Abstract
Human growth hormone (GH) binds and activates GH receptor (GHR) and prolactin (PRL) receptor (PRLR). LNCaP human prostate cancer cells express only GHR. A soluble fragment of IGF-1 receptor (IGF-1R) extracellular domain (sol IGF-1R) interacts with GHR and blocks GH signaling. We now explore sol IGF-1R’s specificity for inhibiting GH signaling via GHR vs. PRLR and test GHR and PRLR extracellular domain inhibition determinants. Although T47D human breast cancer cells express GHR and PRLR, GH signaling is largely PRLR- mediated. In T47D, sol IGF-1R inhibited neither GH- nor PRL-induced STAT5 activation. However, sol IGF-1R inhibited GH-induced STAT5 activation in T47D-shPRLR cells, which harbor reduced PRLR. In MIN6 mouse β-cells, bovine GH (bGH) activates mouse GHR, not PRLR, while human GH activates mouse GHR and PRLR. In MIN6, sol IGF-1R inhibited bGH-induced STAT5 activation, but partially inhibited human GH-induced STAT5 activation. These findings suggest sol IGF-1R’s inhibition is GHR-specific. Using a cellular reconstitution system, we compared effects of sol IGF-1R on signaling through GHR, PRLR, or chimeras in which extracellular subdomains 2 (S2) of the receptors were swapped. Sol IGF-1R inhibited GH-induced STAT5 activation in GHR- expressing, not PRLR-expressing cells, consistent with GHR specificity of sol IGF-1R. Interestingly, we found that GHR S2 (which harbors the GHR-GHR dimer interface) was required, but not sufficient for sol IGF-1R inhibition of GHR signaling. These results suggest sol IGF-1R specifically inhibits GH-induced GHR-mediated signaling, possibly through interaction with GHR S1 and S2 domains. Our findings have implications for GH antagonist development.
Keywords: Growth hormone receptor, Prolactin receptor, IGF-1 receptor, Signaling
1. Introduction
Growth hormone (GH), a 22 kDa polypeptide hormone derived mostly from the anterior pituitary gland, signals powerful anabolic and metabolic actions (Isaksson et al., 1985; Waters et al., 2006; Moller and Jorgensen, 2009). Cell surface GH receptor (GHR) is a 620 residue transmembrane glycoprotein member of the cytokine receptor superfamily (Bazan, 1990; Huising et al., 2006; Waters and Brooks, 2011). GH binds to preformed GHR dimers and induces a conformational change that results in the activation of GHR, the GHR-associated tyrosine kinase, JAK2, and downstream signaling pathways, including the STAT5 pathway (Herrington and Carter-Su, 2001; Frank and Messina, 2002; Brooks et al., 2014; Liu et al., 2014). Previous crystal structure analysis revealed a 1:2 GH:GHR stoichiometry of the ligand-receptor complex and that the receptor dimerization interface resides on the GHR extracellular domain (ECD) subdomain 2 (S2) (Ultsch et al., 1991).
Prolactin (PRL) signaling, which mainly impacts breast development and lactation (Goffin et al., 2002), shares features with GH signaling, such as the utilization of JAK2/STAT5 pathway (Campbell et al., 1994; Rui et al., 1994; Huang et al., 2006). PRL receptor (PRLR) also belongs to the cytokine receptor superfamily and has similarities with GHR in both sequence and folded structure (Boutin et al., 1989). Similar to GHR, PRLR is predimerized, mediated by the interface between S2 domains of the dimer partners (Broutin et al., 2010; van Agthoven et al., 2010).
In humans, GH activates both GHR and PRLR, while PRL binds PRLR but not GHR. The physiological significance of this interesting feature is incompletely understood, but might be relevant for the diverse effects of GH in humans (Hughes and Friesen, 1985; Cunningham et al., 1990; Somers et al., 1994; Fu et al., 1992). Development and characterization of novel GHR-specific antagonist of GH signaling and actions might be of particular interest to allow more precise therapeutic targeting approaches. One such example is our anti-GHRext-mAb, a monoclonal antibody (mAb) whose epitope resides largely within the S2 domain of GHR. Treatment with anti-GHRext-mAb does not greatly affect GH binding, but inhibits the GH-induced GHR conformational change(s) required for signaling (Jiang et al., 2004, 2011).
Type 1 insulin-like growth factor-1 receptor (IGF-1R) is cell surface tyrosine kinase receptor that transduces IGF-1 signaling. IGF-1R exists as heterotetramer that consists of two α- and two β-chains (Ullrich et al., 1986; LeRoith, 2000; Nakae et al., 2001). Interestingly, our recent studies suggested that, in addition to binding IGF-1, IGF-1R functions as a component of the GH signaling pathway in an IGF-1-independent fashion (Huang et al., 2004a; Gan et al., 2010, 2013, 2014a). Further, we found that a soluble fragment of the IGF-1R ECD (sol IGF-1R) that includes the L1-CR-L2 region of the α-chain interacts with GHR upon GH treatment and inhibits GH-induced signaling in multiple cell lines, including mouse primary calvarial cells, mouse 3T3-F442A preadipocyte fibroblasts, and human LNCaP prostate cancer cells (Gan et al., 2014a, 2014b). In the current study, we use several cell systems to examine the specificity of the inhibitory effect of sol IGF-1R. Comparison of its effects on GH-induced signaling mediated by GHR vs. PRLR strongly suggests that sol IGF-1R is a GHR-specific GH signaling inhibitor, competing with endogenous IGF-1R for GHR interaction in a dominant-negative manner. We also explore the GHR ECD component (s) required for sol IGF-1R’s action. Our results indicate both the S1 and S2 of GHR ECD are required for sol IGF-1R to inhibit GH signaling.
2. Materials and methods
2.1. Materials
Routine reagents were purchased from Sigma Aldrich Corp. (St. Louis, MO) unless otherwise noted. Fetal bovine serum, gentamicin sulfate, penicillin, and streptomycin were purchased from BioFluids (Rockville, MD). Recombinant hGH was kindly provided by Eli Lilly & Co. (Indianapolis, IN). Bovine GH (bGH; lot number APF11182B) was kindly provided by Dr. A. F. Parlow, Pituitary Hormones and Antisera Center, Harbor-UCLA Medical Center (Torrance, CA) and the NIDDK, National Institutes of Health National Hormone and Pituitary Program. Recombinant human PRL was obtained from the National Hormone and Pituitary Program.
2.2. Antibodies
Polyclonal anti-pSTAT5 was purchased from Cell Signaling Technology, Inc. (Danvers, MA). Polyclonal anti-STAT5 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-phosphotyrosine (pY, 4G10) was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Polyclonal anti-pJAK2 antibody was purchased from EMD Millipore (Darmstadt, Germany). Polyclonal anti-GHRcytAL-47 (referred to as anti-GHR) against the intracellular domain of GHR (Jiang et al., 1998), polyclonal anti-PRLRcytAL-84 (referred to as anti-PRLR) against the intracellular domain of PRLR (Chen et al., 2015), polyclonal anti-JAK2AL33 (referred to as anti-JAK2) (Jiang et al., 1998), monoclonal anti-GHRext-mAb against the extracellular domain of GHR (Jiang et al., 2004, 2011; Alele et al., 1998; Kim et al., 1998; Zhang et al., 1999; Liu et al., 2017), monoclonal anti-GHRcyt-mAb against the intracellular domain of GHR (Zhang et al., 1999), and monoclonal anti-PRLRext-mAb against the extracellular domain of the long form of human PRLR (Liu et al., 2017) were described previously.
2.3. Cell culture and stable transfection
Human T47D breast cancer and human LNCaP prostate cancer cells were purchased from American Type Culture Collection (Manassas, VA). T47D cells were cultured in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS), 50 μg/ml gentamicin sulfate, 100 units/ml penicillin, and 100 μg/ml streptomycin. LNCaP cells were cultured in RPMI 1640 medium, supplemented with 10% FBS, 1.5 g/ liter sodium bicarbonate, 1.0 mm sodium pyruvate, 50 μg/ml gentamicin sulfate, 100 U/ml penicillin, and 100 μg/ml streptomycin.
T47D-SCR and T47D-ShPRLR cells and their derivation and preparation have been previously described (Plotnikov et al., 2009). The cells were cultured in DMEM/F12 medium, supplemented with 10% FBS, 50 μg/ml gentamicin sulfate, 100 U/ml penicillin, and 100 μg/ml streptomycin and puromycin, as described (Plotnikov et al., 2009).
