This study suggests that the combination therapy of intravitreal ranibizumab and 577-nm subthreshold micropulse photocoagulation can treat macular edema secondary to macular edema secondary to branch retinal vein occlusion effectively, by decreasing the frequency of intravitreal ranibizumab injections than intravitreal ranibizumab monotherapy while maintaining good visual acuity at 6 months.
Key words: macular edema, branch retinal vein occlusion, intravitreal ranibizumab, 577-nm subthreshold micropulse photocoagulation
Abstract
Purpose:
To determine the efficacy of the combination therapy of intravitreal ranibizumab (IVR) and 577-nm yellow laser subthreshold micropulse laser photocoagulation (SMLP) for macular edema secondary to branch retinal vein occlusion cystoid macular edema.
Methods:
Retrospective, consecutive, case–control study. Forty-six eyes of 46 patients with treatment-naive branch retinal vein occlusion cystoid macular edema were enrolled. The IVR + SMLP group consisted of 22 patients who had undergone both SMLP and IVR. Intravitreal ranibizumab group consisted of 24 patients who had undergone IVR monotherapy. Intravitreal ranibizumab therapy was one initial injection and on a pro re nata in both groups, and SMLP was performed at 1 month after IVR in the IVR + SMLP group. Preoperatively and monthly, best-corrected visual acuity and central retinal thickness were evaluated using swept source optical coherence tomography.
Results:
Best-corrected visual acuity and central retinal thickness significantly improved at 6 months in IVR + SMLP and IVR groups. Best-corrected visual acuity and central retinal thickness were not significantly different between the two groups at any time points. The number of IVR injections during initial 6 months in IVR group (2.3 ± 0.9) was significantly greater (P = 0.034) than that in IVR + SMLP group (1.9 ± 0.8).
Conclusion:
The combination therapy of IVR and SMLP can treat branch retinal vein occlusion cystoid macular edema effectively, by decreasing the frequency of IVR injections while maintaining good visual acuity.
Branch retinal vein occlusion (BRVO) is the second most common cause of retinal vascular abnormality after diabetic retinopathy and a frequent cause of visual loss.1 Branch retinal vein occlusion presents as venous dilatation or tortuosity, intraretinal hemorrhage, cystoid macular edema (CME), capillary nonperfusion, and serous retinal detachment (SRD),2,3 and the most common vision-threatening pathogenesis are macular edema with/without SRD in acute phase. Visual acuity is affected by all of these conditions, but visual loss is frequently ascribed to CME.4 Therefore, various treatments for BRVO-CME are being addressed.
Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, is believed to play an important role in the pathogenesis of BRVO-CME.2,5–7 Recently, anti-VEGF is recognized as the main treatment for BRVO-CME, and it has been reported that intravitreal ranibizmab (Lucentis; Genentech, San Francisco, CA) (IVR) treatment is highly effective at suppressing macular edema.8–10 However, according to several studies,11,12 the rate at which edema can be controlled with a single injection of anti-VEGF therapy was less than 30% and other cases require multiple additional injections because of persistent or recurrent edema. Increasing the frequency of vitreous injections may increase the risk of endophthalmitis and retinal detachment, and the problem of medical expense will increase in the future. Therefore, attention has recently been focused on the development of new therapeutic methods that suppress the recurrence of edema rather than on anti-VEGF monotherapy.
However, traditional retinal laser photocoagulation in the macula lesion has long been recognized as the optimal treatment of macular edema by the Branch Retinal Vein Occlusion Study.13 However, the visual improvement after laser treatment occurred slowly and was limited, and conventional laser photocoagulation may lead to several complications over the long term, such as enlargement of laser scar,14 subretinal fibrosis,15 choroidal neovascularization,16 and field sensitivity deterioration,17 which can severely affect visual function. To reduce these laser complications, advances in laser technology have led to the development of selective photocoagulation for the retinal pigment epithelium (RPE) through the subthreshold micropulse laser photocoagulation (SMLP) method. This is designed to target the RPE, while having a minimal effect on the sensory retina and choroid. The principle is to shorten the coagulation time and save the total amount of energy to reduce invasiveness. Friberg and Karatza18 first reported the clinical application of 810-nm diode SMLP for diabetic macular edema in 1997. After that, several clinical studies have since demonstrated the efficacy of this method for diabetic macular edema19–22 and BRVO-CME.23–25
One of the more recent developments is the incorporation of micropulse laser technology in a 577-nm yellow laser system, which offers both continuous wave and micropulse modes and facilitates the confirmation of the threshold coagulation spot because of a yellow wavelength. Although this laser system was recently released domestically in October 2011, few clinical studies of 577-nm SMLP have been reported for diabetic macular edema to date.26,27 This laser is effective for mild macular edema and has a sustained effect without visible damage. To the best of our knowledge, there have been no previous reports on anti-VEGF drugs and 577-nm SMLP combination therapy for BRVO-CME. The purpose of this study is to verify the therapeutic effects and safety of edema recurrence suppression effect using 577-nm SMLP in combination with IVR.
