Abstract
Late onset Alzheimer’s disease is the most common form of dementia for which about 30 susceptibility loci have been reported. The aim of the current study is to identify novel genes associated with Alzheimer’s disease using the largest up-to-date reference single nucleotide polymorphism (SNP) panel, the most accurate imputation software and a novel gene-based analysis approach which tests for patterns of association within genes, in the powerful genome-wide association dataset of the International Genomics of Alzheimer’s Project Consortium, comprising over 7 million genotypes from 17,008 Alzheimer’s cases and 37,154 controls. In addition to earlier reported genes, we detected three novel gene-wide significant loci PPARGC1A (p = 2.2 × 10−6), RORA (p = 7.4 × 10−7) and ZNF423 (p = 2.1 × 10−6). PPARGC1A and RORA are involved in circadian rhythm; circadian disturbances are one of the earliest symptoms of Alzheimer’s disease. PPARGC1A is additionally linked to energy metabolism and the generation of amyloid beta plaques. RORA is involved in a variety of functions apart from circadian rhythm, such as cholesterol metabolism and inflammation. The ZNF423 gene resides in an Alzheimer’s disease-specific protein network and is likely involved with centrosomes and DNA damage repair.
Introduction
Late Onset Alzheimer’s disease (LOAD) is a devastating neurodegenerative condition with significant genetic heritability [1]. The apolipoprotein E (APOE) gene is the strongest genetic risk factor for LOAD [2]. Subsequently, more genes were found to be associated with AD development. The Genetic and Environmental Risk in Alzheimer’s Disease (GERAD) Consortium published a Genome-Wide Association Study (GWAS) that identified novel variants in CLU and PICALM which were associated with AD [3]. Concurrently, the European Alzheimer’s Disease Initiative (EADI) identified an association between the CR1 and CLU loci and AD [4]. Subsequent publications by GERAD, the Alzheimer’s Disease Genetic Consortium (ADGC) and Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium identified a further 5 novel loci [5] [6] [7]. The International Genomics of Alzheimer’s Project (IGAP) [4] Consortium is an amalgamation of these four different genetic groups (GERAD, EADI, ADGC and CHARGE). Meta-analysis of the 4 GWAS datasets determined 11 novel variants associated with AD. A gene-based analysis has been undertaken in the IGAP AD data using Brown’s method [8]. This approach determined two additional novel genes; TP53INP1 and IGHV1-67 [9]. Additionally, low frequency risk variants have been identified through next generation sequencing (TREM2) [10] and a whole-exome association study (PLCG2, TREM2 and ABI3 [11]).
Gene-based analysis is an alternative to GWAS analyses, which considers the association of an individual single nucleotide polymorphism (SNP) with disease. Gene-based analyses provide more power due to the aggregate effect of multiple SNPs being larger than that of individual SNPs. For example, determining the association of genes rather than SNPs, is beneficial since genes are more robust across different populations, this is due to the linkage disequilibrium (LD) between SNPs resulting in different SNPs being associated in different populations [12]. Gene-based analyses are being widely used in the field and as expected, are able to identify novel genes or pathways associated with disease. Pathways clustering in eight areas of biology have been found to be associated with AD using the ALIGATOR [13] algorithm [14] [15].
The aim of the current study is to identify novel genes associated with AD using the largest up-to-date reference SNP panel, the most accurate imputation software and a novel gene-based analysis approach. In this study, we used the GERAD data [3] which have been imputed using the latest Haplotype Reference Consortium data (HRC). Polygenic Linkage disequilibrium- Adjusted Risk Score (POLARIS) [16] is a powerful gene-based method which produces a risk score per person per gene, adjusts for LD between SNPs and informs the analysis with summary statistics from an external data set. POLARIS, unlike standard Polygenic Risk Score (PRS) does not require data to be pruned for LD prior to analysis, so it is able to incorporate information from a larger number of SNPs. We employed the POLARIS approach [16] and using the individual genotypes in the GERAD imputed data, produced the risk score for each individual for every gene considered. The IGAP [4] SNP summary statistic data, where the individuals from GERAD were excluded, were used to generate the gene-based PRS.
