Abstract
PURPOSE
We evaluated the clinical features and outcomes of invasive breast cancer (BC) among different age groups by analyzing a modern BC registry including subtypes and treatment information.
METHODS
This was a retrospective cohort study of 6,405 women aged 18 years or older with pathologically confirmed stage I, II, or III BC who underwent curative surgery followed by adjuvant therapy at a university-based hospital in Seoul, South Korea, between January 2003 and December 2011. The study end point was all-cause mortality. We used Cox proportional hazards models and hazard ratios (HRs) with 95% CIs calculated after adjusting for age, body mass index, stage, subtype, and treatment, including type of surgery and use of chemotherapy, radiation therapy, hormone therapy, and targeted therapy.
RESULTS
During 36,360 person-years of follow-up (median follow-up: 5.45 years; interquartile range, 4.3-7.1), 256 deaths were reported (mortality rate, 7.0/1,000 person-years). The adjusted HR for all-cause mortality was higher in patients older than 40 years (HR, 2.03; 95% CI, 1.44 to 2.87) and older than 60 years (HR, 2.35; 95% CI, 1.63 to 3.39) than in patients aged 40 to 49 years. Across age groups, advanced stage at diagnosis, luminal type as well as triple-negative BC, and not receiving adjuvant treatment were associated with increased risk of mortality.
CONCLUSION
A strong J-shaped relationship was observed between age and mortality, indicating worse clinical outcomes in young and old patients. This study suggested a possible benefit of personalized BC screening examination and precise and active treatment strategies to reduce BC-related mortality.
INTRODUCTION
Breast cancer (BC) is the most common type of cancer in women worldwide, with approximately 1,670,000 new cases globally.1 Although 90% of patients with BC survive for over 5 years, up to 10% of patients experience disease recurrence and die of disease progression after curative surgery.2,3 TNM stage, tumor grade, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status are major predictive markers for recurrence4; they have been incorporated into management guidelines and are used to personalize treatment regimens with targeted agents. Moreover, age at diagnosis,5 alcohol consumption,6 smoking,7 and obesity8 have been associated with prognosis in some studies, but young age at diagnosis was consistently associated with poor prognosis.9,10 Most studies of clinical outcomes and prognostic factors of BC have been conducted in Western countries before the introduction of modern tumor subtyping and targeted treatments. Moreover, the results from Western studies may not be applicable to Asian women, because of the major differences in clinical characteristics of BC in these women.
BC is the second most common cancer in Korea after thyroid cancer (crude incidence rate, 72.1 per 100,000 people in 2014).11 Up to 50% of Korean patients with BC receive the diagnosis before 50 years of age, and most Korean women with BC are premenopausal, one of the risk factors for disease progression and poor clinical outcomes.9 Some studies have investigated the clinical characteristics and outcomes of Korean patients with BC by age; however, these studies only evaluated a specific age group—either the very young (< 40 years) or very old (> 70 years).12,13 In addition, like previous studies in Western countries, they had limited information about subtype and detailed treatments, including hormone or targeted therapy.13-16 In this study, we aimed to evaluate the clinical features and outcomes of invasive BC among different age groups by analyzing a large hospital-based BC registry including information on modern subtyping and detailed treatment. We specifically hypothesized that the clinical features and outcomes would be different depending on the patient’s age at diagnosis.
METHODS
Study Population
This was a retrospective cohort study of women at least 18 years old with pathologically confirmed stage I, II, or III BC (N = 6,692) who underwent curative surgery followed by adjuvant systemic therapy at Samsung Medical Center, Seoul, Korea, between January 2003 and December 2011. We excluded patients who had missing information on menopausal status (n = 115) or subtype (ER, n = 10; PR, n = 9; and HER2, n = 181). Because study participants could have more than one exclusion criterion, the final sample size was 6,405. The Institutional Review Board of the Samsung Medical Center approved this study and waived the requirement for informed consent because we used only deidentified data routinely collected during clinical care.
Measurements
Detailed information on surgery, adjuvant chemotherapy, radiotherapy, hormone therapy, and targeted therapy were obtained from electronic medical records. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared at the time of BC diagnosis. Women were considered postmenopausal if they were amenorrheic for at least 12 months, had a prior bilateral oophorectomy, or were age 60 years or older.4
Pathologic stage was based on the criteria of the American Joint Committee on Cancer.17 Two pathologists with 13 and 17 years of experience, respectively, reviewed and determined the primary tumor characteristics on the basis of size, axillary nodal status, resection margin, and receptor status (ER, PR, and HER2) by immunohistochemical staining. ER positivity and PR positivity were defined as an Allred score of 3 to 8, on the basis of immunohistochemical staining with antibodies against ER (Immunotech, France) and PR (Novocastra, UK), respectively. HER2 status was evaluated using the appropriate antibody (Dako, Carpinteria, CA) and/or silver in situ hybridization. HER2 grades 0 and 1 indicated a negative result, and grade 3 indicated a positive result. Amplification of HER2 was confirmed by silver in situ hybridization for results of 2+. Triple negative BC (TNBC) was defined as BC with negative ER expression, PR expression, and HER2 overexpression.
