Abstract
Background:
Early mobilization is an important therapeutic goal after total knee replacement and total hip replacement. Orthostatic hypotension and orthostatic intolerance can impede mobilization. Midodrine hydrochloride, an orally administered vasoconstrictor, may improve blood pressure and diminish the prevalence of adverse mobilization events.
Methods:
We conducted a pilot change-of-practice study. Two cohorts, each comprising 10 patients managed with total knee replacement and 10 patients managed with total hip replacement, were managed with blood pressure-adjusted midodrine, which was administered 3 times daily for the first 72 hours postoperatively at either a low dose (2.5 or 5 mg) or a higher dose (5 or 10 mg). These patients were then matched with an equivalent preintervention cohort of patients.
Results:
The midodrine protocol was instituted effectively and with high compliance. Hypotension was uncommon across all groups, with the mean lowest systolic blood pressure ranging from 110 to 121 mm Hg. Moreover, adverse mobilization events were uncommon across all groups (prevalence, 9.6% in the control group, 5.6% in the low-dose group, and 2.9% in the high-dose group) (p = 0.046 for the high-dose group versus the control group). A midodrine dose of 10 mg generated a significant mean dose-related systolic blood pressure increase of 14 mm Hg at 2 hours after administration (p < 0.001). There were no significant differences between the groups in terms of mean systolic blood pressure, biochemical markers, or intravenous therapy administration.
Conclusions:
A dose of 10 mg was found to achieve a significant systolic blood pressure response at 2 hours after administration and, in patients who received higher-dose midodrine, adverse mobilization events appeared less common. Additional investigation with a blinded randomized controlled trial, utilizing 10 mg of midodrine 2 hours before mobilization, would be needed to confirm the efficacy of midodrine therapy.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Early mobilization is important after major orthopaedic surgery to prevent increased morbidity and prolonged hospital stay1. Common barriers to early mobilization include hypotension, orthostatic intolerance (defined as symptoms such as dizziness, blurred vision, nausea, vomiting, or syncope), and orthostatic hypotension (defined as a systolic blood pressure drop of >20 mm Hg on standing)1-3. Previous studies have demonstrated that the prevalence of orthostatic intolerance ranges from 40% to 50% after hip arthroplasty4,5. Orthostatic intolerance and orthostatic hypotension can lead to episodes of failed physiotherapy.
There are multiple causes for postoperative hypotension, with hypovolemia (resulting from dehydration and blood loss) considered to be a cause of major importance3. However, liberal fluid therapy appears to have limited impact on orthostatic intolerance after hip arthroplasty, arguing against hypovolemia5. Recently, it has been suggested that postoperative inflammation and associated vasodilatation could be a pertinent contributor to postoperative hypotension5-7. For example, after hip arthroplasty, inflammatory markers such as interleukin-6 (IL-6) and C-reactive protein (CRP) can increase by up to 15-fold and can correlate with the postoperative systemic inflammatory response syndrome (SIRS)8,9 triggered by damage-associated molecular patterns (DAMPs)7,10. This SIRS response may lead to hypotension due to vasodilatation and increased vascular permeability and is therefore unlikely to be effectively treated with intravenous fluid therapy alone11. We studied the introduction of vasoconstrictive medications for the treatment of postoperative hypotension, orthostatic intolerance, orthostatic hypotension, and failed physiotherapy outside of the intensive care environment.
Midodrine hydrochloride is an oral alpha-adrenoceptor agonist that is approved for the treatment of orthostatic hypotension12. A recent double-blinded randomized controlled trial indicated that midodrine was safe and effective for the treatment of orthostatic hypotension as it was associated with decreased syncopal symptoms during tilt-table testing13. Recently, midodrine was shown to significantly reduce the duration of intravenous vasopressors in patients with septic shock14. Midodrine also has been tested to determine whether it can improve the rates of early mobilization after hip arthroplasty4. However, only two 5-mg doses were administered for the duration of that study, leading to the conclusion that further studies were required to examine optimal dosing. There is a lack of research on the use of midodrine for the above indications. In the present study, we compared 3 groups (low-dose, higher-dose, and matched control groups) to assess the effects of midodrine on adverse mobilization events, mean systolic blood pressure, change in systolic blood pressure 2 hours after midodrine administration, biochemical parameters, and length of stay. We hypothesized that, compared with the control group, either a lower or higher-dose protocol would increase systolic blood pressure and reduce adverse mobilization events.
