Abstract
Alcohol and cannabis are two of the most commonly used substances by adolescents and are associated with adverse medical and psychiatric outcomes. These adverse psychiatric outcomes may reflect the negative impact of alcohol and/or cannabis abuse on neural systems mediating reward and/or error detection. However, work indicative of this has mostly been conducted in adults with Alcohol and/or Cannabis Use Disorder (i.e., AUD and CUD), with relatively little work in adolescent patients. Furthermore, of the work that has been conducted in adolescents, groups were based on categorical diagnoses of AUD and/or CUD, so the relationship between AUD and/or CUD symptom severity in adolescents and neural dysfunction is unclear. We used a Monetary Incentive Delay (MID) task to examine the relationship between AUDIT and/or CUDIT scores and functional integrity of neuro-circuitries mediating reward processing and error detection within 150 adolescents. Our findings indicate that AUDIT score is negatively related to activity in reward processing neuro-circuitry in adolescents. However, CUDIT score is negatively related to activity in brain regions involved in error detection. Each of these relationships reflected a medium effect size (Partial-η2 0.09-0.14). These data suggest differential impacts of AUD and CUD on reward versus error detection neuro-circuitries within the adolescent brain.
Keywords: Adolescent, Alcohol use disorder, Anterior cingulate cortex, Cannabis use disorder, fMRI, Striatum
1. Introduction
The two most commonly used substances by adolescents in the US are alcohol and cannabis (Miech et al., 2016). Epidemiological evidence suggests that alcohol and/or cannabis use during adolescence is associated with increased risk for developing Alcohol Use Disorder (AUD) and/or Cannabis Use Disorder (CUD) during adulthood (Winters and Lee, 2008). Additionally, individuals with AUD and/or CUD who initiated use during adolescence face a more severe disease course, including a greater likelihood of relapse (Babor et al., 1992). This may be due to the adverse neurodevelopmental impact of these substances on the adolescent brain (Filbey et al., 2015; Squeglia et al., 2015).
One structure that is undergoing development during adolescence (Galvan, 2010) and that has been implicated in addiction is the striatum (Volkow et al., 2016). More specifically, two sub-regions of the striatum are thought to be important for the pathophysiology of AUD/CUD: the ventral striatum (VS) and the dorsal striatum (DS; Volkow et al., 2016). In particular, the VS (including nucleus accumbens) is a region critically responsive to the receipt of reinforcement (Diekhof et al., 2012) and is implicated in early stages of AUD/CUD (Volkow et al., 2016). However, the DS (including caudate and putamen) is involved in error processing (Steele et al., 2014; Dugré et al., 2018) and is implicated in late stages of AUD/CUD (Volkow et al., 2016). Distinguishing the degree to which AUD and/or CUD symptom severity are related to VS reward processing versus DS error processing may be critical for the development of targeted interventions specific to AUD or CUD.
Animal and human neuroimaging work suggests that alcohol/cannabis consumption leads acutely to the release of dopamine within VS (Martinez et al., 2005; Bossong et al., 2009). In contrast, chronic substance use has been linked to decreased VS responsiveness to non-drug reinforcements (Koob and Volkow, 2016). In line with this, multiple studies using the Monetary Incentive Delay (MID) task have shown that adults with AUD show reduced VS responses to monetary reward relative to healthy controls (Wrase et al., 2007; Beck et al., 2009) – though it should be noted that some work with other reward-based paradigms has indicated greater VS response to reward anticipation among adult AUD patients than controls (van Holst et al., 2014). The results of studies with patients with CUD have been more mixed. Studies of cannabis-using participants have reported that monetary reward responsiveness within VS is decreased (van Hell et al., 2010; Martz et al., 2016), increased (Nestor et al., 2010) or not significantly different from that of comparison individuals (Filbey et al., 2013).
Despite this, very few studies have examined how substance use in adolescence may impact VS functioning. One study found a positive relationship between overall substance use and striatal response to reward in adolescents (Bjork et al., 2011). However, this study did not include adolescents who met criteria for Substance Use Disorders (SUDs). Another study reported decreased VS responsivity to reward in adolescents ages 14–17 with Alcohol Use Disorder Identification Test (AUDIT) scores≥4 relative to those with AUDIT scores<4 (Nees et al., 2015). Conversely, increased VS responsivity to rewards in children ages 8–12 ha s been identified as a risk factor for alcohol problems at follow-up visits 3–6 years later (Heitzeg et al., 2014). In contrast, adolescent cannabis users have been reported to show heightened VS responsivity to neutral (but not reward) cues relative to controls (Jager et al., 2013).
A second issue relatively neglected in the previous literature concerns co-morbid AUD/CUD. Adolescents often use multiple substances based on availability (Moss et al., 2014). For example, only one study to date has examined the effects of co-morbid AUD/CUD on reward processing in adolescents (Karoly et al., 2015). In contrast to literature exploring AUD or CUD individually (Beck et al., 2009; Jager et al., 2013; Martz et al., 2016; Nees et al., 2015), Karoly and colleagues did not find any differences in VS reward responsiveness between adolescents with AUD or CUD and typically developing adolescents (2015). However, this may reflect a categorical approach to considering the impact of co-morbid AUD/CUD. A dimensional approach to AUD or CUD severity might be more likely to reveal differential neural impacts associated with use of these substances.
A third issue is that the classic measure of reward sensitivity used extensively in this work, the MID, not only identifies regions sensitive to reward but also regions sensitive to response accuracy. Participants receive reward or avoid punishment as a function of their ability to respond rapidly and accurately to a target presented for a short period of time. Incorrect responses can either prevent the receipt of reward or result in the delivery of punishment depending on the MID variant. Incorrect responses across a variety of tasks, including the MID, are associated with activity within dorsal striatum, insula, anterior cingulate/dorsomedial prefrontal cortex (ACC/dmPFC), parietal cortices, and visual cortices (Dugré et al., 2018; Steele et al., 2014). There are indications that error responsiveness is compromised in patients with substance abuse (Carey et al., 2015; Claus et al., 2013; Hester et al., 2009; Wesley et al., 2011). In particular, both alcohol and cannabis abuse have been associated with decreased responses to errors in ACC/dmPFC, parietal cortex and/or putamen (Carey et al., 2015; Claus et al., 2013; Hester et al., 2009; Wesley et al., 2011) although one study has reported increased activity in ACC and parietal cortex to errors in patients with alcohol dependence (Li et al., 2009). However, little previous work has been conducted with adolescent participants or taken a dimensional approach to examine the potentially differential relationships with AUD/CUD.
In the current study, we implemented the MID (Knutson et al., 2001) in adolescents with varying degrees of AUD and CUD symptomatology, including those with co-morbid AUD/CUD. We hypothesized that (i) participants with high levels of AUD symptomatology, although perhaps not participants with high levels of CUD symptomatology, would show reduced VS responsivity to reward; and (ii) participants with high levels of AUD and CUD symptomatology would show reduced within ACC/dmPFC and/or DS responsivity to error feedback.
2. Materials and methods
2.1. Participants
To obtain a wide range of symptom severity, participants included 175 youths aged 14–18 years recruited from both a residential treatment program and the surrounding community (see Table 1). Twenty-five youth were excluded from analysis (due to excessive movement (>15% censored volumes at >0.5 mm motion across adjacent volumes (N = 10), low response rate (N = 4), technical difficulties during scanning (N = 4), and/or incidental neurological findings (N = 7)). This resulted in a final sample of 150 youths (109 from the residential treatment program and 41 from the community); average age = 16.1 years, (SD = 1.08), IQ = 100.5 (SD = 12.29), and 92 (61%) male. See Table 1 for further details.