Mouse MIN6 cells were cultured in DMEM with 4.5 g/liter glucose, supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 50 μM β-mercaptoethanol. γ2A-JAK2 cells were generated by transfection of γ2A cells (Kohlhuber et al., 1997) (gift from G. Stark, Cleveland Clinic, Cleveland, OH) with pcDNA3.1 (+)/zeo-JAK2 and carried in culture, as described previously (Loesch et al., 2006; Deng et al., 2007). The human GHR complementary DNA (cDNA) in pcDNA1 was a gift provided by R. Ross (University of Sheffield, Sheffield, UK). The human PRLR cDNA in pEF/V5/HIS was a gift provided by C. Clevenger (Virginia Commonwealth University, Richmond, VA). Generation of the plasmids encoding GHR, PRLR, GHR (PRLR-S2) (in which the PRLR-S2 replaces the GHR-S2), and PRLR (GHR-S2) (in which the GHR-S2 replaces the PRLR-S2) has been previously described (Liu et al., 2017). γ2A-JAK2-GHR cells were generated by cotransfection of γ2A-JAK2 cells with pcDNA3.1(+)/zeo-GHR and a hygromycin-encoding plasmid at a weight ratio as 20:1, followed by hygromycin selection and single clone amplification. γ2A-JAK2-PRLR, γ2A-JAK2-GHR(PRLR-S2), γ2A-JAK2-PRLR(GHR-S2) cells were made in the same fashion.
2.4. Adenovirus and lentivirus and conditioned medium preparation
Adenoviruses driving expression of human sol IGF-1R or control human sol IR were amplified and purified, as previously described (Gan et al., 2014b). Details of the preparation of the conditioned medium (CM) containing the sol IGF-1R and control sol IR proteins have been described previously (Gan et al., 2014a). Briefly, HEK293 cells were infected with the appropriate adenoviruses at 70%–80% confluence. For each 10-cm2 dish of cells, complete medium was first replaced with 2 ml of serum-free medium, and each adenovirus was added at 400 multiplicity of infection to the serum-free medium and incubated at 37 °C for 1 h. At the end of incubation, 8 mL of complete medium was added and the infected cells were incubated at 37 °C overnight. The medium was then replaced with 10 mL of serum-free medium for 48 h. Supernatant (referred to as conditioned medium, CM) was collected and clarified by centrifugation and used undiluted.
As an alternative method of CM generation, we employed doxycycline-controlled lentivirus-driven expression of sol IGF-1R or sol IR in stable (HEK-293) cell lines. The respective coding sequences were cloned into a lentiviral vector immediately downstream of the tetracycline (Tet)-responsive element (TRE). The expression strategy further incorporated an internal ribosomal entry site (IRES) and a contiguous downstream sequence comprising the puromycin (puro), T2A, and enhanced green fluorescent protein (EGFP) open reading frame (Hildebrandt et al., 2015; Go et al., 2015). The integrity of the recombinant vectors was confirmed by nucleotide sequencing. Sol IGF-1R and sol IR were produced by expression in 293HEK cells. Briefly, vectors comprising the sol IGF-1R and sol IR sequences were packaged, pseudotyped with vesicular stomatitis virus (VSV) G protein, and used to transduce 293F cells (Invitrogen) that constitutively express the reverse Tet transactivator (rtTA), as we and others have described (Hildebrandt et al., 2015; Urlinger et al., 2000). The vector transduced cells were enriched by incubation for 18 h in a DMEM/F12 culture medium containing 1 μg/ml of doxycycline (Dox) and then for 7 days in a medium supplemented with both Dox (0.5 μg/ml) and puro (25 μg/ ml). The selected cells were adapted for growth in a serum-free suspension culture medium (CDM4HEK293TM, Thermo) supplemented with 1% pluronic (Gibco); 1% Pen-Strep-Glutamine (Gibco); 1% L-Glutamine(Gibco); and 0.1% Fungizong (Gibco). For the production of exogenous sol IGF-1R and sol IR protein, the cells were expanded in suspension culture and treated with 1 μg/ml of Dox after reaching a volume of 250 ml and a density of 5 × 106 cells per ml. After 48 h of culture with Dox, the supernatants (conditioned media: CM) were separated from cells by centrifugation, and cryostored at −80 °C. (We note that indistinguishable results were obtained with CM derived by adenovirus and lentivirus-based expression systems.)
2.5. Cell starvation, cell stimulation, and protein extraction
Serum starvation of cells was accomplished by substitution of 0.5% (w/v) bovine serum albumin (fraction V: Roche Molecular Biochemicals, Indianapolis, IN) for fetal bovine serum in the culture medium for 16–20 h prior to the experiments. Pretreatments and stimulations were carried out at 37 °C in serum-free medium. Stimulations were terminated by washing the cells once with ice-cold phosphate-buffered saline supplemented with 0.4 mM sodium orthovanadate. The cells were then harvested in lysis buffer (1% Triton X-100, 150 mM NaCL, 10% glycerol, 50 mM Tris-HCL, 100 mM NaF, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 10 mM benzamidine, 5 μg/ml aprotinin, and 5 μg/ml leupeptin. Cells were lysed for 30 min at 4 °C in lysis buffer before centrifugation at 15, 000×g for 10 min at 4 °C. The protein concentration was determined and equal amounts of protein extracts (supernatant) were subjected to immunoprecipitation or were directly electrophoresed and immunoblotted as indicated.
2.6. Immunoprecipitation and Western blotting
For immunoprecipitation, 0.5–1 mg protein was incubated with antibody against JAK2 or PRLR overnight at 4 °C. Protein A sepharose (fast flow, Pharmacia Biotech, Providence, RI) was then added, and incubations continued for 1 h at 4 °C. The beads were washed five times with lysis buffer. SDS Sample buffer eluates were resolved by SDS-PAGE under reducing conditions in a similar fashion as were non-immunoprecipitated cell extracts. Resolved proteins were transferred to nitrocellulose membranes (Amersham Biosciences, Pittsburgh, PA), followed by blocking with 2% BSA. Western transfers were immunoblotted with anti-pY (4G10) (1:2000), anti-pJAK2 (1:1000), anti-JAK2AL33 (1:1000), anti-pSTAT5 (1:1000), anti-STAT5 (1:1000), anti-GHRcyt-AL-47 (1:1000), anti-PRLRcyt-AL-84 (1:1000) antibodies.
3. Results
3.1. Effects of soluble IGF-1R on GH signaling in LNCaP and T47D cells
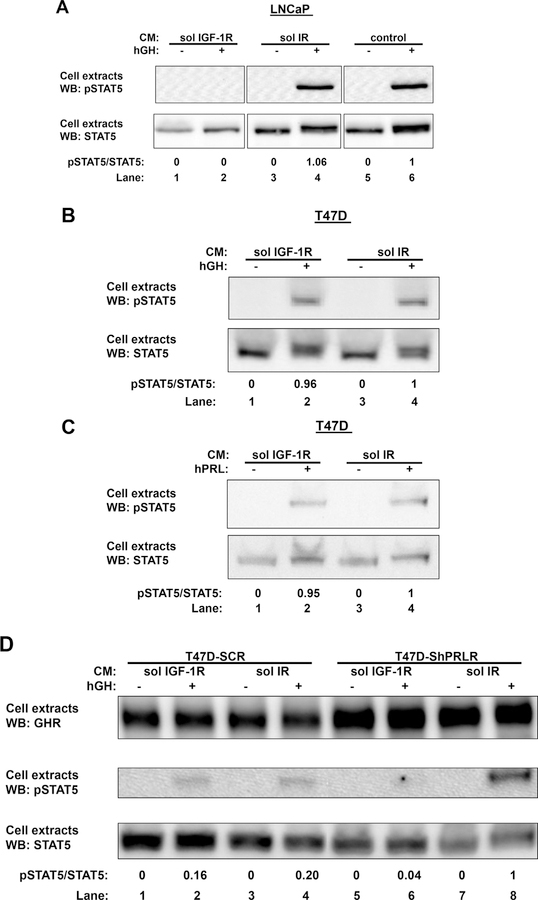
In our previous work, we have reported that a soluble fragment of IGF-1R extracellular domain, solIGF-1R, was able to inhibit GH-induced STAT5 phosphorylation in multiple cell lines, such as mouse primary calvarial cells, mouse 3T3-F442A preadipocyte fibroblasts, as well as human LNCaP prostate cancer cells (Gan et al., 2014a, 2014b). The effect of soluble IGF-IR on GH-induced STAT5 phosphorylation in LNCaP cells is demonstrated in Fig. 1A. Serum-starved LNCaP cells were preincubated with the CM containing either soluble IGF-1R (sol IGF-1R; lane 1 and 2) or soluble insulin receptor (sol IR; lane 3 and 4) or serum-free medium as control (lane 5 and 6). One hour later, cells were treated with vehicle or GH (500 ng/ml) for 5min, after which detergent extracts were resolved by SDS-PAGE and immunoblotted with an antibody that recognizes tyrosine phosphorylated STAT5 (pSTAT5). As expected, GH induced similar STAT5 phosphorylation in cells that were incubated with CM containing sol IR (lane 4) or serum-free medium (lane 6), while GH-induced STAT5 phosphorylation was blocked in cells treated with CM containing sol IGF-1R (lane 2). When the blot was stripped and reprobed with antibody for total STAT5 (STAT5), a clear shift of STAT5 in response to GH was observed in cells treated with sol IR (lane 4) or serum-free medium (lane 6), but not in cells treated with sol IGF-1R (lane 2). These are consistent with previous findings that sol IGF-1R, but not sol IR, was able to inhibit acute GH-induced STAT5 signaling.
