Abstract
Phthalates, compounds commonly used in plastics and personal care products, have been associated with childhood obesity in cross-sectional and some longitudinal studies. Using advanced statistical methods, we characterized the heterogeneity in body mass development patterns over childhood (ages 2 to 14 years) and explored associations with maternal prenatal urinary concentrations of phthalates among 335 children in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort study. Height and weight were measured every one to two years in this cohort, which had a high prevalence of obesity and overweight. Building upon a previous analysis that showed a positive association between prenatal phthalate exposure and body mass index (BMI) in CHAMACOS children, we used three advanced statistical methods: generalized additive models, growth mixture models, and functional principal component analysis with tree-based methods to identify patterns of childhood BMI development and allow for non-linear relationships with the environmental exposures. Our results highlight the heterogeneity in childhood BMI development patterns and suggest a sex-specific non-linear association between prenatal monoethyl phthalate urinary concentrations and BMI level in children, confirmed across a variety of statistical methods. There is also evidence to suggest positive associations between DEHP metabolites and BMI stabilization during puberty for girls.
Keywords: prenatal, phthalate, trajectory, body mass index, development
1. Introduction
There is increasing evidence that certain endocrine disrupting chemicals (EDCs) may act as “obesogens”, chemicals that influence adipocyte differentiation, shift energy balance to favor fat storage, and/or alter mechanisms of appetite and satiety, leading to increased body mass (Katsikantami et al., 2016; Thayer et al., 2012). Early life may be a particularly critical window of exposure for obesogenic effects. Exposure to phthalates, EDCs commonly used in plastics and personal care products, is widespread. Cross-sectional studies show higher urinary phthalate concentrations among obese children (Buser et al., 2014; Trasande et al., 2013; Wang et al., 2013), but these findings may result from reverse causality and can be confounded by energy intake (Campbell et al., 2018), since diet is a significant route of exposure to some phthalates.
A number of prospective studies have examined prenatal phthalate exposure and subsequent body composition during childhood with mixed conclusions (Agay-Shay et al., 2015; Botton et al., 2016; Buckley et al., 2016a, 2016b; De Cock et al., 2014; Maresca et al., 2016; Shoaff et al., 2017; Vafeiadi et al., 2018; Valvi et al., 2015; Yang et al., 2017). In particular, we have previously shown that concentrations of several phthalates in maternal urine during pregnancy were associated with increased body mass index (BMI) of children between ages 5 to 12 years using marginal models (Harley et al., 2017). However, these analyses focused on individual associations at each age point and assumed linear relationships between the metabolite and BMI. Recent advances in statistical methodology and software provide improved approaches to explore and explain the variation in change over time as a longitudinal pattern while allowing for non-linear relationships with metabolite concentrations. In this paper, we use generalized additive models, growth mixture models, and functional principal component analysis with tree-based methods to accomplish this task.
Traditional longitudinal methods, such as linear mixed-effects (Laird and Ware, 1982) and marginal models (Liang and Zeger, 1986), are regression-based methods for estimating the mean outcome while accounting for repeated measures. Interaction terms with exposure can be used to accommodate linear effect modification. However, there is evidence to suggest that EDCs have a non-linear dose response (Lagarde et al., 2015; Vandenberg et al., 2012). While these methods can be modified to assess non-linear exposure-response relationships, non-linear effect modification is not straightforward and the methods do not focus on developmental trajectory patterns.
Generalized additive models (GAM) (Hastie and Tibshirani, 1990) extend linear models by allowing non-linear, smooth relationships between predictors such as metabolite concentration and cross-sectional outcomes using piecewise polynomials (i.e. splines). These models focus on individual associations at age points but can capture complex relationships between exposure and body mass at each age that may be undetected in linear regression models.
Growth mixture model (GMM) (Muthén and Shedden, 1999) can be used to discover distinct longitudinal patterns by estimating group-specific regression parameters while allowing non-monotonic relationships between baseline exposures and group membership. Like GAM’s, basis splines (De Boor, 1976, 1972) can be used as explanatory variables to accommodate non-linear relationships between the response and time in these models (Grajeda et al., 2016; Liem et al., 2013).
Functional principal component analysis (FPCA) (Ramsay and Silverman, 2005, 2002) is a non-parametric approach that can be used to explore the variation in childhood development (Zhang et al., 2017). FPCA, similar to multivariate principal component analysis, provides a set of functions that explain the most variation in the observed trajectories with corresponding subject-specific score coefficients that measure their deviation from the mean functional pattern. Regression models, regression trees, and random forests (Breiman, 2001; Breiman et al., 1984) can be used in conjunction with FPCA to assess relationships between estimated score coefficients and covariates.
In the present analysis, we build on our previous work of prenatal phthalate exposure and childhood BMI from 5 to 12 years (Harley et al., 2017) in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), a prospective birth cohort of low-income primarily Mexican American children with high rates of obesity (Rosas et al., 2011). We introduce, apply, and compare three different statistical methodologies (GAMs, growth mixture models, and FPCA paired with tree-based methodology) to characterize the heterogeneity of childhood BMI development trajectories and explore the association of prenatal phthalate exposure childhood BMI, extended now up to included data from age 14 years.
2. Methods
2.1. Study population and data collection
Pregnant women (N=601) who were living in California’s Salinas Valley, an agricultural area with a large Latino population, were recruited to participate in the CHAMACOS study in 1999 and 2000. Eligible women were 18 years of age or older, spoke English or Spanish, were eligible for low income health insurance (Medicaid), were less than 20 weeks gestation, receiving prenatal care at partnering community clinics that served the farmworker population, and planning to deliver at the county hospital. During pregnancy, women completed interviewer-administered questionnaires and provided two urine samples for measurement of environmental exposures, including phthalates. At delivery, 536 women remained enrolled in the study, and we have conducted periodic follow-up visits with them and their children from infancy through adolescence.
The current analysis uses height and weight data collected at eleven follow-up visits conducted between ages 2 and 14 years (y). At each of these visits, child height was measured in triplicate to the nearest 0.1 cm using a wall-mounted stadiometer. Between age 2y and 7y, children were weighed using a Tanita Mother-Baby Scale (Model 1582) with shoes and coats removed. At ages 9y through 14y, youth were weighed standing barefoot (with coat removed) on a Tanita bioimpedance scale (Tanita TBF-300A Body Composition Analyzer). For purposes of weight calculations, clothing weights were estimated at 0.5 kg for ages 9y-12.75y, and at 1 kg at age 14y. We limited our analysis to 435 children with recorded prenatal phthalate measurements and of those, we focus on 335 children who had standing height and weight measurements at 4 or more visits between 2y and 14y. The analytical sample is not significantly different from the original cohort in terms of child sex and maternal characteristics such as pre-pregnancy BMI, diet and smoking status during pregnancy, gestational weight gain, years in the U.S., marital status, age, and education, but has a higher percentage of mothers that exclusively breastfed for more than 6 months (48% as compared to 40% in the original cohort). All study procedures were approved by the Office for the Protection of Human Subjects at UC Berkeley. Mothers provided written informed consent and parent permission pertaining to every study visit. Children provided formal verbal assent at each visit between 7y and 11y, and provided written assent for 12y to 14y visits.
2.2. Urinary phthalate measurement
Urine samples were obtained from the mothers at the time of the two pregnancy interviews (mean (SD): 14.0 (4.8) and 26.9 (2.5) weeks gestation) and were stored at −80°C until shipment to the Centers for Disease Control and Prevention in Atlanta, GA for analysis. Concentrations of 11 metabolites of 8 parent phthalate compounds were measured using solid phase extraction coupled with isotope dilution high performance liquid chromatography-electrospray ionization-tandem mass spectrometry (Silva et al., 2007). Phthalate metabolites quantified were mono-ethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), mono-isobutyl phthalate (MiBP), mono-benzyl phthalate (MBzP), mono(carboxyoctyl) phthalate (MCOP), mono(3-carboxypropyl) phthalate (MCPP) and four metabolites of diethyl-hexyl phthalate (DEHP): mono-2-ethylhexyl phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP). Limits of detection (LOD) ranged from 0.2–0.6 ng/mL. Concentrations below the limit of detection (LOD) were assigned an imputed value less than LOD randomly selected from the lognormal distribution using maximum likelihood estimation (Lubin et al., 2004). Urinary specific gravity was measured using a hand-held refractometer (National Instrument Company Inc., Baltimore, MD) and metabolite concentrations were corrected for urinary dilution using the formula: metabolite concentration * (mean specific gravity - 1)/(sample specific gravity – 1).
