Supplemental Digital Content is available in the text.
Abstract
Background.
Ex situ normothermic machine perfusion (NMP) can be used to assess viability of suboptimal donor livers before implantation. Our aim was to assess the diagnostic accuracy of bile biochemistry for the assessment of bile duct injury (BDI).
Methods.
In a preclinical study, 23 human donor livers underwent 6 hours of end-ischemic NMP to determine biomarkers of BDI. Livers were divided into groups with low or high BDI, based on a clinically relevant histological grading system. During NMP, bile was analyzed biochemically and potential biomarkers were correlated with the degree of BDI. Receiver operating characteristics curves were generated to determine optimal cutoff values. For clinical validation, identified biomarkers were subsequently included as viability criteria in a clinical trial (n = 6) to identify transplantable liver grafts with low BDI.
Results.
Biliary bicarbonate and pH were significantly higher and biliary glucose was significantly lower in livers with low BDI, compared with high BDI. The following cutoff values were associated with low BDI: biliary bicarbonate greater than 18 mmol/L (P = 0.002), biliary pH greater than 7.48 (P = 0.019), biliary glucose less than 16 mmol/L (P = 0.013), and bile/perfusate glucose ratio less than 0.67 (P = 0.013). In the clinical trial, 4 of 6 livers met these criteria and were transplanted, and none developed clinical evidence of posttransplant cholangiopathy.
Conclusions.
Biliary bicarbonate, pH, and glucose during ex situ NMP of liver grafts are accurate biomarkers of BDI and can be easily determined point of care, making them suitable for the pretransplant assessment of bile duct viability. This may improve graft selection and decrease the risk of posttransplant cholangiopathy.
The gap between the demand and availability of donor livers for transplantation has stimulated the use of extended-criteria donor livers, including steatotic, elderly, and donation after circulatory death (DCD) liver grafts.1 These types of donor livers, however, have a higher risk of developing postoperative complications. Especially posttransplant cholangiopathy, or nonanastomotic biliary strictures (NAS), occur in 13% to 35% of DCD grafts, compared with 1% to 24% of livers donated after brain death.2-7 Although the pathogenesis of NAS is not fully understood, the degree of histological bile duct injury (BDI) at the time of transplantation has been identified as a strong predictor of the development of NAS after transplantation.8-10
In an attempt to expand the donor liver pool, researchers are increasingly using ex situ machine perfusion of liver grafts before transplantation. When performed at 37°C, normothermic machine perfusion (NMP) renders the organ metabolically active, which allows for viability testing and the selection of potentially transplantable organs.11-14 Currently, criteria for the selection of suitable donor livers during NMP include bile production, lactate clearance, vascular flows, macroscopic appearance of the liver, transaminase concentration, and pH buffering capacity.11-14 These parameters, however, mainly reflect hepatocellular function and injury, and do not provide information about cholangiocellular function or injury. Identification and determination of the diagnostic accuracy of biomarkers of BDI is clinically relevant as they may provide a missing tool in the selection of liver grafts with an anticipated low risk of NAS. Low biliary pH, bicarbonate, and high biliary glucose during NMP have recently been associated with the development of NAS in 3 cases, and with histological bile duct stroma necrosis in 5 research livers.14 The authors reported that a biliary pH of 7.4 or less during NMP was able to discriminate between these livers, but have provided no other cutoff values and have not reported on the implementation of these biliary parameters clinically.
Cholangiocytes lining the bile duct lumen and peribiliary glands actively contribute to the composition of bile by secretion of bicarbonate via cystic fibrosis transmembrane regulator (CFTR) and chloride-bicarbonate anion exchanger 2 (AE2).15,16 As a result of this, bile in the extrahepatic bile duct has a pH ranging between 7.5 and 8.1 and bicarbonate concentration of 12 to 55 mmol/L.17,18 Moreover, cholangiocytes actively reabsorb glucose from bile via sodium-dependent glucose transporter, SGLT1, expressed on their apical plasma membrane domain, and another glucose transporter, GLUT1, on their basolateral domain.19,20 This results in very low biliary glucose concentrations under physiological conditions.21,22 These transporters are ATP-dependent, and studies have shown that ischemia leads to diminished function of SGLT1 in cholangiocytes, with subsequent decreased glucose reabsorption.23
To determine the added value of specific biomarkers of biliary viability during NMP, we first examined whether histological BDI correlates with markers of hepatocellular injury. As this correlation was poor, we proceeded to identify biomarkers of BDI that can be easily assessed point-of-care. Our aim was to determine the diagnostic accuracy of biliary pH, bicarbonate, glucose and lactate dehydrogenase (LDH) concentration,24-27 to discriminate between livers with a high or low degree of BDI.
MATERIALS AND METHODS
Donor Livers
Twenty-three human donor livers that were declined for transplantation nationwide and offered for research were used to determine suitable biomarkers of BDI during NMP in a preclinical study. All livers underwent 6 hours of NMP for viability testing after static cold storage, as described previously.28 In brief, livers underwent NMP at 37°C using a pressure-controlled perfusion device (Liver Assist, Groningen, the Netherlands), providing continuous portal flow and pulsatile arterial flow. For the preclinical study, informed consent was obtained from relatives of the donors and the study protocol was approved by the Medical Ethical Committee of the University Medical Center Groningen (METc, 2012.068) and by the Dutch Transplantation Foundation (NTS). For validation purposes, the identified cutoff values were prospectively applied to 6 human livers in an ongoing clinical trial as described below.
