Abstract
Tinnitus, sound perception in the absence of physical stimuli, occurs in 15% of the population, and is the top-reported disability for soldiers after combat. Noise overexposure is a major factor associated with tinnitus but does not always lead to tinnitus. Furthermore, people with normal audiograms can get tinnitus. In animal-models, equivalent cochlear damage occurs in animals with and without behavioral evidence of tinnitus. But, cochlear-nerve recipient neurons in the brainstem demonstrate distinct, synchronized spontaneous firing patterns only in animals that develop tinnitus, driving activity in central brain regions and ultimately giving rise to phantom perception. Examining tinnitus-specific changes in single-cell populations enables us to begin to disctinguish neural changes due to tinnitus from those that are due to hearing loss.
Keywords: Tinnitus, cochlear nucleus, central auditory pathways, spontaneous firing rate, spontaneous neural synchrony, somatosensory-auditory integration, stimulus-timing-dependent plasticity
In Brief
In ‘Mechanisms of noise-induced tinnitus: Insights from cellular studies’
Shore and Wu discuss single-neuron studies showing distinct brainstem signatures in animals with behavioral evidence of tinnitus. Transmission of tinnitus-specific, synchronous activity to cortex is necessary for eventual phantom perception.
Introduction
For those estimated 15 million in the United States and quarter billion people worldwide with debilitating tinnitus (McCormack et al., 2016; Shargorodsky et al., 2010), the sound of silence is a dream out of reach. For decades no gold standard of treatment has existed due to limited understanding of the mechanisms underlying the generation of the phantom sounds, commonly called tinnitus. With the advent of new technologies and innovative approaches some light is now being shed on the neural processes involved and new and innovative treatments are emerging.
Scientists generally agree that tinnitus is generated within the brain in response to a reduction of auditory nerve fiber input from the cochlea to the brain. By far the major cause of cochlear damage resulting in deafferentation is environmental noise overexposure (Agrawal et al., 2008, 2009). Even in cases where there is no measurable change in audiometric thresholds after noise exposure, loss of auditory nerve synapses in the cochlea can occur (see ‘hidden hearing loss’, below). Cochlear synaptopathy can result in tinnitus when accompanied by homeostatic and timing-dependent plasticity beginning in the first brain station that receives input from the cochlea, the cochlear nucleus (CN). As will be described below, noise-induced cochlear synaptopathy appears to be necessary but not sufficient to produce tinnitus because animals with equivalent synaptopathy but lacking the requisite neural plasticity do not show behavioral evidence of tinnitus (Heeringa et al., 2018a; Li et al., 2015; Marks et al., 2018; Wu et al., 2016). Careful study of the mechanisms and time course of changes to neural-plasticity, or metaplasticity, is necessary for the development of treatments to reverse pathological plasticity, as evidenced by recent advances in translating animal models into human clinical trials (De Ridder et al., 2014; Engineer et al., 2011; Marks et al., 2018).
While perception ultimately takes place in the auditory cortex, current theories of cortical involvement in tinnitus presume that the auditory cortex receives decreased excitatory input due to cochlear deafferentation. This assumption is, however, erroneous, because a plethora of literature documents unequivocally that while the output of the cochlea to the CN is decreased, the output of the first auditory brain station is increased after deafferentation due to homeostatic or timing-dependent plasticity. The tinnitus-specific increases that are observed in CN neural spontaneous rates and synchrony are also evident in target nuclei in the medial geniculate body, but so far there is no consensus as to whether these are also conveyed to the inferior colliculus along the ascending auditory pathway (Fig. 1). The alternate route to the auditory cortex would be the direct connection from CN to the thalamus, which bypasses the inferior colliculus. While tinnitus pathophysiology likely involves the descending (corticofugal) pathways as well as the ascending pathways, that evidence is currently lacking. Thus, the focus of this review is on the generation of tinnitus in the CN and its likely route of transmission to the midbrain, thalamus and ultimately cortex.
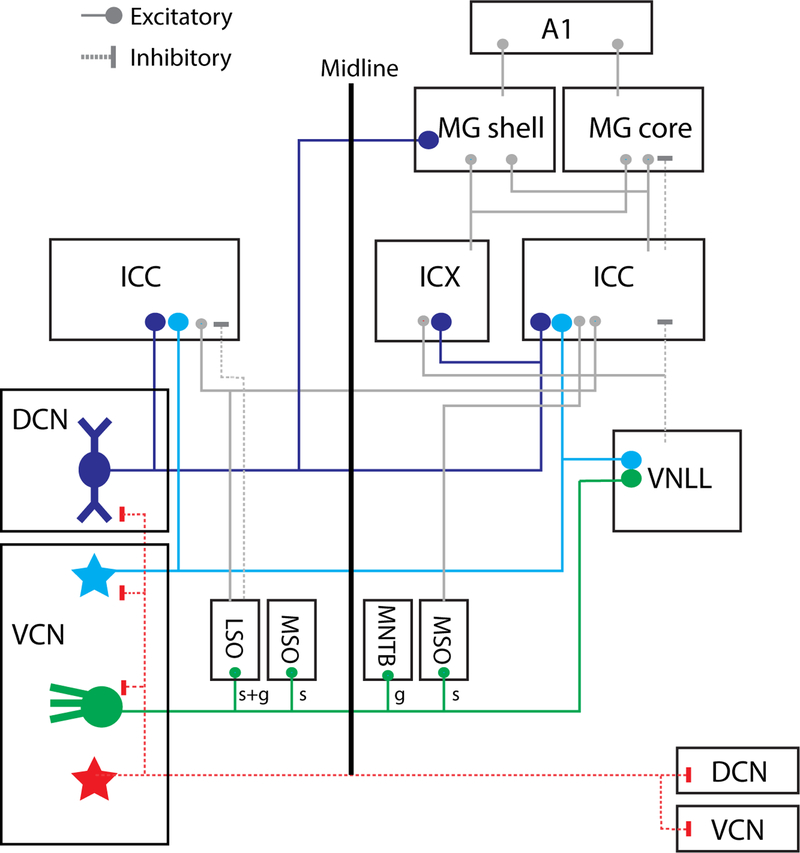
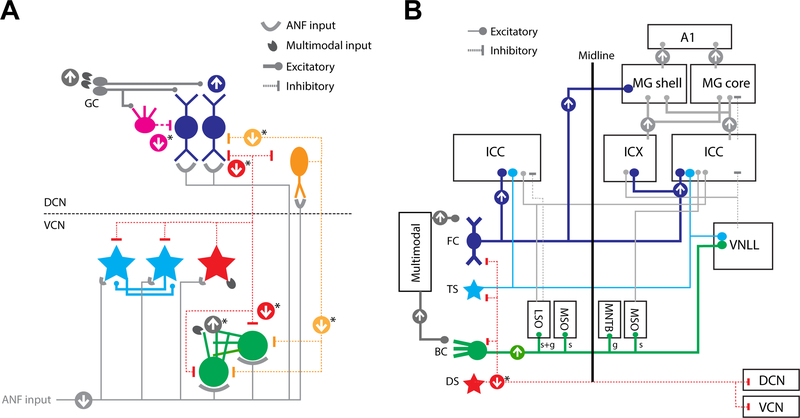
Fig. 1: Ascending projections of CN output neurons.
DCN fusiform cells (FC) send projections to the contralateral central nucleus of inferior colliculus (ICC; predominant), external cortices (ICX; sparse), ipsilateral IC, and contralateral non-lemniscal medial geniculate (MG shell). VCN T-stellate cells (TS) project to the contralateral (predominant) ICC and ventral nucleus of lateral lemniscus (VNLL). Spherical bushy cells (BC; spherical: s) project to the ipsilateral lateral superior olive (LSO), medial superior olive (MSO), and contralateral MSO. Globular BC (g) project to the ipsilateral LSO, and contralateral medial nucleus of trapezoid body (MNTB). D-stellate cells (DS) project to contralateral CN and ipsilateral DCN. Inhibitory projections are shown in red.
Information flow and multimodal integration in the cochlear nuclei
The CN, as the immediate recipient of auditory nerve fiber input to the brain, plays an important task in funneling ascending information to the rest of the brain. Distinct cell types (Fig. 2), with different intrinsic physiological properties and synaptic inputs, form parallel pathways subserving distinct functions (Cant and Benson, 2003). The major excitatory output neurons of the ventral CN (VCN) are spherical and globular bushy cells that project bilaterally to superior olivary nuclei as well as T-stellate cells that project to contralateral SOC and IC. Inhibitory D-stellate neurons project to the contralateral CN and ipsilateral dorsal CN (DCN)(Arnott et al., 2004; Bledsoe et al., 2009)(Fig. 1). Fusiform cells are the principal output neurons of DCN projecting to the contralateral inferior colliculus (IC) and the medial geniculate (MG) (Anderson et al., 2006; Malmierca et al., 2002). Inhibitory inter-neurons in DCN and VCN also play important roles in modulating the temporal and spectral processing of principal neurons (Caspary et al., 1994; Moller, 1975; Wu and Shore, 2018). In addition to processing cochlear input, many cell types in the CN also receive multimodal input from somatosensory, vestibular, and reticular brain areas (Ryugo et al., 2003; Shore and Moore, 1998; Shore et al., 2000; Zhou and Shore, 2004). These projections terminate primarily onto granule cells, whose axons form parallel fibers and synapse on fusiform and cartwheel cells. Bushy cells and D-stellate cells in the anteroventral CN also receive axodendritic somatosensory inputs (Heeringa et al., 2018b; Wu and Shore, 2018), which modulate coding of monaural as well as binaural sound localization.