Materials and Methods
Study Design
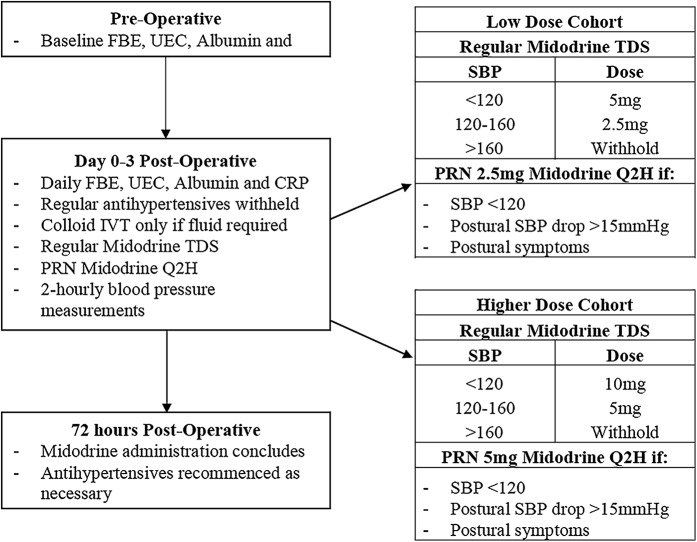
We performed a pilot before-and-after, change-of-practice study that assessed appropriate dosing, feasibility, compliance, safety and physiological efficacy. The study was performed at an elective surgery center of a tertiary hospital from January 2017 until October 2017 and was approved by the Austin Hospital Ethics Committee both for its execution as a pilot change-of-practice study and for the related data collection. It included the introduction of a care package over the first 72 hours postoperatively, which entailed midodrine administration and withholding of each patients’ preexisting regular anti-hypertensive drugs (Fig. 1). Regular anti-hypertensive medications were administered until the day of surgery, when anti-hypertensive medications were ceased and midodrine commenced postoperatively.
Fig. 1.
Diagram illustrating the process of care with the introduction of midodrine. FBE = full blood count; UEC = urea, electrolytes, creatinine; CRP = C-reactive protein; TDS = ter in die (three times daily); PRN = pro re nata (as needed); Q2H = 2-hourly; and SBP = systolic blood pressure.
Regular doses of midodrine were administered at 6:00 a.m., 12:00 p.m., and 6:00 p.m. Dosing of midodrine was dependent on the patient’s recorded systolic blood pressure. For the low-dose cohort, 5 mg was administered if the systolic blood pressure was <120 mm Hg and 2.5 mg was administered if the systolic blood pressure was 120-160 mm Hg. For the higher-dose cohort, 10 or 5 mg was administered, respectively. For both groups, midodrine was withheld if systolic blood pressure was >160 mm Hg.
Additional midodrine was also given as a PRN (pro re nata, or “as needed”) dose as often as every 2 hours (Q2H) if systolic blood pressure was <120 mm Hg, the patient displayed any symptoms of orthostatic intolerance, or the patient had a postural systolic blood pressure drop of >15 mm Hg. The dose was 2.5 mg for the low-dose cohort and 5 mg for higher-dose cohort.
To reduce the chance of overnight supine hypertension, no doses were administered after 6:00 p.m. Nursing staff performed blood pressure measurements every 2 hours, and physiotherapy sessions were unaltered. Reasons to terminate midodrine administration early included a hypertensive episode requiring treatment, urinary retention, unexpected adverse drug reactions, or a significant postoperative complication (e.g., myocardial infarction or pulmonary embolism).
Inclusion/Exclusion Criteria
All patients ≥18 years of age undergoing total hip or knee replacement by a single surgeon were eligible. The exclusion criteria were digoxin treatment, glaucoma, chronic urinary retention requiring treatment, history of autonomic dysfunction or orthostatic hypotension, and renal or hepatic failure.
