Abstract
Background
Inositol is an essential nutrient required by human cells in culture for growth and survival. Inositol promotes maturation of several components of surfactant and may play a critical role in fetal and early neonatal life. A drop in inositol levels in infants with respiratory distress syndrome (RDS) can be a sign that their illness will be severe.
Objectives
To assess the effectiveness and safety of supplementary inositol in preterm infants with or without respiratory distress syndrome (RDS) in reducing adverse neonatal outcomes including: death (neonatal and infant deaths), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), necrotizing enterocolitis (NEC) and sepsis.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 11), MEDLINE via PubMed (1966 to 5 November 2018), Embase (1980 to 5 November 2018), and CINAHL (1982 to 5 November 2018). We searched clinical trial databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials (RCT) and quasi‐randomised trials.
Selection criteria
We included all randomised controlled trials of inositol supplementation of preterm infants compared with a control group that received a placebo or no intervention. Outcomes included neonatal death, infant death, bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intraventricular haemorrhage (IVH), necrotizing enterocolitis (NEC) and sepsis.
Data collection and analysis
The three review authors independently abstracted data on neonatal outcomes and resolved any disagreements through discussion and consensus. Outcomes were reported as typical risk ratio (RR), risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH). We used the GRADE approach to assess the quality of evidence.
Main results
Six published randomised controlled trials were identified, with a total of 1177 infants. Study quality varied for the comparison 'Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control' and interim analyses had occurred in several trials for the outcomes of interest. In this comparison, neonatal death was found to be significantly reduced (typical RR 0.53, 95% CI 0.31 to 0.91; typical RD −0.09, 95% CI −0.16 to −0.01; NNTB 11, 95% CI 6 to 100; 3 trials, 355 neonates). Infant deaths were not reduced (typical RR 0.89, 95% CI 0.71 to 1.13; typical RD −0.02, 95% CI −0.07 to 0.02; 5 trials, 1115 infants) (low‐quality evidence). ROP stage 2 or higher or stage 3 or higher was not significantly reduced (typical RR 0.89, 95% CI 0.75 to 1.06; typical RD −0.04, 95% CI −0.10 to 0.02; 3 trials, 810 infants) (moderate‐quality evidence). There were no significant findings for ROP (any stage), NEC (suspected or proven), sepsis, IVH grade greater than II (moderate‐quality evidence). For the comparison 'Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at less than 30 weeks' postmenstrual age (PMA) compared to placebo for preterm infants at risk for or having respiratory distress syndrome' the results from two studies of high quality were included (N = 760 neonates). Recruitment to the larger study (N = 638) was terminated because of a higher rate of deaths in the inositol group. We did not downgrade the quality of the study. The meta‐analyses of the outcomes of 'Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome', 'Type 1 ROP including adjudicated ROP outcome', 'All‐cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth)' and 'Severe IVH (grade 3 or 4)' did not show significant findings (moderate‐quality evidence). There were no significant findings for the outcomes 'BPD or death by it prior to 37 weeks' postmenstrual age (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth)', 'Late onset sepsis (> 72 hours of age)', and 'Suspected or proven NEC' (high‐quality evidence).
Authors' conclusions
Based on the evidence from randomised controlled trials to date, inositol supplementation does not result in important reductions in the rates of infant deaths, ROP stage 3 or higher, type 1 ROP, IVH grades 3 or 4, BPD, NEC, or sepsis. These conclusions are based mainly on two recent randomised controlled trials in neonates less than 30 weeks' postmenstrual age (N = 760), the most vulnerable population. Currently inositol supplementation should not be routinely instituted as part of the nutritional management of preterm infants with or without RDS. It is important that infants who have been enrolled in the trials included in this review are followed to assess any effects of inositol supplementation on long‐term outcomes in childhood. We do not recommend any additional trials in neonates.
Keywords: Humans; Infant, Newborn; Bronchopulmonary Dysplasia; Bronchopulmonary Dysplasia/prevention & control; Dietary Supplements; Enterocolitis, Necrotizing; Enterocolitis, Necrotizing/prevention & control; Infant, Premature; Inositol; Inositol/therapeutic use; Randomized Controlled Trials as Topic; Respiratory Distress Syndrome, Newborn; Respiratory Distress Syndrome, Newborn/drug therapy; Respiratory Distress Syndrome, Newborn/mortality; Retinopathy of Prematurity; Retinopathy of Prematurity/prevention & control; Sepsis; Sepsis/prevention & control; Vitamin B Complex; Vitamin B Complex/therapeutic use
Plain language summary
Inositol in preterm infants at risk for or having respiratory distress syndrome
Review question Does the administration of supplementary inositol reduce adverse outcomes in preterm infants with or without respiratory distress syndrome (RDS)?
Background Inositol is an essential nutrient for cells, with high concentrations in breast milk (particularly in the breast milk of mothers whose babies have been born early). A drop in inositol levels in babies with respiratory distress syndrome (RDS) can be a sign that their illness will be severe. Inositol is thought to be an important nutrient in development before and after birth.
Search date The relevant searches were conducted on 5 November 2018.
Study characteristics Six published randomised controlled trials met our inclusion criteria, with a total of 1177 infants enrolled. This update includes the results from two high‐quality studies conducted in 760 infants of less than 30 weeks' postmenstrual age (PMA).
Key results In our previous update of our review, in 2015, we found that the initial evidence regarding inositol supplementation in preterm babies with RDS was promising. Inositol supplementation lowered rates of death and bleeding in the brain, with an important reduction in eye problems as well. Inositol did not have serious adverse effects. We suggested that further research was warranted to confirm these preliminary findings. Such research has now been published from two high‐quality studies that included 760 infants of less than 30 weeks' PMA, the most vulnerable population. All results indicate that there are no reductions in adverse outcomes associated with inositol supplementation, including infant death, eye problems, bleeding in the brain, infections, chronic lung problems and gastrointestinal problems. Thus inositol supplementation in preterm infants is not recommended. Infants enrolled in these studies should be followed into childhood for assessment of any neuro‐developmental problems.
Quality of evidence According to GRADE (a method to score the quality of the trials supporting each outcome), the quality of the evidence varied but was moderate to high for the important outcomes in the analyses for repeated high doses of inositol in infants born at less than 30 weeks' postmenstrual age.
Summary of findings
Summary of findings for the main comparison. Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) compared to control for preterm infants at risk for or having respiratory distress syndrome (Comparison 1).
| Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) compared to control for preterm infants at risk for or having respiratory distress syndrome | ||||||
| Patient or population: preterm infants at risk for or having respiratory distress syndrome Setting: NICU Intervention: Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) | |||||
| Infant death (age < 1 year) | Study population | RR 0.89 (0.71 to 1.13) | 1115 (5 studies) | Low | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was high heterogeneity for RR (I² = 80 % ) and for RD (I² = 84%). We downgraded the quality of the evidence by 1 step Directness of the evidence: Studies were conducted in the target population. Precision of estimates: Results from 1115 infants have been reported in the studies to date and the confidence intervals around the point estimates for RR and RD were narrow. Presence of publication bias: As only 5 studies were included in the analysis we did not perform a funnel plot. |
|
| 207 per 1000 | 184 per 1000 (147 to 234) | |||||
| Bronchopulmonary dysplasia (at 36 to 38 weeks' PMA) | Study population | RR 1.04 (0.90 to 1.20) | 737 (2 studies) | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 1 study and unclear in 1 study; The risk of bias for allocation concealment was low in 1 study and unclear in 1 study; the risk of bias regarding performance bias and detection bias was low in 1 study and unclear in 1 study. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) and for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date the results from 737 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot |
|
| 459 per 1000 | 477 per 1000 (413 to 551) | |||||
| ROP, stage ≥ 3 or ≥ 2 | Study population | RR 0.89 (0.75 to 1.06) | 810 (3 studies) | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 1 study and unclear in 2 studies; the risk of bias for allocation concealment was low in 1 study and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 1 study and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 63% ) and none for RD (I² = 23%) Directness of the evidence: Studies were conducted in the target population Precision of estimates: to date the results from 810 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 3 studies were included in the analysis we did not perform a funnel plot |
|
| 368 per 1000 | 328 per 1000 (276 to 390) | |||||
| Sepsis (early or late onset) | Study population | RR 1.21 (0.95 to 1.54) | 1067 (4 studies) | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 2 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 1 study; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 1 study. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency: across studies: There was no heterogeneity for RR (I² = 24% ) and low for RD (I² = 34%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date results from 1067 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 4 studies were included in the analysis we did not perform a funnel plot |
|
| 189 per 1000 | 229 per 1000 (180 to 292) | |||||
| Necrotizing enterocolitis (suspected or proven) | Study population | RR 0.94 (0.64 to 1.39) | 1115 (5 studies) | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date results from 1115 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 5 studies were included in the analysis we did not perform a funnel plot |
|
| 83 per 1000 | 78 per 1000 (53 to 115) | |||||
| Intraventricular haemorrhage, grade > 2 | Study population | RR 0.77 (0.58 to 1.01) | 1103 (5 studies) | Moderate | Design (risk of bias): the risk of bias for random sequence generation was low in 2 studies and unclear in 3 studies; the risk of bias for allocation concealment was low in 3 studies and unclear in 2 studies; the risk of bias regarding performance bias and detection bias was low in 3 studies and unclear in 2 studies. We downgraded the quality of the evidence by 1 step Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 48% ) and for RD (I² = 42%) Directness of the evidence: studies were conducted in the target population Precision of estimates: to date the results from 1103 infants have been reported in the studies and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: As only 5 studies were included in the analysis we did not perform a funnel plot |
|
| 177 per 1000 | 136 per 1000 (103 to 179) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval;PMA: Postmenstrual age; RD: Risk difference; ROP: Retinopathy of Prematurity; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 2. Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at less than 30 weeks' PMA compared to placebo for preterm infants at risk for or having respiratory distress syndrome (Comparison 3).
| Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA compared to placebo for preterm infants at risk for or having respiratory distress syndrome | ||||||
| Patient or population: preterm infants at risk for or having respiratory distress syndrome Setting: Intervention: Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA | |||||
| Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome | Study population | RR 1.28 (0.99 to 1.67) | 679 (2 studies) | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was high heterogeneity for RR (I² = 79 %) and for RD (I² = 85%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 679 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: As only 2 studies were included in the analysis we did not perform a funnel plot |
|
| 222 per 1000 | 284 per 1000 (220 to 371) | |||||
| Type 1 ROP including adjudicated ROP outcome | Study population | RR 1.24
(0.82 to 1.86) |
605 (2 studies) | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 46 %) and moderate for RD (I² = 54%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported on for 605 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot |
|
| 120 per 1000 | 149 per 1000 (99 to 224) | |||||
| All‐cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) | Study population | RR 1.35
(0.91 to 2.00) |
701 (2 studies) | Moderate | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 72%) and high for RD (I² = 84%). We downgraded the quality of the evidence by 1 step Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot |
|
| 110 per 1000 | 148 per 1000 (100 to 219) | |||||
| BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) | Study population | RR 1.01
(0.87 to 1.16) |
616 (2 studies) | High | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population. Precision of estimates: this outcome was reported for 616 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot |
|
| 555 per 1000 | 561 per 1000 (483 to 644) | |||||
| Severe IVH (grade 3 or 4) | Study population | RR 0.92
(0.65 to 1.29) |
690 (2 studies) | Moderate | Design (risk of bias): The risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was moderate heterogeneity for RR (I² = 74%) and high for RD (I² = 82%) Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 690 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot. |
|
| 171 per 1000 | 157 per 1000 (111 to 221) | |||||
| Late‐onset sepsis (> 72 hours of age) | Study population | RR 1.33
(1.00 to 1.75) |
701 (2 studies) | HIgh | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was no heterogeneity for RR (I² = 0%) nor for RD (I² = 0%) Directness of the evidence: studies were conducted in the target population Precision of estimates: this outcome was reported for 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot |
|
| 191 per 1000 | 254 per 1000 (191 to 334) | |||||
| Suspected or proven NEC | Study population | RR 0.88
(0.55 to 1.41) |
701 (2 studies) | High | Design (risk of bias): the risk of bias for random sequence generation, for allocation concealment, for performance bias and detection bias was low in both studies Heterogeneity/consistency across studies: there was low heterogeneity for RR (I² = 36%) and moderate for RD (I² = 53%). Directness of the evidence: studies were conducted in the target population. Precision of estimates: this outcome was reported on in 701 infants and the confidence intervals around the point estimates for RR and RD were narrow Presence of publication bias: as only 2 studies were included in the analysis we did not perform a funnel plot. |
|
| 98 per 1000 | 87 per 1000 (54 to 139) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; PMA: Postmenstrual age; RD: Risk difference; ROP: Retinopathy of prematurity; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Background
Description of the condition
As more preterm infants survive beyond the neonatal period, the incidence of long‐term complications such as bronchopulmonary dysplasia (BPD) and retinopathy of prematurity (ROP) can be expected to increase. The relative contributions of risk factors such as barotrauma, oxygen therapy and nutritional status have yet to be fully understood. Interest has recently focused on the use of myo‐inositol (inositol) supplementation in preterm infants for the prevention of BPD and ROP (Hallman 1986; Hallman 1992). Inositol is a six‐carbon sugar alcohol found widely throughout mammalian tissues in its free form as the phospholipid phosphatidylinositol, and in cell membranes as a phosphoinositide (Dawson 1961; Hasan 1974). Inositol is an essential nutrient required by human cells in culture for growth and survival (Eagle 1957). The effects of deprivation and supplementation in animals have been studied extensively (Egberts 1986; Guarner 1992; Hallman 1984). Inositol promotes maturation of the surfactant phospholipids phosphatidylcholine and phosphatidylinositol, and the synthesis of phosphatidylinositol in type II pneumocytes appears to be dependent on extracellular inositol concentrations (Hallman 1980; Hallman 1984). Compositional changes in fetal rat lung surfactant correlate with changes in plasma inositol levels, and supplementation increases phospholipid levels to normal in the deprived rat pup (Egberts 1986; Guarner 1992; Hallman 1980).
Description of the intervention
Inositol is administered intravenously as long as the infant is not on full oral feeds. When the infant progresses to full feeds inositol is given orally or via an oro‐gastric tube.