Fig. 1. Effects of sol IGF-1R human LNCaP prostate cancer cells and T47D breast cancer cells.
A, Sol IGF-1R blunts GH-induced STAT5 activation in LNCaP cells. Serum-starved LNCaP cells were preincubated with CM containing either sol IGF-1R (lanes 1 and 2) or soluble insulin receptor (sol IR; lanes 3 and 4), or serum-free medium (lanes 5 and 6). One hour later, cells were treated with without or with GH (500 ng/ml) for 5 min, after which detergent cell extracts were resolved by SDS-PAGE and immunoblotted sequentially with anti-pSTAT5 and anti-STAT5. The data shown are representative of three such experiments. Densitometric estimates of the relative intensity of the pSTAT5 band normalized by the abundance of total STAT5 (in arbitrary units with 1 as maximum control condition within the representative experiment) are shown. B and C, Sol IGF-1R has no effect on GH- or PRL-induced STAT5 activation in T47D cells. Serum-starved T47D cells were preincubated with CM containing either sol IGF-1R (lanes 1 and 2) or sol IR (lanes 3 and 4) for 1 h, followed by treatment without or with GH (500 ng/ml) (B) or PRL (500 ng/ml) (C) for 10 min. Detergent cell extracts were resolved by SDS-PAGE and immunoblotted sequentially with anti-pSTAT5 and anti-STAT5. The data shown are representative of two such experiments. Densitometric estimates of the relative intensity of the pSTAT5 band normalized by the abundance of total STAT5 (in arbitrary units with 1 as maximum control condition within the representative experiment) are shown. D, Sol IGF-1R inhibits GH-induced STAT5 activation in T47D-ShPRLR cells. Serum-starved T47D-SCR or T47D-ShPRLR cells were preincubated with CM containing either sol IGF-1R (lanes 1, 2, 5, and 6) or sol IR (lanes 3, 4, 7, and 8) for 1 h, followed by treatment without or with GH (500 ng/ml) for 10 min. Detergent cell extracts were resolved by SDS-PAGE and immunoblotted with anti-GHR, anti-pSTAT5, and anti-STAT5. The data shown are representative of two such experiments. Densitometric estimates of the relative intensity of the pSTAT5 band normalized by the abundance of total STAT5 (in arbitrary units with 1 as maximum control condition within the representative experiment) are shown.
We and others previously demonstrated that human T47D breast cancer cells respond to both GH and PRL (Huang et al., 2006; Xu et al., 2011) (Langenheim and Chen, 2009). In T47D cells, both GHR and PRLR are endogenously expressed, and GH signaling is mostly mediated by PRLR (Xu et al., 2011). We first examined the effect of soluble IGF-IR on GH-induced STAT5 phosphorylation in T47D cells. As shown in Fig. 1B, serum-starved T47D cells were first incubated with the CM containing either sol IGF-1R (lane 1 and 2) or sol IR (lane 3 and 4) for 1 h, followed by acute treatment with vehicle or GH. Detergent extracts were immunoblotted for pSTAT5 and total STAT5 sequentially. In contrast to LNCaP cells, GH was able to induce STAT5 phosphorylation at similar level in the presence of either sol IGF-1R or sol IR (lane 2 vs. 4), indicating sol IGF-1R was not able to inhibit GH-induced signaling mediated by PRLR in T47D cells. In a similar fashion, we tested the effect of sol IGF-1R on prolactin-induced STAT5 phosphorylation (Fig. 1C), and found sol IGF-1R was also not able to block PRL-induced STAT5 phosphorylation, which was mediated by PRLR in T47D cells (Fig. 1C; lane 2 vs. 4). Taken together, these results suggest sol IGF-1R might be able to inhibit GH-induced signaling only when it is mediated by GHR, not PRLR.
We next examined the effect of sol IGF-1R in T47D-ShPRLR cells, in which PRLR expression was reduced by stable short hairpin RNA (shRNA) expression, with T47D-SCR cells as control (Fig. 1D). Serum-starved T47D-SCR cells (Fig. 1D; lane 1–4) or T47D-ShPRLR cells (Fig. 1D; lane 5–8) were incubated with the CM containing either sol IGF-1R (lanes 1, 2, 5, and 6) or sol IR (lanes 3, 4, 7, and 8) for 1 h, followed by vehicle or GH treatment (500 ng/ml) for 5 min. As previously observed (Xu et al., 2013), increased abundance of GHR protein was observed in T47D-ShPRLR cells compared with T47D-SCR cells (lanes 5–8 vs. lanes 1–4). In the presence of sol IR (serving as a negative control CM), enhanced GH-induced STAT5 activation was found in T47D-ShPRLR cells compared with T47D-SCR cells (lane 8 vs. 4). (We note that in data not shown, sol IR pretreatment had no impact compared with buffer alone, on GH-induced STAT5 activation in T47D-ShPRLR cells.) These findings are consistent with our previous studies in which PRLR knockdown has been shown to decrease the rate of GHR proteolytic turnover, resulting in an increase of GHR protein and enhanced GH sensitivity (Xu et al., 2013). In T47D-SCR cells, GH induced STAT5 phosphorylation at similar levels in the presence of either sol IGF-1R or sol IR (lane 2 vs. 4), which is consistent with our findings in T47D cells. In contrast, GH-induced STAT5 phosphorylation was blocked by sol IGF-1R in T47D-ShPRLR cells. We have previously reported enhanced GHR usage by GH upon PRLR knockdown in T47D-ShPRLR cells (Xu et al., 2013). Taken together, we conclude sol IGF-1R is able to inhibit GH-induced signaling only when mediated by GHR.
3.2. Effects of soluble IGF-1R on GH signaling in MIN6 cells
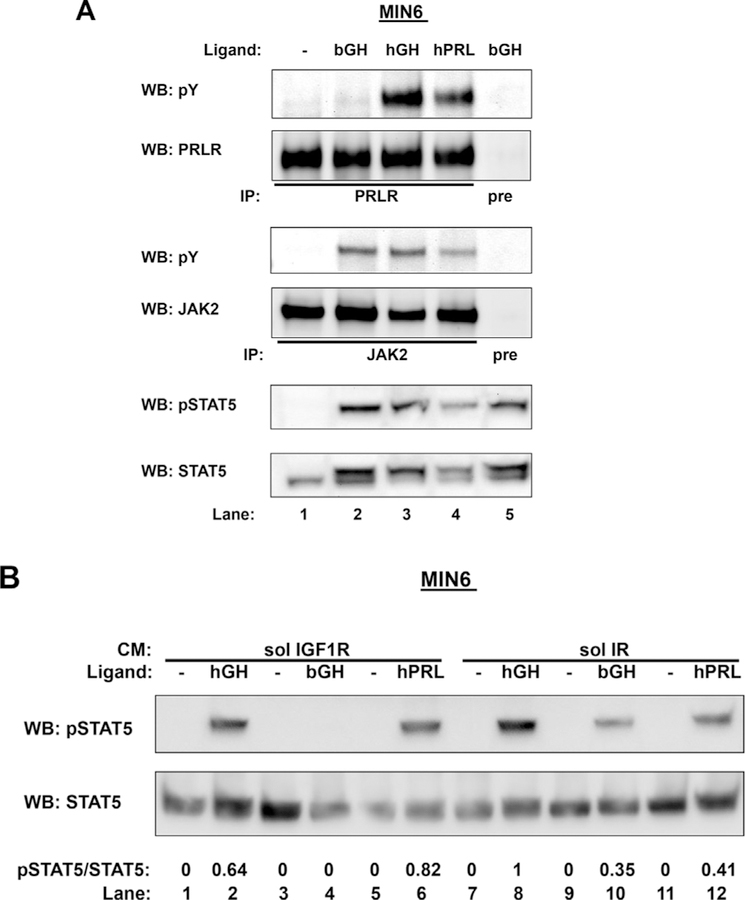
As a well-accepted model system to study β-cell signaling and function (Hohmeier and Newgard, 2004), mouse MIN6 pancreatic β-cells endogenously express GHR. GH treatment has been shown to activate GHR and downstream signaling, including phosphorylation of JAK2 and STAT5 (Ma et al., 2011). Human GH (hGH) activates both GHR and PRLR in rodents, while bovine GH (bGH) only activates GHR (Cunningham et al., 1990; Somers et al., 1994; Fu et al., 1992; Nielsen et al., 2001; Goffin et al., 1996). We sought to exploit this unique feature in MIN6 cells to better understand the specificity of sol IGF-1R action. First, to verify cell responsiveness to bGH, hGH, or PRL treatment, serum-starved MIN6 cells were exposed to vehicle, bGH (500 ng/ ml), hGH (500 ng/ml), or PRL (500 ng/ml) for 15 min before detergent extraction. To assess PRLR tyrosine phosphorylation, extracted proteins were immunoprecipitated with our anti-PRLR and eluates were separated by SDS-PAGE and sequentially immunoblotted with antiphospho-tyrosine (pY) and anti-PRLR. To assess JAK2 tyrosine phosphorylation, extracted proteins were immunoprecipitated with our anti-JAK2, and eluates were separated by SDS-PAGE and sequentially immunoblotted with antiphospho-tyrosine (pY) and anti-JAK2. We also assessed STAT5 tyrosine phosphorylation in response to these ligands. As shown in Fig. 2A, hGH treatment induced tyrosine phosphorylation of PRLR, JAK2, and STAT5 (lane 3 vs. 1). PRL treatment produced a very similar pattern of activation (lane 4 vs. 1). However, bGH treatment was able to induce tyrosine phosphorylation of JAK2 and STAT5 without causing detectable tyrosine phosphorylation of PRLR, consistent with the notion that bGH is able to productively interact with mouse GHR, but not mouse PRLR.
