Abstract
Purpose
To estimate the cost-effectiveness of breast cancer screening in the National Breast and Cervical Cancer Early Detection Program (NBCCEDP).
Methods
Using a modified CISNET breast cancer simulation model, we estimated outcomes for women aged 40–64 years associated with three scenarios: breast cancer screening within the NBCCEDP, screening in the absence of the NBCCEDP (no program), and no screening through any program. We report screening outcomes, cost, quality-adjusted life-years (QALYs), incremental cost-effectiveness ratios (ICERs), and sensitivity analyses results.
Results
Compared with no program and no screening, the NBCCEDP lowers breast cancer mortality and improves QALYs, but raises health care costs. Base-case ICER for the program was $51,754/QALY versus no program and $50,223/QALY versus no screening. Probabilistic sensitivity analysis ICER for the program was $56,615/QALY [95% CI $24,069, $134,230/QALY] versus no program and $51,096/QALY gained [95% CI $26,423, $97,315/QALY] versus no screening.
Conclusions
On average, breast cancer screening in the NBCCEDP was cost-effective compared with no program or no screening.
Keywords: Breast cancer, Screening, Cost-effectiveness, Economic analysis, NBCCEDP
Introduction
Despite documented effectiveness of mammography screening in the early detection and mortality reduction of breast cancer [1-6], not all women receive or have access to breast cancer screening. Uninsured women are less likely to receive mammography screening and more likely to present with advanced-stage cancer than insured women [7-9].
The Centers for Disease Control and Prevention (CDC) National Breast and Cervical Cancer Early Detection Program (NBCCEDP) provides free or low-cost breast cancer screening to medically underserved women aged 40–64 years who have an annual income ≤ 250% of the federal poverty level. Since inception in 1991, the NBCCEDP has provided screening and diagnostic examinations to more than 5.3 million women, diagnosing about 63,000 invasive breast cancers and 20,000 premalignant breast lesions [10]. Through a network of providers, health care systems, and partner organizations, the program fills a gap in education, outreach, and service delivery to uninsured or underinsured women in the United States (U.S.) [11]. A previous study estimated that presence of the NBCCEDP saved 100,800 life-years compared to no program and 369,000 life-years compared to no screening among the 1.8 million women screened through the NBCCEDP between 1991 and 2006 [12]. Program successes have also been evaluated using benchmarks of timeliness of care, provision of effective screening and diagnostic services, case management, and partnerships [11, 13, 14].
While the reported benefits of the program are substantial [11, 12], the operational and service delivery costs of the NBCCEDP are also substantial [15]. During the past decade, the average federal budget for the NBCCEDP was about $144 million per year. Additionally, each grantee matched their federal funding with supplemental contributions and non-federal funds ($1 per every $3 of federal funding received), as required by the Breast and Cervical Cancer Mortality Prevention Act of 1990 (Public Law 101-354) to conduct various, screening, quality assurance, public education, and outreach activities [11]. Over the past 25 years of the program’s existence, the program and its partners have also invested through in-kind donations [15].
Given constrained resources for public health programs and changes in insurance coverage after the implementation of the Affordable Care Act (ACA), it is important to ascertain the baseline value of health care provided from public health programs like the NBCCEDP as a measure of program evaluation. Cost-effectiveness analysis (CEA) is a methodologic tool particularly suited for comparative evaluations in terms of cost and outcomes of programs [16, 17]. In this study, we build on a prior analysis [12] using data from the early years of the NBCCEDP to estimate incremental cost-effectiveness of breast cancer screening within the NBCCEDP compared to scenarios of breast cancer screening with no program or no screening, taking the societal perspective.
Methods
Model structure
We used a decision analytic-simulation model to analyze three scenarios: (1) women who received screening from the NBCCEDP (hereafter Program), (2) women who potentially received screening through another source in the absence of the program (No Program), and (3) women who received no screening at all through any source (No Screening). We modified a breast cancer screening model developed by researchers at Stanford University (Plevritis et al. [20]) as part of the Cancer Intervention and Surveillance Modeling Network (CISNET) [18-20]. The model examines lifetime probability of developing breast cancer, screening, early detection, treatment effects, and mortality from breast cancer and other causes.
