Abstract
Objective
To provide an overview of care in emergency departments (EDs) across Europe in order to interpret observational data and implement interventions regarding the management of febrile children.
Design and setting
An electronic questionnaire was sent to the principal investigators of an ongoing study (PERFORM (Personalised Risk assessment in Febrile illness to Optimise Real-life Management), www.perform2020.eu) in 11 European hospitals in eight countries: Austria, Germany, Greece, Latvia, the Netherlands, Slovenia, Spain and the UK.
Outcome measures
The questionnaire covered indicators in three domains: local ED quality (supervision, guideline availability, paper vs electronic health records), organisation of healthcare (primary care, immunisation), and local factors influencing or reflecting resource use (availability of point-of-care tests, admission rates).
Results
Reported admission rates ranged from 4% to 51%. In six settings (Athens, Graz, Ljubljana, Riga, Rotterdam, Santiago de Compostela), the supervising ED physicians were general paediatricians, in two (Liverpool, London) these were paediatric emergency physicians, in two (Nijmegen, Newcastle) supervision could take place by either a general paediatrician or a general emergency physician, and in one (München) this could be either a general paediatrician or a paediatric emergency physician. The supervising physician was present on site in all settings during office hours and in five out of eleven settings during out-of-office hours. Guidelines for fever and sepsis were available in all settings; however, the type of guideline that was used differed. Primary care was available in all settings during office hours and in eight during out-of-office hours. There were differences in routine immunisations as well as in additional immunisations that were offered; immunisation rates varied between and within countries.
Conclusion
Differences in local, regional and national aspects of care exist in the management of febrile children across Europe. This variability has to be considered when trying to interpret differences in the use of diagnostic tools, antibiotics and admission rates. Any future implementation of interventions or diagnostic tests will need to be aware of this European diversity.
Keywords: infectious diseases, accident & emergency
Introduction
The emergency department (ED) is the setting where reliable care has to be provided for acutely ill patients.1 Children represent a large part of the ED workload, with nearly 40 visits per 100 population.2 General as well as paediatric visits have increased during the last years.2 3
Factors that contribute to this increase are lack of access to 24/7 primary care, lack of paediatric training among primary care physicians4 and parents’ preferences to go directly to the ED.5
Fever is one of the most frequent reason for consultation.2 Although most febrile episodes are self-limiting, infection still remains a common cause of death in children.6 Delayed recognition of potential life-threatening infections may have disastrous implications,7 while overtreatment can be invasive and costly and can lead to increasing antibiotic resistance.8 Caring for this broad spectrum of patients remains an ongoing challenge, and several studies have shown large practice pattern variation in the care for febrile children.9
The organisation of healthcare varies between countries as well as hospitals. For example, healthcare for children can be delivered by general practitioners, primary care paediatricians, and general or paediatric EDs.4
It has been suggested that variation in healthcare organisation accounts for part of the differences in paediatric mortality in Europe.4 For example, death rates from illnesses that rely on first access services such as primary care, for example, pneumonia, are higher in the UK than in Germany and the Netherlands.4
Our aim was to provide an overview of the delivery of care for febrile children at European EDs, which can aid the interpretation of observational studies and the implementation of future interventions.
Methods
Participating hospitals and questionnaire development
This study is embedded in the MOFICHE study (Management and Outcome of Fever in children in Europe), which is part of the European Union-funded PERFORM project (Personalised Risk assessment in Febrile illness to Optimise Real-life Management) (GA:668303, www.perform2020.eu) (online supplemenatary appendix 1). MOFICHE collects information regarding patient characteristics, resource use (diagnostic tests, antibiotic prescription and hospitalisation) and outcome in febrile children.
bmjpo-2019-000456supp001.pdf (55KB, pdf)
An electronic questionnaire (online supplementary appendix 2) was sent by email to the principal investigator of each participating centre, which were 11 European hospitals in eight countries: Austria, Germany, Greece, Latvia, the Netherlands, Slovenia, Spain and the UK. The questionnaire was filled in by the principal investigator in collaboration with the head of the (paediatric) ED or one of the main consultants responsible for the care of febrile children at the ED.
bmjpo-2019-000456supp002.pdf (160.5KB, pdf)
The questionnaire was based on the article by Mintegi et al10 on organisation of paediatric emergency care and was further developed by the MOFICHE research team, consisting of a team of experts with a background in paediatrics, epidemiology, paediatric emergency care, paediatric infectious diseases and health economics. We gathered information on factors influencing case mix as well as resource use.
We focused on local ED quality indicators, regional systems of care and local factors influencing resource use based on Medford-Davis et al1 classification of value-based emergency care.1
The questionnaire was created with Google Forms. Questions consisted of multiple-choice or multiple-option questions, yes/no questions, 5-point Likert-scale questions and open questions.
All analyses were descriptive and performed with SPSS V.21 software. We analysed the correlation between the different setting characteristics using Pearson correlation coefficient.