Fig. 2. Effects of Sol IGF-1R on GH signaling in mouse MIN6 β-cells.
A, GH- or PRL-induced signaling in MIN6 cells. Serum-starved MIN6 cells were treated with vehicle, bovine GH (bGH), human GH (hGH), or PRL at 500 ng/ml for 15 min. Detergent cell extracts were immunoprecipitated with either anti-PRLR or anti-JAK2, and eluates were resolved by SDS-PAGE and immunoblotted sequentially with antiphospho-tyrosine (pY) and anti-PRLR or anti-pY and anti-JAK2. Detergent cell extracts were also resolved by SDS-PAGE and immunoblotted with anti-pSTAT5 and anti-STAT5. B, Sol IGF-1R partially inhibits hGH-induced STAT5 activation and completely blocks bGH-induced STAT5 activation in MIN6 cells. Serum-starved MIN6 cells were preincubated with CM containing either sol IGF-1R (lanes 1–6) or soluble IR (lanes 7–12) for 1 h, followed by treatment without or with hGH, bGH, or PRL at 500 ng/ml for 10 min. Detergent cell extracts were resolved by SDS-PAGE and immunoblotted with anti-pSTAT5 and anti-STAT5 sequentially. The data shown are representative of two such experiments. Densitometric estimates of the relative intensity of the pSTAT5 band normalized by the abundance of total STAT5 (in arbitrary units with 1 as maximum control condition within the representative experiment) are shown.
We next examined the effect of sol IGF-1R on ligand-induced STAT5 activation. Serum starved MIN6 cells were incubated with CM containing either sol IGF-1R (Fig. 2B; lane 1–6) or sol IR (lane 7–12) for 1 h, followed by treatment with vehicle, hGH, bGH, or human prolactin (PRL) at 500 ng/ml for 15 min. Detergent extracts were resolved and immunoblotted for pSTAT5 and total STAT5 sequentially. As expected, there was no difference in PRL-induced STAT5 phosphorylation in the presence of sol IGF-1R vs. sol IR (lane 6 vs. 12), as the signaling was mediated by PRLR. In contrast, bGH-induced STAT5 phosphorylation, mediated by mouse GHR only, was markedly inhibited by sol IGF-1R incubation (lane 4 vs. 10). Interestingly, hGH-induced STAT5 phosphorylation, which was mediated by both GHR and PRLR, was partially inhibited by sol IGF-1R incubation (lane 2 vs. 8). (We note that in data not shown, sol IR pretreatment had no impact compared with buffer alone, on inducible STAT5 activation in MIN6 cells.) These findings further indicate that the inhibitory effect of sol IGF-1R on STAT5 activation is GHR-specific.
3.3. Generation and characterization of stable cells expressing GHR, PRLR, GHR(PRLR-S2), and PRLR(GHR-S2)
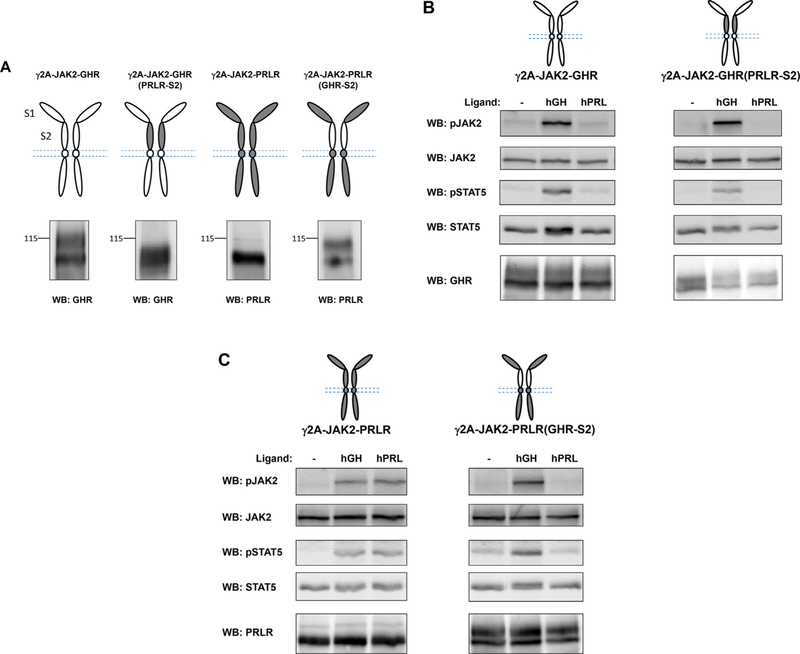
Using both T47D and MIN6 cells, we have established that the inhibition of GH-induced signaling by sol IGF-1R is GHR-specific. Next, we explored the GHR structural requirement(s) for such action using a domain-swapping approach. We engineered two chimera proteins, GHR (PRLR-S2), in which the S2 domain of human GHR (hGHR) was replaced with the same domain of human PRLR (hPRLR), and PRLR(GHR- S2), in which the S2 domain of hPRLR was replaced with the same domain of hGHR. Graphic representation of these chimeras, as well as wild type hGHR and hPRLR, is shown in Fig. 3A. Each of these four proteins was stably transfected into γ2A-JAK2 cells (Loesch et al., 2006; Deng et al., 2007), which are human fibrosarcoma cells that stably express JAK2, but are deficient in GHR and PRLR. Detergent extracts of each stable cell line were resolved by SDS-PAGE under reducing conditions and immunoblotted by antibody specific for the intracellular domain of each protein (Fig. 3A). Similar to our previous findings with rabbit GHR (He et al., 2003, 2005), hGHR was detected in two forms -an approximately 100-kDa form that corresponds to the precursor form of GHR, and a broader 110- to 140-kDa form that corresponds to the mature form of GHR. Replacement of the heavily glycosylated S2 domain of GHR with the S2 domain of PRLR resulted in faster migration of mature GHR(PRLR-S2) compared to the mature GHR. Expression of human PRLR was easily detected, and replacement of its S2 domain with the S2 domain of GHR resulted in slower migration for PRLR(GHR-S2), likely due to the enhanced glycosylation of the GHR S2 domain.
Fig. 3. Generation and characterization of γ2A stable cells expressing GHR, PRLR, GHR(PRLR-S2), and PRLR(GHR-S2).
A, Schematic diagram of domain-swapped human GHR/PRLR chimeras. GHR is in white. PRLR is in gray. GHR(PRLR-S2) is GHR with its S2 replaced with that of PRLR. PRLR(GHR-S2) is PRLR with its S2 replaced with that of GHR. Detergent extracts of γ2A-JAK2 cells stably expressing each receptor were resolved by SDS-PAGE and immunoblotted with antibody specific to the cytoplasmic domain of each receptor. B,C GH- and PRL-induced signaling in γ2A stable cells expressing GHR, PRLR, GHR(PRLR-S2), and PRLR(GHR-S2). Serum-starved cells were treated with vehicle (−), GH, or PRL at 500 ng/ml for 10 min. Detergent extracts were resolved by SDS-PAGE under reducing conditions and sequentially immunoblotted for pJAK2, total JAK2, pSTAT5, and STAT5. Detergent extracts were resolved on a separate SDS-PAGE and immunoblotted by anti-GHR or anti-PRLR to verify receptor expression. The data shown are representative of three such experiments.