The study end point was all-cause mortality. The secondary end point was distant recurrence, including soft tissue or nodal metastases in distant sites, bone metastases, visceral metastases in other organs, and diffuse intra-abdominal metastases.
Statistical Analysis
For all-cause mortality, patients were included in the study at the time of diagnosis and were followed up until death or the end of the study period (May 31, 2016). To determine the clinical features associated with mortality, we used Cox proportional hazards models and hazard ratios (HRs) with 95% CIs calculated after adjusting for age, BMI, stage, subtype, and treatment, including type of surgery and use of chemotherapy, radiation therapy, hormone therapy, and targeted therapy. With regard to distant recurrence, patients were followed until the first recorded evidence of treatment failure or until the last follow-up visit for those alive without recurrence. To account for competing risks due to mortality, we fitted a proportional subdistribution hazards regression model using the Fine and Gray regression model18 with death as the competing event. All reported P values were two sided, and the significance level was set at 0.05. Statistical analyses were performed using STATA, version 14 (StataCorp LP, College Station, TX).
RESULTS
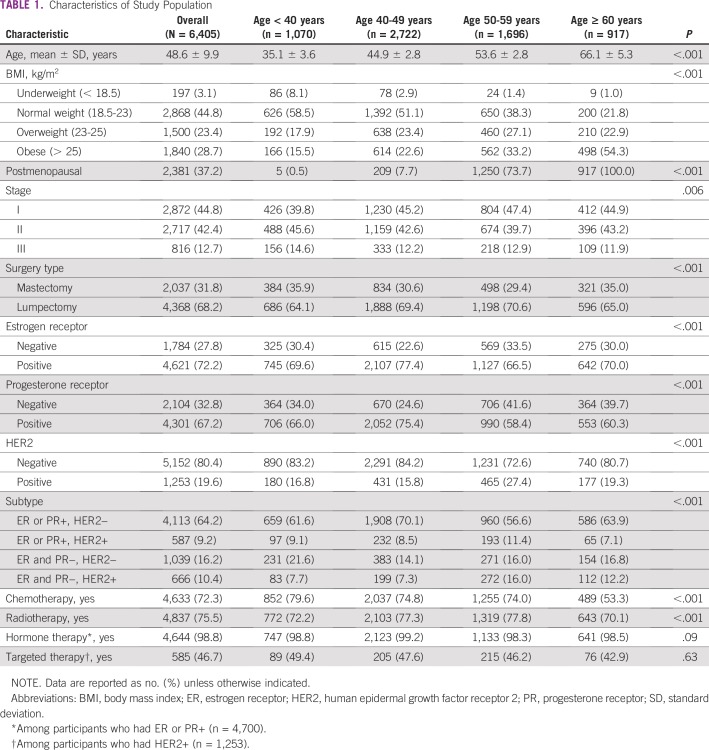
The median age at BC diagnosis was 48.6 (interquartile range, 42-54) years; approximately 16.7%, 42.5%, 26.5%, and 14.3% of women were younger than 40 years old, 40 to 49 years old, 50 to 59 years old, and older than 60 years, respectively; and 37.2% of patients were postmenopausal (Table 1). Altogether, 12.7% of patients were diagnosed with stage III BC, and those younger than 40 years were more likely to be diagnosed with stage III (14.6%) than were older patients (P < .001). The proportion of BC subtype was significantly different by age groups: ER+ or PR+ BC and HER2− BC commonly occurred in women in the 40 to 49 years age group (70.1%), whereas ER−, PR−, and HER2− BC (ie, TNBC) commonly occurred in women younger than 40 years (21.6%) (Table 1). In terms of treatment, 72.3%, 75.5%, 98.8%, and 46.7% of patients received chemotherapy, radiation therapy, hormone therapy, and targeted therapy, respectively; and patients older than 60 years were less likely to receive chemotherapy or radiotherapy (Table 1).
TABLE 1.
