Abstract
Designing therapeutics is a process with many challenges. Even if the first hurdle — designing a drug that modulates the action of a particular biological target in vitro — is overcome, selective delivery to that target in vivo presents a major barrier. Side-effects can, in many cases, result from the need to use higher doses without targeted delivery. However, the established use of macromolecules to encapsulate or conjugate drugs can provide improved delivery, and stands to enable better therapeutic outcomes. In this Review, we discuss how drug delivery approaches have evolved alongside our ability to prepare increasingly complex macromolecular architectures. We examine how this increased complexity has overcome the challenges of drug delivery and discuss its potential for fulfilling unmet needs in nanomedicine.
Nanomaterials have had a key role in delivering drugs, simplifying administration schemes, reducing toxicities and improving disease outcomes. The advancement of nanomedicine (the medical application of nanotechnology) has allowed researchers to evaluate its application in myriad of clinical problems with the goal of developing improved and more efficient therapeutics. In the chemical realm, this includes the integration of multiple functionalities into nanoscaffolds and the development of methods to control the shape and dispersity of macromolecules. In this Review, we highlight how the scope of nanotherapeutics has expanded by moving from linear towards architecturally complex branched and hyperbranched structures, and how continued evolution of macromolecular complexity offers opportunities for future applications of nanomedicine.
The need to deliver a pharmacological dose of a drug to a targeted site with high efficacy, using a route that will facilitate patient compliance, is of critical importance for effective and safe disease management1. An increase in high-mortality diseases means that there is a growing demand to fill the gap between the arsenal of highly potent active agents in our possession and the significant compromise in quality of life with the traditional treatment options2,3. This gap has driven innovation and is fuelling the growth of new technologies for drug delivery — the US market alone is projected to reach several hundred billion dollars by 2021 (REF. 4). Equipping an active pharmaceutical agent with the capacity to overcome physiological obstacles and carry out its function with maximum efficacy comes with considerable design challenges5,6. Macromolecular nanocarriers have the potential to revolutionize therapeutics7, and have provided the scientific community with a platform that has the capacity and potential to evolve with the expanding knowledge and understanding of diseases8,9. It can be tailored to different drug delivery mechanisms and can adapt to the challenges accompanying the delivery of therapeutics. Nanomedicine has witnessed substantial growth over the years (FIG. 1), from micelle-based formulations prepared by the polymerization of monomers that are stabilized in solution using surfactants, to smart and innovative technologies that can deliver small to large molecules by conjugation, encapsulation and combinations thereof, through various administration pathways, including intravenous, local, oral, pulmonary, transdermal and transmucosal, and by responding to varied stimuli10,11. Considering the rapid development of methods for the synthetic elaboration of macromolecular architectures12, it is becoming increasingly easy to improve the shortcomings of well-studied technologies and address the emerging need to perform multiple functions using the same scaffold12,13. As the parameters of maximum efficacy in drug delivery are better understood14, a diverse range of macromolecular structures have been developed. Transporting drugs across various biological barriers15,17 (for example, the skin for topical treatments and the gut-blood barrier for orally dosed drugs) is one of the biggest challenges. Attempts to address this problem has seen the emergence of branched (miktoarm stars) and hyperbranched (dendrimers) macromolecules18. Herein, we present a brief overview of material classes used in therapeutic formulations with the intent of providing a roadmap for the development of new clinical materials.
Figure 1 |. Analysis of macromolecular structure evolution in drug delivery.
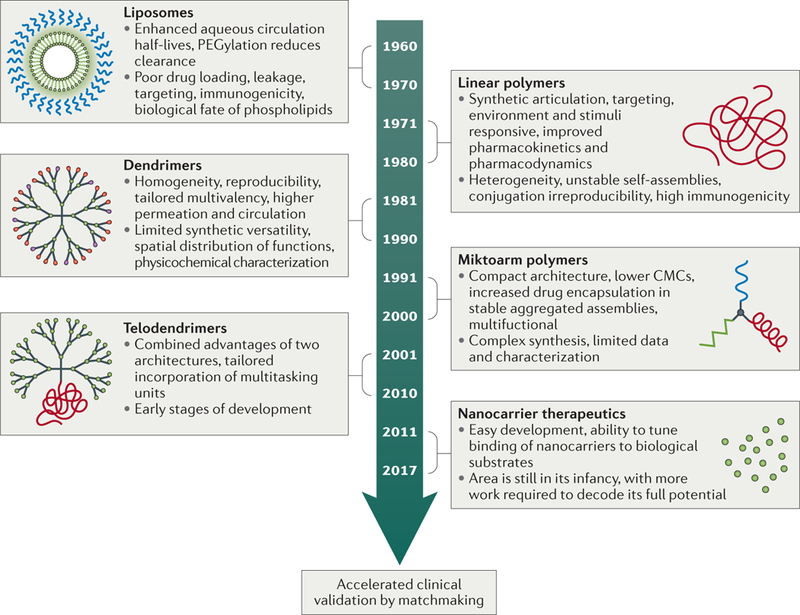
Nanomaterials have had a keyrole in delivering active pharmaceutical agents to the diseased site. Here, we provide a brief summary of the timeline, advantages and disadvantages of each category of nanocarriers, ranging from early discoveries of phospholipids and linear polymers to branched and hyperbranched macromolecules, hybrids thereof, drug-free macromolecules as therapeutics themselves, and strategies to accelerate bench-to-bedside transition. CMC, critical micelle concentration; PEG, poly(ethylene glycol).
Before developing an understanding of how increasing the macromolecular complexity can improve the delivery of active therapeutic agents, it may be advantageous to briefly review the basic requirements of designing a nanocarrier for systemic delivery19–21. First, the nanocarriers should be able to carry a sufficient dose of a hydrophobic or hydrophilic drug and remain in circulation in a physiological medium with tunable leakage. Second, it should target, accumulate and distribute at the desired site in anatomic as well as subcellular compartments. Last, it should be biocompatible. In simplistic terms, a drug delivery vehicle has to solubilize the active pharmaceutical agent, tailor interactions with cells to maximize drug uptake by mechanisms that include, for example, adsorption or endocytosis, and minimize elimination or degradation of its contents before reaching its target. In addition, the drug delivery vehicle should be non-immunogenic, with a cost-effective synthesis that is easy to scale up.
Drug delivery systems
There has been significant effort devoted to designing efficient nanodevices (for example, liposomes, niosomes and solid-lipid nanoparticles) for delivering pharmaceutical agents. Although not strictly macromolecular carriers, liposomes are some of the most extensively investigated drug delivery vehicles and have seen much success in clinics. It is thus appropriate to briefly discuss their use before the discussion of macromolecular nanocarriers.
Liposomes
Liposomes, formed by the self-assembly of amphiphilic phospholipids, have been explored for drug delivery for more than 50 years22. These thermodynamically stable spheres can encage both hydrophilic and hydrophobic drugs, and have become established for their use in clinically approved formulations23. Liposomes have offered distinct advantages and considerable promise in delivering a plethora of otherwise inefficient drugs by modifying their physicochemical properties and biodistribution, and by reducing drug toxicities24. PEGylation (PEG, poly(ethylene glycol)) is a commonly adopted technique for imparting stealth properties, and PEGylated liposomal nanoparticles have generally been considered to be benign and inert carriers25. However, it has been found that PEG itself can elicit an immunogenic response26. More recently, anti-PEG antibodies have been described as a new platform that can further enhance the efficacy of liposomal formulations27,28 by targeting the liposomes towards specific cell types.
There has been immense activity in understanding the main principles of liposomal drug delivery systems and in enhancing their scope and applications by tailoring their preparation method, composition and surface decoration29. The collective scientific efforts surrounding liposomal preparations have culminated into several new nanomedicines30, which are either clinically approved or in clinical trials for the treatment of cancer26,29 (TABLE 1 ). In addition, liposomal technology has also been used for fungal and bacterial infections, as well as for gene therapy27,30.
Table 1 |.
Examples of nanoformulations in clinics and clinical trials
| Trade name | Drug | Formulation | Use |
|---|---|---|---|
| In clinics | |||
| Myocet | Doxorubicin | Liposomal | Metastatic breast cancer |
| Doxil | Doxorubicin | PEGylated liposomal | Leukaemia, lymphoma, carcinoma and sarcomas |
| DaunoXome | Daunorubicin | Liposomal | Kaposi sarcoma, leukaemia and non-Hodgkin lymphoma |
| DepoCyt | Cytarabine | Liposomal | Lymphomatous meningitis |
| Marqibo | Vincristine sulfate | Liposomal | Lymphoblastic leukemia |
| Visudyne | Verteporfin | Liposomal | Macular degeneration |
| Abraxane | Paclitaxel | Albumin-bound paclitaxel | Breast cancer and metastatic adenocarcinoma of the pancreas |
| Adagen | ADA | Linear polymer: monomethoxypoly(ethylene glycol)-ADA conjugate | Enzyme replacement therapy for severe combined immunodeficiency |
| Copaxone | Glatiramer acetate | Random copolymer of amino acids: glutamic acid, lysine, alanine and tyrosine | Multiple sclerosis |
| Oncaspar | L-Asparaginase | PEGylated L-asparaginase | Lymphoblastic leukemia |
| Pegasys | Interferon alfa-2a | PEGylated interferon | Hepatitis B and hepatitis C |
| Renagel | Sevelamer hydrochloride | Poly(allylamine-co-N,N’-diallyl-1,3-diamino-2-hydro xypropane) hydrochloride | Hypocalcemia and chronic kidney disease |
| Clinical trials | |||
| Aroplatin | Bis-neodecanoate diaminocyclohexane platinum |
Liposomal | Mesothelioma and colorectal carcinoma |
| Atragen | All-trans-retinoic acid | Liposomal | Promyelocytic leukemia and hematologic malignancies |
| Lipoplatin | Cisplatin | Liposomal | Non-small cell lung cancer adenocarcinomas |
| LEP-ETU | Paclitaxel | Liposomal | Breast, lung and ovarian cancer |
| Onco-TCS | Vincristine | Liposomal | Relapsed non-Hodgkin lymphoma |
| OSI-211 | Lurotecan | Liposomal | Head, neck and ovarian cancer |
| SPI-077 | Cisplatin | Liposomal | Head, lung and neck cancer |
| Narekt-102 | Irinotecan | PEGylated liposomal | Breast and colorectal cancer |
| Livatag | Doxorubicin | Poly(isohexylcyanoacrylate) | Liver cancer |
| Paclitaxel poliglumex | Paclitaxel | Poly(L-glutamic acid)-paclitaxel conjugate | Breast, lung and ovarian cancer |
| Paclical | Paclitaxel | Micelles from surfactant-based derivative of retinoic acid (XR-17) | Breast, lung and ovarian cancer |
| PEG-docetaxel | Docetaxel | PEGylated docetaxel | Solid tumours |
| VivaGel | SPL7013 astodrimer sodium | Polylysine dendrimer | In phase III trials for the treatment and prevention of bacterial vaginosis |
| DEP | DEP docetaxel | Polylysine dendrimer-docetaxel | In phase I clinical trials for the treatment of breast, lung and prostate cancer |
ADA, adenosine deaminase; PEG, poly(ethylene glycol).
Despite the wide-spread demonstration of versatility in composition, preparation methods and administration routes, of translocation across several barriers and of success in the clinical translation of liposome-based nanotechnology31–33, some basic challenges still remain, such as poor understanding of the biological identity of liposomes and difficulties in structural design. The inability to load sufficient cargo in liposomal formulations, combined with leakage, results in only a very small amount reaching the target34. The formation of the protein corona at the surface of liposomes (even for those that are PEGylated) can change the drug-release profile of the liposomes in vivo. To address these limitations, including fabrication irreproducibility, a different synthetic polymer platform is required. In addition, a careful re-evaluation of the assumed non-cytotoxicity and immunogenicity, as well as the biological fate of phospholipids, is warranted.
Polymers for drug delivery
Natural polymers.
Although the focus of this Review is to evaluate the evolution of structural complexity in synthetic macromolecular nanocarriers, natural polymers have been successfully used in the clinic and are thus worthy of brief mention35. Naturally occurring proteins and polysaccharides have been extensively explored as matrix-based nanoparticles for drug delivery owing to their inherent properties, including biocompatibility, degradability and ease of surface modifications36. There are several protein-based nanoparticles of interest; however, albumin and gelatin are the two most widely studied systems. Albumin is a versatile protein with high aqueous solubility and stability, multiple binding sites and reactive surface functional groups, which make it an attractive nanocarrier for drug delivery37. Abraxane ‒ an albumin-bound nanoparticulate formulation of paclitaxel — is a marketed product in the fight against cancer. It is highly soluble in water and accumulates in tumours using mechanisms that include the enhanced permeation and retention (EPR) effect, as well as the albumin transport pathway38. Natural polymer-based nanocarriers offer considerable advantages, and there is much need to expand their scope and develop multitasking nanomaterials. These systems offer great potential that is yet to be explored. With a large pool of data becoming available on new protein-based nanocarriers, more efficient smart nanotechnologies for drug delivery are expected to emerge.
