Abstract
PURPOSE
The global burden of colorectal cancer (CRC) will continue to increase for the foreseeable future, largely driven by increasing incidence and mortality in low- and middle-income countries (LMICs) such as Nigeria.
METHODS
We used the Wilson-Jungner framework (1968) to review the literature relevant to CRC screening in Nigeria and propose areas for future research and investment.
RESULTS
Screening is effective when the condition sought is both important and treatable within the system under evaluation. The incidence of CRC is likely increasing, although the exact burden of disease in Nigeria remains poorly understood and access to definitive diagnosis and treatment has not been systematically quantified. In high-income countries (HICs), CRC screening builds on a well-known natural history. In Nigeria, a higher proportion of CRC seems to demonstrate microsatellite instability, which is dissimilar to the molecular profile in HICs. Prospective trials, tissue banking, and next-generation sequencing should be leveraged to better understand these potential differences and the implications for screening. Fecal immunochemical test for hemoglobin (FIT) is recommended for LMICs that are considering CRC screening. However, FIT has not been validated in Nigeria, and questions about the impact of high ambient temperature, endemic parasitic infection, and feasibility remain unanswered. Prospective trials are needed to validate the efficacy of stool-based screening, and these trials should consider concomitant ova and parasite testing.
CONCLUSION
Using the Wilson-Jungner framework, additional work is needed before organized CRC screening will be effective in Nigeria. These deficits can be addressed without missing the window to mitigate the increasing burden of CRC in the medium to long term.
INTRODUCTION
The growing incidence of cancer in many low- and middle-income countries (LMICs) has reshaped our understanding of the global burden of cancer care.1,2 Colorectal cancer (CRC) is the third most common cancer globally, and the number of new occurrences is predicted to increase by 77% between 2008 and 2030. The majority of that growth (62%) is projected to occur in LMICs, as the incidence of CRC has stabilized or begun to decline in many high-income countries (HICs).3
Similar to many countries in sub-Saharan Africa (SSA), it is widely held that the incidence of CRC in Nigeria does not justify the establishment of organized screening.4,5 However, recent evidence that suggests a growing incidence has made the issue increasingly relevant to clinicians and policymakers.6-8 The Nigerian National Cancer Strategy (2018 to 2022) has identified CRC screening as a priority and endorses the establishment of a national screening program. We used the Wilson and Jungner (1968) framework to review the literature relevant to CRC screening in Nigeria and propose areas for future research and investment.9
CONTEXT
Key Objective
Are the building blocks in place for colorectal cancer (CRC) screening in Nigeria? We used the Wilson-Jungner framework to examine the concept of colorectal screening in this low-resource environment.
Knowledge Generated
The basic tenets of CRC screening are not met in Nigeria. The true burden of disease, performance of screening instruments, national capacity for endoscopic follow-up, access to diagnostic and treatment infrastructure, quality control, and governance mechanisms for policy creation and program implementation remain undefined or poorly quantified. The Wilson-Jungner paradigm provides an actionable framework for future research and investment.
Relevance
The creation of screening policy and program implementation can take decades. An opportunity now exists to address these deficits without missing the window to mitigate the increasing burden of CRC in the medium to long term.
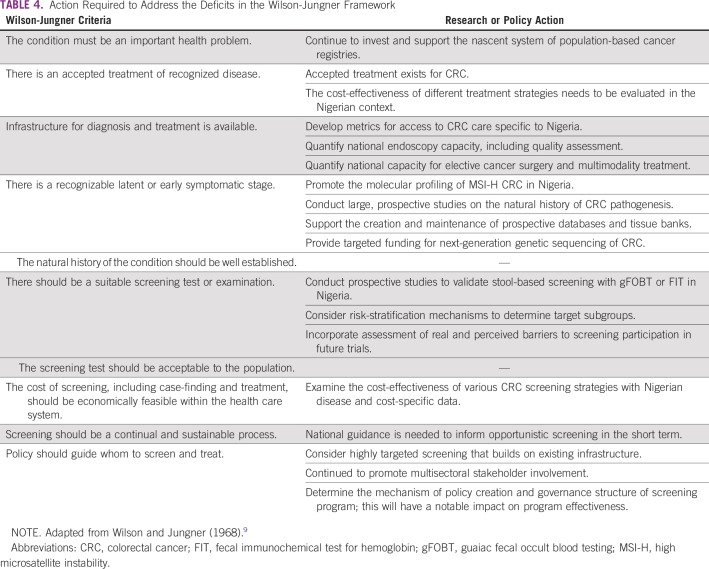
DEFINING THE CRITERIA FOR SCREENING
The seminal work by Wilson and Jungner (1968)9 can be used as a basic framework to evaluate the concept of CRC screening in Nigeria (Table 1). Screening can be discussed as either opportunistic or organized; organized screening can be subcategorized as either population based or targeted. On an opportunistic basis, individuals can undergo screening that is either self-initiated or proposed by a health care professional. Organized screening is a prescribed, supply-driven pathway with a target population (ie, age range or subgroup), specific screening instrument/frequency, follow-up/referral plan, and built-in quality assurance. The development and implementation of organized screening is a complex multisectoral process, which requires the coordination of all aspects of the health care system.10
TABLE 1.