2.3. Statistical methods
Prenatal phthalate exposure was analyzed as the average of the two pregnancy phthalate metabolite concentrations, examined continuously as log2-transformed variables. The main outcomes of interest were BMI, calculated as weight/height2 (kg/m2), and BMI z-scores, obtained using the CDC growth charts, which are based on cross-sectional studies (U. S. Centers for Disease Control and Prevention, 2000). To focus on the similarity in development patterns regardless of level (e.g. high BMI or low BMI), for some analyses we also calculated centered BMI values by subtracting the mean of a child’s BMI measurements from each of their BMI values.
To account for potential confounding between maternal phthalate concentrations and BMI over time, we adjusted our models for the time-stable maternal variables considered in a previous study (Harley et al., 2017): pre-pregnancy BMI, smoking status during pregnancy (smoking vs. no smoking), gestational weight gain (lbs), diet quality index during pregnancy (score of 0–80) (Bodnar and Siega-Riz, 2002), number of years living in the United States (US), age, marital status (not married, married/living as married), and education (≤6th grade, 7th–12th grade, high school graduate) at the time of birth. To allow for sex-specific trajectories and associations, we stratified our models based on child’s sex (male/female). We used R for all of the analysis (R Core Team, 2018).
2.3.1. BMI trajectories
First, we graphically explored the individual trajectories for raw BMI, BMI z-score, and centered BMI using lowess for all children and for boys and girls separately (Cleveland, 1979).
We then used GMM to identify subgroups within the data with similar BMI or BMI z-score longitudinal patterns (Muthén and Shedden, 1999). The growth mixture model is a weighted sum of groups, each characterized with a regression model with their own mean time trend and covariance defined by random effects. We fit the models to the raw BMI, BMI z-score, and centered BMI data so as to detect heterogeneity in the development and the level (Heggeseth and Jewell, 2018). To allow for nonlinear trajectories, we used a piecewise quadratic B-spline with an internal knot at age 9.5 years for BMI and age four for BMI z-scores. Both knots were chosen to minimize the mean squared error when modeling individual trajectories and accommodate different patterns over time. The number of subgroups was chosen using model selection with Bayesian Information Criteria (BIC). GMM’s were estimated using maximum likelihood estimation with the “hlme” function in the “lcmm” package in R (Proust-Lima et al., 2014, p.).
We also examined BMI trajectories with FPCA, which can be used to detect the functional structure that explains the most variability in individual BMI trajectories over time. We approximate an individual trajectory using a linear combination of individual scores and principal component functions. A more thorough introduction to the method can be found in Ramsay and Silverman (Ramsay and Silverman, 2005, 2002). To implement FPCA, we used the “fpca.sc” function in the refund package in R and stratified by sex (Goldsmith et al., 2016).
2.3.2. Associations of phthalates with BMI over time
We first explored the variability in the association of prenatal urinary concentrations of the phthalate metabolites and BMI at various ages using GAMs (Hastie and Tibshirani, 1990). We allowed a non-linear relationship for prenatal maternal phthalate concentrations and years in the US and linear relationships for maternal pre-pregnancy BMI, smoking status, diet quality index in pregnancy, gestational weight gain, age, marital status, and education at delivery, stratifying by child sex. Separate models were constructed for each phthalate metabolite. To implement GAM, we used the “gam” function in the mgcv package in R (Wood, 2004).
Then, returning to the trajectory approach, we included prenatal maternal phthalate concentrations and covariates in the GMM to explain variation in BMI trajectory group membership. Due to model complexity, we constructed separate models for each metabolite and used a stepwise process with BIC to select covariates from the a priori list of possible confounders.
Finally, we used the FPCA scores as a form of numerical summary of the BMI trajectories. We first regressed the individual FPCA scores derived from BMI trajectories on each of the prenatal maternal phthalate concentrations and confounding variables, fitting a separate model for each principal component and stratifying for sex. We then explored associations with all 11 phthalate metabolites simultaneously, accounting for potential copollutant confounding, using tree-based methods to predict the FPCA scores derived from the BMI trajectories. Using the CART algorithm (Breiman et al., 1984), we generated a regression tree for each set of FPCA scores allowing all phthalate concentration measurements and confounding variables to be part of the construction of the sex-specific trees. This algorithm iteratively splits the data into two groups based on covariate and exposure values to create groups that are more homogenous in FPCA scores. While this process provides an interpretable tree, this algorithm is sensitive to small changes to the data because it chooses the splitting variable that minimizes the variance at each local split rather than choosing the set of splits that provide a global solution. To overcome the sensitivity of a single regression tree, we used the tree-based random forest algorithm (Breiman, 2001) to measure the importance of predictors in explaining variation in the scores, thus BMI trajectories.
3. Results
3.1. Sample characteristics
Table 1 describes the analytic sample of children and their mothers at baseline. Over half of the mothers were overweight or obese prior to pregnancy. The population was of relatively low educational status, with only 22.1% of mothers having completed high school, and most mothers were recent immigrants (mean residence in the US at time of pregnancy was 6 years).
Table 1.
Child and Maternal Baseline Characteristics of the Analytic Sample from the CHAMACOS Study.
| Characteristic | Summarya |
|---|---|
| Maternal pre-pregnancy BMI (kg/m2) | 26.4 (24.1, 30.4) |
| Maternal age at delivery (years) | 25.0 (22.0, 29.5) |
| Maternal residence in USA at delivery (years) | 6.0 (2.1, 11.0) |
| Maternal gestational weight gain (kg) | 29.0 (20.0, 37.0) |
| Maternal diet quality index in pregnancy (0–80) | 44.8(38.1, 52.2) |
| Maternal smoking status during pregnancy | |
| Non-smoker | 322 (96.1) |
| Smoker | 13 (3.9) |
| Maternal Education | |
| 6th grade or less | 146 (43.6) |
| 7th – 12th grade | 115 (34.3) |
| High School Graduate | 74 (22.1) |
| Maternal Marital Status | |
| Not Married | 58 (17.3) |
| Married/Living as Married | 277 (82.7) |
| Child sex | |
| Male | 162 (48.4) |
| Female | 173 (51.6) |
Continuous variables are summarized with median (Q1, Q3) and categorical variables are summarized with frequency, n (%).
MEP was the most prevalent phthalate in this analytic sample with a median prenatal concentration of 184 ng/mL (Q1, Q3: 81.8, 410.1 ng/mL). This was over nine times as high as the next highest metabolite, MnBP, with a median concentration of 20.7 ng/mL. A summary of the distribution of phthalate metabolite concentrations is found in Table S1.
3.2. BMI trajectories
Table 2 shows the mean age, BMI, and BMI z-scores with standard deviations for the children over the 11 visits. The average BMI increased with age, with the increases becoming steeper after age 5y. However, mean BMI z-score was fairly stable after age 2y.
Table 2.
Mean and Standard Deviation of Age, Body Mass Index (BMI), and BMI Z-scores of Analytic Sample at Visits in the CHAMACOS Study.
