Abstract
Objective.
Delayed responses observed with immune checkpoint blockade (ICB) present a challenge for patients with peritoneal malignancies, who risk early symptomatic disease progression requiring treatment discontinuation. While efforts are ongoing to define the biomarkers of response, it is equally important to identify patients at risk for early discontinuation. We sought to investigate the timing of disease progression in epithelial ovarian cancer (EOC) patients treated with ICB and to identify pre-treatment clinical parameters associated with early discontinuation.
Methods.
Retrospective analysis was performed on EOC patients treated with ICB at MSKCC from January 2013 to May 2017. Cutoffs for early and very early discontinuation due to disease progression were defined at 12 and 8 weeks, respectively. Univariate and multivariate logistic regression models were built based on pre-treatment clinical variables.
Results.
Of 108 identified patients, 89 were included in the analysis. Forty-six (51.7%) patients discontinued therapy early, 30 of which (33.7%) discontinued therapy very early. Eight patients (9.0%) died within 12 weeks of ICB initiation from disease progression. In multivariate analyses, bulky peritoneal disease (p = 0.009, OR: 4.94) and liver parenchymal metastases (p = 0.001, OR: 8.08) were associated with early discontinuation. Liver parenchymal metastases (p = 0.001, OR 6.64), and high neutrophil-to-lymphocyte ratio (p = 0.021, OR: 3.54), were associated with very early discontinuation.
Conclusions.
Over 50% of EOC patients suffer disease progression requiring early discontinuation of ICB. Pre-treatment prognostic clinical characteristics may identify patients at highest risk for early discontinuation due to disease progression and warrant caution in using these agents in late line patients with advanced disease.
Keywords: Ovarian cancer, Immunotherapy, PD-1, CTLA-4
1. Introduction
Breakthroughs in tumor immunology have led to development of novel therapies targeting the mechanisms governing tumor resistance to the immune system, such as programmed death receptor-1 (PD-1) its ligand PD-L1, and cytotoxic T-lymphocyte-associated antigen-4 receptor (CTLA-4) [1]. Based on these findings, therapy with immune checkpoint blockade (ICB) has been evaluated in several trials in epithelial ovarian cancer (EOC). However, compared to some solid tumors, the response rates of EOC patients have been modest [2–4]. Furthermore, the median progression-free survival reported in all EOC studies is very short, typically coinciding with the first protocol-defined scan, leading to treatment discontinuation in the majority of patients [2–4]. While an effort is ongoing to identify the patients that are more likely to derive benefit from ICB, it is equally important to determine which patients are the ones that are more likely to discontinue before the first scan and thus are unlikely to derive benefit.
Immune checkpoint inhibitor antibodies do not target cancer cells directly, but rather exert actions indirectly through the immune system [5]. Consequently, in comparison to chemotherapy, time to response to ICB can be delayed [4,6,7]. In a recently-reported NRG-GY003 study of nivolumab and nivolumab with ipilimumab in EOC by Burger et al., tumor responses were frequently delayed, with one patient responding only after 6 months of therapy [8]. Moreover, although uncommon, early findings may include an initial increase in tumor burden or appearance of new lesions prior to subsequent disease response or stabilization, a phenomenon referred to as pseudoprogression [6,9]. As such, treatment beyond first radiographic progression, typically performed at 8 to 12 weeks, is commonly recommended in patients receiving ICB, provided that the patients remain asymptomatic.
While delayed response or disease stabilization may be tolerated in some cancer types with isolated tumor lesions, it may present a challenge for advanced EOC patients, whose metastatic disease pattern is often characterized by peritoneal carcinomatosis, the progression of which may lead to early clinical symptoms such as abdominal pain and refractory ascites. Furthermore, these patients may experience irreversible disease-related risks such as bowel obstructions and fistulas, which may make them ineligible to receive further cancer-directed treatment. These symptoms may preclude the patients from treatment beyond the first scan, and some patients may be at risk for discontinuation even prior to the first protocol-scheduled disease assessment. Finally, a phenomenon of hyper-progression on immune checkpoint inhibitors has been recently reported in several cancer types [10–12], where an apparent acceleration in tumor progression was observed upon initiation of ICB.
It is thus of importance to identify patients that are at risk for early treatment discontinuation to avoid exposing these patients to the risks of disease progression and potential drug toxicities.
As such patients are typically not analyzed extensively within the context of efficacy assessment in clinical trials, in the current study we performed a single-institution analysis of the EOC patients that received ICB agents, specifically focusing on the patients that discontinue therapy early. We find that early discontinuation of therapy due to disease progression is very common in these patients. Previous work has identified several clinical characteristics, including ECOG performance status, hemoglobin, and albumin, that could predict early discontinuation in patients enrolled into phase I clinical trials across multiple cancer types and agents [13]. Additional clinical parameters such as absolute lymphocyte count (ALC), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) may indirectly reflect the integrity of the immune system [14–16]. Using these clinical characteristics, we identify several pre-treatment radiographic, and laboratory parameters associated with the risk of early treatment discontinuation of immunotherapy in patients with EOC.
2. Methods
Patients who received ICB for treatment of EOC, primary peritoneal cancer, and fallopian tube cancer from January 1, 2013 to May 20, 2017 at Memorial Sloan Kettering Cancer Center were identified. Patients who received immunotherapy drugs in combination with cytotoxic therapy were excluded, as early kinetics of response to these combinations is likely to be similar to cytotoxic therapy alone. Patients who discontinued early due to drug-related toxicity were excluded. Approval to conduct this study was received from the Memorial Sloan Kettering Cancer Center Institutional Review Board.
