Abstract
Access to near-real time opioid use data is essential to the effective management of the U.S. opioid crisis. Current narcotic data collection methods are limited by time delay and would be complimented by a rapid data acquisition technique. Use of wastewater-based epidemiology (WBE) analysis may offer access to near real-time data on opioid consumption but application in the United States has been limited. From 2015–2017, monthly 24-hour time-weighted composite samples of municipal raw wastewater from two Midwestern U.S. cities were routinely analyzed using liquid chromatography-tandem mass spectrometry for morphine, codeine, oxycodone, heroin, fentanyl, and select opioid metabolites. Concentrations of opioids (ng/L) in raw wastewater from City 1 and 2, respectively, were: morphine (713 ± 38; 306 ± 29; detection frequency (DF): 100%), oxycodone (17.8 ± 1.1; 78 ± 6; DF: 100%), codeine (332 ± 37; 100 ± 27; DF: 93%), heroin (41 ± 16; 9 ± 11; DF: 81%), an d fentanyl (1.7 ± 0.2; 1.0 ± 0.5; DF: 62%). Average opioid consumption rates estimated using WBE ranged between 9 to 2,590 mg/day/1,000 persons. Anticipated overdoses and overdose-deaths calculated from analyte concentrations in wastewater forecasted 200 opioid-related overdoses/year and 39 opioid related overdose-deaths/year across the two cities during the year 2016, which aligned well with observed coroner-reported opioid deaths. This long-term U.S. screening study of opioids in wastewater was the first to utilize wastewater epidemiological data to estimate the number of expected overdose and overdose-deaths, and to identify detectable levels of the powerful synthetic opioid fentanyl in wastewater.
Keywords: Sewage Epidemiology, Heroin, Fentanyl, Opioid Overdose Estimation, Opioid Death Estimation
Graphical Abstract
1. Introduction
The United States is in the midst of an unprecedented opioid epidemic that claims approximately 42,000 U.S. lives annually (Kounang 2017, Rudd 2016, Schuchat et al. 2017). Opioids were responsible for 67% and 63% of all drug overdose fatalities in 2014 and 2015, with death rate increases from 12.3 to 16.3 per 100,000 population being attributable to increased consumption of heroin (+21%) and the 50-times more powerful synthetic opioid, fentanyl (+72%) (Rudd 2016, Warner et al. 2016). In the U.S., 10.3 million residents reported using prescription opioids for nonmedical purposes in 2014, and a nine-fold increase of young adults using heroin has been observed from 2002 to 2014 (Martins et al. 2017). Positive correlations between non-medical opioid use and heroin use have also been observed (Compton et al. 2016). While exact percentages vary by study and city, studies cite that between 39% to 86% of heroin users admitted to nonmedical use of pharmaceutical opioids before beginning heroin use (Lankenau et al. 2012, Mateu-Gelabert et al. 2015, Peavy et al. 2012, Pollini et al. 2011, Siegal et al. 2003). Despite recent successful efforts by public health and medical professionals to curb opioid prescription rates (Dowell et al. 2016, Frieden and Houry 2016, Schuchat et al. 2017), drug related overdose deaths have continued to increase in the United States (Katz 2017).
With such widespread opioid use, obtaining relevant information related to opioid consumption is vital to developing effective substance abuse prevention strategies. Current data analysis involves a combination of population surveys, crime statistics, medical records and narcotic seizure data (Zuccato et al. 2008), but these methods are often costly, cumbersome, and may be subject to bias. Wastewater-based epidemiology (WBE) was first proposed in 2001 as a method for obtaining population health metrics by back-calculating health metrics from concentrations of related biomarkers within composite wastewater samples (Daughton 2001). In 2005 it was tested as a complementary approach to current narcotic data collection methods of cocaine use (Zuccato et al. 2005), and since has experienced widespread use in Europe (Baker et al. 2014, Baz-Lomba et al. 2016, Gatidou et al. 2016, Kankaanpää et al. 2014, Lindberg et al. 2005, Postigo et al. 2011, Terzic et al. 2010, Van Nuijs et al. 2011b, Vuori et al. 2014, Zuccato et al. 2008, Zuccato et al. 2005), Asia (Kim et al. 2015, Lai et al. 2013), Africa (Archer et al. 2018) and Australia (Lai et al. 2016, Tscharke et al. 2016) in order to obtain anonymous prescription and illicit narcotic consumption data in near-real time. The WBE approach has been further expanded under the umbrella of urban metabolism metrology (UMM) (Halden 2016), which studies multiple process flows within the natural and built water environment to obtain diagnostic information on activities, sustainability and the health statistics for a human population. Analysis composited raw sewage samples obtained from wastewater treatment plant (WWTP) may provide important epidemiological insights as usage prevalence statistics could theoretically be obtained for any commonly consumed product within a population (Dove 2006). The validity of this technique has been demonstrated through the comparison of wastewater epidemiological analysis of therapeutic drugs and known amounts consumed by the population (Heberer and Feldmann 2005, Lindberg et al. 2005).
Compared to European and Asian countries, WBE analysis in the United States has seen limited use (Subedi and Kannan 2014). Studies which have examined U.S. wastewaters for drug use prevalence have primarily focused on United States Drug Enforcement Agency (US DEA) schedule I and II narcotics (Banta-Green et al. 2009, Gerrity et al. 2011, Subedi and Kannan 2014). Some U.S. based studies have screened wastewater for prescription and illicit parent opioids and/or metabolites, with positive detections of morphine (Heuett et al. 2015, Subedi and Kannan 2014), codeine (Heuett et al. 2015), oxycodone (Chiaia et al. 2008, Heuett et al. 2015), and heroin (Heuett et al. 2015) being recorded. Despite the recent drastic increase in fentanyl-related deaths (CDC and University 2017), U.S. studies on the occurrence in wastewater of fentanyl are thus far lacking. Furthermore, small U.S. communities have been significantly impacted by the opioid crisis due to additional circumstances which do not impact larger communities, such as: outdated substance abuse infrastructure, shortages in emergency medical technician (EMT) personnel, long travel times of the same, lack of regional coordination, lack of physicians administering programs on substance abuse and medication-assisted treatment, and various administrative barriers (Hancock et al. 2017). Some of these locations have also been identified as areas with strikingly high opioid prescription rates compared to the number of residents within the service area (Whitaker 2017). Therefore, the goal of the present study was to examine opioid abuse trends in two moderately sized (<200,000 population) cities in the American Midwest, a U.S. region that has experienced the highest percentage increase of reported fentanyl abuse from 2014–2015 (CDC 2016). Opioid and metabolite compounds were selected due to their potential for misuse and include: morphine, morphine-3-glucuronide, codeine, norcodeine, oxycodone, noroxycodone, fentanyl, norfentanyl, heroin, and 6-acetylmorphine. Specific objectives of the study were to: (i) obtain the first wastewater monitoring data for U.S. cities to determine city-wide chemical-based fentanyl consumption estimations, (ii) to generate for participating municipalities wastewater-based data on opioid use prevalence for informed decision making, and (iii) to use wastewater epidemiological data as a tool to forecast expected opioid related overdose and overdose-deaths.