Consistent with our previous analysis [12], the model cohort simulated the age distribution of the 1.8 million women who received screening mammograms covered by NBCCEDP between 1997 and 2006. Data on women screened within the NBCCEDP were unavailable prior to 1997. For each scenario, 2 million women were randomly drawn from the NBCCEDP by birth year and age distributions. Two million was the number needed for the model to converge. In each simulation, the model followed an individual woman in the cohort through death from breast cancer or other causes.
Model inputs and key assumptions
The model’s natural history module determines a woman’s lifetime probability of developing invasive breast cancer and the age of clinical detection in the absence of screening [12]. We derived these inputs from CISNET estimates [12, 21].
The screening history module produces a woman’s simulated screening history, starting with age of first mammogram (if any), which were based on the woman’s age and birth cohort. Intervals between subsequent mammograms were determined from distributions based on whether the woman was screened on an annual, biennial, or irregular basis. We defined these categories from the estimated mean time between mammograms; annual screening was < 1.5 year; biennial was 1.5 to < 2.5 year; and irregular was 2.5 year or more. To generate screening history under the NBCCEDP, we modified the CISNET base-case code [18, 22] by combining it with NBCCEDP data to estimate age at first mammography within the program and screening intervals. For mammography screening in the absence of NBCCEDP, we estimated the proportion of uninsured women who received mammograms using data from the 1990–2005 National Health Interview Survey.
Our model incorporated the CISNET model assumptions about early detection and treatment effects [22, 23]. Briefly, we randomly assigned each woman with breast cancer a survival time based on breast cancer survival curves, accounting for age, tumor diameter and stage at detection, estrogen receptor status, and treatment. We assumed the woman died from breast cancer if the sum of her age at detection and survival time was earlier than her estimated date of death from other causes; we randomly assigned dates of death based on general U.S. mortality rates. We subtracted breast cancer mortality rates from general mortality rate using CISNET methods [12].
Our model accounted for the time between screening and diagnostic follow-up for women with abnormal screening results. Consistent with model assumptions of prior studies [12, 24], the rate of diagnostic follow-up for program participants was assumed to be higher (92% vs. 77%) and the wait time until diagnostic test shorter (25 vs. 42 days) than previously estimated by Raich et al. [25] and Hoffman et al. [26] for low-income uninsured women outside the program. Details on these assumptions are described in Allaire et al. [24]. In our CEA, we also added breast cancer screening, diagnostic, and treatment costs and health state utility decrements associated with diagnosed breast cancer.
Costs
We estimated the average cost of breast cancer screening and diagnosis (Table 1) using the NBCCEDP Cost Assessment Tool (CAT) [27]. We averaged total costs and the number of women screened over the 3 years of CAT data collection to minimize the effects of yearly variation. Further details appear in the Online Appendix.
Table 1.
Model parameters for the cost-effectiveness analysis of the NBCCEDP
| Parameter | Baseline estimate mean (standard devia- tion or range) |
Distribution selected for PSA |
Sources |
|---|---|---|---|
| Cost | |||
| Screening costs | $297 ($127) | Gamma | NBCCEDP data |
| Diagnostic costs | $490 ($144) | Gamma | NBCCEDP data |
| Patient navigation | $22 ($10) | Gamma | NBCCEDP data |
| Treatment costs | Varies based on tumor stage at diagnosis, treatment year (initial, continuing, death due to breast cancer, death due to non-cancer costs), and age, with costs adjusted for Medicaid (see Online Appendix tables) (SD assumed to be 15% of baseline costs) | Gamma | Mariotto et al. [28] Fireman et al. [29] Zuckerman et al. [30] |
| Screening and diagnostic follow-up | |||
| Screening frequency | Probability of annual, biannual, or irregular screening varies by age and program participation—see Online Appendix. | Dirichlet | NBCCEDP data, National Health Interview Survey |
| Diagnostic follow-up | |||
| Within program | 92% | Beta | Raich et al. [25] |
| Outside program | 77% | Beta | Raich et al. [25] |
| Diagnostic wait time (days) | |||
| Within program | 25 (2) | Normal | Hoffman et al. [26] |
| Outside program | 42 (4) | Normal | Hoffman et al. [26] |