Methods
Forty-six eyes of 46 patients with treatment-naïve BRVO-CME were enrolled. Patients affected by BRVO observed in the outpatient department of Niigata University Hospital between 2013 August and 2016 June were identified and invited to enroll prospectively. We tried two treatment methods for BRVO-CME at two different treatment periods. In the earlier period (from 2013 August to 2015 January), combination therapy of IVR and 577-nm SMLP was performed on 22 patients (IVR + SMLP group), and in the later period (from 2015 January to 2016 June), IVR monotherapy was performed on the remaining 24 patients (IVR group). This retrospective, consecutive, single-center, nonrandomized, and case–control study was approved by the Research Ethics Committee of Niigata University Hospital before study initiation, and the study followed the tenets of the Declaration of Helsinki. Before treatment, all patients had been followed up for at least 2 months after disease onset, and their CME and/or SRD had persisted. Inclusion criteria were baseline best-corrected visual acuity (BCVA) ranged from 20/400 to 20/25 on Snellen equivalency and central retinal thickness (CRT), defined as the mean thickness of the central 1-mm diameter disk of the retinal map, exceeded 250 μm as determined by swept source optical coherence tomography (SS-OCT). Exclusion criteria were coexistence of any other retinal disorders, uveitis, presence of glaucoma, and severe cataracts. We also excluded patients with previous history of vitreoretinal surgeries, intravitreal therapies, or macular grid laser treatment within 6 months before the study and follow-up period <6 months. Signed informed consent was obtained from all study subjects.
The examinations included measurements of their BCVA and retinal microstructures. All ophthalmologic examinations were performed before and at 1, 2, 3, 4, 5, and 6 months after treatment. Best-corrected visual acuity was measured using the Landolt Chart and was expressed as the logarithm of the minimum angle of resolution for statistical analysis. And to express the changes of BCVA, logarithm of the minimum angle of resolution BCVA was converted to the Early Treatment Diabetic Retinopathy Study letter score. Based on fundus examinations, the patients were classified as “major BRVO” and “macular BRVO.” Retinal microstructures were obtained using SS-OCT (DRI OCT-1; Topcon, Tokyo, Japan) by way of 12-mm radial scan protocols, and CRT was recorded for analysis from the retinal thickness maps. Fluorescein angiographies were performed using the Heidelberg Retina Angiograph system (Heidelberg Engineering, Heidelberg, Germany) at before and 6 months after treatment to evaluate nonperfusion areas and possible atrophic changes to the RPE after SMLP.
All patients received an initial IVR, and additional IVRs were delivered pro re nata (PRN) basis according to the following retreatment criteria: new or persistent cystoid retinal changes and SRD, an increase of CRT >20% after an initial decrease, and a worsening of the BCVA by >0.2 logarithm of the minimum angle of resolution units after an initial improvement. The combination therapy group (IVR + SMLP) was treated with SMLP one month after IVR. Additional SMLP was performed at intervals of at least 3 months, so if there was a recurrence at that time (within 3 months after SMLP), no additional SMLP was performed in combination and this group was treated with only IVR. Subthreshold micropulse laser photocoagulation was provided by a 577-nm yellow laser system (Iridex IQ577; Laser System Iridex Corp, Mountain View, CA). Laser application was performed with an Area-Centralis lens (Volk Optical, Mentor, OH, USA), and the micropulse laser power used in SMLP was derived for each eye from a test burn. The test burn was performed in the continuous-wave mode using a 100-μm spot diameter and 0.2-second exposure outside the vascular arcade with the power titrated from 80 mW upward until a burn became barely visible. The subthreshold treatment was performed by switching the laser from the continuous-wave to the micropulse mode, and the laser parameters were 100-μm laser spot diameter, 0.2-second exposure, and 15% duty cycle. Multiple laser bursts were delivered to the entire area affected by the macular edema, avoiding the foveal center, to provide as tight a coverage as possible without the individual burns actually touching.