Histological Analysis
Two biopsies of the common or proper hepatic bile duct were collected: one during the back-table procedure before NMP and one at the end of NMP (proximally of the biliary catheter). All biopsies were fixed in formalin and paraffin-embedded. Slides were stained with hematoxylin and eosin (H&E) and assessed by light microscopy using an established, clinically relevant, histological BDI grading system (Table 1).10 The presence of stroma necrosis, injury of the extramural peribiliary glands, and injury of the perivascular plexus in pretransplant bile duct biopsies have been found to predict the development of NAS after transplantation.10 For this reason, we selected these 3 histological parameters for the present study. The range of the total BDI score was 0 to 7 (Table 1). Biopsies were independently scored in duplicate by 2 investigators (A.P.M.M, and Y.dV.) without knowledge of clinical or NMP data under supervision of an expert liver pathologist (A.S.H.G.), who resolved any discrepancies between the researchers’ scores.
TABLE 1.
Histological BDI scoring systema

Bile Sample Analyses
During NMP, bile samples were collected every 30 minutes under mineral oil (to prevent the exchange of CO2 between the sample and ambient air, which would influence the pH and bicarbonate concentration) for immediate point-of-care determination of pH, bicarbonate and glucose concentration using an ABL90 FLEX analyzer (Radiometer, Brønhøj, Denmark). Additional bile samples were stored at −80°C and later analyzed for LDH using routine laboratory technique for standard patient care.
Hepatocellular Injury and Function
Alanine aminotransferase (ALT) concentration, lactate clearance and bile production are commonly used parameters to assess hepatocellular injury and function during NMP.11-14 Alanine aminotransferase and lactate concentrations in perfusate were analyzed as described previously.28 A peak ALT concentration less than 6000 U/L was considered to reflect low hepatocellular injury.14 A lactate concentration of 2.5 mmol/L or less and cumulative bile production of 5 mL/kg liver or greater within 2.5 hours of NMP were considered to reflect good hepatocellular function.
Validation Cohort
Identified biomarkers of BDI were subsequently included as selection criteria, in addition to hepatocellular injury and function criteria, in a clinical trial of end-ischemic NMP for viability assessment of high-risk donor livers that were declined for transplantation nationwide based on perceived suboptimal quality (www.trialregister.nl; NTR5972). The study protocol was approved by the Medical Ethical Committee (METc 2017.281), and all patients gave written informed consent.
Statistics
Continuous variables were presented as median with interquartile range (IQR) and were compared between groups using the Mann-Whitney U test. Correlations were calculated using the Spearman rank correlation test. Receiver operating characteristics (ROC) curves were generated for biliary pH, bicarbonate, glucose, and LDH concentration, as well as for the bile/perfusate glucose concentration ratio and the delta between perfusate and biliary glucose concentration (perfusate minus biliary glucose) to illustrate diagnostic ability of the binary BDI score. The first timepoint with an area under the ROC curve (AUC-ROC) greater than 0.80 and a P < 0.05 for each potential biomarker was selected to determine the cutoff value with the highest sensitivity (Sens) and specificity (Spec) to discriminate livers with low BDI from those with high BDI. Positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+) and negative likelihood ratio (LR−) with corresponding 95% confidence interval (CI) were calculated using cross tabulation. Level of significance was set at a P value less than 0.05. All statistical analyses were performed using IBM SPSS version 23.0 (Chicago, IL).
RESULTS
Donor Liver Characteristics
In the preclinical study, 18 livers were from DCD donors and 5 livers were donated after brain death (DBD). The overall median (IQR) cold ischemia time was 8.1 hours (7.0–9.3 hours) and warm ischemia time (from withdrawal of life support until in situ cold flush) in DCD livers was 38 minutes (33–42 minutes).
Bile Duct Histology
The degree of BDI per histological item in livers with low or high BDI is summarized in Table 2. Representative examples of H&E staining of bile ducts with low or high BDI are presented in Figure 1. The median BDI score before NMP was 4.0 (IQR, 3.0–7.0) and 6.0 (IQR, 6.0–7.0) after NMP, with 8 livers showing no change in the BDI score over time. The mean BDI score of the 2 biopsies ranged between 3 and 7, with 4.75 as the median of the range. This value was chosen to divide livers into a group with a low and a group with a high BDI score.
TABLE 2.
Comparison of BDI score per histological item

FIGURE 1.