Screening Criteria for CRC
BURDEN OF CRC IN NIGERIA
For population-based screening to be effective, the condition sought must be an important health problem. In SSA, estimates suggest that the overall crude incidence of CRC may be 4.04 per 100,000 (men, 4.38; women, 3.69).7 However, the true burden of CRC in Nigeria is difficult to estimate, because the data have historically been of insufficient scope and quality.11,12
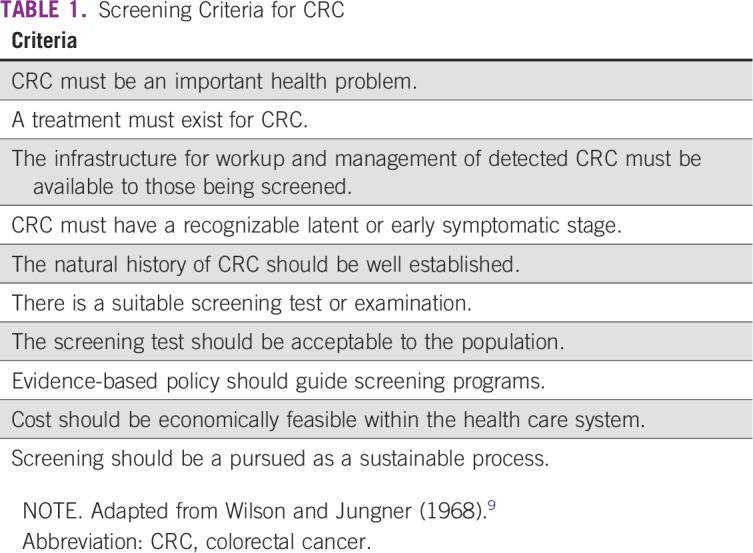
Overall, only 11% of Africans were captured in population-based cancer registries in 2006. When only the highest-quality data are used, as defined by inclusion in the Cancer Incidence on Five Continents (CI5) series (I to XI), the coverage decreases to less than 2%.11 The most recent profile of CRC in SSA from the CI5-XI is summarized in Table 2. The only population-based registries in SSA with longitudinal data from the CI5 series are from Harare, Zimbabwe, and Kampala, Uganda (ie, CI5-X and CI5-XI). The data from these two registries suggest that, in urban SSA, the age-standardized incidence rate (ASR) of CRC may be between 7.6 and 12.9 per 100,000.13 The population-based cancer registry in Ibadan, Nigeria, was only included in the first three editions of the CI5 series and reported an ASR of colon cancer for men of 3.2 per 100,000 from 1960 to 1965.14 The last official CI5 publication of West African incidence and mortality data was from The Gambia in 1997 to 1998 and Bamako, Mali, in 1994 to 1996 (CI5-X).15
TABLE 2.
ASRs for Colorectal Cancer in Sub-Saharan Africa
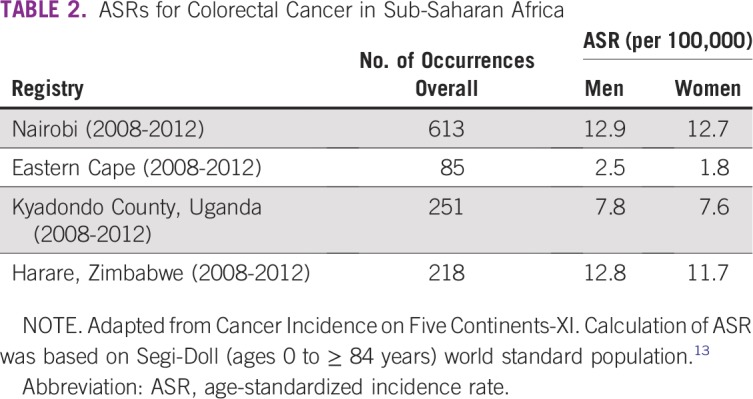
There are now 13 population-based and 20 hospital-based cancer registries in Nigeria, organized under the Nigerian National System of Cancer Registries since 2009. Five of the 16 hospital-based registries queried had incomplete data during an informal audit in 2012.16 Three of the 13 population-based registries were vetted and included in the recent African Cancer Registry Network report (Table 3). Not all of the results in the African Cancer Registry Network report would be of sufficient quality to be included in the CI5 series; however, registries are required to meet a minimum criterion of 70% case ascertainment.17 From Ibadan, Nigeria, the ASRs of CRC were 6.9 and 5.3 per 100,000 for men and women, respectively. Data were also included from Abuja and Calabar, Nigeria. An ASR of CRC of 6.7 per 100,000 for men in Abuja was similar to that in Ibadan, whereas the comparatively rural and impoverished catchment area around Calabar had notably lower incidence rates of 2.8 and 1.7 per 100,000 for men and women, respectively.17
TABLE 3.
ASRs for Colorectal Cancer in Nigeria and West Africa
Several retrospective reviews from single institutions across SSA suggest a steady increase in CRC incidence during the past several decades.6,18,19 In Nigeria, a historical review of retrospective studies reported an ASR that ranged from 1 to 7.2 per 100,000 during the past half century and noted a tripling of incidence at the University College Hospital.19 Despite the limitations of the data, at tertiary care facilities across the country, the incidence of CRC is felt to be increasing.20-22
When the available literature is taken in aggregate, the following conclusions can be put forth: the true burden and trajectory of CRC incidence in Nigeria are relatively unknown; the ASR of CRC is likely quite heterogeneous across the country and depends on level of urbanization and economic development; and, in more affluent urban centers, the ASR of CRC may be closer to the ASR in cities with more robust population-based registry data, such as Harare and Kampala (ie, 10 to 12/100,000).13 Effective cancer control policy must be rooted in unbiased, high-quality, population-level data. Continued investment and expansion of the Nigerian National System of Cancer Registries is needed with an emphasis on total case ascertainment and quality control.
PATHOGENESIS OF CRC IN NIGERIA
CRC is ideally suited to screening, because the majority of occurrences have a long and recognizable latency phase. Most CRC develops along a pathway of chromosomal instability that is characterized by the adenoma-carcinoma sequence.23 In this environment, the role of screening to reduce CRC incidence and mortality has been demonstrated in numerous randomized, controlled trials and robust prospective, cohort studies in HICs.24-26
In Nigeria, the natural history of CRC and the molecular profile of the disease have not been as thoroughly investigated.20 Early reports of colonic pathology reflect the disproportionate burden of communicable disease. In the 1970s, hamartomatous and inflammatory polyps, secondary to schistosomal infection, were the most common endoscopic pathology.27 More contemporary studies reflect a more familiar profile of colonic neoplasia. Of 415 consecutive patients undergoing colonoscopy for a variety of indications at Obafemi Awolowo University Teaching Hospital Complex in Ile-Ife, Nigeria, 16.1% had polyps with an adenoma detection rate of 6.8%.28 In a more recent cohort of patients with rectal bleeding at the same institution, the adenoma detection rate was 8.5%.29 This is congruent with a number of other single-institution series from across the country, which demonstrate a relatively low burden of traditional adenomas.30-32 Unfortunately, there is a dearth of prospective data from asymptomatic individuals from which to draw meaningful conclusions about the true burden and pathogenesis of early colorectal neoplasia in Nigeria.