How the intervention might work
In human infants with respiratory distress syndrome (RDS), a premature drop in serum inositol levels predicts a more severe course of the syndrome (Hallman 1985). Inositol supplementation increases the amount of saturated phosphatidylcholine in surfactant in newborns and produces a rise in serum inositol concentration (Hallman 1987). In humans, free inositol levels in sera from preterm neonates are two to 20 times higher than are levels in maternal or adult sera (Bromberger 1986; Burton 1974; Lewin 1978). Studies in newborns suggest an endogenous synthesis of inositol during fetal life (Bromberger 1986; Pereira 1990). Human milk has a high concentration of inositol, with preterm milk being the richest source. Infants who are breast fed have higher serum inositol levels compared to those that are not breast fed at one to two weeks of life (Bromberger 1986; Pereira 1990). These facts suggest a critical role for inositol in fetal and early neonatal life. Several studies have been published assessing serum inositol levels in the preterm human infant (Bromberger 1986; Hallman 1987; Lewin 1978; Pereira 1990); as well as the effects of inositol supplementation. However, at the time of our original Cochrane Review (Howlett 1997) only two published randomised controlled trials (RCTs) of inositol supplementation (Hallman 1986, and an interim analysis of Hallman 1992 published in 1990) had been subjected to systematic review (Soll 1992). As additional evidence has become available, another critical overview of the use of inositol supplementation that includes all known trials to date was warranted. Maintaining inositol concentrations similar to those occurring naturally in utero may reduce the rates of ROP and BPD in preterm infants.
Why it is important to do this review
This review is an 2019 update, in 2019, of an existing review 'Inositol for respiratory distress syndrome in preterm infants' which was first published in the Cochrane Library in 1997 (Howlett 1997); and updated in 2003, 2012 and 2015 (Howlett 2003; Howlett 2012; Howlett 2015).
Objectives
To assess the effectiveness and safety of supplementary inositol in preterm infants with or without respiratory distress syndrome (RDS) in reducing adverse neonatal outcomes including death (neonatal and infant deaths), BPD, ROP, intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), necrotizing enterocolitis (NEC) and sepsis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials with a control group that received a placebo, low‐dose inositol or no intervention.
Types of participants
Preterm infants (< 37 weeks' postmenstrual age) or low‐birth‐weight (< 2500 grams) infants or both.
Types of interventions
Supplementation with inositol either enterally or intravenously.
Types of outcome measures
Primary outcomes
Death
Neonatal death (death < 28 days postnatal age)
Death during hospital stay (added as an outcome in 2015)
Infant death (death during the first year of life)
Type 1 ROP and death before determination of ROP outcome (added as an outcome in 2019)
All cause mortality (outcomes collected up to 55 weeks' PMA (added as an outcome in 2019)
All‐cause mortality (outcomes collected through the first event; death, hospital discharge, hospital transfer or 120 days after birth (added as an outcome in 2019))
Secondary outcomes
Bronchopulmonary dysplasia (BPD)
BPD oxygen dependency at 28 days of age (including 30 days or one month if that age was used by the authors) with a roentgenogram compatible with BPD
BPD oxygen dependency at 36 weeks' PMA (including 38 weeks' PMA if used by the authors) with a roentgenogram compatible with BPD (Shennan 1988)
BPD requiring oxygen at 36 weeks’ postmenstrual age for oxygen saturation greater than 90% (added as an outcome in 2019)
Retinopathy of prematurity (ROP)
ROP any stage (ICROP 1984)
ROP stage 1 to 2
ROP stage ≥ 3
ROP (number of infants who required surgery for ROP) (added as an outcome in 2015)
Type 1 ROP (defined as meeting the criteria for ophthalmological intervention to prevent retinal detachment) (added as an outcome in 2019)
Necrotizing enterocolitis (NEC) (Bell 1978)
NEC (infants requiring surgery) (added as an outcome in 2015)
Sepsis (clinical signs of sepsis and positive bacterial cultures from normally sterile body fluids or from autopsy material). Early and late sepsis were combined in some analyses
Intraventricular haemorrhage (IVH) any grade (Papile 1978)
IVH grade > 2
Periventricular leukomalacia (PVL)
Developmental impairment at 12 months, 18 months or later in life (assessed using a validated instrument)
Hearing test (failed one or both ears) (added as an outcome in 2015)
Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death (added as an outcome in 2019)
Any adverse effects reported by the authors (added as an outcome in 2015)
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 11) in the Cochrane Library; MEDLINE via PubMed (1966 to 5 November 2018); Embase (1980 to 5 November 2018); and CINAHL (1982 to 5 November 2018).
We used the following search terms: inositol, plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (ClinicalTrials.gov; the World Health Organization’s International Trials Registry and Platform, and the ISRCTN Registry).
We searched trials registries (ClinicalTrials.gov and www.controlled‐trials.com). We searched content on the Pediatric Academic Societies' (PAS) web site published between 2000 and 2018. We searched the Web of Science in August 2011 and in September 2014 using Hallman 1992 as the starting point.
Searching other resources
We searched personal files in August 2011, September 2014 and November 2018. We searched the reference lists of any articles selected for inclusion in this review in order to identify additional relevant articles.
Data collection and analysis
We used the standardized review methods of Cochrane Neonatal to assess the methodological quality of the studies.
For the original review and previous updates of the review the main comparison has been inositol supplementation versus control (Comparison 1) and we included studies under this comparison that provided repeated doses of inositol (by IV or enteral route) to the infants. For the update in 2015, we identified one dose‐finding study in which infants were supplemented with a single dose of inositol (Phelps 2013). We did not consider it appropriate to include the results of this study in the meta‐analyses of repeat doses of inositol and we changed the first comparison to 'inositol supplementation (repeat doses) versus control' (Comparison 1) and added a second comparison, 'inositol supplementation (single dose) versus control' (Comparison 2). These different dosing regimens were not known at the protocol stage and we have made a deviation from the protocol and included a single dose of inositol in our review as those analyses provide important information. The two new studies we included in this update enrolled infants with a PMA up to 29 6/7 weeks (Phelps 2016); and infants with a PMA less than 28 0/7 weeks (Phelps 2018). The three other studies included in Comparison 1 enrolled some infants with a PMA beyond 30 weeks (Friedman 1995; Hallman 1986; Hallman 1992). As most of the adverse outcomes included in this review are highly influenced by PMA with higher rates in infants of low PMA, we therefore performed a separate comparison for studies that used multiple doses of inositol and enrolled infants less than 30 weeks' PMA (Comparison 3).
Selection of studies
We applied machine learning using the Cochrane Classifier tool in the Cochrane Register of Studies (CRS) to remove reports with the least (0% to 2%) probability of being randomised controlled trials and with the least (0% to 100%) probability of having infants in the population.
For this update the three review authors (AH, AO, NP) reviewed the titles (and abstracts when available) in CENTRAL (in the Cochrane Library), MEDLINE, Embase, Web of Science, PAS Abstracts (PAS Abstracts‐AAP.org) and handsearched printouts. We retrieved any article that a review author felt met the inclusion criteria or warranted having its reference list searched. We attempted to locate additional unpublished information from published studies.
Data extraction and management
We developed data extraction forms. The three review authors (AH, AO, NP) independently abstracted information on each study and checked for any discrepancies. AO pooled the results. Data abstraction included: the time period and geographical location of the study, baseline characteristics of the patients, inclusion and exclusion criteria, preparation, route of administration and dosing regime of inositol and placebo. We abstracted information on outcomes and numbers of affected infants. Outcomes included neonatal and infant deaths. We abstracted the total number of infants with 1) BPD at 28 to 30 days of life (oxygen requirements above the concentration in room air at 28 days of life and a chest roentgenogram compatible with BPD) and 2) BPD at 36 to 38 weeks' PMA (oxygen requirements above the concentration in room air at 36 to 38 weeks' PMA and a chest roentgenogram compatible with BPD), as well as information on ROP (stage 0 to 2; ≥ 3); type 1 ROP, IVH (all grades and grade > 2); NEC; and sepsis (early and late onset). We abstracted any adverse effects reported by the authors.
Assessment of risk of bias in included studies
Three review authors (AH, AO, NP) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2011).
• Sequence generation (selection bias) • Allocation concealment (selection bias) • Blinding of participants and personnel (performance bias) • Blinding of outcome assessment (detection bias) • Incomplete outcome data (attrition bias) • Selective reporting (reporting bias) • Any other bias
We resolved any disagreements by discussion or by involving a third assessor to achieve consensus. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
The statistical analyses followed the recommendations of the Cochrane Neonatal Review Group. We calculated a treatment effect using Review Manager 5 software, supplied by Cochrane (Review Manager 2014). The treatment effect estimates included typical relative risk (RR), risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) for dichotomous outcomes; and mean difference (MD) for continuous outcomes. We have reported all estimates of treatment effects with 95% confidence intervals (CI).
Unit of analysis issues
We expected only to encounter data reported as dichotomous or continuous for the whole population randomised.
Dealing with missing data
In the event of missing data, we planned to contact the authors for clarification. For previous updates, we have contacted authors, but for the update in 2012 we found no need to do so. For this 2019 update, we contacted Dr D Phelps, and she and Dr T Nolen provided clarifying information for the Phelps 2016 study.
Assessment of heterogeneity
We performed heterogeneity tests including the I² test to assess the appropriateness of pooling the data. The degree of heterogeneity was roughly categorised according to Higgins and co‐workers as I² less than 25% equals no heterogeneity, 25% to 49% equals low heterogeneity, 50% to 74% equals moderate heterogeneity, and of 75% or more equals high heterogeneity (Higgins 2003).
Assessment of reporting biases
To ascertain the possibility of publication bias, we had planned to perform a funnel plot for the primary outcome of infant death. Because of the small number of studies (< 10) included in all the analyses in the review this was not done.
Data synthesis
Meta‐analyses were performed using Review Manager 2014. For estimates of typical RR and RD we used the Mantel‐Haenszel method. For measured quantities we used the inverse variance method. All meta‐analyses were done using the fixed‐effect model.
Quality of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes.
For Comparison 1 we included the following outcomes in the Table 1: infant death (age < 1 year); bronchopulmonary dysplasia (at 36 to 38 weeks' PMA); ROP (stage ≥ 3 or ≥ 2); sepsis (early or late onset); necrotizing enterocolitis (suspected or proven); and intraventricular haemorrhage grade > 2).
For Comparison two we did not construct a 'Summary of findings' table, as only one study was identified and the study had a small number of infants enrolled (N = 74).
For Comparison three, we included the following outcomes in the Table 2: 'Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome'; 'Type 1 ROP including adjudicated ROP outcome'; 'All‐cause mortality (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth)'; 'BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth)'; 'Severe IVH (grade 3 or 4)'; 'Late‐onset sepsis (> 72 hours of age)'; and 'Suspected or proven NEC'.
The three review authors independently assessed the quality of evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias): consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro Guideline Development Tool (GRADEpro GDT) to create tables to report the quality of evidence.
The GRADE approach yields an assessment of the quality of a body of evidence using one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate.
Subgroup analysis and investigation of heterogeneity
We did perform one subgroup analysis. In Comparison three we included studies that used 'Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA'. As noted under Assessment of heterogeneity we performed heterogeneity tests including the I² test to assess the appropriateness of pooling the data. The degree of heterogeneity was roughly categorised according to Higgins and colleagues as I² less than 25% equals no heterogeneity, 25% to 49% equals low heterogeneity, 50% to 74% equals moderate heterogeneity, and 75% or above equals high heterogeneity (Higgins 2003).
Sensitivity analysis
We did not perform sensitivity analyses.
Results
Description of studies
The results of the searches are shown in the Study flow diagram (Figure 1). For study details please refer to the table Characteristics of included studies.
1.
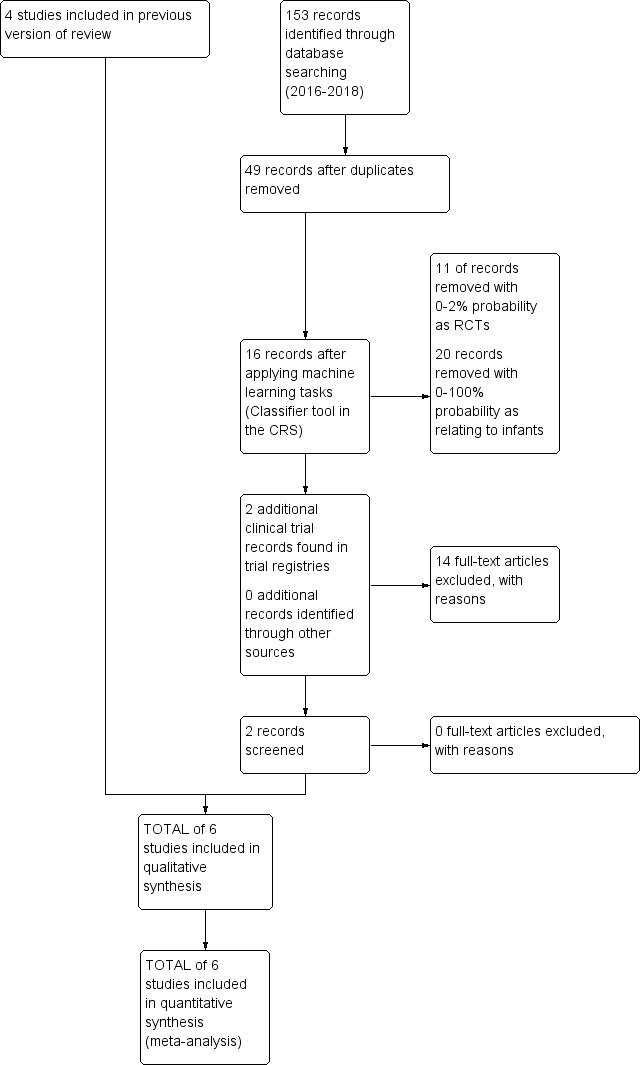
Study flow diagram: review update
Hallman 1986 was a randomised, placebo‐controlled, double blind single‐centre study performed in Helsinki, Finland.
Objective: to assess the effects of inositol supplementation provided for 10 days.
Population: 74 preterm infants (37 in each group) with RDS, birth weight (BW) < 2000 g.
Intervention: both IV (75% of the intragastric dose when enteral inositol could not be given) and enteral (intragastric) (160 mg/kg/day, divided in four doses) inositol were used, and the control group received a placebo (5% glucose).
Outcomes assessed: neonatal death, infant death, BPD (at 28 days), NEC, ROP, IVH, and sepsis.
Hallman 1992 was a placebo‐controlled, randomised, double blind trial conducted in Helsinki, Finland.
Objective: to assess the effects of IV inositol supplementation in the first five days of life in preterm infants with RDS.
Population: 221 infants (24 to 32 weeks' PMA, BW < 2000 g, age two to 10 hours and mechanically ventilated) were enrolled, of which 119 were randomised to receive inositol. All enrolled infants were stratified according to whether they had received surfactant as part of another ongoing study.
Intervention: inositol or placebo (glucose) was given as a 5% solution IV The dosage was 80 mg/kg of body weight per day, given for five days.
Outcomes assessed: neonatal death, infant death, BPD, ROP, patent ductus arteriosus (PDA), IVH, NEC, infection and neurodevelopmental impairment at 12 months' corrected age.
Friedman 1995 was a placebo‐controlled, randomised trial conducted in two units in the USA.
Objective: to examine the relationship between the intake of sugar inositol, serum inositol levels and ROP in LBW infants.