Synthetic polymers.
To facilitate the delivery of a range of small molecules, proteins and nucleic acids, it is essential to be able to engineer the characteristics of polymer-based nanostructures1,9,11,39. Tremendous effort has gone into deciphering how supramolecular assemblies of linear amphiphilic polymers and polymer conjugates can be optimized for specificity40, improved bioavailability, reduced toxicity and desirable pharmacokinetics41. Understanding the interplay between composition and surface properties of such polymers, as well as biology, continues to provide impetus for designing new and improved technologies42–44.
Macromolecule-based drug delivery has come a long way, from humble beginnings using simple and easily accessible materials, to state-of-the-art tailored compositions that take advantage of the diversity of linear, branched and hyperbranched architectures, and hybrids thereof, that are now available45–54. The optimization of the overall structure and properties of these polymers has been achieved by chemical innovation. A tremendous amount of effort has gone into the development of efficient methodologies for polymer synthesis, including, for example, living anionic polymerization, controlled free-radical polymerization (atom transfer radical polymerization (ATRP) and reversible additionfragmentation chain transfer (RAFT)), ring-opening polymerization (ROP), and ring opening metathesis polymerization (ROMP)55–57. A brief outline of these procedures is provided in FIG. 2. Of the available methods for controlled free-radical polymerization methods, RAFT is becoming increasingly popular in synthesizing amphiphilic block copolymers with narrow poly- dispersities, which could encapsulate chemotherapeutics into their core upon self-assembly. An ABC-type triblock copolymer, poly(ethylene glycol)-b-poly(2,4,6-tri- methoxybenzylidene-1,1,1-tris(hydroxymethyl)ethane methacrylate)-b-poly(acrylic acid), was prepared by using PEG-cyanopentanoic acid dithionaphthalenoate as the RAFT agent, and by sequential addition of 2,4,6-tri-methoxybenzylidene-1,1,1-tris(hydroxymethyl)ethane methacrylate and acrylic acid monomers58. Upon aqueous self-assembly, the polymeric vesicles were found to be highly efficient in loading and in pH-dependent intracellular release of doxorubicin hydrochloride. A combination of methodologies provides an ideal platform for the synthesis of complex architectures. Sequential ROP of different monomers, or the combination of ROP with controlled radical polymerization, has been used for the synthesis of miktoarm polymers. Ring opening of 2,2-bis(hydroxymethyl)propionic acid coupled to PEG, with an amine functionalized alkoxy amine, followed by sequential ROP of l- and D-lactides, was used for the synthesis of amphiphilic ABC miktoarm polymers59. The branched polymers, poly(ethylene glycol)-poly(D-lactide)-poly(L-lactide), contain two complementary polymeric arms that are capable of stereoselective interaction. In an aqueous medium, these miktoarm polymers formed stable micelles with a low critical micelle concentration (CMC), and were highly efficient in the encapsulation and sustained release of paclitaxel. The development of ‘click’ chemistry, including alkyne-azide cycloaddition, Diels-Alder reaction, thiol-ene addition60, and other coupling strategies such as thiol-ene Michael addition61, has further increased the diversity of macromolecular architectures that can be accessed. Alkyne-azide cycloaddition is one of the most extensively explored click reactions for the modification of pre-formed macromolecules as well as their synthesis62. Diels-Alder [4+2] cycloaddition has been used to construct a range of macromolecules, and its thermosensitive reversibility has provided a platform to develop degradable nanocarriers for drug delivery63. Cycloaddition reactions have also been used extensively to modify small-molecule inhibitors64,65. The combination of Huisgen alkyne-azide cycloaddition with reversible Diels-Alder adduct formation between furan and maleimide was used to synthesize dendrimers that underwent retro-Diels-Alder disassembly, and that released a surface-functionalized anti-inflammatory drug (lipoic acid) within the physiological and pathological range of temperatures (37–42 °C)66. Thiol-ene coupling is another highly versatile reaction that can be performed under various conditions. It has been used for synthesizing dendrimers in a divergent methodology, starting from a 2,4,6-triallyloxy-1,3,5-triazine core and reacting it with 1-thioglycerol. The solvent-free reaction is initiated with 2,2-dimethoxy-2-phenylacetophenone, and iterative growth is continued by the subsequent generation of terminal alkene moieties on the surface through the addition of 4-pentenoic anhydride; the process is then repeated67. Metal catalyst-free methodology is particularly attractive for developing drug delivery nanocarriers. Thiol-ene addition was used to functionalize a PEG-peptide telodendrimer with carboxylic groups for covalent linking of cisplatin68. The linear dendritic block copolymer could co-deliver cisplatin with encapsulated paclitaxel with variable concentrations of the combination drugs.
Figure 2 |. Macromolecular architectures in drug delivery and examples of the methodologies for their synthesis.
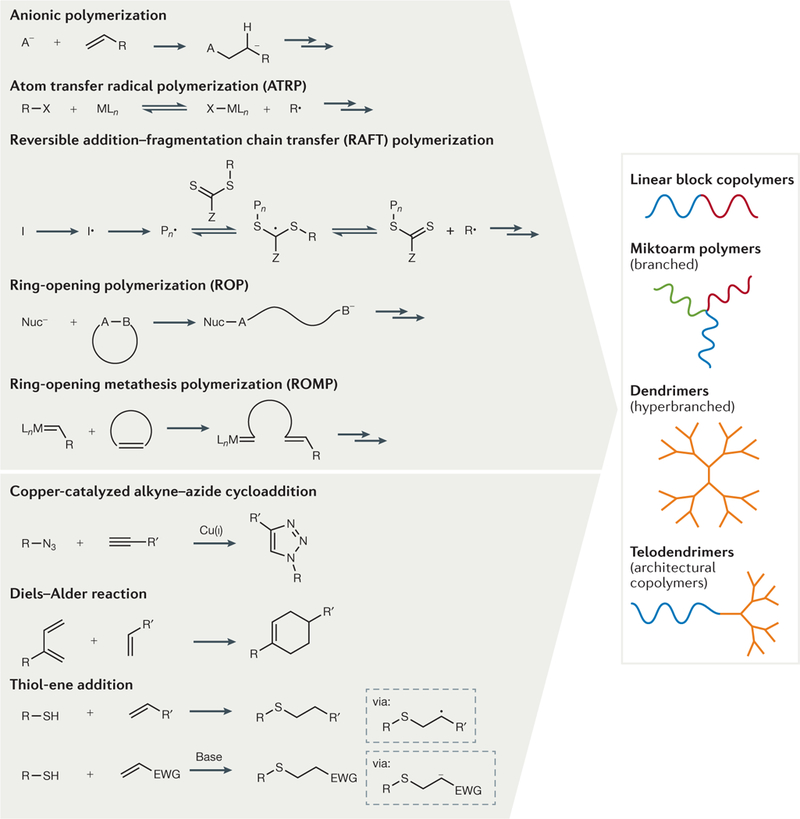
Some of the common synthetic methods (upper left panel), and chemical reactions and coupling strategies (lower left panel) that have been used for the synthesis of linear, branched and hyperbranched macromolecules and hybrids thereof (right panel) are shown. EWG, electron-withdrawing group; L, ligand; M, transition metal; Nuc−, nucleophile.
The versatility of the chemical approaches described in FIG. 2 is key in adjusting the self-assembly behaviour of macromolecular materials with the goal of optimizing loading and release characteristics, as well as keeping them intact and in circulation (by modulating the CMC). The diversity of structures made available by simple and scalable synthetic procedures is helping to address the challenge of designing well-defined constructs that incorporate a balanced combination of functions and that can perform predetermined multiple tasks cooperatively and efficiently69.
Polymeric nanocarriers are being used to address the key issues in drug delivery: loading a sufficient dosage of the active cargo, protecting it from the surrounding environment in vivo, and steadily releasing it at the targeted site without eliciting systemic toxicity (FIG. 3). Many previous studies have focused on characterizing the transport and release mechanisms of these nanocarriers70,71. Considering only the polymeric nanoparticle and drug conjugate-based systems, the release is generally guided by diffusion of the encapsulated drug from its reservoir, erosion of the polymeric nanocarrier (or combinations thereof), or degradation of the carrier-drug links. Thus, for polymeric nanocarriers, drug release can be controlled by diffusion, or by activation of the polymer matrix by solvents, local chemistry or external parameters such as pH or temperature72,73. The composition and morphology of the polymeric nanocarrier can be expected to majorly influence the release kinetics and pharmacokinetic profile of the drug74,75.
Figure 3 |. Common drug delivery methods: oral, intravenous, transderm alandlocal administration.
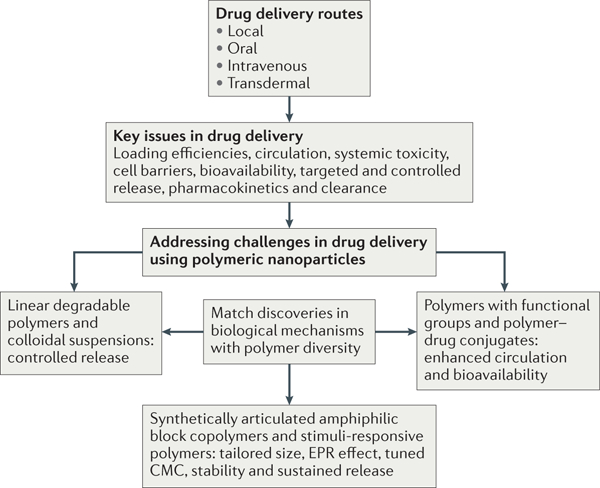
The challenges that we face in our efficacy in treating a particular disease can be resolved by using our understanding of the local biology in designing a suitable macromolecule-based nanocarrier for drug delivery. Advances in synthetic methodologies have allowed the design of linear polymers that can increase blood circulation, bioavailability, active and passive (via the enhanced permeability and retention (EPR) effect) targeting, controlled release, and that can respond to various external stimuli. The CMC refers to the critical micelle concentration of polymeric assemblies from amphiphilic polymers, and it is an important parameter that determines the in vivo stability of drug-loaded micelles.
Colloidal suspensions.
Colloidal nanocarriers that follow a degradation mechanism have been extensively explored for drug delivery. Linear, commercially available polylactide and poly(lactide-co-glycolide) (PLGA), which degrade through hydrolysis of ester linkages, are two of the most commonly used synthetic polymers76,77. This technology offers potential in controlling the drug release profile and minimizing toxicity owing to the bio-degradability and biocompatibility of the matrix (FIG. 3). Despite advances in the microparticle formulations of these polymers, significant success at the nanoscale has not been achieved. Several new polymers containing functional groups that might follow a similar surface or bulk hydrolysis degradation pathway have been prepared using highly efficient synthetic methodologies, some of which are described in FIG. 2: ROP for poly (caprolactone), poly(anhydrides), poly(phosphazenes), poly(phosphoesters); anionic polymerization for poly (cyanoacrylates); and transesterification of orthoesters with diols for poly(orthoesters). Some issues that have limited the clinical translation of PLGA-based formulations to drug delivery are reproducibility and scalability of polymer synthesis, variability in nanoencapsulation methods, toxicity due to premature and excessive release of therapeutic cargo from nanoparticles, and polymer interactions with the encapsulated drug. New synthetic biodegradable polymers have offered promise in addressing these concerns, and some are at different stages of clinical development78–83. Poly(orthoester) IV is one of the members in its family that can be synthesized with reproducible control of its drug release and erosion rates. Injectable formulations of semi-solid poly(orthoe-ster) that were synthesized from a diketene acetal and triethylene glycol or 1,10-decanediol have been evaluated in clinical trials78. The synthesis of poly(alkyl cyanoacrylates) has the potential to be scaled up, and BioAlliance Pharma (France) has pursued poly(hexyl cyanoacrylate)-based nanotechnology for doxorubicin (Doxorubicin Transdrug)84. The bulk erosion properties of PLGA nanocarriers have been well studied, and the resulting non-toxic products (that is, lactic acid and glycolic acid) can be eliminated by the body’s metabolism85–87. However, there is much to be understood in terms of the biological fate of the degradation products from the new polymeric systems.