Immunotherapy targets of interest included PD-1, PD-L1, CTLA-4, and LAG3 alone or in combination. All patients except for 4 were treated on prospective clinical protocols and followed protocol-defined radio-graphic assessment schedules. Discontinuation date was identified as the off-treatment date documented by the treating physician. Patients were defined as having discontinued early or very early if therapy was stopped at or prior to 12 weeks or 8 weeks, respectively, due to clinical or radiographic progression. The selected cutoff times were based on the commonly-used protocol-mandated imaging schedules, as demonstrated in recently published ICB clinical trials in ovarian cancer [17,19].
Clinical and treatment data were retrospectively collected from electronic medical records. The following pre-treatment clinical properties were analyzed: age at diagnosis, body mass index (BMI), prior lines of therapy, histology, disease volume, peritoneal carcinomatosis, ascites, serum albumin, absolute lymphocyte count (ALC), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and the presence of metastases (irrespective of size) in liver parenchyma, lung or pleura, and bone on the basis of prior reports [15,20–24]. A separate univariate analysis was conducted on the presence or absence of germline BRCA (gBRCA), for those patients with gBRCA data available in the EMR. Extent of disease was identified by review of radiologic image reports, and peritoneal carcinomatosis was recorded as present if defined as such by the reading radiologist. Bulky peritoneal disease was defined as the presence of any baseline peritoneal lesion greater than or equal to 5 cm, based on prior studies [25,26]. A larger tumor burden has been shown to be a negative predictor of clinical outcomes and response to PD-1 blockade in melanoma [27]. Ascites was documented as present if defined as greater than or equal to small volume ascites on the pre-treatment CT radiology reports. Presence or absence of symptoms was assessed by the review of EMR documentation at the time of treatment discontinuation.
Descriptive statistics were listed for the clinical factors of interest for the whole cohort as well as the early and late discontinuation groups. Logistic regression was applied to examine the clinical factors predicting early discontinuation in univariate setting first. A multivariable logistic regression model was built based on the univariate results (factors with p value < 0.05 in univariate setting were added in the multivariable model). All patients had a minimum of 12 weeks follow up if they did not discontinue early. The primary analysis considered events only those patients who stopped therapy at or prior to 12 weeks due to clinical or radiographic progression. A secondary analysis focused on early discontinuation defined as stopping therapy at 8 weeks. A supplemental analysis looked at early discontinuation secondary to symptomatic disease progression at 8 weeks. p-Value < 0.05 was considered significant. The receiver operating characteristic (ROC) curves were used to plot the sensitivity along the y-axis and the “1-specificity” along the x-axis for comparing the multivariate model prediction versus the true binary outcome. All analyses were performed using SAS v9.4 and R v3.4.2.
3. Results
3.1. Early discontinuation of immune checkpoint blockade immunotherapy is common in patients with ovarian cancer
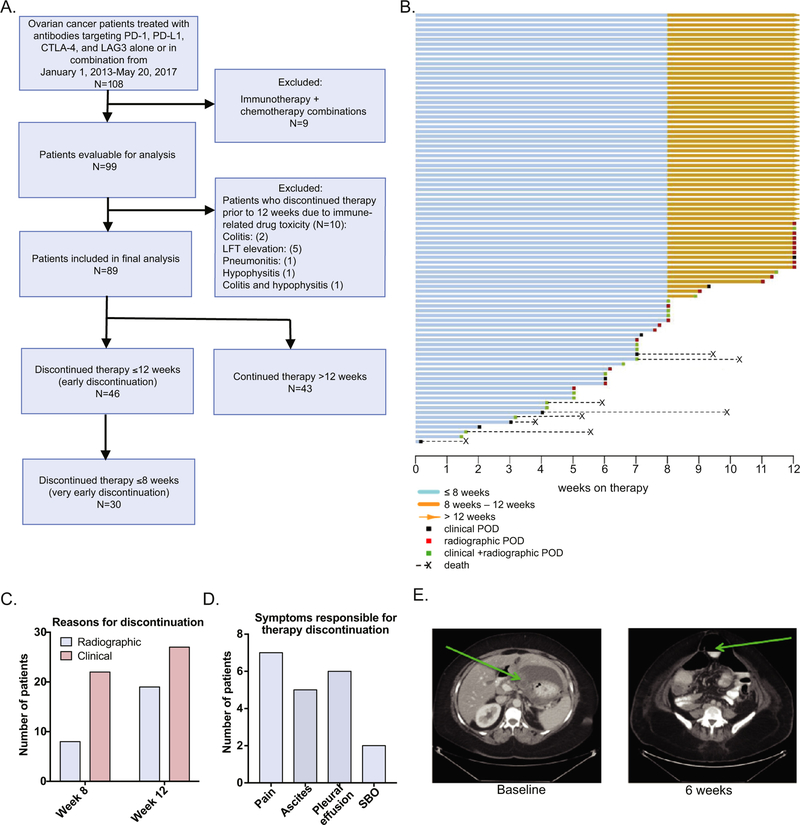
Data on 108 patients with EOC treated at Memorial Sloan Kettering Cancer Center on ICB agents were reviewed (Fig. 1A). Nine (8.3%) patients were excluded for treatment with immunotherapy in combination with chemotherapy. Ten (9.2%) patients were excluded for discontinuation early due to immune-related drug toxicity (Fig. 1A), as disease status (progression vs. non-progression) for these patients was not available. Of the final cohort of 89 patients, the majority (N = 85, 95.5%) received ICB while on a clinical trial, while 4 patients (4.5%) patients were treated with compassionate use therapy. Treatment agents and combinations included single-agent anti-PD-1/PD-L1 (34, 38.2%), anti-CTLA-4 (2, 2.2%), anti-LAG3 (3, 3.4%), combination of anti-CTLA-4 with anti-PD-1/PD-L1 (35, 39.3%), and combination of anti-PD-1/PD-L1 with other immunomodulatory agents (15, 16.9%).