In numerous retrospective series, CRC in Nigeria seems more likely to produce a phenotype characterized by younger age of onset, right-sided and rectal disease, and mucinous differentiation.6,20,21,33,34 This phenotype is more consistent with the molecular profile of microsatellite instability (MSI) than chromosomal instability, which accounts for 85% of CRC in HICs.23 At a single institution, 43% of available paraffin-embedded CRC specimens had high levels of MSI (MSI-H).22 Another analysis demonstrated that 23% of colon cancer specimens were negative on immunohistochemistry for mismatch-repair proteins MSH2 and MLH1.35 From the African Research Group for Oncology prospective database, 24% of patients were MSI-H (personal communication, Alatise and Kingham, 2008). Despite these high rates of MSI, BRAF was only mutated in 4.5% of patient cases in a separate, retrospective series of Nigerian CRC.36 The proportion of MSI-H CRC in Nigeria that is sporadic versus hereditary is unknown, and there has been no peer-reviewed evidence of next-generation genetic sequencing of CRC in Nigeria.
The current data suggest that, compared with the burden of CRC in HIC, a higher proportion of neoplasia in Nigeria is developing outside of the chromosomal instability pathway. However, much of the available literature is likely biased by the inherent flaws of small, retrospective data sets. There are still important questions about the behavior and molecular profile of CRC in Nigeria. These unanswered questions pose a great challenge to clinicians and policymakers interested in establishing opportunistic screening guidelines or organized screening policy.
CRC SCREENING MODALITIES
The National Polyp Study from the United States provided the first robust evidence in support of colonoscopy and polypectomy as a means to reduce the incidence of CRC.37 However, it was the Minnesota Colon Cancer Control Study that demonstrated a survival benefit to organized, population-based CRC screening with the annual guaiac fecal occult blood testing (gFOBT).26 Today, there is considerable variability between well-established national and international screening guidelines.38 The majority recommend starting opportunistic screening for average-risk individuals at age 50 years and consider gFOBT, the fecal immunochemical test for hemoglobin (FIT), flexible sigmoidoscopy, and colonoscopy as core modalities.38 Colonoscopy remains the most commonly used modality for opportunistic screening, whereas most population-based screening programs rely on noninvasive, stool-based instruments, such as gFOBT and FIT.39
In Nigeria, colonoscopy was first introduced in the 1970s, and the first major series of 562 patients was published from Obafemi Awolowo University Teaching Hospital Complex in Ile-Ife.40 Endoscopy units are now established across the country, although they are primarily used for the diagnosis and management of symptomatic patients, which reflects the scarcity of the resource.21,28-32,41,42 Colonoscopy as a primary screening strategy, even among high-risk individuals, is likely beyond the capacity of the limited resources. To date, there has been no systematic, national assessment of endoscopy capacity or quality. It is also unclear which facilities can adequately manage advanced adenomas or malignant polyps that may be discovered during screening.
For population-based screening, FIT has now largely replaced gFOBT as the preferred modality.38,39,43 FIT uses an antibody against human globin to detect occult blood in the stool. Compared with gFOBT, FIT has higher compliance rates as well as superior advanced adenoma and CRC detection.43,44 The higher compliance is associated with a single stool sample (compared with two to three samples for gFOBT) and a lack of dietary restrictions before stool collection. When adopted as a population-based screening modality, FIT is also more cost-effective.45
The most important variable of FIT performance is the threshold for a positive result.43,46,47 Pooled data suggests that a cutoff threshold of 20 μg of hemoglobin per gram of stool has the best performance characteristics, with a sensitivity and specificity for CRC detection of 89% and 91%, respectively.46 However, the sensitivity, specificity, and positive predictive value of the test vary considerably with different cutoff thresholds, and the variation has a large impact on the use of endoscopic resources. From a cutoff of 20 μg/g to 50 μg/g, the sensitivity for advanced neoplasia detection decreases by 39%, and the rate of test positivity almost halves.47 A diagnostic FIT threshold of less than 20 μg/g is currently recommended by the US Multi-Society Task Force on CRC; however, the optimal threshold remains uncertain and is not directly discussed in most guidelines. In LMICs, such as Nigeria, the cutoff must be tailored to the limited endoscopy capacity available to investigate a positive result.43,47
STOOL-BASED CRC SCREENING IN NIGERIA
For countries considering CRC screening, the World Gastroenterology Organization and American Society of Clinical Oncology have resource-stratified screening guidelines, which recommend FIT (or highly sensitive gFOBT in American Society of Clinical Oncology guidelines) in settings with limited endoscopic resources.48,49 In middle-income countries, such as Iran, Argentina, and Chile, organized, stool-based screening has been successfully piloted.50-52 However, there are no organized CRC screening programs or pilot projects in SSA, and little evidence exists to support the efficacy or feasibility of CRC screening with gFOBT or FIT in Nigeria.
In Nigeria, several specific issues related to FIT performance must be examined. For instance, there is conflicting evidence about the impact of temperature on FIT performance, despite the use of sodium azide–containing buffer solution in many newer products.43,53 Symonds et al54 demonstrated a relationship between FIT positivity and ambient temperature, in which a 1.8% decrease in positivity occurred per 1°C increase in temperature (odds ratio [OR], 0.982; 95% CI, 0.973 to 0.991). A retrospective review of almost 200,000 quantitative FIT results from Italy demonstrated an inverse relationship between temperature and fecal hemoglobin concentration and showed a 17% lower probability (odds ratio [OR], 0.83; 95% CI, 0.76 to 0.9) of a positive result in the summer months.55 Positive stool samples from a prospective study by van Roon et al56 in 2012 were sequentially tested over 3 weeks and demonstrated a decrease in the hemoglobin concentration of 5.88% per day (95% CI, 4.78% to 6.96%) at 20°C and more than 18% per day at 30°C. However, in other studies, temperature variation resulted in only a small difference in test positivity that did not significantly alter the rates of adenoma detection.57
There are only a handful of studies that examine the performance of FIT in an environment with an ambient temperature similar to Nigeria. Chiang et al58 in 2011 evaluated 2,796 asymptomatic Taiwanese patients with FIT followed by colonoscopy. At a 50 μg/g cutoff, the rate of FIT positivity was 14.2% and the sensitivity and specificity for CRC detection were 96.4% and 86.6%, respectively. In Thailand, a CRC screening pilot program was launched using a qualitative FIT with a 40 μg/g cutoff. The results demonstrated a low positivity rate of just 1.1%, although the detections of CRC (2.6%) and adenomas (21.4%) as a proportion of the FIT-positive population were well within the range reported from high-incidence countries.59
Most LMICs are still experiencing a double burden of overlapping communicable and noncommunicable disease.4 In Nigeria, intestinal parasitic infection remains endemic.60-62 Studies from South America and SSA have demonstrated a correlation between gFOBT positivity and Schistosoma mansoni infection.63-65 However, two studies from Saudi Arabia and Nigeria demonstrated no difference in gFOBT result between positive and negative stool culture for ova and parasites.66,67 The link between GI occult blood loss and parasitic infection seems to be both pathogen and intensity dependent, and there is no literature specific to FIT performance as a CRC screening tool in the setting of endemic parasitic infection. Two fundamental questions that relate to FIT performance must be examined in the Nigerian context: (1) What is the impact of Nigeria’s high ambient temperature on FIT performance? and (2) Is FIT performance altered in the setting of endemic intestinal parasite infection?