Change-of-Practice Audit
The audited period extended from January 2017 to October 2017 and included 20 sequential patients who underwent total hip replacement and 20 who underwent total knee replacement. Only 1 patient undergoing total knee replacement was excluded because of regular treatment with digoxin. Comparisons were drawn between the higher-dose cohort and the low-dose cohort, between the higher-dose cohort and a historical control cohort, and between the low-dose cohort and the historical control cohort. Patients receiving low-dose midodrine were matched to historical patients from January 2012 to December 2016 on the basis of age (within 5 years), sex, operation, operation location, and whether the patient was receiving regular antihypertensive therapy. These historical control patients were selected manually from the medical record in reverse chronological order with use of the criteria above until a match for each patient in the low-dose cohort was identified from among the 335 possible controls over the 5-year period. The primary outcome was the number and percentage of failed physiotherapy sessions and episodes of orthostatic intolerance or orthostatic hypotension. Orthostatic intolerance was defined as postural symptoms, including nausea, vomiting, dizziness, faintness, or blurred vision. Orthostatic hypotension was defined as a postural systolic blood pressure drop of ≥15 mm Hg. Failed physiotherapy was defined as not being capable of physically separating from the hospital bed.
Secondary and tertiary outcomes included blood pressure values during the first 72 hours and at 2 hours after the administration of a midodrine dose, the volume of intravenous therapy, biochemical parameters (hemoglobin [Hb], white blood-cell count [WCC], creatinine, estimated glomerular filtration rate [eGFR], albumin, and CRP), complications, duration of hospitalization, and discharge destination. An incomplete physiotherapy session was defined as having to cease a session due to hypotension, orthostatic intolerance, or orthostatic hypotension.
Statistical Analysis
Data initially were assessed for normality. Group comparisons were performed with use of chi-square tests for equal proportions (or the Fisher exact test when numbers were small), the Student t test for normally distributed data, and the Wilcoxon rank sum test otherwise, with the results reported as the frequency and percentage, the mean and standard deviation, or the median and interquartile range, respectively. Changes over time were determined by mixed linear modeling, fitting main effects for treatment and time and an interaction between the 2 to determine if groups behave differently over time, with results plotted as least square means with error bars indicating the 95% confidence interval of the mean. All analyses were performed with use of SAS (version 9.4; SAS Institute), and a 2-sided p value of 0.05 was considered to be significant.
Results
Overall, 20 patients were recruited to receive low-dose midodrine therapy and 20 were recruited to receive higher-dose midodrine therapy. Each cohort included 10 patients undergoing total knee replacement and 10 undergoing total hip replacement. There was no significant difference in demographic characteristics between the control group and the low-dose group or between the low-dose group and the higher-dose group (Table I). The protocol was successfully implemented, with patients receiving either low-dose or higher-dose midodrine. As expected, there was a significant difference in the amount of midodrine administered between cohorts on day 0, 1, and 2 (Fig. 2-A). Conversely, there was no significant difference between the groups in terms of intravenous therapy administration over the study period.
Fig. 2-A.
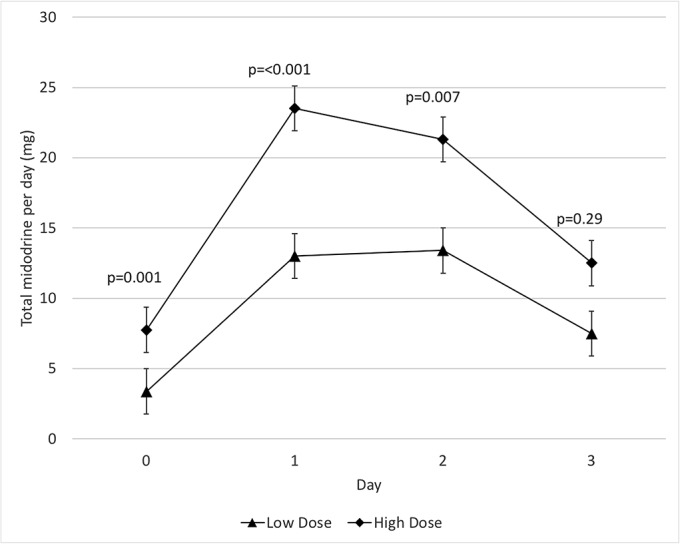
Mean total midodrine administration and 95% confidence interval.