We next examined the GH and prolactin responses in these four stable cells lines (Fig. 3B and C). Serum starved cells were treated with vehicle, hGH, or hPRL at 500 ng/ml for 10 min. Detergent extracts were resolved by SDS-PAGE and immunoblotted for pJAK2, total JAK2, pSTAT5, and total STAT5 sequentially to examine GH/PRL signaling. In both γ2A-JAK2-GHR and γ2A-JAK2-GHR(PRLR-S2) cells, acute GH treatment (500 ng/ml, 10 min) induced tyrosine phosphorylation of both JAK2 and STAT5, consistent with the notion that GH binds GHR mostly via the S1 domain (de Vos et al., 1992). Acute PRL treatment (500 ng/ml, 10 min) did not activate either JAK2 or STAT5, confirming human PRL does not activate human GHR (Fig. 3B). In both γ2A-JAK2-PRLR and γ2A-JAK2-PRLR(GHR-S2) cells, acute GH treatment again activated both JAK2 and STAT5, suggesting hGH is able to activate hPRLR and that only the S1 domain of PRLR is required (Fig. 3C). As expected, acute PRL treatment activated both JAK2 and STAT5 in γ2A-JAK2-PRLR cells. However, PRL treatment failed to activate signaling in γ2A-JAK2-PRLR(GHR-S2) cells (Fig. 3C). In light of these findings, our data suggest the PRLR S2 domain is required for PRLR activation, a key difference compared with GHR activation. Indeed, previous crystal structure analysis of PRL complexed with PRLR extracellular domain (van Agthoven et al., 2010) revealed a 1:2 PRL:PRLR complex, similar as the 1:2 GH:GHR complex. Differences between the two structures were observed in the stem-stem dimerization interface, which encompassed the S2 domains. Due to such structural difference, PRLR S2 domains in the PRLR-PRLR complex are closer than are the S2 domains in the GHR-GHR complex. Detergent extracts from the same experiment were also immunoblotted by antibody specific for the intracellular domain of GHR or PRLR to verify protein expression. Together, these data indicate these wild-type and chimera receptors were expressed at the cell surface and capable of binding their corresponding ligand and activating downstream signaling.
3.4. Effects of anti-GHR and anti-PRLR ECD monoclonal antibodies on GH-induced signaling mediated by GHR, PRLR, GHR(PRLR-S2), and PRLR (GHR-S2)
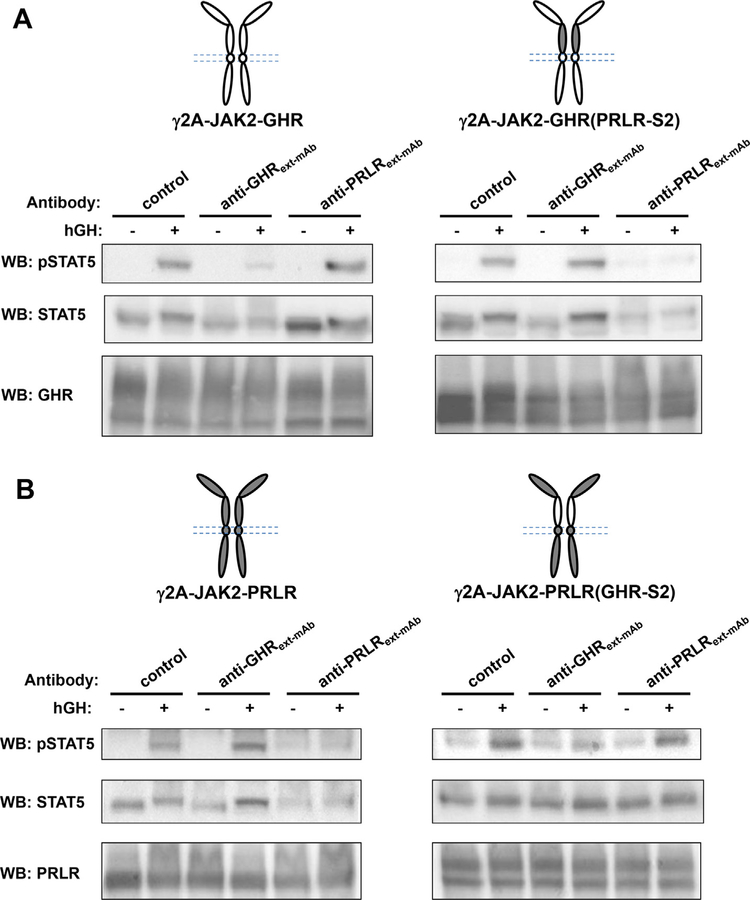
Anti-GHRext-mAb is a monoclonal antibody (mAb) that recognizes largely the S2 domain of GHR (Jiang et al., 2004, 2011). Treatment with anti-GHRext-mAb does not dramatically affect GH binding, but inhibits GH-induced GHR conformational changes required for signaling (Jiang et al., 2004). Anti-PRLRext-mAb is a recently developed monoclonal antibody (mAb) that recognizes the S2 domain of PRLR and inhibits PRLR signaling (Liu et al., 2017). To further characterize the chimera receptors, we compared how anti-GHRext-mAb and anti-PRLRext-mAb would affect GH-induced STAT5 activation mediated through GHR (PRLR-S2) and PRLR(GHR-S2) (Fig. 4AB). Serum starved cells were treated with either a control antibody (anti-GHRcyt-mAb, directed at the GHR intracellular domain), anti-GHRext-mAb, or anti-PRLRext-mAb at 20 μg/ml for 15min, followed by treatment with vehicle or hGH at 500 ng/ml for 10 min. Detergent extracts were immunoblotted for pSTAT5 and total STAT5 sequentially. In each of the four cell lines – expressing GHR, GHR(PRLR-S2), PRLR, or PRLR(GHR-S2) – GH induced tyrosine phosphorylation of STAT5 in the presence of control antibody, as expected. In γ2A-JAK2-GHR cells, GH-induced STAT5 activation was markedly inhibited by anti-GHRext-mAb, whereas anti-PRLRext-mAb had no effect on GHR-mediated STAT5 activation. In γ2A-JAK2-GHR(PRLR-S2) cells, where the GHR S2 domain is replaced by the PRLR S2 domain, anti-GHRext-mAb was no longer able to inhibit GH-induced STAT5 activation. Interestingly, GH-induced STAT5 activation mediated by GHR(PRLR-S2) was markedly inhibited by anti-PRLRext-mAb (Fig. 4A). Similarly, in γ2A-JAK2-PRLR cells, GH-induced STAT5 activation was markedly inhibited by anti-PRLRext-mAb, whereas anti-GHRext-mAb had no effect (Fig. 4B). In γ2A-JAK2-PRLR(GHR-S2) cells, in which the PRLR S2 domain is replaced by the GHR S2 domain, anti-PRLRext-mAb lost its capability to inhibit GH-induced STAT5 activation. In contrast, GH-induced STAT5 activation mediated by PRLR(GHR-S2) was markedly inhibited by anti-GHRext-mAb (Fig. 4B). These results are consistent with our previous results that anti-GHRext-mAb interacts mostly with the S2 domain of GHR while anti-PRLRext-mAb interacts with the S2 domain of PRLR (Jiang et al., 2004, 2011; Liu et al., 2017). Furthermore, our findings indicate the S2 domain of either GHR or PRLR, when swapped, maintains its structural integrity and sensitivity to the corresponding antibody.
Fig. 4.
Effects of anti-GHR and anti-PRLR ECD monoclonal antibodies on GH-induced signaling mediated by GHR, PRLR, GHR(PRLR-S2), and PRLR (GHR-S2). A,B Serum-starved cells were treated with either a control antibody (anti-GHRcyt-mAb, directed at the GHR intracellular domain), anti-GHRext-mAb, or anti-PRLRext-mAb at 20 μg/ml for 15min, followed by treatment with vehicle (−) or hGH at 500 ng/ml for 10 min. Detergent extracts were immunoblotted for pSTAT5 and total STAT5 sequentially. Aliquot of detergent extracts were resolved on a separate SDS-PAGE and immunoblotted by anti-GHR (A) or anti-PRLR (B) to verify receptor expression. The data shown are representative of two such experiments.