Characteristics of Study Population
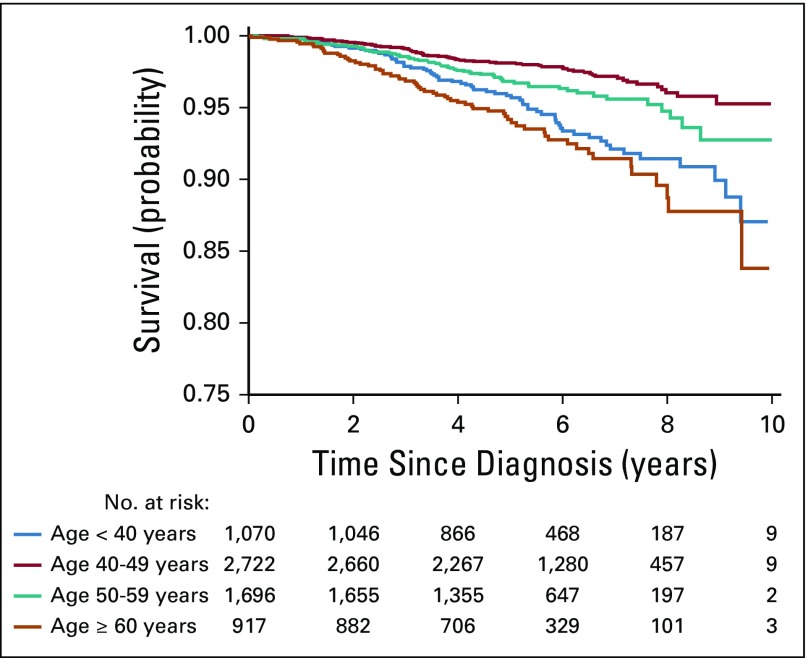
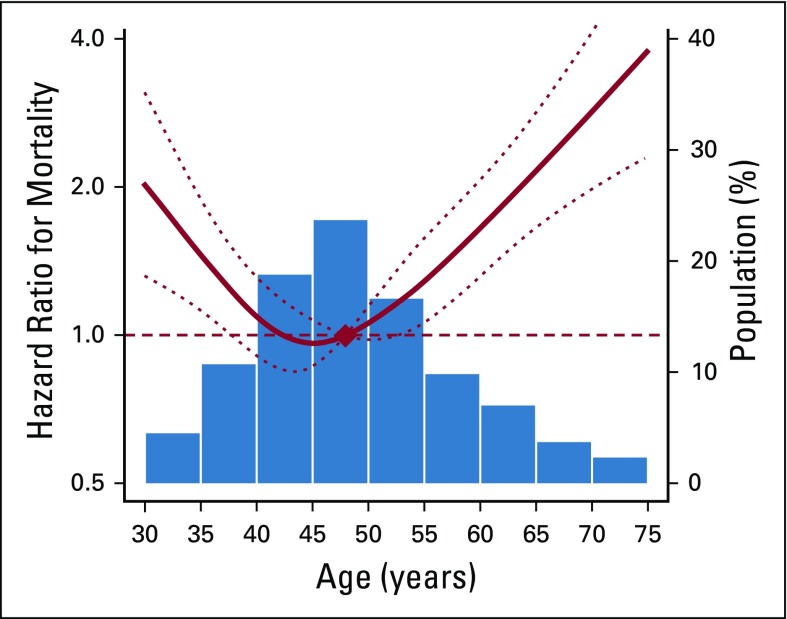
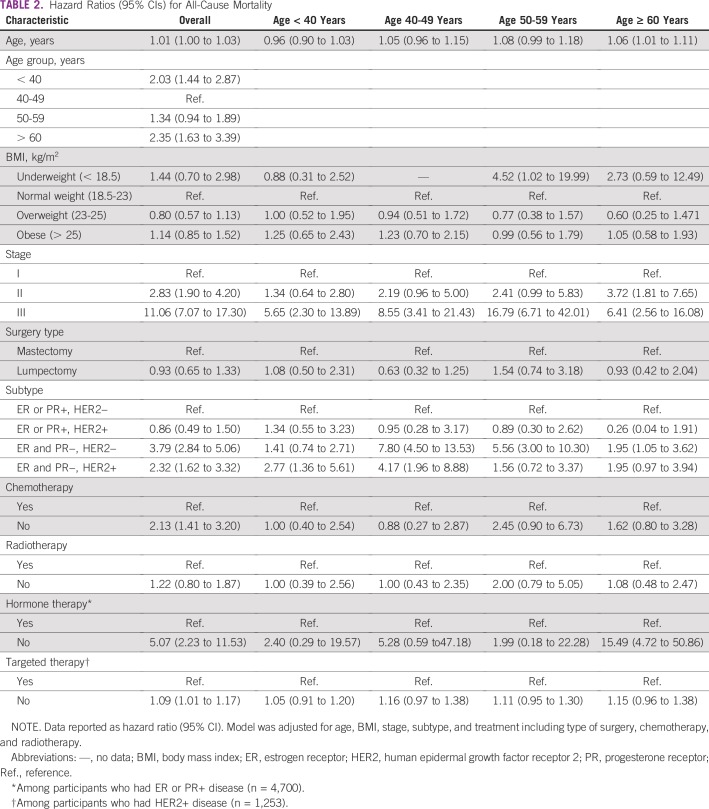
After 36,360 person-years of follow-up (median follow-up: 5.45 years; interquartile range, 4.3-7.1), 256 deaths were reported (mortality rate, 7.0 per 1,000 person-years). In the entire follow-up, the cumulative probability of overall survival was consistently lower in participants younger than 40 years and older than 60 years compared with those 40 to 49 years old (Fig 1). In spline regression models, there was a strong J-shaped association between age and mortality (Fig 2). After adjusting for BMI, stage, and treatment including surgery, chemotherapy, and radiotherapy, the HR for all-cause mortality was significantly higher in patients younger than 40 years (HR, 2.03; 95% CI, 1.44 to 2.87) and in patients older than 60 years (HR, 2.35; 95% CI, 1.63 to 3.39) than in those 40 to 49 years old (Table 2).
FIG 1.
Cumulative probability of surviving according to age.
FIG 2.
Hazard ratios for overall mortality by age. Curves represent adjusted hazard ratios (solid lines) and their 95% CIs (dotted lines) on the basis of restricted cubic splines for age with knots at the fifth, 35th, 65th, and 95th percentiles (34, 44, 51, and, 67 years, respectively) of their sample distributions. The reference values (diamond dot) were set at the 50th percentile (47 years). Adjusted for age, body mass index, stage, and treatment, including surgery, chemotherapy, and radiotherapy.