Fig. 2-B.
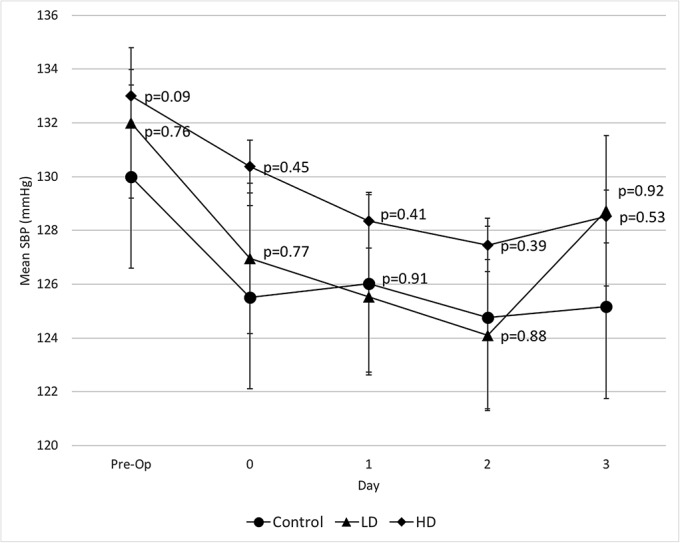
Mean systolic blood pressure (SBP) and 95% confidence interval. The p values on the higher-dose (HD) data points pertain to the comparison between the higher-dose and low-dose (LD) groups, and the p values on the low-dose data points pertain to the comparison between the low-dose group and the control group.
Fig. 2-C.

Mean lowest systolic blood pressure (SBP) and 95% confidence interval. The p values on the higher-dose (HD) data points pertain to the comparison between the higher-dose and low-dose (LD) groups, and the p values on the low-dose data points pertain to the comparison between the low-dose group and the control group.
Fig. 2-D.
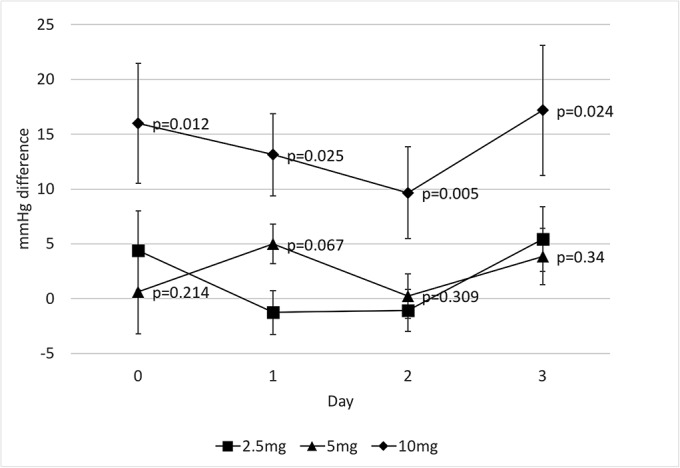
Mean difference in systolic blood pressure 2 hours post midodrine administration and 95% confidence interval. The p values on the 10-mg data points pertain to the comparison between 10 and 5 mg, and the p values on the 5-mg data points pertain to the comparison between 5 and 2.5 mg.
TABLE I.
Demographic and Baseline Characteristics
| Variables | Control Group (N = 20) | Low-Dose Group (N = 20) | P Value (Control Group Versus Low-Dose Group) | Higher-Dose Group (N = 20) | P Value (Low-Dose Group Versus Higher-Dose Group) |
| Age* (yr) | 70.9 ± 7.2 | 70.6 ± 8.3 | 0.89 | 65.7 ± 7.3 | 0.06 |
| Female sex (no. of patients) | 10 (50%) | 10 (50%) | 1.00 | 11 (55%) | 0.75 |
| Hypertension (no. of patients) | 12 (60%) | 12 (60%) | 1.00 | 10 (50%) | 0.53 |
| Preoperative laboratory values | |||||
| Systolic blood pressure* (mm Hg) | 130 ± 16 | 132 ± 20 | 0.76 | 138 ± 13 | 0.09 |
| Hemoglobin* (g/L) | 141 ± 13 | 142 ± 13 | 0.70 | 141 ± 11 | 0.73 |
| Creatinine* (μmol/L) | 73.7 ± 12.6 | 73.5 ± 16.5 | 0.96 | 75.9 ± 18.9 | 0.67 |
| C-reactive protein† (mg/L) | 2 (0.9-3.8) | 3.1 (1.8-5.9) | 0.07 | 2.5 (1.4-5.6) | 0.61 |
The values are given as the mean and the standard deviation.
