Abstract
The approach of tethering together two known receptor ligands, to be used as molecular probes for the study of G protein-coupled receptor (GPCR) systems, has proven to be a valuable approach. Selective ligands that possess functionality that can be used to link to other ligands, are useful in the development of novel antagonists and agonists. Such molecules can also be attached to reporter molecules, such as fluorophores, for the study of GPCR dimerization and its role in signaling. The highly selective serotonin (5-HT) 5-HT2A receptor (5-HT2AR) antagonist M100907 (volinanserin) is of clinical interest in the treatment of neurological and mental health disorders. Here, we synthesized the most active (+)-M100907 enantiomer as well as a series of derivatives that possessed either an alkyne or an azide. The triazole resulting from the dipolar cycloaddition of these groups did not interfere with the ability of the bivalent ligand to act as an antagonist. Thus, we have synthesized a number of compounds which will prove useful in elucidating the role of the 5-HT2AR in the central nervous system.
Keywords: Serotonin, M100907, Volinanserin, Bivalent
M100907 (1), also known as volinanserin, is a highly selective 5-HT2AR receptor antagonist developed initially by Sanofi-Aventis for the treatment of schizophrenia1 and sleep disorders.2 Our group reported that M100907 derivatives substituted at the methoxy group of the catechol ring retain the 5-HT2AR antagonist activity with either the ketone (2) or racemic hydroxyl group (3) at the benzylic position.3,4 Reported here is the installation of a polyethylene glycol (PEG) linker substituted at the methoxy group of M100907 and the chiral resolution of the molecule to provide a version of M100907 possessing an ether tether that is terminated with an azide (4) or an alkyne (5). The azide on (4) will be used for future connection to other molecules such as fluorophores, affinity tags and other receptor ligands (Fig. 1).5,6
Fig. 1.
Structures of M100907 (1) and derivatives (2–5) which retained 5-HT2AR antagonist properties.
The synthetic route to M100907 developed by Rice7 was utilized, however, the chiral resolution was carried out at an earlier stage to provide the possibility of introducing different substituents onto the piperidinyl group. The conditions for this resolution were different from previously reported.7
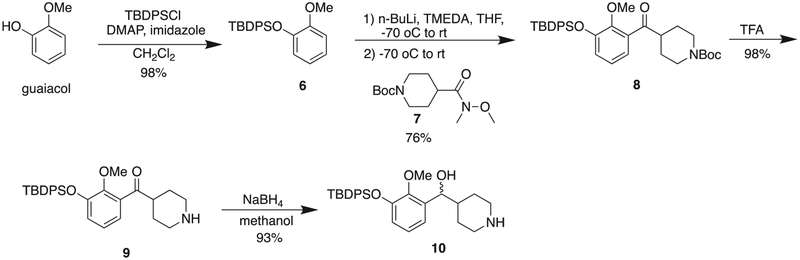
The route to M100907-azide 4, began with the protection of commercially available guaiacol (Scheme 1). Guaiacol was reacted with TBDPSCl, imidazole and catalytic amount of DMAP at room temperature for 24 h to generate compound 6 in 98% yield. The silyl protected 6 was regioselectively ortho-lithiated by n-butyl lithium with TMEDA for 2 h at room temperature. Then Weinreb amide 7 was added at −70 °C and the mixture was stirred at room temperature for 21 h to produce the ketone 8. Following reaction with the lithiated 6, the Boc group of 8 was removed with TFA to give 9. Sodium borohydride reduction then provided the racemic alcohol 10 (Scheme 1).
Scheme 1.
Synthesis of the precursor 10 of M100907 derivative 4.
Weinreb amide 7 was synthesized from isonipecotic acid (Scheme 2). BOC protection of isonipecotic acid by reaction of ditertbutyldicarbonate, in a mixed solvent of 1,4-dioxane, acetonitrile and water in the presence of 1 N NaOH gave compound 12 which was then reacted with N,O-dimethylhydroxylamine hydrochloride, and the coupling reagent HBTU, in the presence of DIPEA to give the desired Weinreb amide 7 in ~80% yield over the two steps.
Scheme 2.
Synthesis of compound 7.