TABLE 2.
Hazard Ratios (95% CIs) for All-Cause Mortality
Obesity was not associated with increased mortality, but underweight was significantly associated with mortality in patients 50 to 59 years old (HR, 4.52; 95% CI, 1.02 to 19.99). Although underweight also increased risk of mortality in patients older than 60 years (HR, 2.73; 95% CI, 0.59 to 12.49), it was not a statistically significant factor.
Multivariable-adjusted HRs were significantly higher in patients with stage II (HR, 2.83; 95% CI, 1.90 to 4.20) and stage III (HR, 11.06; 95% CI, 7.07 to 17.30) BC than in those with stage I. With regard to subtype, multivariable-adjusted HRs were higher in patients with TBNC (HR, 3.79; 95% CI, 2.84 to 5.06) and ER−, PR−, and HER2+ BC (HR, 2.32; 95% CI, 1.62 to 3.32) than in those with ER+ or PR+ and HER2− BC. The positive association among stage, TNBC, and mortality was consistent in all age groups. Patients who did not receive chemotherapy (HR, 2.13; 95% CI, 1.41 to 3.20), hormone therapy (HR, 5.07; 95% CI, 2.23 to 11.53), and targeted therapy (HR, 1.09; 95% CI, 1.01 to 1.17) were more likely to die than those who received each specific therapy. Especially, among patients older than 60 years, those who did not receive hormone therapy had a 15-times higher risk of dying compared with patients receiving hormone therapy (HR, 15.49; 95% CI, 4.72 to 50.86; Table 2).
