Abstract
Purpose:
Breast cancer (BC) has been associated with pregnancy if diagnosed within 5–10 years after delivery (pregnancy-associated BC, PABC). PABC carries a poor prognosis compared to sporadic BC in Western populations. Data are limited regarding PABC in Asian populations, where longer periods of breastfeeding, higher birth rates and a lower median age of BC at diagnosis have been noted, all of which are known to influence prognosis.
Methods:
We used data two datasets of women treated for early BC in Shanghai 1990–2012 (n=10,161 and n=7,411). For the analysis of BC risk after pregnancy we compared the distribution of pregnancy in our dataset to that in Shanghai using age-specific fertility rates. For disease-free survival (DFS) evaluation, we restricted our data to women ≤45 years.
Results:
Women <30 years had a significantly elevated BC risk within 5 years of completing a pregnancy compared to women who had not been pregnant in the previous five years. In women aged 20–24 the relative risk (RR) was 3.33 (P=0.012), and for women aged 25–29 the RR was 1.76 (P=0.0074). For women >30, the RR was decreased. Patients with PABC had a higher risk of recurrence or death (hazard ratio (HR) for DFS 1.72, P=0.019) compared to women with non-PABC by univariable analysis. Age was eliminated from the multivariable model by backward selection, resulting in tumor stage (3 versus 1, HR=3.08, P<.001) and recent pregnancy (HR 1.62, P<0.05) as significant independent prognosticators. Having had a full term pregnancy in the previous 5 years was associated with a 62% increased risk of recurrence.
Conclusions:
We show that recent full-term pregnancy significantly elevates BC risk in women <30 in Shanghai, and that women diagnosed with PABC have a particularly adverse prognosis. Health care providers and women in Asian populations should be made aware of these results.
Keywords: Breast cancer, pregnancy, risk, prognosis, Asian
BACKGROUND
It has been shown repeatedly in Western populations that pregnancy has a dual effect on breast cancer risk, most recognized a decrease in the later risk of postmenopausal breast cancer, and paradoxically an increase in risk during the immediate years after a full-term pregnancy.[1–4].
Breast cancer diagnosed within 5 to 10 years after delivery (pregnancy-associated breast cancer, PABC) carries a poor prognosis compared to sporadic breast cancer [5–8]. Several factors influencing this risk and the adverse prognosis of PABC have been identified in Western populations. Among them age at first childbirth [9–11], the interval between menarche and first birth [12], birth spacing [3] and duration of lactation [13] have been implicated. Data are limited regarding PABC in Asian populations. They are characterized by longer periods of breastfeeding [14–16], higher but rapidly decreasing birth rates [17, 18], a single child policy with multiple terminations of pregnancy, and a lower but increasing median age of breast cancer diagnosis [19, 20]. The effect of a recent pregnancy on breast cancer risk might therefore differ in Asian compared to Western populations.
Here, we report on the risk and prognosis of pregnancy-associated breast cancer in a population of 17,572 women diagnosed with breast cancer at a single medical center in Shanghai between 1990 and 2012.
RESULTS
In Table 1, the relative risk of being diagnosed with breast cancer within five years of a full-term pregnancy (risk of PABC) is shown per age group in comparison to women not having been pregnant in the past five years. Women younger than 30 years had a significantly elevated risk of developing breast cancer within 5 years of completing a pregnancy compared to women who had not been pregnant in the previous five years. In women aged 20–24 the RR was 3.33 (P=0.012), and for women aged 25–29 the RR was 1.76 (P=0.0074). These findings were similar (RR 3.33, P=0.012 and RR 1.51, P=0.02, respectively) when the analysis was restricted to women with a maximum of one previous pregnancy, i.e. for whom the recent pregnancy had been the first pregnancy.