3.5. Effects of soluble IGF-1R on GH-induced signaling mediated by GHR, PRLR, GHR(PRLR-S2), and PRLR(GHR-S2)
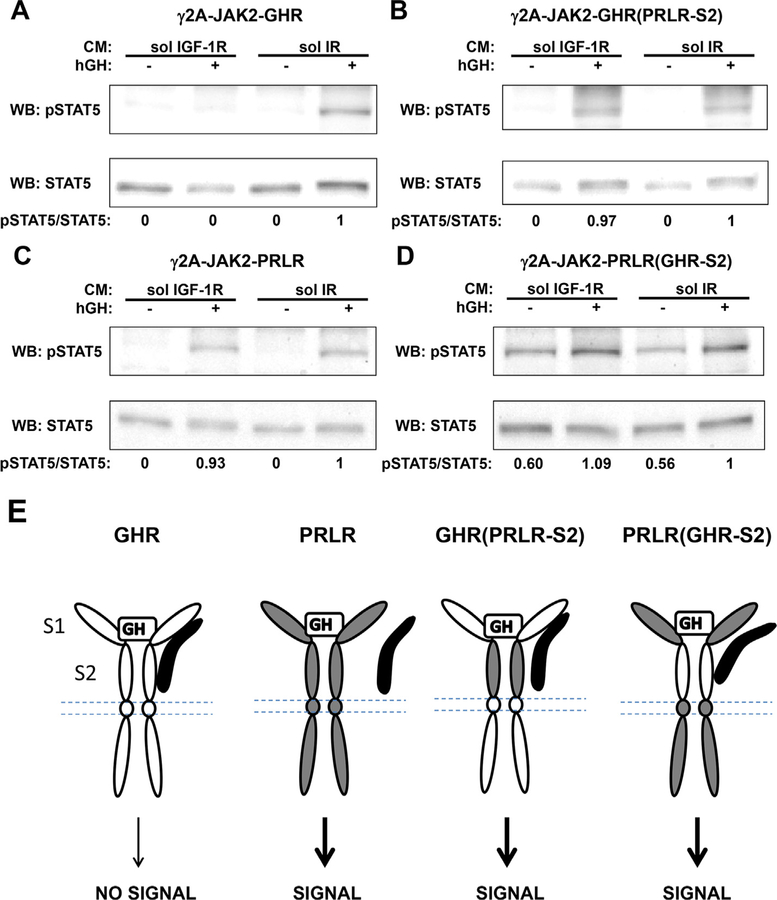
Our findings in both T47D and MIN6 cells have suggested the inhibitory effect of sol IGF-1R to be GHR-specific. Next we examined the effect of sol IGF-1R on GH-induced STAT5 activation mediated by the GHR/PRLR chimera receptors to determine the structural requirement (s) of GHR. Serum-starved cells were incubated with CM containing either sol IGF-1R (Fig. 5; lane 1 and 2) or sol IR (lane 3 and 4), followed by acute treatment with vehicle or hGH at 500 ng/ml for 10 min. Detergent extracts were resolved by SDS-PAGE and sequentially immunoblotted for pSTAT5 and total STAT5. In γ2A-JAK2-GHR cells, acute GH-induced STAT5 activation was completely inhibited by sol IGF-1R (Fig. 5A; lane 2 vs. 4). In γ2A-JAK2-GHR(PRLR-S2) cells, there was no difference in GH-induced STAT5 activation with either sol IGF-1R or sol IR (Fig. 5B; lane 2 vs. 4), suggesting the S2 domain of GHR is required for sol IGF-1R to inhibit GHR-mediated GH signaling. In γ2A-JAK2-PRLR cells, as expected, GH-induced STAT5 activation mediated by PRLR was not inhibited by sol IGF-1R (Fig. 5C; lane 2 vs. 4). Interestingly, in γ2A-JAK2-PRLR(GHR-S2) cells, sol IGF-IR again failed to inhibit GH-induced STAT5 activation (Fig. 5D; lane 2 vs. 4), indicating S2 domain of GHR alone is not sufficient for sol IGF-1R to act. (We note that basal (non-GH-dependent) STAT5 phosphorylation was detected in γ2A-JAK2-PRLR(GHR-S2) cells (Figs. 4B and 5D); we do not know the reason for this basal signal at this time.) Taken together, these findings suggest sol IGF-1R specifically inhibits GH-induced signaling mediated by GHR, possibly through physical and/or functional interaction with both the S1 and S2 domains of GHR.
Fig. 5. Both S1 and S2 of GHR ECD are required for sol IGF-1R to inhibit GH-induced STAT5 activation.
A-D, Serum starved cells expressing GHR, PRLR, GHR(PRLR-S2), or PRLR(GHR-S2) were incubated with CM containing either sol IGF-1R (lanes 1 and 2) or sol IR (lanes 3 and 4), followed by treatment with vehicle (−) or hGH (+) at 500 ng/ ml for 10 min. Detergent extracts were immunoblotted for pSTAT5 and total STAT5 sequentially. The data shown are representative of three such experiments. Densitometric estimates of the relative intensity of the pSTAT5 band normalized by the abundance of total STAT5 (in arbitrary units with 1 as maximum control condition within the representative experiment) are shown.E, Diagrammatic summary and interpretation of findings. GHR is in white. PRLR is in gray. Soluble IGF-1R is in black. GH-induced signaling mediated by GHR, not PRLR, is subject to inhibition by sol IGF-1R. The GHR/sol IGF-1R association is cartooned as involving both the S1 and S2 of the GHR ECD. Such association could be direct or mediated by other unidentified molecule(s). Note that the association between sol IGF-1R and GHR(PRLR-S2) or PRLR (GHR-S2) is depicted as partial in this diagram, which is not sufficient to block downstream signaling. It remains possible that there is no interaction between sol IGF-1R and these chimera receptors.
4. Discussion
GH-induced signaling plays important roles in many cellular and physiological functions. GH induces expression and secretion of IGF-1, a GH effector. IGF-1 signals through IGF-1R and plays important roles in proliferation, anti-apoptosis, and other cellular actions (LeRoith, 2000; Nakae et al., 2001). The relationship between the GH signaling cascade and the IGF-1 signaling cascade has been studied intensively. In particular, we have previously reported a GH-induced complex that includes GHR, JAK2, and IGF-1R in multiple cell systems. Further studies revealed that IGF-1R, independent of its own ligand or tyrosine kinase activity, collaborates with GHR to enhance GH-induced signaling, an action that targets the proximal steps of the GH signaling pathway (Gan et al., 2014a; Ma et al., 2011; Huang et al., 2004b).
IGF-1R is a disulfide-linked heterotetramer consisting of an α2β2 assemblage in which the α-chain is entirely extracellular and the β-chain is a transmembrane protein that harbors tyrosine kinase activity (Ullrich et al., 1986; LeRoith, 2000; Nakae et al., 2001). A soluble fragment of IGF-1R extracellular domain (sol IGF-1R; residues 1–482) has been shown to specifically interact with GHR in response to GH treatment and to reduce GH-induced signaling, including the most proximal steps of GH-induced tyrosine phosphorylation of GHR (Gan et al., 2014a). GH-induced IGF-1 gene expression has been shown to be significantly blunted by sol IGF-1R in primary osteoblasts and LNCaP cells (Gan et al., 2014a). These results suggest sol IGF-1R competes with endogenous IGF-1R for GHR interaction and functions as a dominant-negative inhibitor of GHR-mediated GH signaling. The current study extends these studies substantially and offers more detailed mechanisms for the action of sol IGF-1R.
In the current study, sol IGF-1R (with sol IR as control) pretreatment greatly reduced GH-induced signaling mediated by GHR across several model cell systems. These include: 1) human LNCaP prostate cancer cells, in which PRLR is naturally absent and GH-induced signaling is mostly mediated through GHR; 2) human T47D breast cancer cells, in which PRLR level was reduced by stable shRNA expression and GHR utilization is enhanced compared to control cells; 3) mouse MIN6 β cells, in which GHR can be activated by both bovine and human GH. In contrast, sol IGF-1R did not affect GH-induced signaling when it is mediated by PRLR, as in control T47D cells. These findings strongly suggest that the action of sol IGF-1R is GHR-specific. Furthermore, we note that sol IGF-1R inhibits GH signaling by both mouse and human GHR (this study and (Gan et al., 2014a; Gan et al., 2014b)); this suggests a common element(s) in GHR that allows physical or functional interaction with sol IGF-1R is found across GHR species, but not between GHR and PRLR within species.
GHR and PRLR are cytokine receptors that share topographical features. To determine which domain(s) of GHR is required for sol IGF-1R to exert its inhibitory effect, we exploited a domain swapping approach (Liu et al., 2017) and utilized human fibrosarcoma γ2A-JAK2 cells to stably express GHR, PRLR, GHR(PRLR-S2), or PRLR(GHR-S2), and examined the effect of sol IGF-1R. Sol IGF-1R only inhibited GH-induced signaling when mediated by wild type GHR, but not by GHR (PRLR-S2), in which the GHR S2 is replaced by that from PRLR, suggesting GHR S2 is required for sol IGF-1R to exert its inhibition. Sol IGF-1R did not affect GH-induced signaling mediated by either wild type PRLR or PRLR(GHR-S2), in which PRLR S2 is replaced by that from GHR, indicating that fusion of GHR S2 with PRLR S1 is not sufficient for sol IGF-1R to act. These findings indicate sol IGF-1R clearly distinguishes the ECDs of GHR vs. PRLR and functionally interacts with GHR ECD specifically, either directly or in collaboration with another (unidentified) molecule(s) (Fig. 5E).