Population: 48 preterm infants (BW < 1500 g with severe lung disease) were enrolled, of which 24 were randomised to receive either standard enteral feeds (SC 24 242 µmol/L of inositol) or supplemented formula high in inositol (SC 30 2500 µmol/L of inositol).
Intervention: 24 infants received formula containing 242 µmol/L of inositol (control group) and 24 infants received high‐concentration inositol (2500 µmol/L of inositol). Duration not stated.
Outcomes: neonatal death, infant death, BPD, NEC, IVH and ROP.
Phelps 2013 was a multi‐centre, randomised, placebo‐controlled trial conducted in 10 units belonging to the National Institutes of Child Health and Human Development Neonatal Research Network.
Objective: to describe the pharmacokinetics (PK) of a single IV dose of inositol in 23 to 29 weeks' gestational age infants.
Population: 74 infants were randomised in PMA strata (23 to 26 weeks' PMA (N = 37) or 27 to 29 weeks' PMA (N = 37)) to receive either inositol or 5% glucose.
Intervention: infants received a single dose of 5% myon‐inositol (60 mg/kg or 120 mg/kg) (N = 49) within six days of birth and before enteral feeds began, or 5% glucose (N = 25).
Outcomes: inositol was measured over 96 hours in serum and timed urine collections. "Morbidity and mortality were prospectively recorded through discharge or 120 days of postnatal age." Outcomes included: death during hospital stay, BPD at 36 weeks' PMA, ROP (number of infants, who underwent surgery for ROP), NEC (stage II A or worse), NEC (number of infants who underwent surgery), sepsis (late onset), IVH (grade 3 or 4) and hearing test (failed both ears).
Phelps 2016 was a multi‐centre, randomised, placebo‐controlled trial conducted in 14 units belonging to the National Institutes of Child Health and Human Development Neonatal Research Network.
Objective: to assess the safety and pharmacokinetics of daily inositol to select a dose providing serum levels previously associated with benefit, and to learn if accumulation occurred when administered throughout the normal period of retinal vascularization.
Population: infants ≤ 29 weeks' PMA (23 0/7 to 29 weeks' PMA), who weighed at least 400 g, and could receive study drug by 72 h after birth (N = 122)
Intervention: myo‐inositol (5% solution) at 10, 40 or 80 mg/kg/day was given IV and converted to enteral when feedings were established, and continued to the first of 10 weeks, 34 weeks' PMA, death or discharge. Total number randomised: 10 mg/kg (N = 29); 40 mg/kg (N = 30); 80 mg/kg (N = 28). Placebo: 5% glucose (N = 35).
Outcomes: an unfavourable outcome was defined as either type 1 ROP or worse, in either eye, or surgical intervention for severe ROP in either eye. The final ROP status was judged separately in each eye as 'probably favourable', 'probably unfavourable' or 'cannot be determined', and the majority classification was assigned as the adjudicated outcome. Additional outcomes included: death , BPD, RDS, PDA, IVH. seizures, cystic areas in brain parenchyma, sepsis (early and late onset), NEC (suspected or proven), NEC requiring surgery, spontaneous gastrointestinal (GI) perforation and hearing screen failed (either ear). At 18 to 22 months' corrected age, infants received a set of standardized examinations of neurologic function and development according to the NRN Follow‐Up Protocol (to be reported separately).
Phelps 2018 was a multi‐centre, randomised, placebo‐controlled trial conducted in 18 units belonging to the National Institutes of Child Health and Human Development Neonatal Research Network.
Objective: to test the adverse events and efficacy of myo‐inositol to reduce type 1 ROP among infants younger than 28 weeks' PMA.
Population: 638 infants < 28 weeks' PMA were randomised to receive either myo‐inositol or placebo.
Intervention: A 40 mg/kg dose of myo‐inositol was given every 12 hours (initially IV, then enterally when feeding; N = 317), or placebo (N = 321) for up to 10 weeks.
Outcomes: type 1 ROP or death before determination of ROP outcome was designated as unfavourable. The designated favourable outcome was survival without type 1 ROP. Other included outcomes were: type 1 ROP, any ROP, ROP ≥ 2 ROP, all‐cause mortality to 55 weeks' PMA, all‐cause mortality (outcomes collected to the first event: death, hospital discharge, hospital transfer, or 120 days after birth), BPD defined as requiring oxygen at 36 weeks' PMA for oxygen saturation > 90%, BPD or death caused by it prior to 37 weeks' gestation (outcomes collected through the first event: death, hospital discharge, hospital transfer, or 120 days after birth, severe IVH (grade 3 or 4), late onset sepsis (> 72 h of age), suspected or proven NEC, surgical NEC, spontaneous GI perforation without NEC, pulmonary haemorrhage, PDA, PDA requiring indomethacin, PDA requiring surgery, seizure treatment(≥ 2 days), hearing screen failed (either ear), and cystic areas in brain parenchyma,
We did not identify any ongoing studies.
Results of the search
The searches in November 2018 identified two additional studies (Phelps 2016; Phelps 2018). For details see 'Study flow diagram: review update' (Figure 1).
Included studies
The review currently includes six studies (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2013; Phelps 2016; Phelps 2018). The total number of infants randomised in these studies was 1177, an increase in sample size from the previous update — Howlett 2015 — of 760 infants.
Excluded studies
For this update of the review we did not identify any additional studies for exclusion.
Risk of bias in included studies
For details see 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3). Three studies reported that the randomisation sequence was generated by computer (Phelps 2013; Phelps 2016; Phelps 2018); and in three studies, it was unclear how the randomisation sequence was generated (Friedman 1995; Hallman 1986; Hallman 1992).
2.
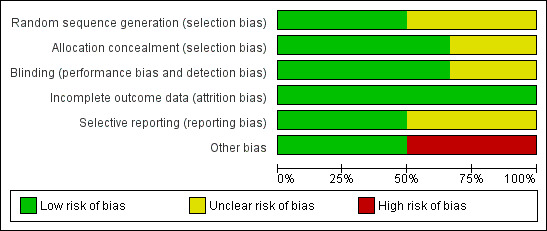
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
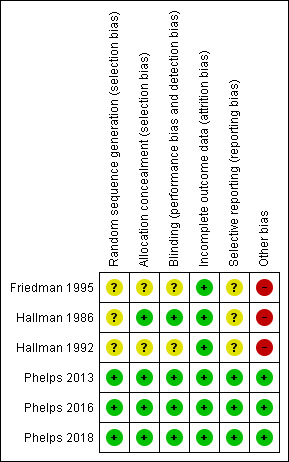
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two reports lacked written information on allocation concealment (Friedman 1995; Hallman 1992). In the studies by Hallman 1986,Phelps 2013, Phelps 2016 and Phelps 2018 the allocation was conducted centrally.
Blinding
Friedman 1995 did not provide any information on whether the clinical staff and the researchers were blinded. In the study by Hallman 1986 the clinicians and the researchers were blinded to which solution (inositol or glucose) the infants received. Only the pharmacist preparing the doses knew the contents of the drug packages. In the study by Hallman 1992, 5% glucose was given as placebo, but no information was provided on whether staff were blinded to study drugs or not. In the study by Phelps 2013 the drug or placebo was dispensed from the respective pharmacies in unit doses labelled as 'inositol study drug', and all clinical and research personnel except the pharmacist were masked to the study drug. In the two most recent studies by Phelps (Phelps 2016; Phelps 2018), care takers and outcome assessors were blinded to the intervention and the outcome assessments.
Incomplete outcome data
Incomplete outcome data were addressed and the reports seemed free of incomplete data in all studies.
Selective reporting
The studies by Phelps 2013,Phelps 2016 and Phelps 2018 were entered into a trials registry and there did not appear to be any differences between the published protocol and the full report.
The study protocols were not available to us for the studies by Friedman 1995,Hallman 1986 and Hallman 1992, so we can not judge if there were any deviations from the study as planned and the final report.
Other potential sources of bias
Three studies undertook interim analyses (Friedman 1995; Hallman 1986; Hallman 1992). Thus the code must have been broken and that might have influenced decisions on when to close the studies. In two studies (Phelps 2013, Phelps 2016), interim analyses were not undertaken. The Phelps 2018 study was terminated early due to a statistically significantly higher mortality rate in the myo‐inositol group. At 18 months, trial enrolment and treatment were suspended because of a manufacturing issue (later identified as glass lamellae in the third lot of drug, which was never used). Glass lamellae were subsequently found in 1.9% of stored vials of lot two of the trial drug. Detailed analyses revealed that there were no differences in the outcomes for infants treated with myo‐inositol between the two lots of the trial drug. Because the trial did not enrol as many infants as the preplanned sample size, it was underpowered to make conclusions regarding the efficacy and safety of myo‐inositol (Phelps 2018). We did not consider this early stopping of the trial a source of bias.
Effects of interventions
Effects of intervention section The updated literature search detected six published reports (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2013; Phelps 2016; Phelps 2018). Hallman 1986, Hallman 1992 and Friedman 1995 have all been published as interim analyses, when fewer neonates were enrolled than in the final publication.
Inositol supplementation IV or enterally (repeat doses of any amount and duration) versus control (Comparison 1)
Primary outcomes
Neonatal death, age < 28 days (Outcome 1.1)
Neonatal death was reported in three studies (N = 355) (Friedman 1995; Hallman 1986; Hallman 1992). There was a significant reduction in death prior to 28 days of age in the inositol compared to the control group (typical RR 0.53, 95% CI 0.31 to 0.91; typical RD −0.09, 95% CI −0.16 to −0.01; NNTB 11, 95% CI 6 to 100); I² was 0% for RR (none) and 58% (moderate) for RD (Analysis 1.1).
1.1. Analysis.
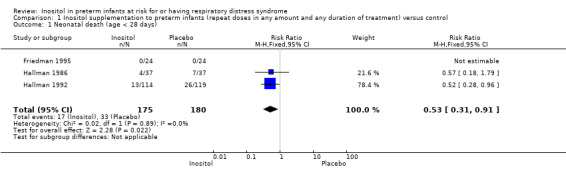
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 1 Neonatal death (age < 28 days).
Infant death, age < one year (Outcome 1.2)
Infant death was reported in five studies (N = 1115) (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2016; Phelps 2018). There was no significant change in infant deaths in the inositol compared to the control group (typical RR 0.89, 95% CI 0.71 to 1.13; typical RD −0.02, 95% CI −0.07 to 0.02). I² was 80% for RR and 84% for RD (both high) (Analysis 1.2). The certainty of the evidence according to GRADE was low.
1.2. Analysis.
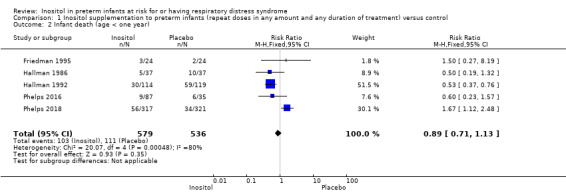
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 2 Infant death (age < one year).
Secondary outcomes
BPD (supplementary oxygen at 36 weeks' PMA or death due to BPD at 36 weeks' PMA) (Outcome 1.3)
BPD according to this definition was reported in two studies (N = 666) (Phelps 2016; Phelps 2018). There was no significant difference in BPD in the inositol compared to the control group (typical RR 1.00, 95% CI 0.87 to 1.14; typical RD −0.00, 95% CI −0.08 to 0.07); I² test was 0% (none) for both RR and RD (Analysis 1.3).
1.3. Analysis.
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 3 BPD (supplementary oxygen ar 36 weeks; PMA or death due to BPD)at 36 week's PMA.
Bronchopulmonary dysplasia (BPD) at 28 to 30 days (Outcome 1.4)
Three studies (N = 343) examined the effect of inositol on BPD at 28 to 30 days (Friedman 1995; Hallman 1986; Hallman 1992). There was no significant difference between the groups (typical RR 0.78, 95% CI 0.54 to 1.13; typical RD −0.06, 95% CI −0.15 to 0.03); I² was 49% (low) for RR and 31% (low) for RD (Analysis 1.4).
1.4. Analysis.
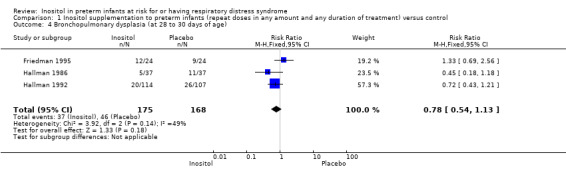
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 4 Bronchopulmonary dysplasia (at 28 to 30 days of age).
Bronchopulmonary dysplasia (BPD) at 36 to 38 weeks' PMA (Outcome 1.5)
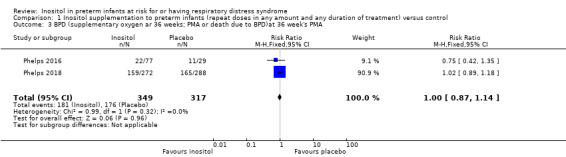
Two studies (N = 737) reported on this outcome (Hallman 1992; Phelps 2018). There was no significant difference between the inositol supplementation group and the control group (RR 1.04, 95% CI 0.90 to 1.20; RD 0.02, 95% CI −0.05 to 0.09); I² was 0% (none) for both RR and RD (Analysis 1.5). The certainty of the evidence according to GRADE was moderate.
1.5. Analysis.
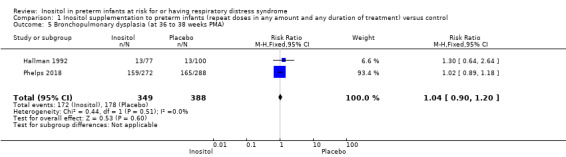
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 5 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA).
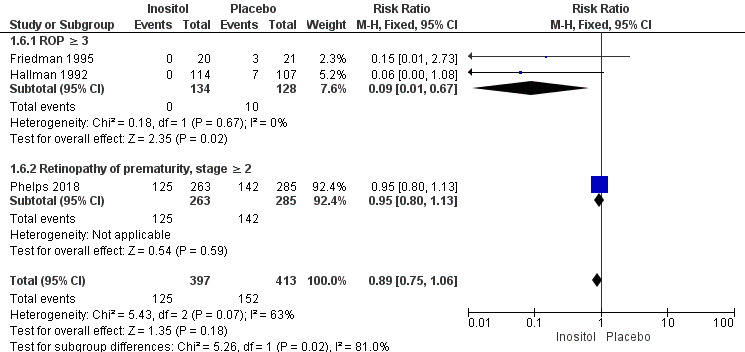
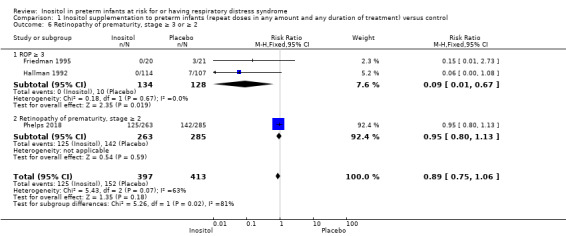
Retinopathy of prematurity (ROP), stage ≥ 3 or ≥ 2 (Outcome 1.6)
Three studies (N = 810) reported on this outcome (Friedman 1995; Hallman 1992; Phelps 2018) (Figure 4). There was no significant difference in ROP stage 3 or more or stage 2 or more in the inositol compared to the control group (typical RR 0.89, 95% CI 0.75 to 1.06; typical RD −0.04, 95% CI −0.10 to 0.02); I² was 63% (moderate) for RR and 23% (none) for RD (Analysis 1.6).
