The human immunodeficiency virus type 1 (HIV-1) envelope proteins (Envs) bind receptors on the host cell and change shape to allow the virus to enter the cell. Most virus-inhibiting antibodies and drugs recognize a particular shape of Env called state 1. Disulfide bonds formed by cysteine residues have been introduced into soluble forms of the flexible envelope proteins in an attempt to lock them into state 1 for use in vaccines and as research tools. We evaluated the effect of these cysteine substitutions on the ability of the membrane Env to support virus entry and on susceptibility to inhibition by antibodies and small molecules. We found that the conformation of the envelope proteins with the cysteine substitutions differed from that of the unmodified membrane envelope proteins. Awareness of these effects can assist efforts to create stable HIV-1 Env complexes that more closely resemble the state 1 conformation.
KEYWORDS: Env, HIV-1, antibody, inhibitor, mutant, retrovirus, structure
ABSTRACT
Most broadly neutralizing antibodies and many entry inhibitors target the pretriggered (state 1) conformation of the human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein (Env). Here we examine two previously reported Env mutants designed to be stabilized in this conformation by the introduction of artificial disulfide bonds: A501C/T605C (called SOS) and I201C/A433C (called DS). SOS Env supported virus entry and cell-cell fusion only after exposure to a reducing agent, dithiothreitol (DTT). Deletion of the Env cytoplasmic tail improved the efficiency with which the SOS Env supported virus infection in a reducing environment. The antigenicity of the SOS Env was similar to that of the unmodified Env, except for greater sensitivity to some state 1-preferring ligands. In contrast, viruses with the DS Env were not infectious, even after DTT treatment. The proteolytic maturation of the DS Env on both cell surfaces and virions was severely compromised compared with that of the unmodified Env. The DS Env exhibited detectable cell-fusing activity when DTT was present. However, the profiles of cell-surface Env recognition and cell-cell fusion inhibition by antibodies differed for the DS Env and the unmodified Env. Thus, the DS Env appears to be stabilized in an off-pathway conformation that is nonfunctional on the virus. The SOS change exerted more subtle, context-dependent effects on Env conformation and function.
IMPORTANCE The human immunodeficiency virus type 1 (HIV-1) envelope proteins (Envs) bind receptors on the host cell and change shape to allow the virus to enter the cell. Most virus-inhibiting antibodies and drugs recognize a particular shape of Env called state 1. Disulfide bonds formed by cysteine residues have been introduced into soluble forms of the flexible envelope proteins in an attempt to lock them into state 1 for use in vaccines and as research tools. We evaluated the effect of these cysteine substitutions on the ability of the membrane Env to support virus entry and on susceptibility to inhibition by antibodies and small molecules. We found that the conformation of the envelope proteins with the cysteine substitutions differed from that of the unmodified membrane envelope proteins. Awareness of these effects can assist efforts to create stable HIV-1 Env complexes that more closely resemble the state 1 conformation.
INTRODUCTION
The entry of human immunodeficiency virus type 1 (HIV-1) into target cells is mediated by the trimeric envelope glycoprotein (Env) complex (1). The Env trimer is composed of three gp120 exterior surface unit Envs (SU) and three gp41 transmembrane Envs (TM). After proteolytic cleavage of the gp160 Env precursor in HIV-1-infected cells, the gp120 and gp41 subunits bond noncovalently. The mature Env trimers are incorporated into budding virions. Interaction with the receptors CD4 and CCR5 or CXCR4 triggers the metastable Env trimer to make transitions from its unliganded conformation (state 1) to lower-energy states (2–14). The initial engagement with CD4 induces an asymmetric Env trimer, in which a default intermediate conformation (state 2) is present (15, 16). Binding of additional CD4 molecules induces the full CD4-bound, prehairpin intermediate (state 3) Env conformation (14–23). The interaction of the state 3 Env with the CCR5 or CXCR4 coreceptor induces the formation of a gp41 six-helix bundle, a process that culminates in fusion of the viral and target cell membranes (24–28).
In addition to conformational triggering by receptors, internal changes in Env that destabilize state 1 can also result in Envs that favor state 2 and/or state 3 (13, 29–33). These viruses become more sensitive to antibody neutralization. In natural HIV-1 infection, the commonly elicited but “weakly neutralizing” antibodies cannot access their Env epitopes in the context of the virus-host cell synapse (34); thus, these antibodies can neutralize only viruses that spontaneously expose state 2 or state 3 conformations. In contrast, less commonly elicited but broadly neutralizing antibodies (bNAbs) can recognize Envs in the pretriggered (state 1) conformation on the virus surface (14, 29, 30, 35–38). Thus, the elicitation of bNAbs for the prevention of HIV-1 transmission may require vaccine immunogens that mimic the state 1 Env structure.
The metastability of Env creates immense challenges for structural studies and for the creation of vaccine immunogens. The pretriggered state 1 Env readily transitions into lower-energy conformational states upon removal from its native membrane environment (39–42). Soluble Env trimers lacking the transmembrane region and cytoplasmic tail are labile and easily dissociate into monomers and dimers (39, 43–46). Moreover, the gp120 glycoprotein readily dissociates from the transmembrane gp41 subunit. Therefore, the introduction of a disulfide linkage (A501C/T605C, the “SOS” change) between gp120 and gp41 along with an I559P change has been employed to stabilize soluble HIV-1 Env trimers truncated at gp41 ectodomain residue 664 (47–56). Structures of these soluble gp140 (sgp140) SOSIP.664 Env trimers have been solved and are generally assumed to represent a state 1 conformation of Env (57–59). To stabilize the sgp140 SOSIP.664 trimer further, another pair of cysteine residues (I201C/A433C, called DS) was designed to cross-link the β3 and β21 strands of gp120; the DS changes are expected to prevent the CD4-induced movement of Env into the CD4-bound conformation (state 3). Indeed, the introduction of the DS changes decreased the recognition of a CD4-induced epitope by the 17b anti-gp120 antibody following incubation with soluble CD4 (sCD4) (60).
In addition to the structural studies discussed above, both the SOS and DS changes are being used in soluble Env trimers for immunization studies (51, 54, 55, 61–71). However, it has been reported that changes in hydrophobic gp41 residues near the viral membrane can disrupt a state 1 HIV-1 Env conformation (13, 72–77), raising the possibility that membrane interactions can influence the conformation of the HIV-1 Env ectodomain. Such evidence has spurred consideration of membrane Envs from cells or viruslike particles (VLPs) as immunogens that are potentially more representative of the functional, state 1 Env. Proteolytically mature Envs better resist the binding of weakly neutralizing antibodies (42, 78–83) and therefore may have advantages as immunogens; however, the noncovalent nature of the gp120-gp41 association can create lability in Env trimer preparations. Moreover, binding to CD4 in humans or monkeys can drive Env immunogens into state 2 or state 3 conformations (14, 84–86), potentially diverting antibody responses away from state 1 Env. Given the potential ability of the SOS and DS changes to address these respective problems, we evaluated the effects of these changes on the conformation and function of membrane Envs from two primary HIV-1 strains.
RESULTS
Infectivity of viruses with SOS- and DS-modified Envs.
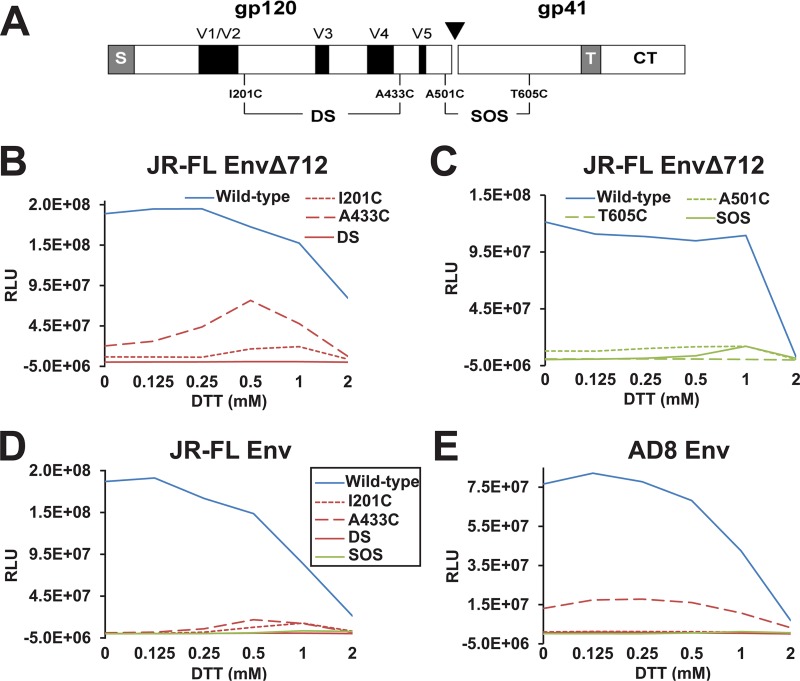
The DS (I201C/A433C) and SOS (A501C/T605C) changes were introduced into the full-length Envs of HIV-1JR-FL and HIV-1AD8 and the cytoplasmic tail-deleted EnvΔ712 of HIV-1JR-FL (Fig. 1A). We also created Env and EnvΔ712 mutants containing the individual cysteine substitution changes I201C, A433C, A501C, and T605C. We assessed the ability of these Envs to complement the single-round infectivity of an Env-defective luciferase-expressing HIV-1 vector in Cf2Th-CD4/CCR5 target cells. The infections were carried out in the absence and presence of different concentrations of the reducing agent dithiothreitol (DTT). The wild-type HIV-1JR-FL and HIV-1AD8 Envs and the HIV-1JR-FL EnvΔ712 efficiently mediated virus entry into Cf2Th-CD4/CCR5 cells (Fig. 1B to E). The infectivity of these viruses decreased at higher concentrations of DTT, presumably due to the reducing effects of DTT on one or more native Env disulfide bonds. The HIV-1JR-FL EnvΔ712 SOS mutant was poorly infectious in the absence of DTT; the infectivity of the EnvΔ712 SOS mutant increased to a detectable level at DTT concentrations of up to 1 mM, as previously reported (87–89) (Fig. 1C). Similar to the EnvΔ712 virus, the EnvΔ712 SOS virus exhibited reduced infectivity at DTT concentrations beyond 1 mM. The full-length HIV-1JR-FL and HIV-1AD8 Env SOS mutants were poorly infectious in the absence of DTT and were only marginally infectious when DTT was present (Fig. 1D and E). Viruses with the Env DS and EnvΔ712 DS Envs were defective for infection and were not activated by incubation with DTT (Fig. 1B, D, and E). Viruses with the individual I201C and A433C changes generally exhibited a low level of infectivity, with some increase in infectivity at higher concentrations of DTT (Fig. 1B). The individual cysteine residues at these positions may promote the formation of aberrant disulfide bonds that interfere with the ability of Env to support virus infection; some level of aberrantly disulfide-bonded molecules forms even for wild-type Envs (90). Viruses with the individual A501C and T605C changes exhibited low infectivity, which was minimally affected by the concentration of DTT (Fig. 1C). These results are consistent with the formation of the disulfide bond between Cys 501 and Cys 605 that impedes Env function in the SOS mutants; reduction of the disulfide bond allows a partial return of EnvΔ712 SOS to a functional state. Even in a reducing environment, the full-length Env SOS mutants were severely compromised in their ability to support virus infection. The DS Env mutants do not apparently function in this virus infectivity assay, regardless of the reducing environment. In summary, in the context of single-cycle virus infections, the DS Env mutants are nonfunctional, and the SOS mutant partially functions in the EnvΔ712 background after the addition of a reducing agent, DTT.