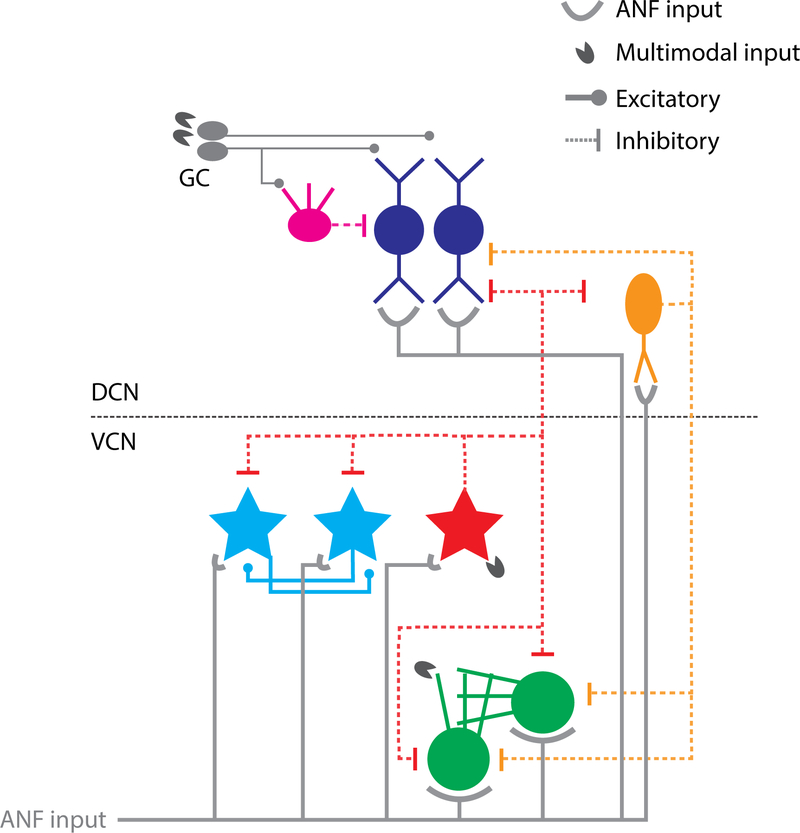
Fig. 2: Circuitry of VCN and DCN neurons putatively involved in tinnitus.
Auditory nerve fibers (ANF) from the cochlea synapse on the basal dendrites of fusiform cells (FC), dendrites of vertical cells (VS) and T-stellate cells (TS), cell bodies and dendrites of D-Stellate cells (DS) and cell bodies of bushy cells (BC). Multimodal projections synapse the dendritic fields of DS and BC in VCN (Heeringa et al., 2018b; Wu and Shore, 2018) and granule cells (GC), which project to the apical dendrites of FC and inhibitory interneuron cartwheel cells (CW). DS and VC provide wide-band and narrow-band inhibition, repectively, to the output neurons of DCN and VCN.
Is it tinnitus or is it hearing loss?
A challenge for the field is the undisputed connection between hearing loss and tinnitus. Because most subjects (animal and human) with tinnitus also have some degree of hearing loss (even if undetectable by the audiogram – see ‘hidden hearing loss’ below) it is often impossible to determine the contribution of hearing loss to tinnitus-related measures. This is particularly the case with human studies because the amount of cochlear damage cannot be experimentally controlled. On the other hand, while it is possible to precisely titrate the noise-exposure to produce expected cochlear damage in animals, the field has been challenged by difficulties with the development of reliable behavioral tests to signal the presence or absence of tinnitus in animals, as they are unable to tell us whether or not they have tinnitus. The increasingly utilized gap prepulse inhibition of the acoustic startle (GPIAS) technique (Berger et al., 2013; Turner et al., 2006), while providing reproducible results and valuable insights into tinnitus pathophysiology in animal models, can be affected by hearing loss, and therefore can only reliably be used if there is no measurable hearing loss in animals subjects. Even ‘hidden’ hearing loss could affect the perception of a silent gap in a background noise (Lauer et al., 2019). Therefore, this review has confined itself to studies in which hearing thresholds have returned to normal by the time of tinnitus assessment, and in which thresholds or suprathreshold hearing are equivalent in animals defined as having, or not having tinnitus. Furthermore, while GPIAS has not yet been fully confirmed in human subjects (Fournier and Hebert, 2016), in a key study, Fournier and Hebert (2013) demonstrated that humans with tinnitus did indeed demonstrate overall decreased startle reflexes in tinnitus subjects, as shown in animals. However, the underlying theory of “tinnitus filling the gap” could not be confirmed (see Galazyuk and Hebert, 2015). A plausible reason for this failure could be the high variability of eye blink startle responses demonstrated in human studies (Wilson et al., 2019). While further evidence in human studies is necessary to fully validate GPIAS in animals, well-controlled animal studies using GPIAS have led to the identification of physiological tinnitus signatures that are reproduced by studies using operant behavioral techniques (Brozoski et al., 2002; Kaltenbach et al., 2004; Zhang et al., 2016). Nonetheless, further studies are necessary to evaluate the extent to which GPIAS is a specific read-out for tinnitus or also reflects other plastic changes in brain circuits
While in studies with humans, it is difficult to separate effects due to hearing loss from those due to tinnitus, animal-model studies are able to carefully titrate noise exposure to produce temporary (TTS) rather than permanent threshold shifts (PTS) as well as limited suprathreshold hearing deficits. These studies can provide valuable insights into the differences between animals that develop tinnitus compared to those that do not develop tinnitus, which are distinct from those produced from hearing loss (Li et al., 2015; Wu et al., 2016).
The distribution of animals with and without evidence of tinnitus after TTS-producing noise exposure varies from 40–80%, across different studies (Kalappa et al., 2014; Li et al., 2015; Longenecker and Galazyuk, 2011; Wu et al., 2016). Examining the neural changes in animals with- and without behavioral evidence of tinnitus has revealed changes in the brain that are then ‘tinnitus-specific’ versus ‘hearing-loss contaminated’. For example, two studies from independent labs demonstrated alterations in CN neural activity that were correlated with tinnitus behavior but not with changes in auditory brainstem response (ABR) thresholds or suprathreshold ABR wave-1 amplitude (i.e., ABR responses to increasing levels of intensity) (Li et al., 2015; Wu et al., 2016). A subsequent study showed that alterations in vesicular glutamate transporters in the cochlear nucleus correlated with tinnitus behavior but not with ABRs or cochlear synaptopathy (Heeringa et al., 2018a)(Fig. 3). These studies suggest that because the changes occurring in the brain appear to be independent of cochlear damage status, the distinct changes that are observed in the brains of animals with behavioral evidence of tinnitus are indeed changes that are related to tinnitus itself and not to the peripheral, cochlear changes. On the other hand, the absence of changes in the brains of animals without behavioral evidence of tinnitus tell us something important about those animals’ resilience to tinnitus.
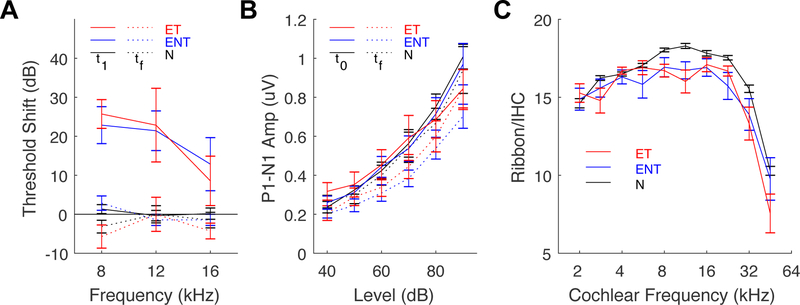
Fig. 3: Measures of cochlear pathophysiology and histopathology do not differ between animals with tinnitus and those without tinnitus.
(A) ABR threshold shifts immediately following noise exposure (t1, solid lines) and 12 weeks following noise exposure (tf, dashed lines). (B) ABR wave-1 amplitude (P1–N1) before (t0, solid lines) and 12 weeks after noise exposure (tf, dashed lines). (C) Ribbon synapse counts per IHC, as sampled at 10 locations along the cochlear spiral, converted to frequency according cochleotopic mapping. Exposed-tinnitus animals - red, exposed no-tinnitus animals (ENT) - blue, and sham-exposed normal animals (N) - black for all panels. Adapted from Heeringa et al. (2018a).
Where does tinnitus begin?
Animal-model studies have demonstrated that after noise exposures that lead to some damage of the cochlea, neurons in both the dorsal and ventral divisions of the first auditory brain station, the cochlear nucleus (Fig. 2), show increased SFR (Bledsoe et al., 2009; Kaltenbach and Afman, 2000; Kaltenbach et al., 1998; Kaltenbach and McCaslin, 1996; Vogler et al., 2011). In order to distinguish whether these effects are simply a reflection of cochlear-damage induced events or whether they are tinnitus-specific, several studies have expanded this approach to distinguish the animals in terms of whether they demonstrate behavioral evidence of tinnitus (Brozoski et al., 2002; Dehmel et al., 2012; Kaltenbach et al., 2004; Koehler and Shore, 2013a; Wu et al., 2016). These studies have provided insights by examining the neural changes in the CN and at different levels of the auditory system that are specific to animals with evidence of tinnitus and absent in those without tinnitus, starting in the DCN.
Dorsal cochlear nucleus
Using recordings from single neurons in the DCN, researchers from several different laboratories have demonstrated increased SFR in fusiform cells with best frequencies (BFs) close to the noise-exposure spectrum that occur immediately (Gao et al., 2016), as well as weeks after the noise damage (Kaltenbach et al., 2000). When behavioral tinnitus tests were employed, several of these studies also demonstrated that the increases in SFR were restricted to animals with behavioral evidence of tinnitus (GPIAS and operant) and correlated with tinnitus severity (Kaltenbach et al., 2004; Wu et al., 2016). A recent study showed that in addition to increases in SFR, there were also increases in cross-unit spike correlations or synchrony in animals with tinnitus but not in those without tinnitus (Wu et al., 2016). The increases in SFR and principal-neuron synchrony correlated strongly with tinnitus severity (Wu et al., 2016). The finding of increased synchrony between neurons in animals with tinnitus is especially important because cross-unit synchrony could potentially create perceptual grouping of auditory objects through neural patterns (Ilin et al., 2013) and thereby be transmitted to higher auditory centers to lead to the perception of a phantom sound in the absence of a physical stimulus.