Patient involvement
Patients were not directly involved in the design of this study.
Quality of care
In the quality of care domain, we included availability and type of triage system, guideline availability, paediatric intensive care unit (PICU) admission criteria, guidelines on maximum time spent in the ED, supervision, and availability of electronic health records (EHR).
Four types of supervision were distinguished11:
Direct supervision, the supervising physician is present on site with the junior doctor and patient.
Indirect supervision (I), the supervising physician is within the hospital and is immediately available for direct supervision.
Indirect supervision (II), the supervising physician is not present within the hospital, but is immediately available by telephone and available for direct supervision within 20–30 min.
Oversight, the supervising physician is available for feedback after care is delivered.
Office hours were defined as daytime from Monday to Friday. Out-of-office hours were defined as evenings, nights, weekends and public holidays according to the local organisation.
Regarding EHR we asked for the electronic availability of history, physical examination, vital signs, diagnostic tests, treatment and disposition.
Regional aspects of care
In the regional aspects domain, we studied the organisation of primary care. We focused on the type of clinician providing primary care, out-of-office hours availability and ED self-referral rates. Information concerning routine immunisation was taken from the website of the European Centre for Disease Prevention and Control.12
We used 2016 WHO data to outline immunisation rates.13
Resource use
In the resource use domain, we studied admission rates and availability of point-of-care tests (POCT). Admission rates were based on annual admission rates of 2016.
Results
General ED characteristics
All 11 hospitals participating in the MOFICHE study filled in the electronic questionnaire. Nine were university hospitals and three were large district general hospitals; seven had a dedicated paediatric ED (table 1). All hospitals had an onsite PICU. Nine settings served mixed inner-city/rural populations, and in 10 settings the population was from a mixed socioeconomic status.
Table 1.
Hospital characteristics
| Hospital | Paediatric upper age limit (years) |
Population, rural/inner city | Type of hospital, paediatric or mixed hospital and ED | Supervising specialist | Paediatric ED visits (n) |
Primary care during out-of-office hours | Self- referral (%) | Triage system | Admission rate (%) |
| AT, MUG* | 17 | Mixed | Tertiary, university hospital mixed, ED paediatric |
Paediatrician | 10 000–20 000 | No | 50–75 | MTS | 12 |
| DE, LMU† | 18 | Mixed | Tertiary university hospital and ED paediatric |
Paediatrician, paediatric emergency physician | 10 000–20 000 | Yes | 50–75 | MTS | 10 |
| GR, NKUA‡ | 16 | Inner city | Tertiary, university hospital and ED paediatric |
Paediatrician | 30 000–40 000 | No | >75 | Local/National | 15 |
| LV, RSU§ | 18 | Mixed | Tertiary, university hospital and ED paediatric |
Paediatrician | >40 000 | No | 20–50 | MTS | 12 |
| NL, RUMC¶ | 18 | Mixed | Tertiary university hospital paediatric, ED mixed |
Paediatrician, emergency physician | <10 000 | Yes | <20 | Local/National | 30 |
| NL, EMC** | 18 | Mixed | Tertiary university hospital paediatric, ED mixed |
Paediatrician | <10 000 | Yes | 20–50 | MTS | 20 |
| SL, UKCL†† | 18 | Mixed | Tertiary university hospital mixed, ED paediatric |
Paediatrician | <10 000 | Yes | <5 | Local/National | 51 |
| SP, SERGAS‡‡ | 15 | Mixed | University hospital mixed, ED paediatric |
Paediatrician | 30 000–40 000 | Yes | >75 | MTS | 4 |
| UK, LIV§§ | 16 | Mixed | Tertiary, university hospital and ED paediatric |
Paediatric emergency physician | >40 000 | Yes | 50–75 | MTS | 20 |
| UK, SMH¶¶ | 16 | Inner city | University hospital mixed, ED paediatric |
Paediatric emergency physician | 20 000–30 000 | Yes | >75 | MTS | 15 |
| UK, UNEW*** | 16 | Mixed | Tertiary, university hospital and ED paediatric |
Paediatrician, emergency physician | 20 000–30 000 | Yes | 50–75 | MTS | 15 |
*Medical University of Graz, Department of General Paediatrics, Graz, Austria.
†Dr von Hauner Children’s Hospital, university hospital, Ludwig-Maximilians-University (LMU), Munich, Germany.
‡National and Kapodistrian University of Athens, Second Department of Paediatrics, P & A Kyriakou Children’s Hospital, Athens, Greece.
§Rīgas Stradiņa universitāte, Children’s Clinical University Hospital, Department of Paediatrics, Riga, Latvia.
¶Amalia Children’s Hospital, Radboudumc, Nijmegen, the Netherlands.