Table 1:
Relative Risk of breast cancer for women having completed a pregnancy in the past 5 years (dataset 1) versus women not pregnant in the past 5 years
| Age of Diagnosis | Approximate relative risk, recently pregnant* versus not** | 95% Confidence Interval | #pregnant < 5 years prior / sample size | p-value |
|---|---|---|---|---|
| 20–24 | 3.33 | (1.32,8.38) | 9 / 18 | .012 |
| 25–29 | 1.76 | (1.27,2.46) | 93 /149 | .00074 |
| 30–34 | 0.87 | (0.73,1.05) | 201/472 | .15 |
| 35–39 | 0.75 | (0.63,0.89) | 159 / 863 | .00092 |
| 40–44 | 0.92 | (0.75,1.14) | 92 / 1391 | .5 |
| 45–49 | 0.73 | (0.5,1.08) | 26 /1639 | .13 |
risk of diagnosis of breast cancer within 5 years of a full-term pregnancy
risk of diagnosis of breast cancer in women not having completed a pregnancy in the past 5 years
For women over 30, the data show a less clear picture: The relative risk of developing breast cancer was decreased in women over the age of 30 if they had been pregnant in the previous 5 years. However, this finding was only significant in the group of women aged 35–39 (RR=0.75, P=0.00092). When the analysis was restricted to women for whom the recent pregnancy had been the first pregnancy, the results were similar, with two age groups (30–34: RR=0.74, P=0.0033 and 35–39: RR=0.51, P<0.0001) showing a significantly decreased risk of being diagnosed with breast cancer if they had been pregnant in the past 5 years.
A Breslow-Day test for homogeneity across all age groups showed that the risk ratios were significantly different across age groups (P = 0.014).
The rate of ER-negative breast cancers was consistently (but not significantly) higher in patients <40 with pregnancy-associated breast cancer (PABC) compared to breast cancer patients who had not completed a pregnancy in the previous five years (non-PABC) (Table 2). However, ER status was missing in a high number of patients in the overall cohort (49.4% and 50.3% of women with PABC and non-PABC, respectively) and this remains to be confirmed.
Table 2:
Primary Tumor ER Status in women with PABC versus non-PABC in dataset 1
| ER negative, n (% of women with ER status in database) | P-value | Missing ER status, n (% of all women) | ||
|---|---|---|---|---|
| Age ≤ 30 | PABC | 32.5 % | .863 | 63/103 (61.2%) |
| Non-PABC | 27.6 % | 39/68 (57.4 %) | ||
| Age > 30 & ≤ 35 | PABC | 28% | .335 | 87/164 (53.5%) |
| Non-PABC | 21.8 % | 145/278 (52.2%) | ||
| Age > 35 & ≤ 40 | PABC | 27.0 % | .324 | 82/156 (52.6%) |
| Non-PABC | 20.9 % | 396/707 (56.0%) | ||
| Age > 40 & ≤ 49 | PABC | 14.5% | .32 | 51/113 (45.1%) |
| Non-PABC | 20.6% | 1388/2623 (52.9%) | ||
| Age ≤ 40 | PABC | 28.5% | .057 | 232/423 (52.8%) |
| Non-PABC | 21.6% | 580/1053 (55.1%) | ||
| Age ≤ 49 | PABC | 25.4% | .096 | 283/536 (52.8%) |
| Non-PABC | 20.8% | 1968/3676(53.5%) |
In the second database (dataset 2), including patient records with longer follow-up, the prognosis of patients with PABC versus those with non-PABC was analyzed. As mentioned, pregnancies recorded in this dataset were only first pregnancies, but based on the fertility rate of ≤1, ‘first’ and ‘most recent’ pregnancies were used synonymously for this analysis. In Table 3, baseline characteristics of patients with PABC and non-PABC in dataset 2 are shown.