Table 1.
Characteristics of the participant sample.
| Boys Town (N = 109) |
Community (N = 41) |
Total (N = 150) |
t/χ2 | ||||
|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | ||
| Age | 16.15 | 1.04 | 16.05 | 1.18 | 16.12 | 1.08 | 0.49 |
| IQ | 99.41 | 12.64 | 103.51 | 10.88 | 100.53 | 12.29 | 1.84 |
| %age Female | 33.02% | 53.66% | 38.67% | 5.35* | |||
| AUDIT | 5.39 | 7.49 | 0.37 | 0.74 | 4.01 | 6.77 | 4.82* |
| CUDIT | 9.91 | 9.78 | 0.41 | 1.30 | 7.31 | 9.37 | 6.29* |
| Smoking | 1.76 | 1.51 | 0.20 | 0.60 | 1.33 | 1.50 | 6.42* |
| ADHD | 66.97% | 14.63% | 52.67% | 32.74* | |||
| CD | 67.89% | 4.88% | 50.67% | 47.33* | |||
| MDD | 30.28% | 24.39% | 28.67% | 0.51 | |||
| GAD | 28.44% | 12.2% | 24% | 3.42 | |||
| CBCL: ADHD | 6.74 | 3.24 | 1.88 | 3.03 | 5.38 | 3.86 | 8.05* |
| CBCL: CD | 13.72 | 6.54 | 1.24 | 2.13 | 9.82 | 8.03 | 11.90* |
| SCARED: GAD | 6.57 | 5.16 | 6.24 | 4.36 | 6.48 | 4.94 | 0.35 |
| SCARED: SAD | 4.90 | 3.88 | 5.83 | 4.09 | 5.16 | 3.95 | 1.29 |
| SCARED: Total | 20.37 | 16.08 | 19.85 | 14.33 | 20.22 | 15.56 | −0.18 |
| MFQ | 18.01 | 13.97 | 3.97 | 6.75 | 13.40 | 13.76 | 6.09* |
Key to Table 1: ADHD/CD/MDD/GAD: Percentage of participants meeting criteria for these psychiatric diagnoses; CBCL: ADHD & CD: CBCL raw scores for ADHD and CD; SCARED: GAD, SAD & Total: GAD and SAD subscales of the SCARED as well as total score. * indicates t-value or χ2-value significant at a threshold of p<.05.
Youths recruited from the Boys Town Campus had been referred for behavioral and mental health problems. Participants from the community were recruited through flyers and were all youth without significant substance abuse histories (i.e., AUDIT < 4 and CUDIT < 8). Clinical diagnoses were assigned by a licensed and board-certified psychiatrist according to DSM-V criteria (American Psychiatric Association, 2013) following clinical interviews with the participants and their parents, to adhere closely to common clinical practice (see Table 1).
Eighty-six youth endorsed having used alcohol and/or cannabis once or more in the past year on the Alcohol Use Disorder Identification Test (AUDIT) and/or the Cannabis Use Disorder Identification Test (CUDIT), respectively (Adamson et al., 2010; Saunders et al., 1993). AUDIT scores ranged from 0 to 34 (M = 4.0; SD = 6.77) and CUDIT scores ranged from 0 to 32 (M = 7.3, SD = 9.37). There were no significant correlations between age, IQ, AUDIT scores, and CUDIT scores (r's<0.155, ns) and there were no significant differences between males and females on AUDIT scores or CUDIT scores (t's<1.91, ns).
Of the youths endorsing alcohol and/or cannabis use during their lifetimes, 22 youths showed subclinical levels of alcohol and/or cannabis use while 64 met the clinical cutoff on the AUDIT and/or CUDIT suggestive of adolescent AUD (AUDIT ≥ 4) or CUD (CUDIT ≥ 8), respectively (Adamson et al., 2010; Adamson and Sellman, 2003; Fairlie et al., 2006). 61 youths showed symptoms of both AUD and CUD. All youths with significant substance abuse histories (AUDIT ≥ 4 and/or CUDIT ≥ 8) were residents of the residential treatment program and were abstinent for at least four weeks prior to scanning. Forty-seven participants had an AUDIT score ≥4 and 56 participants had a CUDIT score≥8. In line with previous work indicating high rates of co-morbid alcohol and cannabis use in adolescents (Moss et al., 2014), 38 participants had both an AUDIT score ≥4 and CUDIT score ≥8.
The Boys Town National Research Hospital institutional review board approved this study. A doctoral level researcher or a member of the clinical research team obtained written informed consent and assent. In all cases, youth had the right to decline participation at any time before or during the study. With respect to community participants, informed consent was obtained from the youths’ parents/legal guardians at the beginning of the on-site screening. At this time, the consent document was reviewed in detail and the parents/legal guardians had the opportunity to have their questions answered before being asked to sign the consent form. After that, informed assent was obtained from the youths themselves. This procedure differed slightly for youth recruited from the Boys Town campus. Consent was typically obtained from parents during or shortly after the child’s arrival at Boys Town. Assent was obtained from the youth in a separate session, 5–10 days after parental consent had been obtained.
Exclusion criteria included IQ < 75, pregnancy, non-psychiatric medical conditions that require the use of medication that may have psychotropic effects (e.g., beta blockers or steroids), current psychosis, pervasive developmental disorders, Tourette’s disorder, neurological disorders, presence of metallic objects in the body (e.g., braces, metal plates, pacemakers), and claustrophobia. Use of psychotropic medications for psychiatric indications (e.g., stimulants, selective serotonin reuptake inhibitors) were not exclusory. However, participants on stimulant medication withheld medication for 24 h prior to scanning.
2.2. Measures
2.2.1. Monetary incentive delay (MID) task
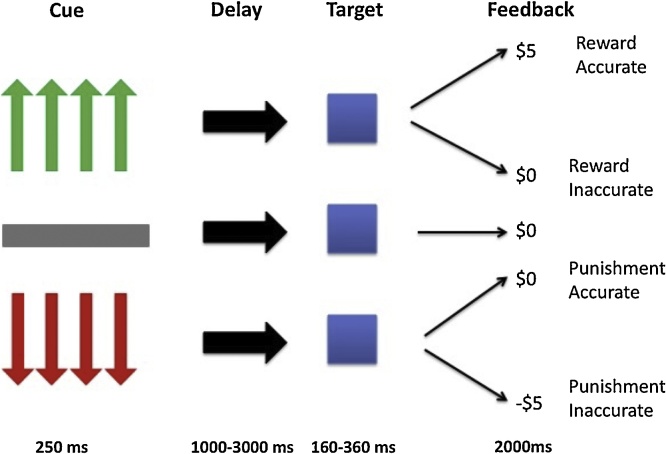
Participants completed a Monetary Incentive Delay (MID) task similar to that used previously (Knutson et al., 2001); see Fig. 1. In this paradigm, the participant’s task is to respond with a button press when a stimulus (an image of superman) is on the screen (depicted as a blue square in Fig. 1). Successful performance can either win, or avoid the loss of, money.
Fig. 1.
