Abstract
Vaccines prevent 2-3 million childhood deaths annually; however, low vaccine efficacy and the resulting need for booster doses create gaps in immunization coverage. In this translational study, we explore the benefits of extended release of licensed vaccine antigens into skin to increase immune responses after a single dose in order to design improved vaccine delivery systems. By administering daily intradermal injections of inactivated polio vaccine according to six different delivery profiles, zeroth-order release over 28 days resulted in neutralizing antibody titers equivalent to two bolus vaccinations administered one month apart. Vaccinations following this profile also improved immune responses to tetanus toxoid and subunit influenza vaccine but not, a live-attenuated viral vaccine, measles vaccine. Finally, using subunit influenza vaccine, we demonstrated that daily vaccination by microneedle patch induced a potent, balanced humoral immunity with an increased memory response compared to bolus vaccination. We conclude that extended presentation of antigen in skin via intradermal injection or microneedle patch can enhance immune responses and reduce the number of vaccine doses, thereby enabling increased vaccination efficacy.
Keywords: vaccine, intradermal delivery, route of administration, response to vaccination, microneedle patch, slow release
Introduction
While vaccines prevent 2-3 million childhood deaths annually, 1.5 million children still die from vaccine-preventable diseases [1]. In developing countries, vaccinations are often delivered by mass campaigns utilizing fixed clinics and house-to-house distribution [2]. Despite these efforts, vaccination coverage remains low in many parts of the world. Low seroconversion rates to some vaccines require multiple costly, resource-intensive vaccinations, and the majority of vaccines recommended by the WHO Expanded Programme on Immunization are delivered by hypodermic needle and syringe [3], which requires trained healthcare providers to deliver injections and dispose of needles safely. Improving vaccination technology to increase immunogenicity, reduce number of vaccinations, and simplify administration could lead to increased immunization efficacy.
One proposed strategy to increase the immune response to vaccination is prolonging antigen presentation [4]. Live-attenuated vaccines, such as measles and varicella, mimic natural infection and can provide lifelong protection after a single administration. However, inactivated/subunit vaccines usually require one or more booster administrations separated by months or years to induce lasting immunity [3]. Extended delivery of an inactivated antigen could present antigen over a prolonged period, thereby generating a stronger immune response to the same dose. However, typical controlled-release delivery systems exhibit a large burst release, followed by low levels of antigen release. While this approach can elicit improved immune responses, other delivery profiles may generate more-robust responses. For example, daily injections administering exponentially increasing doses of immune stimulants gp33 peptide and CpG over 4 days significantly increased CD8+ active T cells [5], but an exponentially increasing release profile is difficult to engineer into a controlled release formulation. Increased antibody responses may be due to increased antigen uptake and retention in lymph nodes [6, 7]. Dendritic cells released higher levels of IL-2 and IL-10 cytokines in vitro when stimulated in an exponentially increasing fashion [5]. Thus, the literature suggests that extended presentation of some model antigens can increase immune responses. However, translational studies are needed that use licensed vaccines, conduct a detailed examination of antigen delivery profiles, and determine if a single dose of vaccine delivered over an extended time can provide protective immunity comparable to that observed after a multi-dose schedule.
Intradermal (ID) delivery can improve immune responses. The skin, unlike muscle and subcutaneous space, is an immunological organ with roles in both innate and adaptive immunity [2]. Epidermis is largely composed of keratinocytes, expressing a variety of receptors specialized in recognizing pathogen-associated molecular patterns and bacterial, fungal, and viral antigens [8]. Antigen-presenting cells (APCs), such as epidermal Langerhans cells and dermal dendritic cells, can effectively capture, process, and present antigens in nearby lymph nodes, activating the adaptive immune response. ID vaccination has led to more efficient antigen migration into draining lymph nodes than intramuscular (IM) injections and higher levels of activated T follicular helper (Tfh) cells and germinal center B (GC-B) cells were identified in lymph nodes following skin vaccination [9]. ID vaccination can enhance immunogenicity, thereby reducing the dose or number of doses needed to seroconvert, as seen for smallpox, rabies and influenza vaccines [3, 10].
Delivering antigen into skin is difficult. ID injections are usually performed using the Mantoux technique, in which the needle is inserted at a shallow angle and requires special training to reliably target the skin [11]. Dissolving microneedle (MN) patches are an emerging technology for ID administration [12-14]. MN patches contain an array of polymer needles <1 mm long and fixed to a hard backing. The patch can be painlessly applied to skin, delivering vaccine in a controlled fashion directly into epidermis and dermis. Once the microneedles dissolve, the patch can be discarded as non-sharps waste. Vaccination with MN patches can induce potent immune responses, often demonstrating dose-sparing. Extended delivery of vaccines from MN patches has received limited attention but shown to increase immunogenicity [15-18].
Guided by these studies, we envision a vaccine delivery system that targets antigen to skin, presents antigen over an extended period to improve immunogenicity, and uses a MN patch that simplifies vaccination. Here, we examined the effect of six different extended-delivery profiles on immunogenicity using four different licensed vaccines administered to skin. Extended-delivery profiles were studied by administering multiple fractional vaccine doses by ID injection or MN patch (i.e., delivery was not continuous but extended over a series of closely spaced boluses). In this context, we compared single doses of extended-delivery vaccination to two-dose bolus vaccination. We showed for the first time that extended delivery for one month further improves immune response compared to extended delivery over shorter times. Additionally, extended delivery achieved through repeated MN patch vaccination further increases the immune response compared to injections. Future studies will address development of formulations that can achieve optimized delivery profiles after application of a single MN patch.
Materials and Methods
Vaccines
Monovalent stock solutions of IPV vaccine types 1, 2 and 3 were generously provided by GlaxoSmithKline Biologics (Rixensart, Belgium). These vaccine antigens are the same as those used in Poliorix. Influenza subunit vaccine bulk (H1N1 A/Brisbane/10/10) was generously provided by Seqirus (Maidenhead, United Kingdom). This vaccine antigen is the same as that used in the 2015-2016 seasonal vaccine Fluvirin. Tetanus toxoid vaccine bulk was kindly supplied by the Serum Institute of India (Pune, India). This vaccine antigen is the same as that used in Tetanus Toxoid Vaccine Adsorbed. The Edmonston-Zagreb strain of measles virus vaccine (kindly supplied by the Serum Institute of India) was grown in Vero cells maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Grand Island, NY) supplemented with 2% fetal bovine serum (FBS, Gibco). At the maximum of the cytopathic effect, cell suspensions were freeze-thawed and centrifuged to eliminate cellular debris, as previously described [19]. This measles virus is the same as that used in M-VAC but propagated using Vero cells at CDC rather than in MRC-5 cells at the Serum Institute of India. All vaccines were diluted in sterile phosphate buffered saline solutions prior to injections.
Microneedle patch
Microneedle (MN) patches were fabricated as described previously [20, 21]. Briefly, influenza vaccine was mixed with 10% w/v sucrose (Sigma Aldrich, St. Louis, MO) and 1% w/v sodium carboxymethyl cellulose (250 kDa, Sigma Aldrich) in deionized water. This solution was cast onto silicone molds under vacuum and dried. A polymer matrix solution of 28% w/w polyvinyl alcohol (78% hydrolyzed, 6 kDa; Acros Organics, Geel, Belgium), 21% w/w sucrose, and water was added to the mold and dried. MN patches were removed from their molds and stored at room temperature (22-25°C) with desiccant until use for vaccination. Placebo patches were fabricated with only the polymer matrix solution.
Animal studies
On vaccination days, rodents were anesthetized either daily for seven days or every other day for fourteen or twenty-eight days. Anesthesia was induced with 5% isoflurane and maintained during the procedure at 2%. Animals were then vaccinated by injection in the skin using a 28 gauge hypodermic needle by the Mantoux method, in the muscle using a 28 gauge hypodermic needles, or by MN patch applied to the skin. All injections administered 20 or 100 μL. Prior to vaccinations with MN patches, the hair was removed with clippers followed by depilatory cream (Nair, Ewing, NJ).