Role of DCN in tinnitus generation and maintenance
In addition to consistent physiological correlates observed in DCN, lesion studies have further established that changes in neural-circuit activity associated with tinnitus initially occur in the DCN after noise-exposure. First, DCN lesions prior to noise exposure prevented tinnitus generation in rodents (Brozoski et al., 2012). Furthermore, after tinnitus-related changes were already established, transection of cochlear and descending inputs to DCN had no effects on the established increases in SFR (Zacharek et al., 2002; Zhang et al., 2006), suggesting that tinnitus arises de novo in DCN. A separate study, however, showed that DCN lesions failed to abolish tinnitus that was establish a priori (Brozoski and Bauer, 2005). The results of Brozoski and Bauer (2005) led to the conclusion that DCN is essential in tinnitus generation but not necessarily tinnitus maintenance. But it is important to note that the DCN lesions that study were incomplete and it was acknowledged by the authors that a small portion of DCN could probably maintain tinnitus signals and transmission to higher stations – as shown subsequently by Manzoor et al. (2012).
What are the factors underlying increased SFR and synchrony in DCN?
Several studies have demonstrated that homeostatic and long-term plasticity are altered in the CN after cochlear damage. Even a partial reduction of auditory nerve input to the DCN or VCN can result in decreased levels of inhibitory neurotransmitters including glycine and GABA as well as in alterations in their receptors on fusiform cells (Middleton et al., 2011; Wang et al., 2009). But importantly, in addition to the decrease in inhibitory neurotransmitters, there are also increases in excitatory neurotransmission as indicated by increases in vesicular glutamate transporters in different regions of CN and increases in glutamate receptors on principal neurons after both severe and partial cochlear damage (Barker et al., 2012; Heeringa et al., 2016; Heeringa et al., 2018a; Wang et al., 2009; Zeng et al., 2009). Furthermore, a recent study demonstrated that the increase in glutamatergic excitatory transmission was restricted to animals with behavioral evidence of tinnitus in CN regions associated with non-auditory pathways (Heeringa et al., 2018a). Other studies revealed that the upregulation of glutamatergic neurotransmission is due to an increased number of excitatory non-auditory projections to the CN (Zeng et al., 2012).
Potassium channels and HCN channels have been shown to be important for tinnitus generation and resilience (Li et al., 2013; Li et al., 2015; Pilati et al., 2012a; Pilati et al., 2012b). In studies on mice, Li et al. (2015) demonstrated that noise-induced tinnitus developed in mice that did not compensate for a reduction in KCNQ2/3, while those that did not develop behavioral evidence of tinnitus (using GPIAS) showed a re-emergence of KCNQ2/3 channel activity as well as a reduction in HCN channel activity. In addition to KCNQ2/3 (also termed Kv7), Pilati et al. (2012b) showed that reduction in Kv3 current underlies increased fusiform-cell bursting. Together with increased excitatory neurotransmission and decreased inhibitory neurotransmission, changes in intrinsic membrane excitability in DCN are associated with the development of tinnitus.
Spike timing dependent plasticity demonstrated in vitro (Tzounopoulos et al., 2004) and its macroscopic in vivo correlate, stimulus timing dependent plasticity (STDP) (Koehler and Shore, 2013b; Wu et al., 2015), are processes regulating neural activity in the DCN. Spike timing dependent plasticity is evaluated by the order of pre-and post-synaptic activity as well as by NMDA and acetylcholine modulation (Stefanescu and Shore, 2015, 2017). In vitro when presynaptic activation of parallel-fiber synapses precedes spikes in fusiform cells, long term potentiation (LTP) occurs (Hebbian plasticity). Similarly, in normal animals in vivo, stimulation of somatosensory nuclei that activate parallel fibers prior to sounds that elicit spikes in fusiform cells demonstrate primarily long term potentiation (LTP) (Koehler and Shore, 2013b). The reversed order elicits long term depression (LTD) in vitro and in vivo (Wu et al., 2015). However, in animals with tinnitus, assessed by GPIAS, fusiform cells show altered STDP with enhanced LTP (Fig. 4). In contrast, animals that did not develop tinnitus showed instead increased LTD (Koehler and Shore, 2013a; Marks et al., 2018). NMDA receptor changes as well as acetylcholine-mediated neuromodulation (Jin et al., 2006; Kaltenbach and Zhang, 2007; Stefanescu and Shore, 2015) play important roles in these effects (D’amour and Froemke, 2015). As postulated by modeling studies (Talathi et al., 2008), spike timing dependent plasticity plays a role in the increased synchronization between fusiform cells observed in animals with tinnitus (Marks et al., 2018).
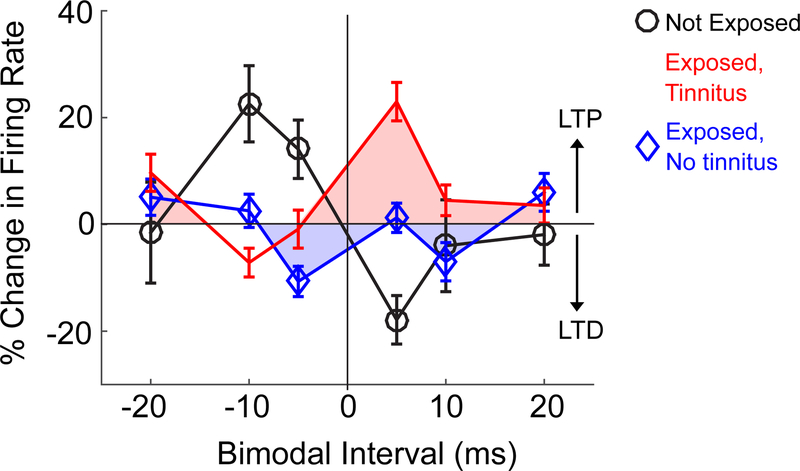
Fig. 4: Stimulus timing dependent plasticity (STDP) of DCN fusiform cells is altered in tinnitus.
Mean evoked firing rate of a population of fusiform cells before and 15 minutes after bimodal stimulation as a function of time intervals between auditory and somatosensory stimuli (somatosensory – auditory; negative intervals indicate auditory-preceding; positive intervals indicate auditory following somatosensory). STDP “timing” rule for the tinnitus group (red squares) is inverted and shifted upward (more long-term potentiation/LTP) relative to the controls (black circles). STDP timing rules for the no-tinnitus group (blue diamonds) is shifted downward (more long-term depression). Modified from Marks et al. (2018).
Ventral Cochlear Nucleus
Like the DCN, recordings from single units in the VCN have revealed increases in SFR after cochlear damage or even conductive hearing loss (Bledsoe et al., 2009; Sumner et al., 2005; Vogler et al., 2011, 2014), supporting the possibility that the VCN could contribute to tinnitus generation in the brainstem along with fusiform cells of the DCN. Human studies demonstrating altered ABR waveforms in tinnitus patients support the view that VCN as well as the DCN might be involved in tinnitus (Gu et al., 2012). Indeed, bushy cells in the VCN, by virtue of their large onset responses to sound, generate a synchronized volume-conducted potential that largely constitutes the summed activity known as the ABR (Melcher and Kiang, 1996).
However, to date, there are no published studies that have demonstrated tinnitus-specific increases in SFR in the VCN using a behavioral tinnitus model. Thus, at the present time, neurons in the DCN can be considered the location where reduced auditory nerve input initiates increased SFR and synchrony as the first physiological hallmarks of tinnitus, which are then conveyed to higher subcortical and cortical areas.
Not everyone with hearing loss develops tinnitus
Although tinnitus is associated with hearing loss, there is a high percentage of people that does not develop tinnitus after a history of noise exposure (Axelsson and Sandh, 1985; Konig et al., 2006) – suggesting that cochlear damage may be necessary for tinnitus to develop, but it is not sufficient. In animal model studies, only about half of noise-exposed animals develop tinnitus, the others are resistant to tinnitus development after the same noise exposures. These studies have demonstrated important differences between these groups: For example, in one study the distribution of glutamatergic inputs from non-cochlear sources to the CN was increased in animals that developed tinnitus but not in those that did not develop tinnitus (Heeringa et al., 2018a). Importantly, the cochleas of the animals in that study, while showing evidence of minor cochlear synaptopathy after a TTS-inducing noise exposure, showed no differences in cochlear synapses between those that did or did not develop tinnitus (Fig. 3). In another study, in which animals with and without tinnitus had equivalent cochlear synaptopathy, also reflected by equivalent ABR thresholds and wave 1 amplitudes, the animals with evidence of tinnitus showed increased SFR and cross-unit synchrony of DCN fusiform cells compared to normal, while the animals that did not develop tinnitus showed normal SFR and synchrony (Wu et al., 2016). In a study from a different laboratory, in which animals with and without evidence of tinnitus had equivalent ABR thresholds and wave 1 amplitudes. the animals with evidence of tinnitus had reduced Kv7.2/3 channel activity in DCN fusiform cells, while those without tinnitus displayed normal channel activity, suggesting that intrinsic membrane properties are altered in animals developing tinnitus, but not in those that do not develop tinnitus after noise exposure (Li et al., 2013), even though cochlear function appeared to be equivalent.
Consistent with findings in humans that tinnitus subjects can have clinically normal audiograms (Schaette and McAlpine, 2011; Xiong et al., 2013), animal models with TTS are efficacious as they enable findings of neural changes that can be more readily attributed to central homeostatic or timing dependent plasticity without confounds of changes in auditory thesholds or suprathreshold hearing. Two studies that report only a temporary threshold shift after noise exposure demonstrated increased levels of long-term depression in animals that did not develop tinnitus. In contrast, the animals with the same cochlear profiles that developed tinnitus showed more long-term potentiation and less long-term depression (Dehmel et al., 2012; Koehler and Shore, 2013a). Furthermore, these studies have shown that tinnitus severity is correlated with physiological or molecular changes so that the more severe the tinnitus, the greater the likelihood of observing increased SFR, synchrony or bursting in the DCN (Heeringa et al., 2018a; Wu et al., 2016). Molecular changes included an upregulation of glutamatergic inputs from non-auditory sources, including the somatosensory system (Zeng et al., 2009; Zeng et al., 2012).