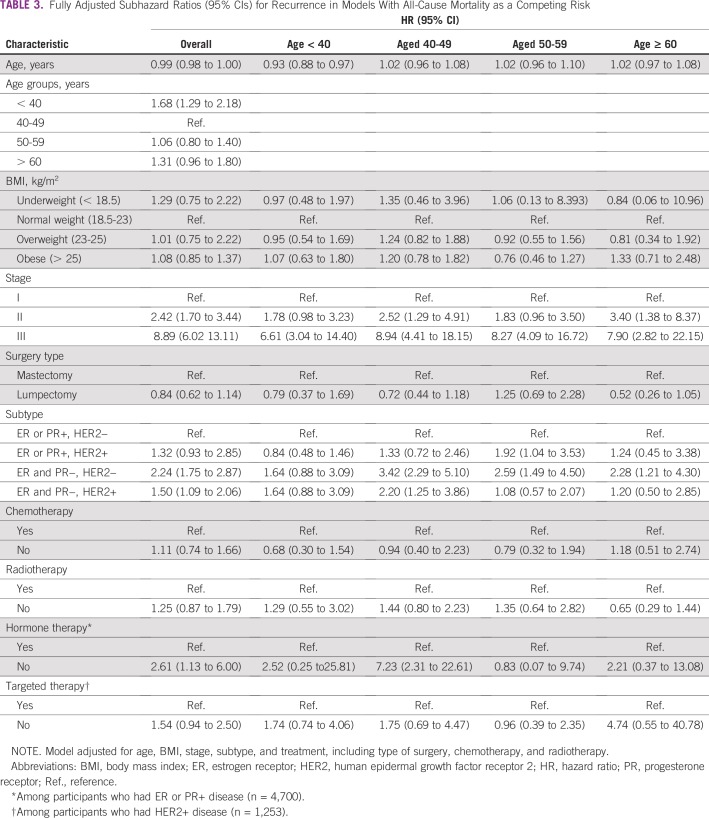
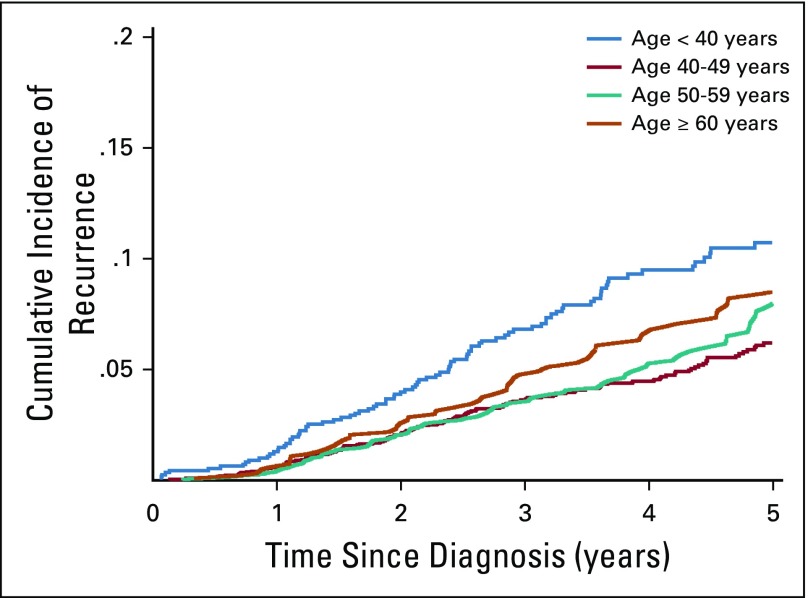
The cumulative distant recurrence rates were consistently higher in participants younger than 40 years (HR, 1.68; 95% CI, 1.29 to 2.18) compared with those 40 to 49 years old (Table 3; Fig 3). In multivariable models with mortality as a competing risk, advanced stage was associated with recurrence in all age groups. Patients with TNBC (HR, 2.24; 95% CI, 1.75 to 2.87) and ER−, PR−, and HER2+ BC (HR, 1.50; 95% CI, 1.09 to 2.06) had higher risk of distant recurrence than those with ER+ or PR+ BC and HER2− BC. Moreover, patients who did not receive hormone therapy had a higher risk of distant recurrence (HR, 2.61; 95% CI, 1.13 to 6.00) than those who received hormone therapy.
TABLE 3.
Fully Adjusted Subhazard Ratios (95% CIs) for Recurrence in Models With All-Cause Mortality as a Competing Risk
FIG 3.
Cumulative incidence function of recurrence according to age.
DISCUSSION
By analyzing this modern BC registry, we found a strong J-shaped association between age and mortality. The HR for all-cause mortality was significantly higher in patients younger than 40 years and older than 60 years than those 40 to 49 years old, after adjusting for all other risk factors. Besides age group, advanced stage at diagnosis, luminal type including TNBC, and not receiving chemotherapy, hormone therapy, and targeted therapy were associated with all-cause mortality. Different clinical features were associated with increased risk of all-cause mortality and distant recurrence, depending on age groups.