The values are given as the mean and the interquartile range.
Adverse mobilization events (failed physiotherapy, orthostatic intolerance, and orthostatic hypotension) appeared to progressively decrease with increased midodrine dose, from no midodrine to low-dose midodrine to higher-dose midodrine, with the rate of such events being significantly lower in the higher-dose group than in the control group (2.9% compared with 9.6%; p = 0.046) (Table II).
TABLE II.
Total Adverse Mobilization Events*
| No. of Adverse Events | |||||
| Group | Day 0 | Day 1 | Day 2 | Day 3 | Total† |
| Control group | 2 | 0 | 6 | 2 | 10 (9.6%) |
| Low-dose group | 2 | 0 | 2 | 1 | 5 (5.6%) |
| Higher-dose group | 2 | 0 | 0 | 1 | 3 (2.9%)‡ |
Failed physiotherapy, orthostatic intolerance, orthostatic hypotension.
The total number of mobilization attempts for the control, low-dose, and higher-dose groups were 104, 90, 105, respectively.
P = 0.046 in comparison with control group (Fisher exact test).
There was no significant difference in mean systolic blood pressure preoperatively or postoperatively between the control, low-dose, and higher-dose groups (Fig. 2-B). The mean lowest systolic blood pressure was similar between the control, low-dose, and higher-dose groups and ranged from 110 to 121 mm Hg (Fig. 2-C). However, there was a significant dose-related systolic blood pressure response after the administration of 10 mg of midodrine when compared with 5 mg (Fig. 2-D). Systolic blood pressure increased by an average of 14 mm Hg at 2 hours after the administration of 10 mg of midodrine (p < 0.001), compared with 2.4 mm Hg after the administration of 5 mg and 3.4 mm Hg after the administration of 2.5 mg.
There were no significant differences between the groups in terms of the median duration of hospital stay (4 days, 4 days, and 4.5 days in the control group, low-dose group, and higher-dose group, respectively). On subgroup analysis, the mean systolic blood pressure was significantly higher both preoperatively and postoperatively in patients undergoing total knee replacement than in those undergoing total hip replacement (Appendix Figure A), with no more than a 5 to 8-mm Hg drop postoperatively. Biochemically, there was no significant difference between the groups. Of note, CRP increased >60-fold from baseline and was similar between the total knee replacement and total hip replacement groups, with a significant difference only on day 1 (Appendix Figure B).
Two male patients who underwent total knee replacement and received higher-dose midodrine developed acute urinary retention requiring catheterization. In this group, 13 of 20 patients underwent preoperative catheterization. One patient who was not catheterized preoperatively developed acute urinary retention on day 1, whereas the other passed their trial of void on day 1 and developed acute urinary retention on day 2. Both achieved a successful trial of voiding at a later date. No patient in the control or low-dose groups were identified to have documented acute urinary retention.
Discussion
Key Findings
We conducted a pilot study of low-dose and higher-dose midodrine administration in patients undergoing total knee replacement and total hip replacement to test whether such treatment would reduce barriers to mobilizing patients. Although hypotension was uncommon among control patients, there was a significant mean dose-related systolic blood pressure increase of 14 mm Hg at 2 hours after treatment with 10 mg of midodrine. Moreover, even though mobilization-associated adverse events were uncommon in the control population, their combined incidence decreased significantly in association with higher-dose midodrine therapy.