Somatosensory pathways contribute to tinnitus
Many people with tinnitus can modulate its intensity and frequency by moving parts of their face or neck (Levine et al., 2007; Simmons et al., 2008). This observation points to a potential role for somatosensory (or motor) projections in tinnitus. Somatosensory projections innervate the peripheral-most sites of the auditory system, beginning at the CN. These projections originate in trigeminal and dorsal column ganglia and brain stem (Haenggeli et al., 2005; Wright and Ryugo, 1996; Zhou and Shore, 2004) and terminate primarily on the CN granule cells (Weedman et al., 1996). The parallel-fiber axons of the granule cells synapse on the apical dendrites of DCN fusiform cells while the cochlear auditory nerve fibers terminate on their basal dendrites, thus rendering fusiform cells ideal integrators of multisensory information. Because the somatosensory system connects indirectly to fusiform cells via their apical dendritic synapses, which are plastic (Fujino and Oertel, 2003), somatosensory influences on these principal cells are plastic and long lasting, while the auditory nerve synapses onto basal dendrites are not (Dehmel et al., 2012; Kanold et al., 2011). Multimodal stimulation of fusiform cells elicits the macroscopic equivalent of spike timing dependent plasticity, or stimulus timing dependent plasticity (Koehler and Shore, 2013b).
Interestingly, after cochlear damage, which reduces auditory nerve activation of fusiform cells, the number of somatosensory projections were shown to be upregulated over a time interval of days (Zeng et al., 2009; Zeng et al., 2011; Zeng et al., 2012), likely contributing to the heightened fusiform-cell responses observed in response to stimulation of brainstem somatosensory nuclei (Shore et al., 2008). This effect is likely due to the increased glutamatergic neurotransmission from somatosensory fibers following loss of input from auditory pathways (Heeringa et al., 2018a; Zhou et al., 2007). Increases in markers of glutamatergic transmission from non-auditory nuclei were found to be tinnitus-specific: animals with behavioral evidence of tinnitus showed increases in VGLUT2 expression in regions receiving somatosensory innervation, while animals without tinnitus did not show these increases (Heeringa et al, 2018). Tinnitus-related changes in auditory-somatosensory integration by the fusiform cells including increased long-term potentiation (Koehler and Shore, 2013a; Marks et al., 2018), were likely mediated by the increased non-auditory glutamatergic innervation (Barker et al., 2012; Zeng et al., 2009). Importantly, animals that did not develop tinnitus instead displayed increased long-term depression at fusiform synapses. The long-term potentiation vs depression outcomes for those animals with, or without tinnitus, involve a complex interplay between multiple mechanisms involved in homeostatic and spike-timing dependent plasticity. These significant alterations in processing of multisensory information in the CN, which are transmitted to the auditory cortex (Basura et al., 2012), likely contribute to the ability of tinnitus sufferers to manipulate the intensity and frequency of their tinnitus by stimulating or moving their face and neck (Levine et al., 2003; Sanchez and Rocha, 2011), regions providing somatosensory innervation of the CN (Zhan et al., 2006; Zhou and Shore, 2004, 2006). This so-called “somatic tinnitus” or “somatosensory tinnitus” occurs in up to 80% of humans with tinnitus (Levine et al., 2003; Sanchez and Rocha, 2011).
Transmission of tinnitus-related SFR and synchrony to higher centers
Inferior colliculus
The inferior colliculus (IC) integrates input from nearly all ascending auditory brainstem nuclei, including second-order neurons in the CN that encode tinnitus-specific neural activity (Fig. 1). One would therefore predict that the tinnitus phenotype would be relayed to neurons in the IC and progressively central auditory centers. Indeed, increased IC activity has been demonstrated by human imaging studies (Lanting et al., 2008; Melcher et al., 2000), which may reflect increased SFR (reviewed by Berger and Coomber, 2015). A series of studies have demonstrated that that the presence of increased SFR in IC is dependent on increases in SFR already present in the DCN (Manzoor et al., 2013; Manzoor et al., 2012). However, intrinsic processes in IC neurons may further contribute to the transmitted activity, through a tinnitus-specific alteration in the excitatory-inhibitory synaptic balance (Sturm et al., 2017). Because neural correlates of tinnitus in IC do not appear to arise independently of CN input, any intrinsic changes in IC related to tinnitus are likely also triggered by the already heightened SFR generated by principal output neurons of the CN.
How tinnitus-specific changes in fusiform cells, bushy cells, or (possibly) stellate cells in the CN are conveyed to the IC would depend on their target cells in the central (ICC) and external nucleus (ICX) neurons. A challenge in identifying tinnitus correlates in IC is that, in contrast to the CN, there are no physiologically- or morphologically-identified cell types that correspond to clearly-defined inputs and outputs (Palmer et al., 2013; Wallace et al., 2012), with the possible exception of the large GABAergic neurons (Geis and Borst, 2013). It is likely that only the IC cells receiving predominantly CN projections and driven strongly by excitatory input would replicate the tinnitus phenotype generated in CN. Furthermore, extensive intrinsic inhibitory connections within the IC (Ito et al., 2015; Sturm et al., 2014) could strongly modulate the spontaneous activity directly transmitted from CN. Thus, recording from a random sample of IC units, without identification of specific cell types or input origins, may not readily reveal tinnitus correlates.
Several studies have attempted to classify ascending projection patterns in the ICC based on their origins in the CN or superior olivary complex (Cant and Benson, 2006; Chen et al., 2018; Loftus et al., 2010; Loftus et al., 2008). Two regions and cell types have been identified as receiving inputs from distinct locations: monaural neurons in rostralateral and caudal locations that receive terminals originating in contralateral VCN, and dorsolateral regions that receive inputs from low BF, binaural neurons in ipsilateral MSO (Loftus et al., 2010). A confound to this picture is that projections from DCN terminate in the same regions (Cant and Benson, 2006). Moreover, the anatomic separation applies only to projections onto glutamatergic ICC neurons (Chen et al., 2018). Since glutamatergic and GABAergic neurons are almost indistinguishable by their physiological properties (Ono et al., 2017), even directing electrode insertions towards specific zones in IC would be unlikely to increase cellular specificity of recordings. In the future, the advent of new research techniques such as optogenetics-assisted circuit mapping and genetic cell-type identification (Cardin et al., 2010; Goyer et al., 2018), will enable recordings from specific neuronal subpopulations to examine potential physiological correlates of tinnitus in the IC.
Like ICC, the ICX also receives projections from CN, but additionally receives numerous descending and non-auditory projections (Coleman and Clerici, 1987) that form a modular structure (Lesicko et al., 2016). Outside of these modules, projections from CN and SOC neurons are diffuse and overlapping (Chen et al., 2018; Loftus et al., 2008). Few studies have examined ICX for tinnitus-specific activity (Bauer et al., 2008), but as a target of both DCN and VCN neurons, transmission of tinnitus correlates to ICX neurons is likely.
Do IC neurons show tinnitus-related activity after noise overexposure?
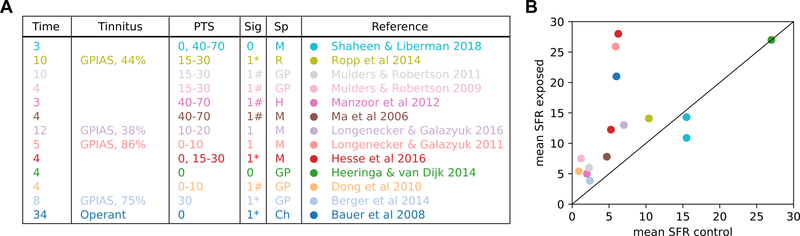
A summary of studies exploring changes in IC after noise damage is shown in Fig. 5A, highlighting the lack of consensus regarding tinnitus-specific changes in IC spontaneous activity. Only a handful of these studies incorporated behavioral tinnitus testing to isolate tinnitus-specific effects. Some of these studies showed that heightened SFR in IC, as in DCN, is present predominantly in animals with behaviorally-confirmed tinnitus (Bauer et al., 2008; Longenecker and Galazyuk, 2011), while others observed increased SFR in all of their noise-exposed animals regardless of tinnitus status (Berger et al., 2014; Longenecker and Galazyuk, 2016) or no significant increases in SFR in either group (Ropp et al., 2014). Furthermore, the degree of SFR increase reported in the IC varies widely across studies. More importantly, recent studies have not replicated the earlier reports of increased SFR after noise exposure (Heeringa and van Dijk, 2014; Shaheen and Liberman, 2018). These inconsistences could arise from different noise-exposure models with different dosages, methods, species, and amount of residual hearing across studies. However, using a generalized mixed model, we found that none of the dependent variables – residual hearing loss (P=0.56), post-exposure time (P=0.16), exposure sound level (P=0.63) and duration (P=0.53), exposure laterality (P=0.55), species (P=0.28), anesthetic (P=0.44), single vs. multiunit recording (P=0.67) – were sufficient to explain disparities in SFR across studies. Exemplary are two studies using the same procedure, same animal models, both with minimal noise damage (synaptopathy): Hesse et al. (2016) reported increased SFR in the exposed group while Shaheen and Liberman (2018) did not.
Fig. 5: Summary of studies documenting changes in IC SFR after noise overexposure.
(A) Time: time between noise exposure and recording (weeks). Tinnitus: behavioral tinnitus test used and percent of tinnitus-positive animals (GPIAS: gap-prepulse inhibition of acoustic startle). PTS: Permanent threshold shift (dB) at time of recording. Sig: whether the study reports increased SFR (0: no significant SFR increase; 1: significant SFR increase; #: SFR increase is frequency specific; *: SFR increase is not frequency-specific). Sp: species (M: mouse; R: rat; H: hamster; GP: guinea pig; Ch: chinchilla). (B) Mean SFR in the exposed group vs mean SFR in the control, unexposed group plotted against each other. Each data point represents a different study indicated by the same colors as (A).
The emerging picture in IC is thus dramatically different from that observed in the DCN, where multiple research groups across several decades have produced highly consistent results demonstrating increased SFR after noise exposure, which indeed correlate with behavioral evidence of tinnitus using both GPIAS and operant conditioning. We surmise that this consistency can be largely attributed to the homogenous population of principal output neurons layered across the DCN. In contrast, the lack of specificity across studies of IC may be due to unresolved circuit heterogeneity (functional zone, cell type, or origin of input). Tinnitus correlates may exist in a subset of IC neurons but these are not readily revealed through random sampling.
