Abstract
Objective:
The objective of this study was to compare sex differences in bone deficits among adolescents with anorexia nervosa (AN) and to identify other correlates of bone health.
Method:
Electronic medical records of all patients 9–20 years of age with a DSM-5 diagnosis of AN who underwent dual-energy x-ray absorptiometry (DXA) scans within three months of initial evaluation by the eating disorders program at Stanford between March 1997 and February 2011 were retrospectively reviewed. Whole body bone mineral content Z-scores and bone mineral density (BMD) Z-scores at multiple sites were recorded using the Bone Mineral Density in Childhood Study (BMDCS) reference data.
Results:
A total of 25 males and 253 females with AN were included, with median age 15 years (interquartile range [IQR] 14–17) and median duration of illness 9 months (IQR 5–13). Using linear regression analyses, no significant sex differences in bone deficits were found at the lumbar spine, total hip, femoral neck, or whole body when controlling for age, %mBMI, and duration of illness. Lower %mBMI was significantly associated with bone deficits at all sites in adjusted models.
Discussion:
This is the first study to evaluate sex differences in bone health among adolescents with AN, using novel DSM-5 criteria for AN and robust BMDCS reference data. We find no significant sex differences in bone deficits among adolescents with AN except for a higher proportion of females with femoral neck BMD Z-scores <−1. Degree of malnutrition was correlated with bone deficits at all sites.
Keywords: anorexia nervosa, eating disorders, dual-energy x-ray absorptiometry, DXA, bone density, bone health, sex differences
Anorexia nervosa (AN), a condition characterized by self-induced weight loss, severe body image distortion, and fear of weight gain (1), affects between 0.7% and 2.2% of young women in Western societies (2,3). The 2013 DSM-5 criteria eliminated specific body weight and amenorrhea requirements for the diagnosis of AN (1); therefore the prevalence of AN is expected to rise (4). Medical complications associated with AN include electrolyte disturbances, gastrointestinal manifestations including pancreatitis and elevated liver enzymes, low bone mineral density (BMD), and increased fracture risk (5–21).
Although 5–15% of patients with AN are male (22), few studies have assessed BMD in males with AN (6,7,23). Prior studies in female adolescents with AN reported significant BMD deficits in the lumbar spine (8,9,13), areas of primarily trabecular bone, greater than deficits in the femoral neck (12). The few studies in males with AN reported significant BMD deficits in the total hip, femoral neck, trochanter, and intertrochanteric regions, areas of primarily cortical bone, compared to healthy male controls (6,7). Misra et al. reported that 11 of 17 (65%) 12–19 year old boys had femoral neck BMD Z-scores < −1 compared to 18% in a sample of normal weight boys in the same age range (6). Recent meta-analyses have confirmed lower BMD at the lumbar spine, total hip, intertrochanteric region, trochanteric region, and femoral neck in subjects with AN versus healthy controls (19,20). However, these systematic reviews and meta-analyses did not stratify results by sex, and were not specific to adolescent populations.
To our knowledge, no studies have examined sex differences in bone deficits in adolescents with AN. Previous studies on bone health in AN used older DSM-IV diagnostic criteria and were published prior to the availability of robust sex-, race-, age-, and height-specific reference data from the Bone Mineral Density in Childhood Study (BMDCS) (24).
The objective of this study was therefore to compare BMD in males and females, as well as to identify correlates of bone deficits among male and female adolescents with AN using DSM-5 criteria and robust BMDCS reference curves. We hypothesized that female adolescents with AN may have greater bone deficits in lumbar spine BMD given their severe estrogen deficiency (5). We also hypothesized that adolescent boys with AN may have greater deficits in total hip and femoral neck BMD than girls due to testosterone deficiency, as testosterone prevents osteoclastic bone resorption following its aromatization to estrogen leading to increased cortical thickness and also contributes to periosteal bone apposition (5). Finally, we hypothesized that degree of malnutrition would be associated with deficits in bone health.