**Erasmus MC-Sophia Children’s Hospital, Department of General Paediatrics, Rotterdam, the Netherlands.
††University Medical Centre Ljubljana, Department of Infectious Diseases, Ljubljana, Slovenia.
‡‡Hospital Clínico Universitario de Santiago de Compostela, Santiago de Compostela, Spain.
§§Alder Hey Children’s NHS Foundation Trust, Liverpool, UK.
¶¶Imperial College of Science, Technology and Medicine, Section of Paediatrics, London, UK.
***Great North Children’s Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK.
ED, emergency department; MTS, Manchester Triage System.
Quality of care
The Manchester Triage System (MTS) was used in eight settings. The other settings used a local or national triage system.
In six settings (Athens, Graz, Ljubljana, Riga, Rotterdam, Santiago de Compostela), the supervising ED physicians responsible for febrile children were general paediatricians, in two (Liverpool, London) these were paediatric emergency physicians, and in three (München, Nijmegen, Newcastle) supervision could take place by either a general paediatrician or an (paediatric) emergency physician (table 1).
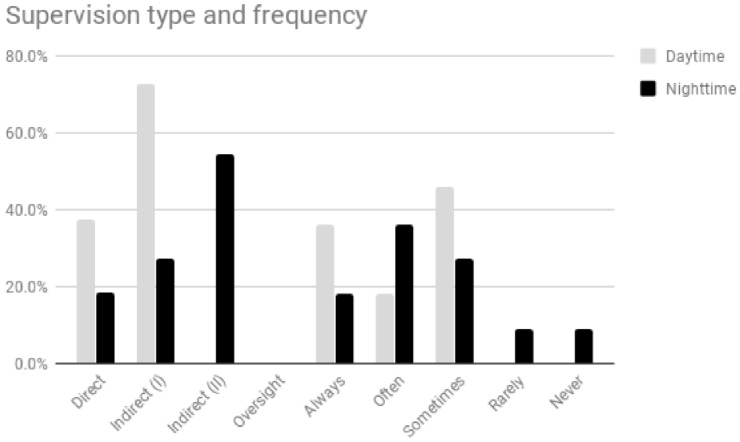
During office hours, three settings used direct supervision, while eight used type I indirect supervision. During out-of-office hours most settings used type II indirect supervision. Oversight was not used in any setting.
According to the study respondents, in four settings all febrile children were discussed with a supervisor during office hours; this number was lower during out-of-office-hours (figure 1).
Figure 1.
Supervision type and frequency: office hours versus out-of-office hours. Direct supervision: the supervising physician is physically present on site with the resident and patient. Indirect supervision (I): with direct supervision immediately available—the supervising physician is physically present within the hospital and is immediately available to provide direct supervision. Indirect supervision (II): with direct supervision available—the supervising physician is not physically present within the hospital or other sites of patient care, but is immediately available by means of telephonic and/or electronic modalities, and is available to provide direct supervision in person within 20–30 min at all times. Oversight: the supervising physician is available to provide review of procedures/encounters with feedback provided after care is delivered.
We studied the availability of guidelines for common infections: fever, respiratory tract infections (RTI), urinary tract infections (UTI) and sepsis.14 All settings had guidelines available for fever and sepsis. Ten settings had guidelines available for RTI and UTI. For fever, three settings used the National Institute for Health and Care Excellence (NICE) guideline, while eight settings used a local or a national guideline. For sepsis, five settings used the NICE guideline.
Invasive ventilation and inotrope use were reasons for PICU admission in all settings, while non-invasive ventilation was a reason for PICU admission in nine out of eleven settings. High-flow oxygen or continuous antiepileptic drugs required PICU admission in five settings and continuous cardiorespiratory monitoring in four (online supplementary appendix 3).
bmjpo-2019-000456supp003.pdf (14KB, pdf)
Six hospitals had guidelines regarding the time a child could stay in the ED, after which they should be admitted or discharged. This varied between 3 and 24 hours.
In four settings all items were available electronically, and in one setting all items were registered on paper; all other settings used a combination of paper and EHR (online supplementary appendix 4). Eight settings had patient data (eg, vital signs) available electronically, while 10 had diagnostic tests available electronically.
bmjpo-2019-000456supp004.pdf (14.8KB, pdf)
Regional systems of care
In six settings, primary care was provided by general practitioners, while in two settings this was offered by primary care paediatricians. In the other settings, primary care could be delivered by either type of physician. Primary care was available during out-of-office hours in eight settings. Overall, self-referral rate was high (table 1).
Immunisation to diphtheria, tetanus and acellular pertussis vaccine/inactivated polio vaccine/measles, mumps, rubella vaccine; Haemophilus influenzae type b vaccine; and pneumococcal conjugate vaccine (PCV) was part of routine care in all areas.
Meningococcal immunisation was part of routine care in six countries. Subtypes of PCV and meningococcal serotype vaccines in use differed. Other routine immunisations that varied are outlined in table 2. In some regions, additional immunisation was offered to specific groups, such as influenza in children with comorbidity or BCG to high-risk infants.12 In Slovenia and Latvia several immunisations are mandatory (table 2).