In all age groups, advanced cancer stage was significantly associated with all-cause mortality and distant recurrence, emphasizing the importance of BC screening and early detection. There was clear evidence that regular screening can help detect early-stage cancer19 and screening examination provides a significant decrease (20% to 35%) in BC mortality.20 In our study, patients younger than 40 years were more likely to be diagnosed with stage III BC than were older patients, resulting in relatively higher mortality. In Korea, like most other countries, BC screening is provided to women older than 40 years.21,22 Therefore, women younger than 40 years would have less chance of undergoing BC screening such as mammography or breast ultrasonography than would older patients who were recommended for routine screening. In fact, many women at an average risk of BC are offered relatively little opportunity to discuss and initiate mammographic screening before the age of 50 years.23 Although regular screening or clinical examination of women younger than 40 years remains controversial, personalized approaches to BC screening have been discussed. Now a clinical trial in the United States is examining efficacy of screening patients at an earlier age, regular performance of mammograms, and continuation of screening until women are older, on the basis of a model including personal history, family history, and genetic testing.24 It would be necessary to conduct similar trials with Asian women, considering that their clinical features are different from those of Western women. In our study, the proportion of patients with stage I BC who were older than 60 years was relatively small, which might be associated with the higher mortality observed in individuals that age group. Previous studies found that patients 60 to 69 years old were 0.61 times less likely to undergo screening mammography than those 40 to 49 years old.25 Although we did not have screening information, the lower proportion of patients with stage I BC who were older than 60 years might be due to the lower screening rate. Older women should be encouraged to undergo regular screening for the early detection of cancer, which would facilitate early treatment and improvement of prognosis.
Beside advanced stage at diagnosis, young patients with BC had worse clinical outcomes, partly because of the over-representation of more aggressive subtypes such as TNBC or HER2+ BC.26 In fact, in our study, higher proportions of women with stage II and III BC and TNBC were younger than 40 years. Considering that patients with TNBC who were from other age groups had a higher mortality rate than those with other subtypes, patients younger than 40 years might have poor prognosis regardless of the subtype. This finding was also consistent with the results of the previous studies. A cohort study of patients with newly diagnosed BC from one of eight National Comprehensive Cancer Network centers in the United States found that progression of TNBC did not differ according to age group. However, patients with luminal BC who were younger than 40 years had higher risk of BC-specific mortality than patients in other age groups.27 Another study, using the Norwegian cancer registry, found that patients younger than 40 years and older patients (70 to 89 years) had higher BC-specific mortality than those 50 to 59 years old regardless of subtype.28 They also found that the influence of age was commonly observed in patients with luminal subtype.28 In our study, among patients younger than 40 years, those with TNBC had a 1.41-times higher risk of mortality than patients with ER+ or PR+ and HER2− BC, but this association was not statistically significant. In other age groups, the mortality rate among patients with TNBC was two-fold higher than among those with luminal type. Accordingly, we expected that patients with ER+ or PR+ and HER2− BC who were younger than 40 years had a relatively poor prognosis. Therefore, young patients with ER+ or PR+ and HER2− BC need more delicate therapeutic strategies to reduce disease recurrence and mortality. Furthermore, a recent genomic study showed that younger patients expressed ER at lower levels along with weaker expression signature for ER signaling, suggesting that their tumors were less dependent on estrogen signaling.29 The TAILORx (Trial Assigning Individualized Options for Treatment) clinical trial also suggested that younger premenopausal women with hormone receptor–positive BC, defined as ER+ or PR+ and HER2– BC, BC benefitted more from adjuvant chemotherapy and had higher disease-free survival rates than did women older than 50 years.30 Moreover, premenopausal women whose estrogen level did not significantly reduce after receiving endocrine treatment had higher BC-specific mortality.31 Therefore, delicate endocrine therapy and other systemic treatments for younger patients can improve survival.
In our study, patients who did not receive adjuvant treatment had worse progression. Patients who did not receive chemotherapy had approximately a two-times higher risk of mortality than patients who received chemotherapy, and patients who did not receive hormone therapy had a five-fold higher risk of mortality than patients who did receive this hormone therapy. Among the patients with HER2+ BC, those who did not receive targeted therapy had worse progression than those who did. These findings are consistent with those of previous studies. Many studies already demonstrated that adjuvant treatment is effective in improving survival.32,33 In our study, a substantially less proportion of patients older than 60 years than younger patients received chemo- and radiation therapy, and patients who did not receive hormone therapy had a 15-times higher risk of mortality than patients who did. Traditionally, the cutoff age of 65 years was considered to define elderly patients, who are considered vulnerable to standard adjuvant chemotherapy. According to a previous study, physicians were less likely to treat elderly women with adjuvant therapy after curative surgery,34 and older patients might be less likely to receive adjuvant therapy because they do not feel the necessity of additional treatment35 or because they have concerns about potential adverse effects.36 In fact, treatment-related symptoms are also a frequent reason for discontinuing therapy (20%).37 Older patients might be less likely to receive adjuvant therapy considering the potential adverse effects, resulting in poor progression. However, age should not be used as the sole criterion in deciding a therapeutic regimen for patients with BC.4 Instead, the estimated absolute benefit, life expectancy, tolerance, and performance of each patient should be considered. Meanwhile, all therapeutic regimens may need to be adapted for the elderly patients to minimize toxicity and achieve favorable long-term outcomes.5 Moreover, patients need to be informed about possible benefits of adjuvant therapy and hormonal therapies. Provision of education and supportive care would also be meaningful for elderly patients to comply with planned therapy.