The mean scores were compared and SD values were calculated for each parameter. Statistical analysis was performed using the Student's t-test (paired and unpaired depending on the groups) to evaluate the changes of BCVA, CRT, and number of IVR. The chi-square test was applied for the comparison of proportion. The level of statistical significance was set at P < 0.05.
Results
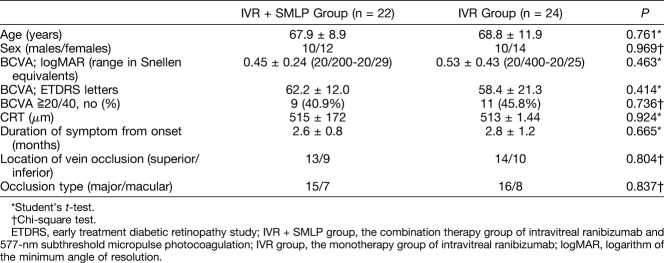
Table 1 shows the baseline clinical characteristics, visual acuity, and OCT parameters in patients with BRVO-CME. No differences were observed between two groups in age, sex, visual acuity, CRT, duration of symptom from onset, location of vein occlusion, and occlusion type. No serious ocular or nonocular complications associated with IVR and SMLP were observed in any of the eyes over the entire observational period. In addition, no laser spots were detected in any treated areas on SS-OCT findings and fluorescein angiographies.
Table 1.
Baseline Clinical Characteristics, Visual Acuity, and OCT Parameters in Patients With Macular Edema Secondary to BRVO
Time Course of Changes in Best-Corrected Visual Acuity and Central Retinal Thickness of IVR + SMLP Group and Intravitreal Ranibizumab Group
Figure 1 reveals time course of changes in visual acuity in both groups. Best-corrected visual acuity significantly improved by the treatment in both group. Best-corrected visual acuity significantly improved at all time points compared with baseline in IVR + SMLP group and IVR group (P < 0.001). Central retinal thickness also showed significant improvement by the treatment in both group. Central retinal thickness significantly improved at all time points compared with baseline in IVR + SMLP group and IVR group (P < 0.001, Figure 2).
Fig. 1.
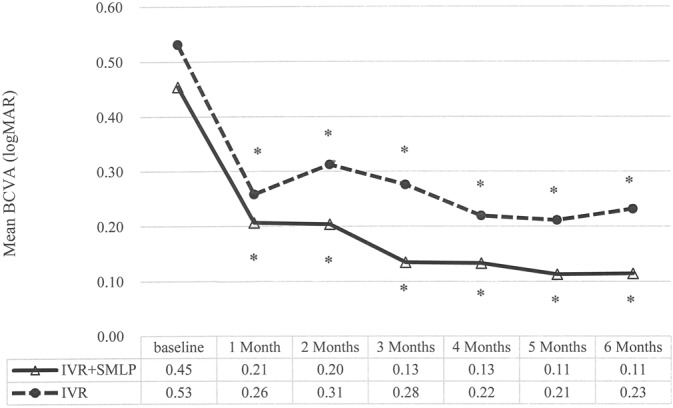
Time course of changes in BCVA of IVR + SMLP group (the combination therapy group of IVR and 577-nm SMLP) and IVR group (the monotherapy group of IVR). Best-corrected visual acuity significantly improved at all time points compared with baseline in IVR + SMLP group and IVR group (*P < 0.001).
Fig. 2.