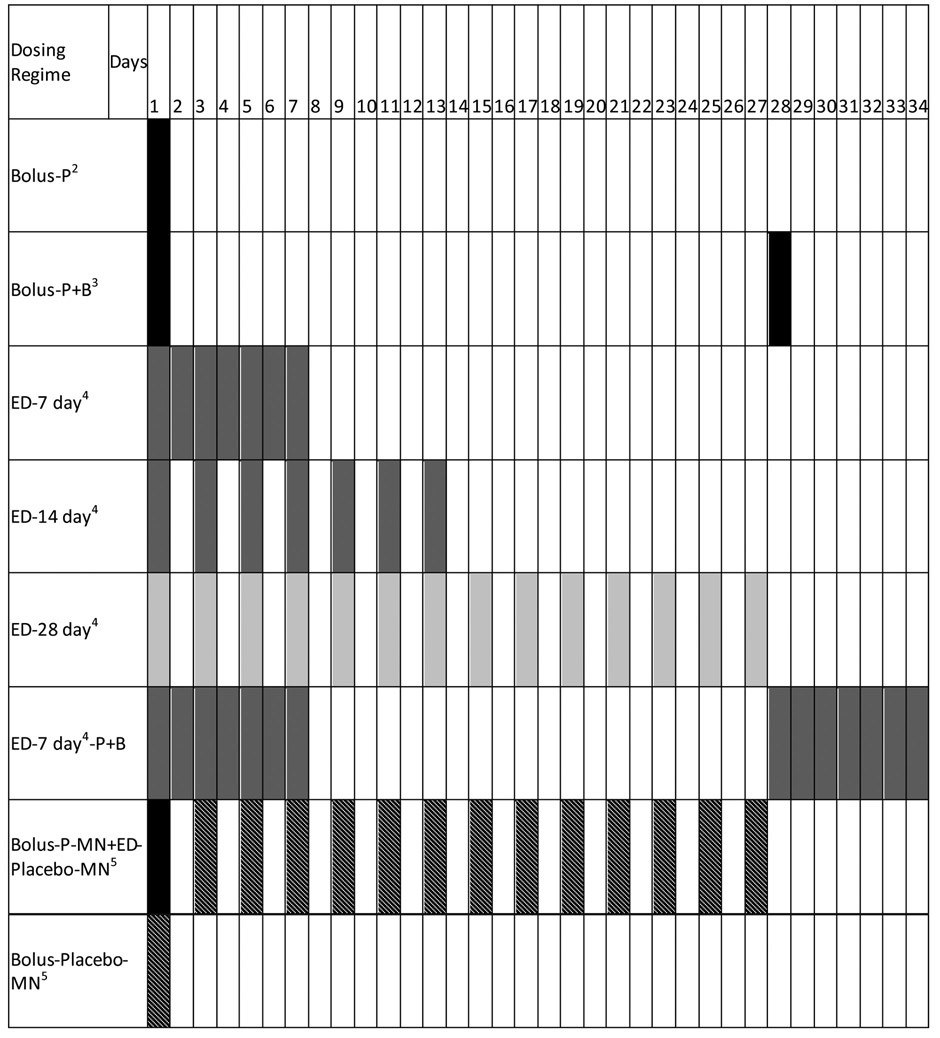
The daily administration of vaccine contained full or fractional vaccine doses such that the cumulative dose received by all animals was the same, as described for each experiment and summarized in Table 1. Bolus groups received a full dose on Day 1 via ID injection, IM injection, or MN patch (Bolus-P). Animals receiving booster doses received a second full dose, such that those animals received a cumulative dose twice that of the non-boosted groups (Bolus-P+B). Extended delivery (ED) groups were dosed every 7, 14, or 28 days via ID injection, IM injection, or MN patch (ED-7 day, ED-14 day, ED-28 day, respectively). The ED-7 day-P+B group received a full dose administered as fractional doses between Day 1 and Day 7 and a second full dose administered as fractional doses between Day 28 and Day 35. The Bolus-P-MN-ED-Placebo-MN group received a full dose via MN patch on Day 1 followed by placebo MN patches (containing no vaccine) every other day until Day 27. In the final group, MN patches were applied once as a placebo on Day 1 (Bolus-Placebo-MN).
Table 1.
Dosing regimens1.
Vaccine dose was held constant across all groups (unless a boost was administered, which provided an additional dose).
P stands for prime dose, indicating a single dose.
B stands for boost, which indicates a group that received 2 full doses. Boosts were administered as a bolus on either day 28 or day 77.
Extended delivery (ED) groups received total dose of antigen equal to a prime dose; however, that dose was administered in smaller increments over 7, 14, or 28 days. Vaccination in ED groups was performed by intradermal, intramuscular or MN patch, as indicated in the text.
Slashes indicate placebo administration using a MN patch containing only inert excipients, i.e., no vaccine was administered
In all cases, MN patches were applied to the skin for 15 min. While MN patch dissolution occurs in water in less than 1 min (data not shown), the dissolution process is slower in skin. While we have not optimized MN patch wear time on the skin, we left them on the skin for 15 min and found that it was sufficient for the MNs to dissolve off the patch backing. It should be noted, however, that complete MN dissolution probably had not occurred with 15 min, but that was enough time for MNs to become sufficiently hydrated and soft that they remained in the skin upon removal of the patch backing. Completion of the dissolution process, release of antigen and antigen uptake by APCs took place over a longer period of time.
All doses were verified by enzyme-linked immunosorbent assay (ELISA) [21-23] or end-point titration, in the case of measles vaccine [21-23]. Blood was collected at set time points; serum separator tubes (Becton Dickinson, Franklin Lakes, NJ) were used to separate sera during centrifugation. All animal studies were approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee (IACUC), and IPV vaccination studies were also approved by the CDC IACUC.
Inactivated polio vaccination
For IPV studies, female Wistar rats (Charles River, Wilmington, MA), age 6-8 weeks, were anesthetized with isoflurane (n=10 per group). Wistar rats are commonly used for IPV vaccination studies [24]. Vaccine was diluted in sterile saline to the proper concentration. Rats were vaccinated with 100 μl either intradermally (ID) on the flank or intramuscularly (IM) in the thigh. In monovalent IPV studies, all rats received IPV type 2 at a cumulative dose of 0.8 D-antigen units (DU). In trivalent IPV studies, rats received 10 DU of IPV type 1, 2 DU of IPV type 2 and 8 DU of IPV type 3 (which is 25% of the conventional IPV dose administered to humans [25]) via 100 μl injections. Blood was collected from the tail vein via laceration biweekly until day 56 or 84; for the longitudinal study, blood draws continued monthly until month 6.
Tetanus toxoid vaccination
Female Balb/c mice (Charles River, age 6-8 weeks) were vaccinated ID with tetanus toxoid vaccine at the base of the tail (n=9 - 10 per group). Balb/c mice are commonly used for tetanus toxoid vaccination studies [26]. The cumulative dose was 5 flocculation units (Lf) (which is equal to the conventional tetanus toxoid dose administered to humans, but without adjuvant [25]), administered either as a full-dose bolus (in 20 μL) or by 1/14th dose (in 20 μL) every other day over 28 days. Blood was collected via the jugular vein on days 0, 14, 28, and 42.
Measles vaccination
Female cotton rats (Harlan, Indianapolis, IN, age 8 weeks) were vaccinated ID with live-attenuated measles vaccine on the flank (n= 10 per group). Cotton rats are commonly used for measles vaccination studies [27]. The total dose was 200 50% tissue culture infective dose (TCID50) (which is 20% of the minimum conventional measles vaccine administered to humans [19]), administered either as a full-dose bolus (in 20 μL) or by 1/14th dose (in 20 μL) every other day over 28 days. Blood was collected via the jugular vein on days 0, 14, 28, 42, and 56.
Influenza vaccination
Female Balb/c mice (Charles River, 6-8 weeks of age) were immunized with influenza vaccine either via ID injection at the base of the tail, IM injection in the thigh muscle or MN patch on the back (n=8 per group). Balb/c mice are commonly used for influenza vaccination studies [28]. A cumulative dose of 1 μg (which is 7% of the conventional influenza vaccine administered to humans [25]) was given either by full-dose bolus (in 20 μL) or by 1/14th dose (in 20 μL) every other day over 28 days. On day 77, groups that had received bolus vaccination were boosted with a second full-dose bolus. Blood was collected via the jugular vein on days 0, 14, 28, 42, 56, 77, 91, and 105.
In a second study, mice were vaccinated ID or by MN patch, receiving 1 μg total dose. Blood was collected on days 0, 7, 14, 21, 28, and 35. On day 28, the boost group received another 1 μg dose by ID injection. On day 35, animals were euthanized; spleen, lymph nodes, and bone marrow were collected, as previously described [29, 30].
