Abstract
Acute pancreatitis is an inflammatory disorder of the exocrine pancreas associated with tissue injury and necrosis. The disease can be mild, involving only the pancreas, and resolve spontaneously within days or severe, with systemic inflammatory response syndrome- associated extrapancreatic organ failure and even death. Importantly, there are no therapeutic agents currently in use that can alter the course of the disease. This article emphasizes emerging findings that stressors (environmental and genetic) that cause acute pancreatitis initially cause injury to organelles of the acinar cell (endoplasmic reticulum, mitochondria, and endolysosomal–autophagy system), and that disorders in the functions of the organelles lead to inappropriate intracellular activation of trypsinogen and inflammatory pathways. We also review emerging work on the role of damage-associated molecular patterns in mediating the local and systemic inflammatory response in addition to known cytokines and chemokine pathways. In the review, we provide considerations for correction of organelle functions in acute pancreatitis to create a discussion for clinical trial treatment and design options.
Keywords: Acute Pancreatitis, Autophagy, Mitochondria, Endolysosomal System, Stimulator of Interferon Genes, Damage-Associated Molecular Patterns, Unfolded Protein Response, Endoplasmic Reticulum Stress, Mitophagy, Macrophage
Acute Pancreatitis: A Disorder With Different Causes Requiring Solutions
Acute pancreatitis is an inflammatory disorder of the exocrine pancreas associated with tissue injury and necrosis.1,2 The disease can be mild, involving only the pancreas, and resolve spontaneously within days, or severe, with systemic inflammatory response syndrome–associated extrapancreatic organ failure and even death. Importantly, there are no therapeutic agents currently in use that can alter the course of the disease. Current management consists of fluid resuscitation and supportive care.2
Acute pancreatitis has been reported as one of the most common reasons for inpatient hospital care in the United States, with an annual incidence of 13–45 cases per 100,000 people.3,4 Gallstones and alcohol abuse are key causative factors in the mechanisms of pancreatitis; and the incidence varies between regions and sexes as a function of the prevalence of gallstone disease and alcohol abuse.4 Gallstones migrating out of the gallbladder and causing transient obstruction of the pancreatic duct and exposure of the pancreas to biliary constituents still represent the most common cause of acute pancreatitis.4,5 The second most common cause of acute pancreatitis is alcohol abuse.4,5 Alcohol abuse as a cause of pancreatitis requires a significant amount of intake over a prolonged period (≥4–5 drinks per day over >5 years).6 The mechanisms of alcohol-induced pancreatitis are complex and involve disorders of the acinar cell and ductal cells of the exocrine pancreas.7–20
Cigarette smoking is common with alcohol consumption and recent studies support a significant role for smoking and the combination of alcohol consumption with smoking.18,21,22 These studies show that smoking is an independent risk factor for pancreatitis (acute, recurrent, and chronic) in addition to heavy alcohol abuse. There also is increasing recognition of the important role of hyper-triglyceridemia in acute pancreatitis. Hypertriglyceridemic pancreatitis is the third leading cause of acute pancreatitis.23 Estimates suggest that 15%–20% of those with severe hypertriglyceridemia (triglyceride levels >1000 mg/dL) will develop acute pancreatitis.24 The clinical course in these patients is more severe with a greater incidence of persistent multiorgan failure.25,26 Of interest are recent studies showing increased severity of pancreatitis in patients with mild (triglyceride levels 150–199 mg/dL) and moderate (triglyceride levels 200–999 mg/dL) increases in serum triglycerides measured during an episode. Other more common causes of acute pancreatitis include complications of endoscopic retrograde cholangiopancreatography and an autoimmune etiology and are idiopathic.4 Of note, recent literature also associates diabetes with an increased risk for development of pancreatitis.27–29
Medication-associated pancreatitis is a less common cause, probably accounting for fewer than 5% of cases, although multiple drugs have been associated with the development of pancreatitis.30 Drugs with strong associations with the development of acute pancreatitis include azathioprine, 6-mercaptopurine, didanosine, valproic acid, angiotensin-converting enzyme inhibitors, and mesalamine.30 Although there has been considerable interest in the association between glucagon-like peptide 1 mimetics (aka incretin mimetics) used for the treatment of diabetes in causing pancreatitis, more thorough investigations show that the increased incidence in these cases is likely due to the underlying diabetes, which increases the risk of acute pancreatitis by 2–3 times, and not to treatment with these agents.31–34
Currently, the consensus is that only a minority of adult patients have pancreatitis resulting from genetic alterations. However, episodes of recurrent acute pancreatitis or unexplained first episodes of acute pancreatitis in patients younger than 35 years have pathogenic genetic variants in nearly half of patients.35 Further, of importance is the recent report that a genetic mutation in claudin 2 can augment the effect of alcohol drinking on the susceptibility for pancreatitis, pointing out the potential interactions between lifestyle factors and genetic susceptibility to pancreatitis.36,37
There are cases in which the cause is not easily discernable. One should not overlook the possibility of a malignancy that can occasionally present as acute pancreatitis, especially in patients older than 50 years.30 In those cases with an unknown cause, especially if there is a recurrent episode, a more detailed examination of the pancreas with imaging procedures, including endoscopic ultrasound, and testing for genetic abnormalities are warranted.35
The diagnosis of acute pancreatitis is less common in the pediatric population and emerging data suggest that most pediatric cases are due to underlying genetic influences involving mutations in key digestive enzymes (hereditary pancreatitis) and genes in the cystic fibrosis family of mutations.38,39
The reason for this discussion is to emphasize that there are multiple causes of acute pancreatitis and that each cause could have unique mechanisms to initiate the disease. A limiting factor in investigating the early stages in the disease process is the rapid progression of the disease so that it is difficult to obtain information about the natural history of the disease process from its inception. Furthermore, it is rare to obtain pancreatitis tissues from patients because of the relative inability to sample pancreatitis tissues because of the location of the pancreas and concern that manipulations can worsen disease severity. Therefore, as discussed below, investigations of mechanisms of pancreatitis initiation and promotion have depended on experimental animal models and ex vivo human acinar tissue. The theme of this article as emphasized in the title is that recent evidence indicates interactions among cell types in the pancreas, organelles in the pancreatic acinar cell, and the inflammatory system that underlie the full manifestations of pancreatitis.