Finally, participation and compliance are core components of effective screening.10 Studies of screening compliance and perceptions of stool-based screening are exclusively from HICs.68 The translatability of these results to the Nigerian or low-resource environment is not known. For instance, many households lack access to basic waste management systems. This may preclude stool capture uncontaminated by urine or other excrement and decrease the participation in endoscopic follow-up that requires bowel preparation. In Nigeria, even basic knowledge about CRC (eg, symptoms) and about the role screening plays in early diagnosis remains poor.69 A more nuanced and comprehensive understanding of the national and regional barriers to FIT-based screening is needed and should be built into future prospective trials.
TARGETED HIGH-RISK SCREENING IN NIGERIA
Given the limited resources and potentially variable disease incidence (eg, urban v rural), future prospective trials and perhaps initial pilot programs should consider targeting high-risk populations. A highly targeted approach addresses some of the logistic challenges that would preclude a more generalized approach in Nigeria at this time. The African Organization for Research and Training in Cancer endorses this approach.70 They recommend targeting individuals with a family history of CRC and suggest that any initial policy of organized screening should be stool based. Individuals with a first-degree relative with CRC are at twice the risk of developing the disease compared with individuals without a family history.71 Given the higher risk, colonoscopy has been the preferred screening modality for this population.72 However, there is growing evidence to support high-risk, stool-based CRC screening as both efficacious and cost-effective.
In a prospective, randomized trial of annual FIT versus one-time colonoscopy, for asymptomatic individuals with CRC in a first-degree relative, FIT had a sensitivity for CRC and advanced adenoma detection of 100% and 61%, respectively. This met a priori equivalency criteria.72 However, conflicting evidence about the use of FIT in high-risk individuals does exist. Evidence from Hong Kong with the use of a single FIT demonstrated a sensitivity of just 25% which resulted in three false-negative results.73 Targeted screening also potentially limits the disability-adjusted life years gained; however, evidence from both high- and middle-income countries suggests that such an approach can remain cost-effective.74,75
A multicenter, prospective trial conducted by the Asian-Pacific Working Group on CRC used a validated risk-scoring system that included positive family history, sex, age, and cigarette use to stratify asymptomatic patients into three tiers. Low- and medium-risk individuals received FIT-based screening, whereas high-risk individuals went straight to colonoscopy. This approach yielded a sensitivity for advanced neoplasia detection of 70.6% (95% CI, 65.6% to 75.1%) and reduced the number of colonoscopies needed to detect one invasive cancer by 57.8% (95% CI, 49.6% to 65.9%).76 A symptom-based predictive model for highly targeted CRC screening has already been developed in Nigeria. Among 362 patients with self-reported rectal bleeding, a symptom-based risk model accurately predicted 89% of CRC (OR, 12.8; 95% CI, 4.6 to 35.4). More than 70% of those individuals had resectable disease, which represented a dramatic stage migration for a CRC cohort in Nigeria.29 Risk stratification, even in traditionally high-risk subgroups (eg, those based on family history), may be required in the Nigerian context while endoscopy capacity remains limited.
ACCESS TO TREATMENT OF RECOGNIZED DISEASE
Screening for a disease is only effective if treatment is available once latent disease is identified. Treatment of early colorectal neoplasia can often be accomplished with endoscopy alone, whereas invasive disease can be effectively treated with high-quality surgery. For patients with more advanced disease, the addition of systemic chemotherapy and radiotherapy greatly improves recurrence-free and overall survival. However, the cost-effectiveness of different adjuvant therapies in a low-resource setting like Nigeria is unknown.49
Across the continent, access to multidisciplinary care for CRC, including endoscopy and radiotherapy, is limited, and even access to basic emergency surgery is not universal.12,41,77,78 According to GLOBOCAN data from East Africa in 2010, Ginsberg79 suggested that population-based CRC screening should not be prioritized in SSA, because treatment levels remain below 50% the most cost-effective intervention is expanding access to patients with known CRC. Ouma et al77 in 2018 reported that 70% of individuals in SSA and 92.3% in Nigeria are within 2 hours of a hospital capable of basic surgical care. However, facilities that offer emergency surgery do not necessarily provide cancer care. In the Federal Capital Territory, more than 80% of individuals can reach a hospital with surgical capacity within 2 hours; however, only 50% of those facilities regularly perform laparotomies, and only 40% were staffed by board-certified general surgeons.80 In more rural regions, the majority of surgical care is performed by general practitioners, and the capacity for elective cancer surgery that requires general anesthesia is quite limited.81 These data are derived from small, survey-based publications. There is currently no national capacity assessment for CRC diagnosis and management. The creation of resource-sensitive guidelines for CRC treatment, by the Society of Gastroenterology and Hepatology in Nigeria, facilitates the standardization of care and lays the foundation for capacity assessment and quality measurement. According to these guidelines, robust cost-effectiveness studies are needed with Nigerian data to guide diagnostic-and treatment-related investment and policy.
Fundamentally, treatment depends on services that are both physically accessible and affordable.82 Although Nigeria has a public health system, only 5% of the population is covered by some form of prepaid health insurance.83 This low coverage is associated with a high rate of catastrophic health expenditure for emergency surgery and the treatment of communicable disease.84,85 Less is known about the cost of cancer care in Nigeria. There is emerging evidence to suggest that screening behavior and cancer management are dictated by income.69,86 Without a robust health insurance mechanism (ie, public, private, or mixed), the efficacy of opportunistic and organized screening may be curtailed by inequitable access to diagnostic and treatment services.
The dearth of data on access to cancer care services in Nigeria poses a large barrier to the creation of meaningful screening policy. At a minimum, metrics for access in the Nigerian context must be proposed and quantified before effective, organized, and opportunistic CRC screening can be established. In most LMICs, access to safe and affordable treatment will need to expand concurrently with screening related research.