The current study had some limitations. First, behavioral risk factors associated with outcomes such as smoking, drinking, and exercise were not evaluated. Second, although we had relatively larger sample size compared with those of previous studies, we still had limited power to evaluate the risk factors in each age group. Third, because the registry was from a single institution in Seoul, Korea, the characteristics of study patients would be different from those at other institutions or other countries. Hence, the results of our study might not be generalizable to other patients with cancer in other settings. Despite these limitations, this is the first comprehensive modern registry to include all subtypes and treatment information, presenting valuable information on the clinical characteristics and outcomes of patients with BC by age groups.
Altogether, we confirmed a strong J-shaped association between age and mortality, presenting worse clinical outcomes of young and old patients. We also confirmed the impact of stage at diagnosis, subtype, and adjuvant treatment on outcomes of BC regardless of age. This study suggested a possible benefit of personalized BC screening examination and precise and active treatment strategies to reduce BC mortality. Yet, considering that the BC epidemiology in Korea differs from that in Western countries,2,38,39 additional multicenter studies are warranted to develop a more precise treatment guideline by age group.
Footnotes
Supported by the National Research Foundation of Korea (Grants No. NRF-2018R1A2B6004690 and NRF-2017R1D1A1B03028446); the Ministry of Health and Welfare, Republic of Korea (Grant No. HA17C0055); and the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (Grant No. 1720150).
AUTHOR CONTRIBUTIONS
Conception and design: Seok Jin Nam, Eliseo Guallar, Juhee Cho, Yeon Hee Park
Administrative support: Juhee Cho
Provision of study material or patients: Ji-Yeon Kim, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Jong Han Yu, Se Kyung Lee, Young-Hyuck Im, Jin Seok Ahn, Yeon Hee Park
Collection and assembly of data: Ji-Yeon Kim, Danbee Kang, Juhee Cho, Yeon Hee Park
Data analysis and interpretation: Ji-Yeon Kim, Danbee Kang, Eliseo Guallar, Juhee Cho, Yeon Hee Park
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Jong Han Yu
Research Funding: Alvogen Korea (Inst)
Jin Seok Ahn
Honoraria: Menarini, Amgen, Pfizer, AstraZeneca, Roche, Janssen, MSD, Bristol-Myers Squibb-Ono Pharmaceutical, Eisai, Boehringer Ingelheim
Consulting or Advisory Role: Samsung Bioepis
Yeon Hee Park
Honoraria: Novartis, Pfizer, Merck
Consulting or Advisory Role: Pfizer
Research Funding: AstraZeneca, Pfizer, Eisai
Travel, Accommodations, Expenses: Merck, Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ferlay J SI, Ervik M, Dikshit R, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer; Lyon, France: 2013. [Internet] [Google Scholar]
- 2.Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines©): Breast Cancer Version 3.2016. Plymouth Meeting, PA, National Comprehensive Cancer Network, 2016. [DOI] [PubMed]
- 5.Adami HO, Malker B, Holmberg L, et al. The relation between survival and age at diagnosis in breast cancer. N Engl J Med. 1986;315:559–563. doi: 10.1056/NEJM198608283150906. [DOI] [PubMed] [Google Scholar]
- 6.Smith-Warner SA, Spiegelman D, Yaun SS, et al. Alcohol and breast cancer in women: A pooled analysis of cohort studies. JAMA. 1998;279:535–540. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds P, Hurley S, Goldberg DE, et al. Active smoking, household passive smoking, and breast cancer: Evidence from the California Teachers Study. J Natl Cancer Inst. 2004;96:29–37. doi: 10.1093/jnci/djh002. [DOI] [PubMed] [Google Scholar]
- 8.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn SH, Son BH, Kim SW, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: Nationwide survival data in Korea--A report from the Korean Breast Cancer Society. J Clin Oncol. 2007;25:2360–2368. doi: 10.1200/JCO.2006.10.3754. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Lim SH, Lee MY, et al. Impact on survival of regular postoperative surveillance for patients with early breast cancer. Cancer Res Treat. 2015;47:765–773. doi: 10.4143/crt.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung K-W, Won Y-J, Oh C-M, et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017;49:292–305. doi: 10.4143/crt.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeRouen MC, Gomez SL, Press DJ, et al. A population-based observational study of first-course treatment and survival for adolescent and young adult females with breast cancer. J Adolesc Young Adult Oncol. 2013;2:95–103. doi: 10.1089/jayao.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan HG, Malmgren JA, Atwood MK. Triple-negative breast cancer in the elderly: Prognosis and treatment. Breast J. 2017;23:630–637. doi: 10.1111/tbj.12813. [DOI] [PubMed] [Google Scholar]