Roles of Acinar and Ductal Cells in Acute Pancreatitis
The exocrine pancreas is designed to carry out functions of digestion of meal macronutrients and neutralization of gastric acid entering the small intestine for optimal pH for digestive enzyme activity.40 To accomplish these functions, the exocrine pancreas synthesizes, stores, and secretes digestive enzymes from the acinar cell; and the secreted digestive enzymes are transported to the small intestine by a ductal system secreting large amounts of bicarbonate-rich fluid. Disorders of the acinar cell and ductal epithelial cells can initiate pancreatitis; and these disorders can initiate and propagate and inflammatory response. Much of our understanding of the mechanism of pancreatitis has come from studies focused on the acinar cell in the initiation of the disease,14,41 but more recently there has been increasing understanding of the role of the ductal epithelium in the initiation of acute pancreatitis.9,16,42 However, the general consensus is that dysfunction in ductal water and bicarbonate secretion mediate pancreatitis through secondary effects on the acinar cell, which generates that pancreatitis response.9
Preclinical Animal Models and Ex Vivo Human Acinar Tissue Used for Mechanistic Insights
Because of the lack of safe access to human tissues, studies addressing pancreatitis disease mechanisms have largely used animal models. There are several rodent models of acute pancreatitis reproducing the spectrum of human disease manifestations.43 These models have greatly advanced our understanding of the cell biology of the disease and the molecular factors involved and allowed testing of potential therapeutic interventions. The most widely used in vivo models of acute pancreatitis include those induced in rodents by administering supraphysiologic doses of cholecystokinin 8 (CCK), cerulein (an orthologue of CCK), or muscarinic agonists (ie, carbachol); treating with bile acids or L-arginine; and feeding mice a choline-deficient ethionine-supplemented diet. Treating isolated acini (functional units of acinar cells of the exocrine pancreas) with supraphysiologic doses of CCK (or cerulein) or with bile acid salts triggers early pathologic responses of acute pancreatitis (trypsinogen activation, dysregulated secretion, vacuole accumulation) and thus is considered an ex vivo disease model.
Regarding human acinar tissue, we recently published a study comparing responses in human cadaveric pancreatic acini with those in rodents.14 We found similar organelle structures and components by electron microscopy, proteomic analysis, and immunohistochemistry and acinar cell secretory responses to the muscarinic agonist carbachol. When we provoked a pancreatitis response ex vivo with high doses of carbachol or with known pancreatitis-causing agents such as bile acid or tauro-lithocholic acid 3-sulfate, acini from the 2 species similarly responded with mitochondrial depolarization, disordered autophagy, and pathologic endoplasmic reticulum (ER) stress. Furthermore, we found inappropriate conversion of preformed trypsinogen to the activated trypsin state, a hallmark of pancreatitis responses from dysfunctional autophagy, and production of proinflammatory cytokines. All these pathobiologic responses are the same as those observed in rodent tissues.14 Thus, at least with respect to acinar cell pancreatitis responses ex vivo, we found no differences between rodent and human tissues.
Organelle Machinery of the Acinar Cell: From Homeostasis to Dysfunction in Pancreatitis
The central physiologic role of the pancreatic acinar cell is to synthesize, transport, store, and secrete digestive enzymes. It relies on normal functions of acinar cell organelles including the ER, mitochondria, and endolysosomal–autophagy system. Recent studies have shown that the functions of these organelles are deranged in pancreatitis and underlie the mechanisms involved in the generation of acute pancreatitis.