FIG 1.
Infectivity of recombinant viruses with HIV-1JR-FL and HIV-1AD8 Env variants. (A) Schematic of the HIV-1 Env, with the gp120-gp41 cleavage site depicted as a black triangle. The gp120 major variable regions (V1 to V5) are shown as black boxes. The cysteine substitutions associated with the DS and SOS mutants are indicated. S, signal peptide; T, transmembrane region; CT, cytoplasmic tail. (B to E) Recombinant luciferase-expressing viruses with the indicated Envs were incubated with Cf2Th-CD4/CCR5 cells in the presence of 0 to 2 mM dithiothreitol (DTT). The levels of the p24 Gag protein in the virus preparations were similar for the different Env variants (Fig. 2A). Forty-eight hours later, the Cf2Th-CD4/CCR5 target cells were lysed, and the luciferase activity was measured. The reported relative light unit (RLU) values reflect the infectivities observed for each Env mutant. The y axis has been scaled so that the RLU values near the background of the assay can be visualized. The data are representative of those obtained in at least three independent experiments. The key in panel D also applies to panel E.
Expression and processing of the SOS- and DS-modified Envs.
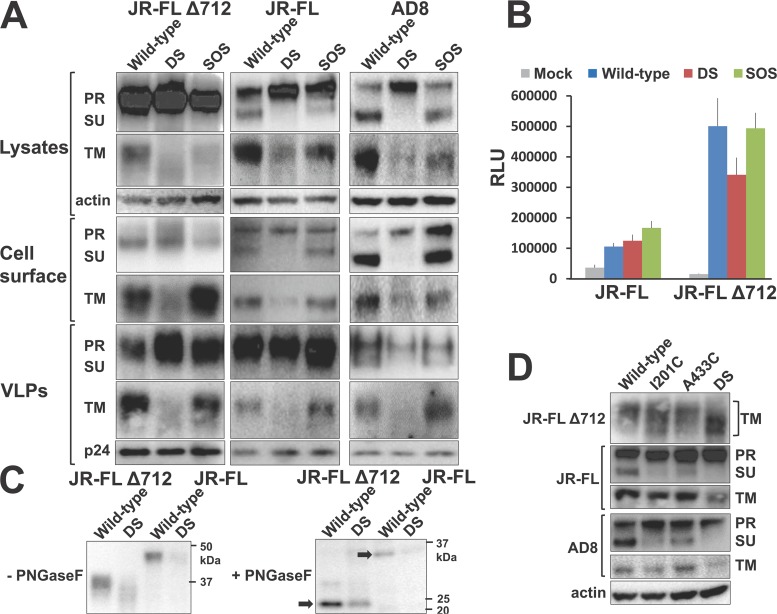
We next evaluated the expression and proteolytic processing of the HIV-1 Env and EnvΔ712 variants. The wild-type, DS, and SOS Env and EnvΔ712 variants were expressed, transported to the cell surface, and incorporated into VLPs (Fig. 2A and B). Consistent with a role of the gp41 cytoplasmic tail in the endocytosis of Env (91–100), the EnvΔ712 variants were expressed at higher levels on the cell surface than the full-length Env (Fig. 2B). The proteolytic processing of the full-length HIV-1JR-FL and HIV-1AD8 Env DS mutants was inefficient relative to the cleavage of the respective wild-type Envs (Fig. 2A). The HIV-1JR-FL EnvΔ712 DS mutant also appeared to be inefficiently processed, and the small amount of processed transmembrane envelope glycoprotein migrated faster than the unmodified EnvΔ712 transmembrane envelope glycoprotein. Treatment of the cell lysates with peptide-N-glycosidase F (PNGase F) resulted in the comigration of the unmodified EnvΔ712 and EnvΔ712 DS transmembrane glycoproteins (Fig. 2C). This result indicates that the EnvΔ712 DS transmembrane envelope glycoprotein is not only inefficiently processed but is also underglycosylated relative to the unmodified EnvΔ712 transmembrane glycoprotein. The inefficient processing of the Env DS and EnvΔ712 DS glycoproteins and the underglycosylation of EnvΔ712 DS were most apparent when both the I201C and the A433C changes were present (Fig. 2D).
FIG 2.
Characterization of cell- and virus-associated Envs. (A) 293T cells were transfected with plasmids encoding the indicated Envs, viral packaging proteins, and a luciferase-expressing HIV-1 vector. Forty-eight-hours later, cell lysates were Western blotted using a goat anti-gp120 antibody (for the Env precursor [PR] and exterior surface unit [SU] glycoproteins) and the 4E10 anti-gp41 antibody (for the transmembrane Env [TM]). Transfected cells were also incubated with the 2G12 antibody, followed by cell lysis and immunoprecipitation to measure cell surface Env expression. Viruslike particles (VLPs) were enriched by pelleting through a 20% sucrose cushion, as described in Materials and Methods. The VLP-containing pellets were Western blotted as described above. Cell lysates and VLPs were also Western blotted for actin and p24 Gag, respectively. (B) The amount of the indicated Envs expressed on the surface of transiently expressing HOS cells was measured by a cell-based ELISA with the 2G12 antibody, as described in Materials and Methods. The means and standard errors of the means derived from 3 to 4 independent experiments are shown. (C) 293T cells were transfected with Env-expressing plasmids using calcium phosphate. Forty-eight hours later, cells were lysed, and clarified lysates were denatured and incubated with PNGase F (NEB) for 1.5 h at 37°C. Samples were mixed with lithium dodecyl sulfate (LDS) buffer and DTT, boiled, and Western blotted with the 4E10 antibody. Black arrows mark the deglycosylated TM. Results of a representative experiment of two independent experiments are shown. (D) Virus-producing 293T cells were prepared as described in Materials and Methods. Forty-eight hours later, cells were lysed, and clarified lysates were Western blotted with a goat anti-gp120 antibody and the 4E10 anti-gp41 antibody, as described above for panel A. The gp160 Env precursor, gp120 surface unit, and gp41 transmembrane glycoprotein are indicated. Data shown are representative of those obtained in at least two independent experiments.
Compared with the DS mutants, the proteolytic processing of the full-length HIV-1JR-FL and HIV-1AD8 Env SOS mutants was efficient, nearly equivalent to that of the wild-type Envs (Fig. 2A). The HIV-1JR-FL EnvΔ712 SOS mutant was cleaved with an intermediate level of efficiency. The proteolytically processed SOS mutants were efficiently incorporated into virions, consistent with the observed retention of some function of these mutants in supporting virus entry in the presence of DTT. However, defects other than those affecting virion incorporation or processing apparently account for the poor infectivity of the full-length Env SOS mutants in a reducing environment (Fig. 1D and E).
Antigenicity of cell surface SOS and DS Envs.
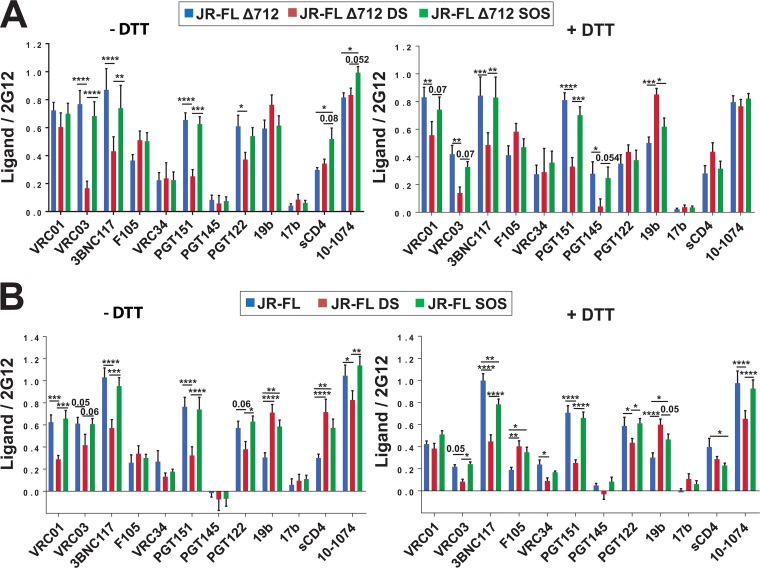
The antigenicity of HIV-1JR-FL Env, EnvΔ712, and the corresponding SOS and DS mutants expressed on the surface of HOS cells was examined. The HIV-1 Env precursor is processed efficiently in HOS cells (78), allowing a better correlation between antibody binding to the cell surface Env and virus neutralization (42, 89, 101). We used a cell-based enzyme-linked immunosorbent assay (ELISA) to measure the recognition of cell surface Envs by a panel of broadly neutralizing antibodies as well as poorly neutralizing antibodies, in the absence and presence of 1 mM DTT. The broadly neutralizing antibodies in our panel included VRC01, VRC03, and 3BNC117 against the CD4-binding site (102–105); VRC34 against the gp41 fusion peptide (106); PGT151 against a gp120-gp41 epitope (107); PGT145 against a quaternary V2 epitope (108); and PGT122 and 10-1074 against V3 glycan epitopes (108, 109). The poorly neutralizing antibodies included F105 against the CD4-binding site (110), 19b against the V3 region (111), and 17b against a CD4-induced epitope (112). We also measured the binding of sCD4 to the Envs. The 2G12 antibody recognizes a glycan-dependent epitope on the gp120 outer domain in a manner that is relatively independent of the Env conformational state (29, 30, 89, 113, 114); thus, 2G12 recognition serves to indicate the relative level of cell surface expression of the Env variants. All three Env and EnvΔ712 variants were efficiently expressed on the cell surface (Fig. 2B). Under both nonreducing and reducing conditions, the antigenic profiles of EnvΔ712 and EnvΔ712 SOS were similar (Fig. 3A). Likewise, the HIV-1JR-FL Env and Env SOS glycoproteins were recognized comparably by the panel of antibodies, in both the absence and presence of DTT (Fig. 3B). In the absence of DTT, the SOS glycoproteins bound sCD4 better than the corresponding unmodified Envs. The binding of the 19b anti-V3 antibody to HIV-1JR-FL Env SOS was greater than the binding to Env, under both reducing and nonreducing conditions. Relative to Env and EnvΔ712, the corresponding DS mutants were recognized less efficiently by the broadly neutralizing antibodies (VRC03 and 3BNC117) against the CD4-binding site and by the PGT151 broadly neutralizing antibody, in both the presence and absence of DTT. At 1 mM DTT, Env DS and EnvΔ712 DS were recognized by the VRC01 antibody slightly less than Env and EnvΔ712, respectively. The 19b antibody against the gp120 V3 loop bound the Env DS and EnvΔ712 DS mutants better than Env and EnvΔ712, respectively, particularly after exposure to DTT. Apparently, the Env DS and EnvΔ712 DS mutants, under reducing or nonreducing conditions, are in conformations distinct from state 1.
FIG 3.