Methods
Study Population
The electronic medical record (EMR) was reviewed for all patients, 9 to 20 years of age, presenting for an initial evaluation to the Eating Disorders Program at Lucile Packard Children’s Hospital, Stanford between March 1997 and February 2011. Patients were initially diagnosed using DSM-IV criteria; therefore, the clinical characteristics were reviewed and patients categorized using DSM-5 criteria for this study.
Inclusion criteria included age 9 to 20 years, DSM-5 diagnosis of AN, and availability of dual-energy x-ray absorptiometry (DXA) results obtained using a Hologic bone densitometer. Females with DXA scans performed after three months of presentation were excluded because of the potential for the change in height (measured at presentation) to affect BMDCS Z-scores. The median interval between the baseline clinical visit and first DXA was 56 days (interquartile range 36 to 95 days). Males with DXA scans performed after three months of presentation were not excluded given the importance of increasing the power and sample size in males, and the imbalance of females compared to males. Patients with a DSM-5 diagnosis of bulimia nervosa, binge eating disorder, other specified feeding or eating disorder (including atypical AN), or unspecified feeding or eating disorder were excluded.
Study Design
This retrospective cross-sectional study included demographics, anthropometry and disease characteristics documented in the EMR. Assessments were completed by clinic and hospital staff in the Eating Disorders Program for the purposes of medical care. They were blind to the purposes of the study. Their clinical assessments in the EMR were then retrospectively reviewed and entered into a database. The duration of illness documented in the EMR was based on self-report of time of onset of symptoms. Body mass index (BMI, kg/m2) was calculated, and mBMI defined as the 50th percentile BMI for age using the Centers for Disease Control and Prevention growth curves (25). Percentage mBMI (%mBMI) on admission was defined as the patient’s BMI on admission divided by the mBMI multiplied by 100 (26).
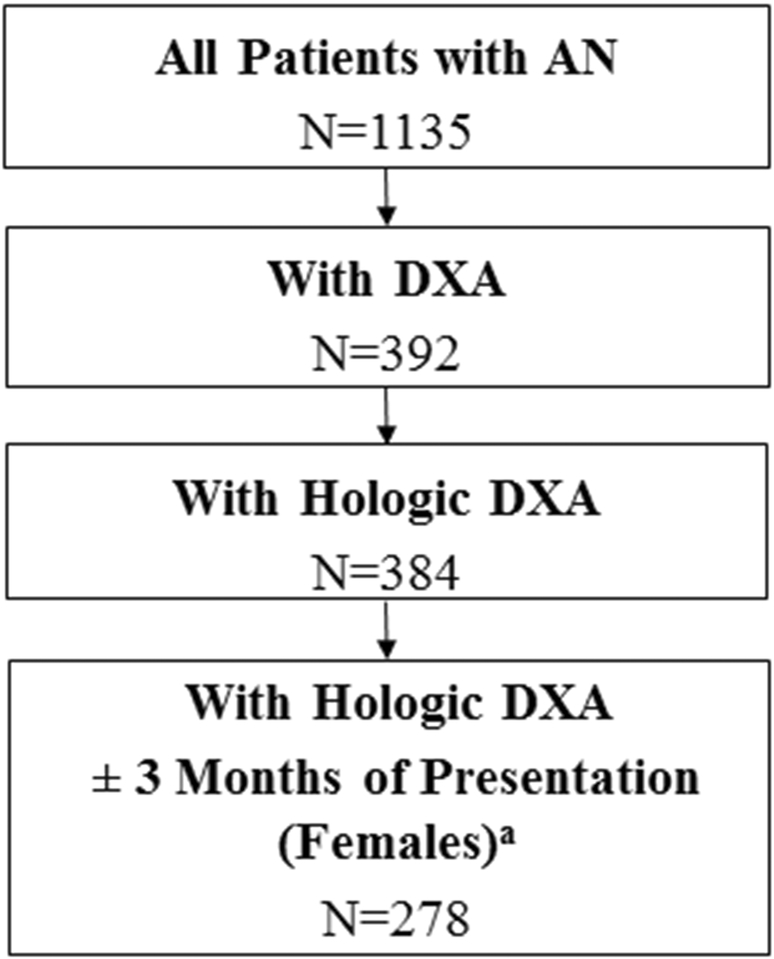
Posteroanterior lumbar spine (L1–L4), total hip, proximal femur and whole body scans were obtained by DXA (Hologic 4500, Hologic, Waltham, MA). All bone density assessments were performed on the same Hologic 4500 densitometer using similar software. We excluded DXAs (n=8) that were performed on different scanners (Figure 1). The coefficient of variation is 0.28% for Hologic DXA scans at Stanford. The DXA whole body bone mineral content (BMC), and lumbar spine, total hip and femoral neck BMD measurements were converted to sex-, race-, and age-specific Z-scores using reference curves generated with the LMS method (27) by the Bone Mineral Density in Childhood Study (24). The Z-scores were further adjusted for height Z-score using the method developed by BMDCS investigators (28).