ER Functions and Ca2+ Signaling and Homeostasis
One of the major ER functions (with mitochondrial participation) is regulation of Ca2+ signaling for secretion and homeostasis in the acinar cell, whereas disorders of Ca2+ signaling and homeostasis lead to pathology and pancreatitis responses. Within a typical eukaryotic cell, ionized calcium in cytosol ([Ca2+]i) is roughly 100 nmol/L, which is approximately 12,000-fold lower compared with the extracellular fluid (as in circulating blood). This gradient is maintained through various calcium pumps and intracellular calcium storage organelles such as the ER.44–46 Hormones and neurotransmitters, such as acetylcholine and CCK, that are involved in inducing acinar cell secretion release small amounts of Ca2+ from the ER, which results in the transient and oscillatory pattern of increases in [Ca2+]i. Increases in [Ca2+]i during physiologic stimulation leads to transient and oscillatory patterns of [Ca2+]i.43–46 Physiologic responses such as secretion occur with these transient increases because Ca2+ is rapidly taken back to the ER and/or removed from the acinar cell by Ca2+ pumps. Also, the mitochondria take up Ca2+ during the transient increase for promoting adenosine triphosphate (ATP) generation. Ca2+ homeostasis is dysregulated in pancreatitis.46 The common change seen in pancreatitis models, such as those induced by supraphysiologic doses of cerulein or bile acids, is the loss of physiologic [Ca2+]i oscillations, which are replaced by the sustained increase of [Ca2+]i. The sustained increase in [Ca2+]i occurs because the pancreatitis-causing stimuli largely deplete the ER calcium stores, and the depletion promotes the entry of calcium into the pancreatic acinar cell through store-operated calcium channels, the most abundant of which is mediated by a protein called Orai1.47 The sustained increase in [Ca2+]i causes mitochondrial Ca2+ overload, resulting in the loss of mitochondrial membrane potential and ability to make ATP.48
Blocking Orai1-mediated Ca2+ channels by genetic and pharmacologic means prevents mitochondrial failure and largely alleviates pancreatitis responses. The results suggest that aberrant global and sustained increases in [Ca2+]i contribute to the pathogenesis of acute pancreatitis and that pharmacologic approaches aimed at decreasing [Ca2+]i can be developed for the treatment of patients with pancreatitis.47
Another critically important function of the ER is protein synthesis and new protein folding and export.49 Of note, ER is highly developed in acinar cells to fulfill their main function of producing and secreting large amounts of protein. When misfolded secretory proteins are sensed at their sites of synthesis, cells first respond by activating an adaptive “unfolded protein response” that generally decreases new protein synthesis and up-regulates levels of chaperones that mediate new protein folding and export.49 The transcription factor spliced X protein-1 (sXBP1) plays a central role in the unfolded protein response. Nunnari and Suomalainen50 demonstrated that complete genetic deletion of XBP1 results in defects exclusively in secretory organs such as the pancreas and salivary glands. The deletion led to apoptosis of acinar cells during embryogenesis, resulting in an atrophic pancreas at birth. We found that when XBP1 is inhibited by heterozygous (ie, not complete) deletion, there is inhibition of the secretory response to neurohumoral stimulation and that there is acinar cell injury and an increase in the expression of transcription factor CCAAT-enhancer-binding protein homologous protein (CHOP) associated with acinar injury and pancreatitis responses.14,51 The role of CHOP has been examined in other tissues and shown effects on mitochondrial pathways and inflammatory signaling.52,53 We found that inhibition of CHOP in acinar cells prevents cell death responses.51
Protein folding involves ER-based chaperones and formation of disulfide groups between cysteine residues in proteins using oxidoreductase enzymes in the ER.12,45,46,54,55 Optimal performance for disulfide bond formation requires a normal redox state in the ER.49 Our studies show that the redox state is altered by alcohol feeding, which makes the ER environment more oxidative.12,55 However, ER maintains homeostasis even with an altered redox state; the main protective mechanism is through sXBP1, which upregulates the folding systems in the ER despite its altered redox states.12,46,47,54,55 Conversely, with decreased sXBP1 expression by genetic means, acinar injury and pancreatitis responses ensue.12,14,51
Most interestingly, we recently reported that although ethanol feeding up-regulates sXBP1, the addition of smoking results in inhibition of the sXBP1 response, associated with an increase in CHOP and pancreatitis responses, in particular acinar cell death.51 These findings are important to our understanding of the effects of alcohol and smoking on the pancreas and explain epidemiologic studies indicating that smoking promotes alcoholic pancreatitis.6,18,21,22,48,49 It is likely that redox alterations in the ER are responsible for the changes in sXBP1 and CHOP because treatment with the antioxidant N-acetylcysteine prevents the increase in CHOP and acinar death responses.51
Mitochondria are responsible for a range of cellular functions; their major physiologic role is generation of ATP.50,51 Mitochondria also are critical in regulating cell survival.52 In terms of pathobiologic responses of pancreatitis, mitochondrial membrane permeabilization is a universal trigger of apoptosis and necrosis. It is mediated by persistent opening of the mitochondrial permeability transition pore (MPTP), a multiprotein nonspecific channel traversing the inner and outer mitochondrial membrane.52–54 In its “open” conformation, MPTP allows unregulated entry of solutes less than 1500 Da (including water) into the matrix, resulting in mitochondrial depolarization and inhibition of mitochondrial ability to synthesize ATP, leading to loss of cellular functions and necrosis. Various stresses, such as mitochondrial Ca2+ overload and excessive reactive oxygen species generation, cause MPTP opening. The MPTP backbone is organized around the mitochondrial resident protein cyclophilin D (CypD); and CypD inhibition by genetic, molecular, or pharmacologic means blocks MPTP opening. Blocking MPTP prevents mitochondrial failure and necrosis.