DISCUSSION
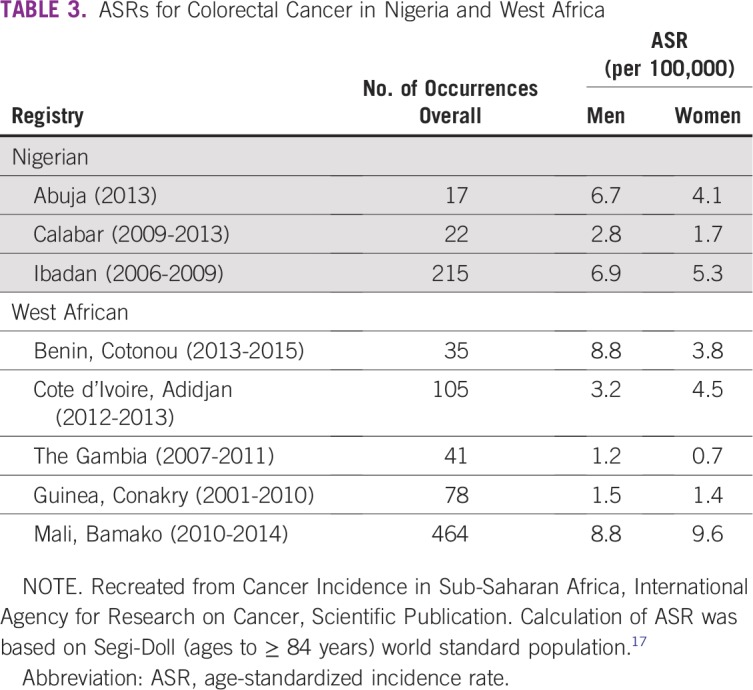
There is insufficient evidence to support a national, population-based CRC screening program in Nigeria at this time. However, the process to design and implement such a program can take decades.3 To move toward evidence-based CRC screening in Nigeria, several areas of research and public investment are required to address the deficits in the Wilson-Jungner framework identified in the literature (Table 4). First, greater emphasis must be placed on establishing, expanding, and maintaining high-quality, population-based cancer registries. In Nigeria, this process is already well underway with the creation of the Nigerian National System of Cancer Registries in 2009. These registries should strive to have their results vetted and included in the CI5 series. A thorough understanding of the incidence and prevalence of disease is essential when the utility of screening is being considered.
TABLE 4.
Action Required to Address the Deficits in the Wilson-Jungner Framework
There is uncertainty in the current literature about the molecular profile of CRC in Nigeria. Prospective databases with tissue banking and large prospective trials to examine the natural history of colorectal neoplasia in Nigeria are needed. The high rate of MSI in Nigerian CRC must be prospectively validated and further characterized. The implications for treatment and screening are consequential. Access to cancer care must be defined and measured in the Nigerian context. In particular, national endoscopy capacity must be quantified before screening recommendations and policy are drafted. Although certain quality measures, such as adequacy of bowel prep and incidence of perforation, are relevant, the predictive value of a specific adenoma detection rate (eg, 20% to 25% in HICs) in Nigeria has not been validated.87,88 Establishment of local criteria and mechanisms for endoscopic quality assessment now will facilitate quality assurance when organized screening is implemented—a vital component of overall program effectiveness.
There is a large body of evidence from HICs that stool-based CRC screening, and FIT in particular, is the preferred modality for population-based screening. Prospective studies are needed to validate the performance, feasibility, and cost-effectiveness of FIT in Nigeria, and these studies should consider concomitant ova and parasite testing. In the interim, for individuals who seek opportunistic screening, national societies and governing bodies should endorse a set of existing, resource-sensitive guidelines.
Nigeria lacks the infrastructure for population-based CRC cancer screening. Integration of screening into existing research and/or delivery platforms may initially offer a pragmatic solution. Highly targeted stool-based screening of individuals with a family history of CRC may be possible in a specific region with a tertiary care facility and a prospective CRC cancer database. Although not comprehensive, this strategy may be appropriate for pilot trials or large prospective studies that can build on the available infrastructure for program implementation, follow-up, and quality control. The development of risk scoring mechanisms, similar to the Asian-Pacific Colorectal Screening score, may further stratify those who would benefit the most from organized screening.
Ultimately, organized screening should be guided by evidence-based policy and pursued as a sustainable, iterative process. National societies and academic institutions responsible for the bulk of professional development, academic research, and service provision must be well represented in this process. The governance mechanism for policy creation and, eventually, program implementation has yet to be defined. This is an opportunity to evaluate how different strategies may affect the screening process and best align with the priorities of the health system.
The global burden of CRC will continue to increase for the foreseeable future. In LMICs, prevention and early detection will be increasingly relevant health policy issues. Using the Wilson-Jungner framework, the basic criteria for organized screening are not yet met in Nigeria. An opportunity now exists to address these deficits without missing the window to mitigate the growing burden of CRC in the medium to long term.
AUTHOR CONTRIBUTIONS
Conception and design: Gregory C. Knapp, Olusegun I. Alatise, Olalekan O. Olasehinde, Omobolaji O. Ayandipo, T. Peter Kingham
Collection and assembly of data: Gregory C. Knapp, Olusegun I. Alatise, Olalekan O. Olasehinde
Data analysis and interpretation: Gregory C. Knapp, Olusegun I. Alatise, Olalekan O. Olasehinde, Ademola Adeyeye, Omobolaji O. Ayandipo, Martin R. Weiser, T. Peter Kingham
Provision of study material or patients: Olusegun I. Alatise
Administrative support: Olusegun I. Alatise, Olalekan O. Olasehinde
Manuscript writing: All authors
Final approval of manuscript: All authors
Agree to be accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Martin R. Weiser
Research Funding: Clinical Genomics
T. Peter Kingham
Consulting or Advisory Role: Physician Education Resource
No other potential conflicts of interest were reported.