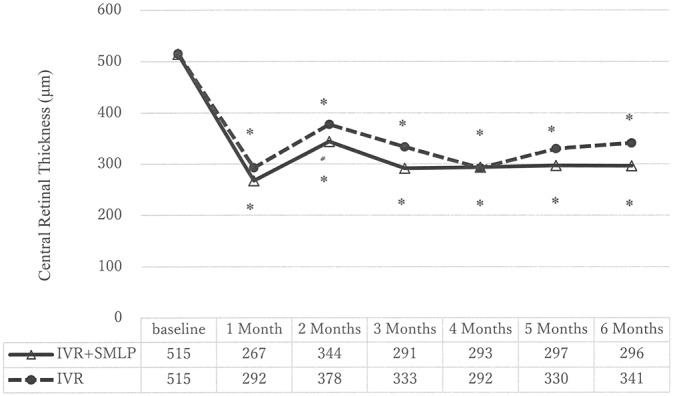
Time course of changes in CRT of IVR + SMLP group (the combination therapy group of IVR and 577-nm SMLP) and IVR group (the monotherapy group of IVR). Central retinal thickness significantly reduced at all time points compared with baseline in IVR + SMLP group and IVR group (*P < 0.001).
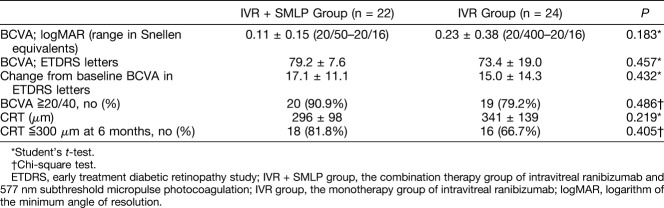
Visual and Anatomical Outcomes at 6 Months After Treatment in IVR + SMLP Group and Intravitreal Ranibizumab Group
Table 2 shows visual acuity and OCT parameters at 6 months after treatment in IVR + SMLP group and IVR group. Best-corrected visual acuity (logarithm of the minimum angle of resolution) at 6 months in the IVR + SMLP group and IVR groups was 0.11 ± 0.15 (range in Snellen equivalents: 20/50–20/16) and 0.23 ± 0.38 (range in Snellen equivalents: 20/400–20/16), respectively, and was not significantly different between the 2 groups. Best-corrected visual acuity changes in Early Treatment Diabetic Retinopathy Study letter score from baseline to 6 months were not significantly different (17.1 ± 11.1 letters in the IVR + SMLP group and 15.0 ± 14.3 letters in the IVR group). Twenty of 22 eyes (90.9%) maintained BCVA >20/40 at 6 months in IVR + SMLP group and 19 of 24 eyes (79.2%) maintained in IVR monotherapy group. The rate of patients with BCVA >20/40 increased in both groups after 6 months. Central retinal thickness at 6 months in the IVR + SMLP group and IVR groups was 296 ± 98 and 341 ± 139, respectively, and was not significantly different between the 2 groups. Eighteen of 22 eyes (81.8%) maintained CRT ≦300 μm at 6 months in IVR + SMLP group and 16 of 24 eyes (66.7%) maintained in IVR group.
Table 2.
Visual and Anatomical Outcomes at 6 Months After Treatment in Patients With Macular Edema Secondary to BRVO
Required Treatments
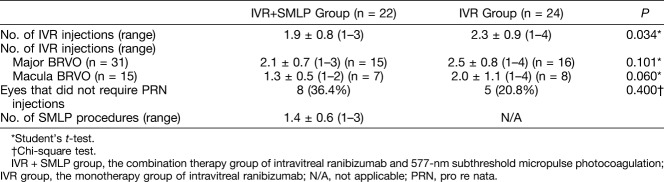
The number of IVR injections was 1.9 ± 0.8 in the IVR + SMLP group and 2.3 ± 0.9 in the IVR group; there was significant difference between the 2 groups (Table 3). In addition, according to the occlusion type result, the number of IVR injections in the major BRVO type (31 eyes) was 2.1 ± 0.7 in the IVR + SMLP group (15 eyes) and 2.5 ± 0.8 in the IVR group (16 eyes); there was no statistically significant difference between the 2 groups. And, in the macula BRVO type (15 eyes), there were no statistically significant differences in the results of seven eyes (1.3 ± 0.5) and eight eyes (2.0 ± 1.1). Eight of the 22 eyes (36.4%) did not require PRN-IVR injections during 6 months (= no recurrence) in IVR + SMLP group and 5 of the 24 eyes (20.8%) in IVR group. The mean number of SMLP procedures was 1.4 ± 0.6 in the IVR + SMLP group.