4.
Forest plot of comparison: 1 Inositol supplementation (repeat doses in any amount and any duration of treatment) versus control, outcome: 1.5 Retinopathy of prematurity, stage ≥ 3.
1.6. Analysis.
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 6 Retinopathy of prematurity, stage ≥ 3 or ≥ 2.
For the subgroup of ROP stage 3 or more, two studies (N = 262) reported on this outcome (Friedman 1995; Hallman 1992). There was a significantly lower incidence of ROP stage 3 or more in the inositol compared to the control group (typical RR 0.09, 95% CI 0.01 to 0.67; typical RD −0.08, 95% CI −0.13 to −0.03; I² was 0% (none) for RR and 0% (none) for RD; NNTB 13 (95% CI 8 to 33) (Outcome 1.61).
For the subgroup of ROP stage 2 or more, one study (N = 548) reported on this outcome (Phelps 2018). There was no significant difference in the incidence of ROP stage 2 or more in the inositol compared to the control group (typical RR 0.95, 95% CI 0.80 to 1.13; typical RD −0.02, 95% CI −0.11 to 0.06); the I² test was not applicable as there was only one study in the analysis (Outcome 1.62). The certainty of the evidence according to GRADE was moderate.
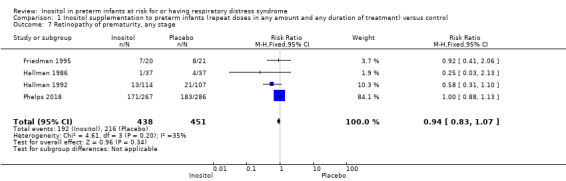
Retinopathy of prematurity (ROP), all (any) stages (Outcome 1.7)
Four trials (N = 889) reported the effect of inositol on the outcome of ROP, all stages (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2018). There was no significant difference in the incidence of ROP, any stage, in the inositol compared to the control group (typical RR 0.94, 95% CI 0.83 to 1.07; typical RD −0.03, 95% CI −0.09 to 0.03). I² was 35% (low) for RR and 0% (none) for RD (Analysis 1.7).
1.7. Analysis.
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 7 Retinopathy of prematurity, any stage.
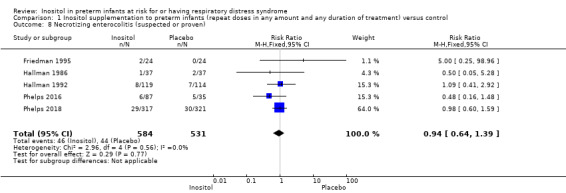
Necrotizing enterocolitis (NEC) ‒ suspected or proven (Outcome 1.8)
Five studies (N = 1115) reported on this outcome (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2016;Phelps 2018). The incidence of NEC was not significantly influenced by the use of inositol supplementation (typical RR 0.94, 95% CI 0.64 to 1.39; typical RD −0.00, 95% CI −0.04 to 0.03); I² was 0% (none) for both RR and RD (Analysis 1.8). The certainty of the evidence according to GRADE was moderate.
1.8. Analysis.
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 8 Necrotizing enterocolitis (suspected or proven).
Sepsis (early and/or late onset (Outcome 1.9))
Four studies (N = 1067) reported on this outcome (Hallman 1986; Hallman 1992; Phelps 2016; Phelps 2018). There was no significant effect of the use of inositol supplementation (typical RR 1.21, 95% CI 0.95 to 1.54; typical RD 0.04, 95% CI −0.01 to 0.09); I² was 24% (none) for RR and 34% (low) for RD (Analysis 1.9). The certainty of the evidence according to GRADE was moderate.
1.9. Analysis.
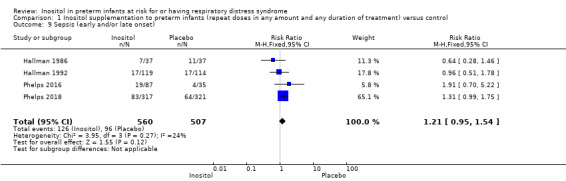
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 9 Sepsis (early and/or late onset).
Intraventricular haemorrhage (IVH), grade > 2 (Outcome 1.10)
Five trials (N = 1103) reported on this outcome (Friedman 1995; Hallman 1986; Hallman 1992; Phelps 2016; Phelps 2018). There was no significant difference in the incidence of IVH grade greater than 2 following treatment with inositol (typical RR 0.77, 95% CI 0.58 to 1.01, P = 0.06; typical RD −0.04, 95% CI −0.09 to 0.00; P = 0.06); I² was 48% (low) for RR and 42% (low) for RD (Analysis 1.10). The certainty of the evidence according to GRADE was moderate.
1.10. Analysis.
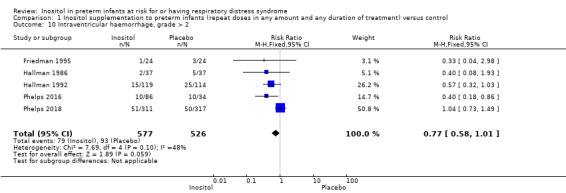
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 10 Intraventricular haemorrhage, grade > 2.
Intraventricular haemorrhage (IVH), all grades (Outcome 1.11)
Three studies (N = 427) reported on this outcome (Hallman 1986; Hallman 1992; Phelps 2016). There was no significant effect of inositol on IVH, all grades (typical RR 0.77, 95% CI 0.59 to 1.00, P = 0.05; typical RD −0.09, 95% CI −0.19 to 0.00); P = 0.05. The I² was 0% (none) for both RR and RD (Analysis 1.11).
1.11. Analysis.
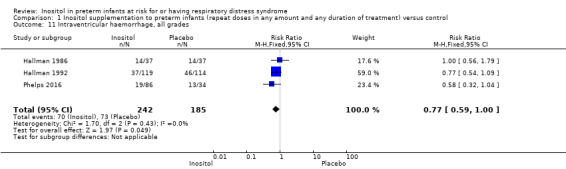
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 11 Intraventricular haemorrhage, all grades.
Minor neural developmental impairment at one year corrected age (impairment defined as sensorimotor abnormality and/or developmental delay) (Outcome 1.12)
One study (N = 169) reported on this outcome (Hallman 1992). There was no significant effect of inositol (RR 0.84, 95% CI 0.38 to 1.86; RD −0.02, 95% CI −0.12 to 0.08). Tests for heterogeneity were not applicable (Analysis 1.12).
1.12. Analysis.
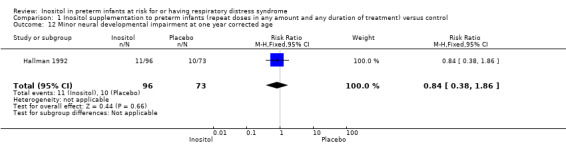
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 12 Minor neural developmental impairment at one year corrected age.
Major neural developmental impairment at one year corrected age (impairment defined as sensory deficit, cerebral palsy, developmental delay, severe hypotonia) (Outcome 1.13)
One study (N = 169) reported on this outcome (Hallman 1992). There was no significant effect of inositol (RR 0.53, 95% CI 0.24 to 1.16; RD −0.08, 95% CI −0.19 to 0.02). Tests for heterogeneity were not applicable (Analysis 1.13).
1.13. Analysis.
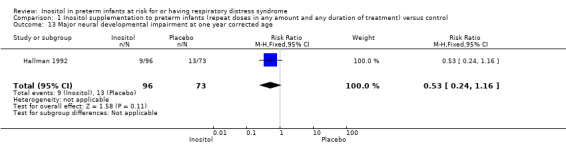
Comparison 1 Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control, Outcome 13 Major neural developmental impairment at one year corrected age.
Periventricular leukomalacia (PVL)
This outcome was not reported in our included studies. Cystic areas in the cerebral parenchyma measured through 28 days of life are reported under Comparison 3 (Analysis 3.12).
3.12. Analysis.
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 12 Cystic areas in the cerebral parenchyma measured through 28 d.
Inositol supplementation (single dose) versus control (Comparison 2)
One study compared inositol supplementation in a single dose of 60 mg/kg or 120 mg/kg with placebo (Phelps 2013). We combined the outcomes for the two groups that received a different dose of inositol. As only one study was included under this comparison, tests for heterogeneity were not applicable for any of the outcomes listed below.
Primary outcome
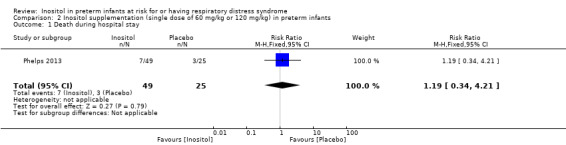
Death during hospital stay (Outcome 2.1)
One study reported on this outcome in 74 infants (Phelps 2013). There was no significant effect of inositol (RR 1.19, 95% CI 0.34 to 4.21; RD 0.02, 95% CI −0.14 to 0.18) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 1 Death during hospital stay.
Secondary outcomes
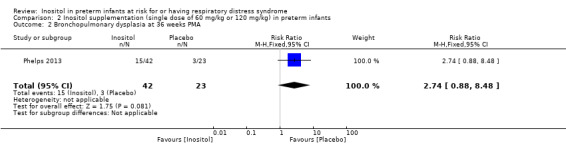
Bronchopulmonary dysplasia (BPD) at 36 weeks' PMA (Outcome 2.2)
One study reported on this outcome in 65 infants (Phelps 2013). There was no significant effect of inositol on this outcome for RR (2.74, 95% CI 0.88 to 8.48; P = 0.08) but the RD was 0.23 (95% CI 0.03 to 0.43; P = 0.03) with NNTB of 4 (95% CI 2 to 33) (Analysis 2.2).
2.2. Analysis.
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 2 Bronchopulmonary dysplasia at 36 weeks PMA.
Retinopathy of prematurity (ROP), infants who underwent surgery for ROP (Outcome 2.3)
One study reported on this outcome in 25 infants (Phelps 2013). There was no significant effect of inositol (RR 0.35, 95% CI 0.10 to 1.22; RD −0.32, 95% CI −0.71 to 0.07) (Analysis 2.3).
2.3. Analysis.
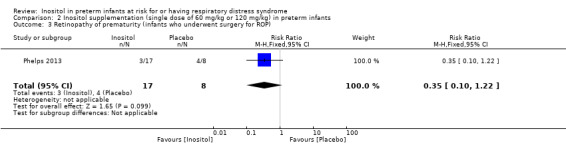
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 3 Retinopathy of prematurity (infants who underwent surgery for ROP).
Necrotizing enterocolitis (NEC), stage 2A or worse (Outcome 2.4)
One study reported on this outcome in 74 infants (Phelps 2013). There was no significant effect of inositol (RR 0.41, 95% CI 0.12 to 1.39; RD −0.12, 95% CI −0.29 to 0.06) (Analysis 2.4).
2.4. Analysis.
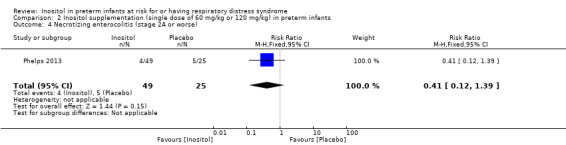
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 4 Necrotizing enterocolitis (stage 2A or worse).
Necrotizing enterocolitis (NEC), infants who underwent surgery for NEC (Outcome 2.5) (Analysis 2.5)
One study (Phelps 2013) reported on this outcome in 74 infants. There was no significant effect of inositol (RR 0.51, 95% CI 0.08 to 3.41; RD −0.04, 95% CI −0.16 to 0.08). Analysis 2.5
2.5. Analysis.
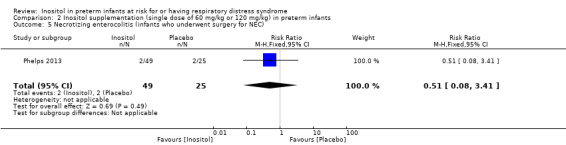
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 5 Necrotizing enterocolitis (infants who underwent surgery for NEC).
Sepsis, late onset (Outcome 2.6)
One study reported on this outcome in 74 infants (Phelps 2013). There was no significant effect of inositol (RR 1.46, 95% CI 0.71 to 2.97; RD 0.13, 95% CI −0.10 to 0.35) (Analysis 2.6).
2.6. Analysis.
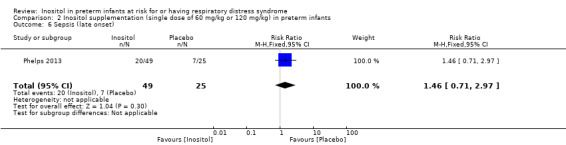
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 6 Sepsis (late onset).
Intraventricular haemorrhage (IVH), grade 3 or 4 (Outcome 2.7)
One study reported on this outcome in 72 infants (Phelps 2013). There was no significant effect of inositol (RR 1.06, 95% CI 0.29 to 3.90; RD 0.01, 95% CI −0.15 to 0.17) (Analysis 2.7)
2.7. Analysis.
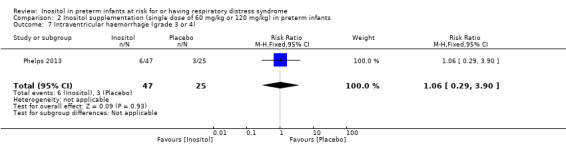
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 7 Intraventricular haemorrhage (grade 3 or 4).
Hearing test (failed both ears) (Outcome 2.8) (Analysis 2.8)
One study reported on this outcome in 57 infants (Phelps 2013). There was no significant effect of inositol (RR 0.58, 95% CI 0.09 to 3.84; RD −0.04, 95% CI −0.19 to 0.11) (Analysis 2.8).
2.8. Analysis.
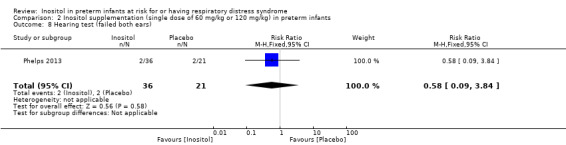
Comparison 2 Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants, Outcome 8 Hearing test (failed both ears).
Inositol supplementation; IV initially, followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA (Comparison 3)
Tests for heterogeneity (I² test) were not applicable for analyses which had only one included study.
Type 1 ROP or death before determination of ROP outcome using adjudicated ROP outcome (Outcome 3.1)
Two studies reported on this outcome (N = 679) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.28, 95% CI 0.99 to 1.67; typical RD 0.06, 95% CI −0.00 to 0.13); I² 79% for RR and 85% for RD (both high) (Analysis 3.1). The certainty of the evidence according to GRADE was moderate.