A subpopulation of IC neurons might encode tinnitus?
Interestingly, we observed a tendency in some studies that reported low SFRs in their control groups to observe higher SFRs in their noise-exposed groups (Fig. 5B). Sampling biases due to different recording techniques are unlikely, as even within the same study (Ropp et al., 2014), greater SFR increase was observed in unit types with low control SFRs. Without direct comparisons before and after noise-exposure, the relationship between baseline SFR and increased SFR following noise damage is difficult to prove, but the trend implies that low SFR neurons could comprise a specific cell type in the IC that encodes tinnitus-related increases in SFR.
Few IC studies have quantified the physiological characteristics of units with increased SFR. But one study (Bauer et al., 2008) performed a multivariate analysis on spontaneous activity patterns and found that increased SFRs were confined to units with regular firing patterns (low ISI variance) and high burst rates (1k spikes per second). A second study (Ropp et al., 2014) showed that increased SFR was more pronounced in units with classical V-shaped receptive fields with strong excitatory areas. In contrast, Vogler et al. (2014), found increased SFR across all IC response types, regardless of input-output functions, temporal response patterns (PSTH), or binaural properties. It is unclear whether these physiological response types reflect specific ascending inputs, or whether noise exposure alters intrinsic physiology, which would complicate comparisons across the normally-defined categories.
In the DCN, increased SFR occurs in neurons that are tuned to behaviorally identified tinnitus frequencies (Wu et al., 2016). However, in IC the frequency-specificity of units with increased SFR is debated. Some studies showed that increased SFR occurred in neurons with BFs overlapping the regions of hearing loss (Ma et al., 2006; Manzoor et al., 2013; Mulders and Robertson, 2009), while others showed pervasive increases in SFR without BF-specificity (Bauer et al., 2008; Berger et al., 2014; Ropp et al., 2014). These inconsistencies suggest that even among studies that reveal noise-induced changes in firing rates, different populations of IC neurons may have been be sampled. Another possibility is that correlates of hyperacusis rather than tinnitus are encoded in the IC (Bakay et al., 2018; Gu et al., 2010; Shaheen and Liberman, 2018).
Tinnitus correlates in the medial geniculate
Increased SFR and bursting in medial geniculate (MG) neurons was identified by Kalappa et al. (2014) who assessed tinnitus behavior in rats after noise exposure that preserved hearing thresholds, and directly correlated tinnitus severity with MG spontaneous activity. Neurons in both the ventral (lemniscal) and dorsal (non-lemniscal) divisions exhibited SFR correlated with tinnitus severity. In addition, the same authors observed increased tonic synaptic and extrasynaptic GABAA receptor current in brain slices from rats with behavioral evidence of tinnitus measured with GPIAS (Sametsky et al., 2015), which likely mediated the increased bursting observed in vivo in the MG neurons. The authors suggested that the tinnitus-specific effects in MG are triggered by increased excitatory inputs from the IC (Caspary and Llano, 2017). Questions remain as to whether MG increases in SFR are frequency-specific, whether they are accompanied by increased synchrony, and whether contributions from various ascending pathways transmit/trigger the increased SFR [e.g. the direct pathway from DCN (Anderson et al., 2006; Malmierca et al., 2002)]. Nevertheless, heightened SFR in MG that is tinnitus specific emphasizes that it is increased thalamocortical input, rather than decreased thalamocortical input that is the driving force in maladaptive auditory cortical plasticity. Furthermore, the finding of tinnitus-specific increased GABA inhibition in MG, in contrast to decreased inhibition in DCN, suggests that the relationship between increased SFR and altered inhibition is more complex than simply “disinhibition”.
Tinnitus correlates in the auditory cortex
The role of auditory cortex (AC) in tinnitus has been inferred primarily from the human imaging and EEG studies. Cortical changes in people with tinnitus include increased fMRI signals (Lanting et al., 2009; Leaver et al., 2011; Lockwood et al., 1998) and increased EEG gammaband power (Ortmann et al., 2011; Sedley et al., 2012; van der Loo et al., 2009; Weisz et al., 2007b). Increased fMRI resting-state activity likely reflects increased SFR observed in animal studies (Chen et al., 2015; Lanting et al., 2009), while increased cross-unit synchrony observed in animal studies may serve as the substrate of enhanced rhythmic firing (Weisz et al., 2007a). Although direct comparisons between single-unit recordings and brain-imaging techniques are limited, increased SFR and synchrony in AC have been described in a few animal model studies of noise-induced tinnitus (Basura et al., 2015; Engineer et al., 2011; Zhang et al., 2016). In addition to spontaneous activity alterations, earlier studies also observed cortical tonotopic reorganization, or expansion/reduction of certain frequency representations (Engineer et al., 2011; Seki and Eggermont, 2003). However, later studies in both humans (Langers et al., 2012) and animals (Yang et al., 2011) dissociated tinnitus from tonotopic reorganization, which was attributed rather to hearing loss and not to tinnitus per se.
Some studies have suggested that increased SFR and synchrony in the AC arise from reduced inhibition within the cortical circuit due to reduced input to the AC (Llano et al., 2012; Yang et al., 2011). The hypothesis that reduced inhibition causes increased SFR and synchrony is plausible, since GABAergic interneurons exert extensive influence on principal neurons and their excitation/inhibition balance plays a crucial role in network information flow (Trevino, 2016) and auditory processing (Isaacson and Scanziani, 2011; Pi et al., 2013; Wehr and Zador, 2003). Cortical disinhibition, however, is unlikely to be due to a reduced thalamocortical input – as previously suggested (Norena and Eggermont, 2003; Weisz et al., 2007a) – in light of accumulating evidence of tinnitus-related increases in SFR and bursting of MG neurons (Kalappa et al., 2014; Sametsky et al., 2015), which provide the input to the AC. The excitatory projections from thalamus innervate both the excitatory principal neurons and the inhibitory interneurons across different cortical layers (Ji et al., 2016), suggesting that the increased thalamocortical activity could have significant impact on the excitation/inhibition balance within the cortical network (Hamilton et al., 2013; Natan et al., 2017). In addition to the thus-altered cortical inhibition, it is also likely that increased SFR and synchrony are directly transmitted by thalamocortical neurons that already encode tinnitus signals through increased SFR and bursting, consistent with the crucial role of thalamus in sensory gating and perception (Portas et al., 1998; Rauschecker et al., 2010; Whitmire et al., 2016).
Recent imaging studies have also identified the involvement of limbic systems (Chen et al., 2015; Leaver et al., 2011) in tinnitus. Increased fMRI signals in brain areas that encode affect, such as the amygdala, nucleus accumbens and hypothalamus, may originate from heightened SFR directly transmitted by MG and the AC (Barry et al., 2015; Clugnet et al., 1990; Kraus and Canlon, 2012; LeDoux et al., 1985). The involvement of the limbic system implies a dissociation and/or interaction between the tinnitus percept and tinnitus “suffering” (Chen et al., 2017). As tinnitus is triggered and transmitted by the ascending auditory pathway, the thalamic, cortical, and limbic structures may ultimately be responsible for the conscious milieu accompanying the sound of tinnitus.
Concluding remarks
To date, there have been a number of pivotal studies of single cell physiology and molecular markers of anatomical changes that have documented the tinnitus-specific neural changes described in this chapter. A summary of neural circuit- and pathway changes occurring in animals with behavioral evidence of tinnitus after noise exposure is presented in Figure 6. Beginning in the CN, decreased auditory nerve innervation of each cell type results in maladaptive homeostatic increases in the activity of the principal output neurons, fusiform cells and bushy cells. Undoubtedly playing a role in these increased activity levels is a decrease in the action of inhibitory interneurons, the D-stellate cells and vertical cells, whose output would be reduced simply because of decreased drive from the auditory nerve fibers but also by decreased levels of glycine. Studies have shown that although cochlear synaptopathy is present in all animals after noise exposure (even with TTS), it is equivalent in animals with and without evidence of tinnitus. In these same animals, dramatic, tinnitus-specific differences arise in second order neurons of the DCN that receive the ANF inputs. The most prominent tinnitus related changes occur in the apical dendritic synapses of the principal output neurons of the DCN, the fusiform cells. Upregulation of multimodal inputs to the CN has been shown to be tinnitus specific – i.e. it only occurs in animals with evidence of tinnitus after noise exposure (Heeringa et al., 2018a). The eventual outcome of increased spontaneous activity in fusiform cells (and perhaps also bushy cells) (Fig. 6A) is transmitted to progressively higher order auditory nuclei, culminating in increased activity in neurons of the AC (Fig. 6B) and perhaps as well as the limbic system (Chen et al., 2017; Kraus and Canlon, 2012; Zhang et al., 2018).
Fig. 6: Changes in neural circuitry in animals with noise-induced tinnitus.
(A) Cochlear nucleus principal cell output is increased. After noise exposure, auditory nerve fiber (ANF) synapses are reduced for both excitatory output neurons (blue/green) and inhibitory interneurons (red) -for animals with and without tinnitus. Multimodal projections (including somatosensory) to the granule cell domain and dendritic fields of DS and BC are upregulated only in animals with tinnitus and not those without tinnitus, increasing input to the apical dendrites of fusiform cells (FC) and inhibitory interneuron cartwheel cells (CW). Reduction in ANF input to DS and VC would reduce wide-band and narrow-band inhibition to output neurons of both DCN and VCN, likely playing a role in increased activity of output neurons. Although the increased output of principal cells is specific to animals with tinnitus and not those without, is not yet known whether the reduction in ANF synapses to inhibitory neurons is tinnitus-specific. Thus, based on the available evidence, the main driver of increased SFR in CN output neurons (FC) is increased somatosensory input driving long term plasticity. (B) Neural activity is increased in ascending projections to IC, MG and auditory cortex. Increased activity from fusiform cells (FC) is conveyed to the nuclei of inferior colliculus (ICC; IC); and contralateral medial geniculate (MG shell). Increased Spherical bushy cells (BC; spherical: s) project to the ipsilateral lateral superior olive (LSO), medial superior olive (MSO), and contralateral MSO. Globular BC (g) project to the ipsilateral LSO and contralateral medial nucleus of trapezoid body (MNTB). It is unknown whether T-Stellate cell activity is increased in tinnitus. D-stellate cell (DS) output to DCN,VCN and contralateral CN is expected to be decreased. Tinnitus-specific increases in neural activity have been reported in DCN, MG and auditory cortex (AI). Increased or decreased activity is indicated by arrows and line thickness. For both panels, asterisks (*) indicate hypothesized effects not yet to be validated by future experiments.