2. Materials and Methods
2.1. Study locations and wastewater sampling methods
Influent from centralized wastewater treatment plants in two Midwestern U.S. cities was collected in 24-hour time-weighted composites using automated samplers by WWTP personnel from March 2015 to March 2017. The WWTP of City 1 serves an approximate 130,000 residents, while that of City 2 serves an approximate 45,000 residents. Demographic data was obtained from the U.S. Census Bureau (SI Table S1) (USCB 2010). Both cities feature a sewer system designed to separate municipal wastewater from stormwater inputs. Both climate range and reported water use per resident were similar across both participating cities. Sampling occurred on one day per month during the 24-month study period; the day of collection varied and was entirely at the discretion of sampling personnel. Samples were stored in polyethylene terephthalate (PET) bottles and shipped to Arizona State University in Styrofoam shipping containers containing either ice or dry ice. Upon receipt, samples were stored at −20°C until analysis.
2.2. Target analytes
Five parent opioids and their respective metabolites were monitored in raw wastewater. The investigated opioids were morphine (MOR), its major metabolite morphine-3-glucuronide (M3G), codeine (COD), its major metabolite norcodeine (NCOD), oxycodone (OXY), its major metabolite noroxycodone (NOXY), fentanyl (FENT), its major metabolite norfentanyl (NFENT), heroin (HER), and its minor but exclusive metabolite 6-acetylmorphine (6-AM). High purity (>97%) standard solutions of the target compounds originated from Sigma Aldrich (Milwaukee, WI) and were prepared by Cerilliant (Round Rock, TX, USA) as solutions in methanol or acetonitrile. Five deuterated compounds, one for each of the parent opioid target compounds were also purchased from Cerilliant for use as internal standards for quantification: heroin-d9 (HER-d9), morphine-d6 (MOR-d6), codeine-d6 (COD-d6), oxycodone-d3 (OXY-d3), and fentanyl-d5 (FENT-d5).
2.3. Isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS)
Briefly, 200 mL of WWTP composite influent was loaded onto Oasis HLB 150 mg solid phase extraction (SPE) cartridges (Waters, Barcelona, Spain) at a rate of 1.5 mL/min using automated extraction with a Dionex Autotrace 280 (Sunnyvale, CA, USA). Prior to extraction, all composite influent samples were spiked with a mixture of the deuterated compounds at a concentration of 5 ng/mL for HER-d9, MOR-d6, COD-d6, OXY-d3, and FENT-d5. Following sample loading, cartridges were washed with water at a rate of 5 mL/min for five minutes and dried under a stream of nitrogen gas for 10 minutes. Drip-wise elution of analytes from the SPE cartridges was accomplished using 4 mL of a 50:50 mixture of acetone and methanol containing 0.5% formic acid.
Mass spectrometric analyses were carried out on an API 4000 instrument (Applied Biosystems, Framingham, MA, USA), in series with a Shimadzu Prominence HPLC (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA) that was controlled by Analyst 1.5 software (Applied Biosystems, Framingham, MA, USA). Chromatographic separation was attained with a Symmetry C18 3.5 µm by 6.4 mm by 75 mm analytical column preced ed by a guard column of the same material, both supplied by Waters (Massachusetts, USA), and a mobile phase consisting of gradient methanol/water with 0.2% formic acid at a 0.35 mL/min flow rate. Samples were introduced into the mass spectrometer using an electrospray ionization probe operating in positive mode. Multiple reaction monitoring (MRM) was used for qualitative analysis (SI Table S2).
2.4. Calculation of opioid mass loadings
Parent opioid compounds were selected as indicators of drug consumption in samples collected over the course of the sampling campaign, lasting from March 2015 to March 2017. Starting in June 2016, metabolite compound concentrations also were tracked as indicators of drug consumption until the end of the monitoring program in March 2017. Opioid mass loadings to the WWTP were calculated from influent wastewater flow and corresponding concentration using equation 1:
| Eq. 1 |
2.5. Estimation of mass and dose per-capita opioid consumption
To determine population normalized mass and dose consumption values (Table 1), the following equations were used:
| Eq. 2 |
| Eq. 3 |
Where M.C. refers to mass consumption, D.C. refers to dose consumption, M.L. refers to mass load, and C.F. refers to the analyte correction factor. Wastewater epidemiological data was then compared to opioid consumption and excretion data obtained from peer-reviewed literature to estimate the number of opioid users. The number of estimated opioid abusers were then compared to national opioid use statistics. Per the National Drug Intelligence Center’s report on Heroin Consumption in the United States (NDIC 2000), average daily use of pure heroin mass was assumed to equal 50 mg/day per user. Prescription opioid mass use was obtained from Mayo Clinic prescription guidelines at an ingestion rate of two doses per day, equaling 60 mg/day for morphine, 60 mg/day for codeine, and 20 mg/day for oxycodone (Mayo 2017). Since unknown exposure to fentanyl is thought to drive the increase in fentanyl use (CDC and University 2017) it is difficult to estimate the average dose a recreational user may receive. Therefore, fentanyl was omitted from dose consumption analysis.
Table 1 -.
Opioid narcotics, respective consumption indicator compounds, excretion rate of respective consumption indicators, correction factors used for each consumption indicator, and average prescribed oral dose per opioid per Mayo Clinic doctor guidelines.
| Drug | Consumption Indicator | Excretion Rate (%) | Correction Factor | Average Dose (mg) |
|---|---|---|---|---|
| Morphine | Morphine | 10 a | 10.0 | 30 g |
| Morphine-3-Glucuronide | 75 a | 0.8 | ||
| Codeine | Codeine | 57.5 b | 1.7 | 30 g |
| Norcodeine | 3.77 c | 27.8 | ||
| Oxycodone | Oxycodone | 8.9 d | 11.2 | 10 g |
| Noroxycodone | 22.1 d | 4.7 | ||
| Fentanyl | Fentanyl | 6 e | 16.7 | 0.1 g |
| Norfentanyl | 91.08 e | 1.6 | ||
| Heroin | Heroin | n/a | n/a | 30 g |
| 6-Acetylmorphine | 1.3 f | 86.8 |
Hasselström, Jan, and Juliette Säwe. “Morphine phar macokinetics and metabolism in humans.” Clinical pharmacokinetics 24.4 (1993): 344–354.
Thai, Phong K., et al. “Refining the excretion factors of methadone and codeine for wastewater analysis—Combining data from pharmacokinetic and wastewater studies.” Environment international 94 (2016): 307–314.
Lafolie, Pierre, et al. “Urine and plasma pharmacokinetics of codeine in healthy volunteers: implications for drugs-of-abuse testing.” Journal of analytical toxicology 20.7 (1996): 541–546.
Lalovic, Bojan, et al. “Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites.” Clinical pharmacology & therapeutics 79.5 (2006): 461–479.
Labroo, Rita B., et al. “Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions.” Drug Metabolism and Disposition 25.9 (1997): 1072–1080.
Postigo, Cristina, Miren López de Alda, and Damià B arceló. “Evaluation of drugs of abuse use and trend s in a prison through wastewater analysis.” Environment international 37.1 (2011): 49–55.