Figure 1.
Flow diagram of included study participants
a Males with DXA scans performed after three months of presentation were not excluded given the importance of increasing the power and sample size in males, and the imbalance of females compared to males.
The study was approved by the Committee on Human Research (IRB) at Stanford University.
Statistical Analysis
Data were analyzed using STATA (StataCorp LP, College Station, TX). Unadjusted differences between males and females in demographic characteristics, %mBMI, duration of illness, BMC and BMD Z-scores and the proportion with Z-scores < −1 or <−2 (29) were calculated using independent samples t-tests or Fisher’s exact tests. Median and interquartile range were reported for age and duration of illness, and differences were analysed using the rank sum test. Linear regression analyses were used to identify risk factors for low BMC and BMD Z-scores, including sex, age, %mBMI, and duration of illness. The duration of illness variable was log-transformed in the regression analysis due to skewness of the original variable. P<0.05 was considered significant. Given a sample size of adolescent girls (N=253) and boys (N=25) with anorexia nervosa, and using an estimated mean BMD Z-score standard deviation of 1.0 (5), our study had statistical power (alpha=0.05, two-sided) of 80% to detect a difference in BMD Z-score of 0.59 or greater (30). Because previous literature has demonstrated that the decrease in BMD in AN depends on duration of emaciation-related bone metabolism (31), we conducted a sub-analyses of a proportion of the sample that had duration of illness > 9 months and BMI < 16 kg/m2.
Results
Clinical Characteristics
A total of 25 males and 253 females with AN met eligibility criteria (Figure 1). Median age was 15.5 years, with an interquartile range of three years (Table 1). Males had younger age at presentation than females (median 13.94 vs.15.53, p=0.01). There were no significant sex differences in median duration of illness or mean BMI. Median duration of illness was nine months and mean BMI was 15.84 ± 1.57 kg/m2.
Table 1.