Table 2.
Routine immunisation in the eight participating countries12
| DTaP/IPV/MMR/Hib | PCV | Men | Hep-A | Hep-B | RV | Varicella | Influenza | BCG | TBE | |
| Austria | + | +10 | +ACWY | − | + | + | − | − | − | − |
| Germany | + | +13 | +C | − | + | + | + | − | − | − |
| Greece | + | +13 | +C, ACWY | + | + | + | + | − | − | − |
| Latvia | ++ | ++10 | − | − | ++ | ++ | ++ | + | ++ | +− |
| The Netherlands | + | +10 | +C | − | + | − | − | − | − | − |
| Slovenia | ++ | +10 | − | − | ++ | − | − | − | − | − |
| Spain | + | +13 | +C | − | + | − | + | − | − | − |
| UK | + | +13 | +B, C, ACWY | − | + | + | − | + | +− | − |
| +Routine immunisation (all children). | ||||||||||
| +−Specific regions only. | ||||||||||
| ++Mandatory immunisation. | ||||||||||
| −Not part of the routine immunisation programme. | ||||||||||
DTaP, diphtheria, tetanus and acellular pertussis vaccine; Hep-A, hepatitis A vaccine; Hep-B, hepatitis B vaccine; Hib, Haemophilus influenzae type b vaccine; IPV, inactivated polio vaccine; Men, meningococcal vaccine; MMR, measles, mumps, rubella vaccine; PCV, pneumococcal conjugate vaccine; RV, rotavirus vaccine; TBE, tick-borne encephalitis vaccine.
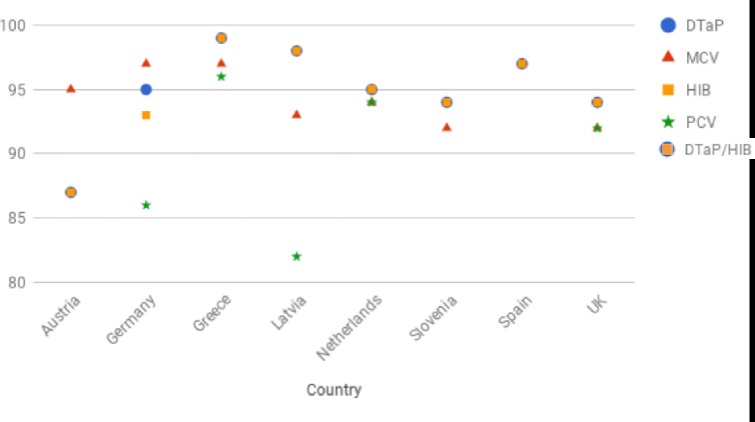
Figure 2 shows the 2016 WHO immunisation data in the participating countries.13 Overall, immunisation rates are estimated to be over 90%–95%; however, in several countries regional data show large differences in immunisation rates. Actual immunisation rates can be lower in specific regions, specific age groups, for specific immunisations and for booster immunisations15; therefore, the shown WHO data might be an overestimation of actual immunisation rates.
Figure 2.
Immunisation rates.13 Slovenia PCV rate (50%) not included in the graphic. PCV immunisation rate not available for Austria and Spain. DTaP: percentage of surviving infants who received the third dose of diphtheria and tetanus toxoid with pertussis-containing vaccine. MCV: percentage of surviving infants who received the first dose of measles-containing vaccine. In countries where the national schedule recommends the first dose of MCV at 12 months or later based on the epidemiology of disease in the country, coverage estimates reflect the percentage of children who received the first dose of MCV as recommended. Hib: percentage of surviving infants who received the third dose of Hib-containing vaccine. PCV: percentage of surviving infants who received the third dose of PCV. In countries where the national schedule recommends two doses during infancy and a booster dose at 12 months or later based on the epidemiology of disease in the country, coverage estimates may reflect the percentage of surviving infants who received two doses of PCV prior to the first birthday. DTaP, diphtheria, tetanus and acellular pertussis vaccine; Hib, Haemophilus influenzae type b vaccine; PCV, pneumococcal conjugate vaccine. MCV, measles-containing vaccine.
Resource use
Paediatric admission rates ranged from 4% to 51% (table 1).
Glucose POCT was available in all settings. Blood gas and urinalysis were available as a POCT in nine settings and C reactive protein (CRP) POCT in six. Nasopharyngeal aspirate tests were available in six settings and a streptococcal antigen test in five.
Correlations between different setting characteristics
We found strong correlations between self-referral rates and admission rates (r=−0.89, p<0.000), and between annual visits and how often febrile children were discussed with a senior doctor during office hours (r=−0.70, p<0.05) or during out-of-office hours (r=−0.82, p<0.05). We found moderate correlations between ED type and how often febrile children were discussed with a senior doctor during out-of-office hours (r=0.63, p<0.05). We did not find any correlation between hospital type and admission rates or hospital type and how often febrile children were discussed with a senior doctor.