Antigenic profile of cell surface DS and SOS Envs. (A and B) The binding of antibodies to the HIV-1JR-FL Env variants expressed on the surface of HOS cells was measured by a cell-based ELISA. (A) Cytoplasmic tail-deleted EnvΔ712 variants. (B) Full-length Env variants. The RLU value associated with the binding of soluble CD4 (sCD4) or the indicated antibody was normalized relative to the detection of the 2G12 antibody. The experiments were conducted in the absence of a reducing agent (left) or in the presence of 1 mM DTT (right). Data represent the averages and standard errors of the means from at least three independent experiments, each performed in quadruplicate. Statistical significance was evaluated using multiple-comparison one-way analysis of variance (ANOVA) or a Kruskal-Wallis test; P values near the level of significance are reported as numerals, and significant P values are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
Cell-cell fusion mediated by the SOS and DS Env mutants.
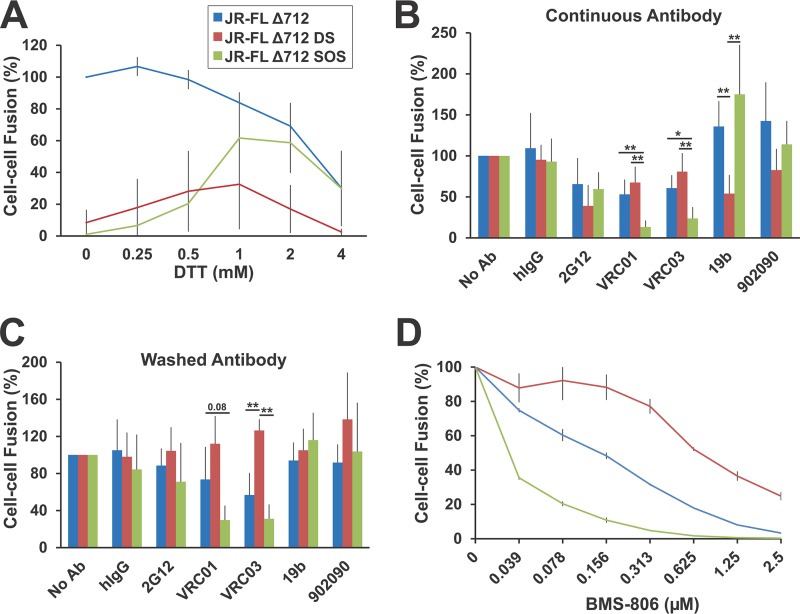
Some HIV-1 Env mutants are unstable and inefficiently support infection by cell-free viruses yet exhibit the ability to mediate cell-cell fusion (29, 115, 116). We therefore measured the ability of the HIV-1JR-FL EnvΔ712 glycoprotein and the SOS and DS EnvΔ712 mutants to induce the formation of syncytia in an α-complementation assay (117). We used the EnvΔ712 variants because their signals were better than those of the full-length Envs (data not shown), consistent with the higher cell surface expression levels of the cytoplasmic tail-deleted Envs (Fig. 2B). In the α-complementation assay, 293T cells expressing these Env variants were cocultivated with Cf2Th cells expressing CD4 and CCR5 for 3 h before measuring galactosidase activity, which is related to the amount of cell-cell fusion (117). In the absence of a reducing agent, EnvΔ712 efficiently mediated cell-cell fusion, whereas the activity of the EnvΔ712 SOS mutant was close to that of the Env-negative control (Fig. 4A). A low level of cell-fusing activity, approximately 8% of that seen for EnvΔ712, was observed for the EnvΔ712 DS mutant in the absence of a reducing agent. As was seen for the virus infection described above, the efficiency of syncytium formation by the unmodified EnvΔ712 glycoprotein decreased with the addition of increasing concentrations of DTT. Presumably, one or more disulfide bonds important for Env function are reduced at high DTT concentrations. In contrast, the cell-fusing activities of the EnvΔ712 SOS and DS mutants increased with the addition of low concentrations of DTT and then decreased as the DTT concentration exceeded 1 mM. In the presence of 1 mM DTT, the EnvΔ712 SOS and DS variants mediated cell-cell fusion at 74% and 39% of the level seen for EnvΔ712, respectively. These results suggest that disulfide bonds decrease the membrane-fusing capacity of the EnvΔ712 SOS and DS mutants and that reduction of these bonds by DTT restores some level of Env function. Presumably, the detrimental effects of high DTT concentrations on the function of HIV-1JR-FL EnvΔ712 also apply to the DS and SOS variants. The DS changes are more detrimental to the EnvΔ712 syncytium-forming ability than the SOS changes, perhaps related to the inefficient processing of the EnvΔ712 DS mutant (Fig. 2A).
FIG 4.
Cell-cell fusion activity of the DS and SOS Envs. (A) 293T cells cotransfected with plasmids expressing the indicated Envs and the α-gal fragment of beta-galactosidase were incubated with Cf2Th cells expressing CD4, CCR5, and the ω-gal fragment of beta-galactosidase for 3 h in the presence of 0 to 4 mM DTT. Cells were then lysed, and galactosidase activity was assessed as a measure of cell-cell fusion. The values shown are normalized to those observed for the unmodified EnvΔ712 glycoprotein in the absence of DTT. The means and standard deviations derived from three independent experiments are shown. As the DTT concentration increased from 0 to 1 mM, cell-cell fusion mediated by the EnvΔ712 DS and SOS mutants exhibited consistent increases in all three experiments. (B) 293T cells expressing the unmodified EnvΔ712 or the EnvΔ712 DS and SOS mutants were preincubated with 50 μg/ml antibodies for 1 h at room temperature before the mixture was added to Cf2Th-CD4/CCR5 target cells in the presence of 1 mM DTT. A polyclonal human immunoglobulin G (hIgG) preparation was used as a negative control. Cell-cell fusion was measured as described above. The means and standard deviations derived from at least three independent experiments are shown. A two-sample t test was used to evaluate significance, which is reported as described in the Fig. 3 legend. The results in panels B, C, and D are color coded according to the key in panel A. (C) 293T cells expressing the unmodified EnvΔ712 or DS or SOS EnvΔ712 glycoproteins were preincubated with 50 μg/ml antibody for 1 h at room temperature. The cells were then washed with PBS to remove unbound antibody before adding them to Cf2Th-CD4/CCR5 target cells in the presence of 1 mM DTT. Cell-cell fusion was measured as described above. Data represent the means and standard deviations derived from at least three independent experiments. Significance was assessed and is reported as described above for panel B. (D) 293T cells expressing EnvΔ712, EnvΔ712 DS, or EnvΔ712 SOS were preincubated with the indicated concentrations of BMS-806 for 30 min at 37°C before the mixture was added to Cf2Th-CD4/CCR5 target cells in the presence of 1 mM DTT. Cell-cell fusion was measured as described above. The means and standard deviations from two measurements of cell-cell fusion, each performed in triplicate, are shown. The 50% inhibitory concentration (IC50) values for EnvΔ712, EnvΔ712 DS, and EnvΔ712 SOS were 0.14 ± 0.015, 0.79 ± 0.21, and 0.036 ± 0.001 μM, respectively. Two-sample t tests indicated significant differences (two-tailed P value of <0.05) between the IC50 values for EnvΔ712 and EnvΔ712 DS and between the IC50 values for EnvΔ712 and EnvΔ712 SOS.
Inhibition of Env-mediated cell-cell fusion.
The modest level of syncytium-forming ability detected for the EnvΔ712 DS mutant in the presence of DTT provided an opportunity to assess the sensitivity of the functional DS-modified envelope glycoprotein to inhibition by antibodies and virus entry blockers. At the DTT concentration of 1 mM used in this assay, the syncytium-forming ability of unmodified EnvΔ712 was only moderately reduced, whereas the syncytium-forming abilities of EnvΔ712 SOS and EnvΔ712 DS were readily detectable (Fig. 4A). We tested some broadly neutralizing antibodies, including VRC01 and VRC03, which are directed against the gp120 CD4-binding site (102, 103) and exhibit a preference for the state 1 Env conformation (29, 30). We also tested the 19b anti-V3 antibody and the 902090 anti-V2 antibody, which exhibit a preference for state 2/3 Env conformations and fail to neutralize most primary HIV-1 isolates (29, 30, 111, 113, 118). The antibodies were tested in two assay formats. In one format, the antibody was preincubated with Env-expressing cells for 1 h at room temperature (RT) and was present throughout the cocultivation of Env-expressing and CD4/CCR5-expressing Cf2Th cells in medium containing 1 mM DTT. In this format, the 2G12 antibody, which efficiently recognizes state 1 and state 2 Envs (29, 30, 114), mildly inhibited cell-cell fusion induced by EnvΔ712 and the SOS and DS mutants (Fig. 4B). The VRC01 and VRC03 antibodies caused a 2-fold reduction in cell-cell fusion by EnvΔ712. The syncytium-forming capacity of the EnvΔ712 SOS mutant was particularly sensitive to inhibition by the VRC01 and VRC03 antibodies. Cell-cell fusion by EnvΔ712 and EnvΔ712 SOS was not inhibited, and even showed mild enhancement, by the state 2-preferring 19b and 902090 antibodies. In contrast, the syncytium-inducing capacity of the EnvΔ712 DS mutant was inhibited by the 19b antibody.
In a second assay format, Env-expressing 293T cells were preincubated with the antibody for 1 h at room temperature, after which the antibody-containing cell supernatants were removed; the Env-expressing cells were then washed once with phosphate-buffered saline (PBS) to remove unbound antibodies and added to Cf2Th-CD4/CCR5 cells in the presence of 1 mM DTT. In this assay format, the ability of the antibody to bind the functional Env trimer in the absence of DTT was assessed. As expected, the VRC01 and VRC03 antibodies were slightly less potent at inhibiting syncytium formation by EnvΔ712 under these circumstances; nonetheless, the cell-fusing activity of the EnvΔ712 SOS mutant was again inhibited by both of these antibodies more efficiently than that of the EnvΔ712 glycoprotein (Fig. 4C). Syncytium formation mediated by EnvΔ712 and EnvΔ712 SOS was resistant to inhibition by the 19b and 902090 antibodies. Of note, in this assay format, cell-cell fusion by the EnvΔ712 DS mutant was not inhibited by any of the antibodies.
These results indicate that the nonreduced EnvΔ712 SOS assumes a conformation that is more susceptible to inhibition by the state 1-preferring VRC01 and VRC03 antibodies than the parental EnvΔ712 glycoprotein. The nonreduced EnvΔ712 DS mutant apparently exists in a conformation that is inefficiently inhibited even by broadly neutralizing antibodies and thus must differ from the unmodified EnvΔ712 conformation. Upon transfer to a reducing environment, during the process of mediating cell membrane fusion, the functional EnvΔ712 DS can be bound and inhibited by the 19b anti-V3 antibody. These properties distinguish EnvΔ712 DS from the unmodified EnvΔ712 glycoprotein. Similar patterns of susceptibility to antibody inhibition were observed when the syncytium formation assay was conducted with full-length HIV-1JR-FL Env, Env DS, and Env SOS; however, the signal in the α-complementation assay was lower than those for the EnvΔ712 variants (data not shown).