3.1. Analysis.
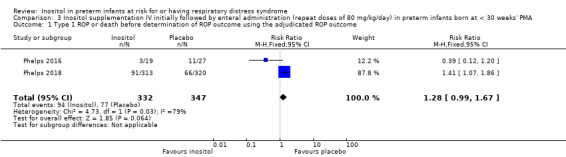
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 1 Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome.
Type 1 ROP (Outcome 3.2)
One study reported on this outcome (N = 511) (Phelps 2018).There was no significant effect of inositol compared to placebo for this outcome (RR 1.41, 95% CI 0.89 to 2.24; RD 0.04, 95% CI −0.01 to 0.10) (Analysis 3.2).
3.2. Analysis.
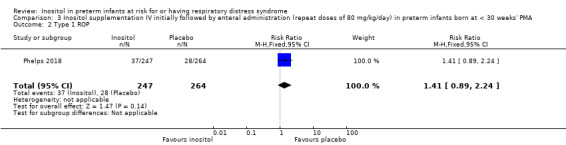
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 2 Type 1 ROP.
Death before determination of ROP outcome (Outcome 3.3)
One study reported on this outcome (N = 638) (Phelps 2018). There was a significantly higher incidence of death before determination of ROP outcome in the inositol group compared with the placebo group (RR 1.53, 95% CI 1.02 to 2.31, P = 0.04; RD 0.05, 95% CI 0.00 to 0.11, P = 0.04); NNTH 33 (95% CI 9 to infinity) (Analysis 3.3).
3.3. Analysis.
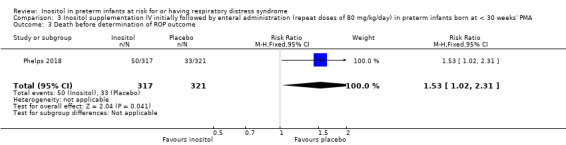
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 3 Death before determination of ROP outcome.
Type 1 ROP including adjudicated ROP outcome (Outcome 3.4)
Two studies reported on this outcome (N = 605) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.24, 95% CI 0.82 to 1.86; typical RD 0.03, 95% CI −0.03 to 0.08); I² 46 % (low) for RR and 54% (moderate) for RD (Analysis 3.4). The certainty of the evidence according to GRADE was moderate (Figure 5).
3.4. Analysis.
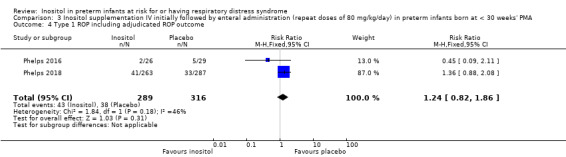
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 4 Type 1 ROP including adjudicated ROP outcome.
5.
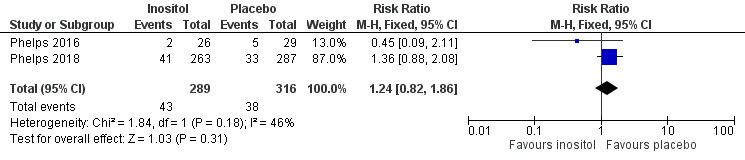
Forest plot of comparison: 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, outcome: 3.4 Type 1 ROP including adjudicated ROP outcome.
Any ROP (Outcome 3.5)
One study reported on this outcome (N = 553) (Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 1.00, 95% CI 0.88 to 1.13; RD 0.00, 95% CI −0.08 to 0.08) (Analysis 3.5).
3.5. Analysis.
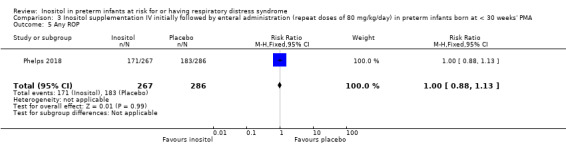
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 5 Any ROP.
ROP stage ≥ 2 ROP (Outcome 3.6)
One study reported on this outcome (N = 548) (Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 0.95, 95% CI 0.80 to 1.13; RD −0.02, 95% CI −0.11 to 0.06) (Analysis 3.6).
3.6. Analysis.
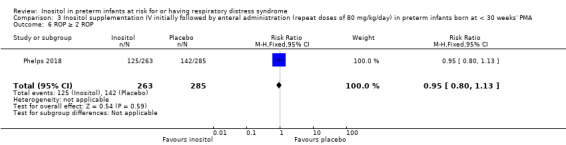
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 6 ROP ≥ 2 ROP.
All‐cause infant mortality to 55 weeks' PMA (Outcome 3.7)
One study reported on this outcome (N = 638) (Phelps 2018). There was a significant higher mortality in the inositol group compared to the placebo group (RR 1.67, 95% CI 1.12 to 2.48; RD 0.07, 95% CI 0.02 to 0.12); NNTH 14 (95% CI 8 to 50) (Analysis 3.7).
3.7. Analysis.
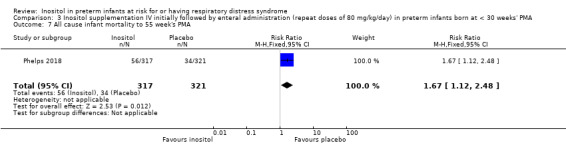
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 7 All cause infant mortality to 55 week's PMA.
All‐cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth (Outcome 3.8))
Two studies reported on this outcome (N = 701) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.35, 95% CI 0.91 to 2.00; typical RD 0.04, 95% CI −0.01 to 0.09); I² = 72% (moderate) for RR; and 84% (high) for RD (Analysis 3.8). The certainty of the evidence according to GRADE was moderate.
3.8. Analysis.
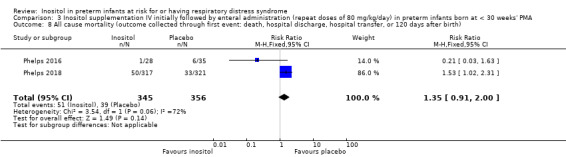
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 8 All cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth).
BPD (requiring oxygen at 36 weeks' PMA for oxygen saturation > 90%) (Outcome 3.9)
One study reported on this outcome (N = 560) (Phelps 2018).There was no significant effect of inositol compared to placebo for this outcome (RR 1.02, 95% CI 0.89 to 1.18; RD 0.01, 95% CI −0.07 to 0.09) (Analysis 3.9).
3.9. Analysis.
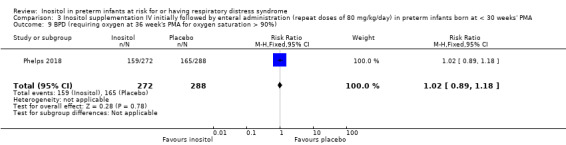
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 9 BPD (requiring oxygen at 36 week's PMA for oxygen saturation > 90%).
BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth (Outcome 3.10)
Two studies reported on this outcome (N = 616) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.01, 95% CI 0.87 to 1.16; typical RD 0.00, 95% CI −0.07 to 0.08); I² = 0% (none) for both RR and RD (Analysis 3.10). The certainty of the evidence according to GRADE was high.
3.10. Analysis.
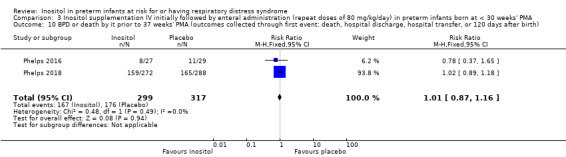
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 10 BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth).
Severe IVH (grade 3 or 4) (Outcome 3.11)
Two studies reported on this outcome (N = 690) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 0.92, 95% CI 0.65 to 1.29; typical RD −0.01, 95% CI −0.07 to 0.04); I² = 74% (moderate) for RR and 82% (high) for RD (Analysis 3.11). The certainty of the evidence according to GRADE was moderate.
3.11. Analysis.
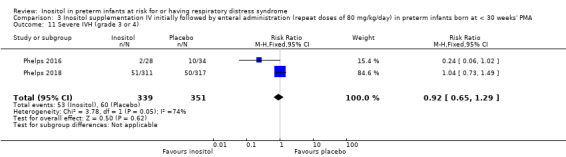
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 11 Severe IVH (grade 3 or 4).
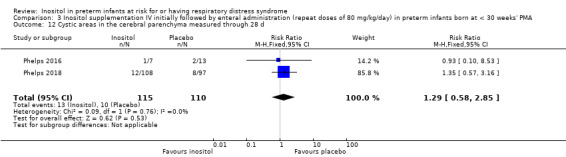
Cystic areas in the cerebral parenchyma measured through 28 d (Outcome 3.12)
Two studies reported on this outcome (N = 225) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR, 1.29, 95% CI 0.58 to 2.85; typical RD 0.03, 95% CI −0.05 to 0.10); I² = 0% for both RR and RD (Analysis 3.12).
Early onset sepsis (Outcome 3.13)
One study reported on this outcome (N = 63) (Phelps 2016). There was no significant effect of inositol compared to placebo for this outcome (RR, not estimable as there were no outcomes in either group; RD 0.00, 95% CI −0.06 to 0.06) (Analysis 3.13).
3.13. Analysis.
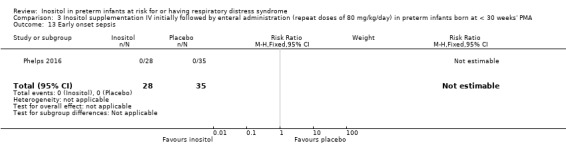
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 13 Early onset sepsis.
Late onset sepsis (> 72 hours of age) (Outcome 3.14)
Two studies reported on this outcome (N = 701) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.33, 95% CI 1.00 to 1.75; typical RD 0.06, 95% CI 0.00 to 0.12; P = 0.05 for both RR and RD); I² = 0% (none) for both RR and RD (Analysis 3.14). The certainty of the evidence according to GRADE was high. (Figure 6)
3.14. Analysis.
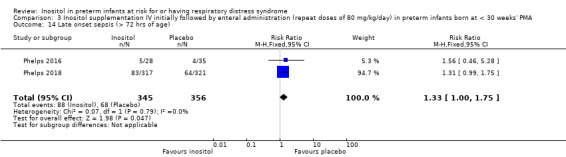
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 14 Late onset sepsis (> 72 hrs of age).
6.
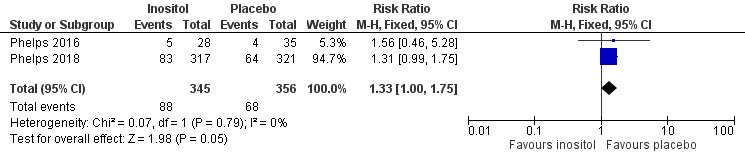
Forest plot of comparison: 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, outcome: 3.14 Late onset sepsis (> 72 hours of age).
Suspected or proven NEC (Outcome 3.15)
Two studies reported on this outcome (N = 701) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 0.88, 95% CI 0.55 to 1.41; typical RD −0.01, 95% CI −0.05 to 0.03); I² = 36% (low) for RR; and 53% (moderate) for RD (Analysis 3.15). The certainty of the evidence according to GRADE was high.
3.15. Analysis.
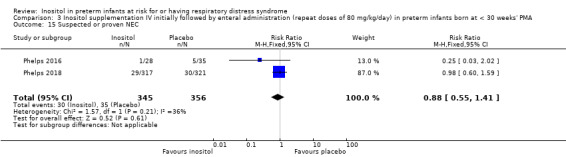
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 15 Suspected or proven NEC.
Surgical NEC (Outcome 3.14) (Analysis 3.16)
Two studies reported on this outcome (N = 701) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.21, 95% CI 0.57 to 2.58; typical RD 0.01, 95% CI −0.02 to 0.04); I² = 51% (moderate) for RR; and 69% (moderate) for RD. Analysis 3.16
3.16. Analysis.
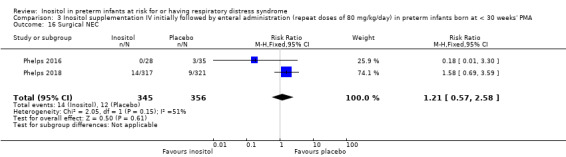
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 16 Surgical NEC.
Spontaneous gastro‐intestinal perforation (Outcome 3.17)
Two studies reported on this outcome (N = 701) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 0.86, 95% CI 0.48 to 1.52; typical RD −0.01, 95% CI −0.05 to 0.03); I² = 0% (none) for both RR and RD. Analysis 3.17
3.17. Analysis.
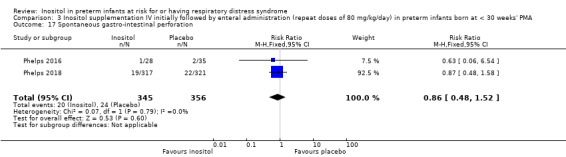
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 17 Spontaneous gastro‐intestinal perforation.
Pulmonary haemorrhage (Outcome 3.18)
One study reported on this outcome (N = 638) (Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 0.98, 95% CI 0.59 to 1.62; RD −0.00, 95% CI −0.05 to 0.04) (Analysis 3.18).
3.18. Analysis.
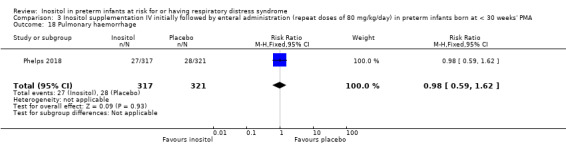
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 18 Pulmonary haemorrhage.
PDA (Outcome 3.19)
Two studies reported on this outcome (N = 700) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 0.98, 95% CI 0.85 to 1.14; typical RD −0.01, 95% CI −0.08 to 0.07); I² = 0% (none) for both RR and RD (Analysis 3.19).
3.19. Analysis.
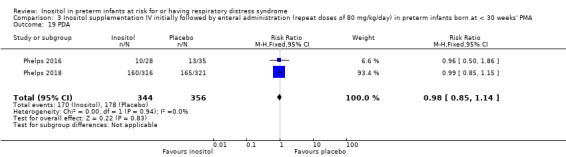
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 19 PDA.
PDA requiring indomethacin (Outcome 3.20)
One study reported on this outcome (N = 637) (Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 0.90, 95% CI 0.67 to 1.22; RD −0.02, 95% CI −0.09 to 0.04) (Analysis 3.20).
3.20. Analysis.
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 20 PDA requiring indomethacin.
PDA requiring surgery (Outcome 3.21)
Two studies reported on this outcome (N = 700) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 0.96, 95% CI 0.65 to 1.42; RD −0.00, 95% CI −0.05 to 0.04); I² = 0% (none) for both RR and RD (Analysis 3.21).
3.21. Analysis.
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 21 PDA requiring surgery.
Seizures (treatment for ≥ 2 days) (Outcome 3.22)
Two studies reported on this outcome (N = 700) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.04, 95% CI 0.43 to 2.56; typical RD 0.00, 95% CI −0.02 to 0.02); I² = 7% (none) for RR and 38% (low) for RD (Analysis 3.22).