- 14.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 15.Spitale A, Mazzola P, Soldini D, et al. Breast cancer classification according to immunohistochemical markers: Clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol. 2009;20:628–635. doi: 10.1093/annonc/mdn675. [DOI] [PubMed] [Google Scholar]
- 16.Lee W-S, Lee JE, Kim JH, et al. Analysis of prognostic factors and treatment modality changes in breast cancer: A single institution study in Korea. Yonsei Med J. 2007;48:465–473. doi: 10.3349/ymj.2007.48.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge SB BD, Compton CC, Fritz AG, et al.(eds)AJCC Cancer Staging Manual 7th ed.Paris, France: Springer; 2010 [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Choi KS, Yoon M, Song SH, et al. Effect of mammography screening on stage at breast cancer diagnosis: Results from the Korea National Cancer Screening Program. Sci Rep. 2018;8:8882. doi: 10.1038/s41598-018-27152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher SW, Elmore JG. Clinical practice. Mammographic screening for breast cancer. N Engl J Med. 2003;348:1672–1680. doi: 10.1056/NEJMcp021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bevers TB, Helvie M, Bonaccio E, et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362–1389. doi: 10.6004/jnccn.2018.0083. [DOI] [PubMed] [Google Scholar]
- 22. Korean Breast Cancer Society: Breast Cancer Facts & Figures 2018. Seoul, Korea, 2018. [Google Scholar]
- 23.Smith P, Hum S, Kakzanov V, et al. Physicians’ attitudes and behaviour toward screening mammography in women 40 to 49 years of age. Can Fam Physician. 2012;58:e508–e513. [PMC free article] [PubMed] [Google Scholar]
- 24.Darabi H, Czene K, Zhao W, et al. Breast cancer risk prediction and individualised screening based on common genetic variation and breast density measurement. Breast Cancer Res. 2012;14:R25. doi: 10.1186/bcr3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee K, Lim HT, Park SMJBC: Factors associated with use of breast cancer screening services by women aged ≥ 40 years in Korea: The Third Korea National Health and Nutrition Examination Survey 2005 (KNHANES III). BMC Cancer 10:144, 2010. [DOI] [PMC free article] [PubMed]
- 26.Assi HA, Khoury KE, Dbouk H, et al. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013;5(Suppl 1):S2–S8. doi: 10.3978/j.issn.2072-1439.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partridge AH, Hughes ME, Warner ET, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34:3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 28.Johansson ALV, Trewin CB, Hjerkind KV, et al. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int J Cancer. 2019;144:1251–1261. doi: 10.1002/ijc.31950. [DOI] [PubMed] [Google Scholar]
- 29.Kan Z, Ding Y, Kim J, et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018;9:1725. doi: 10.1038/s41467-018-04129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 33.Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2010;73:156–166. doi: 10.1016/j.critrevonc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Bergman L, Kluck HM, van Leeuwen FE, et al. The influence of age on treatment choice and survival of elderly breast cancer patients in south-eastern Netherlands: A population-based study. Eur J Cancer. 1992;28A:1475–1480. doi: 10.1016/0959-8049(92)90547-f. [DOI] [PubMed] [Google Scholar]
- 35.Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor--positive breast cancer. J Clin Oncol. 2004;22:3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 36.Grunfeld EA, Hunter MS, Sikka P, et al. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns. 2005;59:97–102. doi: 10.1016/j.pec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 37. Khan QJ, O'Dea AP, Sharma P: Musculoskeletal adverse events associated with adjuvant aromatase inhibitors. J Oncol 2010:Article ID 654348, 2010. [DOI] [PMC free article] [PubMed]
- 38.Howlader N, Noone AM, Krapcho M.(eds)SEER Cancer Statistics Review, 1975-2012. 2014. National Cancer Institute, Bethesda, MD, https://seer.cancer.gov/archive/csr/1975_2012/
- 39.Canadian Cancer Society . Statistics Canada and Provincial/Territorial Cancer Registry. Canadian Cancer Statistics; 2014. [Google Scholar]