Mayo Clinic Guidelines
The following assumptions were factored into every portion of the study analysis: (i) no sewage loss due to leaks or pipe degradation; (ii) no transformation or degradation within sewer lines; and (iii) no direct drug addition to the sewer system (Zuccato et al. 2008). In most cases, the major drug metabolite was selected as the consumption indicator – morphine-3-glucoronide was selected to estimate morphine consumption, noroxycodone was selected to estimate oxycodone consumption, norcodeine was selected to estimate codeine consumption, and norfentanyl was selected to estimate fentanyl consumption. The major metabolite of heroin is morphine, but occurrence of morphine in wastewater can be the result of a number of occurrences and doesn’t necessarily indicate heroin consumption, therefore the minor but human specific heroin metabolite, 6-acetylmorphine, was used to estimate heroin consumption (Postigo et al. 2011).
2.6. Overdose-death and black-market value estimates
In order to estimate the number of overdose-deaths from wastewater data, data provided from state dashboards related to opioid abuse, overdoses, and deaths was compiled into a spreadsheet, and ratios between opioid related overdose deaths and overdoses (SI Table S3) were computed (AZDHS 2017, CCPDAP 2017, MNDH 2017, OHA 2017, RIPO 2017, VDH 2017). The average of this ratio (5.35 overdoses/death) was then compared to data related to overall U.S. opioid abuse prevalence (heroin: 3.8 million persons (Martins et al. 2017); prescription opioids: 11.5 million persons (Thompson 2017)) in order to estimate the ratio of opioid-related deaths and overdoses compared to a single opioid user in the United States. This ratio of opioid users to opioid-related overdoses and fatalities was then compared to the estimated number of heroin and fentanyl users computed from the original opioid consumption indicator compound concentrations observed in wastewater to estimate the number of overdoses and overdose deaths from the chemical measurements. These estimated opioid-related overdoses and deaths were then compared to coroner data from the two cities for opioid-related overdose deaths to check for method accuracy – opioid-related overdose informati on was not available for either city so this comparison could not be attempted. The black-market value of heroin was calculated by comparing the observed mass load of heroin to its street value (NBC 2017).
2.7. Statistical analysis
Statistical analysis of the data was performed with a combination of Microsoft Office suite products, Analyst 1.5 software (Applied Biosystems, Framingham, MA, USA), JMP Pro 12.1.0 (SAS, Phoenix, Arizona), and IBM SPSS 25 (IBM, Armonk, NY). Normality of the datasets was determined through two analyses run in IBM SPSS 25; (1) an analysis of skewness and kurtosis z-values, and (2) the Shapiro-Wilk test for normality. Following previously outlined WBE statistical testing (Brewer et al. 2016, Tscharke et al. 2016), two-tailed t-tests were used for comparison of parent-metabolite excretion rates, as well as opioid concentrations in raw wastewater between study locations.
3. Results and discussion
3.1. Method performance
Method detection limits (MDLs) for the various opioids and metabolites ranged between 0.3 and 1.1 ng/L (SI Table S4, Figures S1–S11), data that were in line with previous U.S. studies (Heuett et al. 2015, Subedi and Kannan 2014). All MDLs were determined based on EPA guidelines described in 40 CFR 136, Appendix B (EPA 1986). Potential loss of opioids and metabolites from wastewater during sample extraction was corrected for by using labeled internal standards and the isotope dilution method. Absolute and relative recoveries from 10-sample matrix spike experiments for the various analytes averaged 85% (range: 66%−104%) and 114% (range: 91%−139%) (SI Table S5), respectively. Analysis precision expressed as relative percent difference (RPD) for non-blinded duplicates of composite wastewater samples averaged ±30% (range: 1%−200%). A detailed explanation of procedures to experimentally determine recovery rates is available in the supporting information (Section S-1.1).
3.2. Concentrations of opioids and metabolites in raw wastewater
Opioid parent compounds were identified in composite wastewater samples for each city once per month from March 2015 to April 2017 (SI Table S6). Ratios of concentrations in raw wastewater (in ng/L) of the parent drug and its metabolite compounds were observed to be similar across both cities (Figure S12). Morphine concentrations in raw wastewater were determined to be 713 ± 38 ng/L (City 1) and 306 ± 29 ng/L (City 2), and morphine-3-glucoronide concentrations in raw wastewater were determined to be 7.0 ± 2.5 ng/L (City 1) and 7.6 ± 1.8 ng/L (City 2). Morphine presence in wastewater can be attributed to consumption of morphine (Hasselström and Säwe 1993), consumption of heroin (Cone et al. 1993), consumption of codeine (Vree and Wissen 1992), or as result of narcotic disposal (Daughton and Ruhoy 2009). Further analyte degradation (Skopp et al. 2001) and metabolization in the sewer system is likely and may influence parent-metabolite ratios (O’Brien et al. 2017). The discrepancy between the morphine parent and metabolite concentrations in raw wastewater suggest that the morphine concentrations are influenced by one of the alternative sources of morphine occurrence in wastewater and could suggest illicit drug use. Average concentrations in raw wastewater of codeine were determined to be 322 ± 37 ng/L (City 1) and 100 ± 27 ng/L (City 2); average concentrations in raw wastewater of oxycodone were determined to be 17.8 ± 1.1 ng/L (City 1) and 78 ± 6 ng/L (Cit y 2); the codeine metabolite norcodeine concentrations were determined to be 162 ± 27 ng/L City 1) and 48 ± 8 ng/L (City 2); observed noroxycodone concentrations were determined to be 73 ± 5 ng/L (City 1) and 105 ± 7 ng/L (City 2). Both codeine and oxycodone showed similar detection frequencies for both parent and metabolite compounds.
Average concentrations in wastewater of heroin were determined to be 41 ± 16 ng/L (City 1), and 19 ± 11 ng/L (City 2), and observed fentanyl concentrations were found to be 1.7 ± 0.2 ng/L (City 1) and 1.0 ± 0.5 ng/L (City 2). The corresponding fentanyl metabolite norfentanyl concentrations were found to be 30 ± 2 ng/L (City 1) and 48 ± 2 ng/L (City 2), and 6-acetylmorphine concentrations were found to be 43 ± 15 ng/L (City 1) and 21 ± 3 ng/L (City 2). Both of these metabolites were detected at a higher frequency than their respective parent compounds (Table 2). In both cities, concentrations of the fentanyl metabolite norfentanyl were significantly larger (2-times and 48-times) than the corresponding concentrations of parental fentanyl, a finding that potentially could be due to the previously observed rapid in vivo degradation and transformation of fentanyl following administration (Labroo et al. 1997).
Table 2 -.
Detection frequency, average analyte concentrations in raw wastewater ± standard deviations (SD), and concentration range per opioid consumption indicator of all sample concentrations.
| Consumption Indicator | Frequency of Detection (%) | Concentration (ng/L) |
|
|---|---|---|---|
| Average ± SD | Range | ||
| Morphine | 100 (n=45) | 514 ± 268 | 159 – 1,310 |
| Morphine-3-glucuronide | 90 (n=21) | 7.3 ±6.6 | <MDL - 26 |
| Codeine | 93 (n=45) | 218 ± 154 | <MDL - 571 |
| Norcodeine | 95 (n=21) | 107 ± 90 | <MDL - 397 |
| Oxycodone | 100 (n=45) | 47 ± 52 | 3 – 251 |
| Noroxycodone | 100 (n=21) | 88 ± 34 | 47 – 171 |
| Fentanyl | 62 (n=45) | 1 ± 0.9 | <MDL - 4.4 |
| Norfentanyl | 100 (n=21) | 38 ± 49 | 11 – 198 |
| Heroin | 81 (n=21) | 27 ± 30 | <MDL - 120 |
| 6-Acetylmorphine | 100 (n=21) | 32 ± 28 | 7 – 115 |
Most opioids show a relatively consistent concentration pattern when compared over the two-year period. An exception of this is the dataset obtained for City 2 codeine concentrations from March 2015 – January 2016 where concentrations varied from 260 ng/L to below the method detection limit. This variation was not observed from June 2016 to March 2017 for City 2, but this observation lacks a definitive explanation. The concentration data was then converted to mass load data for further analysis outlined in the subsequent sections of this manuscript (Table S7, Figure S13).
