Abstract
Background
Growing evidence shows links between septicaemia and non-multiple sclerosis demyelinating syndromes (NMSDS); nevertheless, epidemiological data are still very limited. This study aimed to explore the relationship between septicaemia and NMSDS in a general population.
Methods
The study included 482 781 individuals diagnosed with septicaemia and 1 892 825 age/sex-matched non-septicaemia patients for the comparison. Data were drawn from a population-based nationwide National Health Insurance Research Database Taiwan, from 1 January 2002 to 31 December 2011. The two cohorts of patients with and without septicaemia were followed up for the occurrence of NMSDS. The Cox-proportional hazard regression model was performed to estimate adjusted HR after multivariate adjustment.
Results
Individuals with septicaemia had a 4.17-fold (95% CI 3.21 to 5.4, p < 0.001) higher risk to develop NMSDS compared with those without septicaemia. Patients aged <65 years had a greater NMSDS risk (<45 years: HR = 6.41, 95% CI 3.65 to 11.3, p < 0.001; 45–64 years: HR = 6.66, 95% CI 3.98 to 11.2, p < 0.001). Furthermore, females with septicaemia and individuals with higher severity of septicaemia were associated with increased risks of developing NMSDS.
Conclusions
Our results indicated that patients with septicaemia were likely to develop NMSDS. A possible contributing role of septicaemia in increasing the hazard of NMSDS is proposed, based on the outcome that individuals with higher severity of septicaemia carried elevated threat of encountering NMSDS.
Keywords: septicaemia, transverse myelitis, acute disseminated encephalomyelitis, demyelination
Introduction
Oligodendrocytes are responsible for myelin production in the central nervous system (CNS).1 The myelin sheaths cover and insulate axons which assist electrical conduction between nerves. Demyelinating disorders following oligodendrocyte injuries denote the loss of the myelin sheath that encloses and defends axons in the CNS. Demyelination is likely to be patchy and segmented, with diverse distinctive areas being involved chronologically or concurrently. Individuals with demyelinating diseases present with various symptoms and signs, such as blurred vision, muscle weakness, poor balance and neurocognitive impairment.2 The neural function might recover gradually through regeneration and repair of myelin as remyelination does occur. Nevertheless, widespread loss of myelin commonly results in irremediable degeneration of the axon and the cell body. A comprehensive investigation on risk factors of CNS demyelinating diseases might provide better solutions to disease prevention.
Sepsis is a globally common, lethal and costly disease. Based on the third international consensus, the current definition of sepsis is life-endangering organ dysfunction triggered by a poorly controlled host immune response to infection.3 Septic shock, a subset of sepsis in which predominantly overwhelming circulatory, cellular and metabolic malformations, has increased hazard of mortality compared with sepsis alone.3 Septicaemia is sepsis in the presence of multiplication of pathogenic microorganisms in the circulating blood. Several cases with CNS demyelinating diseases, such as transverse myelitis and acute disseminated encephalomyelitis (ADEM), were reported to be associated with septicaemia.4 5 However, the association between septicaemia and demyelinating diseases remains to be elucidated, with a lack of an appropriate longitudinal follow-up survey.
In this study, we focused on non-multiple sclerosis demyelinating syndromes (NMSDS), which mainly comprised neuromyelitis optica spectrum disorders (NMOSD) and antimyelin oligodendrocyte glycoproteinantibody (MOG) associated spectrum, after septicaemia. We excluded multiple sclerosis (MS) because previous studies demonstrated that MS is a complex disease.6 Genetic susceptibility7 and various environmental factors, such as vitamin D deficiency,8 parental smoking9 and adverse socioeconomic position,10 have been proposed to be connected to the pathophysiology of MS. Therefore, we aimed to explore the association between septicaemia and NMSDS using the nationwide 10-year follow-up data (2002–2011) in Taiwan. We then theorised that individuals with septicaemia had a greater risk of developing subsequent NMSDS.
Methods
Subject enrolment
To evaluate the association between septicaemia and the subsequent NMSDS, Longitudinal Health Insurance Database (LHID) Taiwan was applied to this study. It is a subdata set of National Health Insurance (NHI) programme which contains over 99% of Taiwanese citizens (23 million). The LHID from 2002 to 2011 encloses the records of sociodemographic status, dates of clinical visits, medications and disease diagnosis codes of inpatients and outpatients in the format of the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). All data have been deidentified before available for research. The data of all patients with an index date, that is, the first hospitalisation due to septicaemia, between 2002 and 2011 were followed up and completed until the end of 2014. All protocols in this study were approved by Institutional Review Board of China Medical University Hospital (CMUH104-REC2-115(CR-2)).
Patient and public involvement
Patients and public were not directly involved in the development of this study.