Results
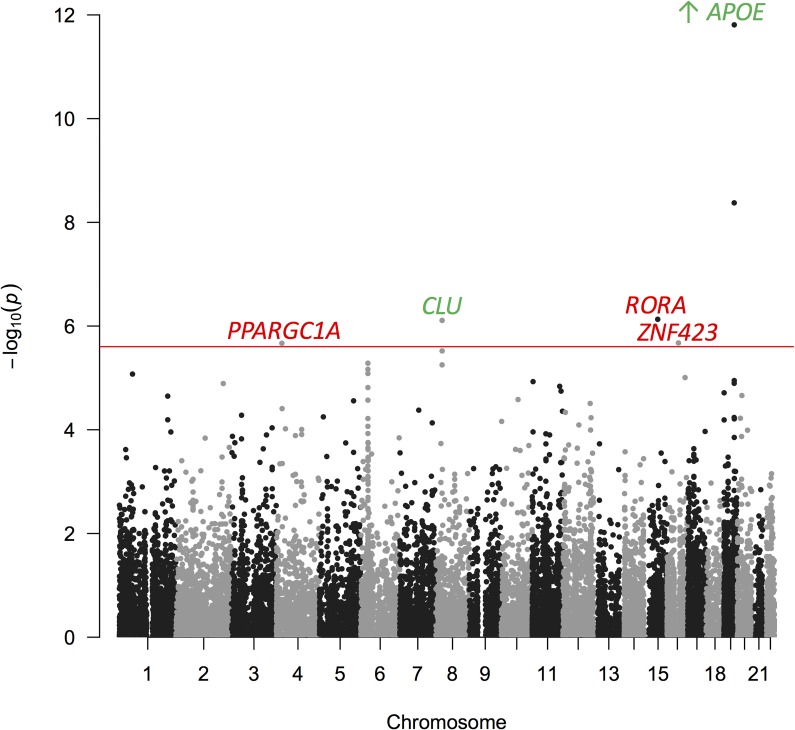
For the imputed GERAD data, using a window around the gene of 35kb upstream and 10kb downstream [17], SNPs are assigned to 18,087 genes which are plotted on a Manhattan plot in Fig 1. The 12 gene-wide significant genes from this analysis are shown in Table 1, where gene-wide significance is defined as p < 2.5 × 10−6 [18]. A large number of genes reside on chromosome 19 and these are likely influenced by the large effect of APOE. Three novel genes have been identified from this analysis: PPARGC1A, RORA and ZNF423. PPARGC1A (peroxisome proliferator-activated receptor gamma co-activator 1alpha) is a master regulator that mainly regulates energy metabolism [19] [20]. It has been linked to the generation of amyloid beta plaques [21] and circadian rhythm [22]. RORA (Retinoic acid receptor-related orphan receptor alpha) is involved in a variety of functions such as circadian rhythm, cholesterol metabolism and inflammation [23]. Its expression is also upregulated in the AD hippocampus [24]. The ZNF423 gene resides in an AD-specific protein network which also includes other AD-related genes such as APOE, CLU, ABCA7, TREM2 etc. [25]. ZNF423 is likely involved with centrosomes and DNA damage repair [26]. The SCARA3 gene overlaps CLU which has previously been identified as being associated with AD [3] [4]. The POLARIS gene-based results for the genes previously identified as being associated with AD are seen in S1 Table, these genes contain genome-wide significant SNPs (p < 5 × 10−8). Table 1 additionally shows the POLARIS gene-based results conditioned on the APOE gene, this is done by including the POLARIS APOE gene risk score into the logistic regression model. ZNF423 conditioned on APOE no longer reaches gene-wide significance, but PPARGC1A and RORA remain significant, suggesting an association independent of APOE. In addition, BCAM, PVRL2 and APOC4-APOC2 on chromosome 19 remain gene-wide significant, even after adjusting for APOE in the model, suggesting a potential signal beyond APOE. To investigate this, we additionally conditioned on BCAM (the most significant chromosome 19 gene after adjusting for APOE) to determine whether this explains the remaining effect. Results are shown in S2 Table; when conditioning on APOE and BCAM the only remaining gene-wide significant gene on chromosome 19 is APOC4-APOC2, suggesting that the majority of signals on chromosome 19 are explained by APOE and BCAM. We were unable to condition on APOE genotype since these are not available for all subjects, so removal of an association may be due to reduced sample size.