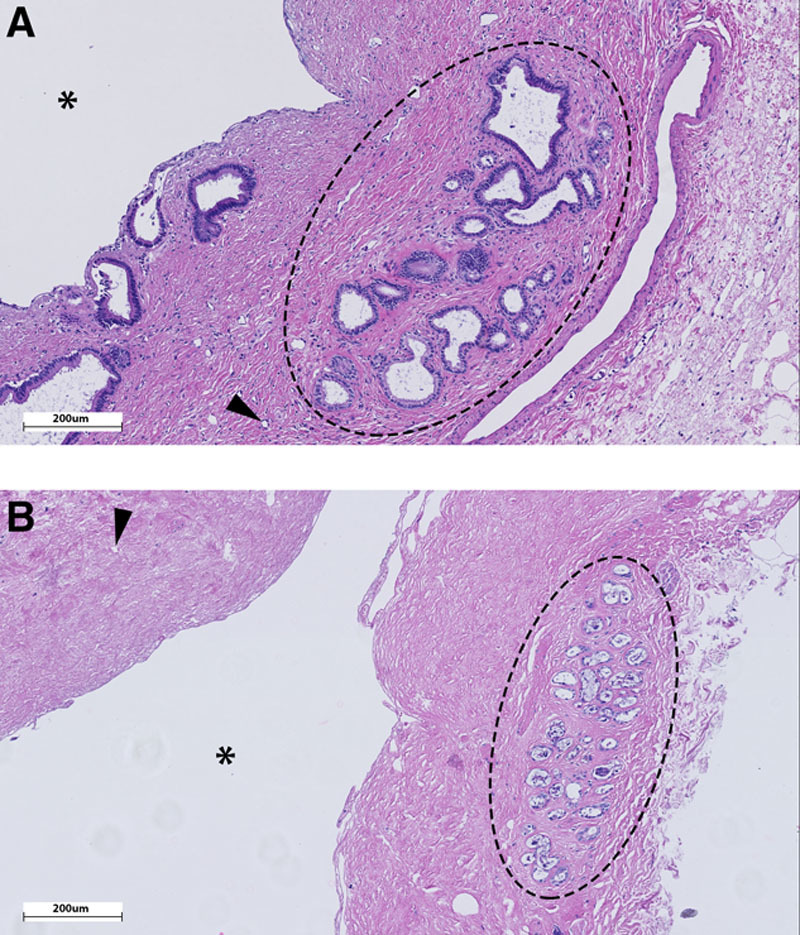
Representative histological H&E staining of an extrahepatic bile duct biopsy with a low histological BDI score (A) and a high BDI score (B). A, Intact extramural peribiliary glands (encircled), intact vasculature (eg, arrowhead pointing to vital arteriole in stroma) and no signs of stroma necrosis. Note that also the periluminal peribiliary glands are largely intact. B, Severe injury to the extramural peribiliary glands with loss of cells (encircled), necrotic (arrowhead) or absent vessels and diffuse stroma necrosis. Note the denuded epithelial lining of the bile duct lumen (asterisk), as is the case in >90% of all donor bile ducts before transplantation.8-10 BDI, bile duct injury.
Hepatocellular Function and Injury Correlate Poorly With BDI
Overall, there was a weak correlation between peak ALT concentration in perfusate during NMP, a marker of hepatocellular injury, and the degree of BDI (Spearman r, 0.424; P = 0.044) (Figure 2A). In livers with extremely high ALT levels (>6000 U/L), the BDI score was also high in 7 (88%) of 8 cases. However, when ALT levels were less than 6000 U/L and livers could potentially be considered for transplantation based on an acceptable degree of hepatocellular injury,14 the correlation between ALT and BDI was very poor (Spearman r, 0.192; P = 0.493) with 7 (47%) of 15 livers still having a high BDI score.
FIGURE 2.
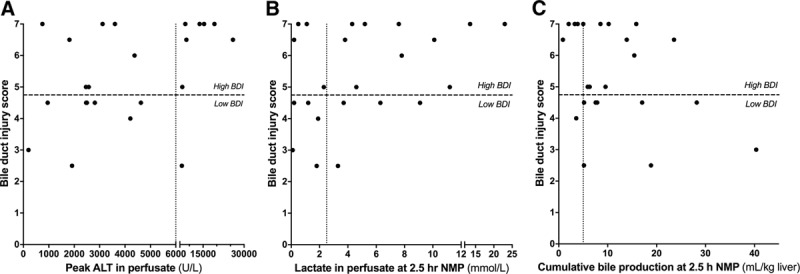
Poor correlation between hepatocellular injury and function and BDI. A, Peak ALT concentration in perfusate during NMP correlated poorly with BDI. This was especially true for livers with ALT <6000 U/L, which are potentially transplantable based on this hepatocellular criterion, of which nearly half had simultaneous high BDI. Livers with very high ALT (>6000 U/L) frequently had very high BDI scores. B, Nearly half of the livers with good lactate clearance, also had a high BDI score. C, There was no correlation between bile production and BDI, with over half of livers with high bile production also having high BDI. ALT, alanine aminotransferase; BDI, bile duct injury; NMP, normothermic machine perfusion.
Similarly, 4 (44%) of 9 livers with good lactate clearance also had high BDI (Figure 2B). There was no correlation between BDI and lactate concentration (Spearman r, 0.349; P = 0.103). Likewise, 10 (56%) of 18 livers with high bile production also had high BDI. There was no correlation between BDI and cumulative bile production (Spearman r, −0.291; P = 0.178) (Figure 2C). Furthermore, there was no correlation between (cumulative) bile production and biliary pH, bicarbonate, LDH and the glucose and bile/perfusate glucose ratio (Figure S1, SDC, http://links.lww.com/TP/B649). These findings indicate that markers of hepatocellular injury and function poorly predicted the degree of BDI, especially in cases that could potentially be considered for transplantation based on hepatocellular criteria. This supports the need for specific biomarkers of BDI during NMP.
Biliary pH and Bicarbonate Correlate Significantly With BDI
Biliary pH and bicarbonate concentration during NMP were significantly higher in livers with low BDI, compared with livers with high BDI (Figures 3A-B). As expected, biliary pH and bicarbonate were strongly correlated (Figure 3C). The line of best fit (R2 = 0.7377) demonstrated that in the lower range of biliary bicarbonate small increases lead to a relatively large increase in pH, whereas at higher bicarbonate concentrations (>30 mmol/L), pH remained relatively stable.
FIGURE 3.