REFERENCES
- 1.Washington, DC: International Bank for Reconstruction and Development/The World Bank; 2015. Bray F, Soerjomataram I: (The changing global burden of cancer: Transitions in human development and implications for cancer prevention and control, in Gelband H, Jha P, Sankaranarayanan R, et al (eds): Cancer: Disease Control Priorities (ed 3) [Google Scholar]
- 2.Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008-2030): A population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 3.Karsa LV, Lignini TA, Patnick J, et al. The dimensions of the CRC problem. Best Pract Res Clin Gastroenterol. 2010;24:381–396. doi: 10.1016/j.bpg.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Lambert R, Sauvaget C, Sankaranarayanan R. Mass screening for colorectal cancer is not justified in most developing countries. Int J Cancer. 2009;125:253–256. doi: 10.1002/ijc.24371. [DOI] [PubMed] [Google Scholar]
- 5.Eguzo K, Camazine B. Beyond limitations: Practical strategies for improving cancer care in Nigeria. Asian Pac J Cancer Prev. 2013;14:3363–3368. doi: 10.7314/apjcp.2013.14.5.3363. [DOI] [PubMed] [Google Scholar]
- 6.Irabor DO. Emergence of colorectal cancer in West Africa: Accepting the inevitable. Niger Med J. 2017;58:87–91. doi: 10.4103/0300-1652.234076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham A, Adeloye D, Grant L, et al. Estimating the incidence of colorectal cancer in Sub-Saharan Africa: A systematic analysis. J Glob Health. 2012;2:020404. doi: 10.7189/jogh.02.020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishola F, Omole O. A vision for improved cancer screening in Nigeria. Lancet Glob Health. 2016;4:e359–e360. doi: 10.1016/S2214-109X(16)30062-6. [DOI] [PubMed] [Google Scholar]
- 9.Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. Geneva: WHO; 1968. [Google Scholar]
- 10.Sullivan T, Sullivan R, Ginsburg OM, editors. Washington, DC: International Bank for Reconstruction and Development / The World Bank; 2015. Screening for cancer: Considerations for low- and middle-income countries. in Gelband H, Jha P, Sankaranarayanan R, et al (eds): Cancer: Disease Control Priorities (ed 3), [PubMed] [Google Scholar]
- 11.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 12.Morhason-Bello IO, Odedina F, Rebbeck TR, et al. Challenges and opportunities in cancer control in Africa: A perspective from the African Organisation for Research and Training in Cancer. Lancet Oncol. 2013;14:e142–e151. doi: 10.1016/S1470-2045(12)70482-5. [DOI] [PubMed] [Google Scholar]
- 13. Bray F, Colombet M, Mery L, et al: Cancer Incidence in Five Continents: Vol. XI. Lyon, France, International Agency for Research on Cancer, 2017. [Google Scholar]
- 14.Doll R. Cancer in five continents. Proc R Soc Med. 1972;65:49–55. [PMC free article] [PubMed] [Google Scholar]
- 15.Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents: Vol. X. Lyon, France: International Agency for Research on Cancer, 2013. [Google Scholar]
- 16.Jedy-Agba EE, Curado MP, Oga E, et al. The role of hospital-based cancer registries in low- and middle-income countries: The Nigerian Case Study. Cancer Epidemiol. 2012;36:430–435. doi: 10.1016/j.canep.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkin DM, Ferlay J, Jemal A, et al. Cancer in Sub-Saharan Africa. Lyon, France, International Agency for Research on Cancer, Scientific Publication No. 167, 2018. [Google Scholar]
- 18.Saidi H, Nyaim EO, Githaiga JW, et al. CRC surgery trends in Kenya, 1993-2005. World J Surg. 2008;32:217–223. doi: 10.1007/s00268-007-9301-2. [DOI] [PubMed] [Google Scholar]
- 19.Chokunonga E, Borok MZ, Chirenje ZM, et al. Trends in the incidence of cancer in the black population of Harare, Zimbabwe, 1991-2010. Int J Cancer. 2013;133:721–729. doi: 10.1002/ijc.28063. [DOI] [PubMed] [Google Scholar]
- 20.Irabor D, Adedeji OA. Colorectal cancer in Nigeria: 40 years on—A review. Eur J Cancer Care (Engl) 2009;18:110–115. doi: 10.1111/j.1365-2354.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim KO, Anjorin AS, Afolayan AE, et al. Morphology of colorectal carcinoma among Nigerians: A 30-year review. Niger J Clin Pract. 2011;14:432–435. doi: 10.4103/1119-3077.91750. [DOI] [PubMed] [Google Scholar]
- 22.Irabor DO, Oluwasola OA, Ogunbiyi OJ, et al. Microsatellite instability is common in colorectal cancer in native Nigerians. Anticancer Res. 2017;37:2649–2654. doi: 10.21873/anticanres.11612. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham D, Atkin WF, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 24.Hardcastle JD, Chamberlain JO, Robinson M, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 25.Towler B, Irwig L, Glasziou P, et al. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ. 1998;317:559–565. doi: 10.1136/bmj.317.7158.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood: Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 27.Mabogunje OA, Subbuswamy SG, Lawrie JH. Rectal polyps in Zaria, Nigeria. Dis Colon Rectum. 1978;21:474–479. doi: 10.1007/BF02586730. [DOI] [PubMed] [Google Scholar]
- 28.Alatise OI, Arigbabu AO, Agbakwuru AE, et al. Polyp prevalence at colonoscopy among Nigerians: A prospective observational study. Niger J Clin Pract. 2014;17:756–762. doi: 10.4103/1119-3077.144391. [DOI] [PubMed] [Google Scholar]
- 29.Alatise OI, Ayandipo OO, Adeyeye A, et al. A symptom-based model to predict colorectal cancer in low-resource countries: Results from a prospective study of patients at high risk for colorectal cancer. Cancer. 2018;124:2766–2773. doi: 10.1002/cncr.31399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oluyemi A, Awolola N, Oyedeji O. Clinicopathologic review of polyps biopsied at colonoscopy in Lagos, Nigeria. Pan Afr Med J. 2016;24:333. doi: 10.11604/pamj.2016.24.333.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olokoba AB, Obateru OA, Bojuwoye MO, et al. Indications and findings at colonoscopy in Ilorin, Nigeria. Niger Med J. 2013;54:111–114. doi: 10.4103/0300-1652.110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ismaila BO, Misauno MA. Gastrointestinal endoscopy in Nigeria: A prospective two year audit. Pan Afr Med J. 2013;14:22. doi: 10.11604/pamj.2013.14.22.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim OK, Afolayan AE, Adeniji KA, et al. Colorectal carcinoma in children and young adults in Ilorin, Nigeria. West Afr J Med. 2011;30:202–205. [PubMed] [Google Scholar]