Of note, individual mitochondria interact with each other forming a tubular–circular dynamic network, which largely determines mitochondrial activity.55 Processes of mitochondria fission and fusion are highly regulated and enable the cell to adapt to metabolic stresses. Disorders of acinar cell mitochondrial dynamics occur in pancreatitis, resulting in inadequate ATP production, increased mitochondrial reactive oxygen species, and impaired Ca2+ transport.56
Mitochondrial dysfunction occurs across various models of pancreatitis.19,56–59 Its main manifestation is persistent opening of MPTP, resulting in loss of mitochondrial membrane potential and mitochondrial fragmentation. Mechanisms of MPTP opening in experimental pancreatitis are model specific. In models induced by high doses of cerulein, the aberrant increases in [Ca2+]i lead to mitochondrial Ca2+ overload, resulting in MPTP opening.58 Conversely, MPTP opening in arginine-induced pancreatitis is mediated by inhibition of ATP synthase56 and in alcohol-induced pancreatitis is mediated by a decrease in the ratio of nicotinamide adenine dinucleotide to nicotinamide adenine dinucleotide plus hydrogen resulting from oxidative alcohol metabolism.19 Importantly, independent of the underlying mechanisms, MPTP opening in all models of pancreatitis is CypD dependent. CypD genetic or pharmacologic inactivation prevents mitochondrial depolarization, resulting in restoration of mitochondrial function and greatly decreased local (pancreatic), systemic, and distant (pulmonary) pathologic responses in various experimental models of pancreatitis.19,56–59
Endolysosomal–Autophagy System
Autophagy (macroautophagy) is the principal cellular pathway for degradation and recycling of organelles, lipids, and long-lived proteins. It begins with sequestration of the material destined for degradation into autophagosomes, which then fuse with lysosomes forming the autolysosomes in which cargo is degraded by lysosomal hydrolases.60,61 Impaired autophagy is a characteristic feature of various models of experimental pancreatitis, caused by the decreased ability of lysosomes to degrade cargo and a concomitant increase in autophagosome formation.56,62–65 Experimental pancreatitis is associated with severe defects in lysosomes.41,57,62,63,66 These include defective processing (maturation) of cathepsins and major lysosomal proteases, manifested by a lower level of fully processed and accumulated intermediate forms of cathepsins.65 Concomitantly, cathepsins’ enzymatic activities decrease in lysosome-enriched pancreatic subcellular fractions from animals with pancreatitis.65,67 Pancreatitis causes alterations in the localization of lysosomal vacuolar proton ATPase, which maintains acidic pH in the lysosomal lumen.68 Levels of lysosomal-associated membrane proteins (LAMPs), which are critical for maintaining the structure and function of lysosomes, dramatically decrease across various experimental models of nonalcoholic and alcoholic pancreatitis.69 Accumulation in acinar cells of abnormally large autolysosomes containing poorly degraded cargo, a key manifestation of defective autophagy, has long been recognized as an early marker of pancreatitis.62
Studies using genetic models targeting the endolysosomal–autophagy system provide mechanistic insights into the role of this system in maintaining pancreatic function and homeostasis. The role of autophagy was analyzed in detail in mice with pancreas-specific knockouts of key mediators of autophagosome formation, the autophagy-related proteins (ATG) ATG5 or ATG7.70,71 Atg5Δpan and Atg7Δpan mice developed spontaneous pancreatitis, with trypsinogen activation, fibrosis, inflammation, acinar-to-ductal metaplasia, and pancreas atrophy. In addition, the impaired lysosomal function in LAMP2-null mice resulted in spontaneous pancreatitis, starting with acinar cell vacuolization and progressing to severe pancreas damage characterized by trypsinogen activation, macrophage-driven inflammation, and acinar cell death.69 Further, LAMP2 deficiency increased the severity of experimental pancreatitis induced by cerulein treatment.69 Importantly, administration of trehalose, a natural disaccharide known to enhance autophagy, largely prevented trypsinogen activation, necrosis, and other parameters of pancreatic injury in mice in arginine- and cerulein-induced pancreatitis models.56 Together, these findings show the essential role of dysfunction of the endolysosomal- autophagy system in pancreatitis development and suggest pharmacologic approaches to enhance autophagy efficiency for human pancreatitis treatment.
Of note, tissue specimen analysis from patients with pancreatitis shows manifestations of endolysosomal–autophagy system disorders in human disease similar to those in rodent models.56,64,65,72
Interrelations Between Acinar Cell Organelle Disorders in Pancreatitis
Several studies indicate that organelles in the acinar cell form an interconnected system and that pathology of one organelle can lead to failure of the entire network. For example, blocking autophagy by genetic ablation of ATG5 or ATG7 or by genetic deletion of the inhibitor of nuclear factor kB kinase subunit a result in ER stress and accumulation of dysfunctional mitochondria unable to generate ATP.64,70,71 Conversely, restoring mitochondrial function by CypD genetic ablation alleviates ER stress and increases performance of the endolysosomal–autophagy system in experimental pancreatitis.56 As another example, XBP1 deficiency causing ER stress also causes mitochondrial dysfunction manifest by decreased oxidative phosphorylation. Abnormal increases in [Ca2+]i from ER calcium depletion and excessive Ca2+ influx into the acinar cell cause mitochondrial depolarization and failure and defects in endocytosis.73 Although these examples show interaction between organelles of the acinar cell, the complete extent of the mechanisms of interaction is yet to be shown.
In sum, these studies indicate that acinar cell organelle dysfunctions play a key role in the pathogenesis of acute pancreatitis, and that there are interactions between organelles so that dysfunctions in one can lead to disorders of others. Future work should focus on determining the mechanisms of these interactions because it is likely that interventions to prevent the spread of disorders across organelles will limit the severity of the disease.
How Do Injury Signals from Acinar Cells Cause Local and Systemic Inflammation?