Diagram of the MID. The cue indicates the amount of money the participant is playing to win (green) or avoid losing (red); after the cue disappears, there is a variable delay; after the delay a target (superman- depicted in the figure as the blue square) appears and participants respond; participants are then provided one of five types of feedback: reward accurate, reward inaccurate, punishment accurate, punishment inaccurate, or neutral feedback (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
On each trial, participants first saw a cue for 250 ms that indicated whether the trial was a win or lose trial and the amount of money that was at stake. Green arrows indicated that successful task performance would win money (reward trials) while red arrows (punishment trails) indicated that they could lose money if they did not respond quickly enough. A grey bar indicated that no money was at stake (neutral trials). On reward and punishment trials, the number of arrows depicted the amount of money that could be won/lost: one = 20 cents; two=$1; three=$3; four=$5. There was then a jittered interval (1000–3000 ms) between the cue and the presentation of the target. The target was then presented for 160–360 ms based on performance on a practice run performed prior to scanning. This ensured a success rate of approximately 66%. Responding within the response window engendered the expected money reward (reward trials) or avoided punishment (punishment trials). Too slow responses either results in no reward (reward trials) or money lost (punishment trials). They were also provided with a running total of money they had won throughout the task. There were 48 reward trials, 48 punish trials, and 12 neutral trials, yielding 108 total trials. All participants completed one run of the MID and often at least one other task.
2.2.2. Substance use disorder assessments
Youths completed the Alcohol Use Disorder Identification Test (AUDIT) and the Cannabis Use Disorder Identification Test (CUDIT). These scales assess overall symptomatology of AUD and CUD, respectively, including overall quantity/frequency of use, abuse symptoms, and dependence symptoms. These scales show high validity, as elevated scores on these scales are associated with a high likelihood of an AUD and/or CUD diagnosis (Adamson et al., 2010; Saunders et al., 1993). Smoking status was determined using the Monitoring the Future Survey (Miech et al., 2016). As can be seen in Table 2, both AUDIT and CUDIT scores were equivalently correlated with tobacco usage.
Table 2.
Zero-Order Correlations Across Demographic and Clinical Variables.
| Age | IQ | Sex | AUDIT | CUDIT | Smoking | ADHD | CD | MDD | GAD | CBCL:ADHD | CBCL: CD |
SCARED:GAD | SCARED:SAD | SCARED: Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||||||||
| IQ | 0.18* | ||||||||||||||
| Sex | 0.01 | −0.01 | |||||||||||||
| AUDIT | 0.15 | −0.16 | −0.16 | ||||||||||||
| CUDIT | 0.13 | −0.15 | −0.01 | .70** | |||||||||||
| Smoking | .20* | −0.11 | 0.09 | .69** | .67** | ||||||||||
| ADHD | −0.16 | 0.05 | .21* | .18* | .22** | .27** | |||||||||
| CD | −0.14 | −0.07 | 0.12 | .30** | .34** | .43** | .45** | ||||||||
| MDD | 0.15 | .25** | −.19* | 0.06 | −0.01 | 0.03 | 0.13 | −0.08 | |||||||
| GAD | 0.14 | 0.05 | −.16* | .28** | .24** | .23** | .22** | −0.01 | .40** | ||||||
| CBCL-ADHD | −0.08 | −0.05 | 0.11 | .27** | .27** | .33** | .63** | .42** | 0.11 | .17* | |||||
| CBCL-CD | −0.03 | −0.10 | 0.08 | .33** | .33** | .43** | .46** | .67** | 0.03 | .17* | .70** | ||||
| SCARD-GAD | .16* | .18* | −.35** | .21** | .18* | 0.08 | 0.11 | −0.04 | .35** | .62** | 0.08 | 0.05 | |||
| SCARED-SAD | 0.15 | 0.13 | −.27** | 0.05 | 0.09 | −0.07 | −0.03 | −0.10 | .37** | .38** | −0.04 | −0.11 | .68** | ||
| SCARED Total | 0.11 | 0.11 | −.36** | .20* | 0.14 | 0.04 | 0.10 | −0.02 | .42** | .60** | 0.07 | 0.04 | .91** | .79** | |
| MFQ | 0.07 | −0.11 | −0.10 | .33** | .32** | .25** | .32** | .32** | .26** | .44** | .50** | .45** | .32** | 0.14 | .29** |
Key to Table 2: ADHD/CD/MDD/GAD: Diagnoses of these psychiatric diagnoses; CBCL: ADHD & CD: CBCL raw scores for ADHD and CD; SCARED: GAD, SAD & Total: GAD and SAD subscales of the SCARED as well as total score. * indicates correlation coefficient significant at p<.05; ** indicates correlation coefficient at p<.01.
2.2.3. Psychiatric symptomatology assessments
In order to provide more dimensional descriptions of psychiatric co-morbidities (externalizing and anxiety/ depressive symptomatology), parents completed the Childhood Behavior Checklist (CBCL; Achenbach and Rescorla, 2001) and the Mood and Feelings Questionnaire (MFQ) (Angold et al., 1995). The self-report version of the Screen for Child Anxiety and Related Disorders (SCARED) was used to assess levels of anxiety symptoms from the past three months (Birmaher et al., 1997). IQ was assessed with the Wechsler Abbreviated Scale of Intelligence – 2nd edition (WASI-II; Wechsler, 2011).
2.3. MRI parameters
All data were collected on a 3 T Siemens Skyra scanner. A total of 274 functional images were taken with a T2* weighted gradient echo planar imaging (EPI) sequence (repetition time = 2500 ms; echo time = 27 ms; 240 mm field of view; 94 × 94 matrix; 90° flip angle). Whole-brain coverage was obtained with 43 axial slices (thickness, 2.5 mm; voxel size 2.6 × 2.6 × 2.5 mm3). A high-resolution T1 anatomical scan (MP-RAGE, repetition time = 2200 ms; echo time = 2.48 ms; 230 mm field of view; 8° flip angle; 256 × 208 matrix; thickness, 1 mm; voxel size 0.9 × .9 × 1 mm3) in register with the EPI data set was obtained covering the whole brain with 176 axial slices.
2.4. fMRI analysis: data preprocessing and individual level analysis
Functional MRI data were preprocessed and analyzed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). The first four volumes in each scan were discarded. The anatomical scan for each participant was registered to the Talairach and Tournoux atlas (Talairach and Tournoux, 1988) using the TT_N27 template and each participant’s functional EPI data were registered to their Talairach anatomical scan in AFNI. Functional images were motion corrected and spatially smoothed with a 6-mm full width half maximum Gaussian kernel. The data then underwent time series normalization by dividing the signal intensity of a voxel at each time-point by the mean signal intensity of that voxel for each run and multiplying by 100. Therefore, the resultant regression coefficients are representative of a percentage of signal change from the mean.
Afterward, nine regressors were generated: punishment trial cue, reward trial cue, neutral trial cue, inaccurate punishment trial feedback, accurate punishment trial feedback, neutral feedback, inaccurate reward trial feedback, accurate reward trial feedback, and trials with no responses (e.g., trials where participants failed to respond to the target). Furthermore, the incentive amount for each individual trial was used to modulate the percent signal change at each voxel and time point. For reward and punishment trials, the incentive magnitude for each trial was used to modulate the percent signal change at each voxel and time-point. Every volume and its predecessor on which motion exceeded 0.5 mm (Euclidean Norm) was censored. Regressors were generated by convolving the train of stimulus events with a gamma-variate hemodynamic response function to account for the slow hemodynamic response. GLM fitting was performed with the nine regressors listed; six motion regressors, and a regressor modeling a first-order baseline drift function. This process produced an unmodulated β-coefficient and an associated t-statistic for each voxel and regressor. Modulated β-coefficients and associated t-statistics were produced for the punishment cue, reward cue, inaccurate punishment feedback, accurate punishment feedback, inaccurate reward feedback, and accurate reward feedback regressors.