| Tumor detection threshold | 5 mm (Range 5–15 mm)cancer | Uniform | Hoerger et al. [12] |
| Utility decrements for diagnosed breast | |||
| Initial | 0.07 (0.01) | Beta | Yabroff et al. [31] |
| Continuing | 0.04 (0.01) | Beta | Yabroff et al. [31] |
| Terminal | 0.08 (0.04) | Beta | Yabroff et al. [31] |
| Disease parameters | |||
| Breast cancer mortality hazard multiplier | 1 (Range 0.994–1.016) | Uniform | Variation from Cho et al. [32] |
| Reduction in breast cancer incidence (DCIS) | 4.5% | Beta | Cronin et al. Supplementary Table 2 (2006) [22] |
DCIS ductal carcinoma in situ, NBCCEDP National Breast and Cervical Cancer Screening Program, PSA probabilistic sensitivity analysis, SD standard deviation, SEER surveillance, epidemiology, and end results program
Breast cancer treatment costs were based on treatment stage (initial, continuing, and terminal), tumor stage at diagnosis (localized, regional, and distant), and age.Baseline attributable costs for breast cancer treatments covered by Medicare, by treatment stage, were derived from Mariotto et al. [28]. We adjusted costs for tumor stage using ratios obtained from Fireman et al. [29]; and we adjusted treatment costs for women aged < 65 years to reflect Medicaid rates, as most women diagnosed with breast cancer in the NBCCEDP are treated by Medicaid, as covered under the Breast and Cervical Cancer Prevention and Treatment Act of 2000 [11]. Derivation of treatment costs is detailed in Online Appendix.
Quality of life/health state utilities
Health state utility decrements vary by time frames since detection (initial, continuing, and final year of life); these were derived from Yabroff et al. [31]. Decrements were 0.07 in the first year after detection, 0.04 in subsequent non-terminal years (up to a maximum of 10 years), and 0.08 in the final year for women who die from breast cancer.
Outcomes
Building on Hoerger et al. [12], the main outcomes of this CEA include: estimates of screening history, breast cancer incidence, screening or clinical detection, stage at detection, costs, life-years, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs). The model was developed and analyzed in TreeAge Pro 2009 (TreeAge Software Inc, Williamstown, MA). Costs are reported in 2018 U.S dollars; costs and QALYs are discounted at a 3% annual rate. This model takes the societal perspective [17].
Sensitivity analyses
We conducted deterministic and probabilistic sensitivity analyses (PSA) to test whether the main results of the model changed when key model assumptions and inputs were varied. We performed one-way sensitivity analyses on select parameters: screening frequency, median tumor diameter for screening detection, breast cancer incidence, efficacy of treatment, potential benefits from earlier detection with symptoms, treatment follow-up rates, screening and diagnostic costs, treatment costs, and patient utilities. Each sensitivity analysis was based on cohort size of 500,000 women.
To determine the sensitivity of the model to uncertainty, we conducted PSAs, which simultaneously vary the input parameters according to specified distributions. Probabilities were modeled using beta or Dirichlet distributions. Costs were assumed to follow gamma distributions. Uniform distribution was used for median detection threshold and beta distributions for patient utility decrements [16]. PSA was conducted with 200 random samples from input distributions and the microsimulation model evaluated with 10,000 simulated women. Results from these analyses are presented as cost-effectiveness acceptability curves (CEACs) in the Online Appendix. Although an appropriate cost-effectiveness threshold continues to be a subject of debate, $50,000/QALY is a conservative threshold often cited in the U.S [17]. Other thresholds of $200,000/QALY to $300,000/QALY have also been suggested based on trends in healthcare spending [17]. The U.S. Department of Health and Human Services does not advocate for or abide by any specific threshold.
Results
Base-case results
In the base-case analysis (Table 2), breast cancer mortality was 3.53, 3.93, and 4.97% under the Program, No Program, and No Screening scenarios, respectively. Overall, a slightly higher proportion of women within the Program were diagnosed with breast cancer (13.82%) than women under the No Screening scenario (13.30%). About 62% (8.55%/13.82%) of breast cancers were screen-detected in the Program compared to about 48% (6.56%/13.73%) screen-detected in the No Program scenario. The stage at diagnosis for the majority of the screen-detected cancers in the Program and No Program scenarios was local.
Table 2.