Fig. 1.
Study flow diagram and summary of 12-week outcomes in the study cohort. A. CONSORT diagram. B. Swimmers plot for the entire study cohort summarizing length of therapy, reason for treatment discontinuation, and disease-related deaths occurring before 12 weeks. C. Number of patients who discontinue therapy secondary to asymptomatic (radiographic) or symptomatic disease progression at or before 8 and 12 weeks. D. Top reasons for discontinuation due to clinical progression before 12 weeks. E. Clinical disease progression in the setting of baseline bulky disease. Baseline CT scan demonstrates bulky disease with 8 × 3 cm metastatic implant along the lesser curvature of the stomach. A CT scan at approximately 6 weeks of treatment demonstrated disease progression resulting in small bowel obstruction. SBO: small bowel obstruction.
Over half of the patients (N = 46, 51.6%) discontinued treatment early (less than or equal to 12 weeks after treatment initiation) due to radiographic or clinical disease progression (Fig. 1B). Of note, 8 patients (9.0%) died from complications related to disease progression within the first 12 weeks of receiving ICB therapy. Baseline patient characteristics are shown in Table 1. The median patient age at start of therapy was 58 years (range, 24–77 years). The majority of the patients had high grade serous carcinoma (83.1%) and were heavily-pretreated, with a median of 4 prior lines of therapy (range, 1–12). All patients had platinum-resistant disease, defined by platinum-free interval of <6 months from the most recent platinum-based therapy.
Table 1.
Baseline patient characteristics.
| Clinical characteristic | Whole cohort N = 89 |
≤12 weeks N = 46 (51.7%) |
>12 weeks N = 43 (48.3%) |
≤8 weeks N = 30 (33.7%) |
>8 weeks N = 59 (66.3%) |
|---|---|---|---|---|---|
| Enrollment period | |||||
| 2013–2015 | 27 (30.3%) | 14 (51.9%) | 13 (48.1%) | 9 (33.3%) | 18 (66.7%) |
| 2016–2017 | 62 (69.7%) | 32 (51.6%) | 30 (48.4%) | 21 (33.9%) | 41 (66.1%) |
| Age at diagnosis [years] | |||||
| <63 | 64 (71.9%) | 36 (56.2%) | 28 (43.8%) | 24 (37.5%) | 40 (62.5%) |
| ≥63 | 25 (28.1%) | 10 (40%) | 15 (60%) | 6 (24%) | 19 (76%) |
| ECOG screening, n (%) | |||||
| 0 | 48 (53.9%) | 23 (48%) | 25 (52%) | 15(31.3%) | 33(68.8%) |
| 1 | 41 (46.1%) | 23 (56%) | 18 (44%) | 15(36.6%) | 26(63.4%) |
| Median number of prior lines of therapy (range) | 4 (1–12) | 4 (1–12) | 4 (1–11) | 4 (1–11) | 4 (1–12) |
| Histology | |||||
| High grade serous | 74 (83.1%) | 39 (52.7%) | 35 (47.3%) | 26 (35.1%) | 48 (64.9%) |
| Clear cell carcinoma | 8 (9.0%) | 3 (37.5%) | 5 (62.5%) | 2 (25%) | 6 (75%) |
| Othera | 7 (7.9%) | 4 (57.1%) | 3 (42.9%) | 2 (28.6%) | 5 (71.4%) |
| Germline BRCA mutation | |||||
| gBRCA1mut | 11(12.4%) | 8 (72.7%) | 3 (27.3%) | 5 (45.5%) | 6 (54.5%) |
| gBRCA2mut | 6(6.7%) | 5 (83.3%) | 1 (16.7%) | 3 (50.0%) | 3 (50.0%) |
| gBRCAwt_ | 51(57.3%) | 26 (51.0%) | 25 (49.0%) | 16 (31.8%) | 35 (68.6%) |
| Unknown | 21(23.6%) | 12 (57.1%) | 9 (42.9%) | 6 (28.6%) | 15 (71.4%) |
| Body mass index (BMI) [kg/m2] | |||||
| <25 | 39 (43.8%) | 23 (59%) | 16 (41%) | 12 (30.8%) | 27 (69.2%) |
| 25–30 | 24 (27%) | 10 (41.7%) | 14 (58.3%) | 10 (41.7%) | 14 (58.3%) |
| >30 | 26 (29.2%) | 13 (50%) | 13 (50%) | 8 (30.8%) | 18 (69.2%) |
| Prior lines of therapy | |||||
| 0–2 | 14 (15.7%) | 7 (50%) | 7 (50%) | 3 (21.4%) | 11 (78.6%) |
| 3 | 17 (19.1%) | 8 (47.1%) | 9 (52.9%) | 4 (23.5%) | 13 (76.5%) |
| ≥4 | 58 (65.2%) | 31 (53.4%) | 27 (46.6%) | 23 (39.7%) | 35 (60.3%) |
| Disease volume at study entry | |||||
| Non-bulky | 65 (73%) | 27 (41.5%) | 38 (58.5%) | 18 (27.7%) | 47 (72.3%) |
| Bulky | 24 (27%) | 19 (79.2%) | 5 (20.8%) | 12 (50%) | 12 (50%) |
| Carcinomatosis at study entry | |||||
| Not present | 59 (66.3%) | 28 (47.5%) | 31 (52.5%) | 18 (30.5%) | 41 (69.5%) |