Polymeric micelles
Amphiphilic block copolymers.
Synthetic articulation (that is, the chemical manipulation of separate polymer blocks) in polymer fabrication has led to the development of linear amphiphilic block copolymers. These macromolecules can self-assemble into a range of architectures, including micelles, depending on the medium. Pharmaceutical agents are often poorly soluble in water; therefore, polymeric micelles with a hydrophobic core can encapsulate drugs efficiently, while presenting a hydrophilic corona to interact with the biological medium. This synthetic polymer platform addresses the key issues noted before for liposomes86–88. The choice of hydrophobic blocks (for maximum efficiency in drug solubilization) and hydrophilic blocks (for stealth and enhanced circulation) is made to balance the competing demands of drug loading capacity and controlled/ sustained release at a specific gastrointestinal region for maximum bioavailability with oral delivery89. One of the important parameters of micelle formation is the CMC ‒ the concentration beyond which the polymeric chains associate to minimize the free energy ofthe system. CMC is directly related to the stability of the self-assembled structures, and a high CMC will imply disassembly upon dilution in biological fluids90. Synthetic advances in controlled radical polymerization methods and click chemistry (FIG. 2) have helped to expand the complexity of available macromolecules and to tailor their selfassembly to more well-defined structures. For example, RAFT polymerization has offered an avenue for developing block copolymers with precisely controlled polymer block lengths that could influence the CMC of the resulting micelles — an important parameter for the stability of drug-loaded nanocarriers91. Despite a large collection of amphiphilic block copolymer architectures at the disposal of chemical engineers, much work in drug delivery has been carried out using only amphi-philic diblock (AB) and triblock (ABA) polymers. PEG is the most commonly used hydrophilic block in these systems owing to its high affinity for water and non-toxic nature88. Significant success has been achieved in reaching the goal of solubilizing lipophilic drugs using amphiphilic block copolymers. However, important issues related to instability over long periods of time, the short duration of sustained release and poor bioavailability still remain.
Sustained release.
The release of cargo from a polymeric assembly is strongly influenced by the CMC (which determines the overall stability of the structure in the biological medium) and by the strength with which drug molecules are bound in the core. Both are dependent on the amphiphile structure92. A lower CMC is desirable for sustained release. One way to achieve this is to increase the hydrophobic content of the copolymer93, but using new polymeric combinations in diblock (poly(ethylene glycol)-b-poly(valerolactone), poly(phos-phazenes)-b-poly(N-isopropylacrylamide)) and triblock (polylactide-b-poly(ethyleneoxide)-b-polylactide) copolymers has also sucessfully reduced CMCs94,95. For example, the CMC of self-assembled structures obtained from a diblock copolymer that was prepared using ROP of valerolactone initiated by PEG could be fine-tuned by increasing the molecular weight of the poly(valerol-actone) fragment94. Slow release of a drug from a micelle can be engineered by increasing the strength of interactions between the drug molecule and the hydrophobic core83,95. Micelles obtained from copolymers containing the same hydrophilic blocks (PEG) but different hydrophobic segments of similar chain length, poly (caprolactone) (PEG-b-PCL) and poly(L-lactide) (PEG-b-PLLA), were shown to have different drug-loading capacity. In one study using the drug quercetin96, the loading capacity of micelles made from a PEG-b-PLLA polymer was found to be higher than that for micelles made from a PEG-b-PCL polymer. It was found that the drug interacted most strongly with the PLLA core through hydrophobic interactions, whereas in the PCL-based copolymer, the drug interacted mainly through hydrogen bonding.
Responsive micelles for better bioavailability.
The desire to deliver pharmaceutical agents to the site of action through several different mechanisms and in doses that will maximize efficiency requires manipulation of polymer composition and has led to the development of ‘smart polymers’ (REF 97). These macromolecules can sense biological environmental changes and respond to various physical and chemical stimuli — including, for example, pH, temperature, ultrasound and ionic strength — by altering their physicochemical proper-ties98,99. Systems that can respond to changes in pH offer opportunities to maximize absorption at a defined point along the gastrointestinal tract, or at inflamed and cancerous tissue where the physiological pH is known to differ from surrounding tissue. Numerous copolymers that incorporate a pH-responsive block, and thus enhance the bioavailability of drugs through this mechanism, have been synthesized. For example, using PEG as a hydrophilic macroinitiator, block copolymers containing variable lengths of hydrophobic fragments, poly(ethylene glycol)-b-poly(alkyl acrylate-co-methacrylic acid) were prepared using ATRP. The presence of pendant COOH groups in these polymers led to pH-dependent aggregation, and their critical aggregation behaviour was found to be tunable by varying the hydrophobic chain length100. Drug release from these aqueous assemblies could also be controlled by pH, and increased upon the change from highly acidic to basic medium. This strategy of using pendant acidic groups to produce pH-responsive polymers has been extensively used for controlled release of encapsulated cargo. For example, micelles that could respond to pH changes along the gastrointestinal tract were synthesized by copolymerization of acrylic acid with poly(ethylene glycol)-b-(4-(2-vinylbenzyloxy) -N,N-(diethylnicotinamide))101, and a multifunctional micelle system with varied pH sensitivity under different environments was prepared from a mixture of two block copolymers, poly(L-histidine)-b-poly(ethylene glycol) and poly(L-lactide)-b-poly(ethylene glyclol)-b-polyhistidine102. Poloxamers are block copolymers containing a hydrophobic central segment, end capped with hydrophilic blocks. The self-assembly behaviour of such block copolymers containing PEG and poly (propylene oxide) in an ABA composition is temperature sensitive. The CMC, which decreases with temperature, and thermoreversible aggregation (gelation) of these assemblies have been utilized for the controlled release of drug cargo103. Block copolymers ofpoly(N-iso-propylacrylamide) and PEG, and polylactide-b-poly (ethylene glycol)-b-polylactide have also been widely studied as thermoresponsive systems104. Micelle formulations based on thermosensitive polymers, such as non-ionic pluronics (for example, SP1049C developed by Supratek Pharma for the delivery of doxorubicin) have shown great promise in clinical trials for the treatment of gastric and oesophageal cancer105. Poly (N-isopropylacrylamide) (PNIPAM) has a low cloud point (32 °C) and is insoluble at body temperature in an aqueous medium. In block copolymers containing hydrophilic PEG, this temperature-dependent solubility allows the facile preparation of micelles, which could deliver their cargo at the cancerous site using local hypothermia106. In general, for in vivo applications, the lower critical solution temperature (LCST) of PNIPAM needs to be raised to avoid aggregation of micelle formulations upon administration. Much effort has been devoted recently to fine-tune the LCST of PNIPAM by introducing hydrophilic segments in its block copolymers. Using free-radical copolymerization of N-isopropyl amide and acrylamide, followed by tin-catalysed ROP of lactide, the block copolymer poly(N-isopropylacrylamide-fo-poly(acrylamide)-fo-poly(D,L-lactide) had an LCST of 41°C (REF 107). The docetaxel-loaded micelles were thermosensitive, and the drug release could be triggered by hyperthermia. Similar strategies of adjusting the LCST by copolymerization with hydrophilic monomers have been used to prepare block copolymers poly(N-isoprpylacrylamide-N-hydroxymethylamide)-fo-poly(methyl methacrylate) (LCST 42.8 °C)108, and poly(N-isopropylacrylamide-N,N-dimethylacrylamide)-fo-poly(caprolactone) (40 °C)109. Advances in synthetic methodologies have made it possible to tailor triggering parameters, such as pH and temperature, in polymeric assemblies. Only through more systems getting to clinical evaluations can such adjustments be assessed for reaching the peak of their efficacy.
Polymer-drug conjugates.
Chemically linking the drug to a polymer can influence its stability, solubility and bioavailability, and this concept has been extensively explored110. With the guidance provided by the work of Ringsdorf111, and the other research that followed, it was suggested that polymer-drug conjugates can help to address several of the key issues facing drug delivery, including long blood circulation times, targeting, accumulation and retention (FIG. 3). The concept of solubilizing the drug by chemically linking it to a macromolecule has been widely applied112,113, with PEGylation being the most commonly used method. Several such conjugates have successfully made it into the clinic or are currently under clinical evaluation (TABLE 1). Progress in this area is dominated by, and will continue to be made, using synthetic evolution114 — especially in linker methodologies, for example, click chemistry115,116 (FIG. 2). It is expected that the development of new conjugates that exploit our increasing knowledge of biology at disease sites can help to advance the field117.
Clinical translation
Since the inception of the field of nanomedicine, there has been an expectation that parallel advances in disease understanding and nanocarrier engineering would lead to rapid progress in the development — both preclinical and clinical — of nanoparticle-based drug formulations118. Clinical translation is a complex and time-intensive process, and a recent review article highlights these challenges119. An overview of nanoparticle-based drug delivery vehicles that have been commercialized, as well as candidates that have demonstrated promise in clinical trials, shows that these are dominated by liposomal for-mulations119–121 (TABLE 1). Polymers have offered synthetic versatility, tunability, adaptability and have been explored for a very long time. Their modest bench-to-bedside translation can be criticized, but the cautious approach towards their use must also be commended. One of the main challenges today is that when systems fail, we rarely know why. The use of new polymers in nanomedicine requires a detailed investigation of their toxicity. In general, the interaction of nanoparticles with biological systems in the human body has not been fully explored and is currently poorly understood. We believe that a deeper understanding of the biological identity of nanoparticles is needed for more predictive clinical translation122. The evolution of available synthetic methods has produced a diverse library of these macromolecules; however, only a handful of these have been intensely investigated and ultimately approved for use. For example, polymer-drug/ antibody/protein conjugates may be easily accessible with advances made by chemists and may perform admirably in laboratory settings. However, even when an already approved pharmaceutical is modified in this way, a further lengthy testing protocol is required.
The slow progress to clinical trials can be difficult to evaluate. It is entirely expected that a higher burden of proof is placed on formulations to be used in the clinic. It is clear that scaling up synthesis to the quantities needed with quality controls and batch-to-batch reproducibility, while obtaining detailed toxicity and safety profiles, are just the basic requirements. Nanocarriers need to cross several complex biological barriers and reach the target site. Addressing these challenges with a detailed evaluation is crucial for the successful clinical translation of any drug delivery vehicle123. In addition, the small-animal model studies that are used as clinical benchmarks may be of limited scope, and effects may not be reproduced in human patients because of far more individual complexity and disease diversity. Future studies may need to be evaluated on a broader animal platform.
Polymers have evolved synthetically, but their versatility is evaluated by chemical engineers, who consider how the strengths and failures of each can guide the successful implementation of these polymers into technology in the future124,125. Imaging at the single cell level has had an important role in deciphering how nanomaterials work and fail in vivo126. As the biological mechanisms of debilitating diseases, su ch as cancer, become better understood, the arsenal of available polymer structures will need to increase further to match our expectations.
Emerging macromolecular complexity
The limited clinical translation of polymeric systems that have proved successful in vitro has engaged chemists, biologists and engineers in determining the key issues that lead to lower efficacy in vivo127. Significant effort is being made to fully explore the roles of nanostructure assembly, morphology, surface characteristics, mode of delivery and intracellular accumulation128,129. For their part, chemists have developed synthetic routes to more elaborate and adaptable structures that could provide further flexibility in engineering smart nanomaterials130.
Studying the variety of linear macromolecules used in polymer therapeutics, it is increasingly clear that their compositions must be carefully assessed to tune their efficacy as drug carriers. The molecular weight and overall shape can significantly influence their biological interactions and cellular uptake mechanisms. New platforms with variable self-assembly characteristics, enhanced drug-loading capacity, plasma stability, and which can incorporate desirable traits such as multivalent surfaces or homogeneity, may offer promise for new delivery vehicles131. This can be achieved by the evolution of polymer structure from linear to branched and hyperbranched architectures with unique and tailored physical and biomedical properties132,133. The latest developments in synthetic methodologies have resulted in the production of nanocarriers for drug and gene delivery, and in the introduction of multiple functions into a single scaffold52,134.
Branched polymer nanotechnology
The application of branched and hyperbranched polymer structures in drug formulations, relative to their linear counterparts, remains in its infancy135. These polymer architectures offer the potential to tailor the dispersity and functionalization of structural compositions, and to introduce multifunctional character using orthogonal chemistries136. With rapid progress in synthesis for easy access and large-scale production137, there exist clear opportunities for such structurally flexible macromolecules in addressing key issues related to drug delivery and theranostics.