IPV neutralizing antibody assay
Assay of poliovirus neutralizing antibodies was performed at the CDC Polio and Picornavirus Laboratory using their standard protocols [31]. Briefly, diluted serum samples were incubated with polioviruses types 1, 2, and 3 (Sabin) at 35°C for 3 h prior to addition to HEp-2(C) cells. After incubation for five days at 35°C, cells were stained with crystal violet and cell viability was measured by optical density. Titers were determined as the reciprocal of the dilution that was able to inhibit 50% of virus binding [23, 32]. Seropositivity was defined as antibody titers greater than or equal to 3.0. Two-way ANOVAs were used to determine significance.
Tetanus toxoid-specific antibody assay
Tetanus toxoid-specific IgG titers were determined via ELISA, as described previously [20]. Briefly, tetanus toxoid was coated on a microwell plate at 0.5 Lf/mL. Diluted serum samples followed by horseradish peroxidase (HRP)-tagged anti-mouse antibody (Southern Biotechnology Associates, Birmingham, AL) were allowed to bind to the plate. Optical density values were compared to a standard curve of mouse IgG to determine titers. Two-way ANOVAs were used to determine significance.
Measles neutralizing antibody assay
Measles titers were determined using a plaque-reduction neutralization assay, as described previously [33]. Briefly, diluted serum samples were mixed with measles challenge virus (University of California, Davis, CA) and incubated for 2 h at 37°C. The samples were then plated onto 24-well plates with a confluent monolayer of Vero cells (American Type Culture Collection, Manassas, VA) and incubated for an additional 2 h at 37°C, after which an overlay media of 0.8% CMC was added to the cells. Five to seven days later, media was removed, and crystal violet solution was added for 20 min. Plaques were counted in each well, and titers were determined according to the Third WHO International Standard Reference Serum (97/648). Two-way ANOVAs were used to determine significance.
Influenza hemagglutination inhibition assay
Hemagglutination inhibition (HAI) assay was used to determine influenza-specific antibodies as a correlate to immune response, as described previously [34]. Briefly, serum was treated with receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) at 37°C overnight. The following day, samples were inactivated at 56°C for 30 min. Packed turkey red blood cells (Lampire Biological Laboratories, Pipersville, PA) were added and incubated with samples overnight. Light centrifugation was used to remove the red blood cells. Supernatant was serially diluted in phosphate-buffered saline (PBS). Samples were then mixed with A/California/07/2009 influenza virus for 30 min. Red blood cells (0.5%) were added and incubated for approximately 10 min until the titer at which agglutination was inhibited could be determined. The highest dilution titer that inhibited hemagglutination was read as the HAI titer. Seroprotection was defined as antibody titers greater than or equal to 40. Two-way ANOVAs were used to determine significance.
Influenza-specific antibody assay
Purified mouse IgG, IgG1, and IgG2a and goat anti-mouse-HRP (Southern Biotechnology Associates) were used to determine titers via ELISA, as previously described [35]. Briefly, subunit influenza vaccine was coated on microwell plates. Diluted serum samples, HRP-tagged antibody, and SureBlue Reserve TMB Microwell Peroxidase Substrate (KPL, Gaithersburg, MD) were subsequently added to the plate. Approximately 15 min later once sufficient color had developed, reaction was stopped using TMB BlueSTOP Solution (KPL); plates were analyzed using absorbance at 620 nm. Two-way ANOVAs were used to determine significance.
Flow cytometry
Lymph nodes (LN; inguinal and axillary) were harvested from mice and single cell suspensions were prepared and stained with fluorescent anti-mouse antibodies for detection of germinal center B cells (B220+GL7+), T follicular helper cells (CD3+CD4+CXCR5+PD1+) and plasmablast cells (CD3-CD138+) by flow cytometry. For antibody staining, the cells were incubated with fluorescent anti-mouse antibodies in FACS buffer for 30 min at 4°C, washed with FACS buffer and fixed with Cytofix (Becton Dickinson, Franklin Lakes, NJ). Flow cytometry data were acquired using a BD LSRII flow cytometer (Becton Dickinson) and analysed by Flow Jo software (Becton Dickinson).
ELISPOT assay
Subunit influenza vaccine-specific antibody-secreting plasma cells in bone marrow were detected by ELISPOT assay. Multiscreen HTS-IP filter 96 well plates (Millipore, Billerica, MA) were coated with antigen (1 μg/ml) overnight at 4°C, and next day, 106 bone marrow cells (collected from femur and tibia) without red blood cells in complete RPMI 1640 cell culture medium were added to each well and incubated overnight in a 37°C incubator with 5% CO2. Secreted anti-hemagglutinin-IgG1 and IgG2a were then detected by alkaline phosphatase (AP)-conjugated goat anti-mouse IgG1 and IgG2a antibodies (Southern Biotech), and the antibody spots were developed by Vector Blue AP substrate (Vector Laboratories, Burlingame, CA). An ELISPOT reader was used to image and count the spots in the well.
Antigen restimulation studies
Antigen restimulation assay was performed to evaluate antigen-specific Th1 immune response. Single-cell suspensions of splenocytes were first prepared by collagenase digestion of spleen and then 106 splenocytes in complete RPMI 1640 medium were incubated with antigen at 1 μg/ml concentration in a 96-well plate for 72 h. IFN-gamma level in the restimulation media was determined by ELISA using a ready-set-go IFN-gamma ELISA kit (EBioscience, San Diego, CA).
Results
Optimal delivery profile
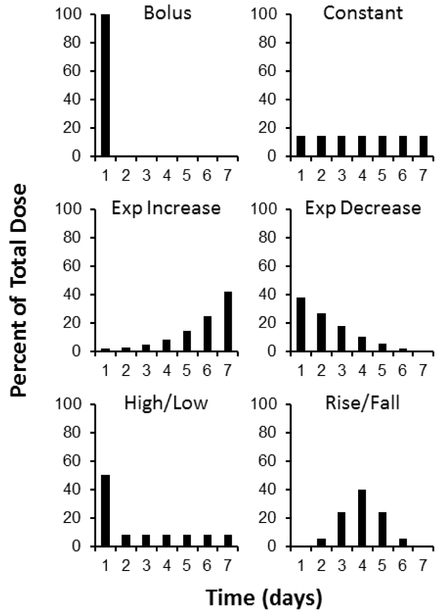
Vaccines are conventionally administered as a bolus, which may not be optimal. Our initial studies were designed to identify the optimal release profile for delivery to skin. First, we studied six different delivery profiles of 0.8 D-antigen units (DU) of monovalent inactivated polio vaccine type 2 (mIPV2) administered over the course of seven days to understand the effect of time course of antigen presentation (Figure 1).
Figure 1:
Extended antigen delivery profiles. Daily intradermal injections were administered over seven days, following six different profiles. The total antigen delivered was held constant in each group at 0.8 DU of monovalent IPV type 2. A bolus injection served as the control for standard vaccinations. Constant and Exponentially Increasing profiles continually reinforce the immune response with equal or increasing stimulus. An initial prime dose was followed by either a steady decrease in dose (Exponentially Decreasing profile) or a constant dose (High/Low profile) to provide a strong initial activation of the immune system followed by continued antigen presentation. Finally, the Rise/Fall profile provides a more gradual increase and decrease of antigen presentation over the course of a week.
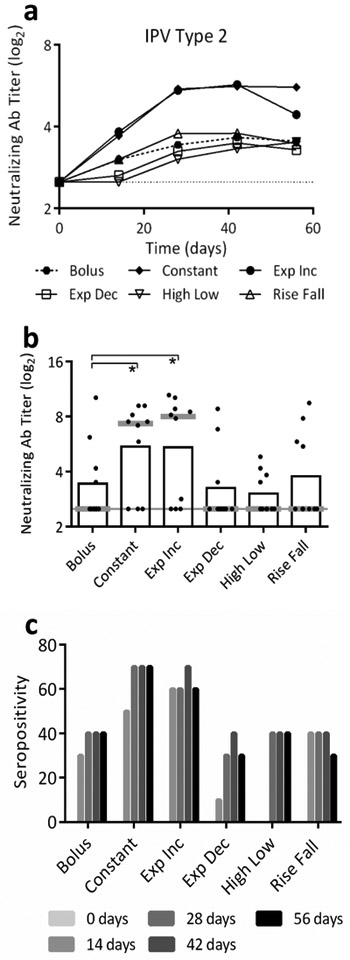
Bolus vaccination showed the expected increase in neutralizing antibody titers followed by a plateau in titers (Figure 2a) [3]. Exponential Decreasing, High/Low and Rise/Fall profiles did not yield significantly different antibody titers compared to bolus delivery (p>0.90). However, titers following Constant and Exponentially Increasing delivery were significantly greater than bolus delivery titers (p=0.011 and 0.005, respectively). Titers on day 28 were 2-3-times higher after Constant and Exponentially Increasing delivery compared to bolus delivery (Figure 2b). Seropositivity was also greater after Constant and Exponentially Increasing delivery (Figure 2c). This indicates that maintaining or increasing dose over time was beneficial, while profiles with initial burst followed by lower levels of antigen presentation did not increase the antibody response to mIPV2. We selected Constant delivery for further characterization; it appeared equivalent to Exponentially Increasing release, with the added benefit of being easier to convert to a single-dose, controlled release delivery system.