Discussion
We found several differences between the participating EDs regarding the care for febrile children in all three domains: quality indicators, regional aspects of care and resource use.
Based on previous literature, we will discuss which of these factors are likely to influence outcome measures such as resource use (table 3).
Table 3.
Factors that can potentially influence resource use, based on previous literature
| Diagnostic tests | Antibiotic prescription rates | Admission rates | |
| Triage | ± | − | ± |
| Supervision and physician specialty | ± | + | + |
| Guideline implementation | + | + | + |
| Electronic health records | + | − | − |
| Criteria for paediatric intensive care unit admission | − | − | + |
| Time spent in the emergency department | − | − | + |
| Primary care and pre-hospital services | + | + | + |
| Immunisation | + | + | + |
| Point-of-care tests | + | +* | + |
+Possible influence.
±Influence not clear.
−No influence expected.
*In adults.
Although the discussed domains are based on a study looking into the US healthcare systems, we believe these domains are similarly relevant for European healthcare systems.4 10
Factors influencing resource use directly
We found variability in physician specialty and supervision; both have been previously found to influence resource use.16 For example, supervised ED visits—as opposed to consultant alone—have been linked to higher resource use and longer ED stay.17
In our study we asked whether guidelines were available but did not assess actual use or adherence. Guideline adherence can improve the quality of care18 and has been shown to reduce resource use and lead to more appropriate antibiotic use without adversely affecting outcomes.19 However, despite guideline availability, there is considerable practice pattern variation, as guideline availability does not automatically lead to adherence.19
In around half of the EDs, a CRP POCT was available. POCT provides results quickly and therefore can reduce time to treatment initiation and ED length of stay.20 CRP has shown to be of value in ruling in or out bacterial infections.21 A Cochrane review concluded that the use of a CRP POCT could reduce antibiotic prescription in adults22; however, this was not confirmed in a paediatric primary care population.23 In their discussion, the authors emphasise that performing a CRP POCT in all children with fever will not reduce antibiotic use, as it can lead to false positive values.23 Which children will benefit most from a CRP POCT and how this will affect resource use still has to be evaluated.
There were marked differences in electronic healthcare records (EHR) availability in our study. Use of EHR can increase efficiency in ordering diagnostic tests, reduce errors, improve overview, reduce duplicate testing and admissions, and improve information exchange between healthcare providers.24
From a researcher’s perspective, it is important to recognise that paper-based records can be incomplete and lack standardisation. Conducting research in settings using EHR potentially offers benefits and new opportunities,25 although aspects such as accuracy, completeness, standardisation, comparability and anonymity need to be addressed.24 25
Triage aims to prioritise patients who need immediate care from those who can safely wait in order to improve outcomes.26 Most settings used the MTS, but local systems were common as well. Research has shown that the performance of MTS is ‘moderate to good’ in children; however, its performance is lower in young children and children with comorbidities, who are at risk for undertriage (ie, they are assigned a lower priority level than they should).27 Most local systems have not been thoroughly validated in children.28
The impact of triage on resource use is not straightforward. As expected, a higher triage category is linked to higher resource use.29 There is some evidence that ‘down-triaging’ of non-ill-appearing children to a lower category can reduce resource use without increasing adverse outcomes.30 On the other hand, as undertriage can cause treatment delay, it has been hypothesised that undertriage can increase resource use31 and improved triage can lead to more appropriate resource use.32 However, it is possible that triage influences resource use, and use of different triage systems can explain practice pattern variation. More research is needed on this topic to understand this exact relationship.
Six hospitals had local guidelines on how long a child could stay in the ED. In the UK, it is a national target that 95% of all ED patients should be discharged or admitted within 4 hours.3 Limiting ED stay can have a large impact on children and their families. It has been shown to decrease crowding and mortality and to improve patient satisfaction.33 However, it has also been suggested that the introduction of the 4-hour target has increased (short-duration) admission rates, but consistent data are lacking.3 This leads to the discussion of a possible role of short-stay units, as these can potentially reduce ED length of stay as well as admissions.34
We found marked variation in PICU admission criteria. This can influence general admissions as well as PICU admissions. PICU admissions are frequently used as an outcome measure for disease severity, and it is important to realise this can be influenced by other factors than disease severity alone.
Factors influencing resource use indirectly through case mix
Even though primary care was available in all settings, not all settings offered out-of-office primary care. Furthermore, the self-referral rate at the ED was high in many settings, suggesting that other factors than merely primary care availability influence self-referral. A high rate of self-referred patients can lead to a higher proportion of non-urgent patients who can delay resources being given to those who need it more urgently.35
In their systematic review, Kraaijvanger et al5 identified several reasons for self-referral, such as patient expectations and accessibility to primary care. In some settings urgent primary care has been made available at the same site as the ED, and this has been shown to reduce self-referral rates, especially of low-urgent patients.5 In our study, we found a strong correlation between self-referral rates and admission rates.