Relationship to Previous Studies
To our knowledge, there has been only 1 other study of the use of midodrine after major orthopaedic surgery4. In that randomized controlled trial, 5 mg of midodrine was administered 6 and 24 hours after total hip replacement, approximately 1 hour prior to mobilization with physiotherapy. The authors reported no improvement in terms of postoperative mobilization, orthostatic hypotension, or orthostatic intolerance but concluded that additional investigation into the dose and timing of midodrine was warranted. We increased the frequency and dose, with our patients receiving up to 10 mg of midodrine per dose and up to 50 mg per day. We were able to demonstrate that 10 mg was necessary to have a dose-related systolic blood pressure response at approximately 2 hours after administration. We also showed that the rates of hypotension and the degree of systolic blood pressure drop postoperatively were less than those demonstrated in the above study4. In particular, the previous study demonstrated a collective 16 to 20-mm Hg drop in systolic blood pressure postoperatively, compared with a 5 to 8-mm Hg drop in our cohort. Interestingly, we also demonstrated that postoperative CRP in our cohorts increased >60-fold, compared with approximately 15-fold in the literature8,9. The previous study also demonstrated that 39.7% of patients in the control group who underwent total hip replacement experienced orthostatic hypotension4, compared with 22.5% in our equivalent cohort who experienced any adverse mobilization event (failed physiotherapy, orthostatic hypotension, and orthostatic intolerance combined).
Implications of Study Findings
Our pilot study implies that a postoperative midodrine administration protocol with both regular and systolic blood pressure-adjusted PRN dosing can be implemented in an elective health-care setting but may increase the risk of urinary retention. However, by showing that 10 mg of midodrine can elicit a clear dose-related response in systolic blood pressure at 2 hours after administration, our study implies that a regimen in which midodrine is administered 2 hours before planned mobilization would be physiologically logical and would be likely to reduce the frequency of midodrine administration. Moreover, by demonstrating a decrease in mobilization-associated adverse events, our study implies that a midodrine-based approach to improve mobilization success in these patients has promise. However, it must be noted that hypovolemia also may be a cause of orthostatic hypotension and orthostatic intolerance; therefore, treatment of hypovolemia should always be considered in the first instance. Finally, the observation that 10% of our higher-dose cohort experienced urinary retention raises concern about the risks versus possible benefits associated with midodrine administration at such doses. Therefore, it seems logical that confining midodrine use to the 2 hours before mobilization may be the safest approach to alpha-agonist therapy for these patients and should be explored in future randomized trials.
Strengths and Limitations
In this single-surgeon, single-institution, before-and-after change-of-practice study, we aimed to minimize confounding variables. We demonstrated that midodrine therapy can be effectively implemented following orthopaedic procedures and obtained information to guide an optimal approach to such therapy in the future. This study was not a randomized controlled trial, and only a small group of patients underwent the intervention. Such small numbers expose the study to a high risk of type-II error, especially in relation to demographic variables, which can only be overcome by studying many more patients. The use of a retrospective control group that was matched only to the low-dose cohort via manual matching through chart review rather than via a statistical matching method is also a major limitation because of the possibility of bias. However, conducting a large-scale randomized controlled trial of an oral vasopressor can only be justified if pilot data provide evidence of feasibility, physiological effect, and safety. The small group of patients in conjunction with the low rate of adverse mobilization events across all cohorts also made significance far less achievable. Nurses and physiotherapists were aware of treatment with midodrine, which could have biased patient care. Conversely, control patients may have had vital observations, failed physiotherapy, and orthostatic hypotension or orthostatic intolerance recorded less diligently. However, despite some intraobserver variability in recording, signals such as blood pressure are relatively independent of such biases. In addition, all antihypertensive therapy was withheld for the duration of the study for the patients who received the intervention, whereas such therapy could be reinitiated at the physician’s discretion for the patients in the control group; however, typical practice in patients undergoing major orthopaedic surgery had been and continues to be that antihypertensive therapy is withheld. This intervention in itself may reduce adverse mobilization events.
Conclusions
In conclusion, we conducted a pilot study of a postoperative midodrine administration protocol with both regular and systolic blood pressure-adjusted PRN dosing. We found that such a protocol could be implemented successfully, that 10 mg of midodrine was necessary to see a systolic blood pressure response 2 hours after administration, and that such a high dose regimen appeared to decrease the overall number of mobilization-associated adverse events. We also found that midodrine therapy may increase the risk of urinary retention. Given the findings of this pilot study, a larger double-blinded randomized controlled trial involving the use of a minimum of 10 mg of midodrine given only 2 hours before attempted mobilization appears justified.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJSOA/A97).