Miktoarm polymers
The lessons learnt while studying polymeric micelles suggest that there are still some basic issues related to the efficiency of drug incorporation, stability (disassembly) of the nanocarrier while travelling in a biological medium and its release at a specific site. These may be addressed by designing new carriers in which the chemical structure of the polymers and hydrophobic core, and the CMC of the resulting micelles, can be tailored. Branched architectures, such as miktoarm polymers, constitute one such example of macromolecules, in which a number of polymeric arms with different (desired) chemical compositions, molecular weights or functions, are tethered to the core, resulting in unique physicochemical properties. These polymers have been prepared using similar methodologies that have been widely explored in the synthesis of linear block copolymers138–140 (FIG. 2). Owing to their shape and programmable amphiphilic architecture, a range of self-assemblies of structural diversity and complexity, including micelles, compartmentalized micelles and vesicles (polymersomes), have been fabricated139–144 (FIG. 4). Although a relatively new area of research, synthetic evolution has made such structures accessible for detailed investigation. It is clear that self-assemblies from miktoarm polymers have narrow size distributions and lower CMCs, which could translate into higher stability in physiological medium, and better loading efficiencies and release characteristics145,146.
Figure 4 |. Self-assembled structures from an ABC-type amphiphilic miktoarm polymer.
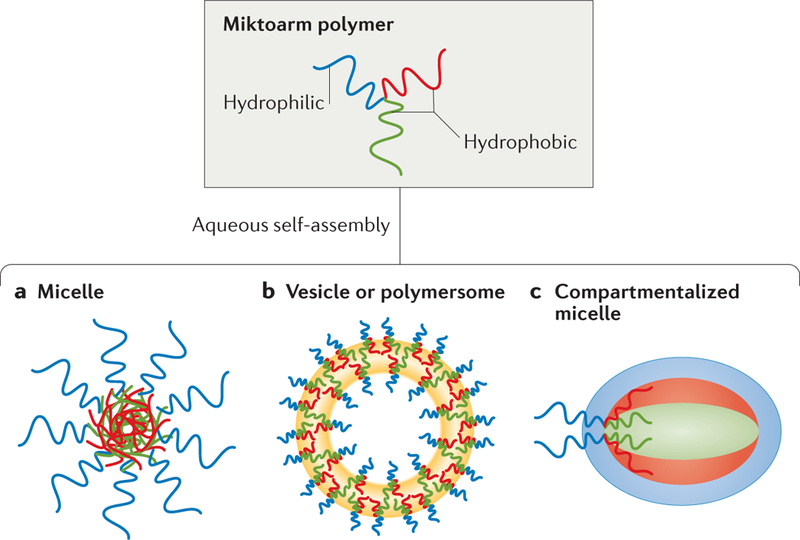
In this ABC-type amphiphilic miktoarm polymer, A is hydrophilic, and the B and C arms have variable hydrophobicity. a | Mitkoarm polymers can form spherical aggregates, known as micelles. b | Mitkoarm polymers can also form vesicles (hollow spheres) or polymersomes (polymer-based liposomes). c | A compartmentalized micelle ‒a spherical aggregate in which each arm of the miktoarm polymer leads to its own hydrophilic and hydrophobic regions — can also be assembled.
Because it is the branched architecture that is being evaluated in developing more efficient nanoformulations, using polymeric arms that are composed of US Food and Drug Administration (FDA)-approved materials may help to expedite their clinical implementation. Miktoarm polymers that are being explored for drug delivery often incorporate such FDA-approved or biocompatible polymers, including PEG, PCL and polylactides, into their arms. Such amphiphilic AB2- or ABC-type architectures have been synthesized from a core on which sequential coupling of PEG arms of predetermined molecular weights, using copper catalysed alkyne-azide click chemistry, is followed by ROP of caprolactone (FIG. 5a). These miktoarm polymers have shown promise in enhancing solubility and drug loading efficiencies of lipophilic drugs, such as nimodipine and curcurmin147,148, and in targeting specific cell organelles149. These interesting properties of branched macromolecules have been further extended with synthetic elaboration into stimuli- responsive systems. A3B3C3-type miktoarm star polymers were synthesized using a combination of ROP and activators regenerated by electron transfer atom transfer radical polymerization (ARGET ATRP)150 (FIG. 5b). Selfassembled micelles from these pH-responsive miktoarm polymers could incorporate sufficient quantities of doxorubicin and were shown to change the morphology upon variations in pH, which led to an enhancement of the rate of doxorubicin delivery150.
Figure 5 |. Synthesis of architecturally complex branched macromolecular structures.
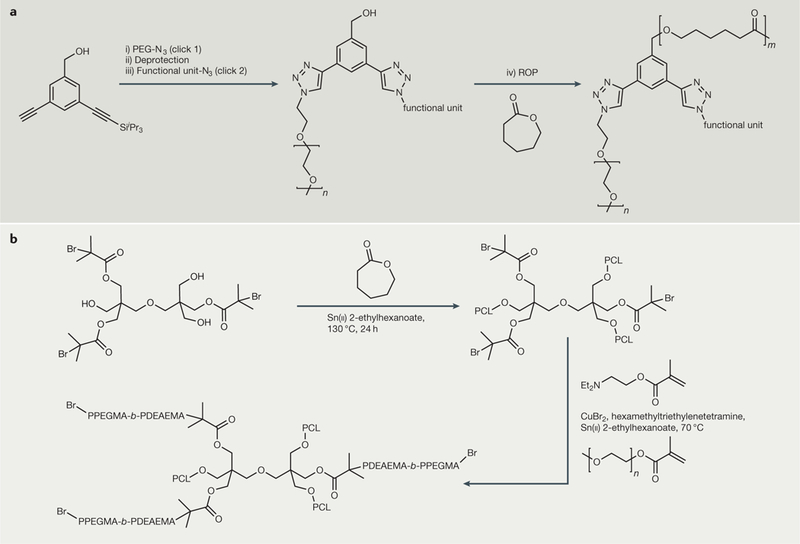
a | Synthesis of mikto arm polymers using combined copper-catalysed click and ring-opening polymerization (ROP) reactions. The core with three orthogonal functional groups (3-(triisopropylsilylethynyl)-5-ethynylbenzyl alcohol) was prepared from 3-bromo-5-iodobenzyl alcohol by sequential Sonogoshira coupling of triisopropylsilylacetylene and trimethylsilylacetyl- ene: (i) click 1 with PEG-N3, CuSO4-5H2O/sodium ascorbate, H2O/tetrahydrofuran (THF), room temperature, overnight; (ii) tetrabutylammoniumfluoride/THF, overnight; iii) click 2 with another functional entity with an azide group, CuSO4-5H2O/ sodium ascorbate, H2O/THF/dimethylformamide, room temperature, overnight; iv) toluene, Sn(ii) 2-ethylhexanoate, reflux, 24 h. b | Synthesis of A3B3 miktoarm polymer using ROP and continuous activators regenerated by electron transfer atom transfer radical polymerization (ARGET ATRP). The difunctional initiator, prepared from dipentaerythritol and 2-bromoisobutyryl bromide, was used to carry out sequential ROP of caprolactone. This was followed by continuous ARGET ATRP of 2-(diethylamino)ethyl methacrylate (DEAEMA) and poly(ethylene glycol) methyl ether methacrylate (PEGMA) in sequence to yield the miktoarm polymer. PCL, poly(caprolactone).
Using articulation of the available synthetic methodologies, the composition of miktoarm stars could be varied to develop multiple stimuli-responsive branched copolymers151,152 (FIG. 6). The flexibility in the fabrication of miktoarm polymers may also offer opportunities to combine polymer-drug conjugation with encapsulation, yielding smart nanoassemblies for multiple drug therapy153.
Figure 6 |. Examples of smart nanoassemblies constructed using a combination of differentsynthetic methodologies.
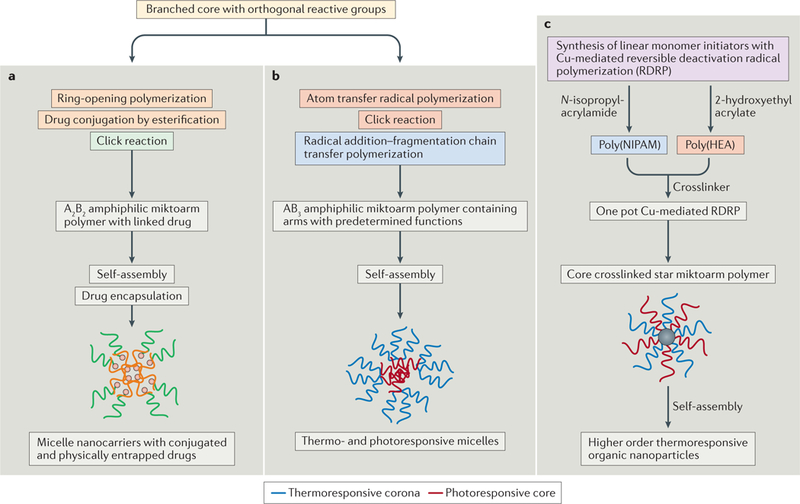
a | Starting with 2,2-bis(bromomethyl)propane-1,3-diol, and carrying out sequential reactions — i) ring-opening polymerization of caprolactone, ii) azidation of bromides at the core, iii) esterification to covalently link ibuprofen, iv) Cu-catalysed click reaction of alkyne-terminated poly(ethylene glycol) — led to the formation of an amphiphilic A2B2 miktoarm polymer with conjugated ibuprofen. This drug-conjugated polymer formed spherical micelles upon self-assembly in water, which showed enhanced efficacy in the encapsulation of ibuprofen. This methodology could be expanded to combination drug therapy. b | Azobenzene-functionalized methacrylate was polymerized using atom transfer radical polymerization on a core with three azide arms and one with bromoisobutyryl bromide. An alkyne-func-tionalized chain transfer agent was subsequently clicked to the core, followed by radical addition-fragmentation chain transfer reaction to give an AB3 miktoarm polymer. This then self-assembles into dual thermo- and photoresponsive micelles. c | N-isopropylacrylamide and 2-hydroxyethyl acrylate were first individually polymerized in an aqueous medium using Cu-mediated reversible deactivation radical polymerization (RDRP). The resulting solutions were subsequently mixed with N,N’-methylenebis(acrylamide) initiator, and RDRP continued to yield core crosslinked star mikotarm polymers. These easy-to-synthesize core crosslinked macromolecules self-assemble into stimuli-responsive higher order nanostructures.
Dendrimers
Detailed studies of therapeutics that use linear polymers have highlighted several common problems. Structural heterogeneities that arise from self-assembly result in differences in drug loading, and there is limited scope for drug conjugation and for introducing multiple functionalities. Dendrimers — constructed using controlled divergent and convergent reaction sequences — yield globular, well-defined, hyperbranched, monodisperse and multivalent architectures154; thus, they provide a new platform to address these issues155. In addition, dendrimers may offer enhanced permeation across barriers for better drug distribution because of their shape and multivalent surface156. Since the very first reports of poly(amidoamine) (PAMAM) dendrimers, a large range of synthetic methodologies have been developed, which have provided access to a plethora of backbones with any desired number of generations. In drug delivery, PAMAM dendrimers stand out as one of the most extensively explored dendrimers for applications in biology and are commercially available157.
The dendrimer architecture offers the potential for these systems to be used as unimolecular micelles for the encapsulation of drugs158. In addition, their multivalency can be exploited in dendrimer-drug conjugates by surface modification159. Considering the stability of unimo-lecular micelles at varying concentrations, dendrimers were initially considered an alternative to polymeric micelles, and several studies looked at encapsulating various drugs into the internal cavities of dendrimers160–162. However, difficulties with drug release, improved performance of micelles prepared from linear and branched polymers, and higher costs made these systems unattractive for further evaluation and translation into drug delivery technology.
Covalent linking of drugs to dendrimers is an area that has been well developed considering: the availability of various synthetic methodologies (including the chemical reactions and coupling methodologies described in FIG. 2) for dendrimer synthesis, as well as surface and internal functionalization; multivalency tailored to specific needs with generation number; and the possibility of controlled release using degradable linkers with drugs163,164. Much of the work in dendrimer-drug conjugation has been done on commercially available PAMAM dendrimers using a statistical approach to functionalizing the periphery of different generation dendrimers. This strategy has significant issues related to irreproducibility and heterogeneity in the formulation, which once again has hindered translation of this technology into clinical trials. The challenge as with drug encapsulation into dendrimers is one for chemists, and there have been several advances in the field in which new backbones and better linker chemistries have been developed165–168.