- 34.Adebamowo CA, Adeyi O, Pyatt R, et al. Case report on hereditary non-polyposis colon cancer (HNPCC) in Nigeria. Afr J Med Med Sci. 2000;29:71–73. [PubMed] [Google Scholar]
- 35.Duduyemi BM, Akang E, Adegboyega PA, et al. Significance of DNA mismatch repair genes and microsatellite instability in colorectal carcinoma in Ibadan, Nigeria. American Journal of Medical and Biological Research. 2013;1:145–148. [Google Scholar]
- 36.Abdulkareem FB, Sanni LA, Richman SS, et al. KRAS and BRAF mutations in Nigerian colorectal cancers. West Afr J Med. 2012;31:198–203. [PubMed] [Google Scholar]
- 37.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 38.Bénard F, Barkun AN, Martel M, et al. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J Gastroenterol. 2018;24:124–138. doi: 10.3748/wjg.v24.i1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navarro M, Nicolas A, Ferrandez A, et al. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol. 2017;23:3632–3642. doi: 10.3748/wjg.v23.i20.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arigbabu AO, Odesanmi WO. Colonoscopy: First experience in Nigeria. Dis Colon Rectum. 1985;28:728–731. doi: 10.1007/BF02560287. [DOI] [PubMed] [Google Scholar]
- 41.Hassan C, Aabakken L, Ebigbo A, et al. Partnership with African countries: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endosc Int Open. 2018;6:E1247–E1255. doi: 10.1055/a-0677-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obonna G, Arowolo A, Agbakwuru A. Experience with colonoscopy in the riverine southwestern Nigeria. J West Afr Coll Surg. 2012;2:80–90. [PubMed] [Google Scholar]
- 43.Robertson D. J., Lee J. K., Boland C. R., et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: A consensus statement by the US multi-society task force on colorectal cancer. Gastroenterology. 2017;152:1217–1237.e3. doi: 10.1053/j.gastro.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 44.van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135:82–90. doi: 10.1053/j.gastro.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Sharp L, Tilson L, Whyte S, et al. Cost-effectiveness of population-based screening for colorectal cancer: A comparison of guaiac-based faecal occult blood testing, faecal immunochemical testing and flexible sigmoidoscopy. Br J Cancer. 2012;106:805–816. doi: 10.1038/bjc.2011.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JK, Liles EG, Bent S, et al. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann Intern Med. 2014;160:171–xxxx. doi: 10.7326/M13-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenner H, Werner S. Selecting a cut-off for colorectal cancer screening with a fecal immunochemical test. Clin Transl Gastroenterol. 2017;8:e111. doi: 10.1038/ctg.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winawer SJ, Krabshuis J, Lambert R, et al. Cascade colorectal cancer screening guidelines: A global conceptual model. J Clin Gastroenterol. 2011;45:297–300. doi: 10.1097/MCG.0b013e3182098e07. [DOI] [PubMed] [Google Scholar]
- 49.Lopes G, Stern MC, Temin S, et al. Early detection for colorectal cancer: ASCO resource-stratified guideline. J Glob Oncol. 2019;5:1–22. doi: 10.1200/JGO.18.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salimzadeh H, Bishehsari F, Sauvaget C, et al. Feasibility of colon cancer screening by fecal immunochemical test in Iran. Arch Iran Med. 2017;20:726–733. [PubMed] [Google Scholar]
- 51.Washington, DC: International Bank for Reconstruction and Development / The World Bank; 2015. Rabeneck L, Horton S, Zauber AG, et al: Colorectal cancer, in Gelband H, Jha P, Sankaranarayanan R, et al (eds): Cancer: Disease Control Priorities (ed 3). [Google Scholar]
- 52.López-Kostner F, Zárate AJ, Ponce A, et al. Results of a multicentric colorectal cancer screening program in Chile [in Spanish] Rev Med Chil. 2018;146:685–692. doi: 10.4067/s0034-98872018000600685. [DOI] [PubMed] [Google Scholar]
- 53.Daly JM, Xu Y, Levy BT. Which fecal immunochemical test should I choose? J Prim Care Community Health. 2017;8:264–277. doi: 10.1177/2150131917705206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Symonds EL, Osborne JM, Cole SR, et al. Factors affecting faecal immunochemical test positive rates: Demographic, pathological, behavioural and environmental variables. J Med Screen. 2015;22:187–193. doi: 10.1177/0969141315584783. [DOI] [PubMed] [Google Scholar]
- 55.Grazzini G, Ventura L, Zappa M, et al. Influence of seasonal variations in ambient temperatures on performance of immunochemical faecal occult blood test for colorectal cancer screening: Observational study from the Florence district. Gut. 2010;59:1511–1515. doi: 10.1136/gut.2009.200873. [DOI] [PubMed] [Google Scholar]
- 56.van Roon A. H., Hol L, van Vuuren A. J., et al. Are fecal immunochemical test characteristics influenced by sample return time? A population-based colorectal cancer screening trial. Am J Gastroenterol. 2012;107:99–107. doi: 10.1038/ajg.2011.396. [DOI] [PubMed] [Google Scholar]
- 57.Cha JM, Lee J.IJoo KR, et al. Performance of the fecal immunochemical test is not decreased by high ambient temperature in the rapid return system Dig Dis Sci 572178–2183.2012 [DOI] [PubMed] [Google Scholar]
- 58.Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183:1474–1481. doi: 10.1503/cmaj.101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khuhaprema T, Sangrajrang S, Lalitwongsa S, et al. Organised colorectal cancer screening in Lampang Province, Thailand: Preliminary results from a pilot implementation programme. BMJ Open. 2014;4:e003671. doi: 10.1136/bmjopen-2013-003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Efunshile AM, Olawale T, Stensvold CR, et al. Epidemiological study of the association between malaria and helminth infections in Nigeria. Am J Trop Med Hyg. 2015;92:578–582. doi: 10.4269/ajtmh.14-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adeoye GO, Osayemi CO, Oteniya O, et al. Epidemiological studies of intestinal helminthes and malaria among children in Lagos, Nigeria. Pak J Biol Sci. 2007;10:2208–2212. doi: 10.3923/pjbs.2007.2208.2212. [DOI] [PubMed] [Google Scholar]