Table 3:
Patient and tumor characteristics in patients with PABC versus patients with non-PABC (dataset 2)
| Breast Cancer Patients ≤ age 45 | Patients with PABC (%), n = 109 | Patients with non-PABC (%), n = 1274 | Missing (%), n=416 | All Patients (%), n= 1799 | P-value (PABC vs non-PABC |
|---|---|---|---|---|---|
| N (%) | |||||
| Age | <.001 | ||||
| ≤ 30 | 64 (58.7) | 25 (2.0) | 51 (12.3) | 121 (6.7) | |
| > 30 | 45 (41.3) | 1249 (98.0) | 365 (87.7) | 1678 (93.3) | |
| Stage | 0.341 | ||||
| 0–1 | 11 (10.1) | 194 (15.2) | 61 (14.7) | 266 (14.8) | |
| 2 | 52 (47.7) | 695 (54.6) | 198 (47.6) | 945 (52.5) | |
| 3 | 11 (10.1) | 102 (8.0) | 41 (9.9) | 154 (8.6) | |
| Missing | 35 (32.1) | 283 (22.2) | 116 (32.1) | 434 (24.1) | |
| ER status | 0.731 | ||||
| Positive | 58 (53.2) | 666 (52.3) | 207 (49.8) | 931 (51.8) | |
| Negative | 28 (25.7) | 359 (28.1) | 126 (30.3) | 513 (28.5) | |
| Missing | 23 (21.1) | 249 (19.5) | 83 (20.0) | 355 (19.7) | |
| BC Family History | 0.899 | ||||
| Yes | 7 (6.4) | 61 (4.8) | 14 (3.4) | 82 (4.6) | |
| No | 71 (65.1) | 715 (56.1) | 189 (45.4) | 975 (54.2) | |
| Missing | 31 (28.4) | 498 (39.1) | 213 (51.2) | 742 (41.2) | |
| Age at menarche | 0.142 | ||||
| < 13 | 17 (15.6) | 134 (10.5) | 28 (6.7) | 179 (9.9) | |
| ≥13 | 91 (83.5) | 1127 (88.5) | 366 (88.0) | 1584 (88.0) | |
| Missing | 1 (0.9) | 13 (1.0) | 22 (5.3) | 36 (2.0) | |
| PgR Status | 0.859 | ||||
| Positive | 39 (35.8) | 501 (39.3) | 176 (42.3) | 716 (39.8) | |
| Negative | 34 (31.2) | 406 (31.9) | 128 (30.8) | 568 (31.6) | |
| Missing | 36 (33.0) | 367 (28.8) | 112 (26.9) | 515 (28.6) | |
| Age of 1st gestation | <.001 | ||||
| Never | 0 | 93 (7.3) | 0 | 93 (5.2) | |
| <25 | 9 (8.3) | 385 (30.2) | 0 | 394 (21.9) | |
| 25–29 | 67 (61.5) | 658 (51.6) | 0 | 725 (40.3) | |
| 30–34 | 23 (21.1) | 133 (10.4) | 0 | 156 (8.7) | |
| 35+ | 10 (9.2) | 5 (0.4) | 0 | 15 (0.8) | |
| Missing | 0 | 0 | 416 (100.0) | 416 (23.1) | |
| HER2 | 0.355 | ||||
| Positive | 12 (11.0) | 195 (15.3) | 106 (25.5) | 313 (51.0) | |
| Negative | 58 (53.2) | 664 (52.1) | 196 (47.1) | 918 (17.4) | |
| Missing | 39 (35.8) | 415 (32.6) | 114 (27.4) | 568 (31.6) | |
| Grade | 0.1673 | ||||
| I | 2 (1.8) | 7 (0.5) | 11 (2.6) | 20 (1.1) | |
| II | 22 (20.2) | 329 (25.8) | 126 (30.3) | 477 (26.5) | |
| III | 10 (9.2) | 125 (9.8) | 56 (13.5) | 191 (10.6) | |
| Missing | 75 (68.8) | 813 (63.8) | 223 (53.6) | 1111 (61.8) | |
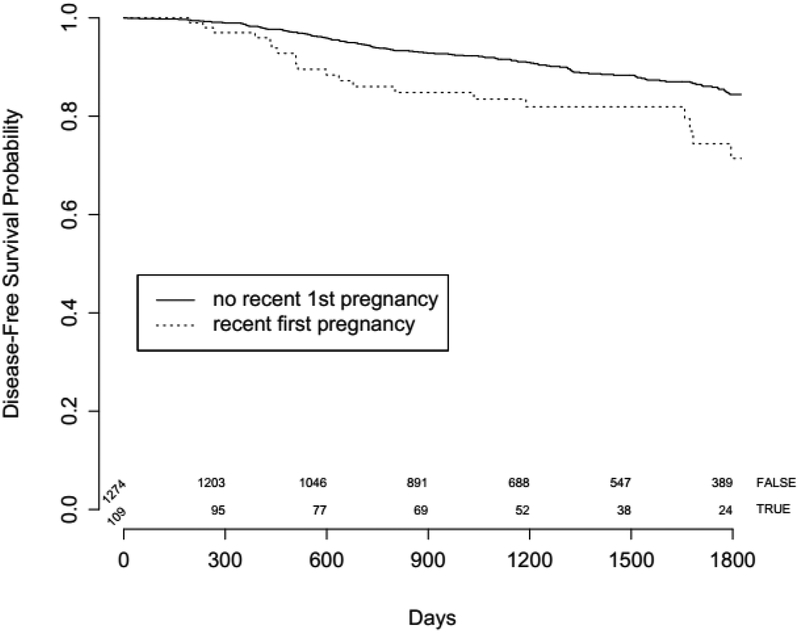
Patients with PABC had an elevated risk of recurrence or death (HR for DFS 1.72, P=0.019) compared to women with non-PABC in univariable analysis (n=1,383). The DFS curve of patients below 45 years of age with PABC versus those with non-PABC is shown in Figure 1. For multivariable analysis, a model allowing multiple imputation of missing values was used in order to account for missing values of disease stage, and of age at first pregnancy. Age was eliminated from the multivariable model in a backward selection process as it was not significantly associated with recurrence, resulting in tumor stage (stage 3 versus 1, HR=3.08, P<.001) and recent pregnancy (HR 1.62, P<0.05) as significant independent prognosticators of disease-free survival (Table 4). Having had a full term pregnancy in the previous 5 years was thus associated with a 62% increased risk of recurrence.