| Boys | Girls | ||||
|---|---|---|---|---|---|
| Visit | Age (years) | BMI (kg/m2) | BMI Z-score | BMI (kg/m2) | BMI Z-score |
|
2 year (n = 308) |
2.05 (0.09) | 17.45 (1.95) | 0.44 (1.17) | 17.31 (2.03) | 0.5 (1.19) |
|
3.5 years (n = 297) |
3.61 (0.18) | 17.69 (2.56) | 1.23 (1.38) | 17.55 (2.51) | 1.12 (1.08) |
|
5 years (n = 305) |
5.04 (0.17) | 17.81 (2.97) | 1.21 (1.27) | 17.92 (3.16) | 1.17 (0.98) |
|
7 years (n = 322) |
7.11 (0.23) | 18.85 (3.81) | 1.13 (1.04) | 18.99 (3.74) | 1.10 (1.00) |
|
9 years (n = 321) |
9.14 (0.22) | 20.80 (4.38) | 1.21 (0.98) | 20.72 (4.80) | 1.01 (1.12) |
|
9.75 years (n = 265) |
9.84 (0.14) | 21.5 6(4.71) | 1.24 (0.95) | 21.68 (5.25) | 1.07 (1.07) |
|
10.5 years (n = 305) |
10.6 (0.17) | 22.35 (5.04) | 1.23 (0.96) | 22.21 (5.57) | 1.01 (1.09) |
|
11.25 years (n = 289) |
11.33 (0.13) | 22.66 (4.93) | 1.20 (0.90) | 23.37 (5.94) | 1.09 (1.07) |
|
12 years (n = 321) |
11.98 (0.18) | 23.53 (5.26) | 1.23 (0.92) | 23.91 (6.09) | 1.08 (1.08) |
|
12.75 years (n = 291) |
12.78 (0.16) | 24.21 (5.57) | 1.22 (0.91) | 24.66 (6.46) | 1.07 (1.06) |
|
14 years (n = 315) |
14.17 (0.27) | 24.98 (6.20) | 1.09 (1.06) | 25.30 (6.43) | 1.00 (1.04) |
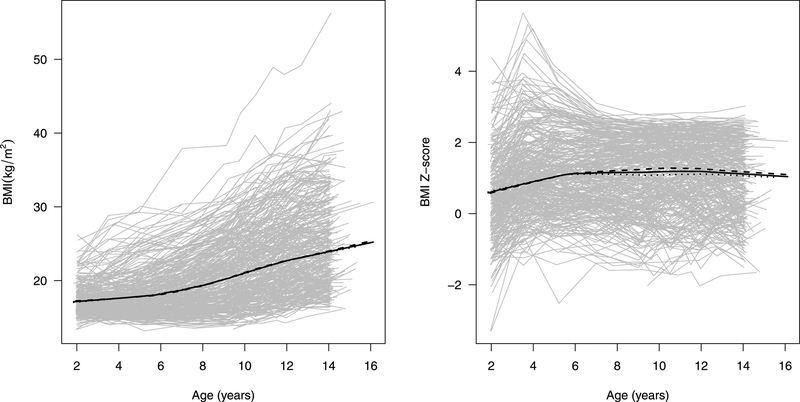
Figure 1 shows the BMI and BMI z-score trajectories for each individual child in gray with overall and sex-specific means over time, estimated using lowess, in black. There are no noticeable differences in average BMI between sexes during this age range. Consistent with Table 2, the overall mean BMI gradually increased between ages 2 and 14 with increased growth rate starting around age 5 – 6 years old. The individual BMI trajectories illustrate noticeable variability in development pattern with greater variability in later childhood. In contrast, the mean BMI z-score trajectory was relatively flat and was stable around 1.1, indicating that this sample had an average BMI about 1.1 standard deviation higher than the CDC growth charts. Looking at individual trajectories, we note that many individuals have a peak z-score value around age 4 before settling back to values between about −1 and 3.
Figure 1.
Individual BMI and BMI z-score trajectories between 2 and 14 years of age for children in analytic sample from the CHAMACOS study (in grey) with overlaid lowess estimates of the mean BMI over time (in black; solid: all, dashed: boys, dotted: girls).
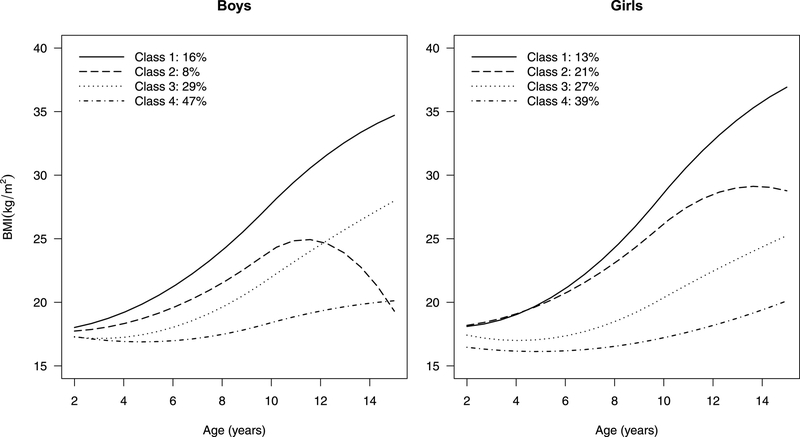
Figure 2 shows the four distinct mean trajectories for boys and girls identified using the centered BMI variable. We similarly estimated four-group GMM’s using BMI (Figure S1) and BMI z-scores (Figure S2), with a random intercept and no covariates. The group means for the centered BMI were similar to those from a model fit to the raw BMI data but were more homogeneous in development pattern. We note that 70–80% of the children were classified into groups with a relatively stable (Class 4) or a moderately increasing pattern (Class 3). About 15% of the children were classified with the steep linear increase in BMI (Class 1). A small proportion of the children had a BMI trajectory pattern that increased steeply in childhood but seemed to level off (girls) or decrease (boys) around puberty (Class 2).
Figure 2.
Sex-specific BMI mean trajectories determined by a four-group growth mixture model with a random intercept and no covariates, fit to centered BMI data on the analytic sample from the CHAMACOS study. Since the centered BMI has mean zero, we shifted the group mean trajectories so that the value at age two reflects the group mean BMI at age 2.
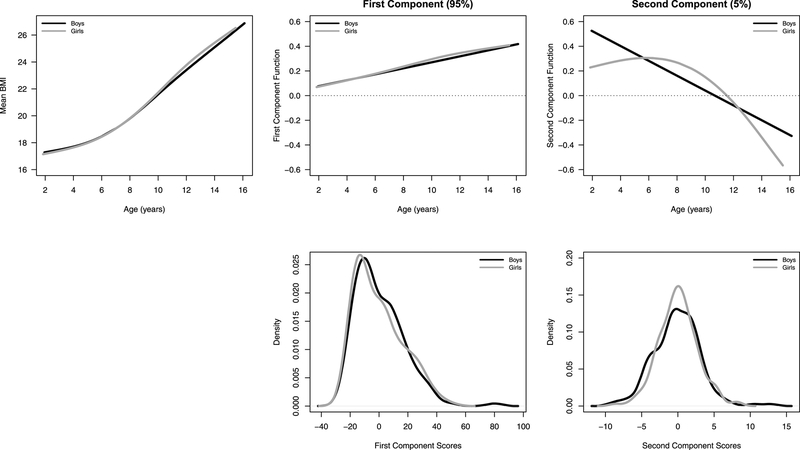
In Figure 3, we use FPCA to visualize the BMI trajectory in boys and girls, which can be decomposed into the mean trajectory plus the principal component functions that explain variation in BMI, weighted by their individual scores. Individuals with a high positive score for the first component would have a higher than average BMI level at age 2 as well as an increased rate of change in BMI across childhood. This first component scores can account for about 95% of the variation in the observed BMI data. The next 5% of variation in BMI is explained by a function that decouples the BMI level from the development rate. The second component function allows an increase in the starting level to be associated with a slight decrease in the growth rate. After accounting for the first component, individuals with a higher score for the second component would have increased BMI levels at age 2 with a lower than expected growth rates during early childhood resulting in a lower BMI at age 14.
Figure 3.
Estimated mean BMI trajectory across age for CHAMACOS data, the first two principal component functions, and the distribution of the estimated scores which weight the functions, stratified by sex (boys: black, girls: grey).
3.3. Association of phthalates and BMI trajectories
3.3.1. Generalized additive models
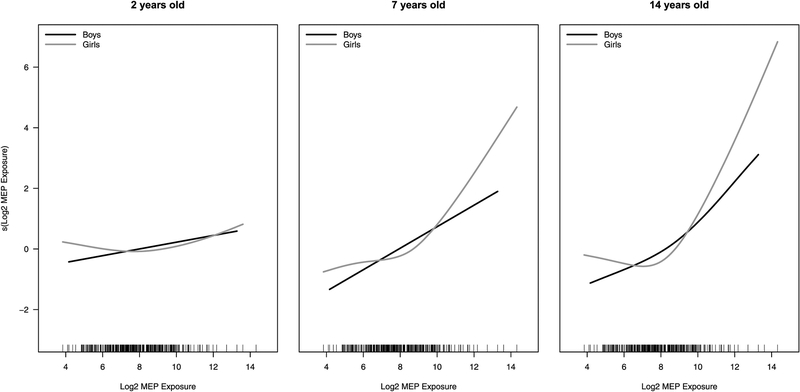
To measure the relationship between each phthalate metabolite concentration and BMI, we first fit a GAM for each age-specific visit stratifying by sex. Table 3 shows the results from models for MEP, the metabolite with the strongest association with BMI at various ages. Results for the models of the other metabolites are presented in Table S2. Table 3 suggests maternal pre-pregnancy BMI has a strong, positive linear association with a child’s BMI throughout childhood, as we also observed using more traditional statistical methodologies (Harley et al., 2017) although the association is stronger in boys than girls. The data suggest a significant sex-specific association (boys: negative, girls: positive) for maternal marital status at birth and BMI levels at ages 3.5y-9y. We observed significant positive, linear associations of prenatal maternal MEP exposure with BMI for boys at several points throughout early childhood and a non-linear association for girls starting at age 7y. Figure 4 includes the estimated association between MEP exposure with BMI at ages 2, 7 and 14 years for boys and girls. Among girls, we note a non-monotonic relationship among girls with a slightly negative or absence of an association with BMI at low levels of exposure and a positive association with middle to high levels of exposure.