Figure 2:
Serological responses following extended delivery of mIPV2. Effect of antigen delivery profile extended over seven days. The total dose of antigen delivered was held constant in each group at 0.8 DU of mIPV2 administered intradermally to Sprague Dawley rats. Data based on a0 replicates. (a) Mean neutralizing antibody titers show Constant and Exponentially Increasing profiles induced higher titers compared to bolus vaccination by 42 days after vaccination. (b) Mean (black bar) and median (grey line) titers at day 28 post initial vaccination; the dotted line at a titer of 2.5 indicates the assay baseline (*p<0.05). Dots represent individual rats. (c) Seropositivity is the percent of animals in each group with titers ≥3.0.
Optimal length of delivery and boosting
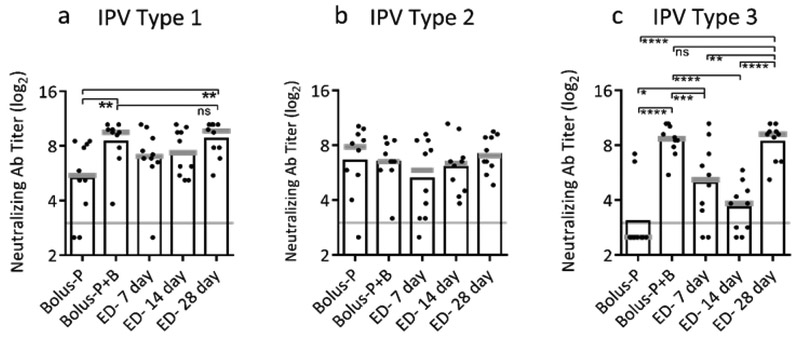
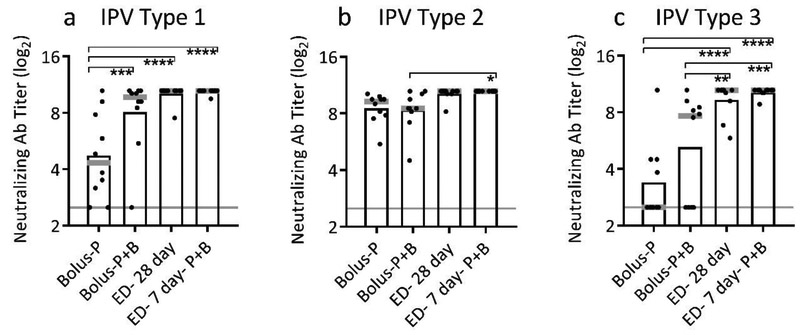
Next, we investigated the effect of duration of delivery on immune response, and conducted this analysis using trivalent IPV to more broadly assess effects of extended delivery. Experimental groups received a single dose distributed as fractional doses over 7, 14 or 28 days (Table 1). These results were compared to a single bolus priming dose (Bolus-P) or a two-dose prime+boost regimen (Bolus-P+B). The neutralizing antibody response to IPV type 2 was similarly strong in all vaccination groups at day 84 following initial dosing, possibly because the response to the bolus prime vaccination (Bolus-P) was so strong that even conventional boosting with a second full vaccine dose (Bolus-P-B) did not improve immune response further (Figure 3b). For IPV types 1 and 3, 28-day exposure to antigen produced significantly better neutralizing antibody responses than a single priming dose (p<0.003) and was statistically indistinguishable from the two-dose regimen (p≥0.999) (Figure 3a,c). Moreover, only 28 days of constant dosing and the group achieved 100% seropositivity across all three serotypes (Supplemental Figure 1). This indicates that one dose of month-long IPV delivery can produce immunity similar to a two-dose prime+boost regimen, potentially reducing the dose delivered and the number of visits to a healthcare provider.
Figure 3:
Effect of duration of extended delivery on neutralizing antibody titers. Extended delivery over 28 days induced neutralizing antibody titers equivalent to two doses administered one month apart for trivalent IPV: (a) IPV type 1, (b) IPV type 2 and (c) IPV type 3 (ns- p>0.05; * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001). Total antigen delivered was held constant for each dose at 10 DU of IPV type 1, 2 DU of IPV type 2, and 8 DU of IPV type 3 administered intradermally to Sprague Dawley rats. A bolus prime dose (Bolus-P) or a two-dose prime+boost regimen (Bolus-P+B) were compared to extended delivery of IPV administered over 7 days (ED-7 day), 14 days (ED-14 day), or 28 days (ED-28 day). Neutralizing antibody titers were determined on day 84. Data show mean (black bar) and median (grey line) titers based on 10 replicates.
To further understand the relationship between extended delivery and boosting, rats received trivalent IPV as a two-dose 7-day extended-delivery regimen to combine the benefits of extended delivery and boosting. This approach yielded neutralizing antibody titers greater than either a single bolus dose or a two-dose prime+boost regimen for IPV types 1 and 3 (Figure 4, p=0.047 and p=0.001). However, the two-dose extended-delivery regimen was not significantly different from a single 28-day extended delivery for any of the three serotypes. These data are consistent with the initial observation that extended delivery can improve bolus prime or two-dose regimens and that 28-day delivery is superior to 7-day delivery.
Figure 4:
Effect of extended delivery during prime+boost regimens on neutralizing antibody titers. Extended intradermal delivery over seven days during the prime and boost increased immune response compared to bolus delivery for both doses for trivalent IPV: (a) IPV type 1, (b) IPV type 2 and (c) IPV type 3 (* p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001). Total antigen delivered was held constant for each dose at 10 DU of IPV type 1, 2 DU of IPV type 2, and 8 DU of IPV type 3 in Sprague Dawley rats. Neutralizing antibody titers were determined on day 56. Data show mean (black bar) and median (grey line) titers based on 10 replicates.
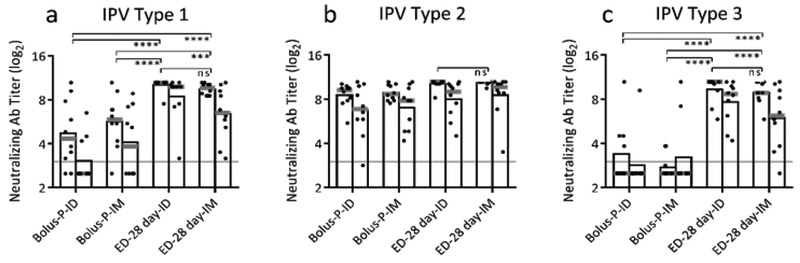
Effect of route of administration
In addition to ID vaccination studied above, we next examined if extended delivery would benefit IM vaccination. Comparing ID and IM vaccination, bolus delivery responses were equivalent and extended-delivery responses were also equivalent for all three IPV serotypes (Figure 5, p>0.662 and p>0.858). However, titers in both extended-delivery groups were significantly higher than their bolus counterparts for IPV types 1 and 3 (Figure 5a,c, p<0.001). This indicates that extended delivery is beneficial with both IM and ID vaccination. Furthermore, antibody titers remained high six months after vaccination (Figure 5, right bar). Extended delivery into skin was the only group to maintain 100% seropositivity six months after vaccination (Supplemental Figure 3). This shows that extended-delivery vaccination can induce long-lasting antibody responses.
Figure 5:
Effect of route of administration on neutralizing antibody titers. Extended delivery to skin or muscle induced potent immune response compared to bolus vaccination with no difference between the two routes of administration for trivalent IPV: (a) IPV type 1, (b) IPV type 2 and (c) IPV type 3 (ns- p>0.05; * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001). Total antigen delivered was held constant for each dose at 10 DU of IPV type 1, 2 DU of IPV type 2, and 8 DU of IPV type 3 in Sprague Dawley rats. Neutralizing antibody titers were determined on day 56 (left bar) and day 168 (right bar). Data show mean (black bar) and median (grey line) titers based on 10 replicates.