Table 3.
Analysis of Required Treatments
Discussion
The IVR & SMLP combination therapy and the IVR monotherapy had an immediate effect, and all edema rapidly disappeared at 1 month after treatment and significantly improved visual acuity for 6 months in BRVO-CME eyes in this study. Visual acuity of combination therapy group was better than that of the monotherapy group throughout the observation periods, although there was no significant difference between the two groups. In addition, CRT was significantly decreased after 1 month from IVR, and the rates of macular edema (that was controlled at 300 μm or less at 6 months) were 82% in the combination therapy group and 62% in the monotherapy group. However, there was no significant difference between two groups. According to Parodi et al,23 threshold grid laser treatment had a more rapid effect in promoting the resolution of macular edema because a reduction in both foveal thickness and total macular volume could be detected at 6 months from treatment, whereas a similar decrease was obtained after 12 months in the group treated with subthreshold grid laser treatment with an infrared micropulse diode laser, that is, the effect of SMLP appears more slowly than the conventional laser treatment. Therefore, as the full effects of SMLP are generally not apparent until closer to 12 months after treatment, this study cannot show those effects as clearly because of the shorter 6-month study period and smaller number of cases, although this study certainly showed that this combination therapy had a synergistic effect in promoting macular edema resolution.
Interestingly, the number of IVR injections required at 6 months was much lower in the combination therapy group (1.9 ± 0.8) than that in the IVR monotherapy group (2.3 ± 0.9). In addition, the number of cases that did not recur at 6 months and where macular edema was able to be controlled with only one IVR treatment was 36.4% in the combination therapy group as compared with 20.8% in the IVR monotherapy group. In addition, there were fewer cases of early recurrence (2–3 months) after the first IVR in the combination therapy group (54.5%) than in the monotherapy group (75.0%). Also, the classification of occlusion type (major or macular BRVO) seems to affect the response to treatment. Therefore, we compared the number of IVR injections by occlusion type, but although there was no statistically significant difference, combination therapy was less frequent than IVR monotherapy regardless of occlusion type. Early recurrence was considered to have been suppressed using SMLP in combination, which led to a decrease in the number of IVR injections. If we evaluate it further for a period longer than 6 months, we may be able to expect a further decrease in the number of relapses (reduction in the number of IVR injections) by combination therapy.
According to the BRIGHTER study,28 the number of IVR injections required for 6 months was 4.8 ± 1.0 times in the 0.5-mg IVR monotherapy group and 4.5 ± 1.2 times in the IVR + conventional grid laser treatment group. The administration methods were different between this study and the BRIGHTER study. The protocol of this study was one injection + PRN; if there was a loss of visual acuity and the macula status met the recurrence criteria, only one injection was administered to the patients in the ranibizumab group. However, in the BRIGHTER study, if there was a loss of visual acuity due to disease activity as judged by the investigator, monthly injections were again administered to the patients in the ranibizumab (with or without laser) groups until stability was achieved for three consecutive months; this required at least two consecutive injections. Change in visual acuity from baseline to 6 months was higher in both groups (IVR + SMLP group and IVR group) of this study (+17.1 letters and +15.0 letters, respectively) compared with the BRIGHTER study (+14.8 letters). However, there were differences in baseline visual acuity in the two studies; patients of the IVR + SMLP group in this study had a higher mean baseline BCVA score (62.2 letters in IVR + SMLP vs. 59.5 letters in the BRIGHTER study). Although it cannot be compared simply because there are differences in administration method and baseline visual acuity, a similarly good visual acuity in this combination therapy was obtained as with the BRIGHTER study and with a smaller number of IVR injections.
The administration method and subsequent readministration criteria are key to decreasing the number of IVR injections. It was confirmed at this time that vision can be sufficiently maintained even with administration of 1 + PRN, rather than 3 times in a row. Regarding readministration criteria, at present, there is no unified standard and there are many differences between facilities. The readministration criteria adopted this time was performed not only with an increase in macular edema but also in cases involving deterioration of visual acuity and SRD recurrence. Often, clinical practice demonstrates that morphological change and functional impairment at the time of recurrence of BRVO are not necessarily parallel. Regarding BRVO, unlike the recurrence criteria of age-related macular degeneration,29,30 the necessity of prophylactic administration is low and we believe the PRN method to be enough for macular edema secondary to BRVO.