Figure 1:
Disease-free survival in women < 45 years with PABC (first pregnancy) compared to women with non-PABC.
Table 4:
Univariable and multivariable analyses of parameters influencing disease-free survival in dataset 2
| Coefficient | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Unadjusted Model | ||
| Recent pregnancy | 1.66 (1.05,2.62) | 0.03 |
| Unadjusted Model - Multiple Imputation | ||
| Recent pregnancy | 1.66 (1.06,2.60) | 0.027 |
| Adjusted Model - Missing Indicator | ||
| Recent pregnancy (≤ 5 years prior surgery) vs no recent pregnancy | 1.64 (1.04–2.60) | .034 |
| Stage 2 vs Stage 0–1 | 2.02 (1.14–3.60) | .017 |
| Stage 3 vs Stage 0–1 | 6.72(3.59–12.58) | <.001 |
| Missing Stage vs Stage 0–1 | 1.64 (0.87–3.10) | .13 |
| Adjusted Model*- Multiple Imputation | ||
| Recent pregnancy (≤ 5 years prior surgery) vs no recent pregnancy | 1.624 (1.04,2.54) | .034 |
| Stage 2 vs Stage 0–1 | 1.38 (0.90,2.11) | .137 |
| Stage 3 vs Stage 0–1 | 3.08 (1.90,5.01) | <.001 |
age was eliminated from the model in a backward elimination process
DISCUSSION
Women from Shanghai below the age of 30 had an increased risk of breast cancer if they had completed a pregnancy in the previous five years. This finding was independent of the number of previous pregnancies. For women over 30 years of age, we found a decreased risk of breast cancer within five years of completing a pregnancy. A test for homogeneity showed that the risk ratios for PABC were significantly different across age groups.
Our results in women < 30 years are consistent with findings from Western populations, where a transient increase in breast cancer risk after delivery has been reported [3, 21]. However, our results in older women are somewhat contrary with data coming from Western populations, where the peak in the transient increase in risk shortly after delivery is actually strongest after a late first birth [3, 21]. A possible reason for this discrepancy might be the fact that the incidence rate ratio of breast cancer for uniparous compared to nulliparous women varies considerably over time, especially for women > 30 years [3]. In fact, Albrektsen et al reported a decreased breast cancer risk shortly after delivery in women > 30, with a steep increase thereafter and a peak in risk at five years that slowly decreased over time [3]. Our estimated relative risk over a five-year period after delivery includes the assumed period of decreased risk in the first few years, but only partly includes the subsequent period of elevated risk. This might explain why our numbers for women aged > 30 are lower than the previously reported short-term peak at five years post-delivery.
Despite these considerations we believe it is possible that the influence of recent full-term pregnancy on breast cancer is indeed different in Asian compared to Western populations. Several confounding risk factors might play a role in this regard, most importantly the duration of breastfeeding, which is longer in Asian than in Western populations and is associated with a decreased risk of breast cancer in both populations [14–16, 22].