Applicability to other vaccines
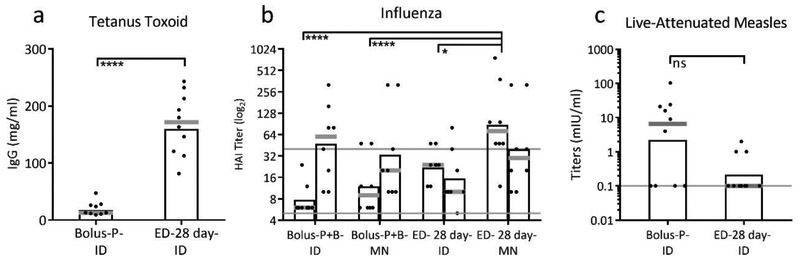
To determine if the extended-delivery profile studied using IPV would broadly apply to other vaccines, we replicated this experiment using tetanus toxoid, live-attenuated measles and subunit influenza vaccines administered ID. After tetanus toxoid vaccination, anti-tetanus toxoid IgG titers were increased 15-fold following 28-day extended delivery compared to bolus delivery in Balb/c mice (Figure 6a, p<0.0001). This shows that extended delivery can improve immune responses to a toxoid vaccine as well as inactivated virus vaccine.
Figure 6:
Applicability of extended delivery to multiple vaccines. Antibody responses to extended-delivery vaccination were improved relative to bolus vaccination for tetanus toxoid and subunit influenza vaccines, but not for live-attenuated measles vaccine. (a) Anti-tetanus toxoid IgG determined 42 days after initial vaccination using 5 Lf tetanus toxoid vaccine in Balb/c mice (**** p<0.0001) based on 10 replicates. (b) Hemagglutination inhibition (HAI) titers determined 56 days and 107 days after initial vaccination using 1 μg of subunit inactivated influenza vaccine (H1N1 A/Brisbane/10/10) administered by ID injection or microneedle patch (MN) in Balb/c mice (*p<0.05, ***p<0.001). When boost doses were given (Bolus-P+B groups), they were administered on day 77. The solid, grey line at a log2 titer of 5 indicates the assay detection limit, and the line at 40 represents the titer considered seropositive. Data based on 8 mice per group. (c) Measles virus neutralizing antibody titers determined 28 days after vaccination with 200 TCID50 live-attenuated measles vaccine (Edmonston-Zagreb strain) in cotton rats (ns p>0.05). Data show mean (black bar) and median (grey line) titers based on 10 rats. The solid, grey line at a titer of 0.1 mIU/ml indicates the assay detection limit.
Vaccination using a subunit influenza vaccine elicited hemagglutination inhibition (HAI) titers after extended-delivery vaccination (ED-28 day-ID) that were significantly higher than after bolus vaccination in Balb/c mice (Bolus-P+B-ID) (Figure 6b, p=0.006). After the bolus group received an additional boost dose, HAI titers were comparable to the single-dose extended-delivery group (Figure 6b, p=0.695). These data indicate that extended delivery can also improve responses to a subunit vaccine, thereby enabling a single extended-delivery dose to produce an immune response equivalent to a two-dose prime+boost regimen.
Finally, when live-attenuated measles vaccine was administered to cotton rats, the bolus and 28-day extended delivery vaccination were indistinguishable from each other (Figure 6c, p=0.700), indicating that extended delivery did not improve immunogenicity of this live-attenuated vaccine. This result was expected, because the live-attenuated measles vaccine replicates in the host with extended presentation of antigen.
Delivery using microneedle patches
For possible future applications, MN patches offer logistical advantages and have been shown to increase immune responses compared to injections. We therefore administered influenza subunit vaccine by MN patch as a bolus or 28-day extended delivery to Balb/c mice. After a single dose, extended delivery using MN patches induced higher HAI titers compared to bolus vaccination by either route (Figure 6b, p<0.001) and compared to extended delivery by ID injection (Figure 6b, p<0.05). After boosting with a second bolus dose, HAI titers increased to a level comparable to the single-dose extended-delivery MN patch group (p>0.6). This demonstrates that extended delivery of a single dose of influenza vaccine given by MN patch can elicit immunity similar to two doses of vaccine.
Extended-delivery enhancement of humoral immunity
To further understand effects of long-term antigen presentation on immune response, we compared extended delivery of influenza vaccine using MN patches to bolus MN patch, bolus ID injection and prime+boost bolus ID injection in Balb/c mice (Figure 7). As additional controls, two groups of mice received either a “bolus” placebo MN patch (as a naïve control, Bolus-Placebo-MN) or a bolus MN-patch vaccination followed by repeated placebo MN patches for the remainder of the 28 days (to account for possible effects of repeated injury by MN patch, Bolus-P-MN + ED-Placebo-MN) (Table 1).
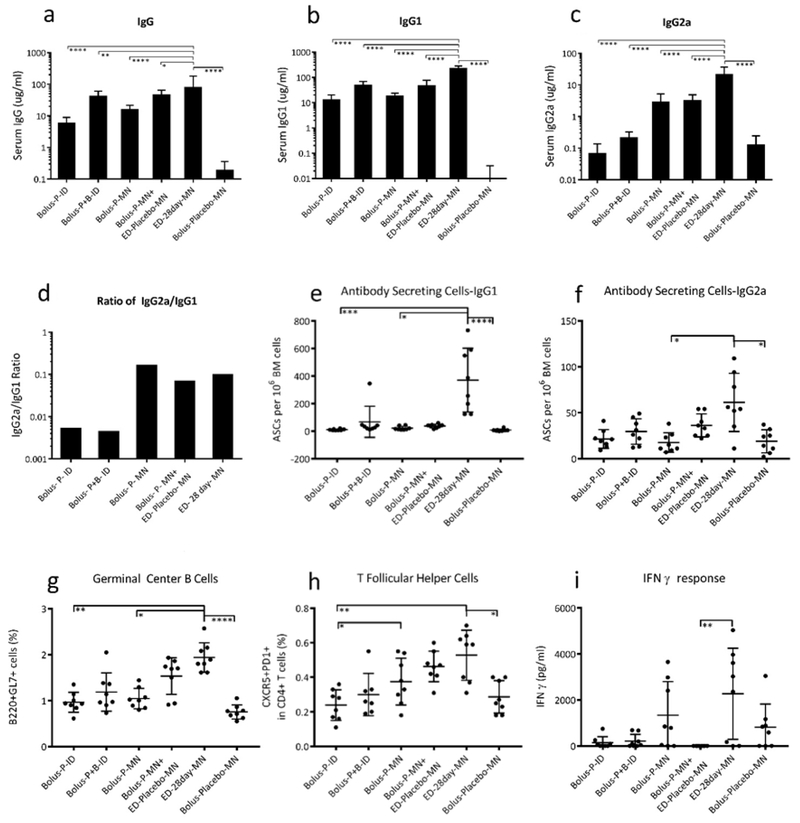
Figure 7:
Immune responses to extended-delivery of influenza vaccines with microneedle (MN) patches in mice. Influenza-specific (a) total IgG, (b) IgGa, and (c) IgG2a. Extended delivery with MN patches induced higher antibody titers compared to all other groups. (d) The ratio of IgG1 to IgG2a was approximately equivalent for all MN groups. Antibody secreting cells for (e) IgG1 and (f) IgG2a were present in higher numbers in the bone marrow of mice vaccinated with extended-delivery than bolus MN-patch vaccination. Lymph nodes of mice receiving extended-delivery MN-patch vaccination had higher percentage of (g) germinal center B cells and (h) T follicular helper cells compared to mice vaccinated by intradermal injection, but not higher than those receiving a MN prime dose followed by placebo patches. (i) Splenocytes that were re-stimulated with antigen secreted higher concentrations of IFN ɣ after extended-delivery vaccination (* p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001). Vaccination groups: bolus intradermal (ID) (Bolus-P-ID), bolus prime + boost on day 28 given ID (Bolus-P+B-ID) bolus MN patch (Bolus-P-MN), bolus MN patch followed by placebo MN patches for the remainder of the 28 days (Bolus-P-MN + ED-Placebo-MN), 28-day extended delivery by MN patch (ED-28day-MN) and placebo MN patches (Bolus-Placebo-MN), which were interpreted as the negative control group. Serum antibody titers and cellular responses were measured at day 35, which was one week after final vaccination for the ED group and one week after the boost. Total antigen delivered was held constant for each dose at 1 μg of inactivated influenza vaccine (H1N1 A/Brisbane/10/10). Data show mean ± S.D. based on 8 replicates.