2.5. fMRI analysis: group analysis
To reduce skewness and kurtosis, a Rankit Transformation was applied to participants' AUDIT and CUDIT scores (Bliss et al., 1956). The Rankit-Transformed standardized AUDIT and CUDIT scores were used for all analyses. For AUDIT scores, pre-transformation skewness and kurtosis values were 2.19 and 4.75, respectively (right-skewed). Post-transformation, skewness and kurtosis values for AUDIT scores were 0.84 and -0.28. For CUDIT scores, pre-transformation skewness and kurtosis values were 1.01 and -0.19, respectively (right-skewed). Post-transformation skewness and kurtosis values for CUDIT scores were 0.80 and -0.44, respectively.
There were two group-level analyses performed on the feedback-phase data. First, we ran a univariate ANCOVA on the BOLD response data modulated by the value of the reward received. This analysis used the modulated accurate reward feedback regressor. Secondly, we ran a 2 (Reinforcement: Punishment, Reward)-by-2 (Accuracy: Inaccurate, Accurate) repeated-measures ANCOVA on the unmodulated BOLD response feedback data. This analysis used the unmodulated inaccurate punishment, accurate punishment, inaccurate reward, and accurate reward feedback regressors. In both ANCOVAs, AUDIT scores, CUDIT scores, and the AUDIT-by-CUDIT interaction were entered as continuous covariates. Follow-up correlations and Steiger’s-Z tests were performed within SPSS 22.0 and using freely available online tools (Lee and Preacher, 2013).
Following much of the previous literature using the MID task (Wrase et al., 2007; Helfinstein et al., 2013; Beck et al., 2009; Martz et al., 2017; Van Hulst et al., 2015) and given our a priori hypotheses, regions of interest (ROI) for the striatum and ACC/dmPFC were obtained. The striatum ROI was generated by creating a mask that included all voxels labeled as caudate, putamen, or accumbens in the Desai atlas (Desikan et al., 2006). The ACC/dmPFC ROI was created by combining two 10-mm spheres centered on coordinates identified from a previous error-processing study (Talairach Coordinates: +4, -16, 33 and -4, -16, 33; Nee et al., 2011). These masks were then combined to create a single mask for the purposes of extent thresholds for multiple comparison correction (see Figures S1 and S2). For completion, an exploratory whole-brain analysis was conducted. Correction for multiple comparisons was performed using a spatial clustering operation in AFNI’s 3dClustSim utilizing the autocorrelation function (-acf) with 10,000 Monte Carlo simulations for the combined striatal and ACC/dmPFC ROI and the whole-brain analysis. Following the advice of recent statistical modeling work (Cox et al., 2017), the initial threshold was set at p = .002. A cluster-wise small-volume correction (SVC) applied to the combined striatal and ACC/dmPFC ROI mask (corrected p = .05) provided a threshold of 6 voxels. For the whole-brain analysis, correction for multiple comparisons yielded an extent threshold of 26 voxels. Follow-up analyses were conducted on the percent signal change using SPSS 22.0 (IBM SPSS Statistics For MacOSX, 2012). In line with recent recommendations (Chen et al., 2016), effect sizes are reported for each analysis.
3. Results
3.1. Clinical correlations
Correlation analyses were conducted to determine the relationships between AUD, CUD, probability of psychiatric diagnoses, and psychiatric symptom levels dimensionally (see Table 2). These analyses revealed significant positive correlations between AUDIT and CUDIT scores and both and ADHD, CD, and GAD (but not MDD) diagnoses (see Table 2). Importantly, there were no significant differences in correlation strengths between AUDIT and CUDIT scores and probability of diagnoses [Steiger’s-Z’s=-0.60-1.10, p’s>.05] or psychiatric symptom assessments [Steiger’s-Z's=-0.46-0.95, p's>.05]; see Table 2. There were also no differential correlations between AUDIT and CUDIT scores and level of smoking [Steiger’s-Z=-0.39, p > .05]. Given the high correlation between AUDIT and CUDIT scores, variance inflation factors (VIF) were calculated for these in our analyses. The VIFs for AUDIT, CUDIT, and the AUDIT-by-CUDIT interaction were all <2.5, indicating acceptable levels of collinearity.
3.1.1. Behavioral results
We ran two one-way (Reinforcement: Punishment, Reward) repeated measures ANCOVAs on both the accuracy and response time (RT) data with Rankit-transformed AUDIT and CUDIT scores as continuous covariates. For accuracy, the main effect of reinforcement was not significant [F(1,147) = 0.12, ns]. There was no significant effect of AUDIT [F(1,147) = 3.51, ns]. However, there was a significant effect of CUDIT score [F(1,147) = 6.13, p < .05; CUDIT scores were positively associated with accuracy; r = .200, p < .05]. There were no significant reinforcement-by-covariate interaction effects.
With regards to RT, there was a main effect of reinforcement [F(1,147) = 7.12, p < .05; participants were faster on reward relative to punishment trials] and CUDIT scores [F(1,147) = 5.82, p < .05]; CUDIT scores were inversely associated with RT [r=-.195, p < .05]. However, there was no significant effect of AUDIT [F(1,146) = 4.57, p < .05] or significant reinforcement-by-covariate interactions.
One note of caution, however, follow-up, single covariate ANCOVAs (AUDIT or CUDIT scores alone) did not reveal a significant effect of CUDIT score on accuracy though all other findings remained the same (including the inverse relationship between CUDIT scores and RT).
3.2. Movement data
Fourteen participants were excluded due to excessive motion (N = 10) or low response rate (N = 4) on the task. Within the final sample (N = 150), volumes were censored if there was >0.5 mm motion across adjacent volumes. Participants were excluded if they had >15% censored volumes. There were no significant correlations between AUDIT scores and CUDIT scores and censored volumes, average motion per volume, and maximum displacement during scanning within the final sample [r's=-.02--.13, p's>.05].
3.3. fMRI results
The goals of this study were to examine the extent to which adolescent AUD and/or CUD symptomatology related to: (i) dysfunction in brain regions responding to reward; and (ii) regions responsive to reinforcement received and accuracy (significant task effects may be found in Tables S1 and S2). These goals were examined via two analyses:
3.3.1. The impact of AUD and/or CUD symptomatology on regions responsive to reward
This was examined by a univariate ANCOVA on the BOLD response data modulated by reward received during the feedback phase of accurate reward trials. Rankit-transformed AUDIT and CUDIT scores were included as continuous covariates.
3.3.1.1. Main effect of AUDIT
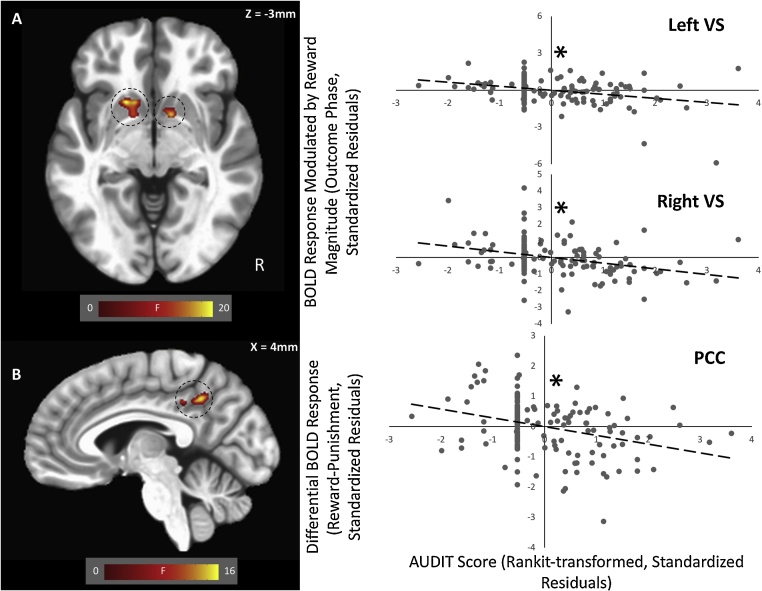
There was a significant main effect of AUDIT scores on BOLD response modulated by reward received within bilateral ventral striatum (Fig. 2A, Table 3). Within both clusters, there was an inverse relationship between AUDIT scores and modulated BOLD response [left VS: r=-.329, p < .001, partial η2 = 0.108, Cohen's d = 0.69; right VS: r=-.341, p < .001, partial η2 = 0.116, Cohen's d = 0.72]; i.e., increasing AUD symptomatology was associated with decreased striatal modulation by reward received. No effects survived correction for multiple comparisons within the whole-brain analysis.