One area in which the traits of dendrimers might be more fully exploited is in the introduction of multiple functionalities for targeting, imaging and stealth. There are opportunities for chemists to expand the synthetic complexity of dendrimers using high-yield reactions166,167, such that they have a controlled spatial distribution of different functionalities at their surface. Combination therapies may also be improved by the ability to deliver a precise ratio of two different agents using the same scaffold. Higher generation dendrimers have been widely explored for introducing multiple functions on their surfaces. With the detailed analysis of dendrimer-based drug delivery, it is becoming clear that there may be issues related to homogeneity of higher generation dendrimers and to the statistical distribution of surface-bound moieties in post-functionalization methods, which lead to irreproducible pharmacokinetics. However, lower generations with well-defined and reproducible synthesis may be more useful for technological advances. New synthetic methodologies based on orthogonality of functional groups are beginning to emerge for the preparation of bi- and tri-functional dendrimers, which have shown promise in studies carried out both in vitro and in vivo168–172.
Several products based on dendrimers have been marketed18, including Ocuseal, SuperFect, Alert Ticket and Stratus CS. Examples of clinical translation of dendrimer- based nanotechnology are also beginning to emerge. For example, Vivagel, a polyanionic dendrimer-based topical microbicide developed by Starpharma, is currently in phase III trials for the treatment and prevention of bacterial vaginosis. Starpharma has also developed a dendrimer-based drug delivery system, DEP, for the anticancer drug docetaxel, which is in phase I clinical trials. The potential of dendrimers has not been fully exploited yet, and as the synthetic challenges facing dendrimer structure build-up and subsequent functionalizations are addressed, it is hoped that more products will enter clinical evaluations.
Telodendrimers
Linear, branched and hyperbranched macromolecu- lar architectures have offered distinct properties, which have been individually well explored for applications in biology. Attempts have also been made to address their limitations within their own space. However, the solution may lie in developing hybrid macromolecular architectures that could combine their useful traits and offer opportunities to collectively address challenges in drug delivery to advance the field at a fast pace. Architectural copolymers, also referred to as telodendrimers, are such macromolecules, in which the ease of synthesis and the self-assembly of linear polymers are coupled with the uniformity and multivalency of dendrimers173 (FIG. 7). The driving force for interest in telodendrimers for biological applications is the synthetic ability to tailor their chemical composition, molecular weight and number of generations of the hyperbranched block, and to control the morphology of the self-assemblies that result and their stability. Because the synthetic methodologies for constructing linear and dendrimer blocks of these copolymers are well established (FIG. 2), the evolution in architectural complexity oftelodendrimers has directly arisen from the ability to stitch dendrimer fragments together in a predictable and reproducible manner — that is, the development of click chemistry174. The entry of these architectural copolymers into the field of drug delivery is relatively new175. There have been some interesting studies that have explored the role of the morphology of linear-dendrimer block copolymers on the micelle assemblies and the effect that the latter has on drug encapsulation, circulation times and cellular uptake of the nanocarriers176–178. The scope of these hybrid materials can be expanded further by, for example, making them respond to external stimuli, including pH and temperature. Such smart hybrid materials will be of interest because the response could be triggered through changes in either block179. Much remains to be done in terms of understanding and exploiting the potential of these promising materials, and progress will come, once again, from synthetic expansion in laboratories, as well as recently explored computational design and combinatorial chemistry180.
Figure 7 |. Telodendrimers combine important characteristics of hyperbranched and monodisperse dend ronswith those of linear polymers.
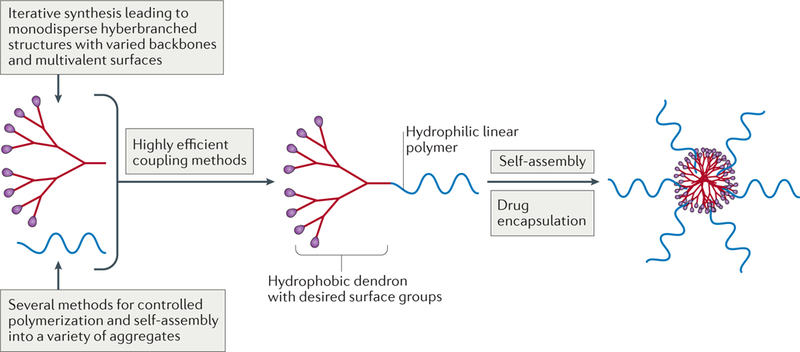
Dendrons and linear polymers with varied backbones can be synthesized using a large number of highly efficient synthetic methods described in FIG. 2. The multivalent surface of dendrons offers opportunities for conjugating any desired functional group, including therapeutics, imaging agents, and so on. Self-assembly into micelles then allows another drug to be physically encapsulated.
Macromolecules as therapeutics
Advances in understanding the interactions of polymers themselves with biological systems have given rise to increasing interest in developing novel methodologies to cure diseases. This has led to the emergence of a field commonly referred to as macromolecular therapeutics181,182. These macromolecules, which do not carry any encapsulated or covalently linked pharmaceutical agent, offer a new direction in combating ever-evolving pathologies183. Drug-free macromolecules in various shapes and sizes have demonstrated therapeutic efficiencies and have been suggested to be of particular value, because they do not carry pharmaceutical agents that could themselves invoke immune response and systemic toxicity. In some of the earlier studies, water soluble and long circulating N-(2-hydroxypropyl) methacrylamide copolymers with grafted biorecognition motifs were shown to induce programmed cell death184. Macromolecules containing carefully designed structural motifs bind to non-internalized or slowly internalizing receptors on the cell surface and cause receptor coupling or clustering, leading to cell death185 (FIG. 8). The cell-surface biorecognition principles that are responsible for apoptosis and information from biological mechanisms offer potential and guidelines for designing novel macromolecular therapeutics that target specific diseases.
Figure 8 |. Polymer backbones are grafted with pendant targeting moieties that contain conjugated antiparallel coiled-coil peptides.
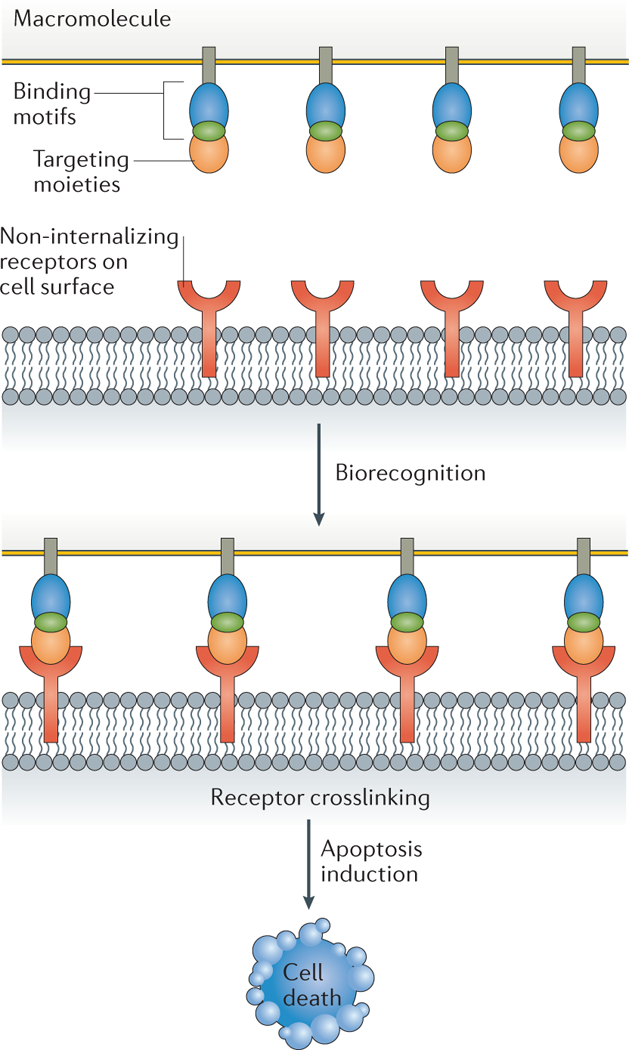
Biorecognition of these by the non-internalizing receptors on the cell surface causes cell receptor clustering (crosslinking), leading to cell death.
Hyperbranched drug-free PAMAM generation 4 and 5 dendrimers containing amine, hydroxyl and carboxylic acid surface groups have been shown to possess anti-inflammatory properties, which in some cases has been higher than the drug indomethacin. It was hypothesized that the origins of anti-inflammatory effects may be related to the inhibition of cyclooxygenase (COX-2) activity; however, a detailed evaluation of the specific mechanism(s) is needed to help establish structure-property relationships in these dendrimers186. Much smaller dendrimers (generation 1) synthesized using alkyne-azide click chemistry, containing hydroxyl terminal groups and no (other) active pharmaceutical agent, were shown to have concentration-dependent anti-inflammatory behaviour187. An evaluation using computer-assisted molecular docking simulations, performed using molecular operating environment software, has suggested that the mechanism of action may involve dendrimer interaction with inducible nitric oxide synthase (iNOS) and COX-2 enzymes, with reduction in nitric oxide release and inhibition of prostaglandin synthesis. Anti-inflammatory activity was found to be dependent on dendrimer size and terminal functional groups, and lower generation hydroxyl terminated dendrimers were found to have a better fit to the enzyme binding active site, inhibiting their activity (FIG. 9). Micelles assembled from branched A2B miktoarm polymers containing PEG and PCL arms, and that are absent of any encapsulated drug, have also been shown to reduce nitric oxide release at a level comparable with that of the drug-loaded (nimodipine) micelles146. It is anticipated that the scope of macromolecules as therapeutics will be further expanded by more detailed evaluations of the mechanisms by which naked nanocarriers provide a pathway for biological interference.
Figure 9 |. Anti-inflammatory activity of drug-free macromolecules.
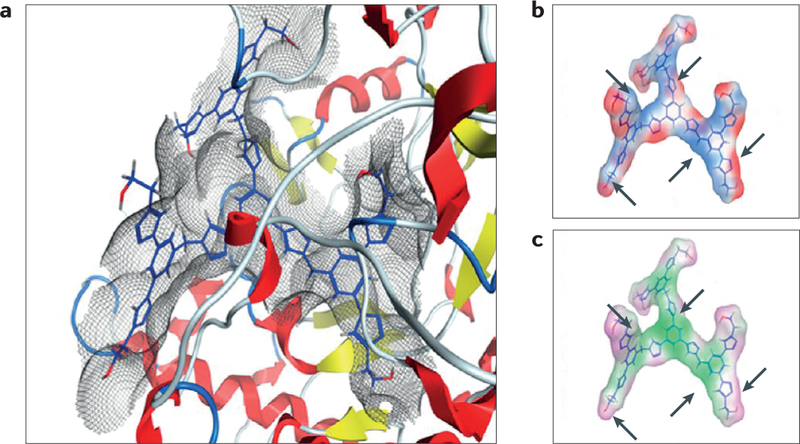
Generation 1 dendrimer containing terminal hydroxyl groups fits into the active site of inducible nitric oxide synthase, and has favourable binding interactions: van der Waals (part a), electrostatic (part b; red is electronegative and blue is electropositive) and lipophilic (part c; green is lipophilic and purple is hydrophilic) surface maps. Part a depicts the lowest energy docking conformations, and parts b and c depict a comparison of the electrostatic and lipophilic surfaces. Arrows indicate the correlation between electropositive (blue) and lipophilic regions (green) of the molecule. Figure is adapted with permission from REF 187, American Chemical Society.
Several elegant studies have explored designing polymer-based nanoparticles that could emulate aptamers and antibodies in interacting with biomacromolecules to cloister toxins188–190. Considering the diversity of macro-molecular architectures that is currently at our disposal and that can be easily synthetically tailored, this approach offers enormous potential in fine-tuning binding capabilities for a range of biomedical applications. The area of macromolecules as therapeutics is still in its infancy, and a detailed understanding of this platform may provide novel pathways to engineer new technologies.