Results of the three scenarios modeled for women receiving breast cancer screening in the NBCCEDP (n = 2,000,000 in each scenario)
| Parameter | Scenario |
||
|---|---|---|---|
| Program | No program | No screening | |
| Mammograms (per woman) over lifetime | 17.61 | 12.34 | 0.00 |
| Mammograms (per woman) age 40–64, 1991–2006 | 4.23 | 2.41 | 0.00 |
| Died of breast cancer | 3.53% | 3.93% | 4.97% |
| Clinically detected rate | 5.27% | 7.17% | 13.30% |
| Screen detected rate | 8.55% | 6.56% | 0.00% |
| Overall detected rate | 13.82% | 13.73% | 13.30% |
| Clinically detected | |||
| Localized | 52.0% | 50.8% | 48.0% |
| Regional | 41.7% | 42.7% | 44.7% |
| Distant | 6.3% | 6.5% | 7.3% |
| Screen detected | |||
| Localized | 81.1% | 78.8% | |
| Regional | 17.5% | 19.6% | |
| Distant | 1.4% | 1.6% | |
| Missed diagnostic follow-ups (per woman) | 0.049 | 0.059 | 0.000 |
| Costing information (per woman) | |||
| Screening costs | $2,635 | $1,771 | $0 |
| Diagnostic costs | $342 | $233 | $0 |
| Treatment costs | $5,689 | $5,560 | $5,258 |
| Patient navigation costs | $126 | $0 | $0 |
| Total costs | $8,791 | $7,564 | $5,258 |
| Life-years (per woman) | 21.990 | 21.965 | 21.915 |
| QALYs (per woman) | 21.969 | 21.945 | 21.898 |
| Incremental cost-effectiveness ratios (ICERs) | |||
| $ per life-year | |||
| Versus no screening | $47,489 | $46,580 | |
| Versus no program | $49,296 | ||
| $ per QALY | |||
| Versus no screening | $50,223 | $49,443 | |
| Versus no program | $51,754 | ||
Life-years and QALYs were rounded in the TreeAge software
NBCCEDP National Breast and Cervical Cancer Early Detection Program, QALYs quality-adjusted life-years
Overall costs per woman were higher in the Program ($8,791) and No Program ($7,564) scenarios compared to No Screening ($5,258) scenario (Table 2). The largest cost differences between scenarios were for screening, followed by treatment and diagnostic costs.
In the base-case, we found that the Program improved life-years (incremental life-years gained was 0.025 and 0.075) and QALYs (incremental QALYs gained was 0.024 and 0.071) compared to No Program and No Screening scenarios, respectively. The ICER for the Program was $51,754 per QALY compared to the No Program and $50,223 per QALY compared to the No Screening. ICERs per discounted life-year were slightly lower than the ICERs per QALY since estimated life-years gained exceed QALYs gained.
Sensitivity analyses
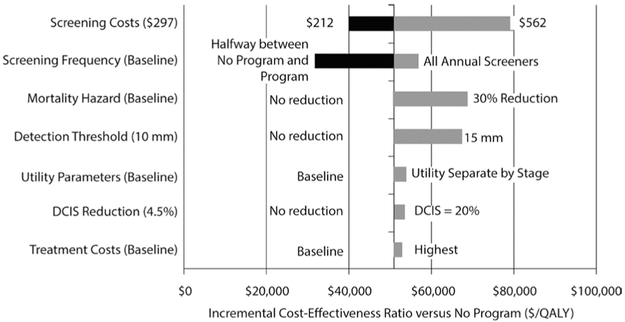
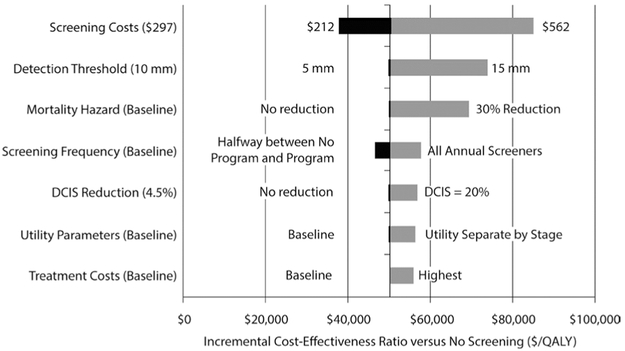
Results of one-way sensitivity analyses are presented in Figs. 1 and 2. The Program ICER versus No Program (Fig. 1) was most sensitive to screening costs and screening frequency. Lower screening cost reduced incremental costs of the Program to where the ICER fell below $40,000 per QALY. Similarly, higher screening costs increased the ICER (because of the assumption that there are more screens in the Program versus No Program scenario). If all Program participants received annual screening, both incremental costs and incremental QALYs increased, and the ICER was > $50,000 per QALY. In examining sensitivity to recent improvements in therapies and treatment effects, we reduced the mortality hazard by 30% for both clinical and screen-detected cancers at every tumor volume and clinical stage. The result was an increased Program ICER ($72,801 per QALY). Increasing the detection threshold to 15 mm also increased the ICER to $70,959 per QALY. Utility parameters, the ductal carcinoma in situ (DCIS) reduction factor, and treatment costs impacted the ICER minimally. Compared with the No Screening scenario (Fig. 2), Program ICER was most sensitive to changes in screening costs and screening detection threshold.