Regarding safety and side effects of these treatments, no sight-threatening ocular adverse events related to IVR and SMLP were recorded. Subthreshold micropulse photocoagulation was performed 1.4 times for 6 months in the combination therapy group, in all cases, including cases where 577-nm SMLP was performed more than once, scarring of the laser as seen in normal retinal photocoagulation throughout 6 months was not detected in color fundus photograph, fluorescein angiography, and SS-OCT. In addition, no patients complained of ocular discomfort after treatment with SMLP. Laser scarring by SMLP was not confirmed either in the flattering image at RPE level in the en face image by SS-OCT. Intravitreal ranibizmab had an immediate effect, and at 1 month after treatment, all edema disappeared. By implementing 577-nm SMLP systematically at the stage where the edema disappeared, there was a possibility that the RPE could be coagulated more selectively and efficiently.
Limitations of this study included being nonrandomized and open label, a nonparallel group study with a short duration, and a small population. Despite this, the results of this study suggest that 577-nm SMLP is an effective option of IVR treatment of macular edema secondary to BRVO, and randomized clinical trials are warranted to determine the optimal treatment interval, duration, duty cycle, and laser power. And, the difference in the number of injections was statistically significant, although only compared during a short period of 6 months, so the effects of significantly reducing the number of IVR by combination therapy of SMLP are not yet perfectly understood. However, we hope that further study can identify better indications in the future.
In conclusion, the combination therapy of IVR and 577-nm SMLP can treat macular edema secondary to BRVO effectively and safely, by decreasing the frequency of IVR while maintaining good visual acuity.
Footnotes
None of the authors has any financial/conflicting interests to disclose.
The authors have full control of all primary data and agree to allow Investigative and Ophthalmology of Visual Sciences to review their data upon request.
References
- 1.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol 1996;114:1243–1247. [DOI] [PubMed] [Google Scholar]
- 2.Glacet-Bernard A, Coscas G, Chabanel A, et al. Prognostic factors for retinal vein occlusion: prospective study of 175 cases. Ophthalmology 1996;103:551–560. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein D. Ischemic macular edema. Recognition and favorable natural history in branch vein occlusion. Arch Ophthalmol 1992;110:1427–1434. [DOI] [PubMed] [Google Scholar]
- 4.Wallow IH, Danis RP, Bindley C, Neider M. Cystoid macular degeneration in experimental branch retinal vein occlusion. Ophthalmology 1988;95:1371–1379. [DOI] [PubMed] [Google Scholar]
- 5.Kreutzer TC, Alge CS, Wolf AH, et al. Intravitreal bevacizumab for the treatment of macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol 2008;92:351–355. [DOI] [PubMed] [Google Scholar]
- 6.Noma H, Funatsu H, Yamasaki M, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol 2005;140:256–261. [DOI] [PubMed] [Google Scholar]
- 7.Kriechbaum K, Michels S, Prager F, et al. Intravitreal avastin for macular oedema secondary to retinal vein occlusion: a prospective study. Br J Ophthalmol 2008;92:518–522. [DOI] [PubMed] [Google Scholar]
- 8.Campochiaro PA, Heier JS, Feiner L, et al. BRAVO investigators. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1102–1112. [DOI] [PubMed] [Google Scholar]
- 9.Kamei M, Terasaki H, Yoshimura N, et al. Short-term efficacy and safety of ranibizumab for macular oedema secondary to retinal vein occlusion in Japanese patients. Acta Ophthalmol 2017;95:e29–e35. [DOI] [PubMed] [Google Scholar]
- 10.Kinge B, Stordahl PB, Forsaa V, et al. Efficacy of ranibizumab in patients with macular edema secondary to central retinal vein occlusion: results from the sham-controlled ROCC study. Am J Ophthalmol 2010;150:310–314. [DOI] [PubMed] [Google Scholar]