Demographic and bone characteristics of adolescents with anorexia nervosa by sex
| Malea | Femalea | Effect size | p (unadjusted) | P (adjusted)b | |
|---|---|---|---|---|---|
| n | 25 | 253 | |||
| Age, years | 13.94 (13.04, 15.84) | 15.53 (14.11, 16.89) | −1.59c | 0.01 | |
| Height, cm | 163.05 ± 10.05 | 160.06 ± 8.79 | 2.99 ± 8.91 | 0.11 | |
| Height Z-score | −0.09 ± 0.93 | −0.11 ± 1.05 | 0.02 ± 1.04 | 0.92 | |
| BMI, kg/m2 | 15.88 ± 1.40 | 15.83 ± 1.58 | 0.05 ± 1.57 | 0.87 | |
| %mBMId | 80.78 ± 5.55 | 78.73 ± 7.06 | 2.05 ± 6.95 | 0.18 | |
| Duration illness (months) | 9 (7, 14) | 9 (5, 13) | 0c | 0.52 | |
| Whole body BMC Z-score | −0.60 ± 0.94 | −0.42 ± 0.93 | −0.18 ± 0.93 | 0.36 | 0.12 |
| Whole body BMC Z-score less than -1 | 9 (36.00%) | 81 (32.02%) | 0.66 | ||
| Whole body BMC Z-score less than -2 | 3 (12.00%) | 33 (13.04%) | 1.00 | ||
| Lumbar spine BMD Z-score | −0.19 ± 0.95 | −0.51 ± 1.00 | 0.32 ± 0.99 | 0.20 | 0.77 |
| Lumbar spine BMD Z-score less than -1 | 12 (48.00%) | 113 (44.66%) | 0.83 | ||
| Lumbar spine BMD Z-score less than -2 | 8 (32.00%) | 59 (23.32%) | 0.33 | ||
| Total hip BMD Z-score | −0.96 ± 0.93 | −0.81 ± 1.07 | −0.15 ± 1.06 | 0.51 | 0.28 |
| Total hip BMD Z-score less than -1 | 11 (44.00%) | 115 (45.45%) | 1.00 | ||
| Total hip BMD Z-score less than -2 | 5 (20.00%) | 45 (17.79%) | 0.79 | ||
| Femoral neck BMD Z-score | −0.70 ± 0.91 | −0.77 ± 1.02 | 0.06 ± 1.01 | 0.79 | 0.76 |
| Femoral neck BMD Z-score less than -1 | 10 (40.00%) | 162 (64.03%) | 0.03 | ||
| Femoral neck BMD Z-score less than -2 | 6 (24.00%) | 112 (44.27%) | 0.06 |
Results are presented as mean ± SD, median (interquartile range), or n (%).
multiple linear regression adjusted for age, %mBMI, and duration of illness
median difference (p-value shows if the difference is significant or not)
%mBMI = percentage median body mass index
BMC and BMD Measurements
Table 1 shows DXA results in total and divided by sex. There were no significant sex differences in BMC or BMD Z-scores at any of the sites we evaluated using independent samples t-tests in unadjusted analyses. However, there was a greater percentage of females with BMD Z-scores less than −1 (64%) compared to males (40%, p=0.03) at the femoral neck, in unadjusted analyses. Sex was not significantly associated with BMC or BMD Z-score at any of the sites in multivariate models when controlling for %mBMI, age, and duration of illness. For both males and females, there were significant deficits in Z-scores compared to zero including whole body BMC (p=0.005 for males, p<0.0001 for females), total hip BMD (p<0.0001 for males, p<0.0001 for females), femoral neck BMD (p=0.002 for males, p<0.0001 for females), and lumbar spine BMD (p<0.0001 for females). Lumbar spine BMD Z-score for males was the only site not significantly less than zero (p=0.43).
Correlates of Bone Deficits in AN in Subset of Sample
A total of 16 males and 227 females were included in the subset of the sample with duration of illness > 9 months and BMI < 16 kg/m2. Multivariate regression models of the sample subset are summarized in Table 2. Sex was not significantly associated with BMC or BMD Z-score at any of the sites in multivariate models when controlling for %mBMI, age, and duration of illness. Lower %mBMI was significantly associated with bone deficits in whole body BMC Z-score (β=0.03, p<0.0001) and BMD at the lumbar spine (β=0.03, p<0.001), total hip (β=0.03, p<0.001), and femoral neck (β=0.02, p<0.0009) when controlling for sex, age, and duration of illness. Younger age was associated with greater deficits in whole body BMC Z-score (β= 0.07, p=0.04) but not at other sites, when controlling for sex, %mBMI, and duration of illness.
Table 2.