Definition of septicaemia and demyelinating disease by ICD classification
The study population included all individuals who had first time hospitalisation for septicaemia with specific ICD-9-CM codes 003.1, 036.2 and 038 from 2002 to 2011 (N=482 781). The definition of ‘first time’ is that those patients were never hospitalised for septicaemia before by searching throughout the National Health Insurance Research Database (NHIRD) Taiwan. The Inpatient Expenditures by Admissions was from 1996 to 2013. In this study, we selected patients with newly diagnosed septicaemia so that we could know the complete history of the patients after having septicaemia. NHIRD is a database maintained by the government, and the missing rate is very low. The data we used in the present study were all completed.
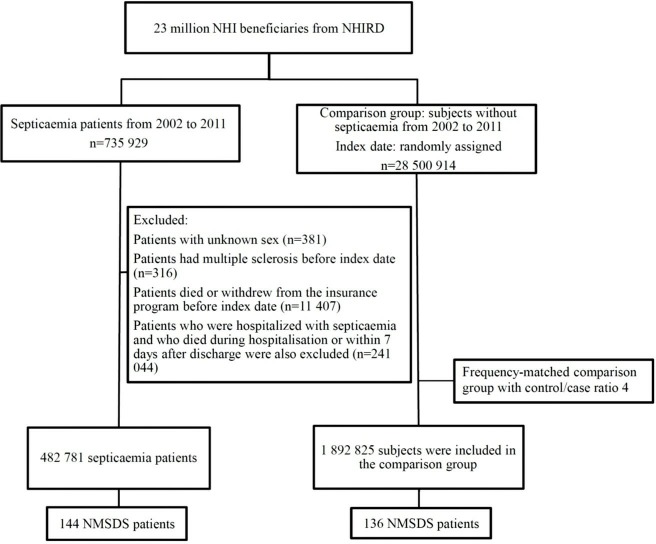
We excluded patients with a diagnosis of MS (ICD-9-CM code 340) and those with other demyelinating diseases (ICD-9-CM code 341) before the index date. The NMSDS here mainly comprise NMO and NMOSD, which are mediated by aquaporin-4 antibodies, as well as anti-MOG associated spectrum, which often presents as an anti-MOG autoimmune encephalomyelitis, chronic relapsing inflammatory optic neuritis, ADEM or acute haemorrhagic leukoencephalitis. Some patients may be classified as unspecified demyelinating diseases of CNS, instead of currently identified specific demyelinating diseases. We arbitrarily recruited an age/sex-matched control group, 1 892 825 individuals (a sample size 4-fold that of the septicaemia group), from the LHID (2002–2011). Each subject in both groups was followed up from the index date to the event of being diagnosed as demyelinating diseases. For individuals who did not develop demyelinating diseases, the follow-up ended on the date of insurance withdrawal, death or the final day of the research period (31 December 2014) (figure 1).
Figure 1.
Flowchart represents the steps of sample selection from National Health Insurance Research Database (NHIRD) Taiwan. NHI, National Health Insurance; NMSDS, non-multiple sclerosis demyelinating syndromes.
The associated comorbidities were selected as the following ICD codes: systemic lupus erythematosus (ICD-9-CM code 710), depression (ICD-9-CM codes 296.2, 296.3, 300.4, 301.12, 309.0, 309.1, 311), peripheral vascular disorders (ICD-9-CM codes 440, 441.2, 441.4, 441.7, 441.9, 443, 444, 447.1), deficiency anaemias (ICD-9-CM codes 280 and 281), rheumatoid arthritis (ICD-9-CM code 714), fluid and electrolyte disorders (ICD-9-CM code 276), tobacco use disorders (ICD-9-CM codes 305.1 and 649.0) and infectious mononucleosis (ICD-9-CM code 075).
Statistical analyses
SPSS V.22.0 was used for the statistical analyses. Group differences in continuous variables were investigated using independent samples t-tests. Pearson’s χ2 tests were used to compare the distribution of categorical variables between the septicaemia and non-septicaemia groups. Furthermore, the multivariate Cox proportional hazards model was applied to estimate the risk factors of NMSDS, and the outcome was shown as HRs with 95% CIs. To examine the interaction between covariates associated with septicaemia and NMSDS, we computed adjusted HRs, stratified by length of hospitalisation due to septicaemia (1–5 days or more than 5 days) and severity of septicaemia. Age (<45 years, 45–64 years and ≥65 years), sex and comorbidities were included in a regression model. All tests were two-sided, and significant differences were indicated when the two-sided p values were less than 0.05.