Our findings that sol IGF-1R dampens GH signaling specifically via the GHR and not via the PRLR have implications for potential development of antagonists of GH action. Such antagonists occupy an important place, for example, in the therapy of acromegaly, in which excessive GH emanates from a pituitary tumor; pegvisomant, a PEG-ylated form of the recombinant GH mutant, B2036, is a useful primary and/or adjunctive therapy for acromegaly (Molitch, 2017; Giustina et al., 2017) that binds GHR (and not PRLR (Goffin et al., 1999)) specifically and with high affinity largely to subdomain 1, but cannot activate receptor signaling. Our anti-GHRext-mAb also specifically inhibits GHR-mediated signaling in vitro and in vivo (Liu et al., 2014, 2017; Jiang et al., 2004, 2011; Yang et al., 2007, 2008); in contrast to pegvisomant, however, anti-GHRext-mAb interacts with GHR subdomain 2 to exert its antagonistic effect on GH signaling, likely in a fashion mechanistically distinct from that of pegvisomant. Our findings in this study suggest that sol IGF-1R may emerge as a bona fide specific GHR antagonist, at least in the setting of coexpression of GHR and IGF-1R. The mechanism(s) by which sol IGF-1R inhibits GH action remains uncertain, but is likely to differ from those of both pegvisomant and anti-GHRext-mAb (Gan et al., 2014a). Given that GHR may mediate effects of local GH in certain cancers and that deficiency of GHR may protect against cancer development (Chesnokova et al., 2016; Guevara-Aguirre et al., 2011), it will be of interest to develop sol IGF-1R as a potentially novel antagonist by further refining its determinants of inhibition.
Acknowledgements
The authors acknowledge the generous provision of reagents by those named in the text. We also appreciate the helpful conversations with members of the Frank laboratory. This work was supported by a Veterans Affairs Merit Review Award, NIH grants DK107441, and DK58259 (to S.J.F.). Parts of this work were presented at the 100th Annual Endocrine Society Meeting in Chicago, IL, in 2018.
Funding
This work was supported by NIH grants DK107441(S.J.F.) and DK58259 (S.J.F.) and a VA Merit Review grant (S.J.F.).
Footnotes
Disclosure statement
The authors have nothing to disclose.
References
- Alele J, Jiang J, Goldsmith JF, Yang X, Maheshwari HG, Black RA, Baumann G, Frank SJ, 1998. Blockade of growth hormone receptor shedding by a metalloprotease inhibitor. Endocrinology 139, 1927–1935. [DOI] [PubMed] [Google Scholar]
- Bazan JF, 1990. Structural design and molecular evolution of a cytokine receptor superfamily. Proc. Natl. Acad. Sci. U. S. A 87, 6934–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin JM, Edery M, Shirota M, Jolicoeur C, Lesueur L, Ali S, Gould D, Djiane J, Kelly PA, 1989. Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol. Endocrinol 3, 1455–1461. [DOI] [PubMed] [Google Scholar]
- Brooks AJ, Dai W, O’Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, Gardon O, Tunny KA, Blucher KM, Morton CJ, Parker MW, Sierecki E, Gambin Y, Gomez GA, Alexandrov K, Wilson IA, Doxastakis M, Mark AE, Waters MJ, 2014. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science 344, 1249783. [DOI] [PubMed] [Google Scholar]
- Broutin I, Jomain JB, Tallet E, van Agthoven J, Raynal B, Hoos S, Kragelund BB, Kelly PA, Ducruix A, England P, Goffin V, 2010. Crystal structure of an affinity-matured prolactin complexed to its dimerized receptor reveals the topology of hormone binding site 2. J. Biol. Chem 285, 8422–8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GS, Argetsinger LS, Ihle JN, Kelly PA, Rillema JA, Carter-Su C, 1994. Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc. Natl. Acad. Sci. U. S. A 91, 5232–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, Parsons R, Jiang J, Zhang Y, Liu H, Wang P, Bender AS, Frank SJ, Stewart AF, 2015. Augmented Stat5 signaling bypasses multiple impediments to lactogenmediated proliferation in human beta-cells. Diabetes 64, 3784–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V, Zonis S, Zhou C, Recouvreux MV, Ben-Shlomo A, Araki T, Barrett R, Workman M, Wawrowsky K, Ljubimov VA, Uhart M, Melmed S, 2016. Growth hormone is permissive for neoplastic colon growth. Proc. Natl. Acad. Sci. U. S. A 113, E3250–E3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham BC, Bass S, Fuh G, Wells JA, 1990. Zinc mediation of the binding of human growth hormone to the human prolactin receptor. Science 250, 1709–1712. [DOI] [PubMed] [Google Scholar]
- de Vos AM, Ultsch M, Kossiakoff AA, 1992. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255, 306–312. [DOI] [PubMed] [Google Scholar]
- Deng L, He K, Wang X, Yang N, Thangavel C, Jiang J, Fuchs SY, Frank SJ, 2007. Determinants of growth hormone receptor down-regulation. Mol. Endocrinol 21, 1537–1551. [DOI] [PubMed] [Google Scholar]
- Frank SJ, Messina JL, 2002. Growth hormone receptor. In: Oppenheim JJ, Feldman M (Eds.), Cytokine Reference On-Line Academic Press, London, UK, pp. 1–21 Harcourt. [Google Scholar]
- Fu YK, Arkins S, Fuh G, Cunningham BC, Wells JA, Fong S, Cronin MJ, Dantzer R, Kelley KW, 1992. Growth hormone augments superoxide anion secretion of human neutrophils by binding to the prolactin receptor. J. Clin. Investig 89, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Zhang Y, Digirolamo DJ, Jiang J, Wang X, Cao X, Zinn KR, Carbone DP, Clemens TL, Frank SJ, 2010. Deletion of IGF-I receptor (IGF-IR) in primary osteoblasts reduces GH-induced STAT5 signaling. Mol. Endocrinol 24, 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Zhang Y, Buckels A, Paterson AJ, Jiang J, Clemens TL, Zhang ZY, Du K, Chang Y, Frank SJ, 2013. IGF-1R modulation of acute GH-induced STAT5 signaling: role of protein tyrosine phosphatase activity. Mol. Endocrinol 27, 1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Buckels A, Liu Y, Zhang Y, Paterson AJ, Jiang J, Zinn KR, Frank SJ, 2014a. Human GH receptor-IGF-1 receptor interaction: implications for GH signaling. Mol. Endocrinol 28, 1841–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Paterson AJ, Zhang Y, Jiang J, Frank SJ, 2014b. Functional collaboration of insulin-like growth factor-1 receptor (IGF-1R), but not insulin receptor (IR), with acute GH signaling in mouse calvarial cells. Endocrinology 155, 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustina A, Arnaldi G, Bogazzi F, Cannavo S, Colao A, De Marinis L, De Menis E, Degli Uberti E, Giorgino F, Grottoli S, Lania AG, Maffei P, Pivonello R, Ghigo E, 2017. Pegvisomant in acromegaly: an update. J. Endocrinol. Investig 40, 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go EP, Herschhorn A, Gu C, Castillo-Menendez L, Zhang S, Mao Y, Chen H, Ding H, Wakefield JK, Hua D, Liao HX, Kappes JC, Sodroski J, Desaire H, 2015. Comparative analysis of the glycosylation profiles of membrane-anchored HIV-1 envelope glycoprotein trimers and soluble gp140. J. Virol 89, 8245–8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin V, Shiverick KT, Kelly PA, Martial JA, 1996. Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr. Rev 17, 385–410. [DOI] [PubMed] [Google Scholar]
- Goffin V, Touraine P, Pichard C, Bernichtein S, Kelly PA, 1999. Should prolactin be reconsidered as a therapeutic target in human breast cancer? Mol. Cell. Endocrinol 151, 79–87. [DOI] [PubMed] [Google Scholar]
- Goffin V, Binart N, Touraine P, Kelly PA, 2002. Prolactin: the new biology of an old hormone. Annu. Rev. Physiol 64, 47–67. [DOI] [PubMed] [Google Scholar]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD, 2011. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med 3 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Wang X, Jiang J, Guan R, Bernstein KE, Sayeski PP, Frank SJ, 2003. Janus kinase 2 determinants for growth hormone receptor association, surface assembly, and signaling. Mol. Endocrinol 17, 2211–2227. [DOI] [PubMed] [Google Scholar]
- He K, Loesch K, Cowan JW, Li X, Deng L, Wang X, Jiang J, Frank SJ, 2005. Janus kinase 2 enhances the stability of the mature growth hormone receptor. Endocrinology 146, 4755–4765. [DOI] [PubMed] [Google Scholar]