In this review, we have focused on the ascending auditory system and discussed the generation of tinnitus in the CN after noise-induced deafferentation and the likely route of transmission of tinnitus signals along the ascending nuclei. However, the descending system (Bajo et al., 2010; Nakamoto et al., 2008) may play an important role in tinnitus pathology and significantly modulate the tinnitus transmission throughout the brain (Shulman and Strashun, 1999). For instance, a recent study has demonstrated increased AC-IC projections after cochlear deafferentation (Asokan et al., 2018), which suggests that tinnitus-related increases in IC activity could arise from heightened descending input as well as ascending CN input. Corticofugal projections from the AC to CN have also been documented (Meltzer and Ryugo, 2006; Schofield and Coomes, 2005; Weedman and Ryugo, 1996), but their function remains inconclusive. Descending projections might thus modulate CN plasticity and influence tinnitus generation. The interplay between ascending and descending systems in tinnitus generation and transmission points to an exciting direction for future work.
Studies of single neurons and how ensembles of neurons produce population responses are likely to be the most effective route to unraveling the mechanisms of this elusive disease. To enable us to develop methods to alleviate the bothersome or debilitating symptoms of tinnitus, we need to understand the cellular underlying mechanisms. Animal model studies that employ single cell recordings as well as population responses that can be directly compared with results of human studies of brain imaging (Chen et al., 2015) would be particularly useful in this regard. Already, these approaches have delivered some initially promising treatments that aim to reverse the pathological neural activity representing phantom perception of sounds, that are based on neuronal measurements in animal models. Vagal nerve stimulation (VNS) targets cholinergic innervation of the AC, which contributes to neural plasticity in this region purported to give rise to the percept of tinnitus (Engineer et al., 2011; Vanneste et al., 2017). The combination of VNS with sound stimulation is designed to alter cortical plasticity to increase the representation of sounds outside the tinnitus region to normalize the activity across the primary AC. Another promising method aiming to reverse tinnitus activity directly targets maladaptive plasticity in the fusiform cells in the DCN by using bimodal (auditory-somatosensory) stimulation to induce long term depression in the neurons that show increased long-term potentiation linked to tinnitus (Marks et al., 2018). Both of these treatments, which emerged from precise single-neuron studies, and using GPIAS to assess tinnitus, are currently in clinical trials. These approaches, which target maladaptive plasticity in brain regions in animals with tinnitus, are more likely to provide reliable therapies compared to trial and error treatments that have been rife in the field for decades, with no gold standard-of-treatment outcome (Attarha et al., 2018).
Acknowledgements
This work was supported by grants from the NIH 1RF1MH114244-01 (SES), R01-DC004825 (SES), T32-DC00011 (CW). We thank Michael Roberts and Adam Hockley for insightful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests. SES is an inventor on US patent no. 3A9252067 “Personalized auditory-somatosensory stimulation to treat tinnitus”
References
- Agrawal Y, Platz EA, and Niparko JK (2008). Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med 168, 1522–1530. [DOI] [PubMed] [Google Scholar]
- Agrawal Y, Platz EA, and Niparko JK (2009). Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol 30, 139–145. [DOI] [PubMed] [Google Scholar]
- Anderson LA, Malmierca MS, Wallace MN, and Palmer AR (2006). Evidence for a direct, short latency projection from the dorsal cochlear nucleus to the auditory thalamus in the guinea pig. Eur J Neurosci 24, 491–498. [DOI] [PubMed] [Google Scholar]
- Arnott RH, Wallace MN, Shackleton TM, and Palmer AR (2004). Onset neurones in the anteroventral cochlear nucleus project to the dorsal cochlear nucleus. J Assoc Res Otolaryngol 5, 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan MM, Williamson RS, Hancock KE, and Polley DB (2018). Sensory overamplification in layer 5 auditory corticofugal projection neurons following cochlear nerve synaptic damage. Nat Commun 9, 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attarha M, Bigelow J, and Merzenich MM (2018). Unintended Consequences of White Noise Therapy for Tinnitus-Otolaryngology’s Cobra Effect: A Review. JAMA Otolaryngol Head Neck Surg 144, 938–943. [DOI] [PubMed] [Google Scholar]
- Axelsson A, and Sandh A (1985). Tinnitus in noise-induced hearing loss. Br J Audiol 19, 271–276. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Moore DR, and King AJ (2010). The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nat Neurosci 13, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakay WMH, Anderson LA, Garcia-Lazaro JA, McAlpine D, and Schaette R (2018). Hidden hearing loss selectively impairs neural adaptation to loud sound environments. Nat Commun 9, 4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker M, Solinski HJ, Hashimoto H, Tagoe T, Pilati N, and Hamann M (2012). Acoustic overexposure increases the expression of VGLUT-2 mediated projections from the lateral vestibular nucleus to the dorsal cochlear nucleus. PLoS One 7, e35955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry KM, Paolini AG, Robertson D, and Mulders WH (2015). Modulation of medial geniculate nucleus neuronal activity by electrical stimulation of the nucleus accumbens. Neuroscience 308, 1–10. [DOI] [PubMed] [Google Scholar]
- Basura GJ, Koehler SD, and Shore SE (2012). Multi-sensory integration in brainstem and auditory cortex. Brain Res 1485, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basura GJ, Koehler SD, and Shore SE (2015). Bimodal stimulus timing-dependent plasticity in primary auditory cortex is altered after noise exposure with and without tinnitus. J Neurophysiol 114, 3064–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, and Brozoski TJ (2008). Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res 86, 2564–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JI, and Coomber B (2015). Tinnitus-related changes in the inferior colliculus. Front Neurol 6, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JI, Coomber B, Shackleton TM, Palmer AR, and Wallace MN (2013). A novel behavioural approach to detecting tinnitus in the guinea pig. J Neurosci Methods 213, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JI, Coomber B, Wells TT, Wallace MN, and Palmer AR (2014). Changes in the response properties of inferior colliculus neurons relating to tinnitus. Front Neurol 5, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SC Jr., Koehler S, Tucci DL, Zhou J, Le Prell C, and Shore SE (2009). Ventral cochlear nucleus responses to contralateral sound are mediated by commissural and olivocochlear pathways. J Neurophysiol 102, 886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, and Bauer CA (2005). The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res 206, 227–236. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, and Caspary DM (2002). Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci 22, 2383–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Wisner KW, Sybert LT, and Bauer CA (2012). Bilateral dorsal cochlear nucleus lesions prevent acoustic-trauma induced tinnitus in an animal model. J Assoc Res Otolaryngol 13, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant NB, and Benson CG (2003). Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull 60, 457–474. [DOI] [PubMed] [Google Scholar]
- Cant NB, and Benson CG (2006). Organization of the inferior colliculus of the gerbil (Meriones unguiculatus): differences in distribution of projections from the cochlear nuclei and the superior olivary complex. J Comp Neurol 495, 511–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, and Moore CI (2010). Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc 5, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Backoff PM, Finlayson PG, and Palombi PS (1994). Inhibitory inputs modulate discharge rate within frequency receptive fields of anteroventral cochlear nucleus neurons. J Neurophysiol 72, 2124–2133. [DOI] [PubMed] [Google Scholar]
- Caspary DM, and Llano DA (2017). Auditory thalamic circuits and GABAA receptor function: Putative mechanisms in tinnitus pathology. Hear Res 349, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Cheng M, Ito T, and Song S (2018). Neuronal Organization in the Inferior Colliculus Revisited with Cell-Type-Dependent Monosynaptic Tracing. J Neurosci 38, 3318–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Li X, Liu L, Wang J, Lu CQ, Yang M, Jiao Y, Zang FC, Radziwon K, Chen GD, et al. (2015). Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife 4, e06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Xia W, Chen H, Feng Y, Xu JJ, Gu JP, Salvi R, and Yin X (2017). Tinnitus distress is linked to enhanced resting-state functional connectivity from the limbic system to the auditory cortex. Hum Brain Mapp 38, 2384–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugnet MC, LeDoux JE, and Morrison SF (1990). Unit responses evoked in the amygdala and striatum by electrical stimulation of the medial geniculate body. J Neurosci 10, 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JR, and Clerici WJ (1987). Sources of projections to subdivisions of the inferior colliculus in the rat. J Comp Neurol 262, 215–226. [DOI] [PubMed] [Google Scholar]
- D’amour JA, and Froemke RC (2015). Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron 86, 514–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Engineer ND, and Kilgard MP (2014). Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: a case series. Neuromodulation 17, 170–179. [DOI] [PubMed] [Google Scholar]
- Dehmel S, Pradhan S, Koehler S, Bledsoe S, and Shore S (2012). Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus--possible basis for tinnitus-related hyperactivity? J Neurosci 32, 1660–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, and Kilgard MP (2011). Reversing pathological neural activity using targeted plasticity. Nature 470, 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P, and Hebert S (2013). Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: does tinnitus fill in the gap? Hear Res 295, 16–23. [DOI] [PubMed] [Google Scholar]
- Fournier P, and Hebert S (2016). The gap-startle paradigm to assess auditory temporal processing: Bridging animal and human research. Psychophysiology 53, 759–766. [DOI] [PubMed] [Google Scholar]
- Fujino K, and Oertel D (2003). Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci U S A 100, 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazyuk A, and Hebert S (2015). Gap-Prepulse Inhibition of the Acoustic Startle Reflex (GPIAS) for Tinnitus Assessment: Current Status and Future Directions. Front Neurol 6, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Manzoor N, and Kaltenbach JA (2016). Evidence of activity-dependent plasticity in the dorsal cochlear nucleus, in vivo, induced by brief sound exposure. Hear Res 341, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis HR, and Borst JG (2013). Large GABAergic neurons form a distinct subclass within the mouse dorsal cortex of the inferior colliculus with respect to intrinsic properties, synaptic inputs, sound responses, and projections. J Comp Neurol 521, 189–202. [DOI] [PubMed] [Google Scholar]