We found a consistently higher rate of estrogen receptor negativity in PABC versus non-PABC breast cancer across all age groups in dataset 1. This finding was not significant, but it is in line with previous data indicating an increased risk of developing ER negative breast cancer after parity [23]. In dataset 2 the rates of ER and PR positivity did not differ between PABC and non-PABC, nor did estrogen receptor status influence prognosis. Conflicting results on the question whether parity influences the risk of hormone receptor positive or negative breast cancers, and in which direction, have been reported in numerous other studies and a meta-analysis on PABC [23–33]. Some of these differences are due to varying definitions of pregnancy-associated breast cancer and whether the analysis was restricted to PABC or was in fact looking at life-time risk of breast cancer after parity. Based on preclinical research demonstrating that the pro-tumorigenic effect of the involuting breast is confined to ER negative lesions [34, 35], a dual effect has recently been suggested where the tumor-promoting environment of the remodeling breast increases the risk of unspecified tumors [4], whereas the later, protective effect of pregnancy applies to hormone-dependent tumors [4]. This is in line with our observation, suggesting a stronger short-term influence of pregnancy on ER negative tumors.
Analysing further tumor and patient characteristics between women < 45 years with PABC and non-PABC in dataset 2, we found no significant differences in family or reproductive history or tumor stage, grade and HER2 status. Importantly, women with PABC were significantly younger at breast cancer diagnosis, while having a significantly higher age at first full-term pregnancy (median age at first full-term pregnancy 28 versus 26 years for PABC and non-PABC, respectively). This finding might indicate that the known increase in age at first childbirth in Asian populations [36] might be linked to the increase in breast cancer incidence in these populations.
Women with PABC had a significantly decreased disease-free survival compared to those with non-PABC in our dataset (HR 1.66, P=0.03 in a model without imputation, and HR 1.66, P=0.027 with multiple imputation). In multivariable analysis including tumor characteristics and patient age, an independent negative prognostic influence of recent pregnancy on breast cancer recurrence was confirmed. This result is in line with several previous studies reporting an impaired prognosis in women diagnosed with breast cancer shortly after childbirth [5–8]. Limitations of our data include the high number of missing values for some variables, which might have influenced our findings, in particular with respect to estrogen receptor status. Although the number of patients < 45 years diagnosed with stage IV breast cancer is extremely low [37], we cannot rule out the possibility that excluding patients with stage IV disease might have biased our results, as the respective numbers might not have been balanced between patients with PABC and non-PABC. In addition, when calculating the risk of PABC we did not have a comparator group without breast cancer, forcing us to use age-specific fertility rates for relative risk estimates. Using fertility rates form the 2010 census (published in five-year intervals), and weighing the exposure time women spent in each age group with a known fertility rate, we believe to have reached a sufficiently accurate approximation of the relative risk estimate.
CONCLUSIONS
Taken together we show that - similar to Western populations - recent full-term pregnancy significantly elevates breast cancer risk in young women < 30 in Shanghai, and that women diagnosed with PABC at any age have a particularly bad prognosis. These data need to be confirmed prospectively and in a wider berth of Chinese women but in the interim health care providers and women in Asian populations should be made aware of these results.
METHODS
PATIENTS
We used two datasets pertaining to women treated for early breast cancer at the Fudan Medical Center in Shanghai between 1990 and 2012. The Fudan Medical Center is a major breast cancer center in Shanghai drawing patients from all areas of the city and socioeconomic levels. Our datasets represent all women diagnosed with early breast cancer at the Fudan Medical Center during the time periods stated below. The two available datasets contained slightly different definitions of exposure and follow-up times (see below). The first, more recent dataset (dataset 1) included 10,161 women diagnosed with breast cancer between June 2007 and July 2012. Dataset 1 was used to analyze whether a recent full-term pregnancy is associated with an elevated risk for breast cancer. The second dataset (dataset 2) included 7,411 women diagnosed with breast cancer between January 1990 and July 2007. Dataset 2 provided longer follow-up and information on disease-free survival. It was used to analyze whether women diagnosed with breast cancer within 5 years of having completed a pregnancy have a worse prognosis compared to women not having completed a pregnancy in the 5 years prior to breast cancer diagnosis. As only age of first full-term pregnancy was available in dataset 2, all analyses are using first full-term pregnancy (and not the most recent full-term pregnancy) as the exposure. Due to the fact that the total fertility rate of Shanghai has been ≤ 1 since 1990 [19], assuming that the first full-term pregnancy was also the most recent one in almost all cases was deemed appropriate for data interpretation and the following definitions:
“Recent pregnancy” (and “recently pregnant”) is thus defined as having completed a full-term pregnancy within 5 years prior to a breast cancer diagnosis. Pregnancy-associated breast cancer (PABC) is defined as breast cancer diagnosed within five years after a full-term pregnancy. ‘Non-PABC’ includes all other diagnoses of breast cancer.