Results
Demographics of the study population
There were 482 781 patients with a diagnosis of septicaemia and 1 892 825 non-septicaemia controls recruited in this retrospective cohort study. The demographic characteristics were shown in table 1. The mean age was about 59 years old and most individuals in both cohorts were male (52.1% and 52.4% in septicaemia and non-septicaemia groups, respectively). The age distribution differed from 0.1% to 1% in each group and the mean age difference was only 0.7 years, which was very similar between the two groups. However, the population in this study was very large, so even the age distribution was very close between the two groups, the p-value was still significant. There was a higher prevalence of all the comorbidities including systemic lupus erythematosus, depression, peripheral vascular disorders, deficiency anaemias, rheumatoid arthritis, fluid and electrolyte disorders, smoking, infectious mononucleosis in the septicaemia group.
Table 1.
Baseline characteristics
| Septicaemia | Comparison group | P value | |
| (n=482 781) | (n=1 892 825) | ||
| Age, years | |||
| <18 | 44 198 (9.2) | 176 792 (9.3) | <0.0001 |
| 18–64 | 185 350 (38.4) | 741 400 (39.2) | |
| ≥65 | 253 233 (52.5) | 974 633 (51.5) | |
| Mean (SD) | 59 (24.7) | 58.3 (24.5) | |
| Sex, n (%) | |||
| Female | 231 254 (47.9) | 901 921 (47.6) | 0.0018 |
| Male | 251 527 (52.1) | 990 904 (52.4) | |
| Comorbidity, n (%) | |||
| Systemic lupus erythematosus | 2660 (0.6) | 1409 (0.1) | <0.0001 |
| Depression | 17 834 (3.7) | 22 843 (1.2) | <0.0001 |
| Peripheral vascular disorders | 24 252 (5.0) | 31 928 (1.7) | <0.0001 |
| Deficiency anaemias | 53 278 (11.0) | 62 885 (3.3) | <0.0001 |
| Rheumatoid arthritis | 5301 (1.1) | 7354 (0.4) | <0.0001 |
| Fluid and electrolyte disorders | 181 843 (37.7) | 191 102 (10.1) | <0.0001 |
| Smoking | 61 (0.0) | 110 (0.0) | <0.0001 |
| Infectious mononucleosis | 734 (0.2) | 657 (0.0) | <0.0001 |
The result showed that 144 septicaemia patients developed subsequent NMSDS with an overall rate of 6.91 cases per 100 000 person-years. There were 136 patients having NMSDS in the comparison group with an overall rate of 1.10 cases per 100 000 person-years. The data disclosed that patients with septicaemia had 4.17 times (95% CI 3.21 to 5.4, p<0.001) higher risk of acquiring NMSDS compared with those without septicaemia (table 2). A list of different diagnoses of NMSDS is provided in online supplementary table 1.
Table 2.
Incidence of subsequent demyelinating diseases of the central nervous system, rather than multiple sclerosis, stratified by severity of septicaemia
| N | Event | PYs | Rate | Crude HR (95% CI) | Adjusted HR (95% CI) | |
| All | ||||||
| Comparison group | 1 892 825 | 136 | 12 352 780 | 1.10 | Reference group | Reference group |
| Septicaemia | 482 781 | 144 | 2 082 900 | 6.91 | 5.99 (4.73 to 7.58)*** | 4.17 (3.21 to 5.4)*** |
| Length of stay due to septicaemia* | ||||||
| Comparisons | 1 892 825 | 136 | 12 352 780 | 1.10 | Reference group | Reference group |
| 1–5 days | 91 289 | 16 | 523 515 | 3.06 | 2.73 (1.63 to 4.59)*** | 2.17 (1.27 to 3.69)*** |
| >5 days | 391 492 | 128 | 1 559 385 | 8.21 | 7.06 (5.54 to 9.01)*** | 4.73 (3.62 to 6.18)** |
| P for trend | <0.0001 | <0.0001 | ||||
| Severity of septicaemia | ||||||
| Comparisons | 1 892 825 | 136 | 12 352 780 | 1.10 | Reference group | Reference group |
| Mild (T1) | 144 548 | 14 | 1 115 282 | 1.26 | 1.12 (0.64 to 1.93) | 0.83 (0.47 to 1.45) |
| Moderate (T2) | 145 160 | 34 | 657 778 | 5.17 | 4.92 (3.36 to 7.19)*** | 3.35 (2.25 to 4.98)*** |
| Severe (T3) | 193 073 | 96 | 309 840 | 31.0 | 28.5 (21.4 to 38.0)*** | 18.7 (13.7 to 25.6)*** |
| P for trend | <0.0001 | <0.0001 |
Models adjusted for age, sex and comorbidities listed in table 1. Severity = (total length of hospital stay due to septicaemia during the follow-up duration) ÷ (length of follow-up duration). T1, the first tertile:<0.44%; T2, the second tertile: 0.44%–1.58%; T3 the third tertile:>1.58%.