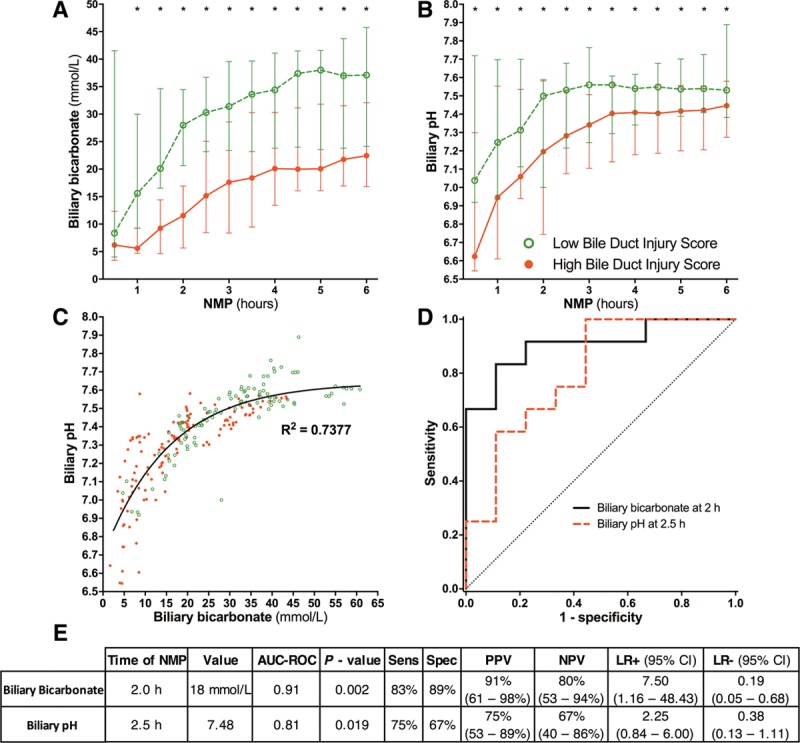
Biliary bicarbonate and pH produced during NMP correlated significantly with BDI. Biliary bicarbonate concentration (A) and biliary pH (B) were significantly higher in livers with a low BDI score, compared with livers with a high BDI score at each timepoint during NMP. C, Biliary pH and bicarbonate concentration were strongly correlated with each other. The line of best fit shows that in the lower range of biliary bicarbonate, small increases in biliary bicarbonate led to relatively large increases in biliary pH. At biliary bicarbonate greater than 30 mmol/L, biliary pH remained relatively stable despite further increases in bicarbonate concentration. (D) ROC curves and (E) statistical analyses of biliary bicarbonate and pH used to discriminate high and low BDI at the earliest timepoints. * P < 0.05. More detailed results, including calculations for all timepoints of bile collection during 6 hours of normothermic machine perfusion (NMP), are provided in Table S1, SDC (http://links.lww.com/TP/B649). AUC-ROC, area under the ROC curve; BDI, bile duct injury; CI, confidence interval; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristics.
Because bile production can be low or absent during the first 2 hours of NMP, we only used bile samples collected after 2 hours to determine optimal cutoff values to discriminate livers with low BDI from those with high BDI (Table S1, SDC, http://links.lww.com/TP/B649). Already after 2 hours, a biliary bicarbonate concentration of 18 mmol/L discriminated between low and high BDI with an AUC-ROC of 0.91 (P = 0.002) and a Sens, Spec, PPV, NPV greater than 80% (Figure 3D-E).
The earliest timepoint at which biliary pH discriminated livers with low BDI from those with high BDI was 2.5 hours (Table S1, SDC, http://links.lww.com/TP/B649). At this timepoint, the optimal cutoff value for pH was 7.48, with an AUC-ROC of 0.81 (P = 0.019) (Figure 3D-E).
Biliary Glucose and Bile/Perfusate Glucose Ratio Correlate Significantly With BDI
Glucose concentrations were lower in bile of livers with low BDI, compared with livers with high BDI score (Figure 4A). Although biliary glucose gradually decreased in livers with low BDI, biliary glucose increased in livers with high BDI, generally remaining greater than 20 mmol/L. In livers with high BDI, the median bile/glucose concentration ratio increased to 1 and remained stable throughout perfusion. In contrast, in livers with low BDI the median ratio was always 0.7 or less and gradually declined over time (Figure 4B-C). Another way to study the relation between biliary and perfusate glucose is by calculating the difference between perfusate and biliary glucose concentration (delta).14 In livers with high BDI, the delta was lower compared with livers with low BDI, reaching significance at only 3 and 3.5 hours NMP (Figure 4C). After several hours of NMP, negative delta values were reached.
FIGURE 4.
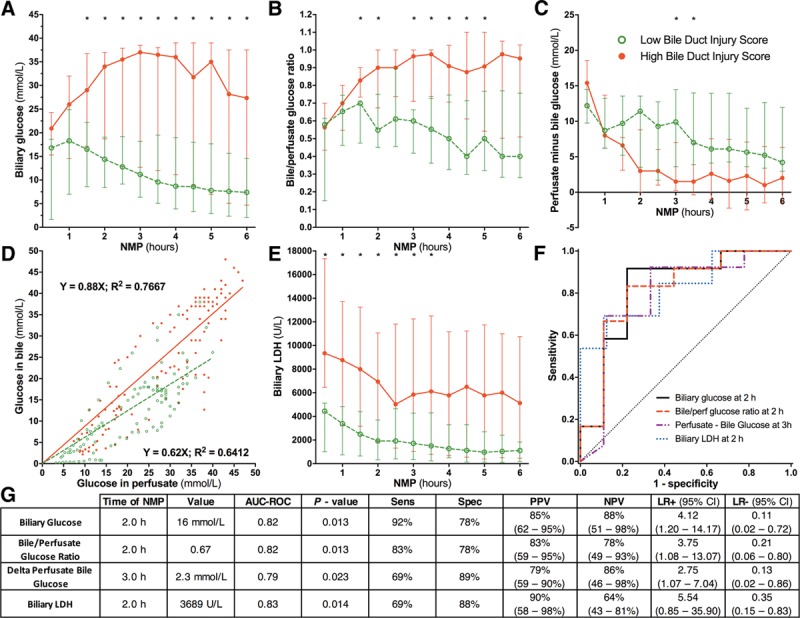
Biliary glucose, bile/perfusate glucose ratio and biliary LDH correlated significantly with BDI during NMP. A, Biliary glucose concentration, inversely reflecting biliary epithelial cell function, was significantly lower in livers with low BDI, compared with livers with high BDI at nearly each timepoint during NMP. B, In livers with high BDI, the bile/perfusate glucose concentration ratio was higher compared with livers with low BDI. C, The delta between perfusate and biliary glucose concentration was lower in livers with high BDI, even reaching negative values at the end of NMP. D, Livers with high BDI have relatively higher biliary glucose in relation to perfusate glucose concentrations, compared with livers with a low BDI. This resulted in a steeper slope in high BDI livers (slope 0.88), compared with low BDI livers (slope, 0.62). E, Biliary LDH concentration, a marker of biliary epithelial cell injury, was significantly higher at each timepoint in livers with a high BDI score compared with livers with a low BDI score. (F) ROC curves and (G) Statistical analyses of biliary glucose, bile/perfusate glucose ratio, perfusate-biliary glucose delta and biliary LDH used to discriminate high and low BDI at the earliest timepoints. * P < 0.05. More detailed results, including calculations for all timepoints of bile collection during 6 hours of NMP, are provided in Table S1, SDC (http://links.lww.com/TP/B649). AUC-ROC, area under the ROC curve; BDI, bile duct injury; CI, confidence interval; LDH, lactate dehydrogenase; LR, likelihood ratio; NMP, normothermic machine perfusion; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristics.