Fig. 1.
Tornado diagram displaying results from one-way sensitivity analyses for program versus no program. Largest range of incremental cost-effectiveness values are shown on top; smallest ranges are at the bottom. The values at either end of the horizontal bar are the high and low parameter values considered in the one-way sensitivity analysis. The vertical line represents the base-case incremental cost-effectiveness ratio of the model
Fig. 2.
Tornado diagram displaying results from one-way sensitivity analyses for program versus no screening. Largest range of incremental cost-effectiveness values are shown on top; smallest ranges are at the bottom. The values at either end of the horizontal bar are the high and low parameter values considered in the one-way sensitivity analysis. The vertical line represents the base-case incremental cost-effectiveness ratio of the model
PSA results are presented in Table 3. The estimated mean Program ICER versus No Program was $56,615 per QALY [95% CI: $24,069, $134,230/QALY] and $51,096 per QALY [95% CI: $26,423, $97,315/QALY] versus No Screening. The CEACs from the PSA (in the Online Appendix) show the probability that the Program was cost-effective based on the societal willingness-to-pay thresholds per QALY.
Table 3.
Probabilistic sensitivity analysis results from the NBCCEDP
| Scenario | Incremental costs (95% CI) | Incremental QALYs (95% CI) | ICER (95% CI) |
|---|---|---|---|
| Versus no program | $1,228 ($654, $2,147) | 0.024 (0.011, 0.040) | $56,615 ($24,069, $134,230) |
| Median | [$1,189] | [0.024] | [$50,097] |
| Versus no screening | $3,533 ($1,807, $5,766) | 0.07 (0.052, 0.088) | $51,096 ($26,423, $97,315) |
| Median | [$3,340] | [0.070] | [$48,049] |
Medians in brackets
ICER incremental cost-effectiveness ratio expressed as $ per QALY, NBCCEDP National Breast and Cervical Cancer Early Detection Program; QALYs quality-adjusted life-years; 95% confidence intervals (CI) presented in parentheses
Discussion
We examined the cost-effectiveness of breast cancer screening in the NBCCEDP. Our results from the CEA suggest that compared to scenarios in which screening occurs in the absence of the program and where no screening occurs, breast cancer screening in the NBCCEDP improves QALYs and was cost-effective among the target population of low-income, uninsured women aged 40–64 years. The base-case ICER for the program was $51,754/QALY compared to no program and $50,223/QALY compared to no screening; there was a wide variation in PSA ICERs and some ICERs fell outside of the conservative threshold [17]. However, even at the conservative willingness-to-pay decision threshold of $50,000/QALY, the probability that the program is cost-effective is about 0.7 (compared to no program and compared to no screening). Our findings are among the limited number of economic evaluations to date to quantify the estimated gains of breast cancer screening in the NBCCEDP [3, 12, 33-35], especially for women who would otherwise not have access to screening if the program did not exist. This study provides a retrospective look at the health and economic outcomes of the program’s recent past to inform future population-based economic analyses of the program.