We first analyzed IgG levels and found that total influenza-specific IgG titers were highest in mice that received extended delivery with MN patches compared to all other groups, including the bolus prime+boost group (Figure 7a, p<0.05). These mice also had the highest IgG1 and IgG2a titers (Figure 7b,c, p<0.0001). The ratio of IgG1/IgG2a was similar among all of the MN patch groups, which were ~10-fold higher than ID vaccination groups (Figure 7d).
After euthanasia on day 35, bone marrow, lymph nodes, and spleen were analyzed for cellular responses. This date was chosen to identify the response 7 days following the final vaccination in the extended delivery regime and 7 days following the boost. Extended delivery with MN patches generated the strongest GC-B and Tfh cellular responses, which were significantly higher than the ID and MN-patch bolus groups (Figure 7g,h p<0.01). Strong GC-B cell and Tfh cellular responses are desirable for high-affinity antibody and long-lived antibody-secreting plasma-cell (ASC) responses.
ASCs travel to bone marrow following vaccination or infection, and are associated with longterm memory response and antibody production. Both IgG1 and IgG2a ASCs in bone marrow were most prevalent after extended delivery by MN patch, which was significantly higher compared to bolus vaccination and placebo by MN patch (Figure 7e,f, p<0.025). While ASC, GC-B, and Tfh cellular responses were stronger after extended delivery by MN patch (ED-28day-MN) compared to bolus MN patch followed by repeated placebo patches (Bolus-P-MN+ED-Placebo-MN), this difference was not statistically different in this small data set (p>0.9), which suggests that repeated injury from MN placebo patch application following skin vaccination may affect immune response. [36]. Plasmablast levels in lymph nodes were not significantly different across all groups after vaccination (Supplemental Figure 6), which may be because measurement at day 35 was too late in the immune response process to see a difference [6].
Finally, IFN-gamma levels in splenocytes re-stimulated with antigen were highest following extended delivery with MN patches, indicating a strong Th1 cellular response, although this difference was not significant in most cases (Figure 7i). In some cases, the various cellular immune responses to vaccination using non-ED protocols were no stronger than the placebo MN patch negative control. Taken together, extended-delivery vaccination with MN patches amplified the humoral immune response through increased antigen-specific antibody, GC-B, Tfh, ASC, and Th1 responses.
Discussion
While vaccines provide a powerful, cost-effective tool in public health, insufficient vaccination coverage limits their impact. Approximately 1.5 million lives could be saved by improved vaccination coverage, both in terms of first-dose vaccination and completing vaccination series [1]. One way to improve coverage is streamlining the vaccine schedule such that a single vaccination can provide full protection without the need for booster doses. Vaccination could also improve public health impact by increasing vaccine immunogenicity without added cost of more antigen or addition of adjuvants. Finally, the reach of vaccines could be improved by simplifying logistics, such as less reliance on cold-chain refrigeration, avoidance of biohazardous sharps waste, and reduced need for highly trained healthcare personnel to administer vaccines.
With these goals in mind, this study sought to identify ways to increase vaccine immunogenicity without increasing vaccine dose, to reduce number of doses needed to achieve protective immunity, and to assess possible use of simplified vaccination methods. We did so by studying effects of six different kinetic profiles of vaccine release into skin on immune response during extended delivery using four licensed vaccines of significance to public health to assess the potential to increase immunogenicity, reduce the number of doses, and further understand immune response to extended vaccine delivery.
Vaccine delivery profile
Most prior studies extended vaccine delivery using an initial burst followed by low levels of release, providing limited information on different antigen presentation profiles [4, 37, 38]. An initial burst followed by a second burst 20 – 30 days later from a controlled-release formulation was able to generate immune responses similar to giving the vaccine as two boluses [39]. Here, we compared six vaccine delivery profiles and found that Constant and Exponentially Increasing profiles induced higher neutralizing antibody titers compared to single bolus injection of IPV. These results indicate that vaccination profiles that continually stimulate the immune response with at least equal dosing are more immunogenic than bolus or profiles with an initial burst, which is consistent with prior observations using model protein antigens [6, 7, 40]. These profiles induce better T-cell stimulation and prolonged T-cell memory, indicating immune activation of both cellular and humoral arms [37]. We considered that repeated exposure could induce tolerance to the antigen, which would result in suppressed responses compared to bolus vaccination. However, neutralizing antibody levels in all extended delivery groups were at least equivalent to the bolus vaccination, indicating that tolerance does not fully explain differences in extended delivery profiles.
We also determined that IPV delivery over one month performed better than release over 1-2 weeks. Even longer exposures could induce stronger reactions, although extremely slow release for longer times could be insufficient to generate an immune response, and possibly induce tolerance [41,42].
While prior studies demonstrated that controlled-release vaccination given IM was comparable to subcutaneous and intranasal delivery [43, 44], no studies have directly compared IM and ID vaccination with extended delivery. ID delivery is of increasing interest due to enhanced immune response to some vaccines and ease of dermal delivery using novel devices such as MN patches [14, 44, 45]. Here, we showed that IM and ID vaccination with extended delivery induced comparable immune responses that were superior to bolus doses. This finding motivates development of a controlled release ID injection or MN patch. The observation that continuous delivery provided an optimal immune response can facilitate development of controlled release formulations, because it is generally more difficult to formulate for more complex continuous delivery profiles or to simulate a prime+boost using pulsatile-release.
Unlike inactivated vaccines, live-attenuated vaccines induce potent immune responses and require fewer boosters for long-lasting protection. These vaccines infect and replicate in their host, inherently providing extended exposure to antigen for days or weeks [46]. Therefore, we predicted that prolonging antigen delivery would not benefit a live-attenuated vaccine, which is what we observed for live-attenuated measles vaccine. In contrast, extended delivery was found to enhance immune responses to all three inactivated or protein-based vaccines.
Improved immunogenicity of licensed vaccines
Extended delivery improved immunogenicity of three licensed vaccines: IPV (GlaxoSmithKline Biologics), tetanus toxoid (Serum Institute of India) and subunit influenza (Seqirus) vaccines. This suggests that our findings may be applicable to a broad range of non-replicating vaccines.
Inactivated polio vaccine.
Recent vaccine shortages have led WHO to recommend a one-fifth dose of IPV administered ID as a two-dose regimen rather than conventional IM administration of full-strength doses [47]. Extended-delivery vaccination could enable further dose reduction and/or reduce number of vaccine doses.
Tetanus toxoid.
Tetanus toxoid is currently given in 5–6 doses during childhood, sometimes followed by repeated boosters in adulthood and pregnancy [3]. Extended-delivery vaccination could reduce the number of doses and/or frequency of boosters, thereby simplifying the vaccination schedule that includes older children and adults outside the primary childhood vaccination schedule.
Influenza vaccine.
Influenza vaccine is given as a two-dose regimen upon first vaccination of children, followed by annual re-vaccination. Extended-delivery vaccination could eliminate the need for two doses upon first vaccination and reduce antigen doses needed during annual vaccination campaigns including high-dose vaccine administered to the elderly [3, 10, 11].
Microneedle patches with extended delivery
ID vaccination is often highly immunogenic [3, 10, 11] and MN patches inherently target skin, while offering logistical advantages that simplify vaccination [12-14]. In contrast to ID injections by experienced providers using the Mantoux method, MN patches are designed to easily vaccinate large numbers of people and induce a potent immune response. Prior studies have shown that MN patches are painless [48], easily administered [2], thermostable [21], free of biohazardous sharps waste [12], and found to be safe, immunogenic and well-accepted in a phase 1 clinical trial of bolus influenza vaccination [49].
Here, we demonstrated that extended-delivery influenza vaccination with MN patches produced the highest HAI titers and overall strongest humoral immune responses compared to bolus and extended-delivery ID injections. We hypothesize that this is because antigen is quickly cleared from skin after ID injection, making this dosing more like a series of closely space boluses. In contrast, MN patches are dry formulations that slowly hydrate, form a viscous gel of sugars and polymers and eventually clear from skin, thereby lengthening antigen presentation [17, 50]. The clearance rate of vaccine from the skin has been shown previously to depend on the antigen and formulation used, as well as the uptake and migration of APCs in the skin [17, 18, 51-53].