The earliest timepoint at which biliary glucose and the bile/perfusate glucose ratio could discriminate livers with low BDI from those with high BDI was 2 hours (Table S1, SDC, http://links.lww.com/TP/B649). At this timepoint, the optimal cutoff values were 16 and 0.67 mmol/L, respectively (both AUC 0.82; P = 0.013) (Figure 4F-G). The delta between perfusate and biliary glucose did not result in an AUC greater than 0.80 at any timepoint, though at 3 hours NMP an AUC of 0.79 resulted in a cutoff value of 2.3 mmol/L (P = 0.030, Figure 4F-G, and Table S1, SDC, http://links.lww.com/TP/B649).
Biliary LDH Correlates Significantly With BDI
Biliary LDH concentration, a marker of biliary epithelial cell injury, was more than twofold higher in livers with high BDI, compared with livers with low BDI (Figure 4E). In both groups, biliary LDH concentration declined gradually during NMP. The earliest timepoint with a significant AUC-ROC for biliary LDH was 2 hours NMP (Table S1, SDC, http://links.lww.com/TP/B649), with an optimal cutoff value of 3689 U/L and an AUC of 0.83 (P = 0.014) (Figures 4F-G).
Clinical Validation of Biliary Biomarkers During NMP
Based on the preclinical research data, we included biliary pH as one of the criteria for hepatobiliary viability assessment in a clinical trial of end-ischemic NMP of high-risk livers that were initially declined nationwide for transplantation. So far, 6 DCD livers (median Eurotransplant Donor Risk Index,29 2.9; IQR, 2.7–2.9) have been included in this trial of which 4 (median Eurotransplant Donor Risk Index, 2.9; IQR, 2.8–3.0; median United Kingdom-DCD risk score,30 6.0; IQR, 5.8–7.5) met all selection criteria for transplantation during NMP, including a biliary pH greater than 7.48 within 2.5 hours of NMP. In addition, biliary bicarbonate and glucose bile/perfusate ratio were within the ranges identified in the preclinical study (Figure 5). The recipients of these 4 liver grafts had an uneventful recovery and none of them developed clinical evidence of posttransplant cholangiopathy at a median follow-up of 8.3 months (IQR, 7.6–10.1 months). Of the 2 livers that were declined for transplantation, the first fulfilled all hepatocellular criteria and was declined only on the basis of bile biochemistry (at 2.5 hours NMP: cumulative bile production 45 mL [≥10 mL within 2.5 hours NMP and ≥4 mL in preceding hour]; perfusate lactate of 0.3 mmol/L [<1.7 mmol/L]; perfusate pH 7.38 [7.35–7.45]).The second liver was also declined based on hepatocellular criteria (cumulative bile production, 57 mL; perfusate lactate, 3.5 mmol/L; perfusate pH, 7.25). At 2.5 hours NMP, the perfusate ALT concentration was approximately 4000 and 6000 U/L, respectively. The retrospectively determined median BDI score for the transplanted livers was 3.2 (IQR, 2.8–3.5) and 4.3 (3.0–5.5) for the nontransplanted livers.
FIGURE 5.
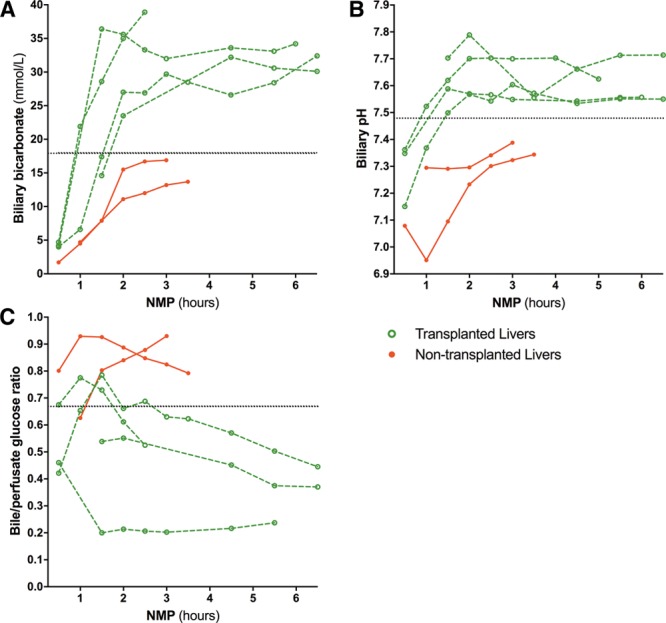
Clinical validation of biliary biomarkers during ex situ NMP of transplanted and nontransplanted livers. Six livers that were declined for transplantation nationwide underwent end-ischemic NMP to assess their viability for transplantation in a clinical trial. According to protocol, viability assessment occurred within 2.5 hours NMP. Four livers fulfilled the hepatobiliary criteria and were transplanted. There was no clinical evidence of posttransplant cholangiopathy at a median of 8.3 months (IQR, 7.6-10.1) follow-up. Dotted lines indicate biliary biomarker cutoff values established in the preclinical study. IQR, interquartile range; NMP, normothermic machine perfusion.