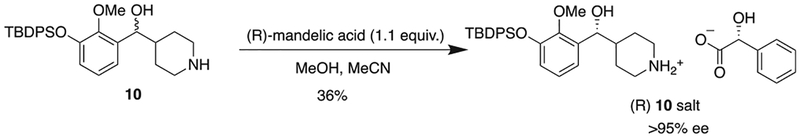
For the chiral resolution of a later synthetic intermediate by the Rice group,7 methanol was used as the solvent to obtain the resolved salt with (R)-mandelic acid. However, the solubility of the diastereomeric salt formed from compound 10 and (R)-mandelic acid was sufficiently high in methanol that the yield of recovered material was low. Using a 1:1 ratio of acetonitrile:methanol for the first recrystallization and 1:2 ratio for the second recrystallization, provided one diastereomeric salt in 36% overall yield as white crystals (Scheme 3). Aqueous workup of the diastereomeric salt with ammonium hydroxide afforded the enantiomer in a purity of >95% ee (Fig. 2).
Scheme 3.
Chiral resolution of compound 10.
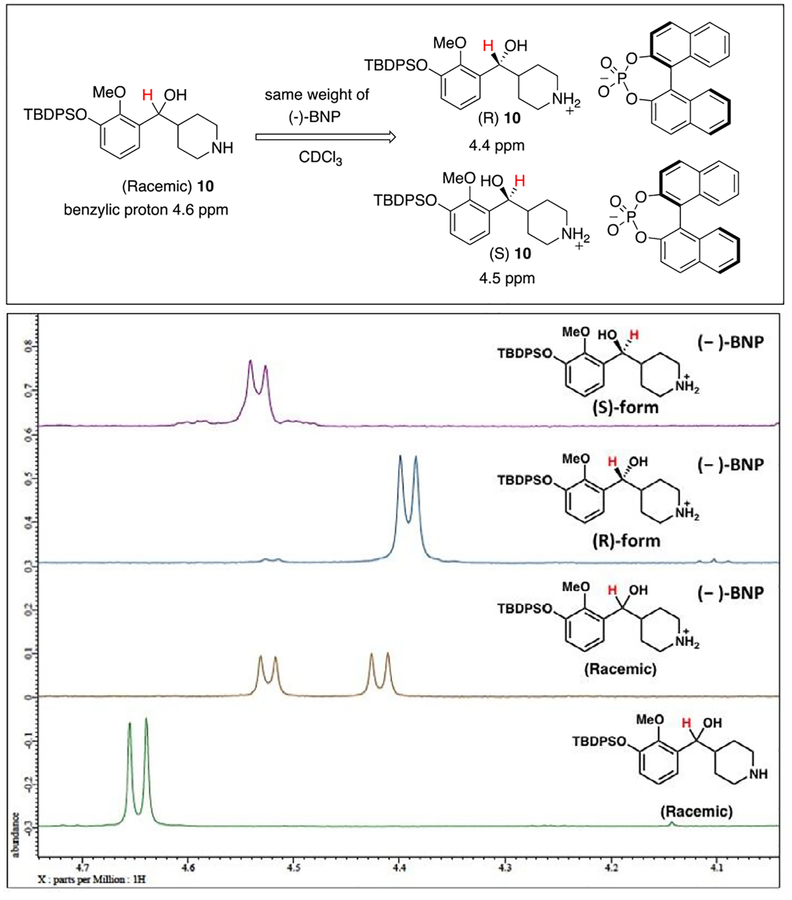
Fig. 2.
Determination of the optical purity of 10. The proton NMR shift of benzylic proton of compound 10 after mixing with same weight of (−)-BNP in CDCl3.