Accumulating evidence indicates that acinar cell organelle damage triggers the inflammatory response of pancreatitis, although the underlying mechanisms remain to be investigated. As an example, autophagy blockade through disruption of genes encoding ATG5, ATG7, LAMP2, or inhibitor of nuclear factor κB kinase subunit α stimulates activation in acinar cells of proinflammatory transcription factors, such as nuclear factor κB and signal transducer and activator of transcription 3, resulting in up-regulation of cytokines and chemokines and inflammatory cell infiltration in the pancreas.71,72 The mechanisms of these processes likely involve increases in reactive oxygen species owing to defective clearance of damaged or depolarized mitochondria or ER dysfunction. Organelle damage also can mediate inflammasome formation in pancreatitis.71
Acute pancreatitis is associated with significant acinar cell death.41 Dying and necrotic cells release damage-associated molecular patterns (DAMPs) and other molecules that stimulate and activate inflammatory responses.74–76 DAMPs, such as the high mobility group box 1 (HMGB1) chromatin protein released from damaged cells, have been shown to play an important role in experimental pancreatitis77; and circulating HMGB1 levels have been correlated with severity of clinical acute pancreatitis.74.78 However, intracellular HMGB1 mitigates the inflammatory response and pancreatic damage,79 suggesting that DAMPs released extracellularly activate inflammatory signals and exacerbate acute inflammation locally and in distant sites, whereas intracellular localization of HMGB1 is anti-inflammatory.
In addition to HMGB1, DNA released from dying and necrotic cells is a well-described DAMP. In fact, DNA released from necrotic cells has been shown to be a potent activator of the innate immune system involving dendritic cells and macrophages.75,80,81 Moreover, DNA in the circulation contributes to autoimmune disease such as systemic lupus erythematosus82 and is associated with severity of clinical acute pancreatitis.78 Pathogen- and host-derived DNA activates cyclic guanosine and adenosine mono-phosphate synthase and generates the second-messenger cyclic guanosine and adenosine monophosphate, which activates stimulator of interferon genes (STING) signaling and generation of interferon type I.83,84 STING activation in macrophages by DNA derived from dying acinar cells and generation of proinflammatory cytokines including inter-feron β and tumor necrosis factor α was recently reported in experimental acute pancreatitis and exacerbated the disease.85 In the absence of STING signaling, macrophages did not mount the observed interferon β response to dying acinar cells, indicating the direct link between acinar cell death-released DNA and immune activation leading to the generation of proinflammatory cytokines. These findings are consistent with reports of STING activation and DNA sensing from dying and necrotic cells that promote inflammatory diseases.86
DNA also can be sensed by Toll-like receptors, such as Toll-like receptor 9, and activate inflammasome pathways (eg, nucleotide-binding oligomerization domain-like receptor protein 3) and promote inflammation in acute pancreatitis.87 Thus, inflammation in acute pancreatitis might be triggered by nuclear components released from damaged and dying acinar cells through different mechanisms described earlier. Additional mechanisms that initiate inflammation include cytokines and chemokines initially released by acinar cells in response to injury that have been extensively reviewed recently.63,88
Summary
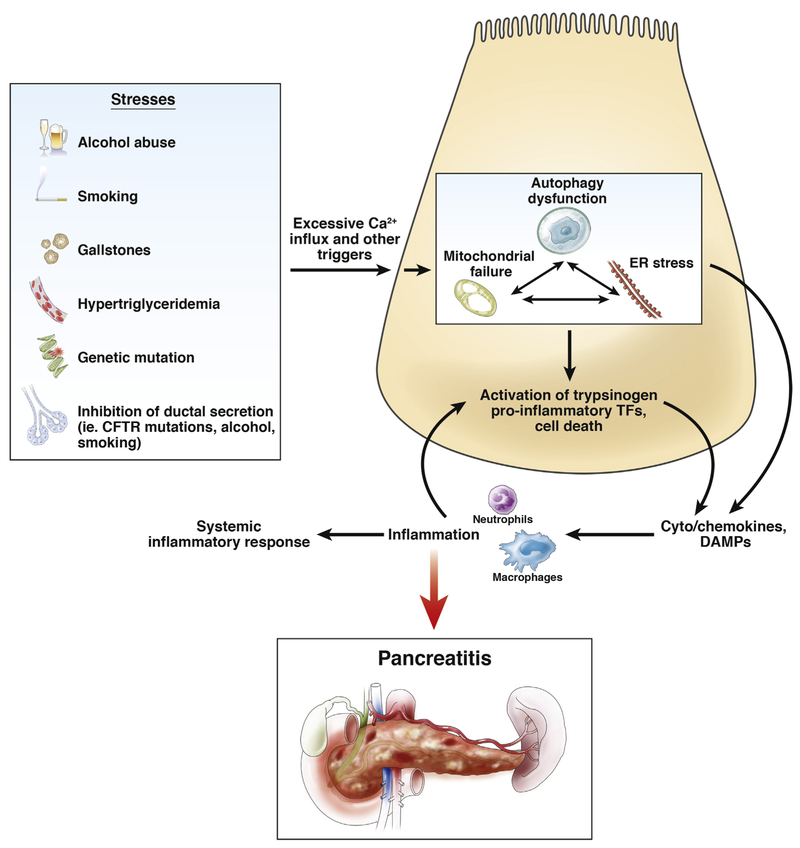
Figure 1 presents a summary of this article in showing environmental stressors and genetic factors known to increase the risk for pancreatitis. As pointed out earlier, some risk factors act in combination to promote the disease as exemplified to alcohol abuse and smoking; and it is likely that genetic alterations also can increase susceptibility when combined with a lifestyle factor such as alcohol abuse.37 Although not completely explored, stressors such as gallstones and the effect their passage has on reflux of bile acids into the pancreas could act through increased calcium influx to cause mitochondrial de-energinization.89–91
Figure 1.
Summary of environmental stressors and genetic factors known to increase the risk for pancreatitis. CFTR, cystic fibrosis transmembrane conductance regulator; TFs, transcription factors.