Linear regression analysis of covariates associated with increased dual-energy x-ray absorptiometry (DXA) Z-scores on subset of sample with duration of illness > 9 months and BMI < 16
| β (95% CI) | p | ||
|---|---|---|---|
| Whole body BMC Z-score | Female (vs. male) | −0.02 (−0.47, 0.43) | 0.44 |
| Age | 0.07 (0.01, 0.13) | 0.04 | |
| %mBMIa | 0.03 (0.02, 0.04) | <0.0001 | |
| Duration of illnessb | −0.13 (−0.28, 0.02) | 0.09 | |
| Lumbar spine BMD Z-score | Female (vs. male) | −0.26 (−0.87, 0.35) | 0.41 |
| Age | −0.08 (−0.16, 0.00) | 0.05 | |
| %mBMIa | 0.03 (0.01, 0.05) | <0.001 | |
| Duration of illnessb | −0.13 (−0.32, 0.06) | 0.19 | |
| Total hip BMD Z-score | Female (vs. male) | −0.22 (−0.76, 0.32) | 0.43 |
| Age | 0.06 (−0.01, 0.13) | 0.11 | |
| %mBMIa | 0.03 (−0.36, −0.02) | <0.001 | |
| Duration of illnessb | −0.19 (−0.36, −0.02) | 0.03 | |
| Femoral neck BMD Z-score | Female (vs. male) | −0.42 (−0.91, 0.07) | 0.1 |
| Age | 0.03 (−0.05, 0.11) | 0.39 | |
| %mBMIa | 0.02 (0.01, 0.03) | 0.0009 | |
| Duration of illnessb | −0.08 (−0.26. 0.10) | 0.39 |
%mBMI = percentage median body mass index
Duration has been log-transformed due to the skewness of the original data
Discussion
This is the first study to assess sex differences in bone health among adolescent males and females with AN, finding deficits in whole body BMC, and BMD at the lumbar spine, total hip, and femoral neck in both sexes. A greater proportion of female adolescents with AN had femoral neck BMD Z-scores <−1 than males; however, the degree of malnutrition appeared to be the primary factor accounting for this difference. There were no significant differences between sexes in whole body BMC or total hip BMD in adjusted models.
There has been an increased recognition of the need to study the skeletal status of males with AN since it is not clear if observations from female patients can be extrapolated to males. Two previous studies compared boys with AN to healthy male controls but not to females with the disorder (6,7). A published letter compared the prevalence of osteoporosis and osteopenia in adult men and women with eating disorders, but did not include adolescents (32). The paucity of studies of bone health in males with AN likely reflects the challenges of recruiting large numbers of male participants. Among males there may be delays in diagnosis or failure to recognize AN in part because males do not have amenorrhea, which alerts clinicians to the diagnosis of AN in females (7).
We report significant deficits in BMD and BMC among both males and females with AN at all sites except for males at the lumbar spine. This is consistent with recent meta-analyses which found significant BMD deficits in subjects with AN compared to healthy controls (19,20). However, the bone deficits in our male sample were less profound at all sites than those reported in previous studies in males. Our whole body BMC Z-score of −0.52 was greater than the previously reported −0.80 among 17 adolescent boys with AN (6). We observed a femoral neck Z-score of −0.52 as compared with the mean of −1.17 in the same previously reported sample (6). In our sample, the spine was not significantly affected compared to the mean for age in males; however, the hip did demonstrate significant deficits. This is consistent with previous reports of the hip being affected more than the spine in adolescent males with AN (6). The use of more inclusive DSM-5 criteria for the diagnosis of AN allowed for the inclusion of participants with higher BMI and %mBMI. However, the mean age and other demographic factors of our sample compared to the previous studies were similar.
Previous studies in adolescent males indicated that deficits in BMD were greatest at the hip, femoral neck, and intertrochanteric regions, as compared to the spine (6,7). In contrast, prior studies in adolescent females indicated that bone density was most affected at the lumbar spine as compared to the hip (8–10,13). Though both adolescent males and females had bone deficits at the total hip and femoral neck, sex differences at those locations were not statistically significant. These findings are similar to those from the one previous study in adults with AN, which found no significant sex differences in BMD at the lumbar spine or femoral neck (32).