Accelerate clinical validation by matchmaking
Macromolecular nanoparticles have had a key role in delivering drugs, simplifying administration schemes, reducing toxicities and improving disease outcomes. Several new platforms have been developed in which the structural dispersity of macromolecules has evolved. This enormous scientific investment has been made with the intention of inventing better and more efficient therapeutic interventions for diseases such as cancer. However, clinical evaluation of nanotechnology suggests that patients suffering from the same cancer type respond differently to a nanoparticle-based drug formulation. One of the common methods to deliver drug-loaded nanoparticles to the tumour site relies on the EPR effect. A heterogeneous response to nanoparticle therapy could thus be related to variability in the EPR effect in the tumour tissue of the same cancer type in different individuals. This suggests that the design of macromolecular nanoparticle cancer therapeutics needs to be strongly influenced by tumour structural features and local biology191. The solution to this problem may lie in a shift towards personalized medicine and by investing in understanding the properties of tumours in a particular set of patients. Imaging tools continue to aid in expanding our knowledge ofthese local facets192. To facilitate clinical efficiency of nanoparticles and significantly improve quality oflife in cancer patients, individual EPR effects may need to be evaluated in patients first by, for example, using imaging-enabled nanoparticles before matching the effect with the drug payload193. In a recent study, such a protocol was demonstrated in which fluorescently labelled Fe2O3 magnetic particles were used to predict co-localization of therapeutic nanoparticles constructed from block copolymers, poly(D,L- lactic-co-glycolic acid)-b-poly(ethylene glycol)193. Such a novel platform offers significant potential194 and can evolve with polymer complexity to accelerate clinical validation of nanoparticle technology1,2,29,116,121,157,195.
Conclusions
Significant progress in engineering polymer-based nanocarriers has been made as a result of the converging efforts from different scientific disciplines. Biologists and pharmacologists continue to provide an understanding of the biological mechanisms involved in different diseases. This offers scientists a platform to develop nanotechnology to deliver active pharmaceutical agents efficiently to desired sites. Chemistry has a crucial role in equipping them with tools for the relevant nanotechnology, and macromolecules are key components in this regard. A complete picture has not yet emerged, but it is clear that the size, shape, functional groups and overall morphology of the nanocarriers are crucial parameters for their efficacy in drug delivery. Design of macromolecular nanoparticles through a detailed understanding of these parameters will help the invention of better and more efficient therapeutic interventions, as well as facilitate the process to expeditious clinical translations. Miktoarm polymers are relatively new and the scientific community has begun to evaluate their efficacy as nanocarriers in detail. It will be necessary to carry out a detailed comparative analysis of these branched systems with block copolymers to develop better macromolecule-based nanotechnologies for drug delivery. Dendrimers have been well studied, although efforts have concentrated on PAMAM-based dendrimers — perhaps justifiably so given their commercial availability. New backbones are now appearing on the market, and it is hoped that any shortcomings noted earlier, for example, homogeneity of higher generation dendrimers and the statistical distribution of surface-bound moieties in post-functionalization methods leading to irreproduc-ible pharmacokinetics, will be addressed to expedite their entry into clinical translations. Advances in drug delivery will be made by the synthetic adaptation of polymer complexity that has been made available and interfacing it with biology. The scientific community understands the need to direct unique and advantageous characteristics of each macromolecule to address specific unmet needs of a desired pathology. Rapid advances in clinical evaluations and a move towards personalized medicine will arise from a better understanding of the successes and failures of macromolecule-based drug delivery technologies. Ultimately, this will spur chemists to improve on their designs for macromolecular nanocarriers, and continued collaboration among multidisciplinary teams will subsequently help expedite realization of their potential.
Acknowledgements
The authors thank the Prostate Cancer Foundation, National Cancer Institute (NIH Grant No. 1U54CA151884), and Natural Sciences and Engineering Research Council of Canada (A.K.) for financial support.
Footnotes
Competing interests
O.C.F. and R.L. declare competing interests in Selecta Biosciences and Tarveda Therapeutics.
Publisher’s note:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hubbell JA & Langer R Translating materials design to the clinic. Nat. Mater. 12, 963–966 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Duncan R & Gaspar R Nanomedicines under the microscope. Mol. Pharm. 8, 2101–2141 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Curry F-RE Drug delivery: redefining tumour vascular barriers. Nat. Nanotechnol. 11, 494–496(2016). [DOI] [PubMed] [Google Scholar]
- 4.Drug Delivery Technology Market by Route of Administration (Oral (Solid), Pulmonary (Nebulizer), Injectable (Device), Ocular (Liquid), Topical (Solid), Implantable (Active), Transmucosal (Oral)), Patient Care Setting (Hospital, ASC) - Global Forecast to 2(021 Markets and Markets http://www.marketsandmarkets. com/Market-Reports/drug-delivery-technologies-market-1085.html (2015).
- 5.Pamies P & Stoddart A Materials for drug delivery. Nat. Mater. 12, 957 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Kannan RM, Nancy E, Kannan S & Tomalia DA Emerging concepts in dendrimer based nanomedicine: from design principles to clinical applications. J. Intern. Med. 276, 579–617 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Elsabahy M, Heo GS, Lim S-M, Sun G &Wooley, K. L. Polymeric nanostructures for imaging and therapy. Chem. Rev. 115, 10967–11011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng CJ, Tietjen GT, Saucier-Sawyer JK & Saltzman WM A holistic approach to targeting disease with polymeric nanoparticles.Nat. Rev. DrugDiscov. 14, 239–247 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelikin AN, Ehrhadrt C & Healy AM Materials and methods for delivery of biological drugs. Nat. Chem. 8, 997–1007 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Mitragotri S, Burke PA & Langer R Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 13, 655–672 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamaly N, Yameen B, Wu J & Farokhzad OC Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 116, 2602–2663 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong R et al. Smart chemistry in polymeric nanomedicine. Chem. Soc. Rev. 43, 6982–7012(2014). [DOI] [PubMed] [Google Scholar]
- 13.Maeda H, Nakamura H & Fang J The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Delivery Rev. 65, 71–79 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Gao H & Bao G Physical principles of nanoparticle cellular endocytosis. ACS Nano 9, 8655–8671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pridgen EM et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci. Transl. Med. 5, 213ra167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J et al. Development of multinuclear polymeric nanoparticles as robust protein nanocarriers. Angew. Chem. Int. Ed. 53, 8975–8979 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark AJ & Davis ME Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc. Natl Acad. Sci. USA 112, 12486–12491 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabanov AV & Batrakova EV New technologies for drug delivery across the blood brain barrier. Curr. Pharm. Design 10, 1355–1363 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peppas N Historical perspective on advanced drug delivery: how engineering design and mathematical modeling helped the field mature. Adv. Drug Deliv Rev. 65, 5–9 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Couvreur P Nanoparticles in drug delivery: past, present and future. Adv. Drug Deliv Rev. 65, 21–23(2013). [DOI] [PubMed] [Google Scholar]
- 21.Kamaly N, Xiao Z, Valencia PM, Radovic- Moreno AF & Farokhzad OC Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev 41, 2971–3010 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregoriadis G The carrier potential of liposomes in biology and medicine. N. Engl. J. Med. 295, 765–770 (1976). [DOI] [PubMed] [Google Scholar]
- 23.Torchilin VP Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4, 145–160 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Lasic DD & Papahadjopoulos D Liposomes revisited. Science 267, 1275–1276 (1995). [DOI] [PubMed] [Google Scholar]
- 25.çagdas M, Sezer AD & Bucak S in Applications of Nanotechnology in Drug Delivery Ch. 1(INTECH, 2014). [Google Scholar]
- 26.Garay RP, El-Gewely R, Armstrong JK,Garratty G & Richette P Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin.Drug Deliv. 9, 1319–1323 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Tung HY et al. Selective delivery of PEGylated compounds to tumor cells by anti-PEG hybrid antibodies. Mol. Cancer Ther. 14, 1317–1326 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Knop K, Hoogenboom R, Fischer D &Schubert US Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 49, 6288–6308 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Samad A, Sultana Y & Aqil M Liposomal drug delivery systems: an update review. Curr. Drug Deliv. 4, 297–305 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Pattni BS, Chupin VV & Torchilin VP New developments in liposomal drug delivery. Chem. Rev. 115, 10938–10966 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Allen TM & Cullis PR Liposomal drug delivery systems: from concept to clinical applications. Adv.Drug Deliv. Rev. 65, 36–48 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Sercombe L et al. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 6, 286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helm F & Fricker G Liposomal conjugates for drug delivery to the central nervous system. Pharmaceutics 7, 27–42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Astruc D Introduction to nanomedicine. Molecules 21, 1–6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S, Yuan W & Jin T Formulating protein therapeutics into particulate forms. Expert Opin. Drug Deliv. 6, 1123–1133 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Bogdansky S in Biodegradable Polymers as Drug Delivery Systems (eds Chasin M& Langer R) 231–259 (Marcel Dekker, 1990). [Google Scholar]
- 37.Elzoghby AO, Samy WM & Elgindy NA Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 157, 168–182 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Gradishar WJ Albumin-bound paclitaxel: a next generation taxane. Expert Opin. Pharmacother. 7, 1041–1053 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Bader RA & Putnam DA Engineering Polymer Systems for Improved Drug Delivery (Wiley, 2014). [Google Scholar]
- 40.Traverso G & Langer R Perspective: special delivery for the gut. Nature 519, S19 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Wong PT & Choi SK Mechanisms of drug release in nanotherapeutic delivery systems. Chem. Rev. 115, 3388–3432 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Elsabahy M & Wooley K Data mining as a guide for the construction of cross-linked nanoparticles with low immunotoxicity via control of polymer chemistry and supramolecular assembly. Acc. Chem. Res. 48, 1620–1630 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delplace V & Nicolas J Degradable vinyl polymers for biomedical applications. Nat. Chem. 7, 771–784(2015). [DOI] [PubMed] [Google Scholar]
- 44.Salvador-Morales C, Zhang L, Langer R & Farokhzad OC Immunocompatibility properties of lipid-polymer hybrid nanoparticles with heterogeneous surface functional groups. Biomaterials 30, 2231–2240 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langer RS & Peppas NA Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials 2, 201–214 (1981). [DOI] [PubMed] [Google Scholar]
- 46.Langer R Drug delivery and targeting. Nature 392, 5–10 (1998). [PubMed] [Google Scholar]
- 47.Sinha VR, Khosla L Bioadsorbable polymers for implantable therapeutic systems. Drug Dev Ind. Pharm. 24, 1129–1138 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Duncan R Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 6, 688–701 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Peppas NA in Smart Polymers: Applications in Biotechnology and Biomedicine 2nd edn (eds Galaev IM & Mattiasson B) (CRC, 2008). [Google Scholar]