Fig. 2.
(A) Main Effect of AUDIT score on modulation of BOLD response by reward value within bilateral ventral striatum (VS); and (B) AUDIT-by-Reinforcement interaction on unmodulated BOLD response within the PCC. Scatterplots depict significant partial correlations and standardized residuals for each of the regions. Adjusted residuals for the Rankit transformed z-scored AUDIT scores (x-axis) are plotted against adjusted residuals for: (A) the modulated BOLD responses to reward (bilateral ventral striatum); and (B) BOLD responses to reward versus punishment receipt (PCC). * indicate the significant inverse relationships.
Table 3.
Brain regions demonstrating significant effects of AUDIT scores on BOLD Response Modulation by Reward Value.
| Coordinates of Peak Activationb | ||||||||
| Regiona | Hemisphere | BA | x | y | z | F | Partial η2 | Voxels |
| Striatum ROI | ||||||||
| Ventral Striatumc | L | – | −16 | 14 | −4 | 17.72 | 0.108 | 20 |
| Ventral Striatumc,d | R | – | 11 | 5 | −4 | 19.25 | 0.116 | 9 |
Note: a According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon/), b Based on the Tournoux & Talairach standard brain template, cSignificant at SVC threshold, BA = Brodmann’s Area, d Significant activity within this region at p < 0.001 when using only AUDIT score as a covariate (11, 8, -1).
3.3.2. The impact of AUD and/or CUD symptomatology on regions responsive to reinforcement received and accuracy
This was examined with a 2 (Reinforcement: Punishment, Reward)-by-2 (Accuracy: Inaccurate, Accurate) repeated-measures ANCOVA on the unmodulated BOLD response data. Rankit-transformed AUDIT and CUDIT scores were entered as continuous covariates.
3.3.2.1. AUDIT-by-reinforcement interaction
There was a significant AUDIT-by-Reinforcement interaction effect in the posterior cingulate cortex (Fig. 2B, Table 4). There was a significantly stronger negative relationship between AUDIT scores and BOLD response on reward trials relative to punishment trials [rrew=-.11, rpun = .18; Steiger’s-Z = 3.93, p < .001, partial η2 = 0.104, Cohen's d = 0.68].
Table 4.
Brain regions demonstrating significant AUDIT-by-CUDIT, AUDIT-by-reinforcement, AUDIT-by-accuracy, CUDIT-by-accuracy, and CUDIT-by-reinforcement-by-accuracy interaction effects on unmodulated BOLD responses.
| Coordinates of Peak Activationb | ||||||||
| Regiona | Hemisphere | BA | x | y | z | F | Partial η2 | Voxels |
| AUDIT-by-Reinforcement | ||||||||
| PCC | R/L | 31 | 8 | −46 | 35 | 16.95 | 0.104 | 39 |
| AUDIT-by-Accuracy | ||||||||
| Lingual Gyrus | R | 19 | 26 | −67 | −4 | 20.86 | 0.125 | 67 |
| Cuneus | L | 18/30 | −10 | −64 | 8 | 15.78 | 0.098 | 30 |
| CUDIT-by-Accuracy | ||||||||
| Putamenc,d | R | – | 23 | −4 | 8 | 13.93 | 0.087 | 9 |
| Lingual Gyrus | R | 18 | 11 | −70 | −4 | 20.60 | 0.124 | 94 |
| Lingual Gyrus | L | 18 | −13 | −67 | 5 | 15.33 | 0.095 | 35 |
| CUDIT-by-Reinforcement-by-Accuracy | ||||||||
| Putamenc,e | R | – | 32 | −13 | 2 | 15.87 | 0.098 | 6 |
| ACC/dmPFCc,f | L | 32 | −13 | 17 | 35 | 20.55 | 0.123 | 13 |
Note: a According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon/), b Based on the Tournoux & Talairach standard brain template, cSignificant at SVC threshold, BA = Brodmann’s Area, d Significant activity within this region at p < 0.001 when using only CUDIT score as a covariate (23, -8, 6), e Significant activity within this region at p < 0.001 when using only CUDIT score as a covariate (-11, 14, 33).
3.3.2.2. AUDIT-by-accuracy interaction
There was a significant AUDIT-by-Accuracy interaction effect in lingual gyrus and cuneus. In lingual gyrus, there was a significantly stronger negative relationship between AUDIT scores and BOLD response on during feedback on accurate trials relative to inaccurate trials [Steiger’s-Z = 3.86, p < .001, partial η2 = 0.125, Cohen's d = 0.75]. In cuneus, there was a significantly more positive relationship between AUDIT scores and BOLD response during feedback on inaccurate trials relative to accurate trials [Steiger’s-Z = 3.80, p < .001, partial η2 = 0.098, Cohen's d's = 0.65].
3.3.2.3. CUDIT-by-accuracy interaction
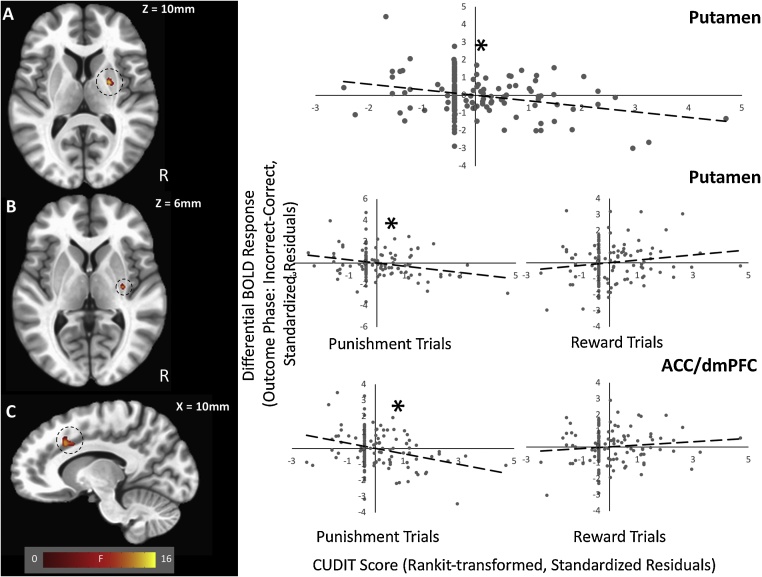
There was a significant CUDIT-by-Accuracy interaction within two regions of lingual gyrus and putamen (SVC; Fig. 3A, Table 4). Within all regions there was a significantly stronger negative relationship between CUDIT scores and BOLD response during feedback for inaccurate trials relative to feedback for accurate trials [r'sinacc=-.26 to -.18, r'sacc = .05 to 0.14; Steiger’s-Z's=-3.47 to -4.30, p's<.001, partial η2's = 0.087-0.124, Cohen's d's = 0.62-0.75].