Our analysis was performed using data prior to the full implementation of the ACA in 2014. Still, in the current environment and even as the discussions around health care delivery continue to shift, there are important lessons to garner from our findings. In perspective, the NBCCEDP is a unique public health initiative and the only organized, national cancer screening program for low-income, uninsured, or underinsured women in the U.S [11]. The NBCCEDP supports a comprehensive range of services that help women access high-quality healthcare (i.e., public education, outreach, case management/patient navigation, clinical service delivery, follow-up, and treatment referrals) to systems-relevant operational activities that include data management, evaluation, tracking, and quality assurance of clinical and service delivery data [11]. A key service of the NBCCEDP is ensuring that high-quality screening and diagnostic services are provided appropriately and within a reasonable timeframe. For example, per predefined NBCCEDP quality standards, a woman with an abnormal screening test should have complete diagnostic evaluation within 60 days and subsequently should initiate treatment within 60 days after diagnosis [14]. This is critical given that uninsured women, ethnic minorities, and women with lower socioeconomic status are more likely to have delayed and incomplete follow-up after an abnormal screening result [14]. Although our study found the NBCCEDP to be cost-effective to society, the main intent of the NBCCEDP is to address health disparities and provide a service to medically underserved groups who may not have access to the current health care system. The two decades of experience of the NBCCEDP in both reaching and tracking women offer insight into relevant and transferable lessons-learned about proven strategies that can guide other health systems across the U.S. The program has demonstrated success in reducing mortality from breast cancer [33, 34] and improving quality of life [24]. Our findings show that breast cancer screening in the program can be cost-effective at the $50,000/QALY threshold (though these thresholds are somewhat arbitrary and are often exceeded in clinical practice). Given the increasing incidence rate of breast cancer among U.S. women [36] and the rising cost of healthcare, evaluating cost-effectiveness of programs and interventions are important and intrinsic to understanding value gained from such “what is” and “what if” scenarios [17].
National data suggest that health insurance reform has reduced the number of uninsured persons in the U.S. [37, 38], with 60% of the reduction in uninsured rate being attributed to expanded Medicaid coverage [38]. Even still, given that about one-third of U.S. states elected not to expand Medicaid, a sizable proportion of low-income adults are likely in a coverage gap and potentially in need of services like those provided by the NBCCEDP [39]. Even if coverage is available through the program, there are layers of challenges for women to obtain breast (and cervical) cancer screening such as limited reach of the program, geographic isolation, transportation issues, limited health literacy, or language proficiency [3, 10, 11, 34]. Building upon its infrastructure and community-clinical linkages, the NBCCEDP focus and population-based approach of reaching the difficult-to-reach women for these services are significant hallmarks of the program. Moving forward, various elements of the NBCCEDP model (such as patient navigation) will likely continue to be valuable in offering timely, quality cancer screening to medically underserved women and in improving life-years and health outcomes. A study by Allaire and colleagues reported on the beneficial effects and cost-effectiveness of patient navigation services within the NBCCEDP [24].
Our analysis uniquely estimates overall NBCCEDP cost-effectiveness of breast cancer screening for low-income, uninsured women. A major strength of our study is that we modified a validated CISNET breast cancer screening model [12, 18-20] and used program-specific cohort and cost information as model inputs. This study also has several limitations. First, as with all CEA, findings are limited by gaps in evidence on model parameters and the assumptions adopted in the study [16, 17]. Input data are largely from earlier years of the program, 1991–2006, and there is a wide variation between programs funded by the NBCCEDP and cost data by state. Second, the reported results are based on a simulation model, not on results from a clinical trial or a longitudinal study. Randomization in a clinical trial that denies screening to some uninsured women is unlikely to occur for ethical reasons [12]. Long-term follow-up data are not currently collected on program participants. Third, we focused on screening mammograms for asymptomatic women; the NBCCEDP also provides diagnostic mammograms and other diagnostic testing for women who have symptoms of potential breast cancer. Including these services would increase the overall benefit attributable to the NBCCEDP, although it would not affect our conclusions about the cost-effectiveness of the breast cancer screening component of the program. Fourth, we do not consider benefits or costs from cervical cancer screening under the NBCCEDP. Some NBCCEDP participants are eligible for both breast and cervical cancer screening, and there are potentially economies of scope for providing the services as a bundle [40]. Estimating the costs and benefits associated with cervical cancer screening was beyond the scope of the study. Lastly, we do not account for the full societal costs and benefits of the program. We used QALYs to measure health-related importance in quality of life, but this utility measure—although commonly used— has inherent limitations [16, 17]. One example of such limitation is the assumption of fairness that the assessed value of life-years gained in an intervention strategy is directly proportional to a participant’s ability to benefit from it [17].