3.3. Estimated opioid consumption
Opioid consumption (in mg/day/1,000 persons) was estimated from opioid mass loads (Table 3) and determined to be stable throughout the sampling campaign for all opioids (SI Table S8) aside from City 1’s oxycodone consumption which showed a statistically significant increase (565%, p-value: <0.01) from the March 2015 – Jan 2016 to th e June 2016 – March 2017 sampling periods. In order to compare estimated opioid consumption rates between the two cities, two tailed t-tests were computed for all available estimated opioid mass loads normalized to the contributing population at the α=0.05 confidence interval. Population normalized mass loads for morphine in City 1 were found to be statistically higher (p-value = 0.048) than those observed in City 2, and this observation was also mirrored by the population-normalized mass load data for morphine-3-glucoronide data (p-value = 0.016). Population normalized mass loads for codeine in City 1 were also statistically higher (p-value = 0.0002) than those calculated for City 2, which was also mirrored by the metabolite norcodeine population normalized mass loads (p-value = 0.023). Calculated oxycodone mass loads normalized to the contributing population were determined to be statistically higher in City 2 (p-value = 0.00002) than those computed for City 1, which was again mirrored in the data computed for the metabolite noroxycodone (p-value = 0.0006). Interestingly, neither parent fentanyl (p-value = 0.734), the fentanyl metabolite norfentanyl (p-value = 0.155), nor the heroin metabolite 6-acetylmorphine (p-value = 0.629) showed any statistical differences between the two cities, which may inform on the possible uniformity of usage patterns of these black market specific narcotics within these two cities.
Table 3 -.
Average ± standard error, minimum, and maximum analyte population normalized mass load consumption across the two cities.
| City 1 | |||
|---|---|---|---|
| Analyte | Average Concentration | Minimum Concentration | Maximum Concentration |
| all concentration in mg/day/1,000 population | |||
| Morphine | 2,590 ± 157 | 1,170 | 4,603 |
| Oxycodone | 72 ± 12 | 14 | 192 |
| Codeine | 204 ± 13 | 111 | 341 |
| Fentanyl | 10 ± 1.2 | 4 | 21 |
| Morphine-3-Glucuronide | 26 ± 8 | <MDL | 103.8 |
| Noroxycodone | 124 ± 6 | 105 | 171 |
| Norcodeine | 1,630 ± 284 | 169 | 4,169 |
| Norfentanyl | 18 ± 7 | 7 | 87 |
| 6-Acetylmorphine | 1,294 ± 296 | 441 | 3,257 |
|
City 2 | |||
| Analyte | Average Concentration | Minimum Concentration | Maximum Concentration |
| all concentration in mg/day/1,000 population | |||
| Morphine | 1,970 ± 255 | 974 | 6,754 |
| Oxycodone | 556 ± 89 | 147 | 1,859 |
| Codeine | 102 ± 21 | 0.9 | 398 |
| Fentanyl | 9 ± 2.7 | 0.9 | 34 |
| Morphine-3-Glucuronide | 3.8 ± 1 | <MDL | 11.5 |
| Noroxycodone | 300 ± 35 | 128 | 487 |
| Norcodeine | 790 ± 180 | <MDL | 1,726 |
| Norfentanyl | 47 ± 18 | 10 | 191 |
| 6-Acetylmorphine | 1,127 ± 163 | 404 | 1,844 |
Estimated morphine consumption values (2,590 ± 157 mg/day/1,000 persons, 1,970 ± 255 mg/day/1,000 persons) were in-line with other WBE values from a New York study (range: 1,610–2,240 mg/day/1,000 persons) (Subedi and Kannan 2014) but higher than international consumption estimates (range: 13.8–310 mg/day/1,000 persons) (Baker et al. 2014, Baz-Lomba et al. 2016, Tscharke et al. 2016, Vuori et al. 2014, Zuccato et al. 2008). Estimated morphine consumption from morphine-3-glucuronide (26 ± 8 mg/day/1,000 persons, 3.8 ± 1 mg/day/1,000 persons) was lower than estimated consumption values from the New York study, but in line with international studies conducted. Morphine excretion as a result of codeine (0–15%) (Thorn et al. 2009) or heroin (7%) (Yeh et al. 1976) consumption likely influenced wastewater morphine concentrations (Cone et al. 1993, Cone et al. 1991), and suggest that a stable morphine specific metabolite would be preferable for morphine consumption estimations.
Codeine consumption estimated from parent codeine was 2-times higher in City 1 (204 ± 13 mg/day/1,000 persons) compared to City 2 (102 ± 21 mg/day/1,000 persons), and oxycodone consumption estimated from parent oxycodone was nearly 8-times higher in City 2 (556 ± 89 mg/day/1,000 persons) compared to City 1 (72 ± 12 mg/day/1,000 persons). When compared to a multi-regional U.S. study, oxycodone consumption estimates for City 1 were in-line with other U.S. consumption estimates (U.S. range: 8–170 mg/day/1,000 persons) (Chiaia et al. 2008), but estimates for City 2 were significantly higher than previously reported values. When compared to international studies, oxycodone consumption estimates were higher across both cities (international range: 20–50.5 mg/day/1,000 persons) but codeine consumption estimates were in-line with international studies (international range: 164–927 mg/day/1,000 persons) (Tscharke et al. 2016, Vuori et al. 2014). Using norcodeine for consumption estimation purposes resulted in higher codeine consumption estimations across both cities (8-times higher) compared to parent codeine which resulted in U.S. consumption estimation exceeding international values. This observation was not mirrored with the noroxycodone:oxycodone relationship, as both values provided similar results (RPD: 53–60%).
Heroin consumption estimates obtained from the metabolite 6-acetylmorphine (1,294 ± 296 mg/day/1,000 persons, 1,127 ± 163 mg/day/1,000 persons) were between 10 to 281 times higher than other estimated consumption values obtained from literature (range: 4.6–115 mg/day/1,000 population) (Heuett et al. 2015, Tscharke et al. 2016). This suggests that heroin consumption within these two midwestern cities may exceed both international and U.S. estimated use rates. While only one study has reported positive detection of fentanyl and its metabolite in U.S. wastewater (Gushgari et al. 2018), the estimated consumption unearthed by this analysis from both fentanyl (10 ± 1.2, 9 ± 2.7 mg/day/1,000 persons) and norfentanyl (18 ± 7, 47 ± 18 mg/day/1,000 persons) are still higher than data published in a Southwestern campus study (REF and range) and the average fentanyl consumption (0.5 mg/day/1,000 persons) estimated by WBE data from Adelaide, South Australia (Tscharke et al. 2016). While fentanyl concentrations were consistently the lowest of any analyte detected in this study, any detectable presence of synthetic fentanyl or its analogs should be considered significant due to the strength of the opioid (Donner et al. 1996), its prevalence in opioid-related fatalities (CDC and University 2017), and its ties to the illicit drug trade (CDC 2016). Furthermore, this study has identified the first frequent detections of fentanyl (DF: 62%) and norfentanyl (DF: 100%) in U.S. wastewater which is necessary for comparison purposes of future U.S. opioid-related wastewater epidemiological work. Population normalized mass load data was then converted to dose-estimated data for further data analysis of user count and estimated overdose-deaths (Table S9).