Fig. 3.
(A) CUDIT-by-Accuracy interaction within the putamen; (B) CUDIT-by-Reinforcement-by-Accuracy interaction effect within the putamen; and (C) ACC/dmPFC. Scatterplots depict significant partial correlations and standardized residuals for each of the regions. Adjusted residuals for the Rankit transformed z-scored AUDIT scores (x-axis) are plotted against adjusted residuals for BOLD responses to Incorrect versus Correct trials (Outcome phase; y-axis). Note that the CUDIT-by-Reinforcement-by-Accuracy interactions within the (B) putamen and (C) ACC/dmPFC are broken down according to whether the trials were for reward or punishment. * indicate the significant inverse relationships.
3.3.2.4. CUDIT-by-reinforcement-by-accuracy interaction
There was a significant CUDIT-by-Reinforcement-by-Accuracy interaction within the right putamen and the left ACC/dmPFC (SVC; Fig. 3, Table 4). Within all regions, there was a significantly stronger negative relationship between CUDIT scores and BOLD response during feedback on inaccurate punishment trials [r's=-.29 to -.24] relative to all other outcomes [all other r's=-0.05 to .12; Steiger’s-Z's=-2.13 to -3.62, p's<.05, partial η2's = 0.098-0.123, Cohen's d's = 0.66-0.75].
3.4. Potential confounds
Calculation of Mahalanobis Distances revealed three multivariate outliers; therefore, the same analysis was repeated with these outliers removed from the dataset (Tables S3-S4). To rule out the possibility that smoking may have influenced our results, this analysis was repeated with participants who endorsed current regular smoking removed from the sample (Tables S5-S6). Since MID performance was related to AUDIT scores and CUDIT scores, the same analysis was repeated with MID accuracy as a covariate (Tables S7-S8). Additionally, there is evidence that males and females may be differentially affected by alcohol and cannabis (Caldwell et al., 2005; Ketcherside et al., 2016; Peters et al., 2015); therefore, the same analysis was repeated with gender was entered as a covariate (Tables S9-S10). Furthermore, since participants were not asked to withhold antidepressant medication prior to scanning, the same analysis was repeated with antidepressant use as a covariate (Tables S11-S12). Finally, to rule out the possibility that placement may have influenced our results, this analysis was repeated with placement (Boys Town versus Community) as a covariate (Tables S13-S14). These analyses yielded similar patterns of results. Since there were no differential correlations between AUDIT/CUDIT scores and ADHD symptoms, conduct problems, depressive symptoms, and anxiety symptoms, we did not repeat these analyses with these measures entered as covariates.
4. Discussion
This study examined the relationships between AUDIT and CUDIT scores and dysfunction in neural systems underlying reinforcement outcomes in adolescents with varying degrees of AUD and CUD symptoms. There were three main findings that revealed distinct relationships between AUD or CUD symptoms and neural dysfunction. First, increasing AUDIT score was associated with decreasing striatal modulation by magnitude of reward received. Second, increasing AUDIT score was associated with reduced PCC recruitment during feedback for reward relative to punishment trials. Third, increasing CUDIT score was associated with decreased activation in putamen during feedback on inaccurate trials relative to accurate trials and putamen and ACC/dmPFC during feedback on inaccurate punishment trials relative to all other trials.
In line with our predictions, increased AUDIT scores were associated with decreased BOLD response modulation by magnitude of reward receipt in the ventral striatum (VS). The VS is a brain region that is sensitive to reward receipt (Diekhof et al., 2012; Oldham et al., 2018) and has been implicated in SUDs (Koob and Volkow, 2016). Previous work has shown has shown that alcohol dependence in rats is associated with decreased dopaminergic activity in the striatum and decreased sensitivity to non-drug rewards (Koob and Volkow, 2016) and that alcohol dependence in human adults is associated with reduced dopamine transmission in the striatum (Martinez et al., 2005). As such, the current study is in line with previous work in alcoholic adults (Beck et al., 2009; Wrase et al., 2007) and adolescents with elevated AUDIT scores (Nees et al., 2015) in finding that increasing AUDIT scores were associating with progressively blunted striatal responses to reward (Beck et al., 2009; Nees et al., 2015; Wrase et al., 2007). Importantly, the current study extends these previous findings by: (i) revealing that this deficient response to reward is seen within other regions implicated in reward processing (PCC); and (ii) showing that this association was selective for AUDIT but not CUDIT scores (see also below).
Our second finding was that there was a significantly stronger negative relationship between AUDIT scores and PCC activation on feedback during reward trials relative to punishment trials; increasing level of AUD symptomatology was associated with decreased responsiveness to reward within PCC. The PCC has been reliably linked to the representation of subjective value; increasing responsiveness as a function of increasing reward level (Clithero and Rangel, 2013). Moreover, PCC may use this information to guide attention to external stimuli (Gusnard and Raichle, 2001). The processes underlying attention to external stimuli are thought to be dysfunctional in individuals with SUDs (DeWitt et al., 2015). Individuals with SUDs are thought to be hyper-attentive to drug-related stimuli but hypo-attentive to non-drug-related stimuli (DeWitt et al., 2015). In adults and adolescents with AUD, there is increased activity in PCC and precuneus when viewing alcoholic beverages compared to controls (Tapert et al., 2003; Wrase et al., 2007), but reduced responses in these brain regions during monetary loss avoidance (Wrase et al., 2007) and during monetary gain (Crowley et al., 2010). We suggest that hypo-reward sensitivity within VS reduces reward-related activity within PCC potentially compromising attention to stimuli associated with (non-drug) reward receipt. In short, the current data indicate that increasing AUDIT scores in adolescents are associated with decreasing reward responsiveness and consequent decreased attention to stimuli associated with (non-drug) reward.
It should be noted that CUDIT score, unlike AUDIT score, was unrelated to striatal modulation by reward. In some respects, this is unexpected. One prominent model of substance abuse suggests that substance use leads to dependence through the down-regulation of striatal dopamine receptors (Koob and Volkow, 2016). The current data indicate that this component of the model at least is less applicable to understanding cannabis abuse. However, while somewhat unexpected theoretically, it is less unexpected on the basis of the previous literature. Thus, there has been marked inconsistency regarding level of reward responsiveness in cannabis-using adults with studies indicating hypo-responsiveness (Martz et al., 2016; van Hell et al., 2010) but many finding no group differences (Filbey et al., 2013; Karoly et al., 2015). It is possible that acute cannabis relative to acute alcohol use has a lesser impact on striatal dopamine release (and thus less down-regulation of striatal dopamine receptors following cannabis abuse relative to alcohol abuse).
Our third finding was that there was a stronger negative relationship between CUDIT scores and BOLD responses while receiving feedback for inaccurate trials within the putamen and inaccurate punished trials within ACC/dmPFC. The putamen and ACC/dmPFC have been implicated in the error detection network (Steele et al., 2014). Neuroimaging data indicate that adults with chronic cannabis use histories show reduced activations in these brain regions during error detection, and that these dysfunctions are related to decreased error awareness (Hester et al., 2009) and impaired learning (Carey et al., 2015; Wesley et al., 2011). In particular, Wesley et al. reported that adult chronic cannabis users showed decreased ACC/dmPFC activation to loss outcomes and impaired learning during the Iowa Gambling Task compared to healthy controls (2011). This suggests that chronic cannabis users may be less sensitive to negative feedback. Consistent with adult fMRI work, our data indicate that increasing CUDIT scores are associated with decreased striatal and ACC/dmPFC responsivity to errors, particularly those resulting in negative outcomes, in an adolescent sample.