This article addresses the effect of acinar cell stressors on the function of intracellular organelles and their dysfunction during disease development and focuses on mitochondria, ER, and autophagy. An important point to stress is that our studies show that once one of the organelles fails, the others likely follow.58,65,71 This observation has significance related to development of therapeutics. Pancreatitis is an inflammatory disease, which is local for milder disease and involves distant organs for more severe disease.88,92,93 This review presents newly developed information about the inflammatory mechanisms related to DAMPs. An important observation with relevance to therapeutic application is that the inflammatory response can promote further acinar cell injury, including necrosis, creating a feed-forward process to create more inflammation.94 Thus, early intervention to address the mechanisms discussed in this article is expected to prevent the feed-forward inflammation and necrosis process.
Gaps and Future Directions to Improve Outcome in Patients with Acute Pancreatitis
This review points out that stressors that cause pancreatitis initially cause organelle disorders in the acinar cell of the pancreas leading to a subsequent inflammatory response that is localized to the pancreas in milder disease or systemic in severe disease. Further, this review shows key pathobiologic processes that are the foundation for therapeutics development.
The key questions that remain are whether treatments are needed to address more than 1 organelle disorder simultaneously (eg, resolving autophagy and mitochondrial failure at the same time) and whether treatment must be initiated at the earliest stages of the disease to prevent the full manifestation of pancreatitis. It will be important to identify the mechanisms whereby organelle dysfunctions and pathologic interactions between organelles promote cell death (necrosis) and generate pancreatitis responses—inflammation in particular. Further, there could be a consideration for treating organelle failure and the inflammatory cascade simultaneously. Some of these questions can be addressed in preclinical models to provide better clinical strategies for the field.
However, much of our progress will come from observing responses to agents in human clinical trials with an emphasis on timing of delivery and methods of measurement of organelle function and inflammatory pathways, possibly during different stages of the disease. That is, there could be therapies during the early stage that reestablish organelle homeostasis and treatments later in the disease that address inflammation and the systemic in-flith an e response, which could be more important.
Progress will come from keeping these concepts in mind during trial designs, including exploratory measures performed to continue to advance our understanding, and using this information for subsequent trial designs and measurements.
Funding
NIH grants: P50 AA11999, P01 DK98108, U01 DK108314, U01 DK108300, R01 DK092421, R01 DK59936, R01 AA024464, DCP NWU2014-04-01. DOD grants: W81XWH-17-1-0339, W81XWH-15-1-0258, W81XWH-17-1-0138. Department of Veterans Affairs Merit Review.
Abbreviations used in this paper:
- ATG
autophagy-related protein
- ATP
adenosine triphosphate
- [Ca2+]i
ionized calcium in cytosol
- CCK
cholecystokinin-8
- CHOP
CCAAT-enhancer-binding protein homologous protein
- CypD
cyclophilin D
- DAMP
damage-associated molecular pattern
- ER
endoplasmic reticulum
- HMGB1
high mobility group box 1
- LAMP
lysosomal-associated membrane protein
- MPTP
mitochondrial permeability transition pore
- STING
stimulator of interferon genes
- sXBP1
spliced X protein-1
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 2007;132:1127–1151. [DOI] [PubMed] [Google Scholar]
- 2.Vege SS, DiMagno MJ, Forsmark CE, et al. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology 2018;154:1103–1139. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–1187.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013; 144:1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med 2016;375:1972–1981. [DOI] [PubMed] [Google Scholar]
- 6.Cote GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011; 9:266–273; quiz e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosen-Binker LI, Lam PP, Binker MG, et al. Alcohol/cholecystokinin-evoked pancreatic acinar basolateral exocytosis is mediated by protein kinase C alpha phosphorylation of Munc18c. J Biol Chem 2007; 282:13047–13058. [DOI] [PubMed] [Google Scholar]
- 8.Gukovskaya AS, Mareninova OA, Odinokova IV, et al. Cell death in pancreatitis: effects of alcohol. J Gastroenterol Hepatol 2006;21(Suppl 3):S10–S13. [DOI] [PubMed] [Google Scholar]
- 9.Hegyi P, Pandol S, Venglovecz V, Rakonczay Z Jr. The acinar-ductal tango in the pathogenesis of acute pancreatitis. Gut 2011;60:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam PP, Cosen Binker LI, Lugea A, et al. Alcohol redirects CCK-mediated apical exocytosis to the acinar basolateral membrane in alcoholic pancreatitis. Traffic 2007;8:605–617. [DOI] [PubMed] [Google Scholar]
- 11.Lugea A, Gong J, Nguyen J, et al. Cholinergic mediation of alcohol-induced experimental pancreatitis. Alcohol Clin Exp Res 2010;34:1768–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lugea A, Tischler D, Nguyen J, et al. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 2011;140:987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lugea A, Waldron RT, French SW, Pandol SJ. Drinking and driving pancreatitis: links between endoplasmic reticulum stress and autophagy. Autophagy 2011;7:783–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lugea A, Waldron RT, Mareninova OA, et al. Human pancreatic acinar cells: proteomic characterization, physiologic responses, and organellar disorders in ex vivo pancreatitis. Am J Pathol 2017;187:2726–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lugea A, Waldron RT, Pandol SJ. Pancreatic adaptive responses in alcohol abuse: role of the unfolded protein response. Pancreatology 2015;15:S1–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maleth J, Balazs A, Pallagi P, et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology 2015;148:427–439 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satoh A, Gukovskaya AS, Reeve JR Jr, et al. Ethanol sensitizes NF-kappaB activation in pancreatic acinar cells through effects on protein kinase C-epsilon. Am J Physiol Gastrointest Liver Physiol 2006;291:G432–G438. [DOI] [PubMed] [Google Scholar]