| Present | 30 (33.7%) | 18 (60%) | 12 (40%) | 12 (40%) | 18 (60%) |
| Ascites at study entry | |||||
| Not present | 69 (77.5%) | 33 (47.8%) | 36 (52.2%) | 20 (29%) | 49 (71%) |
| Present | 20 (22.5%) | 13 (65%) | 7 (35%) | 10 (50%) | 10 (50%) |
| Albumin [g/dL] | |||||
| <4 | 33 (37.1%) | 20 (60.6%) | 13 (39.4%) | 16 (48.5%) | 17 (51.5%) |
| ≥4 | 56 (62.9%) | 26 (46.4%) | 30 (53.6%) | 14 (25%) | 42 (75%) |
| Absolute lymphocyte count (ALC) [K/μL] | |||||
| <1.2 | 39 (43.8%) | 18 (46.2%) | 21 (53.8%) | 15 (38.5%) | 24 (61.5%) |
| ≥1.2 | 50 (56.2%) | 28 (56%) | 22 (44%) | 15 (30%) | 35 (70%) |
| Neutrophil-to-lymphocyte ratio (NLR) | |||||
| <4 | 57 (64%) | 28 (49.1%) | 29 (50.9%) | 14 (24.6%) | 43 (75.4%) |
| ≥4 | 32 (36%) | 18 (56.3%) | 14 (43.8%) | 16 (50%) | 16 (50%) |
| Platelet-to-lymphocyte ratio (PLR) | |||||
| <300 | 68 (76.4%) | 34 (50%) | 34 (50%) | 21 (30.9%) | 47 (69.1%) |
| ≥300 | 21 (23.6%) | 12 (57.1%) | 9 (42.9%) | 9 (42.9%) | 12 (57.1%) |
| Disease sites | |||||
| Liver parenchyma | 26 (29.2%) | 22 (84.6%) | 4 (15.4%) | 16 (61.5%) | 10 (38.5%) |
| Lung/pleura metastases | 39 (43.8%) | 21 (53.8%) | 18 (46.2%) | 16 (41%) | 23 (59%) |
| Bone metastases | 12 (13.5%) | 10 (83.3%) | 2 (16.7%) | 4 (33.3%) | 8 (66.7%) |
Mismatch repair protein status was known in 10 out of 15 patients with non-serous histology; 1 patient had mismatch repair deficiency.
Other histologies include: endometrioid adenocarcinoma, adenocarcinoma NOS, carcinosarcoma, mixed serous and endometrioid carcinoma, low grade serous carcinoma.
3.2. Symptomatic disease progression accounts for early therapy discontinuation in the majority of patients
Clinical symptoms were the reason for treatment discontinuation in 22 out of 30 (73%) patients prior to 8 weeks and in 27 out of 46 (59%) patients prior to 12 weeks (Fig. 1C). The most frequent reasons for discontinuation were increasing abdominal pain, ascites, pleural effusion, and small bowel obstruction (Fig. 1D). One representative patient is shown in Fig. 1E. The patient’s baseline CT scan showed bulky disease, demonstrated by a metastatic implant along the lesser curvature of the stomach, measured as 8 by 3 cm, along with prominent lesser sac ascites. Approximately 6 weeks into therapy with a PD-1-blocking agent, this patient was admitted to the hospital for abdominal cramping secondary to increase in bulky implants with extrinsic serosal disease and small bowel tethering, leading to small bowel obstruction (Fig. 1E).
3.3. Clinical parameters associated with early treatment discontinuation (≤12 weeks)
We proceeded to determine whether any pre-treatment characteristics could predict early or very early treatment discontinuation. On univariate analyses, pre-treatment bulky disease (p = 0.003, OR 5.35, CI 1.78–16.09), liver parenchymal (p < 0.001, OR 8.94, CI 2.74–29.09), and bone metastases (p = 0.031, OR 5.69, CI 1.2–27.7) were predictive of early treatment discontinuation (Table 2). Age at diagnosis, clear cell histology, body mass index (BMI), prior lines of therapy, peritoneal carcinomatosis, ascites, serum albumin, absolute lymphocyte count (ALC), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and the presence of metastases in the lung or pleura did not have a statistically significant association with early treatment discontinuation. The statistically significant variables were carried forward with multivariate analyses (Table 2). The presence of pre-treatment bulky disease (p = 0.009, OR 4.94, CI 1.49–16.43) and liver parenchymal metastases (p = 0.001, OR 8.08, CI 2.33–28.00) remained predictive of early discontinuation on multivariate analysis.
Table 2.
Univariate and multivariate predictors of early treatment discontinuation (≤12 weeks) due to clinical or radiographic progression.
