). Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol 99: 367–72. [DOI] [PubMed] [Google Scholar]
- Nelson JW, Leigh NJ, Mellas RE, McCall AD, Aguirre A and Baker OJ (2014). ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am J Physiol Cell Physiol 306: C178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer CE and McGlone JJ (2015). Comment on AAALAC international and compliance with animal welfare laws. J Appl Anim Welf Sci 18: 311–3. [DOI] [PubMed] [Google Scholar]
- Noël A, De Pauw-Gillet M, Purnell G, Nusgens B, Lapiere CM and Foidart J-M (1993). Enhancement of tumorigenicity of human breast adenocarcinoma cells in nude mice by matrigel and fibroblasts. British journal of cancer 68: 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell AC, Baccaglini L, Fox PC, O’Connell BC, Kenshalo D, Oweisy H, Hoque AT, Sun D, Herscher LL, Braddon VR, Delporte C and Baum BJ (1999). Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther 6: 505–13. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Oshima M, Imamura A, Sekine Y, Ishida K, Yamashita K, Nakajima K, Hirayama M, Tachikawa T and Tsuji T (2013). Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun 4: 2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, Obana A, Usami Y, Sakai M, Nohara K, Egusa H and Sakai T (2015). Regenerating Salivary Glands in the Microenvironment of Induced Pluripotent Stem Cells. Biomed Res Int 2015: 293570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkstaff CA (1998). Salivary gland sexual dimorphism: a brief review. Eur J Morphol 36 Suppl: 31–4. [PubMed] [Google Scholar]
- Pradhan-Bhatt S, Harrington DA, Duncan RL, Jia X, Witt RL and Farach-Carson MC (2013). Implantable three-dimensional salivary spheroid assemblies demonstrate fluid and protein secretory responses to neurotransmitters. Tissue Eng Part A 19: 1610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle S, Maimets M, van der Zwaag M, Stokman MA, van Gosliga D, Zwart E, Witjes MJ, de Haan G, van Os R and Coppes RP (2016). Human Salivary Gland Stem Cells Functionally Restore Radiation Damaged Salivary Glands. Stem Cells 34: 640–52. [DOI] [PubMed] [Google Scholar]
- Redman RS (1995). Proliferative activity by cell type in the developing rat parotid gland. Anat Rec 241: 529–40. [DOI] [PubMed] [Google Scholar]
- Reisman M and Adams KT (2014). Stem cell therapy: a look at current research, regulations, and remaining hurdles. P T 39: 846–57. [PMC free article] [PubMed] [Google Scholar]
- Schapiro SA and Everitt JI (2006). Preparation of animals for use in the laboratory: issues and challenges for the Institutional Animal Care and Use Committee (IACUC). ILAR J 47: 370–5. [DOI] [PubMed] [Google Scholar]
- Shubin AD, Felong TJ, Schutrum BE, Joe DS, Ovitt CE and Benoit DS (2017). Encapsulation of primary salivary gland cells in enzymatically degradable poly (ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomaterialia 50: 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J (2017). How should the IACUC balance an efficient approval process with minimizing risk? Lab Anim (NY) 46: 419. [DOI] [PubMed] [Google Scholar]
- Silverman J, Lidz CW, Clayfield J, Murray A, Simon LJ and Maranda L (2017). Factors Influencing IACUC Decision Making: Who Leads the Discussions? J Empir Res Hum Res Ethics 12: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Taubman MA and King WF (1987). Immunological features of minor salivary gland saliva. J Clin Immunol 7: 449–55. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M and Hochedlinger K (2010). Induced pluripotency: history, mechanisms, and applications. Genes Dev 24: 2239–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LC, Schultheiss TE, Price RE, Ang KK and Peters LJ (1991). Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer 67: 1539–43. [DOI] [PubMed] [Google Scholar]
- Thomas PK (1963). The connective tissue of peripheral nerve: an electron microscope study. J Anat 97: 35–44. [PMC free article] [PubMed] [Google Scholar]
- Tran S, Wang J, Bandyopadhyay B, Redman R, Dutra A, Pak E, Swaim W, Gerstenhaber J, Bryant J and Zheng C (2005). Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue engineering 11: 172–181. [DOI] [PubMed] [Google Scholar]
- Treuting PM and Dintzis SM (2011). Comparative Anatomy and Histology: A Mouse and Human Atlas (Expert Consult), Academic Press. [Google Scholar]
- Turner J and Camden J (1990). The influence of vasoactive intestinal peptide receptors in dispersed acini from rat submandibular gland on cyclic AMP production and mucin release. Archives of oral biology 35: 103–108. [DOI] [PubMed] [Google Scholar]
- van der Helm MW, van der Meer AD, Eijkel JC, van den Berg A and Segerink LI (2016). Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 4: e1142493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme TF (2015). Rodent models for human diseases. Eur J Pharmacol 759: 84–9. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zourelias L, Wu C, Edwards PC, Trombetta M and Passineau MJ (2015). Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion. Gene Ther 22: 739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watermann C, Valerius KP, Wagner S, Wittekindt C, Klussmann JP, Baumgart-Vogt E and Karnati S (2016). Step-by-step protocol to perfuse and dissect the mouse parotid gland and isolation of high-quality RNA from murine and human parotid tissue. Biotechniques 60: 200–3. [DOI] [PubMed] [Google Scholar]
- Wicheta S, Van der Groen T, Faquin WC and August M (2017). Minor Salivary Gland Biopsy-An Important Contributor to the Diagnosis of Sjogren Syndrome. J Oral Maxillofac Surg. [DOI] [PubMed] [Google Scholar]
- Yan X, Xu N, Meng C, Wang B, Yuan J, Wang C and Li Y (2016). Generation of induced pluripotent stem cells from human mesenchymal stem cells of parotid gland origin. Am J Transl Res 8: 419–32. [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II and Thomson JA (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–20. [DOI] [PubMed] [Google Scholar]