Although there were large similarities in immunisations, there were also differences. This could explain some of the variation in the population and subsequent resource use between EDs.36 For example, the introduction of the rotavirus immunisation has led to a large reduction of ED visits and admissions for children with gastroenteritis.37
While the introduction of immunisations has led to a great reduction of invasive diseases,36 the presented data show that low immunisation rates still occur in Europe. Low immunisation rates can lead to increased susceptibility by a direct effect as well as indirect effect by decreasing herd immunity.38
In two recent European studies, the burden of life-threatening infections in children, including PICU admission for sepsis, was found to be largely due to vaccine-preventable meningococcal and pneumococcal infections.39 40
Strengths and limitations
The strengths of our study include the comprehensive collaboration of researchers who are part of a large European Union consortium, using a standardised protocol. To our knowledge, this is the first study comparing healthcare for febrile children at European EDs.
The main limitation is the proportional representation as this was a small convenience study and the survey was filled in by a single respondent (the principal investigator). Some of the results are based on clinicians’ reflection on their local practice and few on validated demographic or hospital data. For example, the data regarding socioeconomic status were based on data provided by the survey respondent, while data regarding immunisation grades were based on WHO data.
In most countries only one or two hospitals from several hundreds in most countries contributed to this study, and not all 28 European Union countries participated. Therefore, results can reflect differences between hospitals as well as between countries and are not representative of all hospitals in the participating countries. For example, not all European hospitals have an onsite PICU. Furthermore, all participating hospitals were either a university or a large district general hospital, and results might therefore not be generalisable to smaller hospitals. However, as all participating hospitals were larger hospitals, the standard of care in these hospitals is expected to be high, and therefore diversity might represent practice variability between countries. This study gives an overview of setting-related factors that may influence resource use; however, this is not exhaustive.
Conclusion
Our study shows differences in the emergency care for febrile children across Europe. Resource use such as diagnostic tests, antibiotic prescription and admission rates can be influenced by differences in the organisation of healthcare.
Our study provides an overview of setting-related factors that need to be considered when interpreting results of observational studies. As new interventions are developed, these factors need to be considered to model their potential impact, to accurately plan clinical trials and to conduct health economic analyses.
More indepth research is needed to study the factors described above, of which some will be covered in the MOFICHE study in more detail.
Studying these differences can be used as a starting point to improve paediatric emergency care across Europe.4
What is known about the subject?
Fever is one of the most frequent reasons for paediatric emergency department consultation.
Differences in outcome of paediatric infectious diseases still exist in Europe and have been linked to differences in organisation of healthcare.
What this study adds?
Our study shows marked differences regarding the organisation of emergency care for febrile children across Europe.
Differences in the organisation of healthcare can influence resource use such as diagnostic tests, antibiotic prescription rates and admission rates.
Our study provides an overview of setting-related factors that can influence resource use and thus need to be considered when interpreting results of observational studies.
Footnotes
Contributors: Conceptualisation: DB, SY, IM, FM-T, JAH, EDC, ME, MT, RdG, MvdF, WZ, FS, MP, DZ, UvB, ML, HAM. Funding: SY, IM, FM-T, JAH, EDC, ME, MT, RdG, MvdF, WZ, FS, MP, DZ, UvB, ML, HAM. Data curation: DB, NNH, SY, IM, FM-T, JAH, EDC, ME, MT, RdG, MvdF, WZ, FS, MP, DZ, UvB, ML, HAM, RN, JED, EL, IE, BK, GW, AB. Formal analysis: DB, HAM. Investigation: DB, NNH, SY, IM, FM-T, JAH, EDC, ME, MT, RdG, MvdF, WZ, FS, MP, DZ, UvB, ML, HAM, RN, JED, EL, IE, BK, GW, AB. Project administration: HAM. Supervision: HAM. Writing—original draft: DB. Writing—review and editing: DB, NNH, SY, JED, IM, FM-T, JAH, EDC, ME, MT, RdG, MvdF, WZ, BK, FS, MP, DZ, UvB, ML and HAM.
Funding: This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no 668303. The research was supported by the National Institute for Health Research Biomedical Research Centre based at Imperial College (JAH, ML) and at Newcastle Hospitals NHS Foundation Trust and Newcastle University (EL, ME). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1.Medford-Davis L, Marcozzi D, Agrawal S, et al. . Value-based approaches for emergency care in a new era. Annals of Emergency Medicine 2017;69:675–83. 10.1016/j.annemergmed.2016.10.031 [DOI] [PubMed] [Google Scholar]
- 2.Rasooly IR, Mullins PM, Alpern ER, et al. . Us emergency department use by children, 2001–2010. Pediatric Emergency Care 2014;30:602–7. 10.1097/PEC.0000000000000204 [DOI] [PubMed] [Google Scholar]
- 3.parliament.uk Available: http://researchbriefings.parliament.uk/ResearchBriefing/Summary/CBP-7281 [Accessed 10 Nov 2017].