Table 3.
Results from Sex and Age-Stratified Generalized Additive Models Predicting Child BMI with MEP for Analytic Sample of CHAMACOS Study.
| Boys | Girls | ||||
|---|---|---|---|---|---|
| Child Age | Variables | Coefficient | P-value | Coefficient | P-value |
| 2 years | Maternal BMI | 0.18 | <0.001b | 0.088 | 0.010 |
| Maternal Age | −0.039 | 0.249 | −0.041 | 0.215 | |
| Married | −0.854 | 0.045 | 0.618 | 0.173 | |
| 7–12th Grade | −0.003 | 0.993 | −0.663 | 0.074 | |
| > HS Grad | 0.373 | 0.408 | 0.165 | 0.720 | |
| Gestational Weight Gain | 0.014 | 0.326 | 0.025 | 0.070 | |
| Diet Quality Index | −0.027 | 0.132 | 0.009 | 0.586 | |
| Smoker | 0.743 | 0.415 | −0.002 | 0.998 | |
| s(MEP)a | NA | 0.233c | NA | 0.585 | |
| s(Years US) | NA | 0.331 | NA | 0.657 | |
| 3.5 years | Maternal BMI | 0.254 | O.001 | 0.133 | 0.001 |
| Maternal Age | −0.059 | 0.195 | −0.042 | 0.266 | |
| Married | −1.352 | 0.014 | 1.362 | 0.011 | |
| 7–12th Grade | 0.740 | 0.112 | −0.934 | 0.029 | |
| > HS Grad | −0.060 | 0.920 | −0.532 | 0.322 | |
| Gestational Weight Gain | 0.003 | 0.895 | 0.039 | 0.016 | |
| Diet Quality Index | −0.019 | 0.396 | 0.005 | 0.806 | |
| Smoker | −0.968 | 0.446 | 1.74 | 0.084 | |
| s(MEP) | NA | 0.065 | NA | 0.161 | |
| s(Years US) | NA | 0.091 | NA | 0.747 | |
| 5 years | Maternal BMI | 0.288 | O.001 | 0.183 | O.001 |
| Maternal Age | −0.007 | 0.887 | −0.056 | 0.244 | |
| Married | −1.264 | 0.048 | 1.568 | 0.020 | |
| 7–12th Grade | 0.461 | 0.395 | −0.76 | 0.165 | |
| > HS Grad | 0.272 | 0.691 | −0.194 | 0.775 | |
| Gestational Weight Gain | 0.016 | 0.439 | 0.056 | 0.005 | |
| Diet Quality Index | −0.027 | 0.301 | 0.009 | 0.713 | |
| Smoker | 0.455 | 0.734 | 1.223 | 0.343 | |
| s(MEP) | NA | 0.035 | NA | 0.163 | |
| s(Years US) | NA | 0.029 | NA | 0.980 | |
| 7 years | Maternal BMI | 0.321 | O.001 | 0.296 | O.001 |
| Maternal Age | 0.041 | 0.497 | −0.069 | 0.205 | |
| Married | −1.404 | 0.076 | 1.570 | 0.044 | |
| 7–12th Grade | 0.804 | 0.241 | −0.985 | 0.114 | |
| > HS Grad | −0.007 | 0.993 | −0.430 | 0.573 | |
| Gestational Weight Gain | 0.016 | 0.520 | 0.063 | 0.007 | |
| Diet Quality Index | −0.024 | 0.450 | 0.007 | 0.783 | |
| Smoker | 1.843 | 0.241 | −0.371 | 0.819 | |
| s(MEP) | NA | 0.039 | NA | 0.035 | |
| s(Years US) | NA | 0.024 | NA | 0.808 | |
| 9 years | Maternal BMI | 0.433 | O.001 | 0.464 | <0.001 |
| Maternal Age | 0.038 | 0.575 | −0.073 | 0.272 | |
| Married | −1.932 | 0.029 | 1.899 | 0.048 | |
| 7–12th Grade | 0.593 | 0.443 | −1.073 | 0.156 | |
| > HS Grad | 0.209 | 0.831 | −0.573 | 0.548 | |
| Gestational Weight Gain | 0.029 | 0.318 | 0.07 | 0.012 | |
| Diet Quality Index | −0.02 | 0.604 | 0.018 | 0.577 | |
| Smoker | 4.381 | 0.023 | 1.787 | 0.371 | |
| s(MEP) | NA | 0.070 | NA | 0.076 | |
| s(Years US) | NA | 0.022 | NA | 0.695 | |
| 11.25 years | Maternal BMI | 0.448 | O.001 | 0.58 | <0.001 |
| Maternal Age | 0.137 | 0.106 | −0.14 | 0.113 | |
| Married | −2.12 | 0.055 | 2.234 | 0.068 | |
| 7–12th Grade | 0.862 | 0.353 | −0.836 | 0.391 | |
| > HS Grad | 0.182 | 0.879 | −0.976 | 0.45 | |
| Gestational Weight Gain | 0.047 | 0.179 | 0.096 | 0.008 | |
| Diet Quality Index | −0.002 | 0.959 | 0.023 | 0.595 | |
| Smoker | 5.119 | 0.042 | 1.568 | 0.511 | |
| s(MEP) | NA | 0.225 | NA | 0.026 | |
| s(Years US) | NA | 0.048 | NA | 0.427 | |
| 14 years | Maternal BMI | 0.427 | 0.001 | 0.678 | <0.001 |
| Maternal Age | 0.091 | 0.387 | −0.161 | 0.073 | |
| Married | −2.062 | 0.151 | 1.271 | 0.308 | |
| 7–12th Grade | 1.621 | 0.170 | −0.786 | 0.421 | |
| > HS Grad | −0.755 | 0.621 | −1.161 | 0.356 | |
| Gestational Weight Gain | 0.035 | 0.431 | 0.104 | 0.005 | |
| Diet Quality Index | 0.008 | 0.885 | 0.007 | 0.864 | |
| Smoker | 5.925 | 0.063 | 2.191 | 0.376 | |
| s(MEP) | NA | 0.276 | NA | 0.059 | |
| s(Years US) | NA | 0.209 | NA | 0.441 | |
The s() indicates penalized regression splines to allow for non-linear relationships. The non-linear relationship cannot be summarized in one coefficient (NA = not available).
P-value for individual variables is for a Wald test on the parametric effects of each variable on the outcome (H0: coefficient is zero).
P-value for non-linear smooth functions is for a Wald-type test for whether there is any relationship with the outcome (H0: no relationship with outcome).
Figure 4.
Estimated association between prenatal MEP concentration and BMI level from the GAM models for age 2 (left), 7 (middle) and 14 years of age (right), separately for boys (black) and girls (grey).
3.3.2. Growth Mixture Models
Table 4 shows the group probability ratios estimated from the GMM’s for raw and centered BMI that include baseline phthalate exposure and maternal pre-pregnancy BMI (final covariates chosen with BIC) to model group membership, stratified by sex. Models were fit separately for each phthalate metabolite. The group labels correspond to the groups in Figure 2, with Class 4 being the group with the most stable BMI (i.e. the lowest increase over time). The group probability ratios are estimates of the multiplicative change in the probability of being in Class k for k=1, 2, 3 associated with a doubling of a metabolite concentration relative to the multiplicative change in probability for the reference group, Class 4. We found some evidence of associations of the metabolites of DEHP (i.e. MEHP, MEOHP, MECPP, and MEHHP) with different class membership. For raw BMI, the 95% confidence interval estimate suggests that a doubling of MECPP concentration was associated with between a 3% decrease and a 96% increase in the probability of being in Class 3 (moderately increasing BMI) relative to Class 4 (stable) for boys. After removing the level by centering the BMI, the intervals estimate for boys suggests that a doubling of MECPP concentration is associated with between a 15% decrease and a 71% increase in the probability of being in Class 3 relative to Class 4 (stable). For girls, increased MECPP concentration is associated with between a 6% decrease and 118% increase in the chance of being in Class 2 (large increase in BMI that levels off at puberty) relative to Class 4. Similar relationships are observed for prenatal concentrations of MEHP, MEHHP, and MEOHP among girls.