**p<0.01; ***p<0.001.
*The first time hospitalisation due to septicaemia.
PYs, person-years;Rate, incidence rate, per 100 000 person-years.
postgradmedj-2019-136667supp001.docx (18.1KB, docx)
We further investigated the impact of severity of septicaemia on the risk of developing NMSDS. First, individuals hospitalised due to septicaemia for 1–5 days or more than 5 days have increased risks of developing NMSDS, with HRs 2.17 (95% CI 1.29 to 3.69, p<0.001) and 4.73 (95% CI 3.62 to 6.18, p<0.01), respectively. Second, patients who had been hospitalised due to septicaemia for more days during the follow-up period had an elevated risk of developing NMSDS (severe group: HR=18.7, 95% CI 13.7 to 25.6, p<0.001 and moderate group: HR=3.35, 95% CI 2.25 to 4.98, p<0.001). The risk of septicaemia-related NMSDS declined in the group with mild severity (the first tertile) and showed no significant difference compared with the matched controls (HR=0.83, 95% CI 0.47 to 1.45, p>0.05). In tables 2 and 3, Cox regression models were conducted. One was for all speticaemia patients versus comparison group and the comparison group was the reference group. Another was the length of stay, 1–5 days, >5 days versus the comparison group and the comparison group was the reference group. The other was the severity of speticaemia, mild, moderate, severe versus the comparison group and the comparison group was the reference group.
Table 3.
Risk of demyelinating diseases of the central nervous system, rather than multiple sclerosis, in patients with and without septicaemia, stratified by age, sex and comorbidity
| Variable | Septicaemia cohort | Comparison cohort | Adjusted HR (95% CI) | ||||
| Event | PYs | Rate | Event | PYs | Rate | ||
| Age group | |||||||
| <45 | 47 | 753 379 | 6.24 | 21 | 3 489 202 | 0.60 | 6.41 (3.65 to 11.3)** |
| 45–64 | 51 | 531 048 | 9.6 | 29 | 3 283 699 | 0.88 | 6.66 (3.98 to 11.2)** |
| ≧65 | 46 | 798 474 | 5.76 | 86 | 5 579 879 | 1.54 | 2.45 (1.65 to 3.62)** |
| Sex | |||||||
| Female | 87 | 1 060 254 | 8.21 | 68 | 5 924 056 | 1.15 | 4.43 (3.12 to 6.29)** |
| Male | 57 | 1 022 646 | 5.57 | 68 | 6 428 724 | 1.06 | 3.89 (2.63 to 5.75)** |
| Comorbidities | |||||||
| No | 115 | 1 893 501 | 6.07 | 130 | 12 135 849 | 1.07 | 3.95 (3.01 to 5.2)** |
| Yes | 29 | 189 399 | 15.31 | 6 | 216 931 | 2.77 | 3.74 (1.50 to 9.27)* |
Models adjusted for age, sex and comorbidities listed in table 1.
**p<0.01; **p<0.001.
PYs, person-years;Rate, incidence rate, per 100 000 person-years.
The results of NMSDS risks after adjusting age (<45, 45–64 and ≥65 years), sex and comorbidities are listed in table 3. One regression model for each stratification, and the reference group was the comparison group. Totally, seven Cox regression models were conducted. In tables 2 and 3, all Cox regression models were adjusted for the same covariates which include age, sex and comorbidities listed in table 1. The HR of developing NMSDS was significantly elevated in the septicaemia group, in patient groups, including <45 years (HR=6.41, 95% CI 3.65 to 11.3, p<0.001) and 45–64 years (HR=6.66, 95% CI 3.98 to 11.2, p<0.001). The impact of septicaemia on NMSDS was relatively mild in the group ≥65 years (HR=2.45, 95% CI 1.65 to 3.62, p<0.001). We also investigated whether septicaemia is a sex-dependent risk factor for acquiring NMSDS. The Cox regression analysis demonstrated that the increased risk of NMSDS in septicaemia females (HR=4.43, 95% CI 3.12 to 6.29, p<0.001) was higher than septicaemia males (HR=3.89, 95% CI 2.63 to 5.75, p<0.001).