DISCUSSION
In the present study, we have determined the diagnostic accuracy of the previously described biomarkers of biliary injury and viability, which can be easily assessed point of care during ex situ NMP of human donor livers. Biliary bicarbonate concentration greater than 18 mmol/L, biliary pH greater than 7.48, biliary glucose concentration less than 16 mmol/L, bile/perfusate glucose concentration ratio less than 0.67, and biliary LDH concentration less than 3689 U/L within 2.5 hours of NMP were strongly associated with low histological BDI. These findings have important clinical implications as the proposed biomarkers allow transplant teams to stratify livers grafts during ex situ NMP based on the risk of BDI, and potentially also that of posttransplant cholangiopathy. Biliary bicarbonate had the highest predictive value out of all of the studied biomarkers. This is likely because biliary bicarbonate is less influenced by other nonbiliary factors.
Ex situ NMP is increasingly being applied and explored as a tool to assess viability of liver grafts that were initially declined for transplantation based on a perceived high risk of early graft failure.11-14 In most centers, selection criteria are currently based on hepatocellular injury and function. The risk of posttransplant graft failure, however, is not only determined by the degree of hepatocellular injury, but also by the presence of biliary injury. Especially DCD livers have an increased risk of developing biliary complications.31 In a recent clinical study of 12 initially declined liver grafts that were identified as transplantable during end-ischemic NMP, 25% developed posttransplant cholangiopathy despite adequate hepatocellular function during pretransplant ex situ NMP.13 These clinical data are in line with our observation that some livers with a low degree of hepatocellular injury can still have a high degree of BDI, which can be missed when no specific biliary viability criteria are used. This difference in hepatocellular and cholangiocellular injury can be explained by the greater susceptibility of cholangiocytes to ischemia-reperfusion injury and a slower postischemic recovery of intracellular ATP, compared with hepatocytes.23,32
The parameters we have selected as potential biomarkers of biliary viability were all based on the known physiological function of healthy bile duct epithelium. Two important physiological functions of biliary epithelium are active secretion of bicarbonate and reabsorption of glucose, leading to an alkalotic pH and very low biliary glucose concentrations at the level of the extrahepatic bile duct.17,18 We have previously suggested that these parameters could potentially serve as biomarkers of bile duct viability during machine perfusion.26,33,34 In the current study, we demonstrated that biliary pH, bicarbonate and glucose concentration indeed strongly correlate with histological BDI of liver grafts during NMP, confirming observations made earlier this year.14 In 3 independent clinical studies, the histological degree of BDI before liver transplantation has been identified as a significant predictor of the development of NAS after transplantation.8-10
Our findings suggest that biliary epithelial cells of livers with high BDI are unable to secrete sufficient amounts of bicarbonate to raise the biliary pH. Increasing the biliary pH helps biliary epithelial cells to protect themselves against the toxic effects of hydrophobic bile salts, which is also known as the “biliary bicarbonate umbrella”.35 Low biliary pH and bicarbonate, therefore, not only reflect biliary injury/dysfunction, but may also contribute to additional biliary injury due to an absent bicarbonate umbrella.36 Biliary pH and bicarbonate were not linearly correlated, which is explained by the fact pH varies on a logarithmic scale, whereas bicarbonate does not. The pH values we observed in liver grafts with low BDI are within the normal range of biliary pH, which varies between 7.5 and 8.1 in the common bile duct.17 The same is true for biliary bicarbonate concentration, which normally ranges widely between 12 and 55 mmol/L.18 Interestingly, Watson et al14 recently reported 3 patients that developed cholangiopathy after the transplantation of livers that were unable to produce bile with a pH greater than 7.4 during NMP. This preliminary clinical observation is in line with the identified biomarkers of BDI in the current study in preclinical livers as well as the clinical validation cohort.