3.4. User count, estimated overdose-deaths, and monetary black-market contribution
The number of heroin addicts within the two study locations were estimated at 3,400 (city 1) and 1,000 (city 2) persons. Considering the national average of 0.21% current habitual heroin users (SAMHSA 2013) these values are 1,135% and 982% higher than the calculated expectancy. These values were 61% and 41% higher than the national average of lifetime heroin use of 1.6% (Martins et al. 2017). The number of codeine users were determined to be 3,600 (city 1) and 600 (city 2) persons. Oxycodone users were determined to be 800 (city 1) and 660 (city 2) persons. Number of morphine users estimated from parent morphine were determined to be 5,600 (city 1) and 1,500 (city 2) persons but does not account for morphine occurrence due to heroin or codeine consumption. A cost estimate for black-market heroin consumption was also attempted for the two cities, with the average street value of heroin estimated to be $240/gram (NBC 2017). This analysis resulted in annual black-market contributions of $1.14 million (city 1) and $990 thousand (city 2) from heroin users. These estimates may be overly conservative, as a city with 100,000 individuals and a 0.21 addict rate could theoretically exceed an annual black-market contribution of 11.5 million USD from heroin alone. Cost estimations for the remainder of the opioids were not attempted due to uncertainties with rates of medical vs. nonmedical use, and uncertainties in pharmaceutical vs. black market costs.
For the first time in literature wastewater epidemiological data was used to forecast the number of opioid-related overdose and deaths expected given the concentrations of opioid consumption indicator compounds observed. The number of estimated heroin users were compared to state opioid overdose death data to estimate the number of expected heroin and prescription opioid overdoses. Data obtained from state opioid dashboards suggested that on average one thousand estimated daily heroin users translated to an estimated 3.4 heroin overdose deaths and 18.3 heroin related overdoses. Consequently, one thousand estimated daily synthetic opioid users translated to an estimated 1.8 synthetic opioid overdose deaths and 9.4 synthetic opioid related overdoses.
From this analysis 12 heroin attributable deaths, 62 attributable heroin overdoses, 18 synthetic opioid attributable deaths, and 94 synthetic opioid attributable overdoses were estimated for City 1. City 2 was estimated to incur 4 heroin attributable deaths, 18 attributable heroin overdoses, 5 synthetic opioid attributable deaths, and 26 synthetic opioid attributable overdoses. When compared to reported coroner data, the estimated attributable death counts of both cities were within 30% of the true number identifying a similarity between the statistics unearthed through this analysis and municipal data. The estimated opioid-related overdose deaths calculations performed here considered the opioids heroin and fentanyl, which could have contributed to the lower estimation across both communities. This suggests that WBE data may also be used as a tool to forecast overdoses and deaths based upon narcotic indicators found in wastewater, furthering the value of wastewater analytical data to government and health personnel.
3.5. Study Limitations
While narcotic use and trend data collection via wastewater monitoring has been shown a viable tool both domestically and internationally (Baker et al. 2012, Kankaanpää et al. 2014, Subedi and Kannan 2014), there are shortcomings which factor in a level of uncertainty within the analysis. The most robust data that can be obtained from wastewater monitoring are analyte concentrations in raw wastewater (mass per volume) and daily mass loads (mass per day). These sources of data are subject to the smallest source of possible error mainly stemming from analyte degradation in-sewer and during sample collection but also limit the knowledge that can be obtained from the dataset without further analysis. Previous literature has reported WBE data through usage statistics (in mass or doses per day per population) (Lai et al. 2013, Zuccato et al. 2008), monetary units (black market or overall economic impact) (Zuccato et al. 2011), and health statistics (attributable users, overdoses, or overdose deaths) (Terzic et al. 2010), but these analyses likely increase the associated error. Variations in individual narcotic mass usage (Harocopos et al. 2016, Warner et al. 2016), pharmacokinetic metabolization and narcotic excretion rates (Andes and Craig 2002, Cone et al. 1993, Jenkins et al. 1994, Schwartz 2003), and the extent of in-sewer analyte degradation and/or metabolization/hydrolyzation by fecal bacteria present in untreated wastewater (Postigo et al. 2011) can have a marked effect on estimating drug use statistics from WBE data. This phenomenon has been thoroughly proven with respect to morphine-3-glucoronide (van Nuijs et al. 2011a), and may account for the low concentrations observed with respect to observed morphine concentrations at both locations. It should also be noted that the use of 6-Acetylmorphine for estimation of heroin consumption has been shown to produce higher values of estimated heroin consumption, and therefore the results presented in this manuscript for heroin consumption may constitute overestimations (EMCDDA 2016). While these observations certainly have an impact on results obtained through this method, the alternative use of morphine as an indicator compound is also associated with certain limitations which factor a great deal of uncertainty into WBE analyses. Therefore, instead of abandonment of 6-AM as a heroin indicator compound, it would be more advantageous for the field to fine-tune the parameters used in 6-AM estimations in order to make estimations from this compound more robust. Analysis of specific narcotics with various limiting factors such as low urinary and fecal excretion profiles or rapid in/ex vivo degradation may provide additional challenges for the quantification of certain narcotics in wastewater.
The use of time-weight samplers and the sampling frequency used in this study also constitutes limitations. The use of time-weighted sampling will not account for the diurnal wastewater flow patterns which could result in an underrepresentation of narcotic concentrations in raw wastewater. Due to budgetary constraints participating WWTP operators opted to sample for one randomly selected weekday 24-hour period per month. While this frequency of sampling can provide insights into long-term trends, annual averages, and baseline usage patterns obtaining more intricate trend analyses of the data (i.e. variation in weekly use trends) is not possible. An ideal study would sample for a set number of consecutive days throughout a longer timeframe to obtain data for both short and long-term drug use trends, and administration of self-reporting surveys for comparison purposes (Heuett et al. 2015, Moore et al. 2014). Similar to previous wastewater-based epidemiology studies the particulate phase of collected wastewater was not analyzed for narcotic indicators which could result in the underestimation of observed total consumption within these communities. Large relative percent differences observed for some samples as well as increase in parent oxycodone observed for City 1 between the two sampling campaigns could have been impacted by analyte sorption on storage containers and sampling equipment, in-sewer analyte degradation, matrix interference, and analysis system losses - but the lack of analyte detection and internal standard loss within process and method blanks suggest this is unlikely. While these factors contribute a level of uncertainty in this analysis data derived from these methods should still be considered a powerful analytical tool and considered alongside additional viable methods of data collection that are currently implemented within municipal communities.