| Clinical characteristic | Univariate ORa | 95% CI | p valueb |
|---|---|---|---|
| Age at diagnosis | |||
| ≥63 years vs. <63 years | 0.52 | 0.20–1.33 | 0.17 |
| Histology | |||
| Clear cell vs. HG serous carcinoma | 0.54 | 0.12–2.42 | 0.69 |
| Body mass index | |||
| 25–30 vs. <25 | 0.50 | 0.18–1.40 | 0.41 |
| >30 vs. <25 | 0.70 | 1.88–0.26 | |
| Prior lines of therapy | |||
| 3 vs. 0–2 lines of therapy | 0.89 | 0.22–3.66 | 0.89 |
| >4 vs. 0–2 | 1.15 | 0.36–3.69 | |
| Tumor size | |||
| Bulky disease | 5.35 | 1.78–16.09 | 0.003 |
| Disease properties | |||
| Ascites | 2.03 | 0.72–5.69 | 0.18 |
| Peritoneal carcinomatosis | 1.66 | 0.68–4.05 | 0.27 |
| Disease sites | |||
| Liver parenchyma metastases | 8.94 | 2.74–29.09 | b0.001 |
| Lung metastases | 1.17 | 0.50–2.70 | 0.72 |
| Bone metastases | 5.69 | 1.17–27.72 | 0.031 |
| Baseline laboratory values | |||
| Median albumin ≥ 4.0 (g/dL) | 0.56 | 0.24–1.35 | 0.94 |
| Absolute lymphocyte count (ALC) [K/μL] | |||
| ≥1.2 vs. <1.2 | 1.49 | 0.64–3.45 | 0.36 |
| Neutrophil-to-lymphocyte ratio (NLR) | |||
| ≥4 vs. <4 | 1.33 | 0.56–3.18 | 0.52 |
| Platelet-to-lymphocyte ratio (PLR) | |||
| ≥300 vs. <300 | 1.33 | 0.50–3.58 | 0.57 |
| Clinical characteristic | Multivariate ORa | 95% CI | p-Valueb |
| Tumor size | |||
| Bulky disease | 4.94 | 1.49–16.43 | 0.009 |
| Disease sites | |||
| Liver parenchyma metastases | 8.08 | 2.33–28.00 | 0.001 |
| Bone metastases | 4.47 | 0.78–25.62 | 0.093 |
Odds ratios are modeled for early treatment discontinuation.
p values < 0.05 were considered to be statistically significant.
3.4. Clinical parameters associated with very early treatment discontinuation (≤8 weeks)
A subset of early progressor patients (30 out of 89, 33.7%) discontinued treatment prior to 8 weeks (Fig. 1B), which was defined as very early discontinuation. On univariate analyses, the presence of liver parenchymal metastases (p = 0.001, OR 5.60, CI 2.08–15.05) and NLR ≥ 4 (p = 0.017, OR 3.07, CI 1.23–7.70) were predictive of very early discontinuation (Table 3). There was a small association of very early discontinuation with pre-treatment ascites and pre-treatment bulky disease, although these associations did not reach statistical significance, likely secondary to small sample numbers. On the contrary, serum albumin ≥ 4 g/dL was negatively associated with very early discontinuation (p = 0.026, OR 0.35, CI 0.14–0.88). On multivariate analysis, only liver parenchymal metastases (p = 0.001, OR 6.64, CI 2.21–19.92) and NLR (p = 0.021, OR 3.54, CI 1.21–10.31) were predictive of very early discontinuation (Table 3).
Table 3.
Univariate and Multivariate predictors of very early treatment discontinuation (≤8 weeks) due to clinical or radiographic progression.
| Clinical characteristic | Univariate ORa | 95% CI | p valueb |
|---|---|---|---|
| Age at diagnosis | |||
| ≥ 63 years vs. <63 years | 0.53 | 0.19–1.50 | 0.23 |
| Histology | |||
| Clear cell vs. HG serous carcinoma | 0.62 | 0.12–3.27 | 0.81 |
| Body mass index | |||
| 25–30 vs. <25 | 1.61 | 0.56–4.63 | 0.63 |
| >30 vs. <25 | 1.00 | 2.93–0.34 | |
| Prior lines of therapy | |||
| 3 vs. 0–2 lines of therapy | 1.13 | 0.21–6.17 | 0.28 |
| >4 vs. 0–2 | 2.41 | 0.61–9.59 | |
| Tumor size | |||
| Bulky disease | 2.61 | 0.99–6.87 | 0.052 |
| Disease properties | |||
| Ascites | 2.45 | 0.88–6.79 | 0.085 |
| Peritoneal carcinomatosis | 1.52 | 0.61–3.80 | 0.37 |
| Disease sites | |||
| Liver parenchyma metastases | 5.60 | 2.08–15.05 | 0.001 |
| Lung metastases | 1.79 | 0.74–4.35 | 0.20 |
| Bone metastases | 0.98 | 0.27–3.56 | 0.98 |
| Baseline laboratory values | |||
| ≥Median albumin (4.0 g/dL) | 0.35 | 0.14–0.88 | 0.026 |
| Absolute lymphocyte count (ALC) [K/μL] | |||
| ≥1.2 vs. <1.2 | 0.69 | 0.28–1.66 | 0.40 |
| Neutrophil-to-lymphocyte ratio (NLR) | |||
| ≥4 vs. <4 | 3.07 | 1.23–7.70 | 0.017 |
| Platelet-to-lymphocyte ratio (PLR) | |||
| ≥300 vs. <300 | 1.68 | 0.61–4.59 | 0.31 |
| Clinical characteristic | Multivariate ORa | 95% CI | p valueb |
| Disease sites | |||
| Liver parenchymal metastases | 6.64 | 2.21–19.92 | 0.001 |
| Baseline laboratory values | |||
| ≥Median albumin (4.0 g/dL) | 0.46 | 0.16–1.3 | 0.141 |
| Neutrophil-to-lymphocyte ratio (NLR) | |||
| ≥4 vs. <4 | 3.54 | 1.21–10.31 | 0.021 |
Odds ratios are modeled for very early treatment discontinuation.
p values < 0.05 were considered to be statistically significant.