Acknowledgments
Note: This study would not have been possible without the unwavering support and hard work completed by the nursing, physiotherapy, and pharmacy staff at the Surgery Centre, Austin Health. Special thanks also go to Adj. A/Prof. Glenn Eastwood for guidance and support throughout the ethics approval process.
Footnotes
Investigation performed at the Surgery Centre, Heidelberg Repatriation Hospital, Austin Health, Ivanhoe, Victoria, Australia
Disclosure: The authors indicated that no external funding was received for any aspect of this work. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A96).
References
- 1.Harper CM, Lyles YM. Physiology and complications of bed rest. J Am Geriatr Soc. 1988. November;36(11):1047-54. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008. August;248(2):189-98. [DOI] [PubMed] [Google Scholar]
- 3.Swope R, Adams A. Prevention and treatment of orthostatic hypotension in the orthopedic patient population. Smith KM, editor. Orthopedics. 2012. July 1;35(7):600-3. [DOI] [PubMed] [Google Scholar]
- 4.Jans Ø, Mehlsen J, Kjærsgaard-Andersen P, Husted H, Solgaard S, Josiassen J, Lunn TH, Kehlet H. Oral midodrine hydrochloride for prevention of orthostatic hypotension during early mobilization after hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Anesthesiology. 2015. December;123(6):1292-300. [DOI] [PubMed] [Google Scholar]
- 5.Jans Ø, Bundgaard-Nielsen M, Solgaard S, Johansson PI, Kehlet H. Orthostatic intolerance during early mobilization after fast-track hip arthroplasty. Br J Anaesth. 2012. March;108(3):436-43. Epub 2011 Dec 15. [DOI] [PubMed] [Google Scholar]
- 6.Memtsoudis SG, Valle AGD, Jules-Elysse K, Poultsides L, Reid S, Starcher B, Ma Y, Sculco TP. Perioperative inflammatory response in total knee arthroplasty patients: impact of limb preconditioning. Reg Anesth Pain Med. 2010. Sep-Oct;35(5):412-6. [DOI] [PubMed] [Google Scholar]
- 7.Yassin MMI, Harkin DW, Barros D’Sa AAB, Halliday MI, Rowlands BJ. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. 2002. January;26(1):115-21. Epub 2001 Oct 25. [DOI] [PubMed] [Google Scholar]
- 8.Chen XX, Wang T, Li J, Kang H. Relationship between inflammatory response and estimated complication rate after total hip arthroplasty. Chin Med J (Engl). 2016. November 5;129(21):2546-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannoudis PV, Harwood PJ, Loughenbury P, Van Griensven M, Krettek C, Pape HC. Correlation between IL-6 levels and the systemic inflammatory response score: can an IL-6 cutoff predict a SIRS state? J Trauma. 2008. September;65(3):646-52. [DOI] [PubMed] [Google Scholar]
- 10.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007. January;81(1):1-5. Epub 2006 Oct 10. [DOI] [PubMed] [Google Scholar]
- 11.Bonanno FG. Clinical pathology of the shock syndromes. J Emerg Trauma Shock. 2011. April;4(2):233-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frith J, Parry SW. New Horizons in orthostatic hypotension. Age Ageing. 2017. March 1;46(2):168-74. [DOI] [PubMed] [Google Scholar]
- 13.Smith W, Wan H, Much D, Robinson AG, Martin P. Clinical benefit of midodrine hydrochloride in symptomatic orthostatic hypotension: a phase 4, double-blind, placebo-controlled, randomized, tilt-table study. Clin Auton Res. 2016. August;26(4):269-77. Epub 2016 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitson MR, Mo E, Nabi T, Healy L, Koenig S, Narasimhan M, Mayo PH. Feasibility, utility, and safety of midodrine during recovery phase from septic shock. Chest. 2016. June;149(6):1380-3. Epub 2016 Mar 4. [DOI] [PubMed] [Google Scholar]