Table 4.
Group Probability Ratios (95% confidence intervals) for Doubling of In-utero Phthalate Exposure for Raw and Centered BMI Trajectory Groups Generated from Metabolite and Sex-Stratified Four-Group Growth Mixture Models fit to Analytic Sample of CHAMACOS Study.
| Boy | Girl | ||||
|---|---|---|---|---|---|
| Metabolite | Group | Ratioa (Raw BMI) |
Ratio (Centered BMI) |
Ratio (Raw BMI) |
Ratio (Centered BMI) |
| MnBP | Class 1 | NAb | 1.31 (0.90, 1.90) | NA | 1.06 (0.65, 1.72) |
| Class 2 | NA | 1.21 (0.72, 2.03) | NA | 1.13 (0.77, 1.66) | |
| Class 3 | NA | 1.07 (0.80, 1.43) | NA | 0.93(0.62, 1.40) | |
| Class 4 | NA | 1 | NA | 1 | |
| MEP | Class 1 | 1.13 (0.86, 1.50) | 1.00 (0.76, 1.30) | 1.37 (0.92, 2.04) | 1.12 (0.81, 1.57) |
| Class 2 | 1.28 (0.90, 1.82) | 0.95 (0.65, 1.39) | 1.23 (0.92, 1.63) | 1.19 (0.91, 1.55) | |
| Class 3 | 1.20 (0.94, 1.52) | 1.03 (0.82, 1.28) | 1.09 (0.85, 1.39) | 0.91 (0.71, 1.17) | |
| Class 4c | 1 | 1 | 1 | 1 | |
| MiBP | Class 1 | 0.99 (0.70, 1.42) | 1.06 (0.80, 1.41) | 1.05 (0.586, 1.88) | NA |
| Class 2 | 0.82 (0.51, 1.30) | 0.85 (0.52, 1.41) | 0.99 (0.70, 1.39) | NA | |
| Class 3 | 1.09 (0.84, 1.42) | 1.06 (0.83, 1.35) | 1.00 (0.76, 1.31) | NA | |
| Class 4 | 1 | 1 | 1 | NA | |
| MBzP | Class 1 | 0.89 (0.63, 1.27) | 1.03 (0.73, 1.46) | 1.22 (0.69, 2.16) | 1.10 (0.70, 1.73) |
| Class 2 | 1.03 (0.65, 1.62) | 0.88 (0.56, 1.40) | 1.20 (0.83, 1.74) | 1.07 (0.75, 1.54) | |
| Class 3 | 1.17 (0.88, 1.56) | 1.02 (0.78, 1.34) | 0.96 (0.71, 1.29) | 0.90 (0.65, 1.25) | |
| Class 4 | 1 | 1 | 1 | 1 | |
| MCNP | Class 1 | 0.85 (0.51, 1.42) | 0.98 (0.58, 1.66) | 0.68 (0.35, 1.33) | 0.69 (0.37, 1.30) |
| Class 2 | 0.70 (0.36, 1.35) | 0.56 (0.27, 1.14) | 1.07 (0.71, 1.62) | 1.14 (0.75, 1.73) | |
| Class 3 | 1.18 (0.76, 1.81) | 0.95 (0.62, 1.44) | 0.73‡ (0.51, 1.04) | 0.72‡ (0.50, 1.04) | |
| Class 4 | 1 | 1 | 1 | 1 | |
| MCOP | Class 1 | NA | 1.01 (0.66, 1.56) | 0.73 (0.35, 1.52) | 0.87 (0.50, 1.52) |
| Class 2 | NA | 1.17 (0.62, 2.22) | 1.03 (0.68, 1.56) | 0.97 (0.67, 1.42) | |
| Class 3 | NA | 1.00 (0.82, 1.22) | 0.70‡ (0.49, 1.02) | 0.67‡ (0.42, 1.07) | |
| Class 4 | NA | 1 | 1 | 1 | |
| MCPP | Class 1 | NA | 0.79 (0.54, 1.16) | 0.92 (0.53, 1.60) | 0.88 (0.53, 1.47) |
| Class 2 | NA | 1.16 (0.66, 2.03) | 0.75 (0.52, 1.07) | 0.78 (0.55, 1.10) | |
| Class 3 | NA | 1.21 (0.87, 1.68) | 0.99 (0.70, 1.42) | 0.91 (0.64, 1.28) | |
| Class 4 | NA | 1 | 1 | 1 | |
| MEHP | Class 1 | NA | NA | 1.04 (0.66, 1.66) | 1.06 (0.68, 1.65) |
| Class 2 | NA | NA | 1.45‡ (0.99, 2.12) | 1.38‡ (0.96, 1.99) | |
| Class 3 | NA | NA | 0.98 (0.63, 1.52) | 0.91 (0.66, 1.25) | |
| Class 4 | NA | NA | 1 | 1 | |
| MEHHP | Class 1 | NA | 0.84 (0.56, 1.24) | 1.12 (0.66, 1.89) | 1.17 (0.72, 1.93) |
| Class 2 | NA | 0.90 (0.54, 1.51) | 1.42‡ (0.96, 2.10) | 1.37 (0.93, 2.02) | |
| Class 3 | NA | 1.16 (0.85, 1.58) | 0.88 (0.59, 1.29) | 0.81 (0.57, 1.17) | |
| Class 4 | NA | 1 | 1 | 1 | |
| MECPP | Class 1 | 1.00 (0.84, 1.20) | 0.94 (0.59, 1.51) | 1.17 (0.68, 2.01) | 1.14 (0.66, 1.96) |
| Class 2 | 1.06 (0.60, 1.88) | 0.89 (0.47, 1.68) | 1.51‡ (0.98, 2.34) | 1.43‡ (0.94, 2.18) | |
| Class 3 | 1.38‡ (0.97, 1.96) | 1.20 (0.85, 1.71) | 0.95 (0.63, 1.43) | 0.87 (0.58, 1.32) | |
| Class 4 | 1 | 1 | 1 | 1 | |
| MEOHP | Class 1 | NA | 0.82 (0.55, 1.22) | 1.13 (0.67, 1.90) | 1.14 (0.69, 1.88) |
| Class 2 | NA | 0.90 (0.53, 1.51) | 1.42‡ (0.95, 2.10) | 1.36 (0.93, 2.00) | |
| Class 3 | NA | 1.14 (0.83, 1.55) | 0.91 (0.63, 1.31) | 0.82 (0.57, 1.18) | |
| Class 4 | NA | 1 | 1 | 1 | |
Models adjusted for maternal pre-pregnancy BMI (confounding variables chosen using BIC).
NA values indicate failure of estimation convergence within 500 iterations.
Class 4 is the reference group for every metabolite.
P≤0.01.
P≤0.05.
P≤0.10.
3.3.3. Functional Principal Components Analysis
Table 5 provides the estimated slopes of metabolites from a regression model predicting FPCA scores. MEP was positively associated the first component for boys and girls. For each doubling of maternal MEP concentration, the score for first component significantly increased by about 1.17 units for boys and 1.32 for girls, on average, suggesting both increased BMI level and development rate, after accounting for maternal pre-pregnancy BMI, gestational weight gain, diet quality index, smoking during pregnancy, education, marital status, age, and time in the US. There was no evidence to suggest an association with BMI for other metabolites using this method.
Table 5.