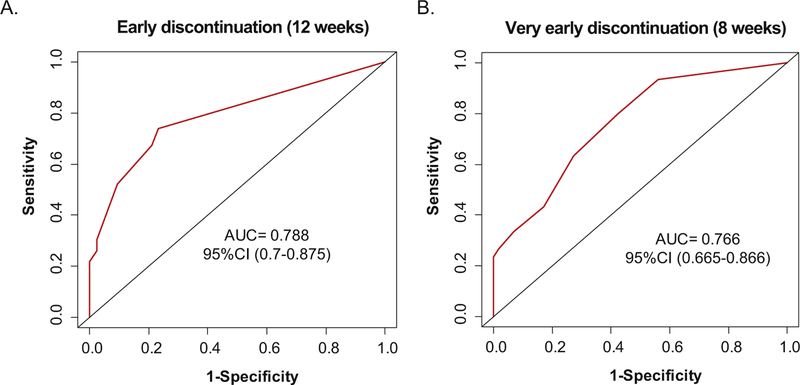
To define the ability of the pre-treatment variables to predict early treatment discontinuation, the receiver-operating characteristic (ROC) curves were used to plot the sensitivity along the y-axis and the “1-specificity” along the x-axis for comparing the multivariate model pre-diction versus the true binary outcome for both 8-week and 12-week variables. For both multivariate models, the AUC of >75 demonstrated to have a good ability to predict early treatment discontinuation in these patients (Fig. 2).
Fig. 2.
Pre-treatment clinical parameters can predict early and very early treatment discontinuation. The receiver-operating characteristic (ROC) curves were used to plot the sensitivity along the y-axis and the “1-specificity” along the x-axis for comparing the multivariate model prediction versus the true binary outcome for both: A. 12-week (bulky disease and liver metastases), and B. 8-week (NLR and liver metastases) variables. The area under the ROC curve (AUC) is a measure of predictive accuracy where a value of 1 corresponds to a perfect prediction and a value of 0.5 to a totally random prediction.
Finally, to determine whether specific baseline factors specifically predict for very early progression (8 weeks) due to clinical symptoms, univariate analyses were performed focusing on the variables previously found to be associated with early discontinuation. Liver parenchymal metastases (p = 0.016, OR 3.47, CI 1.26–9.56), albumin ≥4 g/dL (p = 0.016, OR 0.30, CI 0.11–0.80), and NLR ≥ 4 (p = 0.001, OR 6.30, CI 2.20–18.05) were significantly associated with very early discontinuation secondary to clinical progression (Supplementary Tables 1 and 2).
3.5. Germline BRCA1 and BRCA2 mutational status and early discontinuation
In our study, 68 out of 89 pts had gBRCA mutational status reported in the EMR. Out of the 68 patients, 11 pts had gBRCA1 mutations and 6 pts had gBRCA2 mutations of known significance. On univariate analysis, the presence of gBRCA1 or gBRCA2 mutation was associated with early discontinuation (OR 4.64, CI 1.33–16.23, p = 0.016) but was not statistically significant for very early discontinuation (OR 1.94, CI 0.63–5.97, p = 0.25) in this subset of 68 pts.
4. Discussion
Due to the unique pattern of metastatic disease, characterized by peritoneal carcinomatosis and/or ascites, EOC patients are uniquely vulnerable to symptomatic disease progression and may often be unable to tolerate early disease growth for the treatment duration required to allow time for immune response. Indeed, in our study cohort, over 50% of EOC patients discontinued treatment prior to 12 weeks for clinical and/or radiographic disease progression. Of the patients who stopped therapy early, the majority discontinued due to clinical symptoms (59% at week 12 and 73% prior to week 8). Of note, 8 patients (9.0%) died within 12 weeks of initiation of ICB.
Several studies in other cancer types have recently reported on immunotherapy “hyperprogressors”: patients on immune checkpoint blockade who exhibited early accelerated tumor growth while on immunotherapy [10–12]. While a detailed analysis of tumor growth kinetics was not performed in this study, there is a possibility that some of the patients in the very early discontinuation group may have exhibited this phenomenon.
Such high rates of early discontinuation and even death in the patients that were initially deemed eligible for these clinical trials highlights that the criteria that are used to guide patient selection might not be sufficient when it comes to immune checkpoint blockade. Multiple clinical trials of ICB and combinations are currently ongoing in patients with EOC. Studies reported to date indicate a rather short PFS, with a drop off of the majority of patients prior or at the first protocol-mandated assessment. In a preliminary analysis of anti-PD-1 pembrolizumab in PD-L1+ ovarian cancer, median PFS was 1.9 months, just prior to the protocol response assessment at 8 weeks [19]. Our results thus mirror the findings from the reported studies. However, since none of the trials reported on the characteristics of the early progressors, we thought it would be prudent to examine the clinical characteristics that may be helpful in identifying the patients that are at the highest risk of early discontinuation.
In multivariate analyses, bulky disease and liver parenchyma metastases were identified as predictors of early discontinuation, while liver parenchyma metastases and high NLR were predictive of very early discontinuation. Rather than being specific predictors of immunotherapy non-response, these markers likely reflect the overall disease burden and poor immune health status of these patients prior to initiation of ICB therapies. An increased NLR (≥4) has been suggested to serve as a marker for the host inflammatory response with increased neutrophils potentially serving as a source of VEGF, facilitating angiogenesis and tumor progression [23]. An increased NLR and low serum albumin have been previously shown to be prognostic predictors of poor survival in EOC [22,23,28]. Similarly, presence of liver parenchyma and bone metastases have previously been shown to be indicative of late stage metastatic disease in EOC patients [29,30], and thus may highlight more aggressive tumor biology. Finally, peritoneal bulky disease, as defined in our study by at least one peritoneal tumor measurement over 5 cm, has been used previously as a criterion in other EOC studies evaluating chemotherapy [25,26]. Recognizing the limitations of estimation of disease burden using this definition, this parameter was nevertheless predictive of early discontinuation in our study. Interestingly, it did not prove to be predictive in prior chemotherapy studies, likely secondary to higher early response/disease stabilization rate seen with chemotherapy [25,26]. Advanced studies in radiomics focusing on additional parameters such as other estimators of disease burden, number of sites, and tumor texture may help to further refine the radiographic predictors of early discontinuation.