STATISTICAL METHODOLOGY
Only breast cancer cases were available in our datasets.
We used dataset 1 for the analysis of breast cancer risk after pregnancy, i.e. we aimed to compare the risk of breast cancer in women with a recent full-term pregnancy to that of women without a recent full-term pregnancy. We could not get this information directly from our dataset of women with a diagnosis of breast cancer, but obtained the distribution of pregnancy by age in our dataset compared to the distribution of pregnancy in the population of Shanghai. Then, using Bayes rule, we were able to calculate the relative risk of breast cancer in women by recent pregnancy status. Women aged 50 and older, and women who had at least one full-term pregnancy but were missing age of pregnancy were removed from the analysis, leaving a total of 4,532 subjects. In order to allow for age-adjusted case-control analysis, we acquired age-specific fertility rates in Shanghai from the 2010 census. The Shanghai fertility rates were available in predefined 5-year age groups (e.g. 15–19, 20–24 etc). We weighted the fertility rates of different groups by the amount of exposure time a patient spent in each time period. The final analysis computed these weighted relative risk estimates for all age groups.
These fertility rates were compared with the rates of pregnancy among the breast cancer subjects in our dataset, in the 5-year period before their date of surgery. The estimated relative risk of breast cancer for subjects who had a recent pregnancy versus not, computed using Bayes rule, provided an odds ratio: the odds of recent full-term pregnancy for those who had cancer versus the same odds in the general population (given age). Confidence intervals and P-values were computed based on the normal approximation of the log odds ratio.
We performed this analysis on the full dataset 1. In addition, as some previous publications have suggested that the first full-term pregnancy is a particularly important predictor for breast cancer risk [38, 39] we also specifically analyzed the subset of women in dataset 1 who had at most one full-term pregnancy.
We then analyzed whether the rate of ER-negative breast cancer - as a predictor of poor prognosis - was higher in women with PABC compared to those with non-PABC.
We used dataset 2 for the analysis of disease-free survival of PABC. To analyze the effect of PABC on disease-free survival, we restricted our data to women aged 45 or less, since pregnancies were not observed in any higher age. We also removed subjects who had stage IV disease at diagnosis as we could not exclude the possibility that many of these patients had not been included in this surgical database in the first place. Patients with missing follow-up data were also excluded. There were a total of 1,799 subjects remaining.
We estimated the relative risk of progression (loco-regional or distant recurrence) or death (i.e. DFS) for women with a recent first pregnancy vs. non-recent first pregnancy or no pregnancy, using Cox proportional hazards models. We also adjusted for estrogen receptor, progesterone receptor, and HER2 status, but none of these were statistically significant or meaningfully altered the estimate of our exposure of interest. Age greater than 30 versus 30 or below was not significant in models that included recent pregnancy and stage (p > .75 in each model), so age was removed from the analysis. Our final model therefore adjusted for disease stage (0–1, 2, or 3). Proportional hazards assumptions were assessed with plots of scaled Schoenfeld residuals against time.[40]
To handle missing data on disease stage and recent full-term pregnancy, we used the missing-indicator method, and multiple imputation.[41] For the missing indicator method, we excluded all subjects who were missing data on age of first pregnancy, and created an indicator variable for missing disease stage so that cases with missing values are not deleted from the analysis. Multiple imputations were performed using the mice package in R (version 2.15.2).[42] There were 1,383 subjects (193 events) included in the missing indicator analysis, and 1,799 subjects (245 events) included in the multiple imputation analysis.
All analyses were performed in R version 2.15.2.[43]
ACKNOWLEDGEMENTS
This work was supported be the Avon Foundation, NY.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL STANDARDS
This study complies with the law in Shanghai.