Fig 1. Manhattan Plot for the POLARIS Gene-Based Analysis in Imputed GERAD Data Using a Gene Window 35kb Upstream and 10kb Downstream.
Table 1. Gene-Wide Significant Genes from POLARIS Gene-based Analysis in GERAD Imputed Data Using a Gene Window (35kb Upstream and 10kb Downstream).
| POLARIS | POLARIS, conditioned on APOE | |||||||
|---|---|---|---|---|---|---|---|---|
| Chr | Gene | No. of SNPs | Beta | SE | P-value | Beta | SE | P-value |
| 4 | PPARGC1A | 480 | 0.877 | 0.1851 | 2.2 × 10−6 | 0.920 | 0.1885 | 1.0 × 10−6 |
| 8 | SCARA3 (CLU) | 240 | 0.526 | 0.1064 | 7.8 × 10−7 | 0.537 | 0.1090 | 8.3 × 10−7 |
| 15 | RORA | 1813 | 0.334 | 0.0674 | 7.4 × 10−7 | 0.338 | 0.0688 | 9.1 × 10−7 |
| 16 | ZNF423 | 1056 | 0.551 | 0.1163 | 2.1 × 10−6 | 0.541 | 0.1187 | 5.1 × 10−6 |
| 19 | BCL3 | 88 | 0.377 | 0.0674 | 4.2 × 10−9 | 0.291 | 0.0656 | 8.8 × 10−6 |
| 19 | CBLC | 50 | 0.605 | 0.1161 | 1.8 × 10−7 | 0.455 | 0.1183 | 0.00012 |
| 19 | BCAM | 71 | 0.556 | 0.0543 | 1.4 × 10−24 | 0.492 | 0.0555 | 7.6 × 10−19 |
| 19 | PVRL2 | 160 | 0.546 | 0.0299 | 9.4 × 10−75 | 0.430 | 0.0491 | 2.0 × 10−18 |
| 19 | TOMM40 | 108 | 0.500 | 0.0298 | 3.4 × 10−63 | 0.334 | 0.0891 | 0.00018 |
| 19 | APOE | 55 | 0.520 | 0.0315 | 4.4 × 10−61 | NA | NA | NA |
| 19 | APOC1 | 34 | 0.475 | 0.0315 | 1.5 × 10−51 | -0.249 | 0.1031 | 0.01575 |
| 19 | APOC4-APOC2 | 62 | 0.615 | 0.0871 | 1.6 × 10−12 | 0.419 | 0.0892 | 2.5 × 10−6 |
In order to narrow down the disease-associated SNPs for each of these novel genes, we investigated the gene expression patterns using the BRAINEAC [27] database from the UK Brain Expression Consortium. For the PPARGC1A gene the SNP rs67436520, which is downstream of the PPARGC1A gene, has the best cis-expression quantitative trait loci (eQTL) p-value of 3.3 × 10−4, this is expressed in the hippocampus. The best cis-eQTL p-value in the RORA gene is 1.5 × 10−4, this is for SNP rs113223478 which is 78.5kb upstream of the gene, between the NARG2 and ANXA2 genes, and is expressed in the substantia nigra. This SNP will not be included in the POLARIS score, however, it could be tagged by SNPs included in the score. Finally, SNP rs2270396 has the best cis-eQTL p-value in the ZNF423 gene with a p-value of 3.0 × 10−5 and is expressed in the frontal cortex. These SNPs were checked in RegulomeDB [28] and Variant Effect Predictor [29]. They are all intergenic variants that are not in any well-defined regulatory region of the genome and do not overlap the best risk SNPs, so it is difficult to predict how these SNPs may affect the regulation of the expression of these genes.
Discussion
A gene-based analysis was performed using the individual genotypes in the GERAD imputed data and the summary statistics from IGAP data excluding the GERAD subjects was used to inform the analysis. This analysis expands a gene window around the gene, 35kb upstream and 10kb downstream, which is likely to include transcriptional regulatory elements in the gene [17] and thus contain SNPs influencing gene expression. Three novel genes were found to be associated with AD using the POLARIS method. The novel genes are PPARGC1A, RORA and ZNF423, all of which have credible biological relevance to AD. These results are already adjusted for LD between SNPs in the gene, using the POLARIS methodology. Most of the genes identified before in IGAP data [4] [9] were also identified by POLARIS as statistically significant, however, since previous results are based on IGAP stages 1 and 2, POLARIS p-values were slightly larger.