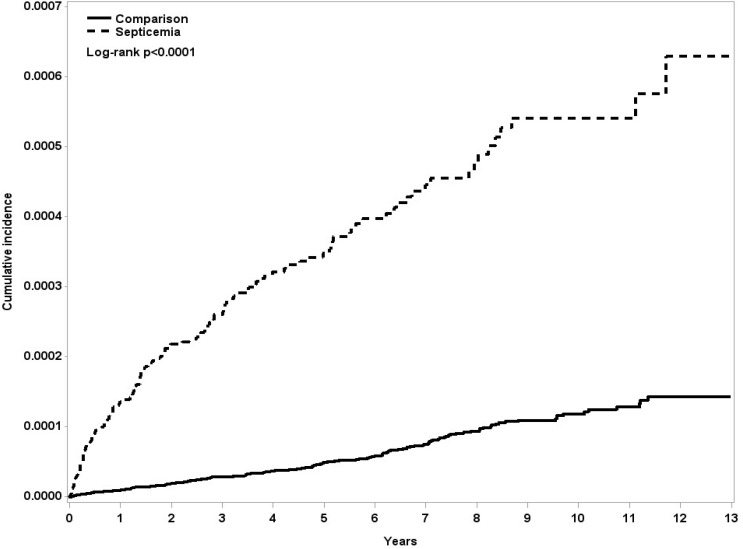
The influence of comorbidities on the risk of developing NMSDS was also investigated. Interestingly, the septicaemia patients with and without comorbidities had similar HR of developing NMSDS compared with the respective control groups (HR=3.74, 95% CI 1.50 to 9.27, p<0.01 and HR=3.95, 95% CI 3.01 to 5.2, p<0.001, respectively). Details of chronic comorbidities including other immunocompromise or autoimmune disease in case and comparison groups are provided in online supplementary table 2. Moreover, the Kaplan–Meier analysis demonstrated that patients with septicaemia had significantly higher incidence of NMSDS compared with the matched controls (log-rank test p<0.0001, figure 2).
Figure 2.
The cumulative incidence curves of non-multiple sclerosis demyelinating syndromes for individuals with and without septicaemia.
Discussion
Postinfectious demyelinating diseases, including myelitis, ADEM and acute transverse myelitis have been reported.4 11 12 However, most of previously reported cases are children and only a few adults. By studying a representative database of Taiwanese population, this 10-year longitudinal study investigated the association between septicaemia and the risk of developing NMSDS. The result suggests that septicaemia patients had an increased risk of developing NMSDS, especially in females, age <65 years and those with higher severities of septicaemia.
Septicaemia is a severe systemic infection that is associated with life-menacing organ dysfunction triggered by unbalanced host immune response. In the present study, we focused on the association between the development of NMSDS and preexisting septicaemia, which was defined as a serious infection which spreads through the entire vascular system of the body. Septicaemia could impair CNS function via a variety of mechanisms, including metabolic dysfunction,13 oxidative stress14 and microcirculatory impairment,15 all of which could instigate sustained damage to neurovascular structure. Currently, septicaemia has been found to be a risk factor for cognitive impairment,16 dementia17 and stroke.18 Our data revealed that septicaemia might have a lasting influence on increasing the risk for NMSDS.
There was a significant higher risk of developing NMSDS in the relatively young age group (<65 years) compared with the elderly (≥65 years) in our study. The NMSDS associated with several autoimmune disorders, such as giant cell arteritis rather than preexisting septicaemia, are relatively common in the elderly.19 In our study, the portion of patients with giant cell arteritis was very low (less than 0.1% in both cohorts, online supplementary table 2). In contrast, fibromyalgia was relatively common in group age 45–64 (1.6% and 0.6% in speticaemia and comparison groups respectively, online supplementary table 2). The finding is in agreement with a previous study.20 In our study population, the prevalence of comorbidity such as fibromyalgia was higher than giant cell arteritis, and these middle age septicaemia patients with fibromyalgia are relatively prone to have demyelinating syndromes, including MS.21 This may explain in part the observation that the 45–64 age group with pre-exsiting septicaemia had the highest adjusted HR of developing NMSDS. Age-related variance of immune response was therefore suggested to be associated with development of subsequent demyelination after systemic infection. We also found that females with septicaemia had a higher risk of developing NMSDS compared with male patients.
Systemic infections may induce damage to the CNS even though there is no evidence of direct invasion. Mawanda et al reported that extra-CNS bacterial infection can trigger diverse cognitive dysfunction with an increased risk for dementia.22 Lindsberg and Grau reported that acute and exacerbating chronic infection may contribute to consequent strokes.23 Various mechanisms have been proposed to explain the impact of systemic infections to CNS inflammation, which may further induce demyelination disease due to oligodendrocyte injuries.