- Goyer D, Silveira MA, George AP, Beebe NL, Edelbrock RM, Malinski PT, Schofield BR, and Roberts MT (2018). A Novel Class of Inferior Colliculus Principal Neurons Labeled in Vasoactive Intestinal Peptide-Cre Mice. bioRxiv, 474312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, and Melcher JR (2010). Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol 104, 3361–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Herrmann BS, Levine RA, and Melcher JR (2012). Brainstem Auditory Evoked Potentials Suggest a Role for the Ventral Cochlear Nucleus in Tinnitus. J Assoc Res Otolaryngol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenggeli CA, Pongstaporn T, Doucet JR, and Ryugo DK (2005). Projections from the spinal trigeminal nucleus to the cochlear nucleus in the rat. J Comp Neurol 484, 191–205. [DOI] [PubMed] [Google Scholar]
- Hamilton LS, Sohl-Dickstein J, Huth AG, Carels VM, Deisseroth K, and Bao S (2013). Optogenetic activation of an inhibitory network enhances feedforward functional connectivity in auditory cortex. Neuron 80, 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa AN, Stefanescu RA, Raphael Y, and Shore SE (2016). Altered vesicular glutamate transporter distributions in the mouse cochlear nucleus following cochlear insult. Neuroscience 315, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa AN, and van Dijk P (2014). The dissimilar time course of temporary threshold shifts and reduction of inhibition in the inferior colliculus following intense sound exposure. Hear Res 312, 38–47. [DOI] [PubMed] [Google Scholar]
- Heeringa AN, Wu C, Chung C, West M, Martel D, Liberman L, Liberman MC, and Shore SE (2018a). Glutamatergic Projections to the Cochlear Nucleus are Redistributed in Tinnitus. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa AN, Wu C, and Shore SE (2018b). Multisensory Integration Enhances Temporal Coding in Ventral Cochlear Nucleus Bushy Cells. J Neurosci 38, 2832–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse LL, Bakay W, Ong HC, Anderson L, Ashmore J, McAlpine D, Linden J, and Schaette R (2016). Non-Monotonic Relation between Noise Exposure Severity and Neuronal Hyperactivity in the Auditory Midbrain. Front Neurol 7, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilin V, Malyshev A, Wolf F, and Volgushev M (2013). Fast Computations in Cortical Ensembles Require Rapid Initiation of Action Potentials. Journal of Neuroscience 33, 2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, and Scanziani M (2011). How inhibition shapes cortical activity. Neuron 72, 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Bishop DC, and Oliver DL (2015). Functional organization of the local circuit in the inferior colliculus. Anat Sci Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XY, Zingg B, Mesik L, Xiao Z, Zhang LI, and Tao HW (2016). Thalamocortical Innervation Pattern in Mouse Auditory and Visual Cortex: Laminar and Cell-Type Specificity. Cereb Cortex 26, 2612–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YM, Godfrey DA, Wang J, and Kaltenbach JA (2006). Effects of intense tone exposure on choline acetyltransferase activity in the hamster cochlear nucleus. Hear Res 216–217, 168–175. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Brozoski TJ, Turner JG, and Caspary DM (2014). Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol 592, 5065–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, and Afman CE (2000). Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res 140, 165–172. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, and Zhang J (1998). Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res 124, 78–84. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, and McCaslin DL (1996). Increases in Spontaneous Activity in the Dorsal Cochlear Nucleus Following Exposure to High Intensity Sound: A Possible Neural Correlate of Tinnitus. Audit Neurosci 3, 57–78. [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, and Frederick S (2004). Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett 355, 121–125. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, and Zhang J (2007). Intense sound-induced plasticity in the dorsal cochlear nucleus of rats: evidence for cholinergic receptor upregulation. Hear Res 226, 232–243. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, and Afman CE (2000). Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res 147, 282–292. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Davis KA, and Young ED (2011). Somatosensory context alters auditory responses in the cochlear nucleus. J Neurophysiol 105, 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler SD, and Shore SE (2013a). Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci 33, 19647–19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler SD, and Shore SE (2013b). Stimulus-timing dependent multisensory plasticity in the guinea pig dorsal cochlear nucleus. PLoS One 8, e59828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig O, Schaette R, Kempter R, and Gross M (2006). Course of hearing loss and occurrence of tinnitus. Hear Res 221, 59–64. [DOI] [PubMed] [Google Scholar]
- Kraus KS, and Canlon B (2012). Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res 288, 34–46. [DOI] [PubMed] [Google Scholar]
- Langers DR, de Kleine E, and van Dijk P (2012). Tinnitus does not require macroscopic tonotopic map reorganization. Front Syst Neurosci 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting CP, De Kleine E, Bartels H, and Van Dijk P (2008). Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol 128, 415–421. [DOI] [PubMed] [Google Scholar]
- Lanting CP, de Kleine E, and van Dijk P (2009). Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res 255, 1–13. [DOI] [PubMed] [Google Scholar]
- Lauer AM, Dent ML, Sun W, and Xu-Friedman MA (2019). Effects of Non-traumatic Noise and Conductive Hearing Loss on Auditory System Function. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, and Rauschecker JP (2011). Dysregulation of limbic and auditory networks in tinnitus. Neuron 69, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, and Reis DJ (1985). Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol 242, 182–213. [DOI] [PubMed] [Google Scholar]
- Lesicko AM, Hristova TS, Maigler KC, and Llano DA (2016). Connectional Modularity of Top-Down and Bottom-Up Multimodal Inputs to the Lateral Cortex of the Mouse Inferior Colliculus. J Neurosci 36, 11037–11050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RA, Abel M, and Cheng H (2003). CNS somatosensory-auditory interactions elicit or modulate tinnitus. ExpBrain Res 153, 643–648. [DOI] [PubMed] [Google Scholar]
- Levine RA, Nam EC, Oron Y, and Melcher JR (2007). Evidence for a tinnitus subgroup responsive to somatosensory based treatment modalities. Prog Brain Res 166, 195–207. [DOI] [PubMed] [Google Scholar]
- Li S, Choi V, and Tzounopoulos T (2013). Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci U S A 110, 9980–9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kalappa BI, and Tzounopoulos T (2015). Noise-induced plasticity of KCNQ2/3 and HCN channels underlies vulnerability and resilience to tinnitus. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Turner J, and Caspary DM (2012). Diminished cortical inhibition in an aging mouse model of chronic tinnitus. J Neurosci 32, 16141–16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, and Murphy BW (1998). The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology 50, 114–120. [DOI] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, and Oliver DL (2010). Differential patterns of inputs create functional zones in central nucleus of inferior colliculus. J Neurosci 30, 13396–13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus WC, Malmierca MS, Bishop DC, and Oliver DL (2008). The cytoarchitecture of the inferior colliculus revisited: a common organization of the lateral cortex in rat and cat. Neuroscience 154, 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker RJ, and Galazyuk AV (2011). Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol 12, 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker RJ, and Galazyuk AV (2016). Variable Effects of Acoustic Trauma on Behavioral and Neural Correlates of Tinnitus In Individual Animals. Front Behav Neurosci 10, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Hidaka H, and May BJ (2006). Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res 212, 9–21. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Merchan MA, Henkel CK, and Oliver DL (2002). Direct projections from cochlear nuclear complex to auditory thalamus in the rat. J Neurosci 22, 10891–10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor NF, Gao Y, Licari F, and Kaltenbach JA (2013). Comparison and contrast of noise-induced hyperactivity in the dorsal cochlear nucleus and inferior colliculus. Hear Res 295, 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor NF, Licari FG, Klapchar M, Elkin RL, Gao Y, Chen G, and Kaltenbach JA (2012). Noise-induced hyperactivity in the inferior colliculus: its relationship with hyperactivity in the dorsal cochlear nucleus. J Neurophysiol 108, 976–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KL, Martel DT, Wu C, Basura GJ, Roberts LE, Schvartz-Leyzac KC, and Shore SE (2018). Auditory-somatosensory bimodal stimulation desynchronizes brain circuitry to reduce tinnitus in guinea pigs and humans. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack A, Edmondson-Jones M, Somerset S, and Hall D (2016). A systematic review of the reporting of tinnitus prevalence and severity. Hear Res 337, 70–79. [DOI] [PubMed] [Google Scholar]
- Melcher JR, and Kiang NY (1996). Generators of the brainstem auditory evoked potential in cat. III: Identified cell populations. Hear Res 93, 52–71. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Sigalovsky IS, Guinan JJ Jr., and Levine RA (2000). Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol 83, 1058–1072. [DOI] [PubMed] [Google Scholar]
- Meltzer NE, and Ryugo DK (2006). Projections from auditory cortex to cochlear nucleus: A comparative analysis of rat and mouse. Anat Rec A Discov Mol Cell Evol Biol 288, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, and Tzounopoulos T (2011). Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A 108, 7601–7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AR (1975). Dynamic properties of excitation and inhibition in the cochlear nucleus. Acta Physiol Scand 93, 442–454. [DOI] [PubMed] [Google Scholar]
- Mulders WH, and Robertson D (2009). Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience 164, 733–746. [DOI] [PubMed] [Google Scholar]
- Nakamoto KT, Jones SJ, and Palmer AR (2008). Descending projections from auditory cortex modulate sensitivity in the midbrain to cues for spatial position. J Neurophysiol 99, 2347–2356. [DOI] [PubMed] [Google Scholar]
- Natan RG, Rao W, and Geffen MN (2017). Cortical Interneurons Differentially Shape Frequency Tuning following Adaptation. Cell Rep 21, 878–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena AJ, and Eggermont JJ (2003). Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res 183, 137–153. [DOI] [PubMed] [Google Scholar]
- Ono M, Bishop DC, and Oliver DL (2017). Identified GABAergic and Glutamatergic Neurons in the Mouse Inferior Colliculus Share Similar Response Properties. J Neurosci 37, 8952–8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmann M, Muller N, Schlee W, and Weisz N (2011). Rapid increases of gamma power in the auditory cortex following noise trauma in humans. Eur J Neurosci 33, 568–575. [DOI] [PubMed] [Google Scholar]