We investigated disease-associated SNPs using expression patterns, which highlighted individual SNPs. A limitation of this analysis is that the POLARIS score tests the aggregated risk across the gene and is unlikely due to a single SNP.
The product of the PPARGC1A gene, PGC-1α (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha) is part of the PGC-1 family of transcriptional coactivators that mainly regulate mitochondrial biogenesis to in turn regulate the cellular energy metabolism [19]. It is also involved in other cellular and physiological functions, including the response to a variety of cellular and external stimuli, cellular glucose homeostasis, circadian rhythm, and the regulation of neuronal apoptosis.
The regulation of this gene is complex; it has multiple isoforms and alternative promoters [30] and gene expression is regulated by a variety of stimuli, including cytokines, insulin, exercise and the cold [31]. PGC-1α can induce ribosomal transcription under stress conditions such as oxidative stress and exercise [32].
Previous animal model work has shown that overexpression of hPGC-1α in APP23 mice improved spatial and recognition memory, along with a significant reduction of Aβ deposition [21]. Furthermore, hPGC-1α overexpression also reduced the levels of proinflammatory cytokines and microglial activation [21] [33]. This suggests a direct link with recent genetic evidence of microglia-mediated innate immune response involvement in AD [11]. In addition, activation of PGC-1α by EKR and p38 inhibitors have been shown to improve spatial and learning memory in Aβ-injected rats [34]. PPARGC1A has also been implicated in the pathogenesis of other neurodegenerative disorders, namely Huntington’s and Parkinson’s diseases [35]. It has been shown that mutated Huntingtin represses PGC-1α, affecting mitochondrial function, hence ribosomal biogenesis may be affected in Huntington’s disease [36]. There is a brain specific promoter 587kb upstream of human PPARGC1A [37], which is located in a genomic region associated with age of onset of Huntington’s disease and relevant here is that hippocampal PGC-1α expression is decreased in the AD brain [38]. A randomised controlled trial of a PPAR-γ agonist, pioglitazone, found improved cognition and regional cerebral blood flow in patients with mild AD [39].
RORA (Retinoic acid receptor-related orphan receptor alpha) is a nuclear hormone receptor with diverse cellular roles [40], for example in immunity, cerebellum development [41], lipid metabolism [42], circadian rhythms and inflammation [23]. RORA regulates its target genes by binding to the ROR response elements (RORE) in the gene regulatory region [43]. It has been shown to regulate more than 3,000 genes in human monocytic and endothelial cell lines [44]. It has a role in the regulation of the BDNF pathway and its expression is upregulated in AD hippocampus [24]. RORA and PPARGC1A are close biological partners, with PGC-1α regulating the expression of a number of clock genes through the coactivation of the ROR family of orphan nuclear receptors [45]. RORA has been shown to be linked to other genes previously implicated in AD [25] and also has been implicated in a large number of neuropsychiatric disorders, such as post-traumatic stress disorder [46] [47] and autism [48]. Furthermore, RORA trans-activates IL-6 and is thought to be neuro-protective in astrocytes and anti-inflammatory in peripheral tissues [49]. The two genes, RORA and PPARGC1A that we report here provide further evidence of the involvement of inflammation in the pathogenesis of AD.
Finally, ZNF423 is a nuclear protein that belongs to the Kruppel-like C2H2 zinc finger proteins. ZNF423 directs bone morphogenetic protein (BMP)-dependent signalling activity and aberrant forms impede B cell differentiation [50]. Furthermore, elevated gene-expression of ZNF423 has been shown to occur in patients with systemic lupus erythematosus, pointing to an impaired function of B cells in human mesenchymal stem cells [51]. ZNF423 resides in an AD-specific protein network [25]. ZNF423 is likely involved with centrosomes and DNA damage repair [26]. It is downregulated in human neuroblastoma and glioma [52] [53] and also has a role in breast cancer [54]. Previously, it also has been shown that missense and LoF variants are likely to be pathogenic for abnormality of brain morphology, Joubert syndrome and Nephronophthisis with autosomal dominant or autosomal recessive inheritance (www.omim.org, https://www.ncbi.nlm.nih.gov/clinvar/). These disorders present with a range of phenotypic characteristics, with the central nervous system being affected too (more specifically the cerebellar vermis). In nur12 mouse model (with introduced nonsense mutation in exon 4 of the mouse Zfp423 gene), Alcaraz et al. [55] observed loss of the corpus callosum, reduction of hippocampus, and a malformation of the cerebellum reminiscent of patients with Dandy-Walker syndrome. Within the cerebellum, Zfp423 was observed to be expressed in both ventricular and external germinal zones. Loss of Zfp423 was also observed to lead to diminished proliferation by granule cell precursors in the external germinal layer and abnormal differentiation and migration of ventricular zone-derived neurons and Bergmann glia [55].