The results of this study must be viewed in light of five caveats. First, we did not conduct urine or Breathalyzer testing at the time of scanning. However, all of the youths with significant substance abuse histories were residents of a highly supervised residential treatment facility and were abstinent for at least four weeks prior to scanning, mitigating this concern. Second, this study was cross-sectional. As such, it is not possible to be certain whether the observed relationships reflected impact of alcohol and/or cannabis abuse on the developing brain or pre-existing risk factors for AUD and/or CUD symptomatology. However, it is important to note given the differential nature of the current findings (AUD relative to CUD), pre-existing neural risk factors could only be driving these results if these risk factors are selective for the emergence of AUD or CUD. While this may be the case, it has not been documented previously. Third, and in line with previous epidemiological work (Kuperman et al., 2001; Moss and Lynch, 2001; Pardini et al., 2007), increasing AUDIT and CUDIT scores were associated with increasing psychiatric psychopathology. However, AUDIT relative to CUDIT scores were not significantly more (or less) related to any psychiatric diagnosis/symptom set. As such, it is unlikely that the observed differential findings could reflect the pathophysiology of co-morbid psychiatric conditions. While it might be possible to investigate participants without co-morbid psychopathology, such participants would be atypical; the vast majority of youths with AUD and/or CUD have at least one co-morbid psychiatric condition (Gattamorta et al., 2017). Fourth, additional indices of substance abuse were not available (e.g., age of first use, cumulative exposure). As such, it is important to note that our findings reflect neural correlates of AUD versus CUD symptom severity, not level of prior alcohol/cannabis use. The current data cannot be used as dose dependent indices of AU/CU. Instead, they indicate that increasing AUD/CUD symptom severity is associated with differentially increasing disrupted neural processing. Fifth, it could be argued that the high correlation between AUDIT and CUDIT scores means that it is difficult to parse out the specific relationships between BOLD responses and AUDIT versus CUDIT scores. However, this concern is mitigated by: (i) our finding that the VIFS for our predictor variables were all <2.5 indicating acceptable levels of collinearity; and (ii) our finding that single covariate analyses (AUDIT or CUDIT) confirmed our core findings with respect to ventral striatum (see Table 3) and putamen and ACC/dmFC (see Table 4). Moreover, there were no significant activations within these regions for the other covariate even at p < 0.05. Thus, while AUDIT scores as a single covariate were associated with significantly reduced ventral striatal reward responsiveness in a highly proximal region to our main analysis, there were no indication of a relationship between CUDIT scores as a single covariate and reduced ventral striatal reward responsiveness.
In summary, we found that AUDIT score was related to reduced reward responsiveness within VS and PCC. However, CUDIT score was particularly related to reduced responsiveness to punished errors within brain regions involved in error detection including ACC/dmPFC and dorsal striatum. These data suggest that there are differential neural correlates of AUD versus CUD symptomatology in adolescents.
Conflicts of interest
All authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgements
We would like to thank Ron Copsey, Kim VanHorn, Michael Wright, Mark Timm, and Rhonda Tuel for their contributions to data collection. We would like to thank all subjects and their families for their participation. This work was supported by Boys Town National Research Hospital. JA was supported by the AACAP Jeanne Spurlock Medical Student Research Fellowship in Substance Abuse and Addiction and a Program of Excellence Fellowship from the University of Nebraska Medical Center. RJB was supported by K22-MH109558 and SFW was supported by K01-MH110643. The funders had no role in study design, data collection, data analysis, decision to publish, or manuscript preparation.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100618.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Achenbach T.M., Rescorla L.A. 2001. Manual for the ASEBA School-Age Forms and Profiles. [Google Scholar]
- Adamson S.J., Sellman J.D. A prototype screening instrument for cannabis use disorder: the Cannabis use Disorders Identification Test (CUDIT) in an alcohol-dependent clinical sample. Drug Alcohol Rev. 2003;22:309–315. doi: 10.1080/0959523031000154454. [DOI] [PubMed] [Google Scholar]
- Adamson S.J., Kay-Lambkin F.J., Baker A.L., Lewin T.J., Thornton L., Kelly B.J., Sellman J.D. An improved brief measure of cannabis misuse: the Cannabis use disorders identification test-revised (CUDIT-R) Drug Alcohol Depend. 2010;110:137–143. doi: 10.1016/j.drugalcdep.2010.02.017. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth edition. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Angold A., Costello E.J., Messer S.C., Pickles A., Winder F., Silver D. The development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int. J. Methods Psychiatr. Res. 1995 https://doi.org/1049-8931/95/040251-12 [Google Scholar]
- Babor T.F., Hofmann M., DelBoca F.K., Hesselbrock V., Meyer R.E., Dolinsky Z.S., Rounsaville B. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch. Gen. Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Beck A., Schlagenhauf F., Wüstenberg T., Hein J., Kienast T., Kahnt T., Schmack K., Hägele C., Knutson B., Heinz A., Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Birmaher B., Khetarpal S., Brent D., Cully M., Balach L., Kaufman J., Neer S.M. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Psychosocial problems and recruitment of incentive neurocircuitry: exploring individual differences in healthy adolescents. Dev. Cogn. Neurosci. 2011;1:570–577. doi: 10.1016/j.dcn.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss C.I., Greenwood M.L., White E.S. A rankit analysis of paired comparisons for measuring the effect of sprays on flavor. Biometrics. 1956;12:381–403. [Google Scholar]
- Bossong M.G., van Berckel B.N., Boellaard R., Zuurman L., Schuit R.C., Windhorst A.D., van Gerven J.M.a, Ramsey N.F., Lammertsma A.a, Kahn R.S. Δ9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Caldwell L.C., Schweinsburg A.D., Nagel B.J., Valerie C., Brown S.A., Tapert S.F. Gender and adolescent alcohol use disorders on BOLD response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey S.E., Nestor L., Jones J., Garavan H., Hester R. Impaired learning from errors in cannabis users: Dorsal anterior cingulate cortex and hippocampus hypoactivity. Drug Alcohol Depend. 2015;155:175–182. doi: 10.1016/j.drugalcdep.2015.07.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Taylor P.A., Cox R.W. Is the Statistic Value All We Should Care about in Neuroimaging? bioRxiv. 2016 doi: 10.1016/j.neuroimage.2016.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus E.D., Feldstein Ewing S.W., Filbey F.M., Hutchison K.E. Behavioral control in alcohol use disorders: relationships with severity. J. Stud. Alcohol Drugs. 2013;74:141–151. doi: 10.15288/jsad.2013.74.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero J.A., Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc. Cogn. Affect. Neurosci. 2013;9:1289–1302. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7:152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T.J., Dalwani M.S., Mikulich-Gilbertson S.K., Du Y.P., Lejuez C.W., Raymond K.M., Banich M.T. Risky decisions and their consequences: neural processing by boys with Antisocial Substance Disorder. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- DeWitt S.J., Ketcherside A., McQueeny T.M., Dunlop J.P., Filbey F.M. The hyper-sentient addict: an exteroception model of addiction. Am. J. Drug Alcohol Abuse. 2015;41:374–381. doi: 10.3109/00952990.2015.1049701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Kaps L., Falkai P., Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Dugré J.R., Dumais A., Bitar N., Potvin S. Loss anticipation and outcome during the Monetary Incentive Delay Task : a neuroimaging systematic review and meta-analysis. PeerJ. 2018;6 doi: 10.7717/peerj.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairlie A.M., Sindelar H.A., Eaton C.A., Spirito A. Utility of the AUDIT for screening adolescents for problematic alcohol use in the emergency department. Int. J. Adolesc. Med. Heal. 2006;18:115–122. doi: 10.1515/ijamh.2006.18.1.115. [DOI] [PubMed] [Google Scholar]