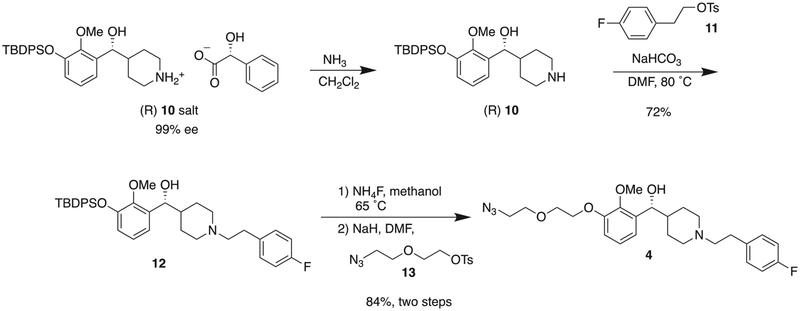
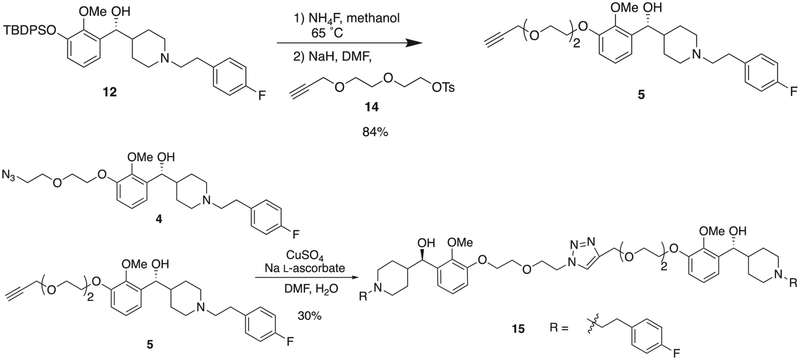
The optical purity was evaluated with (R)-(−)-1,10-binaphthyl-2,2’-diyl hydrogen phosphate [(−)-BNP] as a 1H NMR shift reagent (Fig. 2).8 The benzylic proton adjacent to the hydroxyl group gave a doublet near 4.6 ppm that was resolved from all other aliphatic signals. This peak was followed on adding (−)-BNP. When one equivalent of (−)-BNP was added to the racemic 10 in CDCl3, the benzylic signal separated into 2 doublets, with R-enantiomer at 4.4 ppm and the S-enantiomer at 4.5 ppm. Chemical shift changes of this signal were linear relative to the concentration of amine and (−)-BNP. Higher concentrations of (−)-BNP resulted in more significant proton shifting but with broadening of the proton signal.8 The resolved diastereomeric salt was partitioned between ammonium hydroxide and dichloromethane to obtain a single enantiomer amine (R)-10, which underwent N-alkylation with (2-tosylethyl)-4-fluorobenzene 11 to generate compound 12. Removal of the TBDPS group and reaction with linker 13 provided azide-terminated M100907 (4) (Scheme 4).
Scheme 4.
Synthesis of the target molecule, M100907-azide 4 from the chiral resolved 10.
The alkyne needed to form the homobivalent 15 was synthesized from intermediate 12 through cleavage of the silyl group and alkylation with the tosylated PEG-alkyne 14 (Scheme 5). The bivalent was then synthesized by formation a 1,2,3-triazole ring generated from the dipolar cycloaddition between the azide and the alkyne.5 This reaction was carried out by adding copper sulfate with sodium ascorbate in a mixture of 4 and 5 DMF and water at room temperature to form the triazole homodimer 15.
Scheme 5.
Synthesis of M100908-alkyne 5 and M100907 homodimer 15 with the heterocyclic 1,2,3-triazole ring.
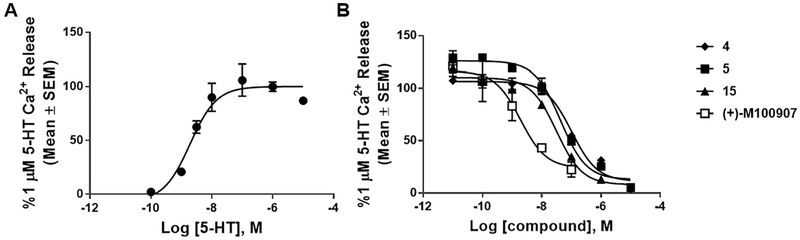
Inhibition of 5-HT2AR-mediated signaling was determined by measuring the reduction of 5-HT (1 μM) stimulated intracellular calcium (Ca2+) release in CHO-K1 cells stably expressing the 5-HT2AR.3,4 Serotonin induces a concentration-dependent increase in Cai2+ release with an EC50 of 4.2 nM (pEC50 = 8.38 ± 0.10) and 1 μM of 5-HT exhibited maximal intracellular Cai2+ release (Fig. 3A). As expected, the active isomer (+)-M1009077 displayed low nanomolar potency in inhibiting 5-HT (1 μM)-evoked Cai2+ release (IC50 = 4.8 nM; pIC50 = 8.32 ± 0.40; Fig. 3B). None of the compounds displayed activity in the absence of 5-HT (data not shown) and all compounds retained sub-micromolar antagonist activity as shown in Table 1. Compound 4 retained antagonist activity suggesting that tethering diethylene-azido linker on the catechol ring of M100907 does not disrupt antagonist properties. Although compounds 5 and 15 displayed slightly lower potency when compared to parent (+)-M100907, these compounds retained comparable potency to a previously published series of 5-HT2AR bivalent ligands which contain 14 and 17 ethylene glycol linkers.3,4 These results indicate that the 1,2,3-triazole ring formed in the click reaction does not significantly affect 5-HT2AR antagonist activity.