A recent meta-analysis demonstrated that reduced BMD is moderated by duration of illness and in females, duration of amenorrhea (20). Our results demonstrate that in a population of adolescents with a relatively short duration of illness, deficits in BMD are already established, both in males and in females. Duration of amenorrhea mediates BMD in females with AN and some guidelines use amenorrhea > 6 months duration as a reason for ordering a DXA scan (11,33–35); however, no such recommendations exist for males. One study of 51 Japanese females with AN demonstrated a significant association between spine BMD and duration of emaciation below a BMI of 15 or 16. The decrease in BMD therefore depended on the duration of emaciation-related abnormal bone metabolism (31). However, in a sub-analysis of patients with BMI < 16 and duration of illness > 9 months, we found no significant sex differences and overall similar results to the full sample.
Limitations of this study include its retrospective nature and cross-sectional design which precludes causal inferences. In addition, we lacked data on sex hormones, growth factors, physical activity, psychotropic medications, comorbid mental health diagnoses, and amenorrhea, which has been shown in a recent meta-analysis to mediate lower BMD in AN (20). A recent meta-analysis demonstrated high levels of smoking in AN (36) and therefore smoking is a potential confounder for low BMD that we were unable to account for. Selection bias is a possible limitation since we only included participants with DXA scans; however, there were no significant differences in demographic or anthropometric data between the total sample of patients with AN and the study sample of participants who were included (Appendix A). We excluded females with DXA scans performed after three months of presentation because of a sufficiently large sample size and the potential for the change in height (measured at presentation) to affect BMDCS Z-scores; however, we did not exclude on this criteria in males given the importance of increasing the power and sample size in males, and the imbalance of females compared to males. The included males with DXA scans performed after three months of presentation may have increased height since their last evaluation and therefore height adjustments may not be accurate. In addition, there are limitations to using DXA in assessing BMD, particularly in populations with abnormal body composition and fat distribution, such as in adolescents with AN (37). Although the hip is not routinely measured in younger participants given concern for changing landmarks, it may still be clinically relevant as total hip as well as femoral neck BMD were shown to be associated with incident fracture in multivariable models using BMDCS data (38). We also note the imbalance in the smaller number of males compared to females in this sample; however, the proportion of males in our study (9% male) is consistent with overall sex differences in AN prevalence nationally (5–15% male) (22). We also did not include healthy controls.
Strengths of this study include the evaluation by a specialized eating disorders team with systematic data collection. We had a relatively large sample size and included males with AN who are an understudied population (39). Furthermore, BMC and BMD Z-scores were calculated using the BMDCS reference curves which are the most robust reference values for Hologic equipment. The data were generated from about 10,000 observations of approximately 2,000 healthy participants from five centers around the United States (24). Prior studies relied upon older reference curves based upon fewer observations. Finally, this study is the first to use new DSM-5 criteria for the diagnosis of AN, as previous studies on bone health in AN collected data using DSM-IV criteria.
Since degree of malnutrition was the most significant predictor of bone deficits at all sites, clinicians may consider a DXA scan to evaluate for low BMD and potential increased fracture risk in the most severely malnourished patients with AN. In addition, from a clinical standpoint, restoration of weight may be essential to improve bone density in patients with AN (40).
Conclusion
To our knowledge, this is the first study to assess sex differences in bone health among adolescents with AN. We found deficits in whole body BMC and BMD at the lumbar spine, total hip, and femoral neck. However, we found no significant sex differences in these bone deficits, except that there was a higher proportion of females with Z-scores <−1 at the femoral neck than males in unadjusted models. Degree of malnutrition was significantly associated with bone deficits at all sites. Future research should evaluate BMD longitudinally to determine the impact of recovery in adolescents with AN and also evaluate sex differences in body composition and fracture risk among adolescents with AN.
Supplementary Material
Appendix A. Comparison of characteristics of adolescents with anorexia nervosa with and without dual-energy x-ray absorptiometry (DXA)
Acknowledgements:
The authors thank Laura Bachrach for advice in study design and Nicole Capdarest-Arest for help with the literature searches. An earlier version of this study was presented at the Society for Adolescent Health and Medicine Annual Meeting 2016 in Washington, D.C. and the International Conference on Eating Disorders 2016 in San Francisco, California.