- Filbey F.M., Dunlop J., Myers U.S. Neural effects of positive and negative incentives during marijuana withdrawal. PLoS One. 2013:8. doi: 10.1371/journal.pone.0061470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F.M., McQueeny T., DeWitt S.J., Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev. Cogn. Neurosci. 2015;16:16–22. doi: 10.1016/j.dcn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front. Hum. Neurosci. 2010;4:1–9. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattamorta K.A., Mena M.P., Ainsley J.B., Santisteban D.A. The comorbidity of psychiatric and substance use disorders among hispanic adolescents. J. Dual Diagn. 2017;13:254–263. doi: 10.1080/15504263.2017.1343965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D., Raichle M. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Heitzeg M.M., Villafuerte S., Weiland B.J., Enoch M.-A., Burmeister M., Zubieta J.-K., Zucker R.a. Effect of GABRA2 genotype on development of incentive-motivation circuitry in a sample enriched for alcoholism risk. Neuropsychopharmacology. 2014;39:1–10. doi: 10.1038/npp.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein S.M., Kirwan M.L., Benson B.E., Hardin M.G., Pine D.S., Ernst M., Fox N.A. Validation of a child-friendly version of the monetary incentive delay task. Soc. Cogn. Affect. Neurosci. 2013;8:720–726. doi: 10.1093/scan/nss057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R., Nestor L., Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM SPSS Statistics For MacOSX . IBM Corp.; Armonk, NY: 2012. IBM SPSS Statistics For MacOSX. [Google Scholar]
- Jager G., Block R.I., Luijten M., Ramsey N.F. Tentative evidence for striatal hyperactivity in adolescent cannabis-using boys: a cross-sectional multicenter fMRI study. J. Psychoact. Drugs. 2013;45:156–167. doi: 10.1080/02791072.2013.785837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly H.C., Bryan A.D., Weiland B.J., Mayer A., Dodd A., Feldstein Ewing S.W. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev. Cogn. Neurosci. 2015;16:1–11. doi: 10.1016/j.dcn.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcherside A., Baine J., Filbey F. Sex effects of marijuana on brain structure and function. Curr. Addict. Rep. 2016;3:323–331. doi: 10.1007/s40429-016-0114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman S., Schlosser S.S., Kramer J.R., Bucholz K., Hesselbrock V., Reich T., Reich W. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. Am. J. Psychiatry. 2001;158:2022–2026. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- Lee I.A., Preacher K.J. 2013. Calculation for the Test of the Difference Between Two Dependent Correlations With One Variable in Common. [Google Scholar]
- Li C.R., Luo X., Yan P., Bergquist K., Sinha R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol Clin. Exp. Res. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D., Gil R., Slifstein M., Hwang D.R., Huang Y., Perez A., Kegeles L., Talbot P., Evans S., Krystal J., Laruelle M., Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol. Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Martz M.E., Trucco E.M., Cope L.M., Hardee J.E., Jester J.M., Zucker R.A., Heitzeg M.M. Association of marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry. 2016;73:838. doi: 10.1001/jamapsychiatry.2016.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R.A., Johnston L.D., O’Malley P.M., Bachman J.G., Schulenberg J.E. Secondary School Students. 2016. Monitoring the Future national survey results on drug use, 1975-2015: Volume I. [Google Scholar]
- Moss H.B., Lynch K.G. Comorbid disruptive behavior disorder symptoms and their relationship to adolescent alcohol use disorders. Drug Alcohol Depend. 2001;64:75–83. doi: 10.1016/s0376-8716(00)00233-7. [DOI] [PubMed] [Google Scholar]
- Moss H.B., Chen C.M., Yi Hye. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 2014;136:51–62. doi: 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Nee D.E., Kastner S., Brown J.W. Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. Neuroimage. 2011;54:528–540. doi: 10.1016/j.neuroimage.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees F., Witt S.H., Dinu-Biringer R., Lourdusamy A., Tzschoppe J., Vollstadt-Klein S., Millenet S., Bach C., Poustka L., Banaschewski T., Barker G.J., Bokde A.L.W., Bromberg U., Buchel C., Conrod P.J., Frank J., Frouin V., Gallinat J., Garavan H., Gowland P., Heinz A., Ittermann B., Mann K., Martinot J.L., Paus T., Pausova Z., Robbins T.W., Smolka M.N., Rietschel M., Schumann G., Flor H. BDNF Val66Met and reward-related brain function in adolescents: role for early alcohol consumption. Alcohol. 2015;49:103–110. doi: 10.1016/j.alcohol.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Nestor L., Hester R., Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. Neuroimage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S., Murawski C., Fornito A., Youssef G., Yücel M., Lorenzetti V. The anticipation and outcome phases of reward and loss processing: a neuroimaging meta-analysis of the monetary incentive delay task. Hum. Brain Mapp. 2018:1–21. doi: 10.1002/hbm.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini D., White H.R., Stouthamer-Loeber M. Early adolescent psychopathology as a predictor of alcohol use disorders by young adulthood. Drug Alcohol Depend. 2007;88:38–49. doi: 10.1016/j.drugalcdep.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Jolles D.J., Van Duijvenvoorde A.C.K., Crone E.A., Peper J.S. The link between testosterone and amygdala-orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinology. 2015;53:117–126. doi: 10.1016/j.psyneuen.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Saunders J.B., Aasland O.G., Babor T.F., de la Fuente J.R., Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Squeglia L.M., Tapert S.F., Sullivan E.V., Jacobus J., Meloy M.J., Rohlfing T., Pfefferbaum A. Brain development in heavy-drinking adolescents. Am. J. Psychiatry. 2015;172:531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele V.R., Claus E.D., Aharoni E., Harenski C., Calhoun V.D., Pearlson G., Kiehl K.A. A large scale (N=102) functional neuroimaging study of error processing in a Go/NoGo task. Behav. Brain Res. 2014;268:127–138. doi: 10.1016/j.bbr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. 1st ed. Thieme; Stuttgart: 1988. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Tapert S.F., Cheung E.H., Brown G.G., Frank L.R., Paulus M.P., Schweinsburg A.D., Meloy M.J., Brown S.A. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch. Gen. Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- van Hell H.H., Vink M., Ossewaarde L., Jager G., Kahn R.S., Ramsey N.F. Chronic effects of cannabis use on the human reward system: an fMRI study. Eur. Neuropsychopharmacol. 2010;20:153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Van Hulst B.M., De Zeeuw P., Lupas K., Bos D.J., Neggers S.F.W., Durston S. Reward anticipation in ventral striatum and individual sensitivity to reward: a pilot study of a child-friendly fMRI task. PLoS One. 2015;10:1–9. doi: 10.1371/journal.pone.0142413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Koob G.F., McLellan A.T. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med. 2016;374:363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. second edition. NCS Pearson; San Antonio, TX: 2011. Wechsler Abbreviated Scale of Intelligence–. [Google Scholar]
- Wesley M.J., Hanlon C.A., Porrino L.J. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. - Neuroimaging. 2011;191:51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters K.C., Lee C.Y.S. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug Alcohol Depend. 2008;92:239–247. doi: 10.1016/j.drugalcdep.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J., Schlagenhauf F., Kienast T., Wüstenberg T., Bermpohl F., Kahnt T., Beck A., Ströhle A., Juckel G., Knutson B., Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.