Although biliary glucose also correlated significantly with the degree of biliary injury, the interpretation of glucose values is slightly more complex. Postischemic reperfusion of a liver graft almost universally results in a pronounced increase of glucose levels in the perfusion fluid due to glycogenolysis.37 This contributes to a higher glucose concentration in the primary bile produced by hepatocytes, which affects the reabsorption of glucose from bile when it passes from the canaliculi to the common bile duct. Even when the biliary epithelium is intact, glucose reabsorption via SGLT1 and GLUT1 becomes insufficient when biliary glucose concentrations are too high, a phenomenon analogous to the renal threshold for glucose in kidney tubuli.22,38 In other words, high glucose concentration in the perfusion fluid (which is frequently seen during NMP) may affect the biliary reabsorption of glucose. Biliary glucose levels should therefore be viewed in relation to glucose levels in the perfusion fluid. To illustrate this, we have correlated glucose concentrations in bile with those in the perfusion fluid. The ratio between glucose in bile and perfusion fluid was almost 1 in livers with high BDI, whereas it was 0.7 or less in livers with low BDI. Others have reported the delta between perfusate and biliary glucose levels,14 though in our hands this did not result in a usable criterion. Our findings regarding biliary glucose are in line with an in vitro study with rat cholangiocytes, in which ATP-depleted cholangiocytes showed prolonged dysfunction of biliary glucose transporter SGLT1 and diminished glucose reabsorption.23 Similarly, in an experimental study in rabbits, higher glucose concentrations were found in bile of livers that had suffered from warm and cold ischemia, compared with livers that had not been ischemic.39
The observations made for biliary bicarbonate, pH, and glucose were supported by the data on biliary LDH concentrations. Based on animal experiments, biliary LDH has previously been proposed as a marker of biliary epithelial injury.24 In the current study, biliary LDH was indeed significantly higher in livers with high BDI, compared with livers with low BDI. In all livers, however, biliary LDH concentration declined gradually during NMP, which can be explained by an early washout of LDH from dead cholangiocytes when bile flow recurs. This is in line with previous research, where biliary LDH correlated with the length of cold ischemia in a rat liver model and previous studies that reported lower LDH concentrations in machine perfused livers compared with cold stored livers.25,27,40
This study has some limitations. We took biopsies from the extrahepatic bile duct to assess the degree of histological injury, whereas the intrahepatic bile ducts can also be involved in posttransplant cholangiopathy. However, in a previous study, we have demonstrated that histological injury (in particular injury to the peribiliary vascular plexus, extramural peribiliary glands and stroma necrosis) assessed at the level of the extrahepatic bile duct also reflects the degree of injury in the intrahepatic bile ducts, as deep as the segmental bile ducts.41 Furthermore, there may have been some degree of sampling error in bile duct biopsies. To minimize this, the average BDI of 2 biopsies was taken. Furthermore, our preclinical data included 5 DBD livers, whereas the clinical livers were all DCD. However, when we excluded the DBD livers in the preclinical study, similar results were obtained. Lastly, some livers produce very little or no recorded bile during NMP. This raises the question as to whether or not such livers can be transplanted safely. We would advocate an approach tailored to the specific donor liver and recipient. For example, when assessing a liver with a high risk of developing NAS, production of good quality bile during NMP would be essential to allow cholangiocyte viability assessment before transplantation. On the other hand, for livers with a low risk of NAS, cholangiocyte viability assessment may not always be necessary, as has been reported in the literature.14
Implementation of the identified biomarkers in larger clinical trials of NMP with longer follow-up, in which both DCD and DBD livers are included, is necessary to confirm their utility in decreasing the incidence of posttransplant cholangiopathy. Machine perfusion has the potential to provide opportunities to ameliorate BDI, for instance through stem cell therapy. These cholangiocellular criteria could be applied to identify livers that require additional pharmacological interventions, and be used to assess the efficacy of such treatments. In conclusion, biliary bicarbonate, pH, glucose, and LDH during ex situ NMP are accurate biomarkers of clinically relevant, histological biliary injury of livers grafts. These biomarkers can be easily determined point-of-care, making them suitable for the assessment of bile duct viability during NMP and the potential identification of donor livers with a low risk of developing posttransplant cholangiopathy.
ACKNOWLEDGMENTS
The authors would like to thank all donors and donor relatives, as well as the Dutch Transplantation Society and its staff, for enabling the use of donor livers for research.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
A.P.M.M., T.L., R.J.P. participated in the research design. A.P.M.M., R.J.P. participated in the writing of the first version of the article. All authors participated in the performance of the research. A.P.M.M., V.E.dM., T.L., R.J.P. participated in data analysis. A.P.M.M., Y.dV., Burlage, R.vR., M.F., V.E.dM., M.T.dB., R.H.dK., H.J.V., A.S.H.G., T.L., R.J.P. critically revised the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Jochmans I, van Rosmalen M, Pirenne J, et al. Adult liver allocation in Eurotransplant. Transplantation. 2017;101:1542–1550.. [DOI] [PubMed] [Google Scholar]
- 2.de Vries Y, von Meijenfeldt FA, Porte RJ. Post-transplant cholangiopathy: classification, pathogenesis, and preventive strategies. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1507–1515.. [DOI] [PubMed] [Google Scholar]
- 3.Meurisse N, Vanden Bussche S, Jochmans I, et al. Outcomes of liver transplantations using donations after circulatory death: a single-center experience. Transplant Proc. 2012;44:2868–2873.. [DOI] [PubMed] [Google Scholar]
- 4.Jay CL, Lyuksemburg V, Ladner DP, et al. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259–264.. [DOI] [PubMed] [Google Scholar]
- 5.Dubbeld J, Hoekstra H, Farid W, et al. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br J Surg. 2010;97:744–753.. [DOI] [PubMed] [Google Scholar]