References
- 1.Bruzzi P, Negri E, La Vecchia C, Decarli A, Palli D, Parazzini F, Del Turco MR: Short term increase in risk of breast cancer after full term pregnancy. BMJ 1988, 297:1096–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layde PM, Webster LA, Baughman AL, Wingo PA, Rubin GL, Ory HW: The independent associations of parity, age at first full term pregnancy, and duration of breastfeeding with the risk of breast cancer. Cancer and Steroid Hormone Study Group. J Clin Epidemiol 1989, 42:963–973. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsen G, Heuch I, Hansen S, Kvale G: Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer 2005, 92:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi S, Sugiura H, Ando Y, Shiraki N, Yanagi T, Yamashita H, Toyama T: Reproductive history and breast cancer risk. Breast Cancer 2012, 19:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, Urquhart A, Schedin P, Borges VF: Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat 2013, 138:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA: Reproductive history and mortality after breast cancer diagnosis. Obstetrics & Gynecology 2004, 104:146–154. [DOI] [PubMed] [Google Scholar]
- 7.Phillips K-A, Milne RL, Friedlander ML, Jenkins MA, McCredie MR, Giles GG, Hopper JL: Prognosis of premenopausal breast cancer and childbirth prior to diagnosis. Journal of clinical oncology 2004, 22:699–705. [DOI] [PubMed] [Google Scholar]
- 8.Olson SH, Zauber AG, Tang J, Harlap S: Relation of time since last birth and parity to survival of young women with breast cancer. Epidemiology 1998, 9:669–671. [PubMed] [Google Scholar]
- 9.Ewertz M, Duffy SW, Adami HO, Kvale G, Lund E, Meirik O, Mellemgaard A, Soini I, Tulinius H: Age at first birth, parity and risk of breast cancer: a meta-analysis of 8 studies from the Nordic countries. Int J Cancer 1990, 46:597–603. [DOI] [PubMed] [Google Scholar]
- 10.Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO: Transient increase in the risk of breast cancer after giving birth. N Engl J Med 1994, 331:5–9. [DOI] [PubMed] [Google Scholar]
- 11.Daling JR, Malone KE, Doody DR, Anderson BO, Porter PL: The relation of reproductive factors to mortality from breast cancer. Cancer Epidemiol Biomarkers Prev 2002, 11:235–241. [PubMed] [Google Scholar]
- 12.Li CI, Malone KE, Daling JR, Potter JD, Bernstein L, Marchbanks PA, Strom BL, Simon MS, Press MF, Ursin G, et al. : Timing of menarche and first full-term birth in relation to breast cancer risk. Am J Epidemiol 2008, 167:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J, Gammon MD, Douglas Thompson W, Bernstein JL: Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat 2011, 130:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalache A, Vessey MP, McPherson K: Lactation and breast cancer. Br Med J 1980, 280:223–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng T, Holford TR, Mayne ST, Owens PH, Zhang Y, Zhang B, Boyle P, Zahm SH: Lactation and breast cancer risk: a case-control study in Connecticut. Br J Cancer 2001, 84:1472–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipworth L, Bailey LR, Trichopoulos D: History of breast-feeding in relation to breast cancer risk: a review of the epidemiologic literature. J Natl Cancer Inst 2000, 92:302–312. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Liu LY, Wang F, Mu K, Yu ZG: The changes in female physical and childbearing characteristics in China and potential association with risk of breast cancer. BMC Public Health 2012, 12:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai JL, Zandecki M, Mary JY, Bernardi F, Izydorczyk V, Flactif M, Morel P, Jouet JP, Bauters F, Facon T: Improved cytogenetics in multiple myeloma: a study of 151 patients including 117 patients at diagnosis. Blood 1995, 85:2490–2497. [PubMed] [Google Scholar]
- 19.Fan L, Zheng Y, Yu KD, Liu GY, Wu J, Lu JS, Shen KW, Shen ZZ, Shao ZM: Breast cancer in a transitional society over 18 years: trends and present status in Shanghai, China. Breast Cancer Res Treat 2009, 117:409–416. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Zhang BN, Fan JH, Pang Y, Zhang P, Wang SL, Zheng S, Zhang B, Yang HJ, Xie XM, et al. : A nation-wide multicenter 10-year (1999–2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer 2011, 11:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Wuu J, Lambe M, Hsieh S-F, Ekbom A, Hsieh C-C: Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden). Cancer Causes & Control 2002, 13:299–305. [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Kim MT, Kim SW, Song MS, Yoon SJ: Effect of lifetime lactation on breast cancer risk: a Korean women’s cohort study. International journal of cancer 2003, 105:390–393. [DOI] [PubMed] [Google Scholar]