Conclusion
POLARIS is a gene-based analysis which produces a genetic risk score per individual per gene, whilst adjusting for LD between SNPs in the gene. This methodology was applied to the latest HRC imputation of the GERAD data, and the summary statistics from IGAP (excluding GERAD subjects) were used as weights in the score. This led to the identification of 3 novel genes associated with AD; these genes are PPARGC1A, RORA and ZNF423. There is evidence that these genes are credible candidates in AD, with PPARGC1A and RORA being linked to circadian rhythm, PPARGC1A is implicated in energy metabolism and the generation of amyloid plaques, RORA is linked to cholesterol metabolism and inflammation and ZNF423 is likely involved in DNA damage repair and resides in an AD-specific protein network.
Materials and methods
The Haplotype Reference Consortium (HRC), version r1.1 2016, was used to impute GERAD genotype data on the Michigan Imputation Server [56], which to date, allows the most accurate imputation of genetic variants. Imputed genotype probabilities (also known as dosages) were converted to the most probable genotype with a probability threshold of 0.9 or greater. SNPs were removed if: their imputation INFO-score< 0.4, minor allele frequency (MAF)< 0.01, missingness of genotypes≥ 0.05 or HWE< 10−6. A total of 6,119,694 variants were retained. To correct for population structure and genotyping differences, all analyses were adjusted for age, gender and the top 3 principal components.
POLARIS was applied to this GERAD (3,332 cases, 9,832 controls; see S3 Table for cohort details) imputed data, using the IGAP [4] data (17,008 cases, 37,154 controls) excluding GERAD subjects (IGAPnoGERAD) as an external dataset to derive weights from the best powered data set avaliable. The IGAP data was imputed using a previous reference panel (1000 genomes, Dec 2010 release). There were 3,169,839 SNPs in common between imputed GERAD and IGAP summary statistics data. The GERAD imputed data contain individual genotypes for every SNP, enabling the production of a risk score per person per gene, and the IGAPnoGERAD data contains effect sizes for every SNP, which are used to weight the risk score. A gene-based risk score was produced for every individual in the GERAD data.
POLARIS adjusts for LD between SNPs and therefore, the SNPs were not pruned for LD and the entire data were used in this analysis. POLARIS adjusts for LD by using spectral decomposition of the correlation matrix between SNPs. Such a matrix was derived for each gene using the individual genotypes from the GERAD imputed data. It was ensured that SNPs had consistent reference alleles across both independent datasets; IGAPnoGERAD and imputed GERAD. If alleles in IGAPnoGERAD were coded in the opposite direction to those in GERAD, the summary effect size for the SNP was inverted. SNPs with alleles AT, TA, CG or GC were excluded.
SNPs were assigned to genes using GENCODE (v19) gene models [57]. Only genes with known gene status and those marked as protein coding were used. A gene window containing SNPs which were within 35kb upstream and 10kb downstream of the gene was considered. This window was used since it is likely to contain transcriptional regulatory elements [17]. SNPs which belong to multiple genes were assigned to all those genes. In the HRC imputed GERAD data, 2,296,690 SNPs were assigned to 18,087 genes.
A POLARIS score was produced for each of these genes, and the overall association of the gene with AD is determined using a logistic regression model, adjusting for population covariates, age and sex.
Supporting information
(DOCX)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank the MRC Centre for Neuropsychiatric Genetics and Genomics for supporting this project and the MRC for supporting author Emily Baker. This project was also supported by the UK Dementia Research Institute.