First, the connections between neurons rely on electrical and chemical signals which are controlled within a narrow and stable homeostatic regulation. The integration of blood brain barrier (BBB) is critical for maintenance of a precise extracellular milieu around synapses and axons. BBB was mainly composed of astroglial foot processes, endothelial cells and pericytes.24 Dysfunction of BBB resulting from the endothelial nitric oxide synthase (e-NOS) related oxidant stress in early stage of systemic inflammation has been demonstrated in animal studies.25 The e-NOS was found to be associated with disturbed cerebral autoregulation in sepsis due to its proinflammatory properties, which contribute to malfunction of cerebrovascular endothelial cells.25 26 Impaired cerebral autoregulation contributes to cerebral hypoperfusion and further ischaemic damage to brain tissues in those with septic shock.26 27 Moreover, lipopolysaccharides could induce oligodendrocyte damage via activation of neuronal NOS in oligodendrocytes and then cause demyelination.28
Second, severe infections cause systemic inflammation with significantly elevated concentrations of inflammatory cytokines, such as tumour necrosis factor-α, interleukin 1β (IL-1β) and IL-6.29 30 Animal studies provided evidence that the circulating cytokines could activate the microglia/macrophages in the CNS, which further induced neuronal damage by secreting inflammatory mediators.31 32 Furthermore, oligodendrocyte injuries evidenced by development of demyelination lesions after direct injection of inflammatory cytokines have been demonstrated in transgenic mice with overexpression of IL-6 and interferon-gamma.33 34 All of these studies supported the hypothesis that cytokines mediate demyelination. Additionally, disruption of BBB allows more inflammatory cytokines crossing into the CNS, which has been proved by the findings of higher concentrations of CSF proteins in septic patients compared with non-septic controls.35
Third, molecular mimicry plays an important role in autoimmunity. Many experiments, using peptides from viral or bacterial pathogen, have successfully activated the autoreactive T cells and caused damage to normal tissues.36 37 Therefore, T cell-mediated autoimmunity could be triggered by microbial peptides that have proper structural resemblance to self-peptides. There are case reports of CNS demyelinating diseases after infection with viral or bacterial pathogens, such as Salmonella bacteremia,12 Epstein-Barr virus4 and influenza virus.5 Moreover, anti-MOG antibodies have been discovered in patients with ADEM5 or longitudinally extensive transverse myelitis4 following viral infection. Notably, the detection of anti-MOG antibodies is currently assumed to be related with NMSDS, rather than MS.38 For patients with a history of septicaemia, detection of anti-MOG and aquaporin-4 antibodies in serum is suggested, in the presence of a suspicion of demyelinating diseases, such as NMO, NMOSD and anti-MOG-associated spectrum. Although it has been proposed that acute viral infection could trigger autoimmunity via molecular mimicry mechanism, it remains unclear whether infection could trigger the NMO-associated autoimmunity.39 In spite of the extensive studies on NMO, NMOSD and anti-MOG-associated spectrum following infections, patients without a history of septicaemia can also develop demyelinating diseases due to other autoimmunity disorders. In summary, septicaemia could cause damages to oligodendrocytes and accordingly increase the risk of developing demyelinating diseases through various mechanisms.
Conclusions
Our results showed that the presence of septicaemia was prominently correlated with developing subsequent NMSDS. The increased risk of NMSDS is greater in septicaemia patients aged below 65 years and those with more hospitalisation days. Notably, the findings of this study point out the importance and necessity of thorough management strategies for septicaemia. Further studies are needed to verify whether the early intervention for septicaemia can reduce the risk of acquiring NMSDS.
Limitations
There are several limitations in this study. First, we could not access the detailed information about life styles of the patients with NMSDS, including intensity of tobacco use, severity of functional disability, laboratory data and image studies because NHI Research Database originating from an administrative coding data set undersupplied above details. Second, we could not evaluate the severity in detail and the prognosis of demyelinating diseases after septicaemia. A prospective and longitudinal study will be required to investigate the association between cytokine change during septicaemia and the severity of NMSDS. Third, the participants were recruited according to ICD-9 codes, and this could give rise to potential misclassification bias in our analyses. A cross-checking system monitoring the correctness of records from diverse hospitals is therefore being intensively conducted by the NHI administration in Taiwan. Furthermore, the diagnosis of several demyelinating diseases such as ADEM can only be made by longitudinally following patients to ensure they do not develop any further CNS demyelinating lesions which would then fulfil the criteria of MS. In fact, the average annual incidence rate of MS in Taiwan was 0.79/100 000 around, lower than many high-latitude areas,40 and we therefore focused on the NMSDS in the present study.
Main messages.
Septicaemia is positively correlated with subsequent non-multiple sclerosis demyelinating syndromes (NMSDS).
The increased risk of NMSDS is greater in septicaemia patients aged below 65 years and those with more hospitalisation days.
This study points out the importance of thorough management strategies for septicaemia, which possibly contributes to increased hazard of NMSDS.
Current research questions.
Could interventions for septicaemia have beneficial effects on onset of non-multiple sclerosis demyelinating syndromes (NMSDS)?