- Palmer AR, Shackleton TM, Sumner CJ, Zobay O, and Rees A (2013). Classification of frequency response areas in the inferior colliculus reveals continua not discrete classes. J Physiol 591, 4003–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, and Kepecs A (2013). Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilati N, Ison MJ, Barker M, Mulheran M, Large CH, Forsythe ID, Matthias J, and Hamann M (2012a). Mechanisms contributing to central excitability changes during hearing loss. Proc Natl Acad Sci U S A 109, 8292–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilati N, Large C, Forsythe ID, and Hamann M (2012b). Acoustic over-exposure triggers burst firing in dorsal cochlear nucleus fusiform cells. Hear Res 283, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, and Frith CD (1998). A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 18, 8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Leaver AM, and Muhlau M (2010). Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66, 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropp TJ, Tiedemann KL, Young ED, and May BJ (2014). Effects of unilateral acoustic trauma on tinnitus-related spontaneous activity in the inferior colliculus. J Assoc Res Otolaryngol 15, 1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Haenggeli CA, and Doucet JR (2003). Multimodal inputs to the granule cell domain of the cochlear nucleus. Exp Brain Res 153, 477–485. [DOI] [PubMed] [Google Scholar]
- Sametsky EA, Turner JG, Larsen D, Ling L, and Caspary DM (2015). Enhanced GABAA-Mediated Tonic Inhibition in Auditory Thalamus of Rats with Behavioral Evidence of Tinnitus. J Neurosci 35, 9369–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez TG, and Rocha CB (2011). Diagnosis and management of somatosensory tinnitus: review article. Clinics (Sao Paulo) 66, 1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R, and McAlpine D (2011). Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci 31, 13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, and Coomes DL (2005). Auditory cortical projections to the cochlear nucleus in guinea pigs. Hear Res 199, 89–102. [DOI] [PubMed] [Google Scholar]
- Sedley W, Teki S, Kumar S, Barnes GR, Bamiou DE, and Griffiths TD (2012). Single-subject oscillatory gamma responses in tinnitus. Brain 135, 3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, and Eggermont JJ (2003). Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res 180, 28–38. [DOI] [PubMed] [Google Scholar]
- Shaheen LA, and Liberman MC (2018). Cochlear Synaptopathy Changes Sound-Evoked Activity Without Changing Spontaneous Discharge in the Mouse Inferior Colliculus. Front Syst Neurosci 12, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, and Farwell WR (2010). Prevalence and characteristics of tinnitus among US adults. Am J Med 123, 711–718. [DOI] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, and Syed S (2008). Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci 27, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, and Moore JK (1998). Sources of input to the cochlear granule cell region in the guinea pig. Hear Res 116, 33–42. [DOI] [PubMed] [Google Scholar]
- Shore SE, Vass Z, Wys NL, and Altschuler RA (2000). Trigeminal ganglion innervates the auditory brainstem. J Comp Neurol 419, 271–285. [DOI] [PubMed] [Google Scholar]
- Shulman A, and Strashun A (1999). Descending auditory system/cerebellum/tinnitus. Int Tinnitus J 5, 92–106. [PubMed] [Google Scholar]
- Simmons R, Dambra C, Lobarinas E, Stocking C, and Salvi R (2008). Head, Neck, and Eye Movements That Modulate Tinnitus. Semin Hear 29, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanescu RA, and Shore SE (2015). NMDA Receptors Mediate Stimulus-Timing-Dependent Plasticity and Neural Synchrony in the Dorsal Cochlear Nucleus. Front Neural Circuits 9, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanescu RA, and Shore SE (2017). Muscarinic acetylcholine receptors control baseline activity and Hebbian stimulus timing-dependent plasticity in fusiform cells of the dorsal cochlear nucleus. J Neurophysiol 117, 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm J, Nguyen T, and Kandler K (2014). Development of intrinsic connectivity in the central nucleus of the mouse inferior colliculus. J Neurosci 34, 15032–15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm JJ, Zhang-Hooks YX, Roos H, Nguyen T, and Kandler K (2017). Noise Trauma-Induced Behavioral Gap Detection Deficits Correlate with Reorganization of Excitatory and Inhibitory Local Circuits in the Inferior Colliculus and Are Prevented by Acoustic Enrichment. J Neurosci 37, 6314–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner CJ, Tucci DL, and Shore SE (2005). Responses of ventral cochlear nucleus neurons to contralateral sound after conductive hearing loss. J Neurophysiol 94, 4234–4243. [DOI] [PubMed] [Google Scholar]
- Talathi SS, Hwang DU, and Ditto WL (2008). Spike timing dependent plasticity promotes synchrony of inhibitory networks in the presence of heterogeneity. J Comput Neurosci 25, 262–281. [DOI] [PubMed] [Google Scholar]
- Trevino M (2016). Inhibition Controls Asynchronous States of Neuronal Networks. Front Synaptic Neurosci 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, and Caspary DM (2006). Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci 120, 188–195. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, and Trussell LO (2004). Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci 7, 719–725. [DOI] [PubMed] [Google Scholar]
- van der Loo E, Gais S, Congedo M, Vanneste S, Plazier M, Menovsky T, Van de Heyning P, and De Ridder D (2009). Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One 4, e7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Martin J, Rennaker RLN, and Kilgard MP (2017). Pairing sound with vagus nerve stimulation modulates cortical synchrony and phase coherence in tinnitus: An exploratory retrospective study. Sci Rep 7, 17345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler DP, Robertson D, and Mulders WH (2011). Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci 31, 6639–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler DP, Robertson D, and Mulders WH (2014). Hyperactivity following unilateral hearing loss in characterized cells in the inferior colliculus. Neuroscience 265, 28–36. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Shackleton TM, and Palmer AR (2012). Morphological and physiological characteristics of laminar cells in the central nucleus of the inferior colliculus. Front Neural Circuits 6, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, and Caspary DM (2009). Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience 164, 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedman DL, Pongstaporn T, and Ryugo DK (1996). Ultrastructural study of the granule cell domain of the cochlear nucleus in rats: mossy fiber endings and their targets. J Comp Neurol 369, 345–360. [DOI] [PubMed] [Google Scholar]
- Weedman DL, and Ryugo DK (1996). Pyramidal cells in primary auditory cortex project to cochlear nucleus in rat. Brain Res 706, 97–102. [DOI] [PubMed] [Google Scholar]
- Wehr M, and Zador AM (2003). Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446. [DOI] [PubMed] [Google Scholar]
- Weisz N, Dohrmann K, and Elbert T (2007a). The relevance of spontaneous activity for the coding of the tinnitus sensation. Prog Brain Res 166, 61–70. [DOI] [PubMed] [Google Scholar]
- Weisz N, Muller S, Schlee W, Dohrmann K, Hartmann T, and Elbert T (2007b). The neural code of auditory phantom perception. J Neurosci 27, 1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire CJ, Waiblinger C, Schwarz C, and Stanley GB (2016). Information Coding through Adaptive Gating of Synchronized Thalamic Bursting. Cell Rep 14, 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CA, Berger JI, de Boer J, Sereda M, Palmer AR, Hall DA, and Wallace MN (2019). Gap-induced inhibition of the post-auricular muscle response in humans and guinea pigs. Hear Res 374, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DD, and Ryugo DK (1996). Mossy fiber projections from the cuneate nucleus to the cochlear nucleus in the rat. J Comp Neurol 365, 159–172. [DOI] [PubMed] [Google Scholar]
- Wu C, Martel DT, and Shore SE (2015). Transcutaneous induction of stimulus-timing-dependent plasticity in dorsal cochlear nucleus. Front Syst Neurosci 9, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Martel DT, and Shore SE (2016). Increased Synchrony and Bursting of Dorsal Cochlear Nucleus Fusiform Cells Correlate with Tinnitus. J Neurosci 36, 2068–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, and Shore SE (2018). Multisensory activation of ventral cochlear nucleus D-stellate cells modulates dorsal cochlear nucleus principal cell spatial coding. J Physiol 596, 4537–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Chen L, Yang H, Li X, Qiu Z, Huang X, and Zheng Y (2013). [Hidden hearing loss in tinnitus patients with normal audiograms: implications for the origin of tinnitus]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 27, 362–365. [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, and Bao S (2011). Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci U S A 108, 14974–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek MA, Kaltenbach JA, Mathog TA, and Zhang J (2002). Effects of cochlear ablation on noise induced hyperactivity in the hamster dorsal cochlear nucleus: implications for the origin of noise induced tinnitus. Hear Res 172, 137–143. [DOI] [PubMed] [Google Scholar]
- Zeng C, Nannapaneni N, Zhou J, Hughes LF, and Shore S (2009). Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci 29, 4210–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Shroff H, and Shore SE (2011). Cuneate and spinal trigeminal nucleus projections to the cochlear nucleus are differentially associated with vesicular glutamate transporter-2. Neuroscience 176, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Yang Z, Shreve L, Bledsoe S, and Shore S (2012). Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J Neurosci 32, 15791–15801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Pongstaporn T, and Ryugo DK (2006). Projections of the second cervical dorsal root ganglion to the cochlear nucleus in rats. J Comp Neurol 496, 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Luo H, Pace E, Li L, and Liu B (2016). Psychophysical and neural correlates of noised-induced tinnitus in animals: Intra- and inter-auditory and non-auditory brain structure studies. Hear Res 334, 7–19. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA, Godfrey DA, and Wang J (2006). Origin of hyperactivity in the hamster dorsal cochlear nucleus following intense sound exposure. J Neurosci Res 84, 819–831. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wu C, Martel DT, West M, Sutton MA, and Shore SE (2018). Remodeling of cholinergic input to the hippocampus after noise exposure and tinnitus induction in Guinea pigs. Hippocampus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, and Shore S (2007). Vessicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol 500, 777–787. [DOI] [PubMed] [Google Scholar]
- Zhou J, and Shore S (2004). Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res 78, 901–907. [DOI] [PubMed] [Google Scholar]
- Zhou J, and Shore S (2006). Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol 495, 100–112. [DOI] [PubMed] [Google Scholar]