There is an ongoing effort to identify molecular and histologic predictors of response to immune checkpoint blockade in EOC and other cancer types. In a recent clinical trial of nivolumab in EOC, one of the two patients who experienced a complete response (CR) had clear cell carcinoma [17]. Larger studies of immune checkpoint inhibitors specifically focusing on clear cell carcinoma are currently ongoing. In our analysis, clear cell histology was not predictive of early or late discontinuation, however, since only 9% of the patients in this cohort had clear cell carcinoma, we acknowledge the possibility of a type II error in this analysis. Association between the discontinuation time and other histologic and molecular parameters such as endometrioid histology and mismatch repair deficiency status likewise could not be performed on this study, since these cancer subtypes were not well-represented in our cohort (Table 1).
A recent study has shown promise for ICB in gBRCA mutated EOC patients, with 4 out of 6 pts demonstrating a partial or complete response [18]. In our analysis of the 68 pts with gBRCA data available, 17 patients had germline deleterious alterations in BRCA1 or BRCA2. Surprisingly, the presence of gBRCA1 or gBRCA2 mutations was associated with early discontinuation. We would like to caution, however, that these findings are based on very small numbers and could be a reflection of a more advanced disease and heavily pre-treated status of the gBRCA mutation carriers. Of note, in the study noted above [18], the two pts who had progression of disease were also heavily pre-treated with N6 prior lines of therapy. These results may reflect the importance of determining gBRCA mutation status and the use of ICB in these patients earlier in the disease setting and several ongoing trials using immune checkpoint blockade alone or in combination with PARP inhibitors in the upfront setting may help to answer this question.
The study has several obvious limitations. First, due to a limited number of patients with EOC that have been treated with ICB to date in our institution, the size of the study cohort is rather small. As a result, the variability in the OR estimates is large and several tested clinical parameters, such as bone metastases, were only present in a small number of patients, which likely influenced their poor predictive ability. Thus, while the individual clinical parameters identified in our study may help guide clinicians to make treatment decisions for individual potentially high-risk patients, the findings clearly need to be validated in a larger patient cohort. Secondly, the study has specifically focused on the patients treated with immunotherapy agents alone. Our findings might not be applicable to the trials utilizing ICB in combination with chemotherapy or targeted agents, where early control of tumor growth by chemotherapy may allow the patient to stay on treatment longer and provide ample time for the development of anti-tumor immune response, ultimately leading to durability of clinical benefit which is frequently seen on such studies [31]. Lastly, the study lacks the detailed molecular characterization of the patients’ tumors. Studies in melanoma, lung, and bladder cancer identified several biomarkers predictive of clinical benefit to PD-1 blockade. These include tumor mutational burden (TMB) [32], HLA diversity [33], expression of PD-L1 [34], presence of tumor-infiltrating lymphocytes (TILs) [35], IFNγ transcriptional signature [36], and intratumoral and peripheral TCR clonality [35,37]. It will be of value to determine whether these parameters would also be predictive of clinical benefit or early discontinuation in ovarian cancer and this effort is currently ongoing. Such information, however, is unlikely to be available for every patient in the near future. In the interim, the use of common clinical parameters described in this study may provide a readily-available resource for the clinicians and may help guide decision for the use of ICB in their patients.
In summary, the study demonstrates that early discontinuation of ICB is common in EOC patients and several clinical characteristics indicative of advanced disease and poor prognosis may identify the patients at the highest risk for early discontinuation. Overall, it is likely that immune therapy should not be reserved as a late line therapy for patients with refractory and bulky disease, but rather should be used earlier in the disease course or in patients with lower disease burden, allowing for the potential delayed benefit in the patients who can tolerate early tumor growth.
Supplementary Material
HIGHLIGHTS.
In ovarian cancer patients treated with immune checkpoint blockade, early discontinuation of therapy is common.
Symptomatic disease progression was common and accounted for early treatment discontinuation in the majority of patients.
Ovarian cancer patients with heavily pretreated, recurrent disease may not be suitable candidates for immunotherapy.
Pre-treatment clinical parameters can identify the patients at risk for early discontinuation and symptomatic progression.
Acknowledgements
M.K.C. and D.Z. are members of the Parker Institute for Cancer Immunotherapy at MSKCC. D.Z. is supported by the Ovarian Cancer Research Foundation Liz Tilberis Award, and the Department of Defense Ovarian Cancer Research Academy (OC150111). Research reported in this publication was supported by the National Cancer Institute Award R25 CA020449 to J.B. This work was supported in part by the Memorial Sloan-Kettering Cancer Center Support Grant P30 CA008748.
Footnotes
Conflicts of interest
The authors report the following potential conflicts of interest. No authors had conflicts of interest related to this work, with the exception of government/foundation funding sources indicated in the Acknowledgements section. Several authors on the study indicated financial activities outside of submitted work, which include personal fees, grants, and intellectual property. All disclosures are indicated in detail in the submitted ICJME Forms for Disclosure of Potential Conflicts of Interest. A.S. is currently an employee of Merck & Co. Inc.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2018.11.025.