What inflammatory mediator(s) responding to septicaemia is correlated to development of NMSDS?
Footnotes
Contributors: Study concept/design: CHC, CKT and JTL; data collection and analysis: CHC, CKT, JTL, IJT, IKW, CHT and CYH; data interpretation: CHC, CKT, LML, JTL, JHY, CCL, YFS, FCY and CLT; manuscript writing: CHC, CKT and JTL. All authors have given their final approval of the version to be published.
Funding: This work was supported in part by grants from the Ministry of Science and Technology (MOST 106-2314-B-016-010, MOST 105-2314-B-016-004 and MOST 106-2314-B-016-007-MY2), Ministry of National Defense Medical Affairs Bureau (MAB-106-041 and MAB-106-044), Tri-Service General Hospital (TSGH-C105-082, TSGH-C107-073, TSGH-C106-068, TSGH-C107-072, TSGH-C107-074 and TSGH-C108-006-007-007-S05), Cheng Hsin General Hospital (CH-NDMC-106-13), the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-123004), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10701010021), Taiwan Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
Competing interests: No, there are no competing interests for any author.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available in a public, open access repository. There are no data in this work. Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. No data are available. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol 2010;119:37–53. 10.1007/s00401-009-0601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev 2014;13:215–24. 10.1016/j.autrev.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 3. Singer M, Deutschman CS, Seymour CW, et al. . The third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakamura Y, Nakajima H, Tani H, et al. . Anti-MOG antibody-positive ADEM following infectious mononucleosis due to a primary EBV infection: a case report. BMC Neurol 2017;17 10.1186/s12883-017-0858-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amano H, Miyamoto N, Shimura H, et al. . Influenza-associated MOG antibody-positive longitudinally extensive transverse myelitis: a case report. BMC Neurol 2014;14 10.1186/s12883-014-0224-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rees J. Complex disease and the new clinical sciences. Science 2002;296:698–700. 10.1126/science.296.5568.698 [DOI] [PubMed] [Google Scholar]
- 7. Lincoln MR, Montpetit A, Cader MZ, et al. . A predominant role for the HLA class II region in the association of the MHC region with multiple sclerosis. Nat Genet 2005;37:1108–12. 10.1038/ng1647 [DOI] [PubMed] [Google Scholar]
- 8. Pierrot-Deseilligny C, Souberbielle J-C. Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain 2010;133:1869–88. 10.1093/brain/awq147 [DOI] [PubMed] [Google Scholar]
- 9. Mikaeloff Y, Caridade G, Tardieu M, et al. . Parental smoking at home and the risk of childhood-onset multiple sclerosis in children. Brain 2007;130:2589–95. 10.1093/brain/awm198 [DOI] [PubMed] [Google Scholar]
- 10. Briggs FBS, Acuña BS, Shen L, et al. . Adverse socioeconomic position during the life course is associated with multiple sclerosis. J Epidemiol Community Health 2014;68:622–9. 10.1136/jech-2013-203184 [DOI] [PubMed] [Google Scholar]
- 11. Eduwu J, Tabasam F, Bastidas AA, et al. . Successful management of methicillin-resistant Staphylococcus aureus bacteremia complicated with diffuse myelitis. Infect Dis 2017;49:234–6. 10.1080/23744235.2016.1212169 [DOI] [PubMed] [Google Scholar]
- 12. Richert ME, Hosier H, Weltz AS, et al. . Acute transverse myelitis associated with Salmonella Bacteremia: a case report. Am J Case Rep 2016;17:929–33. 10.12659/AJCR.900730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Semmler A, Hermann S, Mormann F, et al. . Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation 2008;5 10.1186/1742-2094-5-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barichello T, Fortunato JJ, Vitali AM, et al. . Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med 2006;34:886–9. 10.1097/01.CCM.0000201880.50116.12 [DOI] [PubMed] [Google Scholar]
- 15. Taccone FS, Su F, Pierrakos C, et al. . Cerebral microcirculation is impaired during sepsis: an experimental study. Crit Care 2010;14 10.1186/cc9205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iwashyna TJ, Ely EW, Smith DM, et al. . Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010;304:1787–94. 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tate JA, Snitz BE, Alvarez KA, et al. . Infection hospitalization increases risk of dementia in the elderly. Crit Care Med 2014;42:1037–46. 10.1097/CCM.0000000000000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee J-T, Chung WT, Lin J-D, et al. . Increased risk of stroke after septicaemia: a population-based longitudinal study in Taiwan. PLoS One 2014;9:e89386 10.1371/journal.pone.0089386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watad A, Bragazzi NL, Adawi M, et al. . Autoimmunity in the Elderly: Insights from Basic Science and Clinics - A Mini-Review. Gerontology 2017;63:515–23. 10.1159/000478012 [DOI] [PubMed] [Google Scholar]