Disclosures: MBL has received funding from a NephCure Foundation/American Society of Nephrology Research Grant, Genentech Inc, and the National Kidney Foundation/Amgen Kidney Disease Outcomes Quality Initiative Research Fellowship. MBL has consultancy agreements with Amgen Inc, Johnson & Johnson, and Novartis. She is on the Scientific Advisory Board of Marodyne Medical and an NIH Data Safety and Monitoring Board.
References
- (1).American Psychiatric Association; Diagnostic and Statistical Manual of Mental Disorders. 5th Ed. ed. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- (2).Fairburn CG, Harrison PJ. Eating disorders. The Lancet 2003;361:407–16. [DOI] [PubMed] [Google Scholar]
- (3).Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, et al. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry 2007. August;164(8):1259–1265. [DOI] [PubMed] [Google Scholar]
- (4).Smink FR, van Hoeken D, Hoek HW. Epidemiology, course, and outcome of eating disorders. Curr Opin Psychiatry 2013. November;26(6):543–548. [DOI] [PubMed] [Google Scholar]
- (5).Misra M, Klibanski A. Anorexia nervosa and bone. J Endocrinol 2014. June;221(3):R163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Misra M, Katzman DK, Cord J, Manning SJ, Mendes N, Herzog DB, et al. Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab 2008. August;93(8):3029–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Misra M, Katzman DK, Clarke H, Snelgrove D, Brigham K, Miller KK, et al. Hip structural analysis in adolescent boys with anorexia nervosa and controls. J Clin Endocrinol Metab 2013. July;98(7):2952–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Misra M, Aggarwal A, Miller KK, Almazan C, Worley M, Soyka LA, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics 2004. December;114(6):1574–1583. [DOI] [PubMed] [Google Scholar]
- (9).Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics 1990. September;86(3):440–447. [PubMed] [Google Scholar]
- (10).Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med 2000. November 21;133(10):790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Golden NH. Osteopenia and osteoporosis in anorexia nervosa. Adolesc Med 2003. February;14(1):97–108. [PubMed] [Google Scholar]
- (12).Golden NH, Lanzkowsky L, Schebendach J, Palestro CJ, Jacobson MS, Shenker IR. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol 2002. June;15(3):135–143. [DOI] [PubMed] [Google Scholar]
- (13).Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2002. September;87(9):4177–4185. [DOI] [PubMed] [Google Scholar]
- (14).Lucas AR, Melton LJ 3rd, Crowson CS, O’Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc 1999. October;74(10):972–977. [DOI] [PubMed] [Google Scholar]
- (15).Vestergaard P, Emborg C, Stoving RK, Hagen C, Mosekilde L, Brixen K. Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders--a nationwide register study. Int J Eat Disord 2002. November;32(3):301–308. [DOI] [PubMed] [Google Scholar]
- (16).Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, et al. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord 2014. July;47(5):458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Misra M, Golden NH, Katzman DK. State of the art systematic review of bone disease in anorexia nervosa. Int J Eat Disord 2016. March;49(3):276–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Nagata JM, Park KT, Colditz K, Golden NH. Associations of elevated liver enzymes among hospitalized adolescents with anorexia nervosa. J Pediatr 2015. February;166(2):439–43.e1. [DOI] [PubMed] [Google Scholar]
- (19).Robinson L, Aldridge V, Clark EM, Misra M, Micali N. A systematic review and meta-analysis of the association between eating disorders and bone density. Osteoporos Int 2016. June;27(6):1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Solmi M, Veronese N, Correll CU, Favaro A, Santonastaso P, Caregaro L, et al. Bone mineral density, osteoporosis, and fractures among people with eating disorders: a systematic review and meta-analysis. Acta Psychiatr Scand 2016. May;133(5):341–351. [DOI] [PubMed] [Google Scholar]
- (21).Golden NH, Nagata JM. Starvation in Children, Adolescents and Young Adults: Relevance to Eating Disorders In: Wade T, editor. Encyclopedia of Feeding and Eating Disorders. First ed. Singapore: Springer Singapore; 2016. [Google Scholar]
- (22).Andersen AE, Holman JE. Males with eating disorders: challenges for treatment and research. Psychopharmacol Bull 1997;33(3):391–397. [PubMed] [Google Scholar]
- (23).Castro J, Toro J, Lazaro L, Pons F, Halperin I. Bone mineral density in male adolescents with anorexia nervosa. J Am Acad Child Adolesc Psychiatry 2002. May;41(5):613–618. [DOI] [PubMed] [Google Scholar]
- (24).Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 2011. October;96(10):3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Centers for Disease Control, C.D.C. Growth charts. Available at: http://www.cdc.gov/growthcharts/, 13 January 2014.