The susceptibility of syncytium formation mediated by EnvΔ712 and the SOS and DS mutants to the conformational blocker BMS-806 was also evaluated. BMS-806 inhibits HIV-1 entry by blocking CD4-induced conformational changes in Env (23, 29, 119–122). Specifically, BMS-806 binding stabilizes a state 1 conformation in Env (14), inhibiting Env conformational transitions to downstream conformations (23, 29, 119–122). HIV-1 Env variants that sample conformations other than state 1 typically exhibit less sensitivity to BMS-806 than the wild-type Env; conversely, Env variants that sample state 1 more than the wild-type Env exhibit relative increases in sensitivity to BMS-806 (29, 116). In our experiments, syncytium formation by EnvΔ712 SOS was approximately 3.9-fold more sensitive to BMS-806 inhibition than syncytium formation by EnvΔ712 (Fig. 4D). Syncytium formation by the EnvΔ712 DS mutant was approximately 5.7-fold less sensitive to BMS-806 inhibition than syncytium formation by EnvΔ712. Consistent with the antibody inhibition experiments described above, these results suggest that EnvΔ712 SOS assumes a conformation recognized by ligands with a state 1 preference. On the other hand, the low sensitivity to BMS-806 and the increased sensitivity to the 19b anti-V3 antibody compared with unmodified EnvΔ712 indicate that EnvΔ712 DS assumes a conformation that is distinct from state 1.
DISCUSSION
Stabilizing native conformations of the metastable HIV-1 Env trimer can assist in structural analysis and aid in the design of inhibitors. The introduction of pairs of cysteine residues that form new, artificial disulfide bonds is an effective means of stabilizing particular conformations of a protein. The A501C/T605C changes, generally referred to as the SOS changes, have been used to link gp120 and gp41 covalently in mature soluble and membrane-anchored Env trimers (47–55, 87, 88). Viruses with envelope glycoproteins containing the SOS changes are not infectious, but their infectivity can be restored by incubation with a reducing agent (87–89). In our study, we similarly found that the SOS changes effectively arrested Env-mediated virus entry. The addition of a reducing agent partially restored the infectivity of viruses with the cytoplasmic tail-deleted HIV-1JR-FL EnvΔ712 SOS glycoprotein but was much less effective in reactivating the infectivity of viruses with the full-length HIV-1JR-FL and HIV-1AD8 Env SOS glycoproteins. We note that previous studies of the reversibility of the SOS replication defect reported results only for Envs lacking the gp41 cytoplasmic tail (88, 89). The minimally rescuable infectivity of viruses with full-length SOS Envs recommends caution in employing this artificial disulfide bond in studies of HIV-1 entry. On the other hand, cell-cell fusion mediated by the HIV-1JR-FL EnvΔ712 SOS mutant could be restored nearly to the level seen for the unmodified Env by the addition of a reducing agent. It has been reported that, compared with viruses with the unmodified HIV-1JR-FL EnvΔ712, the SOS mutant virus is sensitive to neutralization by the V3 antibody 447-52D and the 2F5 antibody directed against the membrane-proximal external region of gp41 (88). In another study (89), the EnvΔ712 SOS mutant on the surface of expressing cells bound V3 antibodies and the CD4-induced antibody 17b slightly better than the unmodified HIV-1BG505 EnvΔ712 glycoprotein. The increased binding or sensitivity to these antibodies, which recognize downstream Env conformations better exposed after CD4 binding (29, 35, 112, 123), might indicate that the SOS changes destabilize state 1. Indeed, a recent single-molecule fluorescence resonance energy transfer (smFRET) study (124) found that the introduction of the SOS changes into native virion Envs caused a decreased occupancy of state 1, resulting in Envs that were largely in a state 2-like conformation. In our study, cell-cell fusion mediated by the HIV-1JR-FL EnvΔ712 SOS mutant was not more sensitive to inhibition by the 19b anti-V3 antibody than unmodified EnvΔ712. This argues against a model in which the SOS mutant samples state 2 conformations more frequently than EnvΔ712. On the contrary, the relatively increased sensitivity of the SOS mutant to inhibition by the VRC01 and VRC03 antibodies, which exhibit a preference for state 1 Envs (29, 30), might indicate a gp120 conformational profile enriched in state 1. The EnvΔ712 SOS mutant was also slightly more sensitive to BMS-806 than EnvΔ712, another observation consistent with a state 1 Env (29, 116). The antigenic profiles of the unmodified and SOS Env and EnvΔ712 variants on the surface of expressing cells were nearly indistinguishable, in both the absence and presence of a reducing agent. Some differences were observed in the binding of sCD4 and the 19b anti-V3 antibody to the full-length Env and Env SOS variants. The variation in the reported effects of the SOS changes on Env function and conformation may result from differences in the HIV-1 strain of origin or the contexts in which the Envs were assayed. Investigators using the SOS Env should be cognizant of potential subtle and context-dependent effects of the A501C/T605C changes on the conformation of the Env trimer.
Based on the proximity of Ile 201 and Ala 433 in the sgp140 SOSIP.664 structure (57–59), the I201C/A433C changes in the Env DS mutant were initially designed to prevent the CD4-induced movement of Env into downstream (state 2/3) conformations (60). The design of the Env DS mutant was based on the premise that the sgp140 SOSIP.664 Env structure represents a pretriggered (state 1) conformation (60). Several observations cast doubt upon this assumption. First, differences between the sgp140 SOSIP.664 glycoproteins and native membrane Envs have been documented by analyses of Env function and antigenicity (39, 116, 125). Second, the introduction of the I559P change, and, to a lesser extent, the SOS change, into membrane Envs has been shown to alter Env antigenicity (89, 113, 126). Third, cross-links introduced into an sgp140 SOSIP.664 glycoprotein and a mature membrane Env were analyzed by mass spectrometry (101). The cross-links observed in the sgp140 SOSIP.664 glycoprotein were completely compatible with multiple reported sgp140 SOSIP.664 structures (57–60); thus, the available structural models accurately describe the conformation of sgp140 SOSIP.664 trimers in solution. In contrast, many cross-links observed in the membrane Env trimer could not be explained by the available high-resolution structural models of sgp140 SOSIP.664 trimers (101). Fourth, after comparing the results of double-electron-electron resonance (DEER) spectroscopic analyses of sgp140 SOSIP.664 trimers with the results of smFRET studies of native Env on HIV-1 virions, Stadtmueller et al. speculated that existing high-resolution structures of HIV-1 Env trimers may represent state 2 conformations (127). Indeed, direct smFRET analysis of sgp140 SOSIP.664 trimers recently demonstrated that these are in a state 2-like conformation (124). Fifth, the cryo-electron microscopy (cryo-EM) structure of a cytoplasmic tail-deleted Env, which was bound to the PGT151 bNAb and solubilized from cell membranes, was very similar to the structures of sgp140 SOSIP.664 trimers (128). PGT151 has recently been shown to induce a state 2-like conformation in the HIV-1 virion Env (124). This contrasts with most bNAbs, which recognize and may even stabilize a state 1 conformation (14). Thus, a growing body of evidence indicates that all available high-resolution HIV-1 Env trimer structures represent a default intermediate conformation that resembles state 2 and differs significantly from state 1.
In light of the above-mentioned perspective, we reexamined the evidence used to support the hypothesis that the functional Env DS trimer on a virion is in a state 1 conformation (60). First, smFRET analysis of a full-length HIV-1JR-FL Env with the DS changes on virions suggested a distance between the gp120 V1 and V4 loops compatible with a state 1 conformation (60). The smFRET data even suggested that the DS changes resulted in an increase in the occupancy of state 1 by virion Env, from 47% in the wild-type HIV-1JR-FL Env to 59% (60). Although these results indicate that most Env DS trimers on virions maintain a V1-V4 distance consistent with state 1, they do not rule out other conformational effects of the DS changes that cannot be detected by the particular smFRET probes used in the study. These conformational effects may be manifested in the defectiveness of the DS Envs that we observed with respect to the processing of the Env precursor, cell-cell fusion activity, and infectivity. Second, Kwon et al. (60) documented decreased transitions of Env DS to the CD4-bound conformation (state 3) by elimination of the binding of the 17b antibody, which preferentially recognizes this Env conformation (14, 112, 129); however, these results need to be interpreted cautiously, as residue 433 is proximal to the 17b epitope (129). Third, the formation of a disulfide bond between Cys 201 and Cys 433 has been documented on sgp140 SOSIP.664 and sgp140 NFL glycoprotein trimers (64, 130); this is to be expected since residues 201 and 433 were selected based on their proximity in the sgp140 SOSIP.664 structure (60). However, the presence of this disulfide bond on the virion Env DS glycoprotein was not verified (60). Finally, viruses with Env DS were shown to be defective for replication, but whether the infectivity could be rescued by treatment with a reducing agent was not reported (60). Thus, information necessary to equate the DS disulfide-bonded Env with a functional Env was lacking. Here we found that Env DS and EnvΔ712 DS mutants on the HIV-1 virion are defective and that their infectivity cannot be rescued by incubation with DTT. Therefore, the state 1-like smFRET profile associated with the Env DS mutant on virions cannot be attributed to a functional Env.
A striking feature of the Env DS and EnvΔ712 DS mutants is their poor proteolytic processing. The inefficient maturation of these glycoproteins may reflect some degree of misfolding and likely contributes to their poor functionality. The truncation of the EnvΔ712 DS cytoplasmic tail could contribute to decreased glycan occupancy (131), leading to the observed underglycosylation of the transmembrane Env (TM).
Despite its relatively poor processing, we found that the EnvΔ712 DS mutant could mediate a moderate level of cell-cell fusion in a reducing environment, and this allowed us to investigate the conformation of the functional EnvΔ712 DS glycoprotein. Although these studies were performed with the cytoplasmic tail-deleted EnvΔ712, the ectodomain conformations of the EnvΔ712 and full-length Env from many HIV-1 strains, including HIV-1JR-FL, are similar (42, 101, 132). Consistent with this similarity, the unmodified full-length Env and cytoplasmic tail-deleted EnvΔ712 exhibited nearly identical antigenic profiles (Fig. 4A and B). Moreover, syncytium formation by the unmodified HIV-1JR-FL EnvΔ712 was inhibited by antibodies that recognize state 1 and not by antibodies that recognize state 2/3 (Fig. 4B). In its nonreduced state, EnvΔ712 DS on the surface of cells was insensitive to inhibition of cell-cell fusion by all of the antibodies tested. When the antibodies were present throughout the experiment, the 19b antibody against the gp120 V3 region partially inhibited cell-cell fusion mediated by EnvΔ712 DS. This observation indicates that the DS mutant differs from the unmodified EnvΔ712, which was not inhibited by the 19b antibody. Consistent with this, syncytium formation mediated by EnvΔ712 DS was less sensitive than that of unmodified EnvΔ712 to BMS-806, a state 1-preferring ligand. Together with the antigenicity data, these results suggest that the DS mutants are in a conformation that differs from state 1.
In summary, our results warrant caution in using the DS and SOS disulfide bonds to stabilize pretriggered (state 1) conformations on membrane HIV-1 Env trimers.
MATERIALS AND METHODS
Env glycoprotein constructs.
The codon-optimized genes encoding the HIV-1JR-FL Env and the HIV-1JR-FL EnvΔ712 glycoproteins were subcloned into the pcDNA3.1(−) expression vector (Invitrogen) using the 5′ XbaI and 3′ AflII sites. The HIV-1AD8 Env was coexpressed with the Rev protein in the pSVIIIenv expression vector, using the natural HIV-1 env and rev sequences (13). The mutations specifying the A501C/T605C changes (SOS construct) and the I201C/A433C changes (DS construct), as well as the individual A501C, T605C, I201C, and A433C changes, were introduced by site-directed mutagenesis PCR using Pfu Ultra II polymerase (Agilent Technologies), according to the manufacturer’s protocol.