Estimated Coefficients (95% Confidence Intervals) from a Metabolite and Sex-Stratified Linear Regression Model Predicting Functional Principal Component Scores, PC1 and PC2, Derived from BMI Trajectories of Analytic Sample from CHAMACOS Study.
| Boy | Girl | |||
|---|---|---|---|---|
| Metabolite | PCI Scorea | PC2 Score | PCI Score | PC2 Score |
| MnBP | 1.18 (−0.63, 3.00) | 0.14 (−0.27, 0.55) | 0.57 (−1.38, 2.52) | −0.10 (−0.47, 0.27) |
| MEP | 1.17‡ (−0.24, 2.58) | 0.26‡ (−0.06, 0.58) | 1.32† (0.06, 2.58) | 0.13 (−0.12, 0.37) |
| MiBP | 0.04 (−1.57, 1.65) | −0.12 (−0.48, 0.24) | 0.22 (−1.45, 1.90) | −0.05 (−0.36, 0.27) |
| MBzP | 0.72 (−1.12, 2.57) | −0.11 (−0.52, 0.31) | 0.72 (−0.98, 2.43) | −0.09 (−0.41, 0.23) |
| MCNP | 1.12 (−1.64, 3.87) | 0.39 (−0.23, 1.01) | 0.48 (−1.43, 2.4) | 0.17 (−0.19, 0.53) |
| MCOP | 0.40 (−1.97, 2.76) | −0.23 (−0.76, 0.30) | −0.05 (−1.97, 1.86) | −0.18 (−0.55, 0.18) |
| MCPP | 0.11 (−1.88, 2.10) | 0.07 (−0.37, 0.52) | −1.14 (−2.94, 0.66) | −0.15 (−0.49, 0.19) |
| MEHP | 0.09 (−1.77, 1.95) | 0.14 (−0.28, 0.55) | 0.75 (−0.97, 2.46) | −0.01 (−0.34, 0.31) |
| MEHHP | 0.03 (−1.99, 2.05) | 0.01 (−0.45, 0.46) | 0.98 (−0.91, 2.88) | 0.00 (−0.36, 0.36) |
| MECPP | 0.2 (−2.18, 2.58) | −0.16 (−0.69, 0.38) | 1.46 (−0.63, 3.56) | −0.01 (−0.41, 0.39) |
| MEOHP | −0.13 (−2.17, 1.92) | −0.02 (−0.48, 0.45) | 1.1 (−0.82, 3.01) | 0.01 (−0.36, 0.37) |
Models adjusted for maternal pre-pregnancy BMI, gestational weight gain, diet quality index during pregnancy, smoking during pregnancy, education, marital status, age, and number of years in the US.
P≤0.01.
P≤0.05.
P≤0.10.
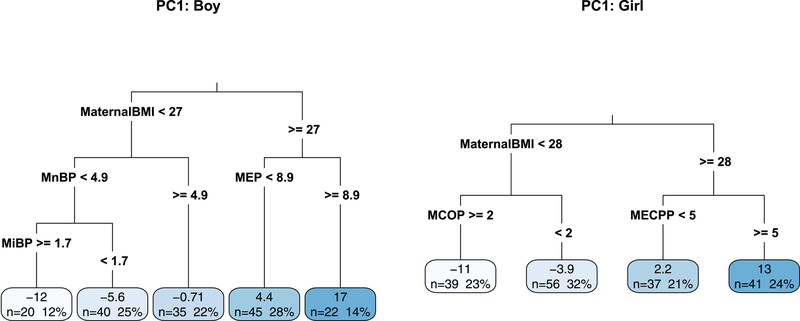
To allow for co-pollutant confounding, we explored relationships between BMI FPCA scores and all metabolites and covariates using regression trees, stratified by sex. Figure 5 shows two regression trees based on the scores for the first component (PC1). For both boys and girls, the tree-building algorithm selected maternal pre-pregnancy BMI as the first splitting variable as it explains the most variation in the childhood BMI trajectories. Once the children were split into groups with lower and higher maternal BMI, there were sex-specific differences in the next chosen split variable. Boys with lower maternal BMI were split based on their exposure to MnBP to create groups more homogeneous in terms of BMI level and development while MCOP reduced variability in BMI trajectory groups among girls with lower maternal BMI. In contrast, different levels of MECPP concentrations explain differences in BMI level and development among girls with mothers with higher BMI and MEP concentrations explain differences in BMI trajectories among boys with mothers with higher BMI. The tree for the second component is Figure S3. However, we want to be careful not to over interpret one regression tree because it cannot provide a full picture of all relationships. In the creation of the tree, if two variables explain about the same amount of variation in the scores, only one can be chosen at each split.
Figure 5.
Regression trees used to explain variation in the FPCA scores for the first component among the individual children of CHAMACOS. Terminal nodes are labels with count (n) and percentage of children as well as the group’s average principal component scores (higher values are associated with a higher starting BMI level and increased development rate). The first component summarizing the majority (~95%) of the variation in the BMI trajectories.
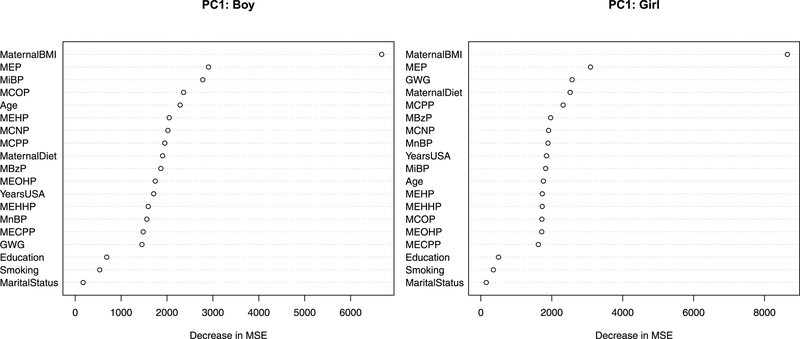
To overcome the limitations of a single tree, we generated a random forest to see which variables are playing a role in the variability in BMI level and development. Figure 6 provides a measure of variable importance calculated from the random forest algorithm defined as the total decrease in group variation in BMI trajectories from splitting on the variable as compared to when the variable values are randomly permuted. For both boys and girls, the variables that can explain the most variation in the first FPCA scores are maternal BMI and MEP. Next few variables in the ranked list are MiBP, MCOP, and maternal age for boys and gestational weight gain (GWG), maternal diet quality index, and MCPP for girls, all with similar values of importance.
Figure 6.
Variable importance plots based on node impurity measures from a random forest used to explain the variation in the FPCA scores for the first component (PC1) among children of CHAMACOS. The first component summarizing the majority (~95%) of the variation in the BMI trajectories and larger values of PC1 are associated with a higher starting BMI level and increased development rate.
Table 6 summarizes the findings of each of these advanced statistical models in addition to a brief description of the method and its strengths and weaknesses.
Table 6.
Summary of Advanced Statistical Methods and Findings based on Analytic Sample from CHAMACOS Study.
| Model Description | Strengths | Weaknesses | Our Findings | |
|---|---|---|---|---|
| Generalized Additive Models (GAM) | The average outcome is a linear combination of linear and nonlinear functions of exposures and covariates | Extends standard cross-sectional regression to allow for non-linear relationships between exposures and outcomes at individual ages | Cannot study longitudinal trajectories | MEP exposure is positively associated with child BMI for boys throughout childhood and in a nonmonotonic way for girls starting in late childhood. No consistently strong relationships detected for other metabolites. |
| Growth Mixture Models | Heterogeneity in outcome can be approximated with subpopulations with distinct average trajectories; group probabilities are modeled as a function of exposures and covariates | Allows for nonlinear relationships over time within groups and nonlinear relationships between exposures and outcome through group membership probabilities | High model complexity and thus reduced power to detect associations | Higher MECPP exposure associated with moderately increasing BMI trajectory for boys. DEHP metabolites associated with initial high increase in BMI that levels of at puberty in girls. |
| Functional Principal Component Analysis (FPCA) | Outcome trajectory over time can be decomposed as mean plus a linear combination of component functions that explain the most outcome variation. | Characterizes the variation in longitudinal outcome with component functions. | Component functions are not chosen to maximize association with exposure. | 95% of variation in BMI can be explained by understanding that higher starting levels are positively associated with higher growth rates. |
| Regression Trees | Extends standard cross-sectional regression to allow for non-linear relationships and potential interactions. | Data-driven variable selection in the process of creating the tree and easy interpretation. | One single tree is sensitive to small changes in the data. | MCOP and MECPP can explain variation in BMI trajectories among girls; MEP and MnBP can explain variation in BMI trajectories among boys. |
| Random Forest | Overcomes issues of one regression tree by creating many trees, each fit to a perturbed bootstrap sample of the original data with restriction of splitting variables to a random subset. | Robust variable importance measured as the change in average prediction error after randomly permuting variable values. | Not as interpretable as a single tree. | MEP can explain variation in BMI trajectories among boys and girls; GWG, maternal diet, and MCPP (girls) and MiBP, MCOP, and maternal age (boys) can next explain the most variation in the BMI trajectories. |
4. Discussion
Our previous study used traditional linear models and found that prenatal urinary concentrations of MEP, and to a certain extent MBP and DEHP metabolites, were associated with increased BMI in boys and girls through 12 years of age. The present study used more sophisticated methods to examine longitudinal BMI trajectories between age 2 and 14 years. Using GAMs, we found that higher prenatal urinary concentrations of MEP metabolites were significantly associated with BMI levels as children get older, but that relationship may not be linear. For girls, we see suggestions of a non-monotonic association with negative to zero association for lower MEP concentrations and a positive association with BMI levels for middle to high MEP concentrations at age 14. For 14-year-old boys, the association was linear and positive for MEP concentrations. Using GMM’s, we characterized the heterogeneity of childhood BMI trajectories by creating data-driven groups and estimated a weak, positive relationship between an increasing then stabilizing BMI development pattern and prenatal concentrations of DEHP metabolites for girls. To allow for sex-specific relationships, we stratified the estimation of the models, which reduced our sample size and reduced our power to detect statistically significant results in these models. Using scores estimated with functional principal component analysis, significant relationships were estimated between MEP prenatal concentrations and the first principal component scores that can explain 95% of the variability in the BMI trajectories.