- 11.Karagiannis DA, Karampelas MD, Soumplis VM, et al. Recurrence of macular edema in retinal vein occlusions after treatment with intravitreal ranibizumab (Lucentis). Can J Ophthalmol 2011;46:486–490. [DOI] [PubMed] [Google Scholar]
- 12.Hanada N, Iijima H, Sakurada Y, Imasawa M. Recurrence of macular edema associated with branch retinal vein occlusion after intravitreal bevacizumab. Jpn J Ophthalmol 2012;56:165–174. [DOI] [PubMed] [Google Scholar]
- 13.Argon laser photocoagulation for macular edema in branch vein occlusion. The branch vein occlusion study group. Am J Ophthalmol 1984;98:271–282. [DOI] [PubMed] [Google Scholar]
- 14.Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol 1991;109:1549–1551. [DOI] [PubMed] [Google Scholar]
- 15.Guyer DR, D'Amico DJ, Smith CW. Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am J Ophthalmol 1992;113:652–656. [DOI] [PubMed] [Google Scholar]
- 16.Lewis H, Schachat AP, Haimann MH, et al. Choroidal neovascularization after laser photocoagulation for diabetic macular edema. Ophthalmology 1990;97:503–510. [DOI] [PubMed] [Google Scholar]
- 17.Ishiko S, Ogasawara H, Yoshida A, Hanada K. The use of scanning laser ophthalmoscope microperimetry to detect visual impairment caused by macular photocoagulation. Ophthalmic Surg Lasers 1998;29:95–98. [PubMed] [Google Scholar]
- 18.Friberg TR, Karatza E. The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology 1997;104:2030–2038. [DOI] [PubMed] [Google Scholar]
- 19.Vujosevic S, Bottega E, Casciano M, et al. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010;30:908–916. [DOI] [PubMed] [Google Scholar]
- 20.Salvetti P, Rosen JM, Reichel E. Subthreshold infrared footprinting with indocyanine green for localizing low-intensity infrared photocoagulation. Ophthalmic Surg Lasers Imaging 2003;34: 44–48. [PubMed] [Google Scholar]
- 21.Lavinsky D, Cardillo JA, Melo LA, Jr, et al. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Opthalmology Vis Sci 2011;52:4314–4323. [DOI] [PubMed] [Google Scholar]
- 22.Ohkoshi K, Yamaguchi T. Subthreshold micropulse diode laser photocoagulation for diabetic macular edema in Japanese patients. Am J Ophthalmol 2010;149:133–139. [DOI] [PubMed] [Google Scholar]
- 23.Parodi MB, Spasse S, Iacono P, et al. Subthreshold grid laser treatment of macular edema secondary to branch retinal vein occlusion with micropulse infrared (810 nanometer) diode laser. Ophthalmology 2006;113:2237–2242. [DOI] [PubMed] [Google Scholar]
- 24.Luttrull JK, Sramek C, Palanker D, et al. Long-term safety, high-resolution imaging, and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina 2012;32:375–386. [DOI] [PubMed] [Google Scholar]
- 25.Inagaki K, Ohkoshi K, Ohde S, et al. Subthreshold micropulse photocoagulation for persistent macular edema secondary to branch retinal vein occlusion including best-corrected visual acuity greater than 20/40. J Ophthalmol 2014;2014:251257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon YH, Lee DK, Kwon OW. The short-term efficacy of subthreshold micropulse yellow (577-nm) laser photocoagulation for diabetic macular edema. Korean J Ophthalmol 2014;28:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inagaki K, Ohkoshi K, Ohde S, et al. Comparative efficacy of pure yellow (577-nm) and 810-nm subthreshold micropulse laser photocoagulation combined with yellow (561–577-nm) direct photocoagulation for diabetic macular edema. Jpn J Ophthalmol 2015;59:21–28. [DOI] [PubMed] [Google Scholar]
- 28.Tadayoni R, Waldstein SM, Boscia F; BRIGHTER Study Group, et al. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion 6-month result in BRIGHTER. Ophthalmology 2016;123:1332–1344. [DOI] [PubMed] [Google Scholar]
- 29.Holz FG, Amoaku W, Donate J; SUSTAIN Study Group, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 2011;118:663–671. [DOI] [PubMed] [Google Scholar]
- 30.Busbee BG, Ho AC, Brown DM; HARBOR study group, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013;120:1046–1056. [DOI] [PubMed] [Google Scholar]