Cell lines.
293T cells (ATCC) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 μg/ml of penicillin-streptomycin. Cf2Th-CD4/CCR5 cells stably expressing the human CD4 and CCR5 coreceptors for HIV-1 were grown in the same medium supplemented with 0.4 mg/ml of G418 and 0.2 mg/ml of hygromycin. HOS cells (ATCC) were grown in DMEM with 5% FBS and 100 μg/ml of penicillin-streptomycin. All cell culture reagents are from Life Technologies.
Virus infectivity.
Single-round virus infection assays were used to measure the ability of the Env variants to support virus entry, as described previously (37). Briefly, 293T cells were cotransfected with an Env-encoding plasmid, a Rev-encoding plasmid (for the HIV-1JR-FL Env-expressing plasmids), or a Tat-encoding plasmid (for the HIV-1AD8 Env-expressing plasmids); the pCMV HIV-1 Gag-Pol packaging construct; and a plasmid containing the luciferase-expressing HIV-1 vector using a standard calcium phosphate transfection protocol. At 48 h posttransfection, virus-containing supernatants were collected, filtered through a 0.45-μm membrane, and incubated with Cf2Th-CD4/CCR5 cells in the presence of 0 to 2 mM DTT. Luciferase activity in the Cf2Th-CD4/CCR5 target cells was measured 48 h later.
Immunoprecipitation of Envs in cell lysates and on cell surfaces.
Virus-producing 293T cells were prepared as described above and used for immunoprecipitation of Env from the cell surface as described previously (101). In brief, at 48 h posttransfection, 293T cells were washed, scraped, resuspended in PBS, and incubated with 10 μg/ml 2G12 antibody for 1 h at room temperature. The cells were then lysed, and clarified lysates were incubated with protein A-agarose beads for another hour at RT. Beads were washed three times, boiled, and analyzed by Western blotting using a 1:2,000 dilution of goat anti-gp120 polyclonal antibody (Thermo Fisher) and a 1:3,000 dilution of horseradish peroxidase (HRP)-conjugated rabbit anti-goat IgG (Thermo Fisher). The transmembrane Env was analyzed by Western blotting with the 4E10 antibody (133) and HRP-conjugated goat anti-human IgG (Santa Cruz).
For analysis of total Env expression in the cell, some of the clarified lysates were saved before the addition of protein A-agarose beads and Western blotted as described above. As a control for sample loading, the cell lysates were Western blotted with a 1:10,000 dilution of mouse anti-β-actin (Abcam) and a 1:10,000 dilution of HRP-conjugated goat anti-mouse IgG (Thermo Scientific).
Characterization of virus-associated Envs.
Virus-producing 293T cells were prepared as described above. Forty-eight hours later, cell supernatants were collected and filtered through a 0.45-μm membrane. Approximately 2.5 ml of the supernatant was layered on a 2.5-ml 20% sucrose cushion (134) and spun in an SW55Ti rotor at 26,000 rpm for a total of 37 min at 4°C. Supernatants were aspirated, and pellets were resuspended in a PBS-LDS-DTT mixture. Samples were boiled and Western blotted with goat anti-gp120 serum, the 4E10 anti-gp41 antibody, or a polyclonal anti-p55/p24/p17 antiserum (Abcam).
α-Complementation assay measuring cell-cell fusion.
An α-complementation assay measuring the fusion of HIV-1 Env-expressing cells with cells bearing the CD4 and CCR5 HIV-1 coreceptors was conducted as previously described (39, 117). Briefly, 293T cells were cotransfected with two plasmids expressing Env and α-gal using the Effectene reagent (Qiagen). Cf2Th-CD4/CCR5 cells were transfected with a plasmid expressing ω-gal using Effectene. At 48 h posttransfection, the 293T cells were washed, scraped, resuspended in PBS–5% FBS, and added to Cf2Th-CD4/CCR5 cells in the presence of 0 to 4 mM DTT. Cells were incubated at 37°C with 5% CO2 for 3 h before cells were lysed and galactosidase activity was measured.
To evaluate the ability of antibodies to inhibit Env-mediated cell-cell fusion in the α-complementation assay, cells were prepared as described above. However, the resuspended 293T cells were preincubated with 50 μg/ml antibody for 1 h at RT before the cells were added to Cf2Th-CD4/CCR5 cells in the presence of 1 mM DTT. In this assay format, the antibody was continuously present throughout the cocultivation of the cells. For the antibody washout assay, the Env-expressing 293T cells were washed once with PBS to remove unbound antibodies before addition to the Cf2Th-CD4/CCR5 target cells in the presence of 1 mM DTT.
The ability of BMS-806 to inhibit Env-mediated cell-cell fusion was evaluated using the α-complementation assay described above. In this case, the resuspended 293T cells were preincubated with 0 to 2.5 μM BMS-806 for 30 min at 37°C before the cells were added to Cf2Th-CD4/CCR5 cells in the presence of 1 mM DTT.
Cell-based ELISA.
The binding of antibodies to the HIV-1JR-FL Env, Env SOS, Env DS, EnvΔ712, EnvΔ712 SOS, and EnvΔ712 DS glycoproteins expressed on the surface of transfected HOS cells was measured by a cell-based ELISA, as described previously (37, 89). Briefly, HOS cells were seeded in T-75 flasks (3 × 106 cells per flask) and transfected the next day with 22.5 μg of Env-expressing plasmids using the standard polyethylenimine (PEI; Polysciences Inc., Warrington, PA, USA) transfection method. Twenty-four hours after transfection, cells were plated in 384-well plates (2 × 104 cells per well). One day later, cells were washed twice with blocking buffer (10 mg/ml nonfat dry milk, 1.8 mM CaCl2, 1 mM MgCl2, 25 mM Tris [pH 7.5], and 140 mM NaCl) and then incubated in the presence or absence of 1 mM DTT for 1 h at room temperature with anti-HIV-1 Env monoclonal antibodies. To assess sCD4 binding, cells were preincubated for 40 min at room temperature with sCD4 (80 nM) before detection with mouse anti-CD4 OKT4 antibody. All ligands were diluted in blocking buffer. A horseradish peroxidase-conjugated antibody specific for the Fc region of human or mouse IgG (Pierce) was then incubated with the cells for 45 min at room temperature. Under all conditions, cells were washed 5 times with blocking buffer and 5 times with washing buffer. HRP enzyme activity was determined after the addition of 20 μl per well of a 1:1 mix of Western Lightning oxidizing and luminol reagents (PerkinElmer Life Sciences). Light emission was measured with an LB 941 TriStar luminometer (Berthold Technologies). Antibody binding was normalized to that observed for the 2G12 antibody to control for any differences in the cell surface expression of the EnvΔ712 variants.
ACKNOWLEDGMENTS
We thank Elizabeth Carpelan for manuscript preparation. The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: anti-HIV-1 gp41 monoclonal antibody (4E10) from Hermann Katinger. Antibodies against HIV-1 Env were kindly supplied by Dennis Burton (Scripps), Peter Kwong and John Mascola (Vaccine Research Center, NIH), and Barton Haynes (Duke).
A.F. is the recipient of a Canada Research Chair on Retroviral Entry. N.A. is the recipient of a King Abdullah scholarship for higher education from the Saudi Government. This work was supported by grants from the National Institutes of Health (AI100645, AI124982, and GM56550) and by a gift from the late William F. McCarty-Cooper. Work performed by N.A. and A.F. was supported by a CIHR foundation grant to A.F.
REFERENCES
- 1.Wyatt R, Sodroski J. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 2.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 3.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 4.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 5.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Broder CC, Kennedy PE, Berger EA. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 7.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 8.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135–1148. doi: 10.1016/S0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 9.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. 1996. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149–1158. doi: 10.1016/S0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 10.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso AA, Desjardin E, Newman W, Gerard C, Sodroski J. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 12.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 13.Haim H, Strack B, Kassa A, Madani N, Wang L, Courter JR, Princiotto A, McGee K, Pacheco B, Seaman MS, Smith AB III, Sodroski J. 2011. Contribution of intrinsic reactivity of the HIV-1 envelope glycoproteins to CD4-independent infection and global inhibitor sensitivity. PLoS Pathog 7:e1002101. doi: 10.1371/journal.ppat.1002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munro JB, Gorman J, Ma X, Zhou Z, Arthos J, Burton DR, Koff WC, Courter JR, Smith AB III, Kwong PD, Blanchard SC, Mothes W. 2014. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 346:759–763. doi: 10.1126/science.1254426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Lu M, Gorman J, Terry DS, Hong X, Zhou Z, Zhao H, Altman RB, Arthos J, Blanchard SC, Kwong PD, Munro JB, Mothes W. 2018. HIV-1 Env trimer opens through an asymmetric intermediate in which individual protomers adopt distinct conformations. Elife 7:e34271. doi: 10.7554/eLife.34271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khasnis MD, Halkidis K, Bhardwaj A, Root MJ. 2016. Receptor activation of HIV-1 Env leads to asymmetric exposure of the gp41 trimer. PLoS Pathog 12:e1006098. doi: 10.1371/journal.ppat.1006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. 2008. Molecular architecture of native HIV-1 gp120 trimers. Nature 455:109–113. doi: 10.1038/nature07159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Cohen AA, Galimidi RP, Gristick HB, Jensen GJ, Bjorkman PJ. 2016. Cryo-EM structure of a CD4-bound open HIV-1 envelope trimer reveals structural rearrangements of the gp120 V1V2 loop. Proc Natl Acad Sci U S A 113:E7151–E7158. doi: 10.1073/pnas.1615939113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozorowski G, Pallesen J, de Val N, Lyumkis D, Cottrell CA, Torres JL, Copps J, Stanfield RL, Cupo A, Pugach P, Moore JP, Wilson IA, Ward AB. 2017. Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Nature 547:360–363. doi: 10.1038/nature23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuta RA, Wild CT, Weng Y, Weiss CD. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol 5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 21.Koshiba T, Chan DC. 2003. The prefusogenic intermediate of HIV-1 gp41 contains exposed C-peptide regions. J Biol Chem 278:7573–7579. doi: 10.1074/jbc.M211154200. [DOI] [PubMed] [Google Scholar]