Conclusion
Our findings suggest that breast cancer screening in the NBCCEDP is cost-effective, although there was a wide range of variability from sensitivity analyses. Given that our model was most sensitive to screening cost and screening frequency, important discussions at the federal, state, and local levels are needed to understand how to maximize services (and utility per unit cost) in an organized, cancer screening program. We offer this study as an example of how economic data can be used to assess the benefits of an organized, national public health program. Looking forward, similar evaluations of the program may be valuable to cancer control planners as improvements in diagnostic tools become available and also to health policy decision-makers as the health care environment continues to evolve.
Supplementary Material
Acknowledgments
The authors thank Wesley Crouse for his assistance in data collection.
Disclaimer The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Funding This research was supported by Contract No. 200-2008-27958 from the Centers for Disease Control and Prevention.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10552-019-01178-y) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bleyer A, Welch HG (2012) Effect of three decades of screening mammography on breast-cancer incidence. New Engl J Med. 367:1998–2005 [DOI] [PubMed] [Google Scholar]
- 2.Mandelblatt JS, Cronin KA, Bailey S et al. (2009) Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med 151:738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melnikow J, Tancredi DJ, Yang Z et al. (2013) Program-specific cost-effectiveness analysis: breast cancer screening policies for a safety-net program. Value Health. 16:932–941 [DOI] [PubMed] [Google Scholar]
- 4.Practice bulletin no. 122: Breast cancer screening. Obstet Gynecol. 118:372–82 [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Fontham ET, Etzioni R et al. (2015) Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 314:1599–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siu AL (2016) Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 164:279–296 [DOI] [PubMed] [Google Scholar]
- 7.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY (2008) Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 9:222–231 [DOI] [PubMed] [Google Scholar]
- 8.Ward E, Halpern M, Schrag N et al. (2008) Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin 58:9–31 [DOI] [PubMed] [Google Scholar]
- 9.Lipscomb J, Fleming ST, Trentham-Dietz A et al. (2016) What Predicts an advanced-stage diagnosis of breast cancer? Sorting out the influence of method of detection, access to care, and biologic factors. Cancer Epidemiol Biomark Prev 25:613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Breast and Cervical Cancer Early Detection Program (NBCCEDP) [cited 2018. March 14, 2018]; https://www.cdc.gov/cancer/nbccedp/about.htm
- 11.Miller JW, Hanson V, Johnson GD, Royalty JE, Richardson LC (2014) From cancer screening to treatment: service delivery and referral in the National Breast and Cervical Cancer Early Detection Program. Cancer 120(Suppl 16):2549–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoerger TJ, Ekwueme DU, Miller JW et al. (2011) Estimated effects of the National Breast and Cervical Cancer Early Detection Program on breast cancer mortality. Am J Prev Med 40:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White MC, Wong FL (2015) Preventing premature deaths from breast and cervical cancer among underserved women in the United States: insights gained from a national cancer screening program. Cancer Causes Control 26:805–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson LC, Royalty J, Howe W, Helsel W, Kammerer W, Benard VB (2010) Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996–2005. Am J Public Health 100:1769–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekwueme DU, Subramanian S, Trogdon JG et al. (2014) Cost of services provided by the National Breast and Cervical Cancer Early Detection Program. Cancer 120(Suppl 16):2604–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briggs A, Claxton K, Schulper M (2006) Decision modelling for health economic evaluation. Oxford University Press, Oxford [Google Scholar]
- 17.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG (2017) Cost-effectiveness in health and medicine, 2nd edn. Oxford University Press, Oxford [Google Scholar]
- 18.Cronin KA, Yu B, Krapcho M et al. (2005) Modeling the dissemination of mammography in the United States. Cancer Causes Control 16:701–712 [DOI] [PubMed] [Google Scholar]
- 19.Feuer EJ (2006) Modeling the impact of adjuvant therapy and screening mammography on U.S. breast cancer mortality between 1975 and 2000: introduction to the problem. J Natl Cancer Inst Monogr. 2006(36):2–6 [DOI] [PubMed] [Google Scholar]