3.22. Analysis.
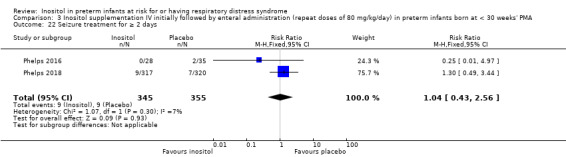
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 22 Seizure treatment for ≥ 2 days.
Negative hearing screen in either ear at discharge (Outcome 3.23)
Two studies reported on this outcome (N = 472) (Phelps 2016; Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (typical RR 1.45, 95% CI 0.92 to 2.29; typical RD 0.05, 95% CI −0.01 to 0.11); I² = 0% (none) for both RR and RD (Analysis 3.23).
3.23. Analysis.
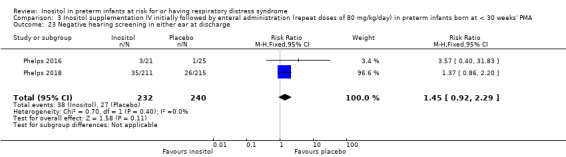
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 23 Negative hearing screening in either ear at discharge.
Respiratory distress syndrome (Outcome 3.24)
One study (Phelps 2016) reported on this outcome (N = 63). There was no significant effect of inositol compared to placebo for this outcome (RR 0.99, 95% CI 0.91 to 1.09; RD −0.01, 95% CI −0.10 to 0.08) (Analysis 3.24).
3.24. Analysis.
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 24 Respiratory distress syndrome.
Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death (Outcome 3.25)
One study reported on this outcome among deaths (N = 83) (Phelps 2018). There was no significant effect of inositol compared to placebo for this outcome (RR 1.36, 95% CI 0.95 to 1.93; RD 0.19, 95% CI −0.01 to 0.40).
Discussion
Summary of main results
In our previous update of this review in 2015 (Howlett 2015), statistically significant reductions in neonatal deaths, infant death and IVH grade higher than 2 were demonstrated with repeat doses of inositol supplementation, and a striking reduction was found in ROP stage 3 or above. There was no significant reduction in BPD and there was no significant increase in potentially adverse effects such as sepsis, NEC or neurological impairment at 12 months' corrected age. We suggested that the results of the review, which showed promising significant results in favour of inositol, should be interpreted with caution — the numbers of neonates enrolled in two of the reviewed trials were small; and the estimates of effect, both in the individual trials and in the meta‐analyses, were not very precise, as indicated by the large confidence intervals. We suggested that future multi‐centre randomised controlled trials of inositol supplementation were required both to confirm the benefits suggested in the review (Howlett 2015); and to assess possible adverse effects of short‐ and long‐term outcomes. One ongoing large study of repeat doses of inositol in preterm infants was identified (NCT01954082) and the study has now been published (Phelps 2018). This study planned to enrol 1760 preterm infants less than 28 0/7 weeks' PMA. The primary outcome of the study was the incidence of survival without severe ROP through acute and final ROP determination up to 55 weeks' PMA. We suggested that this study would confirm or refute the promising results of the repeat inositol supplementation studies included in our 2015 update of this review (Howlett 2015).
In the current update of the review, two additional studies are included. Phelps 2016 enrolled 122 neonates and Phelps 2018 enrolled 638 neonates. The neonates were recruited from 14 and 18 centres in the Eunice Kennedy Shriver NICHD Neonatal Research Network in the US respectively. The sample size in the current review was increased by 760 infants to a total of 1177 infants. The planned enrolment of 1760 participants in the Phelps 2018 study would have permitted a detection of an absolute reduction in death or type 1 ROP of 7% with 90% power. The trial was terminated early due to a statistically significantly higher mortality rate in the myo‐inositol group. The two studies enrolled neonates of less than 30 weeks' PMA, whereas the studies by Friedman 1995,Hallman 1986 and Hallman 1992 enrolled some infants who were more mature. We therefore made two comparisons: 'Inositol supplementation IV or enterally (repeat doses of any amount and duration) versus control) (Comparison 1)' and 'Inositol supplementation; IV initially, followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA' (Comparison 3). We kept a previous comparison from the update of the review: 'Inositol supplementation (single dose) versus control' (Comparison 2). No new trials were identified for that comparison.
There are striking differences in the results for Comparison 1 in this update compared to the 2015 version of the review (Howlett 2015). For 'Inositol supplementation IV or enterally (repeat doses of any amount and duration versus control)' (Comparison 1) the results for neonatal death remained significant as the two new studies — Phelps 2016 and Phelps 2018 — did not report on that outcome. For all other outcomes including infant deaths, IVH grade greater than 2 and ROP 2 or greater or 3 or greater, there were no longer any statistically significant differences between the inositol and the placebo groups.
No new trials were included in Comparison 2 and thus no new findings were noted.
In Comparison 3 − 'Inositol supplementation; IV initially, followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA' − there were no statistically significant differences between the myo‐inositol group (80 mg/kg/day) and the placebo group. There were 24 outcomes included in the analyses including: Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome; Type 1 ROP including adjudicated ROP outcome; All‐cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth); BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth); Severe IVH (grade 3 or 4); Late‐onset sepsis (> 72 hours of age); and Suspected or proven NEC.
Overall completeness and applicability of evidence
The sample size in the current review was increased by 760 infants, who all were less than 30 weeks' PMA at birth. This is the population that is most likely to develop adverse outcomes related to preterm birth. For the outcome 'Infant death' in Comparison 1 there was high heterogeneity for both RR and RD. That comparison included a mixed population of infants of different PMAs and the treatment with inositol varied — therefore heterogeneity can be expected. Comparison 2 included only one trial of small sample size and tests for heterogeneity were not applicable. Comparison 3 included two very similar populations from the Eunice Kennedy Shriver NICHD Neonatal Research Network (Phelps 2016; Phelps 2018). The neonates in a subgroup (treated with 80 mg/kg/d of myo‐inositol) of the Phelps 2016 trial and all the neonates in the Phelps 2018 trial received the same treatment and most outcomes were assessed and reported similarly. The two studies were conducted relatively close in time. It is therefore surprising that the results of the two studies differ with high or moderate heterogeneity for four outcomes: Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome (RR, I² = 79% ‒ high; RD, I² = 85% ‒ high); All‐cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) (RR, I² = 72% ‒ moderate; RD, I² = 84% ‒ high); Severe IVH (grade 3 or 4) (I² = 74% ‒ moderate; RD = 82% ‒ high); and Surgical NEC (I² = 51% ‒ moderate; RD, I² = 69% ‒ moderate).
Quality of the evidence
While all six included studies were randomised controlled trials, three of them had an interim analysis that may have unblinded the researchers before the trial was completed. We felt that the quality of the studies was such that meta‐analyses were appropriate. The two new studies included in this review were of high quality (Phelps 2016; Phelps 2018). However, for these two studies the point estimates for many outcomes go in different directions which explains the heterogeneity that we refer to above under Overall completeness and applicability of evidence. In the analyses, the study by Phelps 2018 carries a higher weight because of its larger sample size (N = 638) compared with the Phelps 2016 study (N = 122). For Comparison 1 the certainty of evidence according to GRADE was low for the outcome Infant death (age < 1 year), and moderate for Bronchopulmonary dysplasia (at 36 to 38 weeks' PMA), ROP (stage ≥ 3 or ≥ 2), Sepsis (early or late onset), Necrotizing enterocolitis (suspected or proven), and IVH (grade > 2). We did not perform GRADE assessments for Comparison 2. For Comparison 3 the certainty of evidence according to GRADE was high for BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth); Late onset sepsis (> 72 hours of age); and for Suspected or proven NEC. The certainty of evidence according to GRADE was moderate for Type 1 ROP or death before determination of ROP outcome using adjudicated ROP outcome, Type 1 ROP including adjudicated ROP outcome, All‐cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth), and for Severe IVH (grade 3 or 4).
The Phelps 2018 study was terminated early due to a statistically significantly higher mortality rate in the myo‐inositol group. At 18 months, trial enrolment and treatment were suspended because of a manufacturing issue (later identified as glass lamellae in the third lot of drug, which was never used). Glass lamellae were subsequently found in 1.9% of stored vials of lot 2 of the trial drug. Detailed analyses revealed that there were no differences in the outcomes for infants treated with myo‐inositol between the two lots of the trial drug. Because the trial did not enrol as many infants as the preplanned sample size, it was underpowered to make conclusions regarding the efficacy and safety of myo‐inositol (Phelps 2018). We did not consider this early stopping of the trial a source of bias.
Potential biases in the review process
We are not aware of any potential bias in our review process.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews or meta‐analyses regarding this topic. The Discussion section of the Phelps 2018 study is directly relevant for this update of our review. We quote (we have inserted the references by the first author instead of the reference numbers Phelps and co‐workers used in their text):
"In this trial of supplemental myo‐inositol to improve survival without type 1 ROP among extremely preterm infants, myo‐inositol did not reduce type 1 ROP rates, and the trial was stopped early for an unexpected significant increase in mortality. The previous beneficial findings of myo‐inositol were not observed in the current trial; however, there are several relevant differences between the current and former inositol studies (Hallman 1990; Hallman 1986; Hallman 1992). Antenatal steroids were not widely used, nor was surfactant available during the earlier trials. The prior studies treated infants for three to 10 days with myo‐inositol during the acute phase of respiratory distress syndrome, whereas the present trial treated infants for up to 10 weeks to support retinal vascular development.
"In the previous trials (Hallman 1990; Hallman 1986; Hallman 1992), infants were more mature at birth (mean, 27 to 29 weeks’ gestational age). One explanation could be that a benefit of myo‐inositol on the rates of intraventricular haemorrhage, bronchopulmonary dysplasia, and ROP during the earlier studies may have resulted from the predicted beneficial effect of myo‐inositol on surfactant function (Hallman 1990; Hallman 1986; Hallman 1987). Reducing the severity of respiratory distress syndrome could be expected to reduce these morbidities. Thus, a myo‐inositol benefit on surfactant function in the current trial may have been outweighed by the beneficial effects of antenatal steroids, exogenous surfactant, and non‐invasive ventilatory support in current use.
"The dose of myo‐inositol to produce serum concentrations was similar to those in the previous studies (Hallman 1987, Hallman 1992, Phelps 2016). However, the combination of longer treatment and the inclusion of infants with younger gestational ages may have resulted in the unexpected increase in mortality through as yet unknown mechanisms. In vitro data have shown that infection of macrophages by some intracellular bacteria is enhanced by their ability to use myo‐inositol as an energy source (Manske 2016).
"An additional issue for this trial was its suspension when particulates (later identified as glass lamellae) were found in the third lot of drug, which was never used. Glass particulates are a commonly cited reason for drug recalls. Delamination within glass vials is affected by both the glass manufacturing process and the chemical characteristics of the drug, particularly if acidic or caustic (Guadagnino 2012; Zhao 2014).
"The glass lamellae subsequently found in 1.9% of stored vials in lot 2 of the trial drug raised the question whether the observed harmful effect of myo‐inositol could have been due to these particles. However, detailed analyses revealed that there were no differences in the outcomes for infants treated with myo‐inositol between the 2 lots of the trial drug".
Phelps 2018 did not discuss why the point estimates for many of the outcomes for the 80 mg/kg/day dose group in the Phelps 2016 study differed from the results for the 80 mg/kg/day dose group used in all infants in Phelps 2018 study. The data from the two studies resulted in heterogeneity for many of the outcomes when included in our meta‐analyses in Comparison 3. However, for the outcome of 'Late onset sepsis (72 hours of age)' the point estimates for the two studies went in the same direction and the typical RR was 1.33 (95% CI 1.00 to 1.75; P = 0.05) and the RD was 0.06 (95% CI 0.00 to 0.12; P = 0.05); I² = 0% for both RR and RD. Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death was higher (not reaching statistical significance) in the myo‐inositol group compared with placebo group in the Phelps 2018 study; RR 1.36 (95% CI 0.95 to 1.93, P = 0.09; RD 0.19 (95% CI −0.01 to 0.40), P = 0.07. In the Phelps 2016 study "Infection was reported as a primary cause of death in the 40 mg/kg group for 17% of subjects; compared with 0 to 3% as the cause of death for other dose groups (P < 0.01 for comparing across all dose groups)". The four groups in the study were inositol 10 mg/kg/d, 40 mg/kg/d, 80 mg/kg/day and placebo 0 mg/kg/d. These results suggest a possible association between myo‐inositol intake and an increased risk of late‐onset sepsis in preterm infants.
The trends for increased risk of late‐onset sepsis and for increased risk of death due to infection in the myo‐inositol group compared with the placebo group may support the in vitro data that have shown that infection of macrophages, by some intracellular bacteria, is enhanced by their ability to use myo‐inositol as an energy source (Manske 2016).
Authors' conclusions
Implications for practice.
Based on the evidence from RCTs to date, inositol supplementation does not result in important reductions in the rates of infant deaths, ROP stage 3 or higher, type 1 ROP, IVH grades 3 or 4, BPD, NEC, sepsis and other neonatal outcomes. These conclusions are based mainly on two recent RCTs in neonates less than 30 weeks' PMA, the most vulnerable population. Currently, inositol supplementation should not be routinely instituted as part of the nutritional management of preterm infants with or without RDS.
Implications for research.
It is important that infants who have been enrolled in the trials included in this review are followed to assess any effects of inositol supplementation on long‐term outcomes in childhood. We do not recommend any additional trials in neonates.
What's new
| Date | Event | Description |
|---|---|---|
| 27 January 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 9 April 2019 | New citation required and conclusions have changed | 2 new studies included. The conclusions have changed. Inclusion of 2 high quality studies (total N = 760) resulted in no significant results for the main outcomes of the review. Conclusions changed to: Based on the evidence from RCTs to date, inositol supplementation does not result in important reductions in the rates of infant deaths, ROP stage ≥ 3, Type 1 ROP, IVH grades 3 or 4, BPD, NEC, and sepsis. |
| 9 April 2019 | New search has been performed | Search updated and 2 new studies found for inclusion. |
| 5 November 2014 | New citation required but conclusions have not changed | This update does not change the conclusions of the previously published version of this review. |
| 5 November 2014 | New search has been performed | Current update This updates the review 'Inositol for respiratory distress syndrome in preterm infants' (Howlett 2012). One additional trial was included (Phelps 2013) and one ongoing trial was identified (NCT01954082). The infants in the study by Phelps 2013 were not included based on whether they had respiratory distress syndrome or not. To justify inclusion of this study we changed the title of the review to 'Inositol in preterm infants at risk for or having respiratory distress syndrome'. We changed the objectives to read: To assess the effectiveness and safety of supplementary inositol in preterm infants with or without respiratory distress syndrome (RDS) in reducing adverse neonatal outcomes. In the previous update of the review the study by Phelps 2012NCT01954082 'Multi‐dose pharmacokinetics and dose ranging of inositol in premature infants' was incorrectly listed under Laptook AR as the primary author (ClinicalTrials.gov: NCT01030575). Previous updates This updates the review 'Inositol for respiratory distress syndrome in preterm infants' (Howlett 2003). In this update, one study that was previously reported in abstract form was now available as a full report (Friedman 1995). |
| 6 February 2008 | Amended | Converted to new review format. |
| 29 December 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are thankful to Dr Friedman who provided us with additional unpublished information related to the abstract publication. For the update in 2015, we are grateful to Dr Phelps who drew our attention to her ongoing study in 2007. For this update, we obtained clarifying information regarding the Phelps 2012NCT01954082, Phelps 2013 and NCT01954082 studies. In 2018 we received additional information on the Phelps 2016 study from Dr Phelps and Dr Nolen. We are thankful to Ms Caitlin O'Connell, Assistant Managing Editor of Cochrane Neonatal, who conducted the literature searches for the current update.