- 4.Wolfe I, Cass H, Thompson MJ, et al. . Improving child health services in the UK: insights from Europe and their implications for the NHS reforms. BMJ 2011;342:d1277 10.1136/bmj.d1277 [DOI] [PubMed] [Google Scholar]
- 5.Kraaijvanger N, van Leeuwen H, Rijpsma D, et al. . Motives for self-referral to the emergency department: a systematic review of the literature. BMC Health Serv Res 2016;16 10.1186/s12913-016-1935-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black RE, Cousens S, Johnson HL, et al. . Global, regional, and national causes of child mortality in 2008: a systematic analysis. The Lancet 2010;375:1969–87. 10.1016/S0140-6736(10)60549-1 [DOI] [PubMed] [Google Scholar]
- 7.Thompson MJ, Ninis N, Perera R, et al. . Clinical recognition of meningococcal disease in children and adolescents. The Lancet 2006;367:397–403. 10.1016/S0140-6736(06)67932-4 [DOI] [PubMed] [Google Scholar]
- 8.Kuehn BM. IDSA: better, faster diagnostics for infectious diseases needed to curb overtreatment, antibiotic resistance. JAMA 2013;310:2385–6. 10.1001/jama.2013.283828 [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Elon LK, Johnson BA, et al. . Physician practice variation in the pediatric emergency department and its impact on resource use and quality of care. Pediatric Emergency Care 2010;26:902–8. 10.1097/PEC.0b013e3181fe9108 [DOI] [PubMed] [Google Scholar]
- 10.Mintegi S, Shavit I, Benito J. REPEM group (research in European paediatric emergency Medicine). Pediatric emergency care in Europe: a descriptive survey of 53 tertiary medical centers. Pediatr Emerg Care 2008;24:359–63. [DOI] [PubMed] [Google Scholar]
- 11.New supervision standards: Discussion and justification. Available: https://www.acgme.org/Portals/0/PDFs/jgme-11-00-39-45%5B1%5D.pdf
- 12.Vaccine schedules in all countries of the European Union Available: https://vaccine-schedule.ecdc.europa.eu [Accessed 1 Nov 2017].
- 13.WHO Available: http://apps.who.int/immunization_monitoring/globalsummary/wucoveragecountrylist.html [Accessed 1 Nov 2017].
- 14.Wing R, Dor MR, McQuilkin PA. Fever in the pediatric pateint. Emerg Med Clin North Am 2013;31:1073–96. 10.1016/j.emc.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 15.Georgakopoulou T, Menegas D, Katsioulis A, et al. . A cross-sectional vaccination coverage study in preschool children attending nurseries-kindergartens: implications on economic crisis effect. Human Vaccines & Immunotherapeutics 2017;13:190–7. 10.1080/21645515.2016.1230577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khine H, Goldman DL, Avner JR. Management of fever in postpneumococcal vaccine era: comparison of management practices by pediatric emergency medicine and general emergency medicine physicians. Emergency Medicine International 2014;2014:1–5. 10.1155/2014/702053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitts SR, Morgan SR, Schrager JD, et al. . Emergency department resource use by supervised residents vs attending physicians alone. JAMA 2014;312:2394–400. 10.1001/jama.2014.16172 [DOI] [PubMed] [Google Scholar]
- 18.Paul R, Melendez E, Stack A, et al. . Improving adherence to PalS septic shock guidelines. PEDIATRICS 2014;133:e1358–66. 10.1542/peds.2013-3871 [DOI] [PubMed] [Google Scholar]
- 19.Breakell R, Thorndyke B, Clennett J, et al. . Reducing unnecessary chest x-rays, antibiotics and bronchodilators through implementation of the NICE bronchiolitis guideline. Eur J Pediatr 2017. [DOI] [PubMed] [Google Scholar]
- 20.Nijman RG, Moll HA, Vergouwe Y, et al. . C-reactive protein bedside testing in febrile children lowers length of stay at the emergency department. Pediatric Emergency Care 2015;31:633–9. 10.1097/PEC.0000000000000466 [DOI] [PubMed] [Google Scholar]
- 21.Van den Bruel A, Thompson MJ, Haj-Hassan T, et al. . Diagnostic value of laboratory tests in identifying serious infections in febrile children: systematic review. BMJ 2011;342:d3082 10.1136/bmj.d3082 [DOI] [PubMed] [Google Scholar]
- 22.Aabenhus R, Jensen J-US, Jørgensen KJ, et al. . Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst Rev 2014;15 10.1002/14651858.CD010130.pub2 [DOI] [PubMed] [Google Scholar]
- 23.Van den Bruel A, Jones C, Thompson M, et al. . C-reactive protein point-of-care testing in acutely ill children: a mixed methods study in primary care. Arch Dis Child 2016;101:382–6. 10.1136/archdischild-2015-309228 [DOI] [PubMed] [Google Scholar]