Data used in the preparation of this article were obtained from the Genetic and Environmental Risk for Alzheimer’s disease (GERAD1) Consortium [3]. The GERAD data used in this paper includes 5770 additional population controls; including the 1958 British Birth Cohort (1958BC), the KORA F4 Study, Heinz Nixdorf Recall Study and controls from the National Blood Service genotyped as part of the Wellcome Trust Case Control Consortium.
We thank the International Genomics of Alzheimer’s Project (IGAP) for providing summary results data for these analyses. The investigators within IGAP contributed to the design and implementation of IGAP and/or provided data but did not participate in analysis or writing of this report.
Data Availability
IGAP data can be downloaded from the following website: http://web.pasteur-lille.fr/en/recherche/u744/igap/igap_download.php Summary data relating to GERAD consortium are available to request by contacting ADresearchoffice@cardiff.ac.uk.
Funding Statement
We thank the MRC Centre for Neuropsychiatric Genetics and Genomics for supporting this project and the MRC for supporting author EB. This project was also supported by the UK Dementia Research Institute. We would like to acknowledge the grants supporting the following authors: UKDRI (UKDRIdata023) EB, DI, MH, NDA, BPM, JW, VEP; MRC Centre for Neuropsychiatric Genetics and Genomics (MR/L010305/1) EB, RS, GL, JH, DG, LJ, PH, VEP; Dementia Platforms UK- DPUK (MR/L023784/2) JH, GL, VEP, JW, DG.
References
- 1. Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining alzheimer disease. Archives of General Psychiatry. 2006;63(2):168–174. 10.1001/archpsyc.63.2.168 [DOI] [PubMed] [Google Scholar]
- 2. Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–81. 10.1073/pnas.90.5.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harold D, Abraham R, Hollingworth P, Sims R, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1088–93. 10.1038/ng.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–8. 10.1038/ng.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hollingworth P, Harold D, Sims R, Gerrish A, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–35. 10.1038/ng.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naj AC, Jun G, Beecham GW, Wang LS, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–41. 10.1038/ng.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–40. 10.1001/jama.2010.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown MB. A Method for Combining Non-Independent, One-Sided Tests of Significance. Biometrics. 1975;31(4):987–992. 10.2307/2529826 [DOI] [Google Scholar]
- 9. Escott-Price V, Bellenguez C, Wang LS, Choi SH, et al. Gene-wide analysis detects two new susceptibility genes for Alzheimer’s disease. PLoS One. 2014;9(6):e94661 10.1371/journal.pone.0094661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guerreiro R, Wojtas A, Bras J, Carrasquillo M, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117–27. 10.1056/NEJMoa1211851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sims R, van der Lee SJ, Naj AC, Bellenguez C, et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nature Genetics. 2017;49:1373 10.1038/ng.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li MX, Gui HS, Kwan JS, Sham PC. GATES: a rapid and powerful gene-based association test using extended Simes procedure. Am J Hum Genet. 2011;88(3):283–93. 10.1016/j.ajhg.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holmans P, Green EK, Pahwa JS, Ferreira MA, et al. Gene ontology analysis of GWA study data sets provides insights into the biology of bipolar disorder. Am J Hum Genet. 2009;85(1):13–24. 10.1016/j.ajhg.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones L, Holmans PA, Hamshere ML, Harold D, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One. 2010;5(11):e13950 10.1371/journal.pone.0013950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones L, Lambert JC, Wang LS, Choi SH, et al. Convergent genetic and expression data implicate immunity in Alzheimer’s disease. Alzheimers Dement. 2015;11(6):658–71. 10.1016/j.jalz.2014.05.1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baker E, Schmidt KM, Sims R, O’Donovan MC, et al. POLARIS: Polygenic LD-adjusted risk score approach for set-based analysis of GWAS data. Genetic Epidemiology. 2018;42(4):366–377. 10.1002/gepi.22117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Dushlaine C, Rossin L, Lee PH, Duncan L, et al. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18(2):199–209. 10.1038/nn.3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kiezun A, Garimella K, Do R, Stitziel NO, et al. Exome sequencing and the genetic basis of complex traits. Nat Genet. 