- 6.Pine JK, Aldouri A, Young AL, et al. Liver transplantation following donation after cardiac death: an analysis using matched pairs. Liver Transpl. 2009;15:1072–1082.. [DOI] [PubMed] [Google Scholar]
- 7.Chan EY, Olson LC, Kisthard JA, et al. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604–610.. [DOI] [PubMed] [Google Scholar]
- 8.Hansen T, Hollemann D, Pitton MB, et al. Histological examination and evaluation of donor bile ducts received during orthotopic liver transplantation—a morphological clue to ischemic-type biliary lesion? Virchows Arch. 2012;461:41–48.. [DOI] [PubMed] [Google Scholar]
- 9.Brunner SM, Junger H, Ruemmele P, et al. Bile duct damage after cold storage of deceased donor livers predicts biliary complications after liver transplantation. J Hepatol. 2013;58:1133–1139.. [DOI] [PubMed] [Google Scholar]
- 10.op den Dries S, Westerkamp AC, Karimian N, et al. Injury to peribiliary glands and vascular plexus before liver transplantation predicts formation of non-anastomotic biliary strictures. J Hepatol. 2014;60:1172–1179.. [DOI] [PubMed] [Google Scholar]
- 11.Sutton ME, op den Dries S, Karimian N, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLOS One. 2014;9:e110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant. 2016;16:3235–3245.. [DOI] [PubMed] [Google Scholar]
- 13.Watson CJE, Kosmoliaptsis V, Randle LV, et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia—important lessons from the first 12 cases. Transplantation. 2017;101:1084–1098.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson CJE, Kosmoliaptsis V, Pley C, et al. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18:2005–2020.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohn JA, Strong TV, Picciotto MR, et al. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993;105:1857–1864.. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Anso E, Castillo JE, Diez J, et al. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–1406.. [PubMed] [Google Scholar]
- 17.Sutor DJ, Wilkie LI. Diurnal variations in the pH of pathological gallbladder bile. Gut. 1976;17:971–974.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3:1035–1078.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazaridis KN, Pham L, Vroman B, et al. Kinetic and molecular identification of sodium-dependent glucose transporter in normal rat cholangiocytes. Am J Physiol. 1997;272:G1168–74.. [DOI] [PubMed] [Google Scholar]
- 20.Masyuk AI, Masyuk TV, Tietz PS, et al. Intrahepatic bile ducts transport water in response to absorbed glucose. Am J Physiol Cell Physiol. 2002;283:C785–91.. [DOI] [PubMed] [Google Scholar]
- 21.Schein CJ, Zumoff B, Kream J, et al. A blood-bile glucose barrier in man. Gastroenterology. 1968;54:1094–1097.. [PubMed] [Google Scholar]
- 22.Guzelian P, Boyer JL. Glucose reabsorption from bile. Evidence for a biliohepatic circulation. J Clin Invest. 1974;53:526–535.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doctor RB, Dahl RH, Salter KD, et al. ATP depletion in rat cholangiocytes leads to marked internalization of membrane proteins. Hepatology. 2000;31:1045–1054.. [DOI] [PubMed] [Google Scholar]
- 24.Vajdová K, Smreková R, Kukan M, et al. Bile analysis as a tool for assessing integrity of biliary epithelial cells after cold ischemia-reperfusion of rat livers. Cryobiology. 2000;41:145–152.. [DOI] [PubMed] [Google Scholar]
- 25.Vairetti M, Ferrigno A, Rizzo V, et al. Correlation between the liver temperature employed during machine perfusion and reperfusion damage: role of Ca2+. Liver Transpl. 2008;14:494–503.. [DOI] [PubMed] [Google Scholar]
- 26.op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13:1327–1335.. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Nassar A, Farias K, et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transpl. 2014;20:987–999.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matton APM, Burlage LC, van Rijn R, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transpl. 2018;24:528–538.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braat AE, Blok JJ, Putter H, et al. The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 2012;12:2789–2796.. [DOI] [PubMed] [Google Scholar]
- 30.Schlegel A, Kalisvaart M, Scalera I, et al. The UK DCD risk score: a new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol. 2018;68:456–464.. [DOI] [PubMed] [Google Scholar]
- 31.Blok JJ, Detry O, Putter H, et al. Longterm results of liver transplantation from donation after circulatory death. Liver Transpl. 2016;22:1107–1114.. [DOI] [PubMed] [Google Scholar]
- 32.Noack K, Bronk SF, Kato A, et al. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation. 1993;56:495–500.. [DOI] [PubMed] [Google Scholar]
- 33.Bruinsma BG, Yeh H, Ozer S, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant. 2014;14:1400–1409.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weeder PD, van Rijn R, Porte RJ. Machine perfusion in liver transplantation as a tool to prevent non-anastomotic biliary strictures: rationale, current evidence and future directions. J Hepatol. 2015;63:265–275.. [DOI] [PubMed] [Google Scholar]
- 35.Beuers U, Hohenester S, de Buy Wenniger LJ, et al. The biliary HCO(3)(-) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 2010;52:1489–1496.. [DOI] [PubMed] [Google Scholar]
- 36.Op den Dries S, Sutton ME, Lisman T, et al. Protection of bile ducts in liver transplantation: looking beyond ischemia. Transplantation. 2011;92:373–379.. [DOI] [PubMed] [Google Scholar]
- 37.Villalobos-Molina R, Saavedra-Molina A, Devlin TM. Effect of hypoxia and reoxygenation on metabolic pathways in rat hepatocytes. Arch Med Res. 1998;29:219–223.. [PubMed] [Google Scholar]
- 38.Lawrence RD. Renal threshold for glucose: normal and in diabetics. Br Med J. 1940;1:766–768.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habib MM, Hafez TS, Parkes HG, et al. A comparison of bile composition from heart-beating and non-heart-beating rabbit organ donors during normothermic extracorporeal liver perfusion: experimental evaluation using proton magnetic resonance spectroscopy. Transplant Proc. 2004;36:2914–2916.. [DOI] [PubMed] [Google Scholar]
- 40.Westerkamp AC, Karimian N, Matton AP, et al. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation. 2016;100:825–835.. [DOI] [PubMed] [Google Scholar]
- 41.Karimian N, Weeder PD, Bomfati F, et al. Preservation injury of the distal extrahepatic bile duct of donor livers is representative for injury of the intrahepatic bile ducts. J Hepatol. 2015;63:284–287.. [DOI] [PubMed] [Google Scholar]