Fig. 3.
Representative Cai2+ release in 5-HT2AR-CHO cells. [A] 5-HT evokes a concentration-dependent elevation of Cai2+ release (pEC50 = 8.38 ± 0.10; EC50 = 4.2 nM) and 1 μM of 5-HT induces maximal Cai2+ release. [B] M100907 derivatives induce a concentration-dependent inhibition of 1 μM of 5-HT. pIC50 and IC50 values are listed in Table 1.
Table 1.
Effect of M100907 derivatives on Intracellular Calcium Release in 5-HT2AR-expressing cells.
Derivatives of (+)-M100907 that possess either an alkyne or an azide have been synthesized. The most active enantiomer was obtained by resolution of a relatively early intermediate in the synthesis. The ability of these molecules to maintain 5-HT2AR antagonist properties as (+)-M100907 was demonstrated together with the ability to use dipolar cycloaddition between the alkyne and azide to link these molecules to form bivalent antagonists. The 1,2,3-triazole ring generated in the dipolar cycloaddition used to link to the M100907 derivatives does not interfere with the ability of the bivalent ligand to act as an antagonist. Thus, functional versions of M100907 have been synthesized with tethers possessing azides or alkynes, allowing the application of these molecules in the synthesis of dimeric ligands as well as conjugation of the 5-HT2AR antagonist to reporter molecules, such as fluorophores, for the study of GPCR dimerization and their role in signaling.
Materials and methods
Cell lines and cell culture
A CHO-K1 cell line stably transfected with the 5-HT2AR (5-HT2AR-CHO cells; FA4 line) was a generous gift of K. Berg and W. Clarke (University of Texas Health Science Center at San Antonio). This line expresses transfected human (h)5-HT2AR in the p198-DHFR Hygro vector containing a hygromycin resistance gene.9 Reverse transcription of RNA followed by a quantitative real time PCR assay confirmed that 5-HT2AR-CHO cells expressed 5-HT2AR mRNA (estimated to be approximately 3–4% of the mRNA level of the housekeeping gene cyclophilin), but not mRNA for other members of the 5-HT2R family (i.e., 5-HT2BR or 5-HT2CR) (data not shown). Levels of 5-HT2AR protein expression (200 fmol/mg protein) which approximates physiological levels in brain, have been reported9 and immunoblot analysis in our hands confirmed moderate 5-HT2AR protein expression (data not shown). Cells were grown at 37 °C, 5% CO2 and 85% relative humidity in GlutaMax α-MEM (Invitrogen, Carlsbad CA), 5% fetal bovine serum (Atlanta Biologicals, Atlanta GA), 100 mg/ml hygromycin (Mediatech, Manassas VA) and were passaged when they reached 80% confluence.
Intracellular calcium assay
Intracellular calcium (Cai2+) release was monitored using FLIPR Calcium 4 Assay Kit (Molecular Devices) according to previously published protocols with minor modifications.3,4,10–13 Cells were plated in serum-replete medium at 16–20 K cells in 150 ml in black-sided, clear bottom 96-well tissue culture plates. Cells were fed 24 h later with serum-free medium and, following 3 h incubation, medium was removed and replaced with 40 μl of fresh serum-free medium plus 40 ml of Calcium 4 dye solution supplemented with 2.5 mM of water-soluble probenicid (Invitrogen, Carlsbad CA) to inhibit extracellular transport of the dye. Plates were incubated for 50 min at 37 °C followed by 60 min at room temperature in the dark. Fluorescence (λex = 485 nm, λem = 525 nm) was measured with a FlexStation 3 (Molecular Devices). Baseline was established for 17 s before addition of 20 ml of vehicle (HBSS without calcium of magnesium) or 5 concentrated test compound and fluorescence was recorded every 1.7 s for a total of 60 s to detect any intrinsic activity of the test compounds. Fifteen minutes after test compound addition, a second 17 s baseline was recorded again immediately before addition of 25 μl of 5 μM 5-HT (final concentration = 1 μM). Fluorescence was subsequently measured for an additional 60 s. Maximum peak height was determined by the FlexStation software (SoftMax Pro 5.2) for each well. Concentration-response curves (10 −10 to 10 −5 M) were performed for each compound. A full 5-HT concentration response curve (10 −10–10 −5 M) was performed in each experiment to establish assay and cell performance.