2012;44(6):623–30. 10.1038/ng.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luo C, Widlund HR, Puigserver P. PGC-1 Coactivators: Shepherding the Mitochondrial Biogenesis of Tumors. Trends Cancer. 2016;2(10):619–631. 10.1016/j.trecan.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23(9):459–66. 10.1016/j.tem.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katsouri L, Lim YM, Blondrath K, Eleftheriadou I, et al. PPARgamma-coactivator-1alpha gene transfer reduces neuronal loss and amyloid-beta generation by reducing beta-secretase in an Alzheimer’s disease model. Proc Natl Acad Sci U S A. 2016;113(43):12292–12297. 10.1073/pnas.1606171113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hood S, Amir S. Neurodegeneration and the Circadian Clock. Front Aging Neurosci. 2017;9:170 10.3389/fnagi.2017.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003 10.1621/nrs.07003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Acquaah-Mensah GK, Agu N, Khan T, Gardner A. A regulatory role for the insulin- and BDNF-linked RORA in the hippocampus: implications for Alzheimer’s disease. J Alzheimers Dis. 2015;44(3):827–38. 10.3233/JAD-141731 [DOI] [PubMed] [Google Scholar]
- 25. Hu YS, Xin J, Hu Y, Zhang L, et al. Analyzing the genes related to Alzheimer’s disease via a network and pathway-based approach. Alzheimers Res Ther. 2017;9(1):29 10.1186/s13195-017-0252-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaki M, Airik R, Ghosh AK, Giles RH, et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 2012;150(3):533–48. 10.1016/j.cell.2012.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trabzuni D, Ryten M, Walker R, Smith C, et al. Quality control parameters on a large dataset of regionally dissected human control brains for whole genome expression studies. J Neurochem. 2011;119(2):275–82. 10.1111/j.1471-4159.2011.07432.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyle AP, Hong EL, Hariharan M, Cheng Y, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22(9):1790–7. 10.1101/gr.137323.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McLaren W, Gil L, Hunt SE, Riat HS, et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17(1):122 10.1186/s13059-016-0974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinez-Redondo V, Pettersson AT, Ruas JL. The hitchhiker’s guide to PGC-1alpha isoform structure and biological functions. Diabetologia. 2015;58(9):1969–77. 10.1007/s00125-015-3671-z [DOI] [PubMed] [Google Scholar]
- 31. Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93(4):884S–90. 10.3945/ajcn.110.001917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jesse S, Bayer H, Alupei MC, Zugel M, et al. Ribosomal transcription is regulated by PGC-1alpha and disturbed in Huntington’s disease. Sci Rep. 2017;7(1):8513 10.1038/s41598-017-09148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nijland PG, Witte ME, van het Hof B, van der Pol S, et al. Astroglial PGC-1alpha increases mitochondrial antioxidant capacity and suppresses inflammation: implications for multiple sclerosis. Acta Neuropathol Commun. 2014;2:170 10.1186/s40478-014-0170-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ashabi G, Ramin M, Azizi P, Taslimi Z, et al. ERK and p38 inhibitors attenuate memory deficits and increase CREB phosphorylation and PGC-1alpha levels in Abeta-injected rats. Behav Brain Res. 2012;232(1):165–73. 10.1016/j.bbr.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 35. Tsunemi T, Spada ARL. PGC-1alpha at the intersection of bioenergetics regulation and neuron function: from Huntington’s disease to Parkinson’s disease and beyond. Prog Neurobiol. 2012;97(2):142–51. 10.1016/j.pneurobio.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cui L, Jeong H, Borovecki F, Parkhurst CN, et al. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127(1):59–69. 10.1016/j.cell.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 37. Soyal SM, Felder TK, Auer S, Hahne P, et al. A greatly extended PPARGC1A genomic locus encodes several new brain-specific isoforms and influences Huntington disease age of onset. Hum Mol Genet. 2012;21(15):3461–73. 10.1093/hmg/dds177 [DOI] [PubMed] [Google Scholar]