References
- [1].Sharma P, Allison JP, The future of immune checkpoint therapy, Science 348 (6230) (2015) 56–61. [DOI] [PubMed] [Google Scholar]
- [2].Hamanishi J, Mandai M, Konishi I, Immune checkpoint inhibition in ovarian cancer, Int. Immunol 28 (7) (2016) 339–348. [DOI] [PubMed] [Google Scholar]
- [3].Zamarin D, Jazaeri AA, Leveraging immunotherapy for the treatment of gynecologic cancers in the era of precision medicine, Gynecol. Oncol 141 (1) (2016) 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Matulonis UA, et al. , Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: interim results from the phase 2 KEYNOTE-100 study, J. Clin. Oncol 36 (2018) (suppl; abstr 5511). [DOI] [PubMed] [Google Scholar]
- [5].Boussiotis VA, Molecular and biochemical aspects of the PD-1 checkpoint pathway, N. Engl. J. Med 375 (18) (2016) 1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wolchok JD, et al. , Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria, Clin. Cancer Res 15 (23) (2009) 7412–7420. [DOI] [PubMed] [Google Scholar]
- [7].Saenger YM, Wolchok JD, The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases, Cancer Immun 8 (2008) 1. [PMC free article] [PubMed] [Google Scholar]
- [8].Burger R, et al. , NRG oncology phase II randomized trial of nivolumab with or without ipilimumab in patients with persistent or recurrent ovarian cancer, 17th Biennial Meeting of the International Gynecologic Cancer Society, 2018. [Google Scholar]
- [9].Beaver JA, et al. , Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis, Lancet Oncol 19 (2) (2018) 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kato S, et al. , Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate, Clin. Cancer Res 23 (15) (2017) 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saada-Bouzid E, et al. , Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma, Ann. Oncol 28 (7) (2017) 1605–1611. [DOI] [PubMed] [Google Scholar]
- [12].Champiat S, et al. , Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1, Clin. Cancer Res 23 (8) (2017) 1920–1928. [DOI] [PubMed] [Google Scholar]
- [13].Hyman DM, et al. , Predictors of early treatment discontinuation in patients enrolled on Phase I oncology trials, Oncotarget 6 (22) (2015) 19316–19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Milne K, et al. , Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes, J. Transl. Med 10 (2012) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Templeton AJ, et al. , Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis, J. Natl. Cancer Inst 106 (6) (2014) dju124. [DOI] [PubMed] [Google Scholar]
- [16].Ma XM, et al. , The platelet-to-lymphocyte ratio as a predictor of patient outcomes in ovarian cancer: a meta-analysis, Climacteric 20 (5) (2017) 448–455. [DOI] [PubMed] [Google Scholar]
- [17].Hamanishi J, et al. , Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer, J. Clin. Oncol 33 (34) (2015) 4015–4022. [DOI] [PubMed] [Google Scholar]
- [18].Matsuo K, et al. , Nivolumab use for BRCA gene mutation carriers with recurrent epithelial ovarian cancer: a case series, Gynecol. Oncol. Rep 25 (2018) 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Frenel JS, et al. , Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial, J. Clin. Oncol 35 (36) (2017) 4035–4041. [DOI] [PubMed] [Google Scholar]
- [20].Zhang W, et al. , Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer, Sci. Rep 7 (1) (2017) 9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Szender JB, et al. , Impact of ascites volume on clinical outcomes in ovarian cancer: a cohort study, Gynecol. Oncol 146 (3) (2017) 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Asher V, Lee J, Bali A, Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer, Med. Oncol 29 (3) (2012) 2005–2009. [DOI] [PubMed] [Google Scholar]
- [23].Yang Z, et al. , Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival of epithelial ovarian cancer: a systematic review and meta-analysis of observational studies, Oncotarget 8 (28) (2017) 46414–46424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Flint TR, et al. , Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity, Cell Metab 24 (5) (2016) 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gordon AN, et al. , Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer, Gynecol. Oncol 95 (1) (2004) 1–8. [DOI] [PubMed] [Google Scholar]
- [26].Gordon AN, et al. , Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan, J. Clin. Oncol 19 (14) (2001) 3312–3322. [DOI] [PubMed] [Google Scholar]
- [27].Huang AC, et al. , T-cell invigoration to tumour burden ratio associated with anti-PD-1 response, Nature 545 (7652) (2017) 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Baert T, et al. , Influence of CA125, platelet count and neutrophil to lymphocyte ratio on the immune system of ovarian cancer patients, Gynecol. Oncol 150 (1) (2018) 31–37. [DOI] [PubMed] [Google Scholar]
- [29].Cormio G, et al. , Distant metastases in ovarian carcinoma, Int. J. Gynecol. Cancer 13 (2) (2003) 125–129. [DOI] [PubMed] [Google Scholar]
- [30].Markman M, Involvement of bone in epithelial ovarian cancer: case report of an uncommon late metastatic event, Case Rep. Oncol 4 (3) (2011) 490–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gandhi L, et al. , Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer, N. Engl. J. Med 378 (22) (2018) 2078–2092. [DOI] [PubMed] [Google Scholar]
- [32].Rizvi NA, et al. , Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer, Science 348 (6230) (2015) 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chowell D, et al. , Patient HLA class I genotype influences cancer response to check-point blockade immunotherapy, Science 359 (6375) (2018) 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rosenberg JE, et al. , Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial, Lancet 387 (10031) (2016) 1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tumeh PC, et al. , PD-1 blockade induces responses by inhibiting adaptive immune resistance, Nature 515 (7528) (2014) 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ayers M, et al. , IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade, J. Clin. Invest 127 (8) (2017) 2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Snyder A, et al. , Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: an exploratory multi-omic analysis, PLoS Med 14 (5) (2017), e1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.