We estimate different associations with these three different statistical methods. Extending standard regression models, GAMs allow for the detection of possible non-linear associations at a particular age, but it does not directly study BMI trajectories over time. Growth mixture models can detect frequent development patterns over time and then estimate associations with baseline exposures. While the GMM can flexibly estimate non-linear relationships using its group structure (Heggeseth and Aleman, 2018), the complexity of the model reduces our power to detect weak signals. Lastly, FPCA provides summary measures of trajectories that optimize explained variation in BMI but those univariate summaries may not differentiate a particular trajectory pattern that has an association with prenatal phthalate exposure. Considering all of the results, there is evidence to suggest weak, non-linear, sex-specific relationships between BMI level and development and prenatal MEP concentrations for both boys and girls. However, the evidence also suggests that the DEHP metabolites may also have with weak sex-specific associations with more subtle BMI patterns, including associations with growth trajectories of intermediate BMI increases in boys or with BMI increases in early childhood that level off in puberty in girls. Larger scale studies are needed to tease out these weak signals.
To the authors’ knowledge, no other study has used these statistical methods to investigate the relationship between prenatal phthalate concentrations and childhood BMI trajectories. Other investigations have studied prenatal urinary phthalate metabolite concentrations and childhood weight and body mass over time (Botton et al., 2016; Buckley et al., 2016a, 2016b; De Cock et al., 2014; Harley et al., 2017; Maresca et al., 2016; Vafeiadi et al., 2018; Valvi et al., 2015), but most of them did not study children through adolescence and used standard least squares regression, mixed-effects models, or marginal models estimated with generalized estimating estimations. Our findings are generally consistent with Harley et al. and Botton et al. (Botton et al., 2016; Harley et al., 2017), who reported a positive relationship between concentrations of MEP and higher BMI. In terms of development patterns, Botton et al. (Botton et al., 2016) found MEP concentrations to be positively associated with growth velocity between age 2 and 5 years old, which is similar to our finding of a positive relationship between MEP concentrations and BMI using the first functional principal component, which is a summary measure of BMI level and development from age 2 to 14. However, it should be noted that most other studies have failed to find positive associations of prenatal phthalate metabolites and childhood BMI, and several studies have found inverse associations with certain phthalate metabolites (Buckley et al., 2016a, 2016b; Maresca et al., 2016; Shoaff et al., 2017; Vafeiadi et al., 2018; Valvi et al., 2015; Yang et al., 2017), which are not consistent with our findings.
Our findings that higher concentrations of several DEHP metabolites were associated with a specific BMI trajectory (i.e. high early childhood weight gain that levels off in puberty) in girls is novel. Because other studies have looked only for associations with higher BMI overall, they lack the ability to detect associations with specific longitudinal BM patterns. Several other studies have observed interaction by sex, with suggestions of different associations of prenatal phthalate exposure and body mass in girls compared to boys (Buckley et al., 2016a; Maresca et al., 2016; Valvi et al., 2015; Yang et al., 2017).
It is plausible that phthalates may impact weight gain differently in boys versus girls due to the endocrine disrupting properties of these compounds (Takeuchi et al., 2005). The main hypothesized biological mechanism by which early life phthalate exposure might influence BMI development in childhood is via activation of peroxisome proliferator-activated receptors (PPAR) (Desvergne et al., 2009). Several phthalates have been shown to activate PPAR-α and PPAR-γ receptors, which are key regulators of fatty acid oxidation, fat storage, and adipogenesis (Hurst and Waxman, 2003). Additionally, exposure to some phthalates has been associated with decreased thyroid hormone levels in rodents (Lv et al., 2016) and humans (Boas et al., 2010) which can impact metabolism and body weight.
As summarized in Harley et al. (Harley et al., 2017), there are many possible reasons for inconsistent findings across studies, including the difficulty in measuring cumulative phthalate exposure over the course of pregnancy, the high variability and short half-lives of urinary phthalate metabolites, and differences in timing of exposure and outcomes. A limitation of this study is that measures of phthalate exposure during childhood were not available. Another reason for inconsistent findings is due to the statistical methods used and the differences in what type of associations they can estimate.
4.1. Conclusions
As compared to previous research, we allowed for non-linear associations between prenatal phthalate concentrations and BMI trajectories using three different advanced statistical methods. Our results highlight the heterogeneity and non-linearity in childhood BMI development patterns, suggest a sex-specific association between prenatal DEHP exposure and BMI development around puberty, and support a non-linear, sex-specific association between prenatal MEP exposure and BMI levels, confirmed across a variety of advanced statistical methods.
Supplementary Material
Highlights:
Phthalates as possible obesogens may explain variability in childhood BMI
Advanced statistical methods allow non-linear relationship with BMI over time
Prenatal MEP positively associated with BMI level across ages
Prenatal DEHP positively associated with increasing and then stabilizing BMI
Acknowledgements
We thank three anonymous reviewers for helpful comments on an earlier draft of the manuscript. We’d also like to thank all of the participants and hard-working staff of the CHAMACOS study.
Funding:
Research reported in this article was supported by the National Institute of Environmental Health Science of the National Institutes of Health under award numbers P01ES009605, R01ES021369, R01ES017054, R24ES028529, 1RC2 ES018792 and R01ES015572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This article was developed under Assistance Agreement No. R82670901, RD83171001, and RD83451301 awarded by the U.S. Environmental Protection Agency. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication by the EPA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Agay-Shay K, Martinez D, Valvi D, Garcia-Esteban R, Basagaña X, Robinson O, Casas M, Sunyer J, Vrijheid M, 2015. Exposure to endocrine-disrupting chemicals during pregnancy and weight at 7 Years of age: A multi-pollutant approach. Environ. Health Perspect 123, 1030–1037. 10.1289/ehp.1409049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebæk NE, Hegedüs L, Hilsted L, Juul A, Main KM, 2010. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ. Health Perspect 118, 1458–1464. 10.1289/ehp.0901331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar LM, Siega-Riz AM, 2002. A Diet Quality Index for Pregnancy detects variation in diet and differences by sociodemographic factors. Public Health Nutr. 5, 801–809. 10.1079/PHN2002348 [DOI] [PubMed] [Google Scholar]
- Botton J, Philippat C, Calafat AM, Carles S, Charles M-A, Slama R, The Eden Mother-Child Cohort Study Group, 2016. Phthalate pregnancy exposure and male offspring growth from the intra-uterine period to five years of age. Environ. Res 151, 601–609. 10.1016/j.envres.2016.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L, 2001. Random forests. Mach. Learn 45, 5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- Breiman L, Friedman J, Stone CJ, Olshen RA, 1984. Classification and regression trees. CRC press. [Google Scholar]
- Buckley JP, Engel SM, Braun JM, Whyatt RM, Daniels JL, Mendez MA, Richardson DB, Xu Y, Calafat AM, Wolff MS, Lanphear BP, Herring AH, Rundle AG, 2016a. Prenatal phthalate exposures and body mass index among 4- to 7-Year-old Children: A pooled analysis. Epidemiology 27, 449–458. 10.1097/EDE.0000000000000436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Engel SM, Mendez MA, Richardson DB, Daniels JL, Calafat AM, Wolff MS, Herring AH, 2016b. Prenatal phthalate exposures and childhood fat mass in a New York City cohort. Environ. Health Perspect 124, 507–513. 10.1289/ehp.1509788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser MC, Murray HE, Scinicariello F, 2014. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007–2010. Int. J. Hyg. Environ. Health 217, 687–694. 10.1016/j.ijheh.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL, Yoon M, Ward PL, Fromme H, Kessler W, Phillips MB, Anderson WA, Clewell HJ, Longnecker MP, 2018. Excretion of Di-2-ethylhexyl phthalate (DEHP) metabolites in urine is related to body mass index because of higher energy intake in the overweight and obese. Environ. Int 113, 91–99. 10.1016/j.envint.2018.01.023 [DOI] [PubMed] [Google Scholar]
- Cleveland WS, 1979. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc 74, 829–836. 10.2307/2286407 [DOI] [Google Scholar]
- De Boor C, 1976. Splines as linear combinations of B-splines. A survey, in: Lorentz GG, Chui CK, Schumaker LL (Eds.), Approximation Theory II. Academic Press, New York, pp. 1–47. [Google Scholar]
- De Boor C, 1972. On calculating with B-splines. J. Approx. Theory 6, 50–62. [Google Scholar]
- De Cock M, De Boer MR, Lamoree M, Legler J, Van de Bor M, 2014. First Year Growth in Relation to Prenatal Exposure to Endocrine Disruptors — A Dutch Prospective Cohort Study. Int. J. Environ. Res. Public. Health 11, 7001–7021. 10.3390/ijerph110707001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvergne B, Feige JN, Casals-Casas C, 2009. PPAR-mediated activity of phthalates: A link to the obesity epidemic? Mol. Cell. Endocrinol 304, 43–48. 10.1016/j.mce.2009.02.017 [DOI] [PubMed] [Google Scholar]
- Goldsmith J, Scheipl F, Huang L, Wrobel J, Gellar J, Harezlak J, McLean MW, Swihart B, Xiao L, Crainiceanu C, Reiss PT, 2016. refund: Regression with Functional Data.