- 20. Vincent A, Lahr BD, Wolfe F, et al. . Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester epidemiology project. Arthritis Care Res 2013;65:786–92. 10.1002/acr.21896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pompa A, Clemenzi A, Troisi E, et al. . Chronic pain in multiple sclerosis patients: utility of sensory quantitative testing in patients with fibromyalgia comorbidity. Eur Neurol 2015;73:257–63. 10.1159/000381211 [DOI] [PubMed] [Google Scholar]
- 22. Mawanda F, Wallace RB, McCoy K, et al. . Systemic and localized extra-central nervous system bacterial infections and the risk of dementia among US veterans: a retrospective cohort study. Alzheimers Dement 2016;4:109–17. 10.1016/j.dadm.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke 2003;34:2518–32. 10.1161/01.STR.0000089015.51603.CC [DOI] [PubMed] [Google Scholar]
- 24. Serlin Y, Shelef I, Knyazer B, et al. . Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol 2015;38:2–6. 10.1016/j.semcdb.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Handa O, Stephen J, Cepinskas G. Role of endothelial nitric oxide synthase-derived nitric oxide in activation and dysfunction of cerebrovascular endothelial cells during early onsets of sepsis. Am J Physiol Heart Circ Physiol 2008;295:H1712–H1719. 10.1152/ajpheart.00476.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terborg C, Schummer W, Albrecht M, et al. . Dysfunction of vasomotor reactivity in severe sepsis and septic shock. Intensive Care Med 2001;27:1231–4. 10.1007/s001340101005 [DOI] [PubMed] [Google Scholar]
- 27. Taccone FS, Castanares-Zapatero D, Peres-Bota D, et al. . Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care 2010;12:35–42. 10.1007/s12028-009-9289-6 [DOI] [PubMed] [Google Scholar]
- 28. Yao SY, Ljunggren-Rose A, Chandramohan N, et al. . In vitro and in vivo induction and activation of nNOS by LPS in oligodendrocytes. J Neuroimmunol 2010;229:146–56. 10.1016/j.jneuroim.2010.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci 2013;50:23–36. 10.3109/10408363.2013.764490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holub M, Lawrence DA, Andersen N, et al. . Cytokines and chemokines as biomarkers of community-acquired bacterial infection. Mediators Inflamm 2013;2013:1–7. 10.1155/2013/190145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hannestad J, Gallezot J-D, Schafbauer T, et al. . Endotoxin-induced systemic inflammation activates microglia: [¹¹C]PBR28 positron emission tomography in nonhuman primates. Neuroimage 2012;63:232–9. 10.1016/j.neuroimage.2012.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moreno B, Jukes J-P, Vergara-Irigaray N, et al. . Systemic inflammation induces axon injury during brain inflammation. Ann Neurol 2011;70:932–42. 10.1002/ana.22550 [DOI] [PubMed] [Google Scholar]
- 33. Horwitz MS, Evans CF, Mcgavern DB, et al. . Primary demyelination in transgenic mice expressing interferon-gamma. Nat Med 1997;3:1037–41. 10.1038/nm0997-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campbell IL. Structural and functional impact of the transgenic expression of cytokines in the CNS. Ann N Y Acad Sci 1998;840:83–96. 10.1111/j.1749-6632.1998.tb09552.x [DOI] [PubMed] [Google Scholar]
- 35. Davies DC. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J Anat 2002;200:639–46. 10.1046/j.1469-7580.2002.00065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Merkler D, Horvath E, Bruck W, et al. . "Viral déjà vu" elicits organ-specific immune disease independent of reactivity to self. J Clin Invest 2006;116:1254–63. 10.1172/JCI27372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Evans CF, Horwitz MS, Hobbs MV, et al. . Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease. J Exp Med 1996;184:2371–84. 10.1084/jem.184.6.2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramanathan S, Dale RC, Brilot F. Anti-MOG antibody: the history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev 2016;15:307–24. 10.1016/j.autrev.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 39. Jasiak-Zatonska M, Kalinowska-Lyszczarz A, Michalak S, et al. . The immunology of neuromyelitis Optica-Current knowledge, clinical implications, controversies and future perspectives. Int J Mol Sci 2016;17 10.3390/ijms17030273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lai C-H, Tseng H-F. Population-based epidemiological study of neurological diseases in Taiwan: I. Creutzfeldt-Jakob disease and multiple sclerosis. Neuroepidemiology 2009;33:247–53. 10.1159/000229779 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
postgradmedj-2019-136667supp001.docx (18.1KB, docx)