4.0. Conclusion
The results of this study indicate that the observed higher opioid consumption in the United States is reflected in opioid analyte concentrations observed in U.S. wastewaters, which have produced some of the highest opioid consumption estimations presented in WBE literature. Implementing WBE monitoring within a community requires minimal adjustment to wastewater infrastructure but would result in pertinent information related to opioid use. This study also provides the first reported U.S. occurrence of fentanyl and its metabolite norfentanyl in wastewater in published literature. Screening for fentanyl and its metabolites should be viewed as a mandatory practice in future U.S. WBE studies due to the association between fentanyl and the rise in opioid-related fatalities in the United States (Warner et al. 2016). This study has also shown that WBE results could be further used to forecast opioid-related overdose and deaths attributable to a measurable concentration of drug analyte within wastewater. While the WBE process may be subject to some uncertainty the technology remains a valuable analytical tool to be used alongside current data acquisition approaches by providing location specific wastewater-based epidemiological data in near real-time.
With the continued increase in national overdose-deaths due to the ongoing opioid crisis, and other health related crises such as the resurgence of methamphetamine consumption, the value of wastewater-based epidemiological data to cities and communities has never been higher. In order to make true change within our communities, community leaders and stakeholders need access to the most up-to-date and pertinent information possible – a need which can be, in part, fulfilled through routine wastewater monitoring for chemical and biological indicators of public health concern. This sentiment has started to be reflected through municipal action, most notably through the recent partnership between the City of Tempe and Arizona State University (Pineda 2019), but the vast majority of municipalities within the United States still do not employ this valuable technology. If WBE were to experience national implementation, public health officials and stakeholders would be able to understand the impact of their decisions on the community much quicker than currently possible, and would also be able to address shortcomings of said education and policy implementation – reducing the significant time and monetary input normally associated with these campaigns, ultimately leading to a healthier community and more lives saved.
Supplementary Material
Figure 1.
Parent opioid concentrations determined in 24-hour time-weighted composite wastewater samples for the two cities over the sampling campaign from March 2015 to April 2017. Non-detects are represented by empty symbols within the graph. Months within the sampling campaign where samples were not received are not represented.
Figure 2.
Estimation of per-capita consumption values for codeine, oxycodone and fentanyl derived from opioid parent compound analysis. Populations were estimated by population served by the wastewater treatment plants, and correction factors used are listed in Table 1. Per-capita consumption estimations are a factor of the observed concentration, wastewater flow, estimation of number of contributing residents, and correction factors based upon excretion rates and pharmacokinetic data.
Figure 3.
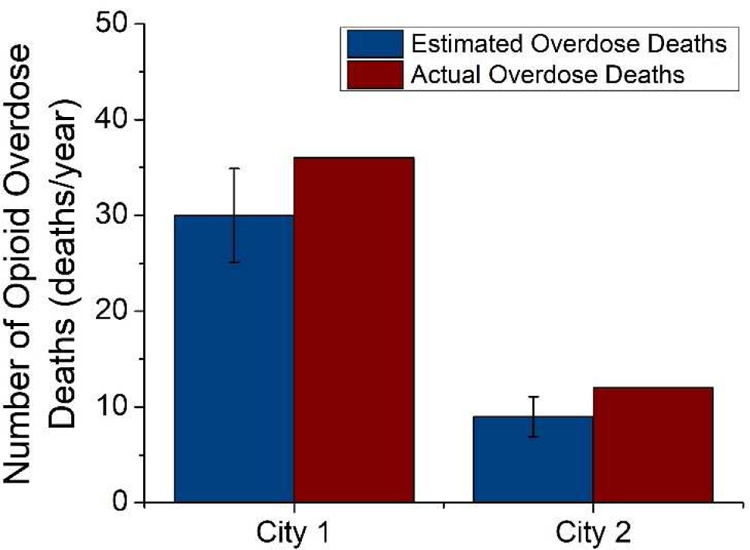
Estimated and actual opioid-related overdose deaths for the two cities. Actual overdose statistics were obtained from state coroner data for confirmed opioid-related fatalities.
Highlights.
Two-year WBE study of opioid use in the American Midwest.
Analysis of cities with known and identified opioid abuse problems.
First estimation of overdoses and deaths from WBE data.
First routine detection of fentanyl and norfentanyl in U.S. wastewater.
5.0. Acknowledgements
The authors of this study would like to thank the participating municipalities for their participation.
7.0 Funding Source Disclosure
This project was supported in part by Award Number R01ES020889 from the National Institute of Environmental Health Sciences (NIEHS) and by award LTR 05/01/12 of the Virginia G. Piper Charitable Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest Statement
The authors are not aware of any substantive or perceived competing interest concerning this work.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author Agreement/Declaration
This certifies that all authors have seen and approved the final version of the manuscript titled “Long-term tracking of opioid consumption in two United States cities using wastewater-based epidemiology approach” for consideration of publication in the journal Water Research.
We warrant that the article is the authors’ original work, hasn’t received prior publication and is not under consideration for publication elsewhere.
8.0 References
- Andes D and Craig W (2002) Animal model pharmacokinetics and pharmacodynamics: a critical review. International journal of antimicrobial agents 19(4), 261–268. [DOI] [PubMed] [Google Scholar]
- Archer E, Castrignanò E, Kasprzyk-Hordern B and Wolfaardt G (2018) Wastewater-based epidemiology and enantiomeric profiling for drugs of abuse in South African wastewaters. Science of the Total Environment 625, 792–800. [DOI] [PubMed] [Google Scholar]
- AZDHS (2017) Opioid Epidemic - Real Time Opioid Data Services, A.D.o.H. (ed).
- Baker DR, Barron L and Kasprzyk-Hordern B (2014) Illicit and pharmaceutical drug consumption estimated via wastewater analysis. Part A: chemical analysis and drug use estimates. Science of the Total Environment 487, 629–641. [DOI] [PubMed] [Google Scholar]
- Baker DR, Očenášková V, Kvicalova M and Kasprzyk-Hordern B(2012) Drugs of abuse in wastewater and suspended particulate matter—further developments in sewage epidemiology. Environment international 48, 28–38. [DOI] [PubMed] [Google Scholar]
- Banta-Green CJ, Field JA, Chiaia AC, Sudakin DL, Power L and De Montigny L (2009) The spatial epidemiology of cocaine, methamphetamine and 3, 4-methylenedioxymethamphetamine (MDMA) use: a demonstration using a population measure of community drug load derived from municipal wastewater. Addiction 104(11), 1874–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baz-Lomba JA, Salvatore S, Gracia-Lor E, Bade R, Castiglioni S, Castrignanò E, Causanilles A, Hernandez F, Kasprzyk-Hordern B and Kinyua J (2016) Comparison of pharmaceutical, illicit drug, alcohol, nicotine and caffeine levels in wastewater with sale, seizure and consumption data for 8 European cities. BMC public health 16(1), 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer AJ, BantaGreen CJ, Ort C, Robel AE and Field J (2016) Wastewater testing compared with random urinalyses for the surveillance of illicit drug use in prisons. Drug and alcohol review 35(2), 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCPDAP (2017) Rates of Drug-Related Poisioning Deaths and ED Visits that Mention Opiates and Heroin Prevention, C.C.f.P.D.A. (ed).