- 24.Ben-Assuli O, records Ehealth. Electronic health records, adoption, quality of care, legal and privacy issues and their implementation in emergency departments. Health Policy 2015;119:287–97. 10.1016/j.healthpol.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 25.Wasserman RC, records Emedical. Electronic medical records (EMRs), epidemiology, and Epistemology: reflections on EMRs and future pediatric clinical research. Academic Pediatrics 2011;11:280–7. 10.1016/j.acap.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron PA, Gabbe BJ, Smith K, et al. . Triaging the right patient to the right place in the shortest time. British Journal of Anaesthesia 2014;113:226–33. 10.1093/bja/aeu231 [DOI] [PubMed] [Google Scholar]
- 27.Zachariasse JM, Kuiper JW, de Hoog M, et al. . Safety of the Manchester triage system to detect critically ill children at the emergency department. The Journal of Pediatrics 2016;177:232–7. 10.1016/j.jpeds.2016.06.068 [DOI] [PubMed] [Google Scholar]
- 28.Zachariasse JM, van der Hagen V, Seiger N, et al. . Performance of triage systems in emergency care: a systematic review and meta-analysis. BMJ Open 2019;9 10.1136/bmjopen-2018-026471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gravel J, Gouin S, Goldman RD, et al. . The Canadian Triage and acuity scale for children: a prospective multicenter evaluation. Annals of Emergency Medicine 2012;60:71–7. 10.1016/j.annemergmed.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 30.Gravel J, Manzano S, Arsenault M. Safety of a modification of the triage level for febrile children 6 to 36 months old using the paediatric Canadian Triage and acuity scale. CJEM 2008;10:32–7. 10.1017/S1481803500009982 [DOI] [PubMed] [Google Scholar]
- 31.Seiger N, van Veen M, Steyerberg EW, et al. . Undertriage in the Manchester triage system: an assessment of severity and options for improvement. Archives of Disease in Childhood 2011;96:653–7. 10.1136/adc.2010.206797 [DOI] [PubMed] [Google Scholar]
- 32.Escobar MA, Morris CJ. Using a multidisciplinary and evidence-based approach to decrease undertriage and overtriage of pediatric trauma patients. Journal of Pediatric Surgery 2016;51:1518–25. 10.1016/j.jpedsurg.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 33.Chang AM, Lin A, Fu R, et al. . Associations of emergency department length of stay with publicly reported quality-of-care measures. Acad Emerg Med 2017;24:246–50. 10.1111/acem.13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karacabeyli D, Meckler GD, Park DK, et al. . System outcomes associated with a pediatric emergency department clinical decision unit. CJEM 2018;15:1–4. [DOI] [PubMed] [Google Scholar]
- 35.Sills MR, Fairclough D, Ranade D, et al. . Emergency department crowding is associated with decreased quality of care for children. Pediatric Emergency Care 2011;27:837–45. 10.1097/PEC.0b013e31822c1382 [DOI] [PubMed] [Google Scholar]
- 36.Gefenaite G, Bijlsma M, Bos H, et al. . Did introduction of pneumococcal vaccines in the Netherlands decrease the need for respiratory antibiotics in children? Analysis of 2002 to 2013 data. Euro Surveill 2014;19 10.2807/1560-7917.ES2014.19.44.20948 [DOI] [PubMed] [Google Scholar]
- 37.Burnett E, Jonesteller CL, Tate JE, et al. . Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis 2017;215:1666–72. 10.1093/infdis/jix186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metcalf CJE, Ferrari M, Graham AL, et al. . Understanding herd immunity. Trends in Immunology 2015;36:753–5. 10.1016/j.it.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 39.Boeddha NP, Schlapbach LJ, Driessen GJ, et al. . Mortality and morbidity in community-acquired sepsis in European pediatric intensive care units: a prospective cohort study from the European childhood life-threatening infectious Disease Study (EUCLIDS). Crit Care 2018;22 10.1186/s13054-018-2052-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinón-Torres F, Salas A, Rivero-Calle I, et al. . Life-threatening infections in children in Europe (the EUCLIDS project): a prospective cohort study. Lancet Child Adolesc Health 2018;2:404–14. 10.1016/S2352-4642(18)30113-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2019-000456supp001.pdf (55KB, pdf)
bmjpo-2019-000456supp002.pdf (160.5KB, pdf)
bmjpo-2019-000456supp003.pdf (14KB, pdf)
bmjpo-2019-000456supp004.pdf (14.8KB, pdf)