- 20.Plevritis SK, Salzman P, Sigal BM, Glynn PW (2007) A natural history model of stage progression applied to breast cancer. Stat Med 26:581–595 [DOI] [PubMed] [Google Scholar]
- 21.Holford TR, Cronin KA, Mariotto AB, Feuer EJ (2006) Changing patterns in breast cancer incidence trends. J Natl Cancer Inst Monogr. 2006(36):19–25 [DOI] [PubMed] [Google Scholar]
- 22.Cronin KA, Mariotto AB, Clarke LD, Feuer EJ (2006) Additional common inputs for analyzing impact of adjuvant therapy and mammography on US mortality. J Natl Cancer Inst Monogr. 2006(36):26–29 [DOI] [PubMed] [Google Scholar]
- 23.Mariotto AB, Feuer EJ, Harlan LC, Abrams J (2006) Dissemination of adjuvant multiagent chemotherapy and tamoxifen for breast cancer in the United States using estrogen receptor information: 1975–1999. J Natl Cancer Inst Monogr 2006(36):7–15 [DOI] [PubMed] [Google Scholar]
- 24.Allaire BT, Ekwueme DU, Hoerger TJ et al. (2018) Cost-effectiveness of patient navigation for breast cancer screening in the National Breast and Cervical Cancer Early Detection Program. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raich PC, Whitley EM, Thorland W, Valverde P, Fairclough D (2012) Patient navigation improves cancer diagnostic resolution: an individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomark Prev 21:1629–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman HJ, LaVerda NL, Young HA et al. (2012) Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomark Prev 21:1655–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekwueme DU, Subramanian S, Khushalani JS, Miller JW, Wong FF, Trogdon JG. Economic Cost of the National Breast and Cervical Cancer Early Detection Program (under review) [Google Scholar]
- 28.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML (2011) Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 103:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fireman BH, Quesenberry CP, Somkin CP et al. (1997) Cost of care for cancer in a health maintenance organization. Health Care Financ Rev. 18:51–76 [PMC free article] [PubMed] [Google Scholar]
- 30.Zuckerman S, Williams A, Stockley K (2009) Trends in medicaid physician fees, 2003–2008. Health Aff 28(3):W510–W519 [DOI] [PubMed] [Google Scholar]
- 31.Yabroff KR, McNeel TS, Waldron WR et al. (2007) Health limitations and quality of life associated with cancer and other chronic diseases by phase of care. Med Care 45:629–637 [DOI] [PubMed] [Google Scholar]
- 32.Cho H, Howlader N, Mariotto A, Cronin K (2011) Estimating relative survival for cancer patients from the SEER Program using expected rates based on Ederer I versus Ederer II method. Surveillance Research Program, NCI, Technical Report #2011-01. http://surveillance.cancer.gov/reports/tech2011.01.pdf. Accessed 26 Feb 2017 [Google Scholar]
- 33.Howard DH, Ekwueme DU, Gardner JG, Tangka FK, Li C, Miller JW (2010) The impact of a national program to provide free mammograms to low-income, uninsured women on breast cancer mortality rates. Cancer 116:4456–4462 [DOI] [PubMed] [Google Scholar]
- 34.Howard DH, Tangka FK, Royalty J et al. (2015) Breast cancer screening of underserved women in the USA: results from the National Breast and Cervical Cancer Early Detection Program, 1998–2012. Cancer Causes Control 26:657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanian S, Ekwueme DU, Gardner JG, Bapat B, Kramer C (2008) Identifying and controlling for program-level differences in comparative cost analysis: lessons from the economic evaluation of the National Breast and Cervical Cancer Early Detection Program. Eval Program Plann. 31:136–144 [DOI] [PubMed] [Google Scholar]
- 36.Jemal A, Ward EM, Johnson CJ et al. (2017) Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst 109(9):djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen RA, Zammitti EP, Martinez ME (2017) Health insurance coverage: early release of estimates from the National Health Interview Survey, 2016. Centers for Disease Control and Prevention, National Center for Health Statistics, Atlanta [Google Scholar]
- 38.Frean M, Gruber J, Sommers BD (2017) Premium subsidies, the mandate, and medicaid expansion: coverage effects of the affordable care act. J Health Econ. 53:72–86 [DOI] [PubMed] [Google Scholar]
- 39.Henry J (2018) Kaiser Family Foundation. Current status of state medicaid expansion decisions. https://www.kff.org/health-reform/slide/current-status-of-the-medicaid-expansion-decision/
- 40.Trogdon JG, Ekweume DU, Subramanian S, Crouse W (2014) Economies of scale in federally-funded, state-organized public health programs: results from the National Breast and Cervical Cancer Early Detection Programs. Health Care Manag Sci 17(4):321–330. 10.1007/s10729-013-9261-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.