- (26).Society for Adolescent Health and Medicine, Golden NH, Katzman DK, Sawyer SM, Ornstein RM, Rome ES et al. Position Paper of the Society for Adolescent Health and Medicine: medical management of restrictive eating disorders in adolescents and young adults. J Adolesc Health 2015. January;56(1):121–125. [DOI] [PubMed] [Google Scholar]
- (27).Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990. January;44(1):45–60. [PubMed] [Google Scholar]
- (28).Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab 2010. March;95(3):1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Gordon CM, Leonard MB, Zemel BS, International Society for Clinical Densitometry. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom 2014. Apr-Jun;17(2):219–224. [DOI] [PubMed] [Google Scholar]
- (30).Chow S, Shao J, Wang H. Sample Size Calculations in Clinical Research 2nd ed. New York: Marcel Dekker, Inc.; 2008. [Google Scholar]
- (31).Hotta M, Shibasaki T, Sato K, Demura H. The importance of body weight history in the occurrence and recovery of osteoporosis in patients with anorexia nervosa: evaluation by dual X-ray absorptiometry and bone metabolic markers. Eur J Endocrinol 1998. September;139(3):276–283. [DOI] [PubMed] [Google Scholar]
- (32).Andersen AE, Watson T, Schlechte J. Osteoporosis and osteopenia in men with eating disorders. Lancet 2000. June 3;355(9219):1967–1968. [DOI] [PubMed] [Google Scholar]
- (33).Golden NH, Katzman DK, Sawyer SM, Ornstein RM, Rome ES, Garber AK, et al. Update on the medical management of eating disorders in adolescents. J Adolesc Health 2015. April;56(4):370–375. [DOI] [PubMed] [Google Scholar]
- (34).Golden NH, Abrams SA, Committee on Nutrition. Optimizing bone health in children and adolescents. Pediatrics 2014. October;134(4):e1229–43. [DOI] [PubMed] [Google Scholar]
- (35).Bachrach LK, Sills IN, Section on Endocrinology. Clinical report-bone densitometry in children and adolescents. Pediatrics 2011. January;127(1):189–194. [DOI] [PubMed] [Google Scholar]
- (36).Solmi M, Veronese N, Sergi G, Luchini C, Favaro A, Santonastaso P, et al. The association between smoking prevalence and eating disorders: a systematic review and meta-analysis. Addiction 2016. May 20. [DOI] [PubMed] [Google Scholar]
- (37).Wren TA, Liu X, Pitukcheewanont P, Gilsanz V. Bone densitometry in pediatric populations: discrepancies in the diagnosis of osteoporosis by DXA and CT. J Pediatr 2005. June;146(6):776–779. [DOI] [PubMed] [Google Scholar]
- (38).Wren TA, Shepherd JA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S, et al. Racial disparity in fracture risk between white and nonwhite children in the United States. J Pediatr 2012. December;161(6):1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Domine F, Berchtold A, Akre C, Michaud PA, Suris JC. Disordered eating behaviors: what about boys? 2009;44:111–117. [DOI] [PubMed] [Google Scholar]
- (40).Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab 2006. August;91(8):2931–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Comparison of characteristics of adolescents with anorexia nervosa with and without dual-energy x-ray absorptiometry (DXA)