- 50.Liechty WB, Kryscio DR, Slaughter BV &Peppas, N. A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 1, 149–173 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duncan R & Vicent MJ Polymer therapeutics- prospects for 21st century: the end of the beginning. Adv. DrugDeliv. Rev. 65, 60–70 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Kurniasih IN, Keilitz J & Haag R Dendritic nanocarriers based on hyperbranched polymers.Chem. Soc. Rev 44, 4145–4164 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Sharma A & Kakkar A Designing dendrimers and miktoarm polymer based multi-tasking nanocarriers for efficient medical therapy. Molecules 20, 16987–17015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tong R & Langer R Nanomedicines targeting the tumor environment. Cancer J. 21, 314–321 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Epps TH III, O’Reilly RK Block copolymers: controlling nanostructure to generate functional materials - synthesis, characterization, and engineering. Chem. Sci. 7, 1674–1689 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schluter DA, Hawker C & Sakamoto J (eds) Synthesis of Polymers: New Structures & Methods (Wiley, 2012). [Google Scholar]
- 57.Theato P & Klok H-A (eds) Functional Polymers by Post-Polymerization Modification: Concepts,Guidelines and Applications (Wiley, 2012). [Google Scholar]
- 58.Du Y, Chen W, Zheng M, Meng F & Zhong Z pH-Sensitive degradable chimaeric polymersomes for the intracellular release of doxorubicin hydrochloride. Biomaterials 33, 7291–7299 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Nederberg F et al. Simple approach to stabilized micelles employing miktoarm terpolymers and stereocomplexes with application in paclitaxel delivery. Biomacromolecules 10, 1460–1468 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Franc G & Kakkar AK Click methodologies: efficient, simple and greener routes to design dendrimers. Chem. Soc. Rev 39, 1536–1544 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Chang W, Wu D & Liu Y Michael addition polymerization of trifunctional amine and acrylic monomer: a versatile platform for development of biomaterials. Biomacromolecules 17, 3115–3126(2016). [DOI] [PubMed] [Google Scholar]
- 62.Arseneault M, Wafer C & Morin J-F Recent advances in click chemistry applied to dendrimer synthesis. Molecules 20, 9263–9294 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brandl FP & Gregoritza M The Diels-Alder reaction: a powerful tool for the design of drug delivery systems and biomaterials. Eur. J. Pharm. Biopharm. 97, 438–453 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Devaraj NK & Weissleder R Biomedical applications of tetrazine cycloadditions. Acc. Chem. Res. 44, 816–827 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang KS, Budin G, Reiner T, Vinegoni C & Weissleder R Bioorthogonal imaging of aurora kinase A in live cells. Angew. Chem. Int. Ed. 27, 6598–6603(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castonguay A et al. Thermosensitive dendrimer formulation for drug delivery at physiologically relevant temperatures. Chem. Commun. 47, 12146–12148 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Killops KL, Campos LM & Hawker CJ Robust, efficient, and orthogonal synthesis of dendrimers via thiol-ene “click” chemistry. J. Am. Chem. Soc. 130, 5062–5064 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Cai L et al. Telodendrimer nanocarrier for co-delivery of paclitaxel and cisplatin: a synergistic combination nanotherapy for ovarian cancer treatment. Biomaterials 37, 456–468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hunter AC & Moghimi SM Smart polymers in drug delivery: a biological perspective, Polym. Chem 10.1039/C6PY00676K(2017). [DOI] [Google Scholar]
- 70.Batycky RP, Hanes J, Langer R & Edwards DA A theoretical model of erosion and macromolecular drug release from biodegrading microspheres. J. Pharm. Sci. 86, 1464–1477 (1997). [DOI] [PubMed] [Google Scholar]
- 71.Brazel CS & Peppas NA Modeling of drug release from swellable polymers. Eur. J. Pharm. Biopharm. 49, 47–58 (2000). [DOI] [PubMed] [Google Scholar]
- 72.Xu X et al. Multifunctional envelope-type siRNA delivery nanoparticle platform for prostate cancer therapy. ACS Nano 11, 2618–2627 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu X et al. Ultra-pH-responsive and tumor- penetrating nanoplatform for targeted siRNA delivery with robust anti-cancer efficacy. Angew. Chem. Int. Ed. 55, 7091–7094 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moghimi SM, Hunter AC & Murray JC Long- circulating and target specific nanoparticles: theory to practice. Pharmacol. Rev. 53, 283–318 (2001). [PubMed] [Google Scholar]
- 75.Farokhzad OC & Langer R Nanomedicine: developing smarter therapeutic and diagnostic modalities. Adv. Drug Deliv. Rev. 58, 1456–1459 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Panyam J & Labhasetwar V Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 55, 329–347 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Soppimath KS, Aminabhavi TM, Kulkarni AR & Rudzinski WE Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 70, 1–20 (2001). [DOI] [PubMed] [Google Scholar]
- 78.Nair LS & Laurencin CT Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32, 762–798 (2007). [Google Scholar]
- 79.Jain JP, Chitkara D & Kumar N Polyanhydrides as localized drug delivery carrier: an update. Expert Opin. Drug Deliv. 5, 889–907 (2008). [DOI] [PubMed] [Google Scholar]
- 80.Heller J & Barr J Poly(ortho esters) — from concept to reality. Biomacromolecules 5, 1625–1632 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Teasdale I & Bruddemann O Polyphosphazenes: multifunctional, biodegradable vehicles for drug and gene delivery. Polymers 5, 161–187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Z, Wang J, Mao HQ & Leong KW Polyphosphoesters in drug and gene delivery. Adv. Drug Deliv. Rev. 55, 483–499 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Vauthier C, Dubernet C, Chauvierre C, Brigger I & Couvreur P Drug delivery to resistant tumors: the potential of poly(alkylcyanoacrylate) nanoparticles. J. Control. Release 93, 151–160 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Nicolas J & Couvreur P Synthesis of poly(alkyl cyanoacrylate)-based colloidal nanomedicine. WIREs Nanomed. Nanobiotechnol. 1, 111–127 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Siegel SJ et al. Effect of drug type on the degradation rate of PLGA matrices. Eur. J. Pharm. Biopharm. 64, 287–293 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Bader H, Ringsdorf H & Schmidt B Watersoluble polymers in medicine. Angew. Makromol. Chem. 123, 457–485 (1984). [Google Scholar]
- 87.Kataoka K, Harada A & Nagasaki Y Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 47, 113–131 (2001). [DOI] [PubMed] [Google Scholar]
- 88.Nishiyama N & Kataoka K Current state achievements and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 112, 630–648 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Kwon GS Polymeric micelles for delivery of poorly water-soluble compounds. Crit. Rev. Ther. Drug Carrier Systems 20, 357–403 (2003). [DOI] [PubMed] [Google Scholar]
- 90.Riess G Micellization of block-copolymers. Prog. Polym. Sci. 28, 1107–1170 (2003). [Google Scholar]
- 91.Matyjaszewski K & Muller AHE (eds) Controlled and Living Polymerizations: From Mechanisms to Applications (Wiley, 2009). [Google Scholar]
- 92.Venkataraman S et al. The effects of polymeric nanostructure shape on drug delivery. Adv. Drug Deliv. Rev. 63, 1228–1246 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Zhang N, Guo SR, Li HQ, Li ZH & Gu JR Synthesis of three types of amphiphilic poly(ethyleneglycol)-block-poly(sebacic anhydride) copolymers and studies of their micellar solutions. Macromol. Chem. Phys. 207, 1359–1367 (2006). [Google Scholar]
- 94.Chang YC & Chu IM Methoxy poly(ethylene glycol)-b-poly(valerolactone) diblock polymeric micelles for enhanced encapsulation and protection of camptothecin. Eur. Pol. J. 44, 3922–3930 (2008). [Google Scholar]
- 95.Trivedi R & Kompella UB Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine 5, 484–505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang X et al. Interactions between an anticancer drug and polymeric micelles based on biodegradable polyesters. Macromol. Biosci. 8, 1116–1125 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Hoffman AS Stimuli-responsive polymers: biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev 65, 10–16 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Schattling P, Jochum FD & Theato P Multi-stimuli responsive polymers — the all-in-one talents. Polym. Chem. 5, 25–36 (2014). [Google Scholar]
- 99.Jochum FD & Theato P Temperature and light- responsive smart polymer materials. Chem. Soc. Rev. 42, 7468–7483 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Sant VP, Smith D & Leroux J-C Novel pH-sensitive supramolecular assemblies for oral delivery of poorly water soluble drugs: preparation and characterization. J. Control. Release 97, 310–312 (2004). [DOI] [PubMed] [Google Scholar]
- 101.Kim S, Kim Y, Huh KM, Acharya G & Park K Hydrotropic polymer micelles containing acrylic acid moieties for oral delivery of paclitaxel. J. Control. Release 132, 222–229 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee ES, Na K & Bae YH Super pH sensitive multifunctional polymeric micelle. Nano Lett. 5, 325–329 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Alexandridis P, Holzwarth JF & Hatton TA Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer association. Macromolecules 27, 2414–2425 (1994). [Google Scholar]
- 104.Bergueiro J & Calderon M Thermoresponsive nanodevices in biomedical applications. Macromol. Biosci. 15, 183–199 (2015). [DOI] [PubMed] [Google Scholar]
- 105.Alakhova DY, Zhao Y, Li S & Kabnov AV Effect of doxorubicin/pluronic SP1049C on tumorigenicity, aggressiveness, DNA methylation and stem cell markers in murine leukemia. PLoS ONE 8, e72238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin S, Geng Y, Discher DE & Yang S Temperature-controlled assembly and release from polymer vesicles of poly(ethylene oxide)-block- poly(N-isopropylacrylamide). Adv. Mater. 18, 2905–2909 (2006). [Google Scholar]
- 107.Yang M et al. Novel thermosensitive polymeric micelles for docetaxel delivery. J. Biomed. Mater. Res. A 81, 847–858 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Cheng C, Wei H, Zhang XZ, Cheng SX & Zhuo RX Thermo-triggered and biotinylated biotin-P(NIPAAm-co-HMAAm)-b-PMMA micelles for controlled drug release. J. Biomed. Mater. Res. A 88, 814–822 (2009). [DOI] [PubMed] [Google Scholar]
- 109.Nakayama M et al. Molecular design of biodegradable polymeric micelles for temperature¬responsive drug release. J. Control. Release 115, 46–56 (2006). [DOI] [PubMed] [Google Scholar]
- 110.Kopecek J Polymer-drug conjugates: origins, progress to date and future directions. Adv. Drug Deliv. Rev. 65, 49–59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ringsdorf H Structure and properties of pharmacologically active polymers. J. Polym. Sci. 51, 135–153 (1975). [Google Scholar]
- 112.Greenwald RB, Choe YH, McGuire J & Conover CD Effective drug delivery by PEGylated drug conjugates. Adv. Drug Deliv. Rev. 55, 217–250(2003). [DOI] [PubMed] [Google Scholar]
- 113.Khandare J & Minko T Polymer-drug conjugates: progress in polymeric prodrugs. Prog. Polym. Sci. 31, 359–397 (2006). [Google Scholar]
- 114.Zou J et al. pH-Sensitive brush polymer-drug conjugates by ring opening metathesis co-polymerization. Chem. Commun. 47, 4493–4495 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Hein CD, Liu X-M & Wang D Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 25, 2216–2230 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pola R, Braunova A, Laga R, Pechar M & Ulbrich K Click chemistry as a powerful and chemoselective tool for the attachment of targeting ligands to polymer drug carriers. Polym. Chem. 5, 1340–1350 (2014). [Google Scholar]
- 117.Kolishetti N et al. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl Acad. Sci. USA 107, 17939–17944 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wessig V, Pettinger TK & Murdock N Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomed. 9, 4357–4373 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Min Y, Caster JM, Eblan MJ & Wang AZ Clinical translation of nanomedicine. Chem. Rev. 115, 11147–11190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pillai G Nanomedicine for cancer therapy: an update of FDA approved and those under various stages of development. SOJ Pharm. Pharm. Sci. 1, 1–13 (2014). [Google Scholar]
- 121.Bamrungsap S et al. Nanotechnology in therapeutics. Nanomedicine 7, 1253–1271 (2012). [DOI] [PubMed] [Google Scholar]
- 122.Shi J, Kantoff PW, Wooster R & Farokhzad OC Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ferrari M Frontiers in cancer nanomedicine, directing mass transport through biological barriers. Trends Biotechnol. 28, 181–188 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De Souza R, Spence T, Huang H & Allen C Preclinical imaging and translational animal models of cancer for accelerated clinical implementation of nanotechnologies and macromolecular agents. J. Control. Release 219, 313–330 (2015). [DOI] [PubMed] [Google Scholar]