- 22.He Y, Vassell R, Zaitseva M, Nguyen N, Yang Z, Weng Y, Weiss CD. 2003. Peptides trap the human immunodeficiency virus type 1 envelope glycoprotein fusion intermediate at two sites. J Virol 77:1666–1671. doi: 10.1128/JVI.77.3.1666-1671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Si Z, Madani N, Cox JM, Chruma JJ, Klein JC, Schon A, Phan N, Wang L, Biorn AC, Cocklin S, Chaiken I, Freire E, Smith AB III, Sodroski JG. 2004. Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc Natl Acad Sci U S A 101:5036–5041. doi: 10.1073/pnas.0307953101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan DC, Fass D, Berger JM, Kim PS. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263–273. doi: 10.1016/S0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 25.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 26.Lu M, Blacklow SC, Kim PS. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol 2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 27.Melikyan GB, Markosyan RM, Hemmati H, Delmedico MK, Lambert DM, Cohen FS. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol 151:413–423. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilen CB, Tilton JC, Doms RW. 2012. Molecular mechanisms of HIV entry. Adv Exp Med Biol 726:223–242. doi: 10.1007/978-1-4614-0980-9_10. [DOI] [PubMed] [Google Scholar]
- 29.Herschhorn A, Ma X, Gu C, Ventura JD, Castillo-Menendez L, Melillo B, Terry DS, Smith AB III, Blanchard SC, Munro JB, Mothes W, Finzi A, Sodroski J. 2016. Release of gp120 restraints leads to an entry-competent intermediate state of the HIV-1 envelope glycoproteins. mBio 7:e10598-16. doi: 10.1128/mBio.01598-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herschhorn A, Gu C, Moraca F, Ma X, Farrell M, Smith AB III, Pancera M, Kwong PD, Schön A, Freire E, Abrams C, Blanchard SC, Mothes W, Sodroski JG. 2017. The beta20-beta21 of gp120 is a regulatory switch for HIV-1 Env conformational transitions. Nat Commun 8:1049. doi: 10.1038/s41467-017-01119-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zolla-Pazner S, Cohen SS, Boyd D, Kong XP, Seaman M, Nussenzweig M, Klein F, Overbaugh J, Totrov M. 2016. Structure/function studies involving the V3 region of the HIV-1 envelope delineate multiple factors that affect neutralization sensitivity. J Virol 90:636–649. doi: 10.1128/JVI.01645-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell RLR, Totrov M, Itri V, Liu X, Fox A, Zolla-Pazner S. 2017. Plasticity and epitope exposure of the HIV-1 envelope trimer. J Virol 91:e00410-17. doi: 10.1128/JVI.00410-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller PW, Morrison O, Vassell R, Weiss CD. 2018. HIV-1 gp41 residues modulate CD4-induced conformational changes in the envelope glycoprotein and evolution of a relaxed conformation of gp120. J Virol 92:e00583-18. doi: 10.1128/JVI.00583-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang CC, Venturi M, Petropoulos CJ, Wrin T, Dimitrov DS, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton DR. 2003. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol 77:10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sattentau QJ, Moore JP. 1991. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med 174:407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fouts TR, Binley JM, Trkola A, Robinson JE, Moore JP. 1997. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol 71:2779–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haim H, Salas I, McGee K, Eichelberger N, Winter E, Pacheco B, Sodroski J. 2013. Modeling virus- and antibody-specific factors to predict human immunodeficiency virus neutralization efficiency. Cell Host Microbe 14:547–558. doi: 10.1016/j.chom.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guttman M, Cupo A, Julien JP, Sanders RW, Wilson IA, Moore JP, Lee KK. 2015. Antibody potency relates to the ability to recognize the closed, pre-fusion form of HIV Env. Nat Commun 6:6144. doi: 10.1038/ncomms7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen HT, Madani N, Ding H, Elder E, Princiotto A, Gu C, Darby P, Alin J, Herschhorn A, Kappes JC, Mao Y, Sodroski JG. 2017. Evaluation of the contribution of the transmembrane region to the ectodomain conformation of the human immunodeficiency virus (HIV-1) envelope glycoprotein. Virol J 14:33. doi: 10.1186/s12985-017-0704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witt KC, Castillo-Menendez L, Ding H, Espy N, Zhang S, Kappes JC, Sodroski J. 2017. Antigenic characterization of the human immunodeficiency virus (HIV-1) envelope glycoprotein precursor incorporated into nanodiscs. PLoS One 12:e0170672. doi: 10.1371/journal.pone.0170672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson J, Zhai Y, Salimi H, Espy N, Eichelberger N, DeLeon O, O’Malley Y, Courter J, Smith AB III, Madani N, Sodroski J, Haim H. 2017. Induction of a tier-1-like phenotype in diverse tier-2 isolates by agents that guide HIV-1 Env to perturbation-sensitive, nonnative states. J Virol 91:00174-17. doi: 10.1128/JVI.00174-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castillo-Menendez LR, Witt K, Espy N, Princiotto A, Madani N, Pacheco B, Finzi A, Sodroski J. 2018. Comparison of uncleaved and mature human immunodeficiency virus membrane envelope glycoprotein trimers. J Virol 92:e00277-18. doi: 10.1128/JVI.00277-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Earl PL, Moss B. 1993. Mutational analysis of the assembly domain of the HIV-1 envelope glycoprotein. AIDS Res Hum Retroviruses 9:589–594. doi: 10.1089/aid.1993.9.589. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Lee J, Mahony EM, Kwong PD, Wyatt R, Sodroski J. 2002. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J Virol 76:4634–4642. doi: 10.1128/JVI.76.9.4634-4642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovacs JM, Noeldeke E, Ha HJ, Peng H, Rits-Volloch S, Harrison SC, Chen B. 2014. Stable, uncleaved HIV-1 envelope glycoprotein gp140 forms a tightly folded trimer with a native-like structure. Proc Natl Acad Sci U S A 111:18542–18547. doi: 10.1073/pnas.1422269112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du SX, Idiart RJ, Mariano EB, Chen H, Jiang P, Xu L, Ostrow KM, Wrin T, Phung P, Binley JM, Petropoulos CJ, Ballantyne JA, Whalen RG. 2009. Effect of trimerization motifs on quaternary structure, antigenicity, and immunogenicity of a non-cleavable HIV-1 gp140 envelope glycoprotein. Virology 395:33–44. doi: 10.1016/j.virol.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanders RW, Schiffner L, Master A, Kajumo F, Guo Y, Dragic T, Moore JP, Binley JM. 2000. Variable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits. J Virol 74:5091–5100. doi: 10.1128/JVI.74.11.5091-5100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Binley JM, Sanders RW, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma DJ, Maddon PJ, Olson WC, Moore JP. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol 74:627–643. doi: 10.1128/JVI.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders RW, Vesanen M, Schuelke N, Master A, Schiffner L, Kalyanaraman R, Paluch M, Berkhout B, Maddon PJ, Olson WC, Lu M, Moore JP. 2002. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol 76:8875–8889. doi: 10.1128/JVI.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schülke N, Vesanen MS, Sanders RW, Zhu P, Lu M, Anselma DJ, Villa AR, Parren PWHI, Binley JM, Roux KH, Maddon PJ, Moore JP, Olson WC. 2002. Oligomeric and conformational properties of a proteolytically mature, disulfide-stabilized human immunodeficiency virus type 1 gp140 envelope glycoprotein. J Virol 76:7760–7776. doi: 10.1128/JVI.76.15.7760-7776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klasse PJ, Depetris RS, Pejchal R, Julien J-P, Khayat R, Lee JH, Marozsan AJ, Cupo A, Cocco N, Korzun J, Yasmeen A, Ward AB, Wilson IA, Sanders RW, Moore JP. 2013. Influences on trimerization and aggregation of soluble, cleaved HIV-1 SOSIP envelope glycoprotein. J Virol 87:9873–9885. doi: 10.1128/JVI.01226-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khayat R, Lee JH, Julien JP, Cupo A, Klasse PJ, Sanders RW, Moore JP, Wilson IA, Ward AB. 2013. Structural characterization of cleaved, soluble HIV-1 envelope glycoprotein trimers. J Virol 87:9865–9872. doi: 10.1128/JVI.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanders RW, Derking R, Cupo A, Julien J-P, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Peña AT, Korzun J, Golabek M, de los Reyes K, Ketas TJ, van Gils MJ, King CR, Wilson IA, Ward AB, Klasse PJ, Moore JP. 2013. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog 9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guenaga J, de Val N, Tran K, Feng Y, Satchwell K, Ward AB, Wyatt RT. 2015. Well-ordered trimeric HIV-1 subtype B and C soluble spike mimetics generated by negative selection display native-like properties. PLoS Pathog 11:e1004570. doi: 10.1371/journal.ppat.1004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guenaga J, Dubrovskaya V, Val N, Sharma SK, Carrette B, Ward AB, Wyatt RT. 2015. Structure-guided redesign increases the propensity of HIV Env to generate highly stable soluble trimers. J Virol 90:2806–2817. doi: 10.1128/JVI.02652-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Georgiev IS, Joyce MG, Yang Y, Sastry M, Zhang B, Baxa U, Chen RE, Druz A, Lees CR, Narpala S, Schön A, Van Galen J, Chuang GY, Gorman J, Harned A, Pancera M, Stewart-Jones GB, Cheng C, Freire E, McDermott AB, Mascola JR, Kwong PD. 2015. Single-chain soluble BG505.SOSIP gp140 trimers as structural and antigenic mimics of mature closed HIV-1 Env. J Virol 89:5318–5329. doi: 10.1128/JVI.03451-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. 2013. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. 2013. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, Stewart-Jones GB, Stuckey J, Bailer RT, Joyce MG, Louder MK, Tumba N, Yang Y, Zhang B, Cohen MS, Haynes BF, Mascola JR, Morris L, Munro JB, Blanchard SC, Mothes W, Connors M, Kwong PD. 2014. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514:455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon YD, Pancera M, Acharya P, Georgiev IS, Crooks ET, Gorman J, Joyce MG, Guttman M, Ma X, Narpala S, Soto C, Terry DS, Yang Y, Zhou T, Ahlsen G, Bailer RT, Chambers M, Chuang GY, Doria-Rose NA, Druz A, Hallen MA, Harned A, Kirys T, Louder MK, O’Dell S, Ofek G, Osawa K, Prabhakaran M, Sastry M, Stewart-Jones GB, Stuckey J, Thomas PV, Tittley T, Williams C, Zhang B, Zhao H, Zhou Z, Donald BR, Lee LK, Zolla-Pazner S, Baxa U, Schon A, Freire E, Shapiro L, Lee KK, Arthos J, Munro JB, Blanchard SC, Mothes W, Binley JM, McDermott AB, Mascola JR, Kwong PD. 2015. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat Struct Mol Biol 22:522–531. doi: 10.1038/nsmb.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramirez M, Back JW, Koff WC, Julien JP, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP. 2015. HIV-1 vaccines. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Taeye SW, Ozorowski G, Torrents de la Pena A, Guttman M, Julien JP, van den Kerkhof TL, Burger JA, Pritchard LK, Pugach P, Yasmeen A, Crampton J, Hu J, Bontjer I, Torres JL, Arendt H, DeStefano J, Koff WC, Schuitemaker H, Eggink D, Berkhout B, Dean H, LaBranche C, Crotty S, Crispin M, Montefiori DC, Klasse PJ, Lee KK, Moore JP, Wilson IA, Ward AB, Sanders RW. 2015. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell 163:1702–1715. doi: 10.1016/j.cell.