Data analysis
Peak responses from each well are expressed as a percent of the maximum Cai2+ response obtained with 1 mM of 5-HT. The pIC50 value for Cai2+ release was determined using 3-parameter nonlinear regression analysis (GraphPad Prism 7.02) and calculated from at least three independent experiments, each conducted in technical replicates of 3–8, and are presented as the mean ± SEM.
Supplementary Material
Funding sources
This work was supported by National Institute on Drug Abuse Grants P50 DA0333935 (K.A.C., S.R.G., and N.C.A.), T32 DA07287 (C.A.S.), and K05 DA020087 (K.A.C.). A portion of this work was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism (K.C.R.).
Footnotes
Conflicts of interest
The authors declare no competing financial interests.
A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bmcl.2018.02.058.
References
- 1.Ebdrup BH, Rasmussen H, Arnt J, Glenthøj B. Serotonin 2A receptor antagonists for treatment of schizophrenia. Expert Opin Invest Drugs. 2011;20:1211–1223. [DOI] [PubMed] [Google Scholar]
- 2.de Paulis T M-100907 (Aventis). Curr Opin Invest Drugs. 2001;2:123–132. [PubMed] [Google Scholar]
- 3.Shashack MJ, Cunningham KA, Seitz PK, et al. Synthesis and evaluation of dimeric derivatives of 5-HT2A receptor (5-HT2AR) antagonist M-100907. ACSChem Neurosci. 2011;2:640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soto CS, Shashack MJ, Fox RG, et al. Novel bivalent 5-HT2A receptor antagonists exhibit high affinity and potency in vitro and efficacy in vivo. ACS Chem Neurosci. 2017. [epub a head of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40:2004–2021. [DOI] [PubMed] [Google Scholar]
- 6.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–2599. [DOI] [PubMed] [Google Scholar]
- 7.Ullrich T, Rice K. A practical synthesis of the serotonin 5-HT2A receptor antagonist MDL 100907, its enantiomer and their 3-phenolic derivatives as precursors for [11C] labeled PET ligands. Bioorg Med Chem. 2000;8:2427–2432. [DOI] [PubMed] [Google Scholar]
- 8.Ravard A, Crooks PA. Chiral purity determination of tobacco alkaloids and nicotine-like compounds by 1H NMR spectroscopy in the presence of 1,1andprime;-binaphthyl-2,2andprime;-diyl phosphoric acid. Chirality. 1996;8:295–299. [Google Scholar]
- 9.Berg KA, Clarke WP, Chen Y, Ebersole BJ, McKay RDG, Maayani S. 5-Hydroxytryptamine type 2A receptors regulate cyclic AMP accumulation in a neuronal cell line by protein kinase C-dependent and calcium/calmodulin-dependent mechanisms. Mol Pharmacol. 1994;45:826–836. [PubMed] [Google Scholar]
- 10.Seitz PK, Bremer NM, McGinnis AG, Cunningham KA, Watson CS. Quantitative changes in intracellular calcium and extracellular-regulated kinase activation measured in parallel in CHO cells stably expressing serotonin (5-HT) 5-HT2A or 5-HT2C receptors. BMC Neurosci. 2012;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding C, Bremer NM, Smith TD, et al. Exploration of synthetic approaches and pharmacological evaluation of PNU-69176E and its stereoisomer as 5-HT2C receptor allosteric modulators. ACS Chem Neurosci. 2012;3:538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham KA, Anastasio NC, Fox RG, et al. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci. 2013;4:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y-C, Hartley RM, Anastasio NC, Cunningham KA, Gilbertson SR. Synthesis and structure–activity relationships of tool compounds based on WAY163909, a 5-HT2C receptor agonist. ACS Chem Neurosci. 2017;8:1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.