Immune responses to extended-delivery MN patches were superior by many measures. Antigen-specific total IgG, IgG1, and IgG2a titers were higher, consistent with prior observations of extended antigen presentation [54, 55]. IgG2a-to-IgG1 ratio was more balanced and less skewed toward a Th2 response; prior studies of controlled-release vaccination were skewed towards Th1 [56, 57] or Th2 [54, 58, 59] response. There were increased levels of GC-B and Tfh cells in lymph nodes and ASCs in bone marrow, which are associated with high-affinity antibody, isotype switching, and lasting immune memory [60]. Elevated GC-B and Tfh cell numbers and serum antibody titers were seen in previous bolus MN patch work [9]. Extended antigen presentation was also previously shown to increase CD8+ T-cell responses. Taken together, extended-delivery vaccination using MN patches elicited superior humoral immunity compared to bolus vaccinations and ID injections.
Limitations and future work
This work demonstrated the impact of extended vaccine delivery utilizing daily ID injections. This approach allowed us to study multiple release profiles with multiple vaccines without the complications of formulating different controlled release systems for each. While repeated injections are not practical clinically, this work motivates development and identifies design parameters for controlled release systems for a single vaccination. Injectable controlled release systems such as polymeric microparticles and thermosensitive hydrogels can stabilize and release proteins over the course of weeks to months [61, 62]. Polymeric microparticles made of poly(lactide-co-glycolide) or chitosan have been used to deliver vaccines into various tissues including the skin [59, 63, 64]. Vaccination via pulsatile hydrogels [65], thermosensitive hydrogels [66], and hydrogel MN patches [67] have also been formulated for improved immune responses. MN patches incorporating controlled release formulations have been formulated [15-18], but elicited significant burst release, which did not result in increased immunogenicity in this study. Techniques such as complexation to the active [62], encapsulation [68], and layer-by-layer deposition [69] have been developed to reduce this burst release and could be applied to MN patches. Novel MN patch formulations that stably incorporate vaccine and control its release without a burst should be developed to achieve single vaccination with extended delivery.
Conclusion
This study showed for the first time that extended-delivery vaccination (i) enables a single vaccination to generate immune responses equivalent to a two-dose vaccination regimen; (ii) enhances immune response to multiple vaccines currently used in licensed products (IPV, tetanus toxoid and influenza); (iii) does not enhance immune response to a live-attenuated vaccine (measles) that already mimics an extended infection; (iv) provides stronger immune responses when administered over the course of one month compared to extended delivery over shorter periods of time; (v) is effective for ID vaccination; and (vi) is further enhanced by using a MN patch compared to injections. We conclude that extended delivery of vaccines to the skin improves immune responses and has the potential for dose sparing and reducing the number of vaccine doses required to reach protective levels.
Supplementary Material
Highlights.
Immune response measured after extended delivery of vaccine for up to 4 weeks
Extended vaccine delivery induced immune response much stronger than bolus delivery
Extended delivery enhanced multiple vaccine types when delivered to skin or muscle
Extended delivery by microneedle patch further increased the immune response
Extended delivery could reduce number of doses and enable dose sparing of vaccines
Acknowledgements
The authors acknowledge support from Donna Bondy and the veterinary staff at Georgia Tech and CDC. JCJ was funded through the NIH/NIGMS-sponsored Cell and Tissue Engineering (CTEng) Biotechnology Training Program (T32GM008433) and National Science Foundation Graduate Research Fellowship (DGE-1148903). This work was also supported by grants from the National Institutes of Health (U01EB012495 to MRP and R01AI111557 to IS). We acknowledge the support of GlaxoSmithKline Biologics, Serum Institute of India and Seqirus for their generous donations of vaccine antigens.
MRP is an inventor of patents licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products (Micron Biomedical). This potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University. JJN's contributions were made prior to his employment at the U.S. Food and Drug Administration (FDA) and do not represent the views and/or policies of the FDA. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy, or views of the Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Immunization coverage. 2016, World Health Organization. [Google Scholar]
- 2.Arya J and Prausnitz MR, Microneedle patches for vaccination in developing countries. J Control Release, 2016. 240: p. 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plotkin SA, et al. , Vaccines 2017: Elsevier Health Sciences. [Google Scholar]
- 4.McHugh KJ, et al. , Single-injection vaccines: Progress, challenges, and opportunities. J Control Release, 2015. 219: p. 596–609. [DOI] [PubMed] [Google Scholar]
- 5.Johansen P, et al. , Antigen kinetics determines immune reactivity. Proc Natl Acad Sci U S A, 2008. 105(13): p. 5189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam HH, et al. , Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci U S A, 2016. 113(43): p. E6639–e6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schipper P, et al. , Repeated fractional intradermal dosing of an inactivated polio vaccine by a single hollow microneedle leads to superior immune responses. J Control Release, 2016. 242: p. 141–147. [DOI] [PubMed] [Google Scholar]
- 8.Teunissen MB, Haniffa M, and Collin MP, Insight into the immunobiology of human skin and functional specialization of skin dendritic cell subsets to innovate intradermal vaccination design. Curr Top Microbiol Immunol, 2012. 351: p. 25–76. [DOI] [PubMed] [Google Scholar]
- 9.Koutsonanos DG, et al. , Enhanced immune responses by skin vaccination with influenza subunit vaccine in young hosts. Vaccine, 2015. 33(37): p. 4675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engel ke L, et al. , Recent insights into cutaneous immunization: How to vaccinate via the skin. Vaccine, 2015. 33(37): p.4663–74. [DOI] [PubMed] [Google Scholar]
- 11.Kim YC, et al. , Delivery systems for intradermal vaccination. Curr Top Microbiol Immunol, 2012. 351: p. 77–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prausnitz MR, Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annu Rev Chem Biomol Eng, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Quinn HL, et al. , The role of microneedles for drug and vaccine delivery. Expert Opin Drug Deliv, 2014. 11(11): p. 1769–80. [DOI] [PubMed] [Google Scholar]
- 14.Marshall S, Sahm LJ, and Moore AC, The success of microneedle-mediated vaccine delivery into skin. Hum Vaccin Immunother, 2016. 12(11): p. 2975–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen MC, et al. , Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials, 2013. 34(12): p. 3077–86. [DOI] [PubMed] [Google Scholar]
- 16.Chu LY and Prausnitz MR, Separable arrowhead microneedles. J Control Release, 2011. 149(3): p. 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demuth PC, et al. , Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv Funct Mater, 2013. 23(2): p. 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeMuth PC, et al. , Implantable silk composite microneedles for programmable vaccine release kinetics and enhanced immunogenicity in transcutaneous immunization. Advanced healthcare materials, 2014. 3: p. 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edens C, et al. , Measles vaccination using a microneedle patch. Vaccine, 2013. 31(34): p. 3403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esser ES, et al. , Tetanus vaccination with a dissolving microneedle patch confers protective immune responses in pregnancy. J Control Release, 2016. 236: p. 47–56. [DOI] [PubMed] [Google Scholar]
- 21.Mistilis MJ, et al. , Long-term stability of influenza vaccine in a dissolving microneedle patch. Drug Deliv Transl Res, 2017. 7(2): p.195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langfield KK, et al. , Manufacture of measles viruses. Methods Mol Biol, 2011. 737: p. 345–66. [DOI] [PubMed] [Google Scholar]
- 23.Edens C, et al. , Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine, 2015. 33(37): p. 4683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bevilacqua JM, et al. , Rat immunogenicity assay of inactivated poliovirus. Dev Biol Stand, 1996. 86: p. 121–7. [PubMed] [Google Scholar]