- 18.Setiawan VW, Monroe K, Lugea A, et al. Uniting epidemiology and experimental disease models for alcohol-related pancreatic disease. Alcohol Res 2017;38:173–182. [PMC free article] [PubMed] [Google Scholar]
- 19.Shalbueva N, Mareninova OA, Gerloff A, et al. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology 2013; 144:437–446 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu S, Chheda C, Ouhaddi Y, et al. Characterization of mouse models of early pancreatic lesions induced by alcohol and chronic pancreatitis. Pancreas 2015;44:882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setiawan VW, Pandol SJ, Porcel J, et al. Prospective study of alcohol drinking, smoking, and pancreatitis: the Multiethnic Cohort. Pancreas 2016;45:819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med 2009; 169:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valdivielso P, Ramírez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med 2014;25:689–694. [DOI] [PubMed] [Google Scholar]
- 24.Adiamah A, Psaltis E, Crook M, Lobo DN. A systematic review of the epidemiology, pathophysiology and current management of hyperlipidaemic pancreatitis. Clin Nutr 2018;37:1810–1822. [DOI] [PubMed] [Google Scholar]
- 25.Nawaz H, Koutroumpakis E, Easler J, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol 2015;110:1497–1503. [DOI] [PubMed] [Google Scholar]
- 26.Sue LY, Batech M, Yadav D, et al. Effect of serum triglycerides on clinical outcomes in acute pancreatitis: findings from a regional integrated health care system. Pancreas 2017;46:874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Perez A, Schlienger RG, Rodríguez LA. Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care 2010;33:2580–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai SW, Muo CH, Liao KF, et al. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol 2011;106:1697–1704. [DOI] [PubMed] [Google Scholar]
- 29.Shafqet M, Sharzehi K. Diabetes and the pancreatobiliary diseases. Curr Treat Options Gastroenterol 2017; 15:508–519. [DOI] [PubMed] [Google Scholar]
- 30.Forsmark ChE Vege SS, Wilcox CM Acute pancreatitis. N Engl J Medicine 2017;376:598–599. [DOI] [PubMed] [Google Scholar]
- 31.Azoulay L, Filion KB, Platt RW, et al. Association between incretin-based drugs and the risk of acute pancreatitis. JAMA Intern Med 2016;176:1464–1473. [DOI] [PubMed] [Google Scholar]
- 32.Forsmark CE. Incretins, diabetes, pancreatitis and pancreatic cancer: what the GI specialist needs to know. Pancreatology 2016;16:10–13. [DOI] [PubMed] [Google Scholar]
- 33.Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab 2017; 19:1233–1241. [DOI] [PubMed] [Google Scholar]
- 34.Storgaard H, Cold F, Gluud LL, et al. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab 2017;19:906–908. [DOI] [PubMed] [Google Scholar]
- 35.Jalaly NY, Moran RA, Fargahi F, et al. An evaluation of factors associated with pathogenic PRSS1, SPINK1, CTFR, and/or CTRC genetic variants in patients with idiopathic pancreatitis. Am J Gastroenterol 2017; 112:1320–1329. [DOI] [PubMed] [Google Scholar]
- 36.Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology 2013;144:1292–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet 2012;44:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S, Ooi CY, Werlin S, et al. Risk factors associated with pediatric acute recurrent and chronic pancreatitis: lessons from INSPPIRE. JAMA Pediatr 2016;170:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uc A, Andersen DK, Bellin MD, et al. Chronic pancreatitis in the 21st century—research challenges and opportunities: summary of a National Institute of Diabetes and Digestive and Kidney Diseases workshop. Pancreas 2016;45:1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandol SJ. The exocrine pancreas. San Rafael, CA: Morgan and Claypool Life Sciences, 2010. [PubMed] [Google Scholar]
- 41.Gukovskaya AS, Pandol SJ, Gukovsky I. New insights into the pathways initiating and driving pancreatitis. Curr Opin Gastroenterology 2016;32:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venglovecz V, Rakonczay Z Jr, Ozsvari B, et al. Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea pig. Gut 2008;57:1102–1112. [DOI] [PubMed] [Google Scholar]
- 43.Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology 2013;144:1180–1193. [DOI] [PubMed] [Google Scholar]
- 44.Kubisch CH, Logsdon CD. Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 2007;292:G1804–G1812. [DOI] [PubMed] [Google Scholar]
- 45.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 2014;21:396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandol SJ, Gorelick FS, Lugea A. Environmental and genetic stressors and the unfolded protein response in exocrine pancreatic function—a hypothesis. Front Physiol 2011;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waldron RT, Su HY, Piplani H, et al. Ethanol induced disordering of pancreatic acinar cell endoplasmic reticulum: an ER stress/defective unfolded protein response model. Cell Mol Gastroenterol Hepatol 2018;5:479–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yadav D, Slivka A, Sherman S, et al. Smoking is under-recognized as a risk factor for chronic pancreatitis. Pancreatology 2010;10:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol 2010; 7:131–145. [DOI] [PubMed] [Google Scholar]
- 50.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell 2012;148:1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spinelli JB, Haigis MC. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol 2018; 20:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 2007; 87:99–163. [DOI] [PubMed] [Google Scholar]
- 53.Baines CP, Gutierrez-Aguilar M. The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore. Cell Calcium 2018; 73:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernardi P, Rasola A, Forte M, Lippe G. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev 2015;95: 1111–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H, Yoon Y. Mitochondrial fission and fusion. Biochem Soc Trans 2016;44:1725–1735. [DOI] [PubMed] [Google Scholar]