- 23.Potter JD, Cerhan JR, Sellers TA, McGovern PG, Drinkard C, Kushi LR, Folsom AR: Progesterone and estrogen receptors and mammary neoplasia in the Iowa Women’s Health Study: how many kinds of breast cancer are there? Cancer Epidemiol Biomarkers Prev 1995, 4:319–326. [PubMed] [Google Scholar]
- 24.Ma H, Bernstein L, Pike MC, Ursin G: Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res 2006, 8:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchbanks PA, McDonald JA, Wilson HG, Burnett NM, Daling JR, Bernstein L, Malone KE, Strom BL, Norman SA, Weiss LK, et al. : The NICHD Women’s Contraceptive and Reproductive Experiences Study: methods and operational results. Ann Epidemiol 2002, 12:213–221. [DOI] [PubMed] [Google Scholar]
- 26.Colditz G, Rosner B, Chen W, Holmes M, Hankinson S: Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst 2004, 96:218 – 228. [DOI] [PubMed] [Google Scholar]
- 27.Britton JA, Gammon MD, Schoenberg JB, Stanford JL, Coates RJ, Swanson CA, Potischman N, Malone KE, Brogan DJ, Daling JR, Brinton LA: Risk of breast cancer classified by joint estrogen receptor and progesterone receptor status among women 20–44 years of age. Am J Epidemiol 2002, 156:507–516. [DOI] [PubMed] [Google Scholar]
- 28.Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, Moorman PG: Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol 2000, 151:703–714. [DOI] [PubMed] [Google Scholar]
- 29.Yoo KY, Tajima K, Miura S, Takeuchi T, Hirose K, Risch H, Dubrow R: Breast cancer risk factors according to combined estrogen and progesterone receptor status: a case-control analysis. Am J Epidemiol 1997, 146:307–314. [DOI] [PubMed] [Google Scholar]
- 30.McCredie MR, Dite GS, Southey MC, Venter DJ, Giles GG, Hopper JL: Risk factors for breast cancer in young women by oestrogen receptor and progesterone receptor status. Br J Cancer 2003, 89:1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cotterchio M, Kreiger N, Theis B, Sloan M, Bahl S: Hormonal factors and the risk of breast cancer according to estrogen- and progesterone-receptor subgroup. Cancer Epidemiol Biomarkers Prev 2003, 12:1053–1060. [PubMed] [Google Scholar]
- 32.Rusiecki JA, Holford TR, Zahm SH, Zheng T: Breast cancer risk factors according to joint estrogen receptor and progesterone receptor status. Cancer Detect Prev 2005, 29:419–426. [DOI] [PubMed] [Google Scholar]
- 33.Ursin G, Bernstein L, Lord SJ, Karim R, Deapen D, Press MF, Daling JR, Norman SA, Liff JM, Marchbanks PA: Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. British Journal of Cancer 2005, 93:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bemis LT, Schedin P: Reproductive state of rat mammary gland stroma modulates human breast cancer cell migration and invasion. Cancer Res 2000, 60:3414–3418. [PubMed] [Google Scholar]
- 35.Polyak K: Pregnancy and breast cancer: the other side of the coin. Cancer cell 2006, 9:151–153. [DOI] [PubMed] [Google Scholar]
- 36.Turati F, La Vecchia C: Risk factors for breast cancer in China: similarities and differences with western populations. Arch Med Sci 2012, 8:179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ries LAG, Eisner MP, Kosary CL: SEER Cancer Statistics Review, 1973–1998. National Cancer Institute; 2001. [Google Scholar]
- 38.Trichopoulos D, Hsieh CC, MacMahon B, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Valaoras VG, Yuasa S: Age at any birth and breast cancer risk. Int J Cancer 1983, 31:701–704. [DOI] [PubMed] [Google Scholar]
- 39.MacMahon B, Cole P, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Valaoras VG, Yuasa S: Age at first birth and breast cancer risk. Bull World Health Organ 1970, 43:209–221. [PMC free article] [PubMed] [Google Scholar]
- 40.SCHOENFELD D: Partial residuals for the proportional hazards regression model. Biometrika 1982, 69:239–241. [Google Scholar]
- 41.Rubin DB: Introduction. In Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons, Inc.; 2008: 1–26 [Google Scholar]
- 42.van Buuren S, Groothuis-Oudshoorn K: mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 2011, 45:1–67. [Google Scholar]
- 43.R: A language and environment for statistical computing [http://www.R-project.org/.]