- 62.Akinbo FO, Omoregie R, Eromwon R, et al. Prevalence of intestinal parasites among patients of a tertiary hospital in Benin city, Nigeria. N Am J Med Sci. 2011;3:462–464. doi: 10.4297/najms.2011.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Betson M, Sousa-Figueiredo JC, Rowell C, et al. Intestinal schistosomiasis in mothers and young children in Uganda: Investigation of field-applicable markers of bowel morbidity. Am J Trop Med Hyg. 2010;83:1048–1055. doi: 10.4269/ajtmh.2010.10-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lehman JS, Jr, Mott KE, Morrow RH, Jr, et al. The intensity and effects of infection with Schistosoma mansoni in a rural community in northeast Brazil. Am J Trop Med Hyg. 1976;25:285–294. doi: 10.4269/ajtmh.1976.25.285. [DOI] [PubMed] [Google Scholar]
- 65.Sousa-Figueiredo JC, Basáñez MG, Mgeni AF, et al. A parasitological survey, in rural Zanzibar, of pre-school children and their mothers for urinary schistosomiasis, soil-transmitted helminthiases and malaria, with observations on the prevalence of anaemia. Ann Trop Med Parasitol. 2008;102:679–692. doi: 10.1179/136485908X337607. [DOI] [PubMed] [Google Scholar]
- 66.Wakid MH. Fecal occult blood test and gastrointestinal parasitic infection. J Parasitol Res. 2010;2010:434801. doi: 10.1155/2010/434801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ugwuoke H, Okonkwo B, Oshilonyah H, et al. Faecal occult blood and intestinal parasites among patients attending outpatient clinic in Agbor, Delta State Nigeria. Archives of Biomedical Sciences and Health. 2013;1:20–28. [Google Scholar]
- 68.Pham R, Cross S, Fernandez B, et al. “Finding the right FIT”: Rural patient preferences for fecal immunochemical test (FIT) characteristics. J Am Board Fam Med. 2017;30:632–644. doi: 10.3122/jabfm.2017.05.170151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alatise OI, Fischer SE, Ayandipo OO, et al. Health-seeking behavior and barriers to care in patients with rectal bleeding in Nigeria. J Glob Oncol. 2017;3:749–756. doi: 10.1200/JGO.2016.006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laiyemo A. O., Brawley O, Irabor D, et al. Toward colorectal cancer control in Africa. Int J Cancer. 2016;138:1033–1034. doi: 10.1002/ijc.29843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor D. P., Burt R. W., Williams M. S., et al. Population-based family history-specific risks for colorectal cancer: A constellation approach. Gastroenterology. 2010;138:877–885. doi: 10.1053/j.gastro.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quintero E, Carrillo M, Gimeno-García AZ, et al. Equivalency of fecal immunochemical tests and colonoscopy in familial colorectal cancer screening. Gastroenterology. 2014;147:1021–1030.e1. doi: 10.1053/j.gastro.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Ng SC, Ching JY, Chan V, et al. Diagnostic accuracy of faecal immunochemical test for screening individuals with a family history of colorectal cancer. Aliment Pharmacol Ther. 2013;38:835–841. doi: 10.1111/apt.12446. [DOI] [PubMed] [Google Scholar]
- 74.Ramsey SD, Wilschut J, Boer R, et al. A decision-analytic evaluation of the cost-effectiveness of family history-based colorectal cancer screening programs. Am J Gastroenterol. 2010;105:1861–1869. doi: 10.1038/ajg.2010.185. [DOI] [PubMed] [Google Scholar]
- 75.Javadinasab H, Daroudi R, Salimzadeh H, et al. Cost-effectiveness of screening colonoscopy in Iranian high-risk population. Arch Iran Med. 2017;20:564–571. [PubMed] [Google Scholar]
- 76.Chiu HM, Ching JY, Wu KC, et al. A risk-scoring system combined with a fecal immunochemical test is effective in screening high-risk subjects for early colonoscopy to detect advanced colorectal neoplasms. Gastroenterology. 2016;150:617–625.e3. doi: 10.1053/j.gastro.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 77.Ouma PO, Maina J, Thuranira PN, et al. Access to emergency hospital care provided by the public sector in sub-Saharan Africa in 2015: A geocoded inventory and spatial analysis. Lancet Glob Health. 2018;6:e342–e350. doi: 10.1016/S2214-109X(17)30488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Washington, DC: International Bank for Reconstruction and Development / The World Bank; 2015. Dare AJ, Anderson BO, Sullivan R, et al: Surgical services for cancer care, in Gelband H, Jha P, Sankaranarayanan R, et al (eds): Cancer: Disease Control Priorities (ed 3) [Google Scholar]
- 79.Ginsberg GM, Lim SS, Lauer JA, et al. Prevention, screening and treatment of colorectal cancer: A global and regional generalized cost effectiveness analysis. Cost Eff Resour Alloc. 2010;8:2. doi: 10.1186/1478-7547-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson JE, Ndajiwo AB, Nuhu SA, et al. Assessment of capacity to meet Lancet Commission on Global Surgery indicators in the federal capital territory, Abuja, Nigeria. World J Surg. 2019;43:704–714. doi: 10.1007/s00268-018-4835-z. [DOI] [PubMed] [Google Scholar]
- 81.Henry JA, Windapo O, Kushner AL, et al. A survey of surgical capacity in rural southern Nigeria: Opportunities for change. World J Surg. 2012;36:2811–2818. doi: 10.1007/s00268-012-1764-0. [DOI] [PubMed] [Google Scholar]
- 82.Gulliford M, Figueroa-Munoz J, Morgan M, et al. What does ‘access to health care’ mean? J Health Serv Res Policy. 2002;7:186–188. doi: 10.1258/135581902760082517. [DOI] [PubMed] [Google Scholar]
- 83.Onwujekwe O, Hanson K, Uzochukwu B. Examining inequities in incidence of catastrophic health expenditures on different health care services and health facilities in Nigeria. PLoS One. 2012;7:e40811. doi: 10.1371/journal.pone.0040811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ukwaja KN, Alobu I, Abimbola S, et al. Household catastrophic payments for tuberculosis care in Nigeria: Incidence, determinants, and policy implications for universal health coverage. Infect Dis Poverty. 2013;2:21. doi: 10.1186/2049-9957-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Urua U, Osungbade K, Obembe T, et al. A cost analysis of road traffic injuries in a tertiary hospital in south-west Nigeria. Int J Inj Contr Saf Promot. 2017;24:510–518. doi: 10.1080/17457300.2016.1278238. [DOI] [PubMed] [Google Scholar]
- 86.Okoronkwo IL, Ejike-Okoye P, Chinweuba AU, et al. Financial barriers to utilization of screening and treatment services for breast cancer: An equity analysis in Nigeria. Niger J Clin Pract. 2015;18:287–291. doi: 10.4103/1119-3077.151070. [DOI] [PubMed] [Google Scholar]
- 87.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72–90. doi: 10.1038/ajg.2014.385. [DOI] [PubMed] [Google Scholar]
- 88.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]