The Methods section of this protocol/review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Cochrane Neonatal standard search strategy
PubMed: ((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies*[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infan*[TIAB] OR neonat*[TIAB]) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase:
#1 (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*).mp
#2 exp infant
#3 (#1 OR #2)
#4 (human not animal) .mp
#5 (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial).mp
#6 (#3 and #4 and #5)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
CENTRAL: infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU
Appendix 2. Risk of bias
‘Risk of bias’ tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
· low risk (any truly random process e.g. random number table; computer random number generator);
· high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
· unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
· low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
· high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
· unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
· low risk, high risk or unclear risk for participants; and
· low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
· low risk for outcome assessors;
· high risk for outcome assessors; or
· unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
· low risk (< 20% missing data);
· high risk (≥ 20% missing data); or
· unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
· low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
· high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
· unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
· low risk;
· high risk; or
· unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal death (age < 28 days) | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.91] |
| 2 Infant death (age < one year) | 5 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.13] |
| 3 BPD (supplementary oxygen ar 36 weeks; PMA or death due to BPD)at 36 week's PMA | 2 | 666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.14] |
| 4 Bronchopulmonary dysplasia (at 28 to 30 days of age) | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.54, 1.13] |
| 5 Bronchopulmonary dysplasia (at 36 to 38 weeks PMA) | 2 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.20] |
| 6 Retinopathy of prematurity, stage ≥ 3 or ≥ 2 | 3 | 810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.06] |
| 6.1 ROP ≥ 3 | 2 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.67] |
| 6.2 Retinopathy of prematurity, stage ≥ 2 | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 7 Retinopathy of prematurity, any stage | 4 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 8 Necrotizing enterocolitis (suspected or proven) | 5 | 1115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.64, 1.39] |
| 9 Sepsis (early and/or late onset) | 4 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.95, 1.54] |
| 10 Intraventricular haemorrhage, grade > 2 | 5 | 1103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.01] |
| 11 Intraventricular haemorrhage, all grades | 3 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.59, 1.00] |
| 12 Minor neural developmental impairment at one year corrected age | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.86] |
| 13 Major neural developmental impairment at one year corrected age | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.16] |
Comparison 2. Inositol supplementation (single dose of 60 mg/kg or 120 mg/kg) in preterm infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death during hospital stay | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.34, 4.21] |
| 2 Bronchopulmonary dysplasia at 36 weeks PMA | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.88, 8.48] |
| 3 Retinopathy of prematurity (infants who underwent surgery for ROP) | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.22] |
| 4 Necrotizing enterocolitis (stage 2A or worse) | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.39] |
| 5 Necrotizing enterocolitis (infants who underwent surgery for NEC) | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.08, 3.41] |
| 6 Sepsis (late onset) | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.71, 2.97] |
| 7 Intraventricular haemorrhage (grade 3 or 4) | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.29, 3.90] |
| 8 Hearing test (failed both ears) | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.09, 3.84] |
Comparison 3. Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome | 2 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.99, 1.67] |
| 2 Type 1 ROP | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.89, 2.24] |
| 3 Death before determination of ROP outcome | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.02, 2.31] |
| 4 Type 1 ROP including adjudicated ROP outcome | 2 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.82, 1.86] |
| 5 Any ROP | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.13] |
| 6 ROP ≥ 2 ROP | 1 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 7 All cause infant mortality to 55 week's PMA | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.12, 2.48] |
| 8 All cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.91, 2.00] |
| 9 BPD (requiring oxygen at 36 week's PMA for oxygen saturation > 90%) | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
| 10 BPD or death by it prior to 37 weeks' PMA (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth) | 2 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.87, 1.16] |
| 11 Severe IVH (grade 3 or 4) | 2 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.29] |
| 12 Cystic areas in the cerebral parenchyma measured through 28 d | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.58, 2.85] |
| 13 Early onset sepsis | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Late onset sepsis (> 72 hrs of age) | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.00, 1.75] |
| 15 Suspected or proven NEC | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.41] |
| 16 Surgical NEC | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.57, 2.58] |
| 17 Spontaneous gastro‐intestinal perforation | 2 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.52] |
| 18 Pulmonary haemorrhage | 1 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.59, 1.62] |
| 19 PDA | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.14] |
| 20 PDA requiring indomethacin | 1 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.22] |
| 21 PDA requiring surgery | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.42] |
| 22 Seizure treatment for ≥ 2 days | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.43, 2.56] |
| 23 Negative hearing screening in either ear at discharge | 2 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.92, 2.29] |
| 24 Respiratory distress syndrome | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.09] |
| 25 Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.95, 1.93] |
3.25. Analysis.
Comparison 3 Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA, Outcome 25 Sepsis, necrotizing enterocolitis, pneumonia or other infection as a cause of death.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Friedman 1995.
| Methods | Randomised, placebo‐controlled trial | |
| Participants | Preterm infants (birth weight < 1500 grams) with a diagnosis of RDS, requiring mechanical ventilation. 24 infants were randomised to high concentration inositol formula (SC 30) (estimated PMA 27.7, SD 1.9) and 24 infants were randomised to a low concentration of inositol formula (SC 24). Randomisation ended when the high‐inositol formula was no longer available. Location: 2 NICUs in the US. Study period: October 1994 to June 1998. |
|
| Interventions | The study group was enterally fed high‐inositol formula (2500 µmol/L inositol), while the control group was given low‐inositol formula (242 µmol/L) enterally. | |
| Outcomes | Neonatal deaths, infant deaths, infants with bacteraemia, necrotizing enterocolitis (radio graphically documented), IVH > grade 2, BPD (oxygen therapy > 30 days), duration of mechanical ventilation, ROP (reported in unpublished data from 1995). | |
| Notes | The results of this study have been reported 3 times; in abstract form in 1995 after 37 infants were enrolled; in a personal communication report to us in 1995 when 41 infants had been enrolled; and in a final published report in 2000 when 48 infants had been entered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Infants were allocated to one of the two groups by sequential random card selection. No information provided whether the cards were enclosed in opaque and numbered envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Infants were blinded but no information provided whether the clinical staff and the researchers were. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes provided for all 48 infants randomised. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot judge if there were any deviations between the protocol and the final report. |
| Other bias | High risk | The results of this study have been reported 3 times: in abstract form in 1995 after 37 infants were enrolled; in a personal communication report to us in 1995 when 41 infants had been enrolled; and in a final published report in 2000 when 48 infants had been entered. |
Hallman 1986.
| Methods | Randomised, placebo‐controlled, double‐blind trial. Enrolment from 1983 to 1985. | |
| Participants | Preterm infants (birth weight < 2000 grams) (mean PMA 29.5, SD 2.0 in the inositol group and 29.5, SD 2.1 in the placebo group) with a diagnosis of RDS, requiring mechanical ventilation.
N = 74; placebo group = 37, inositol group = 37. Location: 1 NICU in Helsinki, Finland. Study period: January 1983 to August 1985. |
|
| Interventions | IV or supplemental inositol (120 to 160 mg/kg/day) or placebo (5% glucose) given daily for 10 days. | |
| Outcomes | Neonatal deaths, infant deaths, BPD (supplemental oxygen at 28 days and x‐ray findings compatible with BPD), IVH, ROP (ophthalmological exam at PMA of 9 and 13 months), NEC (clinical findings and abdominal x‐ray showing pneumatosis intestinalis and air in the portal circulation), and sepsis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Low risk | "Infants were randomly and blindly assigned to be treated with inositol or placebo (glucose) after their parents had consented to their participation". For further details see "Blinding" below. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Each set of solutions, containing either inositol or glucose (5% weight/volume each) had a code number. Only the pharmacist preparing the doses knew the contents of the drug packages. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 83 infants who entered the trial, nine did not fulfil the entrance criteria and were excluded from the final analysis. An explanation was provided for each excluded infant. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot judge if there were any deviations between the protocol and the final report. |
| Other bias | High risk | The present report represents the third interim analysis and the researchers may have been influenced by the results of the two previous interim analyses. The study was not registered in a trials registry. |
Hallman 1992.
| Methods | Randomised, placebo‐controlled, double‐blind trial, occurring between 1985 and 1989. | |
| Participants | Preterm infants (birth weight < 2000 grams and 24.0 to 31.9 weeks' PMA at birth) with evidence of RDS, requiring mechanical ventilation. Total N = 233, placebo group = 114, inositol group = 119. Age at enrolment 2 to 10 hours of life. | |
| Interventions | The study group received IV inositol 80 mg/kg body weight daily for 5 days, with repeated courses at day 10 and day 20 if necessary (infant continued to require ventilation, required supplemental O₂ or did not tolerate enteral feeds). The control group received 5% glucose. | |
| Outcomes | Neonatal death, infant death, BPD (supplemental oxygen at 28 days of age), BPD (supplemental oxygen at 38 weeks' PMA or the week of discharge from hospital), ROP (as per International Classification assessed from 4 to 6 weeks and ending at 12 months), IVH (all grades, grade > 2), NEC (no definition provided), and sepsis (no definition provided). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 5% glucose was given as placebo, but no information provided on whether staff was blinded to study drugs or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 infants in the placebo group and 3 in the inositol group died before receiving any treatment, 2 had lethal malformations (1 in each group), and 3 did not have RDS (2 in the placebo group and 1 in the inositol group). These 12 infants were included only in the safety analysis. |
| Selective reporting (reporting bias) | Unclear risk | This study was not registered in a trials registry so we cannot judge if there were any deviations between the protocol and the final report. |
| Other bias | High risk | Interim analyses were to be performed after enrolment of 100, 200, 300 patients. Early termination of the trial was recommended by the monitoring committee after the second interim analysis, when the Chi² test revealed a significant increase in neonatal survival without BPD and no trend towards serious morbidity in 1 study group. 1 interim analysis previously reported, Hallman 1992 (published in Lung 1990;168 Suppl: 877 to 82). |
Phelps 2013.
| Methods | Randomised, double‐masked, placebo‐controlled pharmacokinetic (PK) study. Enrolment between June 2006 and December 2007. The trial was conducted by the National Institutes of Child Health and Human Development Neonatal Research Network. 10 of the Neonatal Research Network Centers participated. | |
| Participants | Eligible subjects were of 23 0/7 to 29 6/7 weeks' PMA and ≥ 600 G BW, had no major congenital anomalies, were between 12 hours and 6 days of age at randomisation, and had received no human milk or formula feedings since birth. | |
| Interventions | Inositol was given as a single low (60 mg/kg) (N = 25) or high (120 mg/kg) (N = 24) dose of 5% myo‐inositol IV over 20 min in a 1:1:1 randomisation with placebo delivered in 1 of 2 volumes to maintain masking (5% glucose) (N = 25). Drug or placebo was dispensed from the respective pharmacies in unit doses labelled as 'inositol study drug', and all clinical and research personnel except the pharmacist were masked to the study group. | |
| Outcomes | Pharmacokinetic data for inositol (central volume of distribution, clearance, endogenous production, the half‐life, renal inositol excretion during the first 12 H and after 48 H and diuretic side effect. In addition adverse events were reported for the first 7 days as well as neonatal morbidities from birth through hospital discharge (or 120 days if sooner). | |
| Notes | Abbott Nutrition Division, Abbott Laboratories, supplied the inositol drug used in the study. Portions of this study were presented at the 2010 Paediatric Academic Societies Annual Meeting, Vancouver, Canada, May 1–4, 2010 (Abstract 3737.387). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed centrally via computer within two pre‐specified PMA strata (23 0/7 to 26 6/7 weeks and 27 0/7 to 29 6/7 weeks). |
| Allocation concealment (selection bias) | Low risk | There was central allocation to study group. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drug or placebo was dispensed from the respective pharmacies in unit doses labelled as 'inositol study drug', and all clinical and research personnel except the pharmacist were masked to the study group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Consent was obtained for 79 infants, 76 infants were randomised, and 74 infants received study drug. 2 infants did not complete the minimum of 4 specified blood samples (three post drug infusion), and their randomisation were replaced with 2 additional enrollees from the same centre and of the same gestational age (GA) stratum, per protocol. Available data from the 2 replaced infants were included in the PK and safety analyses. 1 infant received placebo instead of the assigned 120 mg/kg dose, and for the PK analysis, this infant’s serum and urine data were included in the placebo group. However, this subject’s data on adverse events and clinical outcomes were included as randomised (intention to treat). |
| Selective reporting (reporting bias) | Low risk | The study was registered with ClinicalTrials.gov (NCT00349726) and there do not appear to be any deviations from the protocol. |
| Other bias | Low risk | Appears free of other bias. |
Phelps 2016.
| Methods | Prospective, parallel, randomised controlled trial. Infants enrolled in 14 centres in the Eunice Kennedy Shriver NICHD Neonatal Research Network. | |
| Participants | Infants ≤ 29 weeks' PMA (23 0/7 to 29 6/7 weeks' PMA), who weighed at least 400 G, and could receive study drug by 72 H after birth. | |
| Interventions | Myo‐inositol provided by Abbott Nutrition, Columbus, Ohio, USA as an isotonic, preservative and pyrogen‐free, sterile, 5% solution at 10, 40 or 80 mg/kg/day. Intravenous administration converted to enteral when feedings were established, and continued to the first of 10 weeks, 34 weeks' PMA, death or discharge. Total number randomised: 10 mg/kg N = 29; 40 mg/kg N = 30; 80 mg/kg N = 28 Placebo: 5% glucose. Total number randomised: N = 35. |
|
| Outcomes | Adverse events were prospectively monitored from 24 hours prior to study drug until 7 days following the final dose (unless discharged sooner), and judged according to a neonatal toxicity table developed for the study. An unfavourable outcome was defined as either type 1 ROP or worse, in either eye, or surgical intervention for severe ROP in either eye. A favourable ROP outcome was assigned if the retinal vessels progressed to full vascularization in both eyes without meeting criteria for severe ROP, or if on 2 consecutive examinations the retinal vessels were in zone III. Infants who did not meet either criterion had all available examinations reviewed by an adjudication committee. Adjudication was conducted by a committee of 3 experienced ophthalmologists not involved with the study and masked to study group assignment. The final ROP status was judged separately in each eye as 'probably favourable', 'probably unfavourable' or 'cannot be determined', and the majority classification was assigned as the adjudicated outcome. At 18 to 22 months' corrected age, infants received a set of standardized examinations of neurologic function and development according to the NRN Follow‐Up Protocol (to be reported separately). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. |
| Allocation concealment (selection bias) | Low risk | Computer generated and communicated to research pharmacist. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Personal communication from the first author indicates blinded performance and detection bias for all outcomes. Ophthalmologists were blinded during the adjudication process. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Severe ROP data presented for 106 surviving infants and there were 15 deaths, which adds up to 121 infants not 122 infants, which was the number enrolled. |
| Selective reporting (reporting bias) | Low risk | The protocol was available to us and we did not notice any major deviations from the planned study. The study was registered as: NCT01030575. |
| Other bias | Low risk | Appears free of other bias. |
Phelps 2018.