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng C, Pancera M, Bossert A, Schmidt SD, Chen RE, Chen X, Druz A, Narpala S, Doria-Rose NA, McDermott AB, Kwong PD, Mascola JR. 2015. Immunogenicity of a prefusion HIV-1 envelope trimer in complex with a quaternary-structure-specific antibody. J Virol 90:2740–2755. doi: 10.1128/JVI.02380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chuang GY, Geng H, Pancera M, Xu K, Cheng C, Acharya P, Chambers M, Druz A, Tsybovsky Y, Wanninger TG, Yang Y, Doria-Rose NA, Georgiev IS, Gorman J, Joyce MG, O’Dell S, Zhou T, McDermott AB, Mascola JR, Kwong PD. 2017. Structure-based design of a soluble prefusion-closed HIV-1 Env trimer with reduced CD4 affinity and improved immunogenicity. J Virol 91:e02268-16. doi: 10.1128/JVI.02268-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torrents de la Pena A, Julien JP, de Taeye SW, Garces F, Guttman M, Ozorowski G, Pritchard LK, Behrens AJ, Go EP, Burger JA, Schermer EE, Sliepen K, Ketas TJ, Pugach P, Yasmeen A, Cottrell CA, Torres JL, Vavourakis CD, van Gils MJ, LaBranche C, Montefiori DC, Desaire H, Crispin M, Klasse PJ, Lee KK, Moore JP, Ward AB, Wilson IA, Sanders RW. 2017. Improving the immunogenicity of native-like HIV-1 envelope trimers by hyperstabilization. Cell Rep 20:1805–1817. doi: 10.1016/j.celrep.2017.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torrents de la Pena A, de Taeye SW, Sliepen K, LaBranche CC, Burger JA, Schermer EE, Montefiori DC, Moore JP, Klasse PJ, Sanders RW. 2018. Immunogenicity in rabbits of HIV-1 SOSIP trimers from clades A, B, and C, given individually, sequentially, or in combination. J Virol 92:e01957-17. doi: 10.1128/JVI.01957-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ringe RP, Ozorowski G, Yasmeen A, Cupo A, Cruz Portillo VM, Pugach P, Golabek M, Rantalainen K, Holden LG, Cottrell CA, Wilson IA, Sanders RW, Ward AB, Klasse PJ, Moore JP. 2017. Improving the expression and purification of soluble, recombinant native-like HIV-1 envelope glycoprotein trimers by targeted sequence changes. J Virol 91:e00264-17. doi: 10.1128/JVI.00264-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kulp DW, Steichen JM, Pauthner M, Hu X, Schiffner T, Liguori A, Cottrell CA, Havenar-Daughton C, Ozorowski G, Georgeson E, Kalyuzhniy O, Willis JR, Kubitz M, Adachi Y, Reiss SM, Shin M, de Val N, Ward AB, Crotty S, Burton DR, Schief WR. 2017. Structure-based design of native-like HIV-1 envelope trimers to silence non-neutralizing epitopes and eliminate CD4 binding. Nat Commun 8:1655. doi: 10.1038/s41467-017-01549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aldon Y, McKay PF, Allen J, Ozorowski G, Felfodine Levai R, Tolazzi M, Rogers P, He L, de Val N, Fabian K, Scarlatti G, Zhu J, Ward AB, Crispin M, Shattock RJ. 2018. Rational design of DNA-expressed stabilized native-like HIV-1 envelope trimers. Cell Rep 24:3324.e5–3338.e5. doi: 10.1016/j.celrep.2018.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang P, Gorman J, Geng H, Liu Q, Lin Y, Tsybovsky Y, Go EP, Dey B, Andine T, Kwon A, Patel M, Gururani D, Uddin F, Guzzo C, Cimbro R, Miao H, McKee K, Chuang GY, Martin L, Sironi F, Malnati MS, Desaire H, Berger EA, Mascola JR, Dolan MA, Kwong PD, Lusso P. 2018. Interdomain stabilization impairs CD4 binding and improves immunogenicity of the HIV-1 envelope trimer. Cell Host Microbe 23:832.e6–844.e6. doi: 10.1016/j.chom.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bale S, Martine A, Wilson R, Behrens AJ, Le Fourn V, de Val N, Sharma SK, Tran K, Torres JL, Girod PA, Ward AB, Crispin M, Wyatt RT. 2018. Cleavage-independent HIV-1 trimers from CHO cell lines elicit robust autologous tier 2 neutralizing antibodies. Front Immunol 9:1116. doi: 10.3389/fimmu.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blish CA, Nguyen MA, Overbaugh J. 2008. Enhancing exposure of HIV-1 neutralization epitopes through mutations in gp41. PLoS Med 5:e9. doi: 10.1371/journal.pmed.0050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lovelace E, Xu H, Blish CA, Strong R, Overbaugh J. 2011. The role of amino acid changes in the human immunodeficiency virus type 1 transmembrane domain in antibody binding and neutralization. Virology 421:235–244. doi: 10.1016/j.virol.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ringe R, Bhattacharya J. 2012. Association of enhanced HIV-1 neutralization by a single Y681H substitution in gp41 with increased gp120-CD4 interaction and macrophage infectivity. PLoS One 7:e37157. doi: 10.1371/journal.pone.0037157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bradley T, Trama A, Tumba N, Gray E, Lu X, Madani N, Jahanbakhsh F, Eaton A, Xia S-M, Parks R, Lloyd KE, Sutherland LL, Scearce RM, Bowman CM, Barnett S, Abdool-Karim SS, Boyd SD, Melillo B, Smith AB III, Sodroski J, Kepler TB, Alam SM, Gao F, Bonsignori M, Liao H-X, Moody MA, Montefiori D, Santra S, Morris L, Haynes BF. 2016. Amino acid changes in the HIV-1 gp41 membrane proximal region control virus neutralization sensitivity. EBioMedicine 12:196–207. doi: 10.1016/j.ebiom.2016.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Rourke SM, Schweighardt B, Phung P, Mesa KA, Vollrath AL, Tatsuno GP, To B, Sinangil F, Limoli K, Wrin T, Berman PW. 2012. Sequences in glycoprotein gp41, the CD4 binding site, and the V2 domain regulate sensitivity and resistance of HIV-1 to broadly neutralizing antibodies. J Virol 86:12105–12114. doi: 10.1128/JVI.01352-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Rourke SM, Schweighardt B, Scott WG, Wrin T, Fonseca DP, Sinangil F, Berman PW. 2009. Novel ring structure in the gp41 trimer of human immunodeficiency virus type 1 that modulates sensitivity and resistance to broadly neutralizing antibodies. J Virol 83:7728–7738. doi: 10.1128/JVI.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haim H, Salas I, Sodroski J. 2013. Proteolytic processing of the human immunodeficiency virus envelope glycoprotein precursor decreases conformational flexibility. J Virol 87:1884–1889. doi: 10.1128/JVI.02765-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herrera C, Klasse PJ, Michael E, Kake S, Barnes K, Kibler CW, Campbell-Gardener L, Si Z, Sodroski J, Moore JP, Beddows S. 2005. The impact of envelope glycoprotein cleavage on the antigenicity, infectivity, and neutralization sensitivity of Env-pseudotyped human immunodeficiency virus type 1 particles. Virology 338:154–172. doi: 10.1016/j.virol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Pancera M, Wyatt R. 2005. Selective recognition of oligomeric HIV-1 primary isolate envelope glycoproteins by potently neutralizing ligands requires efficient precursor cleavage. Virology 332:145–156. doi: 10.1016/j.virol.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 81.Chakrabarti BK, Pancera M, Phogat S, O’Dell S, McKee K, Guenaga J, Robinson J, Mascola J, Wyatt RT. 2011. HIV type 1 Env precursor cleavage state affects recognition by both neutralizing and nonneutralizing gp41 antibodies. AIDS Res Hum Retroviruses 27:877–887. doi: 10.1089/aid.2010.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chakrabarti BK, Walker LM, Guenaga JF, Ghobbeh A, Poignard P, Burton DR, Wyatt RT. 2011. Direct antibody access to the HIV-1 membrane-proximal external region positively correlates with neutralization sensitivity. J Virol 85:8217–8226. doi: 10.1128/JVI.00756-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, O’Dell S, Wilson R, Wu X, Schmidt SD, Hogerkorp CM, Louder MK, Longo NS, Poulsen C, Guenaga J, Chakrabarti BK, Doria-Rose N, Roederer M, Connors M, Mascola JR, Wyatt RT. 2012. HIV-1 neutralizing antibodies display dual recognition of the primary and coreceptor binding sites and preferential binding to fully cleaved envelope glycoproteins. J Virol 86:11231–11241. doi: 10.1128/JVI.01543-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Forsell MN, Dey B, Morner A, Svehla K, O’Dell S, Hogerkorp CM, Voss G, Thorstensson R, Shaw GM, Mascola JR, Karlsson Hedestam GB, Wyatt RT. 2008. B cell recognition of the conserved HIV-1 co-receptor binding site is altered by endogenous primate CD4. PLoS Pathog 4:e1000171. doi: 10.1371/journal.ppat.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forsell MN, McKee K, Feng Y, Mascola JR, Wyatt RT. 2014. HIV-1 envelope glycoprotein trimer immunogenicity elicited in the presence of human CD4 alters the neutralization profile. AIDS Res Hum Retroviruses 30:1089–1098. doi: 10.1089/AID.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Douagi I, Forsell MN, Sundling C, O’Dell S, Feng Y, Dosenovic P, Li Y, Seder R, Lore K, Mascola JR, Wyatt RT, Karlsson Hedestam GB. 2010. Influence of novel CD4 binding-defective HIV-1 envelope glycoprotein immunogens on neutralizing antibody and T-cell responses in nonhuman primates. J Virol 84:1683–1695. doi: 10.1128/JVI.01896-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abrahamyan LG, Markosyan RM, Moore JP, Cohen FS, Melikyan GB. 2003. Human immunodeficiency virus type 1 Env with an intersubunit disulfide bond engages coreceptors but requires bond reduction after engagement to induce fusion. J Virol 77:5829–5836. doi: 10.1128/JVI.77.10.5829-5836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Binley JM, Cayanan CS, Wiley C, Schulke N, Olson WC, Burton DR. 2003. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J Virol 77:5678–5684. doi: 10.1128/JVI.77.10.5678-5684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alsahafi N, Debbeche O, Sodroski J, Finzi A. 2015. Effects of the I559P gp41 change on the conformation and function of the human immunodeficiency virus (HIV-1) membrane envelope glycoprotein trimer. PLoS One 10:e0122111. doi: 10.1371/journal.pone.0122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Owens RJ, Compans RW. 1990. The human immunodeficiency virus type 1 envelope glycoprotein precursor acquires aberrant intermolecular disulfide bonds that may prevent normal proteolytic processing. Virology 179:827–833. doi: 10.1016/0042-6822(90)90151-G. [DOI] [PubMed] [Google Scholar]
- 91.Ohno H, Aguilar RC, Fournier MC, Hennecke S, Cosson P, Bonifacino JS. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238:305–315. doi: 10.1006/viro.1997.8839. [DOI] [PubMed] [Google Scholar]