- 38. Qin W, Haroutunian V, Katsel P, Cardozo CP, et al. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66(3):352–61. 10.1001/archneurol.2008.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sato T, Hanyu H, Hirao K, Kanetaka H, et al. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32(9):1626–33. 10.1016/j.neurobiolaging.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 40. Cook DN, Kang HS, Jetten AM. Retinoic Acid-Related Orphan Receptors (RORs): Regulatory Functions in Immunity, Development, Circadian Rhythm, and Metabolism. Nucl Receptor Res. 2015;2 10.11131/2015/101185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vogel MW, Sinclair M, Qiu D, Fan H. Purkinje cell fate in staggerer mutants: Agenesis versus cell death. Journal of Neurobiology. 2000;42(3):323–337. [DOI] [PubMed] [Google Scholar]
- 42. Kim K, Boo K, Yu YS, Oh SK, et al. RORalpha controls hepatic lipid homeostasis via negative regulation of PPARgamma transcriptional network. Nat Commun. 2017;8(1):162 10.1038/s41467-017-00215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chauvet C, Vanhoutteghem A, Duhem C, Saint-Auret G, et al. Control of gene expression by the retinoic acid-related orphan receptor alpha in HepG2 human hepatoma cells. PLoS One. 2011;6(7):e22545 10.1371/journal.pone.0022545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gulec C, Coban N, Ozsait-Selcuk B, Sirma-Ekmekci S, et al. Identification of potential target genes of ROR-alpha in THP1 and HUVEC cell lines. Exp Cell Res. 2017;353(1):6–15. 10.1016/j.yexcr.2017.02.028 [DOI] [PubMed] [Google Scholar]
- 45. Liu C, Li S, Liu T, Borjigin J, et al. Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism. Nature. 2007;447:477 10.1038/nature05767 [DOI] [PubMed] [Google Scholar]
- 46. Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447(7146):799–816. 10.1038/nature05874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miller MW, Wolf EJ, Logue MW, Baldwin CT. The retinoid-related orphan receptor alpha (RORA) gene and fear-related psychopathology. J Affect Disord. 2013;151(2):702–8. 10.1016/j.jad.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sayad A, Noroozi R, Omrani MD, Taheri M, et al. Retinoic acid-related orphan receptor alpha (RORA) variants are associated with autism spectrum disorder. Metabolic Brain Disease. 2017;32(5):1595–1601. 10.1007/s11011-017-0049-6 [DOI] [PubMed] [Google Scholar]
- 49. Journiac N, Jolly S, Jarvis C, Gautheron V, et al. The nuclear receptor RORα exerts a bi-directional regulation of IL-6 in resting and reactive astrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(50):21365–21370. 10.1073/pnas.0911782106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harder L, Eschenburg G, Zech A, Kriebitzsch N, et al. Aberrant ZNF423 impedes B cell differentiation and is linked to adverse outcome of ETV6-RUNX1 negative B precursor acute lymphoblastic leukemia. J Exp Med. 2013;210(11):2289–304. 10.1084/jem.20130497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Feng X, Che N, Liu Y, Chen H, et al. Restored immunosuppressive effect of mesenchymal stem cells on B cells after olfactory 1/early B cell factor-associated zinc-finger protein down-regulation in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2014;66(12):3413–23. 10.1002/art.38879 [DOI] [PubMed] [Google Scholar]
- 52. Huang S, Laoukili J, Epping MT, Koster J, et al. ZNF423 is critically required for retinoic acid-induced differentiation and is a marker of neuroblastoma outcome. Cancer Cell. 2009;15(4):328–40. 10.1016/j.ccr.2009.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Signaroldi E, Laise P, Cristofanon S, Brancaccio A, et al. Polycomb dysregulation in gliomagenesis targets a Zfp423-dependent differentiation network. Nat Commun. 2016;7:10753 10.1038/ncomms10753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bond HM, Scicchitano S, Chiarella E, Amodio N, et al. ZNF423: A New Player in Estrogen Receptor-Positive Breast Cancer. Front Endocrinol (Lausanne). 2018;9:255 10.3389/fendo.2018.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alcaraz WA, Gold DA, Raponi E, Gent PM, et al. Zfp423 controls proliferation and differentiation of neural precursors in cerebellar vermis formation. Proc Natl Acad Sci U S A. 2006;103(51):19424–9. 10.1073/pnas.0609184103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Das S, Forer L, Schonherr S, Sidore C, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284–1287. 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Harrow J, Frankish A, Gonzalez JM, Tapanari E, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22(9):1760–74. 10.1101/gr.135350.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(PDF)
(PDF)
Data Availability Statement
IGAP data can be downloaded from the following website: http://web.pasteur-lille.fr/en/recherche/u744/igap/igap_download.php Summary data relating to GERAD consortium are available to request by contacting ADresearchoffice@cardiff.ac.uk.