| Methods | Randomised clinical trial included infants enrolled from 18 neonatal intensive care centres throughout the USA from 17 April 2014 to 4 September 2015; final date of follow‐up was 12 February 2016. | |
| Participants | 638 infants < 28 weeks’ PMA, surviving for at least 12 hours, and admitted to 1 of the 18 Neonatal Research Network centres before 72 hours’ postnatal age. | |
| Interventions | A 40 mg/kg dose of myo‐inositol was given every 12 hours (initially intravenously, then enterally when feeding; (N = 317) or for up to 10 weeks. The active drug was an isotonic, sterile, pyrogen‐ and preservative‐free aqueous solution of 5% myo‐inositol (50 mg/mL) at neutral pH and was provided by Abbott Laboratories. A dose of 40 mg/kg every 12 hours was selected to achieve serum concentrations similar to those previously reported. A therapeutic duration of up to 10 weeks was chosen to sustain serum myo‐inositol levels similar to those found in utero throughout the period of normal retinal vascular development and because of the reported benefits in the treatment of ROP and survival. Placebo (N = 321) (5% glucose for IV use from pharmacy stock). |
|
| Outcomes | The unfavourable primary outcome was type 1 ROP, which was defined as meeting the criteria for ophthalmological intervention to prevent retinal detachment, a more severe ROP type than ROP type 1 (e.g. aggressive posterior ROP or Rush disease), or death before the ROP outcome could be determined. | |
| Notes | The planned enrolment of 1760 participants would permit detection of an absolute reduction in death or type 1 ROP of 7% with 90% power. The trial was terminated early due to a statistically significantly higher mortality rate in the myo‐inositol group. The favourable primary outcome was survival with only milder ROP or no ROP. Infants were followed up as outpatients to determine the primary outcome up to a maximum of 55 weeks’ PMA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated and centrally administered randomisation. |
| Allocation concealment (selection bias) | Low risk | With the exception of pharmacists, who prepared the daily unit doses of myo‐inositol or placebo according to randomisation assignment, all other clinical and research personnel and families were blind to group assignment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See above for allocation concealment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome group: of the 317 infants randomised to receive myo‐inositol 313 received the intervention as randomised; 3 died prior to receiving the intervention and 1 was withdrawn prior to intervention. 313 included in primary analysis. Of the 321 infants randomised to placebo 315 received the intervention as randomised; 2 died prior to receiving intervention and 4 received < 2 doses of myo‐inositol. 320 included in the primary analysis of type 1 retinopathy of prematurity or death. 1 excluded from primary analysis. |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available to us and we did not identify any deviations except that the study was terminated prior to reaching the full sample size because of safety concerns. The authors state: “No changes to the protocol occurred during the trial”. Registered as: NCT01954082. |
| Other bias | Low risk | Appears free of other bias. |
BW = birth weight
PMA = postmenstrual age
PK = pharmacokinetics
Differences between protocol and review
For the original review and previous updates of the review the main comparison has been 'inositol supplementation versus control' (Comparison 1) and we included studies under this comparison that provided repeated doses of inositol to the infants. For this update, we identified one dose‐finding study in which infants were supplemented with a single dose of inositol (Phelps 2013). We did not consider it appropriate to include the results of this study in the meta‐analyses of repeated doses of inositol and we have changed the first comparison to read: 'inositol supplementation (repeat doses) versus control' (Comparison 1) and added a second comparison: 'inositol supplementation (single dose) versus control' (Comparison 2). These different dosing regimens were not known at the protocol stage and we have made a deviation from the protocol and included single doses of inositol in our review as those analyses provide important information. The infants in the study by Phelps 2013 were not included based on whether they had respiratory distress syndrome or not. To justify inclusion of this study we changed the title of the review to 'Inositol in preterm infants at risk for or having respiratory distress syndrome'. For this update we changed the objectives to read: "To assess the effectiveness and safety of supplementary inositol in preterm infants with or without respiratory distress syndrome (RDS) in reducing adverse neonatal outcomes including death (neonatal and infant deaths), BPD, ROP, intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), necrotizing enterocolitis (NEC) and sepsis". Outcomes were not reported in an identical manner in the repeat doses of inositol studies and the single dose of inositol study (Phelps 2013). We accepted the outcomes and their definitions reported in the Phelps 2013 study. In this update in 2019 we included an additional comparison 'Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at < 30 weeks' PMA' (Comparison three).
Contributions of authors
All review authors contributed to all stages of this update of the review in 2019.
Sources of support
Internal sources
Izaak Walton Killam Health Centre, Halifax, Nova Scotia, Canada.
Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
Dr. Alexandra Howlett has no interests to declare.
Dr. Arne Ohlsson has no interests to declare.
Dr. Nishad Plakkal has no interests to declare.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Friedman 1995 {published and unpublished data}
- Friedman CA, McVey J, Borne MJ, James M, May WL, Temple DM, et al. Relationship between serum inositol concentration and development of retinopathy of prematurity: A prospective study. Journal of Pediatric Ophthalmology and Strabismus 2000;37(2):79‐86. [PUBMED: 10779265] [DOI] [PubMed] [Google Scholar]
- Friedman CA, Temple DM, Robbins KK, Miller CJ, Rawson JE. Randomized controlled trial of high inositol and calorie supplementation in preterm infants at risk for chronic lung and eye disease. American Academy of Pediatrics Annual Meeting; San Francisco, California, USA. 1995 October.
Hallman 1986 {published data only}
- Hallman M, Jarvanpaa AL, Pohjavuori M. Respiratory distress syndrome and inositol supplementation in preterm infants. Archives of Disease in Childhood 1986;61(11):1076‐83. [PUBMED: 3539028] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallman 1992 {published data only}
- Hallman M, Bry K, Hoppu K, Lappi M, Pohjavuori M. Inositol supplementation in premature infants with respiratory distress syndrome. New England Journal of Medicine 1992;326(19):1233‐9. [DOI: 10.1056/NEJM199205073261901; PUBMED: 1560798] [DOI] [PubMed] [Google Scholar]
- Hallman M, Pohjavuori M, Bry K. Inositol supplementation in respiratory distress syndrome. Lung 1990;168 Suppl:877‐82. [PUBMED: 2117207] [DOI] [PubMed] [Google Scholar]
Phelps 2013 {published data only}
- Phelps DL, Ward RM, Williams RL, Watterberg KL, Laptook AR, Wrage LA, et al. Pharmacokinetics and safety of a single intravenous dose of myo‐inositol in preterm infants of 23‐29 wk. Pediatric Research 2013;74(6):721‐9. [DOI: 10.1038/pr.2013.162; PUBMED: 24067395 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps DL, Ward RM, Williams RL, Watterberg KL, Laptook AR, Wrage LA, et al. on behalf of the Inositol Subcommittee of the Neonatal Research Network. Pharmacokinetics and safety of a single dose of myo‐inositol in preterm infants of 23‐29 wk. E‐PAS2010. 2010:3737.387.
Phelps 2016 {published data only}
- Phelps DL, Ward RM, Williams RL, Nolen TL, Watterberg KL, Oh W, et al. Safety and pharmacokinetics of multiple dose myo‐inositol in preterm infants. Pediatric Research 2016;80(2):209‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phelps 2018 {published data only}
- Phelps DL, Watterberg KL, Nolen TL, Cole CA, Cotten M, Oh W, et al. Effects of myo‐inositol on type 1 retinopathy of prematurity among preterm infants <28 weeks’ gestational age: a randomized clinical trial. JAMA 2018;320(16):1649‐58. [DOI: 10.1001/jama.2018.14996] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bell 1978
- Bell MJ, Ternberg KL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotising enterocolitis: therapeutic decisions based on clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bromberger 1986
- Bromberger P, Hallman M. Myoinositol in small preterm infants: Relationship between intake and serum concentration. Journal of Pediatric Gastroenterology and Nutrition 1986;5(3):455‐8. [PUBMED: 3088251] [DOI] [PubMed] [Google Scholar]
Burton 1974
- Burton LE, Wells WW. Studies on the development pattern of the enzymes converting glucose‐6‐phosphate to myo‐inositol in the rat. Developmental Biology 1974;37(1):35‐42. [PUBMED: 4362962] [DOI] [PubMed] [Google Scholar]
Dawson 1961
- Dawson RM, Freinkel N. The distribution of free mesoinositol in mammalian tissues, including some observations on the lactating rat. Biochemical Journal 1961;78(3):606‐10. [PUBMED: 13720328] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eagle 1957
- Eagle H, Oyama VI, Levy M, Freeman AE. Myo‐inositol as an essential growth factor for normal and malignant human cells in tissue culture. Journal of Biological Chemistry 1957;266(1):191‐205. [PUBMED: 13428752] [PubMed] [Google Scholar]
Egberts 1986
- Egberts J, Noort WA. Gestational age‐dependent changes in plasma inositol levels and surfactant composition in the fetal rat. Pediatric Research 1986;20(1):24‐7. [PUBMED: 3753753] [DOI] [PubMed] [Google Scholar]
Guadagnino 2012
- Guadagino E, Zuccato D. Delamination propensity of pharmaceutical glass containers by accelerated testing with different extraction media. PDA Journal of Pharmaceutical Science and Technology 2012;66(2):116‐25. [DOI: 10.5731/pdajpst.2012.00853; PUBMED: 22492597] [DOI] [PubMed] [Google Scholar]
Guarner 1992
- Guarner V, Tordet C, Bourbon JR. Effects of maternal protein‐calorie malnutrition on the phospholipid composition of surfactant isolated from the fetal and neonatal rat lungs. Compensation by inositol and lipid supplementation. Pediatric Research 1992;31(6):629‐35. [DOI: 10.1203/00006450-199206000-00018; PUBMED: 1635827] [DOI] [PubMed] [Google Scholar]
Hallman 1980
- Hallman M, Epstein BL. Role of myo‐inositol in the synthesis of phosphatidylglycerol and phosphatidylinositol in the lung. Biochemical and Biophysical Research Communications 1980;92(4):1151‐9. [PUBMED: 6245646] [DOI] [PubMed] [Google Scholar]
Hallman 1984
- Hallman M. Effect of intracellular myo‐inositol on the surfactant phospholipid synthesis in the fetal rabbit lung. Biochimica et Biophysica Acta 1984;795(1):67‐78. [PUBMED: 6547857] [DOI] [PubMed] [Google Scholar]
Hallman 1985
- Hallman M, Saugstad OD, Porreco RP, Epstein BL, Gluck L. Role of myo‐inositol in regulation of surfactant phospholipids in the newborn. Early Human Development 1985;10(3‐4):245‐54. [PUBMED: 3838720] [DOI] [PubMed] [Google Scholar]
Hallman 1987
- Hallman M, Arjomaa P, Hoppu K. Inositol supplementation in respiratory distress syndrome: Relationship between serum concentration, renal excretion, and lung effluent phospholipids. Journal of Pediatrics 1987;110(4):604‐10. [PUBMED: 3559811] [DOI] [PubMed] [Google Scholar]
Hallman 1990
- Hallman M, Pohjavuori M, Bry K. Inositol supplementation in respiratory distress syndrome. Lung 1990;168 Suppl:877‐82. [PUBMED: 2117207] [DOI] [PubMed] [Google Scholar]
Hasan 1974
- Hasan SH, Nishigaki I, Tsutsui Y, Yagi K. Studies on myoinositol IX. Morphological examination of the effect of massive doses of myoinositol on the liver and kidney of rat. Journal of Nutritional Science and Vitaminology 1974;20(1):55‐8. [PUBMED: 4836951] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICROP 1984
- Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Archives of Ophthalmology 1984;102(8):1130‐4. [PUBMED: 6547831] [DOI] [PubMed] [Google Scholar]
Lewin 1978
- Lewin LM, Melmed S, Passwell JH, Yannai Y, Brish M, Orda S, et al. Myoinositol in human neonates: serum concentrations and renal handling. Pediatric Research 1978;12(1):3‐6. [DOI: 10.1203/00006450-197801000-00002; PUBMED: 643373] [DOI] [PubMed] [Google Scholar]
Manske 2016
- Mankse C, Schell U, Hibi H. Metabolism of myo‐inositol by Legionella pneumophilia promotes infection of amoebae and macrophages. Applied Environmental Microbiology 2016;82(16):5000‐14. [DOI: 10.1128/AEM.01018-16; PUBMED: 27287324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birthweights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
Pereira 1990
- Pereira GR, Baker L, Egler J, Corcoran L, Chiavacci R. Serum myoinositol concentrations in premature infants fed human milk, formula for infants, and parenteral nutrition. American Journal of Clinical Nutrition 1990;51(4):589‐93. [DOI: 10.1093/ajcn/51.4.589; PUBMED: 2108579] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shennan 1988
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527‐32. [PUBMED: 3174313] [PubMed] [Google Scholar]
Soll 1992
- Soll RF, McQueen MC. Respiratory distress syndrome. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992. [Google Scholar]
Zhao 2014
- Zhao J, Lavalley V, Mangiagalli P, Wright JM, Bankston TE. Glass delamination: a comparison of the inner surface performance of vials and pre‐filled syringes. AAPS PharmSciTech 2014;15(6):1398‐409. [DOI: 10.1208/s12249-014-0167-y; PUBMED: 24938618] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Howlett 1997
- Howlett A, Ohlsson A. Inositol for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD000366] [DOI] [PubMed] [Google Scholar]
Howlett 2003
- Howlett A, Ohlsson A. Inositol for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000366] [DOI] [PubMed] [Google Scholar]
Howlett 2012
- Howlett A, Ohlsson A, Plakkal N. Inositol for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD000366.pub2] [DOI] [PubMed] [Google Scholar]
Howlett 2015
- Howlett A, Ohlsson A, Plakkal N. Inositol in preterm infants at risk for or having respiratory distress syndrome. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD000366.pub3] [DOI] [PubMed] [Google Scholar]