- CDC (2016) Reported Law Enforcement Encounters Testing Positive for Fentanyl Increase Across US Prevention, C.f.D.C.a. (ed), Atlanta, GA. [Google Scholar]
- CDC and University, B. (2017) Increase in overdose deaths involving synthetic opioids other than methadone linked to increase in supply of fentanyl in PBSS states
- Chiaia AC, Banta-Green C and Field J (2008) Eliminating solid phase extraction with large-volume injection LC/MS/MS: analysis of illicit and legal drugs and human urine indicators in US wastewaters. Environmental science & technology 42(23), 8841–8848. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM and Baldwin GT (2016) Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016(374), 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone EJ, Holicky BA, Grant TM, Darwin WD and Goldberger BA (1993) Pharmacokinetics and pharmacodynamics of intranasal “snorted” heroin. Journal of analytical toxicology 17(6), 327–337. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Welch P, Paul BD and Mitchell JM (1991) Forensic drug testing for opiates, III. Urinary excretion rates of morphine and codeine following codeine administration. Journal of analytical toxicology 15(4), 161–166. [DOI] [PubMed] [Google Scholar]
- Daughton CG (2001), ACS Publications. [Google Scholar]
- Daughton CG and Ruhoy IS (2009) Environmental footprint of pharmaceuticals: the significance of factors beyond direct excretion to sewers. Environmental toxicology and chemistry 28(12), 2495–2521. [DOI] [PubMed] [Google Scholar]
- Donner B, Zenz M, Tryba M and Strumpf M (1996) Direct conversion from oral morphine to transdermal fentanyl: a multicenter study in patients with cancer pain. Pain 64(3), 527–534. [DOI] [PubMed] [Google Scholar]
- Dove A (2006) News Feature: Drugs down the drain, Nature Publishing Group. [Google Scholar]
- Dowell D, Haegerich TM and Chou R (2016) CDC guideline for prescribing opioids for chronic pain—United States, 2016. Jama 315(15), 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMCDDA (2016) Assessing illicit drugs in wastewater: asvances in wastewater-based drug epidemiology
- EPA, U. (1986) Appensix B to Part 136 - Definition and Procedure for the Determination of the Method Detection Limit. Code of Federal Regulations, Title 40.
- Frieden TR and Houry D (2016) Reducing the risks of relief—the CDC opioid-prescribing guideline. New England Journal of Medicine 374(16), 1501–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatidou G, Kinyua J, van Nuijs ALN, Gracia-Lor E, Castiglioni S, Covaci A and Stasinakis AS (2016) Drugs of abuse and alcohol consumption among different groups of population on the Greek Island of Lesvos through sewage-based epidemiology. Science of the Total Environment 563–564, 633–640. [DOI] [PubMed] [Google Scholar]
- Gerrity D, Trenholm RA and Snyder SA (2011) Temporal variability of pharmaceuticals and illicit drugs in wastewater and the effects of a major sporting event. Water Research 45(17), 5399–5411. [DOI] [PubMed] [Google Scholar]
- Gushgari AJ, Driver EM, Steele JC and Halden RU (2018) Tracking narcotics consumption at a Southwestern U.S. university campus by wastewater-based epidemiology. Journal of Hazardous Materials 359, 437–444. [DOI] [PubMed] [Google Scholar]
- Halden RU (2016) Planetary Talk: Urban metabolism metrology: A new discipline elucidating the human condition in cities around the world, Philadelphia, PA. [Google Scholar]
- Hancock C, Mennenga H, King N, Andrilla H, Larson E and Schou P (2017) Treating the Rural Opioid Epidemic, National Rural Health Association. [Google Scholar]
- Harocopos A, Allen B and Paone D (2016) Circumstances and contexts of heroin initiation following non-medical opioid analgesic use in New York City. International Journal of Drug Policy 28, 106–112. [DOI] [PubMed] [Google Scholar]
- Hasselström J and Säwe J (1993) Morphine pharma cokinetics and metabolism in humans. Clinical pharmacokinetics 24(4), 344–354. [DOI] [PubMed] [Google Scholar]
- Heberer T and Feldmann D (2005) Contribution of effluents from hospitals and private households to the total loads of diclofenac and carbamazepine in municipal sewage effluents— modeling versus measurements. Journal of Hazardous materials 122(3), 211–218. [DOI] [PubMed] [Google Scholar]
- Heuett NV, Ramirez CE, Fernandez A and Gardinali PR (2015) Analysis of drugs of abuse by online SPE-LC high resolution mass spectrometry: communal assessment of consumption. Science of the Total Environment 511, 319–330. [DOI] [PubMed] [Google Scholar]
- Jenkins AJ, Keenan RM, Henningfield JE and Cone EJ (1994) Pharmacokinetics and pharmacodynamics of smoked heroin. Journal of analytical toxicology 18(6), 317–330. [DOI] [PubMed] [Google Scholar]
- Kankaanpää A, Ariniemi K, Heinonen M, Kuoppasalmi K and Gunnar T (2014) Use of illicit stimulant drugs in Finland: a wastewater study in ten major cities. Science of the Total Environment 487, 696–702. [DOI] [PubMed] [Google Scholar]
- Katz J (2017) Drug Deaths in America Are Rising Faster Than Ever, New York Times, New York. [Google Scholar]
- Kim KY, Lai FY, Kim H-Y, Thai PK, Mueller JF and Oh J-E (2015) The first application of wastewater-based drug epidemiology in five South Korean cities. Science of the Total Environment 524, 440–446. [DOI] [PubMed] [Google Scholar]
- Kounang N (2017) Opioids now kill more people than breast cancer, CNN, Atlanta, GA. [Google Scholar]
- Labroo RB, Paine MF, Thummel KE and Kharasch ED (1997) Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metabolism and Disposition 25(9), 1072–1080. [PubMed] [Google Scholar]
- Lai FY, Bruno R, Leung HW, Thai PK, Ort C, Carter S, Thompson K, Lam PK and Mueller JF (2013) Estimating daily and diurnal variations of illicit drug use in Hong Kong: a pilot study of using wastewater analysis in an Asian metropolitan city. Forensic science international 233(1), 126–132. [DOI] [PubMed] [Google Scholar]
- Lai FY, O’Brien J, Bruno R, Hall W, Prichard J, Kirkbride P, Gartner C, Thai P, Carter S and Lloyd B (2016) Spatial variations in the consumption of illicit stimulant drugs across Australia: a nationwide application of wastewater-based epidemiology. Science of the Total Environment 568, 810–818. [DOI] [PubMed] [Google Scholar]
- Lankenau SE, Teti M, Silva K, Bloom JJ, Harocopos A and Treese M (2012) Initiation into prescription opioid misuse amongst young injection drug users. International Journal of Drug Policy 23(1), 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg RH, Wennberg P, Johansson MI, Tysklind M and Andersson BA (2005) Screening of human antibiotic substances and determination of weekly mass flows in five sewage treatment plants in Sweden. Environmental science & technology 39(10), 3421–3429. [DOI] [PubMed] [Google Scholar]
- Martins SS, Sarvet A, Santaella-Tenorio J, Saha T, Grant BF and Hasin DS (2017) Changes in US lifetime heroin use and heroin use disorder: prevalence from the 2001–2002 to 2012–2013 National Epidemiologic Survey on Alcohol and Related Conditions. Jama psychiatry 74(5), 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu-Gelabert P, Guarino H, Jessell L and Teper A (2015) Injection and sexual HIV/HCV risk behaviors associated with nonmedical use of prescription opioids among young adults in New York City. Journal of substance abuse treatment 48(1), 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo (2017) Drugs and Supplements - Proper Use and Dosing Clinic, M. (ed).