- Grajeda LM, Ivanescu A, Saito M, Crainiceanu C, Jaganath D, Gilman RH, Crabtree JE, Kelleher D, Cabrera L, Cama V, Checkley W, 2016. Modelling subject-specific childhood growth using linear mixed-effect models with cubic regression splines. Emerg. Themes Epidemiol 13, 1 10.1186/s12982-015-0038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Berger K, Rauch S, Kogut K, Claus Henn B, Calafat AM, Huen K, Eskenazi B, Holland N, 2017. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatr. Res 82, 405–415. 10.1038/pr.2017.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ, 1990. Generalized Additive Models. Chapman & Hall/CRC. [Google Scholar]
- Heggeseth BC, Aleman A, 2018. Early-life environmental exposures and childhood growth: A comparison of statistical methods. PLOS ONE 13, e0209321 10.1371/journal.pone.0209321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeseth BC, Jewell NP, 2018. How Gaussian mixture models might miss detecting factors that impact growth patterns. Ann. Appl. Stat 12, 222–245. 10.1214/17-AOAS1066 [DOI] [Google Scholar]
- Hurst CH, Waxman DJ, 2003. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol. Sci. Off. J. Soc. Toxicol 74, 297–308. 10.1093/toxsci/kfg145 [DOI] [PubMed] [Google Scholar]
- Katsikantami I, Sifakis S, Tzatzarakis MN, Vakonaki E, Kalantzi OI, Tsatsakis AM, Rizos AK, 2016. A global assessment of phthalates burden and related links to health effects. Environ. Int 97, 212–236. 10.1016/j.envint.2016.09.013 [DOI] [PubMed] [Google Scholar]
- Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, Guerbet M, Rousselle C, 2015. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ. Health Glob. Access Sci. Source 14, 13 10.1186/1476-069X-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH, 1982. Random-effects models for longitudinal data. Biometrics 38, 963–974. 10.2307/2529876 [DOI] [PubMed] [Google Scholar]
- Liang K-Y, Zeger SL, 1986. Longitudinal data analysis using generalized linear models. Biometrika 73, 13–22. 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- Liem ET, van Buuren S, Sauer PJJ, Jaspers M, Stolk RP, Reijneveld SA, 2013. Growth during infancy and childhood, and adiposity at age 16 years: Ages 2 to 7 years are pivotal. J. Pediatr 162, 287–292.e2. 10.1016/j.jpeds.2012.07.053 [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P, 2004. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environ. Health Perspect 112, 1691–1696. 10.1289/ehp.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z, Cheng J, Huang S, Zhang Y, Wu S, Qiu Y, Geng Y, Zhang Q, Huang G, Ma Q, Xie X, Zhou S, Wu T, Ke Y, 2016. DEHP induces obesity and hypothyroidism through both central and peripheral pathways in C3H/He mice. Obes. Silver Spring Md 24, 368–378. 10.1002/oby.21359 [DOI] [PubMed] [Google Scholar]
- Maresca MM, Hoepner LA, Hassoun A, Oberfield SE, Mooney SJ, Calafat AM, Ramirez J, Freyer G, Perera FP, Whyatt RM, Rundle AG, 2016. Prenatal exposure to phthalates and childhood body size in an urban cohort. Environ. Health Perspect 124, 514–520. 10.1289/ehp.1408750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B, Shedden K, 1999. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics 55, 463–469. 10.1111/j.0006-341X.1999.00463.x [DOI] [PubMed] [Google Scholar]
- Proust-Lima C, Philipps V, Diakite A, Liquet B, 2014. lcmm: Estimation of extended mixed models using latent classes and latent processes.
- R Core Team, 2018. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Ramsay JO, Silverman BW, 2005. Functional Data Analysis, 2nd ed. Springer, New York. [Google Scholar]
- Ramsay JO, Silverman BW, 2002. Applied Functional Data Analysis: Methods and Case Studies. Springer, New York. [Google Scholar]
- Rosas LG, Guendelman S, Harley K, Fernald LCH, Neufeld L, Mejia F, Eskenazi B, 2011. Factors associated with overweight and obesity among children of mexican descent: Results of a binational study. J. Immigr. Minor. Health 13, 169–180. 10.1007/s10903-010-9332-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaff J, Papandonatos GD, Calafat AM, Ye X, Chen A, Lanphear BP, Yolton K, Braun JM, 2017. Early-Life Phthalate Exposure and Adiposity at 8 Years of Age. Environ. Health Perspect 125, 097008 10.1289/EHP1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM, 2007. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B 860, 106–112. 10.1016/j.jchromb.2007.10.023 [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, Kojima H, 2005. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors alpha and beta, and androgen receptor. Toxicology 210, 223–233. 10.1016/j.tox.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA, 2012. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ. Health Perspect 120, 779–789. 10.1289/ehp.1104597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Sathyanarayana S, Spanier AJ, Blustein J, 2013. Race/ethnicity–specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ. Health Perspect 121, 501–506. 10.1289/ehp.1205526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U. S. Centers for Disease Control and Prevention, 2000. CDC growth charts: United States.
- Vafeiadi M, Myridakis A, Roumeliotaki T, Margetaki K, Chalkiadaki G, Dermitzaki E, Venihaki M, Sarri K, Vassilaki M, Leventakou V, Stephanou EG, Kogevinas M, Chatzi L, 2018. Association of Early Life Exposure to Phthalates With Obesity and Cardiometabolic Traits in Childhood: Sex Specific Associations. Front. Public Health 6 10.3389/fpubh.2018.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, Sunyer J, Vrijheid M, 2015. Prenatal phthalate exposure and childhood growth and blood pressure: Evidence from the Spanish INMA-Sabadell birth cohort study. Environ. Health Perspect 123, 1022–1029. 10.1289/ehp.1408887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP, 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev 33, 378–455. 10.1210/er.2011-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou Y, Tang C, He Y, Wu J, Chen Y, Jiang Q, 2013. Urinary phthalate metabolites are associated with body mass index and waist circumference in Chinese school children. PloS One 8, e56800 10.1371/journal.pone.0056800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN, 2004. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc 99, 673–686. 10.1198/016214504000000980 [DOI] [Google Scholar]
- Yang TC, Peterson KE, Meeker JD, Sánchez BN, Zhang Z, Cantoral A, Solano M, Tellez-Rojo MM, 2017. Bisphenol A and phthalates in utero and in childhood: association with child BMI z-score and adiposity. Environ. Res 156, 326–333. 10.1016/j.envres.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou J, Niu F, Donowitz JR, Haque R, Petri WA, Ma JZ, 2017. Characterizing early child growth patterns of height-for-age in an urban slum cohort of Bangladesh with functional principal component analysis. BMC Pediatr. 17 10.1186/s12887-017-0831-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.