- 92.Egan MA, Carruth LM, Rowell JF, Yu X, Siliciano RF. 1996. Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the Pr55gag precursor protein. J Virol 70:6547–6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bültmann A, Muranyi W, Seed B, Haas J. 2001. Identification of two sequences in the cytoplasmic tail of the human immunodeficiency virus type 1 envelope glycoprotein that inhibit cell surface expression. J Virol 75:5263–5276. doi: 10.1128/JVI.75.11.5263-5276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murakami T, Freed EO. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc Natl Acad Sci U S A 97:343–348. doi: 10.1073/pnas.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye L, Bu Z, Vzorov A, Taylor D, Compans RW, Yang C. 2004. Surface stability and immunogenicity of the human immunodeficiency virus envelope glycoprotein: role of the cytoplasmic domain. J Virol 78:13409–13419. doi: 10.1128/JVI.78.24.13409-13419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wyss S, Dimitrov AS, Baribaud F, Edwards TG, Blumenthal R, Hoxie JA. 2005. Regulation of human immunodeficiency virus type 1 envelope glycoprotein fusion by a membrane-interactive domain in the gp41 cytoplasmic tail. J Virol 79:12231–12241. doi: 10.1128/JVI.79.19.12231-12241.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Byland R, Vance PJ, Hoxie JA, Marsh M. 2007. A conserved dileucine motif mediates clathrin and AP-2-dependent endocytosis of the HIV-1 envelope protein. Mol Biol Cell 18:414–425. doi: 10.1091/mbc.e06-06-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Postler TS, Desrosiers RC. 2013. The tale of the long tail: the cytoplasmic domain of HIV-1 gp41. J Virol 87:2–15. doi: 10.1128/JVI.02053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hogan MJ, Conde-Motter A, Jordan APO, Yang L, Cleveland B, Guo W, Romano J, Ni H, Pardi N, LaBranche CC, Montefiori DC, Hu SL, Hoxie JA, Weissman D. 2018. Increased surface expression of HIV-1 envelope is associated with improved antibody response in vaccinia prime/protein boost immunization. Virology 514:106–117. doi: 10.1016/j.virol.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirschman J, Qi M, Ding L, Hammonds J, Dienger-Stambaugh K, Wang JJ, Lapierre LA, Goldenring JR, Spearman P. 2018. HIV-1 envelope glycoprotein trafficking through the endosomal recycling compartment is required for particle incorporation. J Virol 92:e01893-17. doi: 10.1128/JVI.01893-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Castillo-Menendez LR, Nguyen HT, Sodroski J. 2019. Conformational differences between functional human immunodeficiency virus envelope glycoprotein trimers and stabilized soluble trimers. J Virol 93:e01709-18. doi: 10.1128/JVI.01709-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou T, Georgiev I, Wu X, Yang Z-Y, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M Jr, Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang J, Kang BH, Ishida E, Zhou T, Griesman T, Sheng Z, Wu F, Doria-Rose NA, Zhang B, McKee K, O’Dell S, Chuang G-Y, Druz A, Georgiev IS, Schramm CA, Zheng A, Joyce MG, Asokan M, Ransier A, Darko S, Migueles SA, Bailer RT, Louder MK, Alam SM, Parks R, Kelsoe G, Von Holle T, Haynes BF, Douek DC, Hirsch V, Seaman MS, Shapiro L, Mascola JR, Kwong PD, Connors M. 2016. Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth. Immunity 45:1108–1121. doi: 10.1016/j.immuni.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kong R, Xu K, Zhou T, Acharya P, Lemmin T, Liu K, Ozorowski G, Soto C, Taft JD, Bailer RT, Cale EM, Chen L, Choi CW, Chuang G-Y, Doria-Rose NA, Druz A, Georgiev IS, Gorman J, Huang J, Joyce MG, Louder MK, Ma X, McKee K, O’Dell S, Pancera M, Yang Y, Blanchard SC, Mothes W, Burton DR, Koff WC, Connors M, Ward AB, Kwong PD, Mascola JR. 2016. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science 352:828–833. doi: 10.1126/science.aae0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blattner C, Lee JH, Sliepen K, Derking R, Falkowska E, de la Pena AT, Cupo A, Julien JP, van Gils M, Lee PS, Peng W, Paulson JC, Poignard P, Burton DR, Moore JP, Sanders RW, Wilson IA, Ward AB. 2014. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity 40:669–680. doi: 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC, Bjorkman PJ. 2012. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A 109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Posner MR, Hideshima T, Cannon T, Mukherjee M, Mayer KH, Byrn RA. 1991. An IgG human monoclonal antibody that reacts with HIV-1/GP120, inhibits virus binding to cells, and neutralizes infection. J Immunol 146:4325–4332. [PubMed] [Google Scholar]
- 111.Moore JP, Trkola A, Korber B, Boots LJ, Kessler JA II, McCutchan FE, Mascola J, Ho DD, Robinson J, Conley AJ. 1995. A human monoclonal antibody to a complex epitope in the V3 region of gp120 of human immunodeficiency virus type 1 has broad reactivity within and outside clade B. J Virol 69:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thali M, Moore JP, Furman C, Charles M, Ho DD, Robinson J, Sodroski J. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol 67:3978–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alsahafi N, Anand SP, Castillo-Menendez L, Verly MM, Medjahed H, Prevost J, Herschhorn A, Richard J, Schon A, Melillo B, Freire E, Smith AB III, Sodroski J, Finzi A. 2018. SOSIP changes affect human immunodeficiency virus type 1 envelope glycoprotein conformation and CD4 engagement. J Virol 92:e01080-18. doi: 10.1128/JVI.01080-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol 70:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Finzi A, Xiang SH, Pacheco B, Wang L, Haight J, Kassa A, Danek B, Pancera M, Kwong PD, Sodroski J. 2010. Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol Cell 37:656–667. doi: 10.1016/j.molcel.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pacheco B, Alsahafi N, Debbeche O, Prevost J, Ding S, Chapleau JP, Herschhorn A, Madani N, Princiotto A, Melillo B, Gu C, Zeng X, Mao Y, Smith AB III, Sodroski J, Finzi A. 2017. Residues in the gp41 ectodomain regulate HIV-1 envelope glycoprotein conformational transitions induced by gp120-directed inhibitors. J Virol 91:e02219-16. doi: 10.1128/JVI.02219-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holland AU, Munk C, Lucero GR, Nguyen LD, Landau NR. 2004. Alpha-complementation assay for HIV envelope glycoprotein-mediated fusion. Virology 319:343–352. doi: 10.1016/j.virol.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 118.Madani N, Princiotto AM, Easterhoff D, Bradley T, Luo K, Williams WB, Liao H-X, Moody MA, Phad GE, Vázquez Bernat N, Melillo B, Santra S, Smith AB III, Karlsson Hedestam GB, Haynes B, Sodroski J. 2016. Antibodies elicited by multiple envelope glycoprotein immunogens in primates neutralize primary human immunodeficiency viruses (HIV-1) sensitized by CD4-mimetic compounds. J Virol 90:5031–5046. doi: 10.1128/JVI.03211-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ho HT, Fan L, Nowicka-Sans B, McAuliffe B, Li CB, Yamanaka G, Zhou N, Fang H, Dicker I, Dalterio R, Gong YF, Wang T, Yin Z, Ueda Y, Matiskella J, Kadow J, Clapham P, Robinson J, Colonno R, Lin PF. 2006. Envelope conformational changes induced by human immunodeficiency virus type 1 attachment inhibitors prevent CD4 binding and downstream entry events. J Virol 80:4017–4025. doi: 10.1128/JVI.80.8.4017-4025.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pancera M, Lai YT, Bylund T, Druz A, Narpala S, O’Dell S, Schon A, Bailer RT, Chuang GY, Geng H, Louder MK, Rawi R, Soumana DI, Finzi A, Herschhorn A, Madani N, Sodroski J, Freire E, Langley DR, Mascola JR, McDermott AB, Kwong PD. 2017. Crystal structures of trimeric HIV envelope with entry inhibitors BMS-378806 and BMS-626529. Nat Chem Biol 13:1115–1122. doi: 10.1038/nchembio.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Z, Zhou N, Sun Y, Ray N, Lataillade M, Hanna GJ, Krystal M. 2013. Activity of the HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068, against CD4-independent viruses and HIV-1 envelopes resistant to other entry inhibitors. Antimicrob Agents Chemother 57:4172–4180. doi: 10.1128/AAC.00513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Langley DR, Kimura SR, Sivaprakasam P, Zhou N, Dicker I, McAuliffe B, Wang T, Kadow JF, Meanwell NA, Krystal M. 2015. Homology models of the HIV-1 attachment inhibitor BMS-626529 bound to gp120 suggest a unique mechanism of action. Proteins 83:331–350. doi: 10.1002/prot.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sattentau QJ, Moore JP. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med 182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu M, Ma X, Castillo-Menendez LR, Gorman J, Alsahafi N, Ermel U, Terry DS, Chambers M, Peng D, Zhang B, Zhou T, Reichard N, Wang K, Grover J, Carman BP, Gardner MR, Nikíc-Spiegel I, Sugawara A, Arthos J, Lemke EA, Smith AB III, Farzan M, Abrams C, Munro JB, McDermott AB, Finzi A, Kwong PD, Blanchard SC, Sodroski JG, Mothes W. 2019. Associating HIV-1 envelope glycoprotein structures with states on virus observed by smFRET. Nature doi: 10.1038/s41586-019-1101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Madani N, Princiotto AM, Zhao C, Jahanbakhshsefidi F, Mertens M, Herschhorn A, Melillo B, Smith AB III, Sodroski J. 2017. Activation and inactivation of primary human immunodeficiency virus envelope glycoprotein trimers by CD4-mimetic compounds. J Virol 91:e01880-16. doi: 10.1128/JVI.01880-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kesavardhana S, Varadarajan R. 2014. Stabilizing the native trimer of HIV-1 Env by destabilizing the heterodimeric interface of the gp41 postfusion six-helix bundle. J Virol 88:9590–9604. doi: 10.1128/JVI.00494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stadtmueller BM, Bridges MD, Dam KM, Lerch MT, Huey-Tubman KE, Hubbell WL, Bjorkman PJ. 2018. DEER spectroscopy measurements reveal multiple conformations of HIV-1 SOSIP envelopes that show similarities with envelopes on native virions. Immunity 49:235.e4–246.e4. doi: 10.1016/j.immuni.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee JH, Ozorowski G, Ward AB. 2016. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 351:1043–1048. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rizzuto CD, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, Sodroski J. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 130.Guenaga J, Garces F, de Val N, Stanfield RL, Dubrovskaya V, Higgins B, Carrette B, Ward AB, Wilson IA, Wyatt RT. 2017. Glycine substitution at helix-to-coil transitions facilitates the structural determination of a stabilized subtype C HIV envelope glycoprotein. Immunity 46:792–803. doi: 10.1016/j.immuni.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Go EP, Herschhorn A, Gu C, Castillo-Menendez L, Zhang S, Mao Y, Chen H, Ding H, Wakefield JK, Hua D, Liao HX, Kappes JC, Sodroski J, Desaire H. 2015. Comparative analysis of the glycosylation profiles of membrane-anchored HIV-1 envelope glycoprotein trimers and soluble gp140. J Virol 89:8245–8257. doi: 10.1128/JVI.00628-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Samal S, Das S, Boliar S, Qureshi H, Shrivastava T, Kumar N, Goswami S, Bansal M, Chakrabarti BK. 2018. Cell surface ectodomain integrity of a subset of functional HIV-1 envelopes is dependent on a conserved hydrophilic domain containing region in their C-terminal tail. Retrovirology 15:50. doi: 10.1186/s12977-018-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, Steindl F, Katinger H. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses 17:1757–1765. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- 134.Kutner RH, Zhang XY, Reiser J. 2009. Production, concentration and titration of pseudotyped HIV-1 based lentiviral vectors. Nat Protoc 4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]