- MNDH (2017) Indicator Dashboards - Opioid Dashboard Health, M.D.o. (ed).
- Moore DR, Burgard DA, Larson RG and Ferm M (2014) Psychostimulant use among college students during periods of high and low stress: an interdisciplinary approach utilizing both self-report and unobtrusive chemical sample data. Addictive behaviors 39(5), 987–993. [DOI] [PubMed] [Google Scholar]
- NBC (2017) 4 Arrested After NJ Police Seize $9.6 Million of Heroin, NBC Broadcasting, New York, NY. [Google Scholar]
- NDIC (2000) Heroin Consumption in the U.S. Center, N.D.I. (ed).
- O’Brien JW, Banks APW, Novic AJ, Mueller JF, Jiang G, Ort C, Eaglesham G, Yuan Z and Thai PK (2017) Impact of in-sewer degradation of pharmaceutical and personal care products (PPCPs) population markers on a population model. Environmental science & technology 51(7), 3816–3823. [DOI] [PubMed] [Google Scholar]
- OHA (2017) Prescribing and Overdose Data for Oregon - Opiate Data Dashboard Authority, O.H. (ed).
- Peavy KM, Banta-Green CJ, Kingston S, Hanrahan M, Merrill JO and Coffin PO (2012) “Hooked on” prescription-type opiates prior to using heroin: results from a survey of syringe exchange clients. Journal of psychoactive drugs 44(3), 259–265. [DOI] [PubMed] [Google Scholar]
- Pineda P (2019) Tempe and ASU use sewage to pinpoint the opioid problem. Here’s what they found, Arizona’s Family, Arizona. [Google Scholar]
- Pollini RA, Banta-Green CJ, Cuevas-Mota J, Metzner M, Teshale E and Garfein RS (2011) Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Substance abuse and rehabilitation 2, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postigo C, de Alda ML and Barceló D (2011) E valuation of drugs of abuse use and trends in a prison through wastewater analysis. Environment international 37(1), 49–55. [DOI] [PubMed] [Google Scholar]
- RIPO (2017) Prevent Overdose - RI Force, R.O.P.a.I.T. (ed).
- Rudd RA (2016) Increases in drug and opioid-involved overdose deaths—United States, 2010– 2015. MMWR. Morbidity and mortality weekly report 65. [DOI] [PubMed] [Google Scholar]
- SAMHSA (2013) Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings Administration, S.A.a.M.H.S. (ed), U.S. Department of Health and Human Services, Rockville, MD. [Google Scholar]
- Schuchat A, Houry D and Guy GP (2017) New data on opioid use and prescribing in the United States. Jama 318(5), 425–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JB (2003) The influence of sex on pharmacokinetics. Clinical pharmacokinetics 42(2), 107–121. [DOI] [PubMed] [Google Scholar]
- Siegal HA, Carlson RG, Kenne DR and Swora MG (2003) Probable relationship between opioid abuse and heroin use. American family physician 67(5), 942, 945–942, 945. [PubMed] [Google Scholar]
- Skopp G, Pötsch L, Klingmann A and Mattern R. (2001) Stability of morphine, morphine-3-glucuronide, and morphine-6-glucuronide in fresh blood and plasma and postmortem blood samples. Journal of analytical toxicology 25(1), 2–7. [DOI] [PubMed] [Google Scholar]
- Subedi B and Kannan K (2014) Mass loading and removal of select illicit drugs in two wastewater treatment plants in New York State and estimation of illicit drug usage in communities through wastewater analysis. Environmental science & technology 48(12), 6661–6670. [DOI] [PubMed] [Google Scholar]
- Terzic S, Senta I and Ahel M (2010) Illicit drugs in wastewater of the city of Zagreb (Croatia)–estimation of drug abuse in a transition country. Environmental pollution 158(8), 2686–2693. [DOI] [PubMed] [Google Scholar]
- Thompson D (2017) More than 1 in 3 American prescribed opioids in 2015 News, C. (ed).
- Thorn CF, Klein TE and Altman RB (2009) Codeine and morphine pathway. Pharmacogenetics and genomics 19(7), 556–558. [DOI] [PubMed] [Google Scholar]
- Tscharke BJ, Chen C, Gerber JP and White JM (2016) Temporal trends in drug use in Adelaide, South Australia by wastewater analysis. Science of the Total Environment 565, 384–391. [DOI] [PubMed] [Google Scholar]
- USCB (2010) American Fact Finder Bureau, U.S.C. (ed).
- van Nuijs AL, Castiglioni S, Tarcomnicu I, Postigo C, de Alda ML, Neels H, Zuccato E, Barcelo D and Covaci A (2011a) Illicit drug consumption estimations derived from wastewater analysis: a critical review. Science of the Total Environment 409(19), 3564–3577. [DOI] [PubMed] [Google Scholar]
- Van Nuijs AL, Mougel J-F, Tarcomnicu I, Bervoets L, Blust R, Jorens PG, Neels H and Covaci A (2011b) Sewage epidemiology—a real-t ime approach to estimate the consumption of illicit drugs in Brussels, Belgium. Environment international 37(3), 612–621. [DOI] [PubMed] [Google Scholar]
- VDH (2017) Virginia Opioid Addiction Indicators Health, V.D.o. (ed).
- Vree TB and Wissen CP (1992) Pharmacokinetics and metabolism of codeine in humans. Biopharmaceutics & drug disposition 13(6), 445–460. [DOI] [PubMed] [Google Scholar]
- Vuori E, Happonen M, Gergov M, Nenonen T, Järvinen A, Ketola RA and Vahala R (2014) Wastewater analysis reveals regional variability in exposure to abused drugs and opioids in Finland. Science of the Total Environment 487, 688–695. [DOI] [PubMed] [Google Scholar]
- Warner M, Trinidad JP, Bastian BA, Miniño AM and Hedegaard H (2016) Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2010–2014. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System 65(10), 1–15. [PubMed] [Google Scholar]
- Whitaker B (2017) Ex-DEA Agent: Opioid Crisis Fueled by Drug Industry and Congress Minutes (ed), CBS. [Google Scholar]
- Yeh S, Gorodetzky C and McQuinn R (1976) Urinary excretion of heroin and its metabolites in man. Journal of Pharmacology and Experimental Therapeutics 196(2), 249–256. [PubMed] [Google Scholar]
- Zuccato E, Castiglioni S, Tettamanti M, Olandese R, Bagnati R, Melis M and Fanelli R (2011) Changes in illicit drug consumption patterns in 2009 detected by wastewater analysis. Drug and alcohol dependence 118(2), 464–469. [DOI] [PubMed] [Google Scholar]
- Zuccato E, Chiabrando C, Castiglioni S, Bagnati R and Fanelli R (2008) Estimating community drug abuse by wastewater analysis. Environmental health perspectives 116(8), 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato E, Chiabrando C, Castiglioni S, Calamari D, Bagnati R, Schiarea S and Fanelli R (2005) Cocaine in surface waters: a new evidence-based tool to monitor community drug abuse. Environmental Health 4(1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