- 125.Bertrand N, Wu J, Xu X, Kamaly N & Farokhzad OC Cancer nanotechnology: the impact of passive and active targeting in the area of modern cancer biology. Adv. Drug Delivery Rev. 66, 2–25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miller MA et al. Tumor-associated macrophages act as a slow release reservoir of nano-therapeutic Pt(iv) pro-drug. Nat. Commun. 6, 8696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Knudsen KB et al. Differential toxicological response to positively and negatively charged nanoparticles in the rat brain. Nanotoxicology 8, 764–774 (2014). [DOI] [PubMed] [Google Scholar]
- 128.Etheridge ML et al. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine 9, 1–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Truong NP, Whittaker MR, Mak CW &Davis, T. P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Delivery 12, 129–142 (2015). [DOI] [PubMed] [Google Scholar]
- 130.Nguyen KC & Zhao Y Engineered hybrid nanoparticles for on-demand diagnostics and therapeutics. Acc. Chem. Res. 48, 3016–3025 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Vicent MJ, Movellan J & Duro-Castano AS Smart branched polymer drug conjugates as nano¬sized drug delivery systems. Biomater. Sci. 3, 1321–1334 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Wang D, Zhao T, Zhu X, Yan D & Wang W Bioapplications of hyperbranched polymers. Chem. Soc. Rev. 44, 4023–4071 (2015). [DOI] [PubMed] [Google Scholar]
- 133.Wu W, Wang W & Li J Star polymers: advances in biomedical applications. Prog. Polym. Sci. 46, 55–85 (2015). [Google Scholar]
- 134.Khan O & Anderson D Dendrimer-inspired nanomaterials for the in vivo delivery of siRNA to lung vasculature. Nano Lett. 15, 3008–3016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Svenson S The dendrimer paradox — high medical expectations but poor clinical translation. Chem. Soc. Rev. 44, 4131–4144 (2015). [DOI] [PubMed] [Google Scholar]
- 136.Wong C-H & Zimmerman SC Orthogonality in organic, polymer, and supramolecular chemistry: from Merrifield to click chemistry. Chem. Commun. 49, 1679–1695 (2013). [DOI] [PubMed] [Google Scholar]
- 137.Garcia-Gallego S, Hult D, Olsson JV & Malkoch M Fluoride-promoted esterification with imidazolide-activated compounds: a modular and sustainable approach to dendrimers. Angew. Chem. Int. Ed. 54, 2416–2419 (2015). [DOI] [PubMed] [Google Scholar]
- 138.Hadjichristidis N, Iatrou H, Pitsikalis M, Pispas S & Avgeropoulo A Linear and non-linear tri-block terpolymers. Synthesis, self-assembly in selective solvents and in bulk. Prog. Polym. Sci. 30, 725–782 (2005). [Google Scholar]
- 139.Khanna K, Varshney S & Kakkar AK Miktoarm star polymers: advances in synthesis, self-assembly, and applications. Polym. Chem. 1, 1171–1185(2010). [Google Scholar]
- 140.Polymeropoulos G et al. 50th Anniversary perspective: polymers with complex architectures. Macromolecules 50, 1253–1290 (2017). [Google Scholar]
- 141.Kong W, Li B, Jin Q, Ding D & Shi AC Helical vesicles, segmented semi-vesicles and noncircular bilayer sheets from solution state self-assembly of ABC miktoarm star terpolymers. J. Am. Chem. Soc. 131, 8503–8512 (2009). [DOI] [PubMed] [Google Scholar]
- 142.Li Z, Hillmyer MA & Lodge TP Morphologies of multicompartment micelles formed by ABC miktoarm star terpolymers. Langmuir 22, 9409–9417 (2006). [DOI] [PubMed] [Google Scholar]
- 143.Li Z, Kesselman E, Talmon Y, Hillmyer MA & Lodge TP Multicompartment micelles from ABC Miktoarm stars in water. Science 306, 98–101 (2004). [DOI] [PubMed] [Google Scholar]
- 144.Yin H, Kang HC, Huh KM & Bae YH Biocompatible, pH-sensitive AB2 miktoarm polymer- based polymersomes: preparation, characterization, and acidic pH-activated nanostructural transformation. J. Mater. Chem. 22, 19168–19178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alizadeh R & Ghaemy M pH-Responsive ABC type miktoarm star terpolymers: synthesis via combination of click reaction and SET-LRP, characterization, self¬assembly and controlled drug release. Polymer 66, 179–191 (2015). [Google Scholar]
- 146.Soliman GM et al. Tailoring the efficacy of nimodipine drug delivery using nanocarriers based on A2B miktoarm star polymers. Biomaterials 31, 8382–8392 (2010). [DOI] [PubMed] [Google Scholar]
- 147.Sharma A et al. Asymmetric AB3 miktoarm star polymers: synthesis, self-assembly and study of micelle stability using AF4 for efficient and sustained drug delivery. Macromol. Biosci 15, 1744–1754 (2015). [DOI] [PubMed] [Google Scholar]
- 148.Soliman GM et al. Miktoarm star micelles containing curcumin reduce cell viability of sensitized glioblastoma. J. Nanomed. Biother. Discov. 4, 1000124 (2014). [Google Scholar]
- 149.Sharma A et al. Selective delivery of coenzyme Q10 to mitochondria using multifunctional nanocarriers. Biomacromolecules 13, 239–252 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin W et al. Amphiphilic miktoarm star copolymer (PCL)3-(PDEAEMA-b-PPEGMA)3 as pH-sensitive micelles in the delivery of anticancer drug. J. Mater. Chem. B. 2, 4008–4020 (2014). [DOI] [PubMed] [Google Scholar]
- 151.Blasco E, Schmidt BVKJ, Barner-Kowollik C, Pinol M & Oriol L Dual thermo- and photo- responsive micelles based on miktoarm star polymers. Polym. Chem. 4, 4506–4514 (2013). [Google Scholar]
- 152.McKenzie TG et al. Highly efficient and versatile formation of biocompatible star polymers in pure water and their stimuli-responsive self-assembly. Macromolecules 47, 7869–7877 (2014). [Google Scholar]
- 153.Gou P-F, Zhou W-P, Xu N & Shen ZQ Synthesis, self-assembly, and drug-loading capacity of well- defined drug-conjugated amphiphilic A2B2 type miktoarm star copolymers based on poly(s-caprolactone) and poly(ethylene glycol).J. Polym. Sci. A 47, 6962–6976 (2009). [DOI] [PubMed] [Google Scholar]
- 154.Tomalia DA, Christensen JB & Boas U (eds) Dendrimers, Dendrons and Dendritic Polymers (Cambridge Univ. Press, 2012). [Google Scholar]
- 155.Lee CC, Mackay JA, Frechet JM & Szoka FC Designing dendrimers for biological applications. Nat. Biotechnol. 23, 1517–1526 (2005). [DOI] [PubMed] [Google Scholar]
- 156.Jevprasesphant R, Penny J, Attwood D, D’Emanuele A Transport of dendrimer nanocarriers through epithelial cells via the transcellular route.J. Control. Release 97, 259–267 (2004). [DOI] [PubMed] [Google Scholar]
- 157.Kurniasih IN, Keilitz J & Haag R Dendritic nanocarriers based on hyperbranched polymer. Chem. Soc. Rev. 44, 4145–4264 (2015). [DOI] [PubMed] [Google Scholar]
- 158.Sun H-J, Zhang S & Percec V From structure to function via complex supramolecular dendrimer systems. Chem. Soc. Rev. 44, 3900–3923 (2015). [DOI] [PubMed] [Google Scholar]
- 159.Menjoge AR, Kannan RM & Tomalia DA Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov. Today 15, 171–185 (2010). [DOI] [PubMed] [Google Scholar]
- 160.Jansen JFGA, de Brabander-van den Berg, E. M. M. & Meijer, E. W. Encapsulation of guest molecules into a dendritic box. Science 266, 1226–1229 (1994). [DOI] [PubMed] [Google Scholar]
- 161.Liu M, Kono K & Frechet JMJ Water-soluble dendritic unimolecular micelles: their potential as drug delivery agents. J. Control. Release 65, 121–131 (2000). [DOI] [PubMed] [Google Scholar]
- 162.Patri AK, Kukowska-Latallo JF & Baker JR Jr. Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 15, 2203–2214 (2005). [DOI] [PubMed] [Google Scholar]
- 163.Rolland O, Turrin C-O, Caminade A-M &Majoral, J.-P. Dendrimers and nanomedicine: multivalency in action. New J. Chem. 33, 1809–1824 [Google Scholar]
- 164.Carlmark A, Malmstrom E & Malkoch M Dendritic architectures based on bis-MPA: functional polymeric scaffolds for application-driven research. Chem. Soc. Rev. 42, 5858–5879 (2013). [DOI] [PubMed] [Google Scholar]
- 165.Enciso AE, Abid ZM & Simanek EE Rapid, semi¬automated convergent synthesis of low generation triazine dendrimers using microwave assisted reactions. Polym. Chem. 5, 4635–4640 (2014). [Google Scholar]
- 166.Thirumurugan P, Matosiuk D & Jozwia K Click chemistry for drug development and diverse chemical- biology applications. Chem. Rev. 113, 4905–4979 (2013). [DOI] [PubMed] [Google Scholar]
- 167.Hourani R & Kakkar A Advances in the elegance of chemistry in designing dendrimers. Macromol. Rapid Commun. 31, 947–974 (2010). [DOI] [PubMed] [Google Scholar]
- 168.Lim J et al. The role of the size and number of polyethylene glycol chains in the biodistribution and tumor localization of triazine dendrimers. Mol. Pharmaceut. 5, 540–547 (2008). [DOI] [PubMed] [Google Scholar]
- 169.Hourani R, Jain M, Maysinger D & Kakkar A Multi-tasking with single scaffold dendrimers for targeting sub-cellular microenvironments. Chem. Eur.J. 16, 6164–6168 (2010). [DOI] [PubMed] [Google Scholar]
- 170.Ornelas C & Weck M Construction of well-defined multifunctional dendrimers using a trifunctional core. Chem. Commun. 57, 5710–5712 (2009). [DOI] [PubMed] [Google Scholar]
- 171.Sharma A et al. Facile construction of multifunctional nanocarriers using sequential click chemistry for applications in biology. Macromolecules 44,521–529 (2011). [Google Scholar]
- 172.Ornelas C Brief timelapse on dendrimer chemistry: advances, limitations and expectations. Macromol. Chem. Phys. 217, 149–174 (2016). [Google Scholar]
- 173.Wurm F & Frey H Linear-dendritic block copolymers: the state of the art and exciting perspectives. Prog. Polym. Sci. 36, 1–52 (2011). [Google Scholar]
- 174.Dong C-M & Liu G Linear-dendritic biodegradable block-copolymers: from synthesis to application in bionanotechnology. Polym. Chem. 4, 46–52 (2013). [Google Scholar]
- 175.Poon Z et al. Ligand-clustered “patchy” nanoparticles for modulated cellular uptake and in vivo tumor targeting. Angew. Chem. Int. Ed. 49, 7266–7270(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang Y, Hua C & Dong CM Synthesis, selfassembly, and in vitro doxorubicin release behavior of dendron-like/linear/dendron-like poly(s-caprolactone)- b-poly(ethylene glycol)-b-poly(ε-caprolactone) triblock copolymers. Biomacromolecules 10, 2310–2318 (2009). [DOI] [PubMed] [Google Scholar]
- 177.Lundberg P et al. Linear dendritic polymeric amphiphiles with intrinsic biocompatibility: synthesis and characterization to fabrication of micelles and honeycomb membranes. Polym. Chem. 2, 394–402(2011). [Google Scholar]
- 178.Zhou Z et al. Linear-dendritic drug conjugates forming long-circulating nanorods for cancer-drug delivery. Biomaterials 34, 5722–5735 (2013). [DOI] [PubMed] [Google Scholar]
- 179.Blasco E, Pinol M & Oriol L Responsive linear- dendritic block copolymers. Macromol. Rapid Commun. 35, 1090–1115 (2014). [DOI] [PubMed] [Google Scholar]
- 180.Shi C et al. A drug-specific nanocarrier design for efficient therapy. Nat. Commun. 6, 7449–7462(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chu T-W & Kopecek J Drug-free macromolecular therapeutics — a new paradigm in polymeric nanomedicines. Biomater. Sci. 3, 908–922 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Avti P & Kakkar AK Dendrimers as anti¬inflammatory agents. Braz. J. Pharm. Sci. 49, 57–65(2013). [Google Scholar]
- 183.Chen F et al. The development of drug-free therapy for prevention of dental caries. Pharm. Res. 31, 3031–3037 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wu K, Liu J, Johnson RN, Yang J & Kopecek J Drug-free macromolecular therapeutics: induction of apoptosis by coiled-coil-mediated cross-linking of antigens on the cell surface. Angew. Chem. Int. Ed. 49, 1451–1455 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lynn GM et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 33, 1201–1210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chauhan AS, Diwan PV, Jain NK &Tomalia DA Unexpected in vivo anti-inflammatory activity observed for simple, surface functionalized poly(amidoamine) dendrimers. Biomacromolecules 10, 1195–1202 (2009). [DOI] [PubMed] [Google Scholar]
- 187.Neibert K et al. Click dendrimers as anti-inflammatory agents: with insights into their binding from molecular modeling studies. Mol. Pharm. 10, 2502–2508 (2013). [DOI] [PubMed] [Google Scholar]
- 188.Yoshimatsu K, Koide H, Hoshino Y & Shea KJ Preparation of abiotic polymer nanoparticles for sequesteration and neutralization of a target peptide toxin. Nat. Protocols 10, 595–604 (2015). [DOI] [PubMed] [Google Scholar]
- 189.Weisman A, Chen YA, Hoshino Y, Zhang H & Shea KJ Engineering nanoparticle antitoxins utilizing aromatic interactions. Biomacromolecules 15, 3290–3295 (2014). [DOI] [PubMed] [Google Scholar]
- 190.Hu C, Fang R, Copp J, Luk B & Zhang L A biomimetic nanosponge that absorbs pore- forming toxins. Nat. Nanotechnol. 8, 336–340(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Prabhakar U et al. Challenges and key considerations of the enhanced permeability and retention (EPR) effect for nanomedicine drug delivery in oncology. Cancer Res. 73, 2412–2417 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee H, Shin TH, Cheon J & Weissleder R Recent developments in magnetic diagnostic systems. Chem. Rev. 115, 10690–10724 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Miller MA et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 7, 314ra183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Miller MA et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage- mediated vascular bursts. Sci. Transl. Med. 9, eaal0225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang AZ EPR or no EPR? The billion-dollar question. Sci. Transl. Med. 7, 294ec112 (2015). [Google Scholar]