- 56.Biczo G, Vegh ET, Shalbueva N, et al. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology 2018;154:689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gukovsky I, Pandol SJ, Gukovskaya AS. Organellar dysfunction in the pathogenesis of pancreatitis. Antioxid Redox Signal 2011;15:2699–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukherjee R, Mareninova OA, Odinokova IV, et al. Mechanism of mitochondrial permeability transition pore induction and damage in the pancreas: inhibition prevents acute pancreatitis by protecting production of ATP. Gut 2016;65:1333–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Odinokova IV, Sung KF, Mareninova OA, et al. Mechanisms regulating cytochrome c release in pancreatic mitochondria. Gut 2009;58:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016;12:1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol 2014;16:495–501. [DOI] [PubMed] [Google Scholar]
- 62.Yuan J, Liu Y, Tan T, Guha S, Gukovsky I, Gukovskaya A, Pandol SJ. Protein kinase d regulates cell death pathways in experimental pancreatitis. Front Physiol 2012;3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gukovskaya AS, Gukovsky I, Algul H, Habtezion A. Autophagy, inflammation, and immune dysfunction in the pathogenesis of pancreatitis. Gastroenterology 2017; 153:1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li N, Wu X, Holzer RG, et al. Loss of acinar cell IKKalpha triggers spontaneous pancreatitis in mice. J Clin Invest 2013;123:2231–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest 2009;119:3340–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gukovsky I, Gukovskaya A. Nuclear factor-kappaB in pancreatitis: jack-of-all-trades, but which one is more important? Gastroenterology 2013;144:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saluja A, Hashimoto S, Saluja M, et al. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 1987;253:G508–G516. [DOI] [PubMed] [Google Scholar]
- 68.Thrower EC, Diaz de Villalvilla AP, Kolodecik TR, Gorelick FS. Zymogen activation in a reconstituted pancreatic acinar cell system. Am J Physiol Gastrointest Liver Physiol 2006;290:G894–G902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mareninova OA, Sendler M, Malla SR, et al. Lysosome associated membrane proteins maintain pancreatic acinar cell homeostasis: LAMP-2 deficient mice develop pancreatitis. Cell Mol Gastroenterol Hepatol 2015; 1:678–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antonucci L, Fagman JB, Kim JY, et al. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc Natl Acad Sci U S A 2015;112:E6166–E6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diakopoulos KN, Lesina M, Wörmann S, et al. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology 2015;148:626–638.e17. [DOI] [PubMed] [Google Scholar]
- 72.Fortunato F, Burgers H, Bergmann F, et al. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology 2009;137:350–360 e1–5. [DOI] [PubMed] [Google Scholar]
- 73.Chvanov M, De Faveri F, Moore D, et al. Intracellular rupture, exocytosis and actin interaction of endocytic vacuoles in pancreatic acinar cells: initiating events in acute pancreatitis. J Physiol 2018;596:2547–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yasuda T, Ueda T, Takeyama Y, et al. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas 2006;33:359–363. [DOI] [PubMed] [Google Scholar]
- 75.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood 2007; 109:1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDonald B, Kubes P. Innate immune cell trafficking and function during sterile inflammation of the liver. Gastroenterology 2016;151:1087–1095. [DOI] [PubMed] [Google Scholar]
- 77.Sawa H, Ueda T, Takeyama Y, et al. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol 2006; 12:7666–7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kocsis AK, Szabolcs A, Hofner P, et al. Plasma concentrations of high-mobility group box protein 1, soluble receptor for advanced glycation end-products and circulating DNA in patients with acute pancreatitis. Pancreatology 2009;9:383–391. [DOI] [PubMed] [Google Scholar]
- 79.Kang R, Zhang Q, Hou W, et al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology 2014; 146:1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med 1999; 5:1249–1255. [DOI] [PubMed] [Google Scholar]
- 81.Sauter B, Albert ML, Francisco L, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 2000;191:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Napirei M, Karsunky H, Zevnik B, et al. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet 2000;25:177–181. [DOI] [PubMed] [Google Scholar]
- 83.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008;455:674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ishikawa H, Ma Z, Barber GN. STING regulates intra-cellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009;461:788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Q, Wei Y, Pandol SJ, et al. STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology 2018;154:1822–1835 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A 2012;109:19386–19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 2011;141:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Habtezion A Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol 2015;31:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim JY, Kim KH, Lee JA, et al. Transporter-mediated bile acid uptake causes Ca2þ-dependent cell death in rat pancreatic acinar cells. Gastroenterology 2002; 122:1941–1953. [DOI] [PubMed] [Google Scholar]
- 90.Kim MS, Hong JH, Li Q, et al. Deletion of TRPC3 in mice reduces store-operated Ca2þ in flux and the severity of acute pancreatitis. Gastroenterology 2009;137: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petersen OH, Sutton R. Ca2 signalling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci 2006;27:113–120. [DOI] [PubMed] [Google Scholar]
- 92.Hart PA, Bellin MD, Andersen DK, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol 2016;1:226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013;144:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gukovskaya AS, Vaquero E, Zaninovic V, et al. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology 2002;122:974–984. [DOI] [PubMed] [Google Scholar]