- 25.Nijkamp FP and Parnham MJ, Principles of Immunopharmacology. 2005: Springer Basel AG. [Google Scholar]
- 26.Recommendations to assure the quality, safety and efficacy of tetanus vaccines (adsorbed), in WHO Technical Report Series No. 980. 2014, WHO Expert Committee on Biological Standardization. Sixty-third report. Annex 5. [Google Scholar]
- 27.Niewiesk S, Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol, 2014. 5: p. 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodewes R, Rimmelzwaan GF, and Osterhaus ADME, Animal models for the preclinical evaluation of candidate influenza vaccines. Expert Review of Vaccines, 2010. 9(1): p. 59–72. [DOI] [PubMed] [Google Scholar]
- 29.Leleux JA, Pradhan P, and Roy K, Biophysical Attributes of CpG Presentation Control TLR9 Signaling to Differentially Polarize Systemic Immune Responses. Cell Reports, 2017. 18(3): p. 700–710. [DOI] [PubMed] [Google Scholar]
- 30.Madan-Lala R, Pradhan P, and Roy K, Combinatorial Delivery of Dual and Triple TLR Agonists via Polymeric Pathogen-like Particles Synergistically Enhances Innate and Adaptive Immune Responses. Scientific Reports, 2017. 7: p. 2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weldon WC, Oberste MS, and Pallansch MA, Standardized Methods for Detection of Poliovirus Antibodies. Methods Mol Biol, 2016. 1387: p. 145–76. [DOI] [PubMed] [Google Scholar]
- 32.Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. Bull World Health Organ, 1996. 74(3): p. 253–68. [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen BJ, et al. , Plaque reduction neutralization test for measles antibodies: Description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine, 2007. 26(1): p. 59–66. [DOI] [PubMed] [Google Scholar]
- 34.WHO manual on animal influenza diagnosis and surveillance, W.H.O.D.o.E.a.P.A.a. Response, Editor. 2002, World Health Organization: Geneva. [Google Scholar]
- 35.Kang SM and Compans RW, Enhancement of mucosal immunization with virus-like particles of simian immunodeficiency virus. J Virol, 2003. 77(6): p. 3615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Depelsenaire AC, et al. , Colocalization of cell death with antigen deposition in skin enhances vaccine immunogenicity. J Invest Dermatol, 2014. 134(9): p. 2361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachmann MF and Jennings GT, Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol, 2010. 10: p. 787–796. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Z and Leong KW, Controlled delivery of antigens and adjuvants in vaccine development. J Pharm Sci, 1996. 85: p. 1261–1270. [DOI] [PubMed] [Google Scholar]
- 39.Tzeng SY, et al. , Stabilized single-injection inactivated polio vaccine elicits a strong neutralizing immune response. Proc Natl Acad Sci U S A, 2018. 115(23): p. E5269–e5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansen P, et al. , Antigen kinetics determines immune reactivity. Proc Natl Acad Sci U S A, 2008. 105: p. 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinkernagel RM, Localization dose and time of antigens determine immune reactivity. Semin Immunol, 2000. 12: p. 163–344. [DOI] [PubMed] [Google Scholar]
- 42.Zinkernagel RM and Hengartner H, Regulation of the immune response by antigen. Science, 2001. 293: p. 251–253. [DOI] [PubMed] [Google Scholar]
- 43.Singh M, et al. , Biodegradable microparticles with an entrapped branched octameric peptide as a controlled-release HIV-1 vaccine. J Pharm Sci, 1997. 86: p. 1229–1233. [DOI] [PubMed] [Google Scholar]
- 44.Combadiere B and Liard C, Transcutaneous and intradermaivaccination. Hum Vaccin, 2011. 7(8): p. 811–27. [DOI] [PubMed] [Google Scholar]
- 45.Babiuk S, et al. , Cutaneous vaccination: the skin as an immunologically active tissue and the challenge of antigen delivery. J Control Release, 2000. 66(2-3): p. 199–214. [DOI] [PubMed] [Google Scholar]
- 46.Rota PA, et al. , Detection of measles virus RNA in urine specimens from vaccine recipients. J Clin Microbiol, 1995. 33(9): p. 2485–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polio vaccines: WHO position paper – March, 2016, in Weekly epidemiological record. 2016, World Health Organization; p. 145–168. [Google Scholar]
- 48.Norman JJ, et al. , Microneedle patches: Usability and acceptability for self-vaccination against influenza. Vaccine, 2014. 32(16): p. 1856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rouphael NG, et al. , The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulit-Penaloza JA, et al. , A protective role of murine langerin(+) cells in immune responses to cutaneous vaccination with microneedle patches. Sci Rep, 2014. 4: p. 6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, et al. , Rapid kinetics to peak serum antibodies is achieved following influenza vaccination by dry-coated densely packed microprojections to skin. J Control Release, 2012. 158: p. 78–84. [DOI] [PubMed] [Google Scholar]
- 52.Romgens AM, et al. , A theoretical compartment model for antigen kinetics in the skin. Eur J Pharm Sci, 2016. 84: p. 18–25. [DOI] [PubMed] [Google Scholar]
- 53.Raphael AP, et al. , Formulations for microprojection/microneedle vaccine delivery: Structure, strength and release profiles. J Control Release, 2016. 225: p. 40–52. [DOI] [PubMed] [Google Scholar]
- 54.Baras B, et al. , Vaccine properties of antigens entrapped in microparticles produced by spray-drying technique and using various polyester polymers. Vaccine, 2000. 18: p. 1495–1505. [DOI] [PubMed] [Google Scholar]
- 55.Neimert-Andersson T, et al. , Improved immune responses in mice using the novel chitosan adjuvant ViscoGel, with a Haemophilus influenzae type b glycoconjugate vaccine. Vaccine, 2011. 29(48): p. 8965–73. [DOI] [PubMed] [Google Scholar]
- 56.Wheeler AW, Marshall JS, and Ulrich JT, A Thl-inducing adjuvant, MPL, enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy of allergy vaccines. Int Arch Allergy Immunol, 2001. 126(2): p. 135–9. [DOI] [PubMed] [Google Scholar]
- 57.Grenfell RF, et al. , Vaccine self-assembling immune matrix is a new delivery platform that enhances immune responses to recombinant HBsAg in mice. Clin Vaccine Immunol, 2015. 22(3): p. 336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dixit S, et al. , Poly(lactic acid)-poly(ethylene glycol) nanoparticles provide sustained delivery of a Chlamydia trachomatis recombinant MOMP peptide and potentiate systemic adaptive immune responses in mice. Nanomedicine, 2014. 10(6): p. 1311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohanan D, et al. , Administration routes affect the quality of immune responses: A cross-sectional evaluation of particulate antigen-delivery systems. J Control Release, 2010. 147(3): p. 342–9. [DOI] [PubMed] [Google Scholar]
- 60.Mesin L, Ersching J, and Victora GD, Germinal Center B Cell Dynamics. Immunity, 2016. 45(3): p. 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milacic V, et al. , Injectable PLGA Systems for Delivery of Vaccine Antigens, in Long Acting Injections and Implants, Wright JC and Burgess DJ, Editors. 2012, Springer, p. 429–458. [Google Scholar]
- 62.Oak M and Singh J, Controlled delivery of basal level of insulin from chitosan-zincinsulin-complex-loaded thermosensitive copolymer. J Pharm Sci, 2012. 101(3): p. 1079–96. [DOI] [PubMed] [Google Scholar]
- 63.Vora LK, et al. , Novel bilayer dissolving microneedle arrays with concentrated PLGA nano-microparticles for targeted intradermal delivery: Proof of concept. J Control Release, 2017. 265: p. 93–101. [DOI] [PubMed] [Google Scholar]
- 64.Jain S, O'Hagan DT, and Singh M, The long-term potential of biodegradable poly(lactide-co-glycolide) microparticles as the next-generation vaccine adjuvant. Expert Rev Vaccines, 2011. 10: p. 1731–1742. [DOI] [PubMed] [Google Scholar]
- 65.Gübeli RJ, et al. , Pharmacologically triggered hydrogel for scheduling hepatitis B vaccine administration. Scientific reports, 2013. 3: p. 2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, et al. , Thermo-sensitive hydrogel PLGA-PEG-PLGA as a vaccine delivery system for intramuscular immunization. J Biomater Appl, 2017. 31(6): p.923–932. [DOI] [PubMed] [Google Scholar]
- 67.Courtenay AJ, et al. , Novel Hydrogel-Forming Microneedle Array for Intradermal Vaccination in Mice Using Ovalbumin as a Model Protein Antigen. Mol Pharm, 2018. [DOI] [PubMed] [Google Scholar]
- 68.Silva AL, et al. , Optimization of encapsulation of a synthetic long peptide in PLGA nanoparticles: low-burst release is crucial for efficient CD8(+) T cell activation. Eur J Pharm Biopharm, 2013. 83(3): p. 338–45. [DOI] [PubMed] [Google Scholar]
- 69.Shukla A, et al. , Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials, 2010. 31(8): p. 2348–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.