- Herrington J, Carter-Su C, 2001. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol. Metabol 12, 252–257. [DOI] [PubMed] [Google Scholar]
- Hildebrandt E, Mulky A, Ding H, Dai Q, Aleksandrov AA, Bajrami B, Diego PA, Wu X, Ray M, Naren AP, Riordan JR, Yao X, DeLucas LJ, Urbatsch IL, Kappes JC, 2015. A stable human-cell system overexpressing cystic fibrosis transmembrane conductance regulator recombinant protein at the cell surface. Mol. Biotechnol 57, 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmeier HE, Newgard CB, 2004. Cell lines derived from pancreatic islets. Mol. Cell. Endocrinol 228, 121–128. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kim S-O, Yang N, Jiang J, Frank SJ, 2004a. Physical and functional interaction of GH and IGF-1 signaling elements. Mol. Endocrinol 18, 1471–1485. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kim SO, Yang N, Jiang J, Frank SJ, 2004b. Physical and functional interaction of growth hormone and insulin-like growth factor-I signaling elements. Mol. Endocrinol 18, 1471–1485. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li X, Jiang J, Frank SJ, 2006. Prolactin modulates phosphorylation, signaling and trafficking of epidermal growth factor receptor in human T47D breast cancer cells. Oncogene 25, 7565–7576. [DOI] [PubMed] [Google Scholar]
- Hughes JP, Friesen HG, 1985. The nature and regulation of the receptors for pituitary growth hormone. Annu. Rev. Physiol 47, 469–482. [DOI] [PubMed] [Google Scholar]
- Huising MO, Kruiswijk CP, Flik G, 2006. Phylogeny and evolution of class-I helical cytokines. J. Endocrinol 189, 1–25. [DOI] [PubMed] [Google Scholar]
- Isaksson OG, Eden S, Jansson JO, 1985. Mode of action of pituitary growth hormone on target cells. Annu. Rev. Physiol 47, 483–499. [DOI] [PubMed] [Google Scholar]
- Jiang J, Liang L, Kim SO, Zhang Y, Mandler R, Frank SJ, 1998. Growth hormone-dependent tyrosine phosphorylation of a GH receptor-associated high molecular weight protein immunologically related to JAK2. Biochem. Biophys. Res. Commun 253, 774–779. [DOI] [PubMed] [Google Scholar]
- Jiang J, Wang X, He K, Li X, Chen C, Sayeski PP, Waters MJ, Frank SJ, 2004. A conformationally-sensitive GHR (growth hormone (GH) receptor) antibody: impact on GH signaling and GHR proteolysis. Mol. Endocrinol 18, 2981–2996. [DOI] [PubMed] [Google Scholar]
- Jiang J, Wan Y, Wang X, Xu J, Harris JM, Lobie PE, Zhang Y, Zinn KR, Waters MJ, Frank SJ, 2011. Inhibitory GH receptor extracellular domain monoclonal antibodies: three-dimensional epitope mapping. Endocrinology 152, 4777–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SO, Jiang J, Yi W, Feng GS, Frank SJ, 1998. Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J. Biol. Chem 273, 2344–2354. [DOI] [PubMed] [Google Scholar]
- Kohlhuber F, Rogers NC, Watling D, Feng J, Guschin D, Briscoe J, Witthuhn BA, Kotenko SV, Pestka S, Stark GR, Ihle JN, Kerr IM, 1997. A JAK1/JAK2 chimera can sustain alpha and gamma interferon responses. Mol. Cell. Biol 17, 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenheim JF, Chen WY, 2009. Development of a novel ligand that activates JAK2/ STAT5 signaling through a heterodimer of prolactin receptor and growth hormone receptor. J. Recept. Signal Transduct. Res 29, 107–112. [DOI] [PubMed] [Google Scholar]
- LeRoith D, 2000. Insulin-like growth factor I receptor signaling–overlapping or redundant pathways? Endocrinology 141, 1287–1288. [DOI] [PubMed] [Google Scholar]
- Liu Y, Berry PA, Zhang Y, Jiang J, Lobie PE, Paulmurugan R, Langenheim JF, Chen WY, Zinn KR, Frank SJ, 2014. Dynamic analysis of GH receptor conformational changes by split luciferase complementation. Mol. Endocrinol 28, 1807–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jiang J, Lepik B, Zhang Y, Zinn KR, Frank SJ, 2017. Subdomain 2, not the transmembrane domain, determines the dimerization partner of growth hormone receptor and prolactin receptor. Endocrinology 158, 3235–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch K, Deng L, Cowan JW, Wang X, He K, Jiang J, Black RA, Frank SJ, 2006. JAK2 influences growth hormone receptor metalloproteolysis. Endocrinology 147, 2839–2849. [DOI] [PubMed] [Google Scholar]
- Ma F, Wei Z, Shi C, Gan Y, Lu J, Frank SJ, Balducci J, Huang Y, 2011. Signaling cross talk between growth hormone (GH) and insulin-like growth factor-I (IGF-I) in pancreatic islet beta-cells. Mol. Endocrinol 25, 2119–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitch ME, 2017. Diagnosis and treatment of pituitary adenomas: a review. J. Am. Med. Assoc 317, 516–524. [DOI] [PubMed] [Google Scholar]
- Moller N, Jorgensen JO, 2009. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev 30, 152–177. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kido Y, Accili D, 2001. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr. Rev 22, 818–835. [DOI] [PubMed] [Google Scholar]
- Nielsen JH, Galsgaard ED, Moldrup A, Friedrichsen BN, Billestrup N, Hansen JA, Lee YC, Carlsson C, 2001. Regulation of beta-cell mass by hormones and growth factors. Diabetes 50 (Suppl. 1), S25–S29. [DOI] [PubMed] [Google Scholar]
- Plotnikov A, Varghese B, Tran TH, Liu C, Rui H, Fuchs SY, 2009. Impaired turnover of prolactin receptor contributes to transformation of human breast cells. Cancer Res 69, 3165–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui H, Kirken RA, Farrar WL, 1994. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J. Biol. Chem 269, 5364–5368. [PubMed] [Google Scholar]
- Somers W, Ultsch M, De Vos AM, Kossiakoff AA, 1994. The X-ray structure of a growth hormone-prolactin receptor complex. Nature 372, 478–481. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, et al. , 1986. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J 5, 2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultsch M, de Vos AM, Kossiakoff AA, 1991. Crystals of the complex between human growth hormone and the extracellular domain of its receptor. J. Mol. Biol 222, 865–868. [DOI] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W, 2000. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. U. S. A 97, 7963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Agthoven J, Zhang C, Tallet E, Raynal B, Hoos S, Baron B, England P, Goffin V, Broutin I, 2010. Structural characterization of the stem-stem dimerization interface between prolactin receptor chains complexed with the natural hormone. J. Mol. Biol 404, 112–126. [DOI] [PubMed] [Google Scholar]
- Waters MJ, Brooks AJ, 2011. Growth hormone receptor: structure function relationships. Horm Res Paediatr 76 (Suppl. 1), 12–16. [DOI] [PubMed] [Google Scholar]
- Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ, 2006. New insights into growth hormone action. J. Mol. Endocrinol 36, 1–7. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang Y, Berry PA, Jiang J, Lobie PE, Langenheim JF, Chen WY, Frank SJ, 2011. Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex. Mol. Endocrinol 25, 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Sun D, Jiang J, Deng L, Zhang Y, Yu H, Bahl D, Langenheim JF, Chen WY, Fuchs SY, Frank SJ, 2013. The role of prolactin receptor in GH signaling in breast cancer cells. Mol. Endocrinol 27, 266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Wang X, Jiang J, Frank SJ, 2007. Role of the growth hormone (GH) receptor transmembrane domain in receptor predimerization and GH-induced activation. Mol. Endocrinol 21, 1642–1655. [DOI] [PubMed] [Google Scholar]
- Yang N, Langenheim JF, Wang X, Jiang J, Chen WY, Frank SJ, 2008. Activation of growth hormone receptors by growth hormone and growth hormone antagonist dimers: insights into receptor triggering. Mol. Endocrinol 22, 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jiang J, Kopchick JJ, Frank SJ, 1999. Disulfide linkage of growth hormone (GH) receptors (GHR) reflects GH-induced GHR dimerization. Association of JAK2 with the GHR is enhanced by receptor dimerization. J. Biol. Chem 274, 33072–33084. [DOI] [PubMed] [Google Scholar]