Abstract
Marijuana (cannabis) use by pregnant women in the United States is increasing and there is a dire need to understand the beneficial or harmful effects of cannabis during pregnancy. Uterine endometrial stromal cells are fibroblast-like cells that differentiate into secretory cells, a process called decidualization, to create a microenvironment conducive for placenta formation and early embryonic growth. In this study, using model human cell lines, we for the first time demonstrate that Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabinol (CBN) inhibit endometrial stromal cell decidualization and have adverse effects on trophoblast-endometrium cross-talk.
Keywords: Marijuana, cannabinoids, THC, CBD, endometrial stromal cells, decidua, decidualization, trophoblast-endometrium cross-talk, embryo, fertility, reproduction, medicine
Introduction
Endogenous and environmental factors can influence human pregnancy and outcomes and marijuana use during pregnancy is one such factor. Marijuana (from dried flowers) obtained from plants of Cannabis species is the major source of Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabinol (CBN) [1–3]. In the United States, marijuana use during pregnancy increased by 65% over the last decade (2002–2014), with nearly 7.5% of all pregnant women aged 18–25 years reported past-month marijuana use in 2014 [4–8]. The number of pregnant women who use marijuana is likely to increase as more states legalize marijuana. Meta-analysis suggests that marijuana use prior or during pregnancy could be one of the contributing factors associated with lower birth weight, preterm delivery, congenital malformation and neonatal complications [7, 9, 10]. A recent comprehensive review of the health effects of marijuana use during pregnancy by the National Academies of Sciences, Engineering and Medicine (NASEM) found substantial evidence of a statistical association between marijuana use and lower birth weight and have stressed the need of further research to evaluate its effects on other prenatal, perinatal and neonatal outcomes [6].
Cannabinoids activate cannabinoid receptors (CNRs) to trigger intracellular signaling cascades [11]. Cannabinoid receptor 1 (CNR1) is predominantly expressed in the brain and peripheral nervous system and regulates cognitive behavior and hypothalamus-pituitary hormone secretion [12]. Cannabinoid receptor 2 (CNR2) is expressed in lymphoid tissues and regulates inflammatory responses [13]. CNRs belong to the G-protein coupled receptor (GPCR) family and are associated with the inhibitory Gαi subunit which inhibits intracellular cAMP generation upon ligand-mediated activation [11, 14]. While it is known that THC primarily activates CNR1, receptors for CBD and CBN remain largely unclear [15].
Successful pregnancy depends on fruitful reciprocal interactions between a competent embryo and a receptive endometrium [16]. Maternal control of receptivity, exhibited partly via decidualization of uterine fibroblast-like endometrial stromal cells (ESCs), is a unique aspect of human reproduction. ESCs decidualize in the secretory phase of the menstrual cycle under the influence of progesterone into highly exocrine cells which create a conducive microenvironment for early embryonic development, and interruption of ESC decidualization compromises these events [17]. Numerous transcriptomic and proteomic studies using cultured ESCs as well as ESC-derived endometrial biopsy samples obtained from early and late menstrual stage endometrium have revealed that ESC decidualization activates the expression of genes and proteins which play a critical role in embryo implantation, survival and growth [18–23]. Defective or improper decidualization in humans is associated with infertility, recurring pregnancy loss and reduces in vitro fertilization (IVF) success [24–28]. Despite the increasing use of marijuana by pregnant women, the effects of marijuana-derived cannabinoids on the endometrium have never been investigated. Here we report the effects of THC, CBD and CBN on ESC decidualization and trophoblast-endometrium cross-talk using human endometrial stromal model cell lines.
Materials and Methods
Cells and Cell Culture:
Immortalized human endometrial stromal cells (THESCs) were kindly provided by Dr. Gil Mor from Yale University Medical Center, New Haven, CT. THESCs were developed by stably transfecting human telomerase coding gene into primary human endometrial stromal cells from healthy females and can be decidualized by treating them with a cocktail of progesterone, estrogen in low serum media [29]. The cells were maintained in F12-K media (ThermoFisher #21127030) supplemented with 10% charcoal-stripped FBS (Sigma #F6765) and 1X Anti-anti (ThermoFisher #15240062). THESC decidualization was induced by treating a 70% confluent monolayer of cells with a combination of hormones, termed MEF treatment in this report and consisting of 1 μM medroxyprogesterone acetate (MPA) (Sigma #71589), 10 nM β-estradiol (E2) (Sigma #50282) and 10 μM Forskolin (FK) (Sigma #F1141), dissolved in OPTI-MEM, for seven days with media changes every 48 h. BeWo cells obtained from ATCC and HTR8-SV/neo cells (kindly provided by Dr. Cecilia Giachelli, Department of Bioengineering, University of Washington) were cultured in F12-K supplemented with 10% FBS (ThermoFisher #16000044) and 1X Anti-anti. Transmission electron microscopy (TEM) analysis of cells was conducted at the Imaging Core of the Fred Hutchinson Cancer Research Center in Seattle.
Cannabinoids:
(−)-Δ9-tetrahydrocannabinol (THC) (#T-005–1ML), cannabidiol (CBD) (#C6395), and cannabinol (CBN) (#C-046) dissolved in methanol at 1mg/ml were purchased from Sigma and freshly diluted as in respective media prior to treatment.
MTT Assay:
The MTT assay was used to determine cytotoxicity of cannabinoids (CBs) on THESCs. THSECs were plated in 96-well plates and cultured for 48 h in regular media. CBs were diluted in at a concentration of 0.2, 2 and 20 μM in OPTI-MEM +/− MEF and used to culture cells for seven days, with media changes every 48 h. This treatment regimen was used to evaluate the effects of CBs on undecidualized and decidualizing THESCs. On the eight day, 100 μl of 5 mg/ml solution of Thiazolyl Blue Tetrazolium Bromide (MTT) (Sigma #M2128) in OPTI-MEM was added to the culture media and incubated in for 2 h, after which the media was aspirated and the purple crystals were dissolved in 100 μl of 100% DMSO (Sigma #D4540) under gentle agitation. Absorbance was measured at 590 nm (with reference at 620 nm) and used to determine the percentage of surviving cells.
Semi-quantitative real-time PCR:
Total RNA was isolated from undecidualized control and decidualized cells (with and without CB treatments) using Trizol (Invitrogen) according to the manufacturer’s instructions. Two μg of total RNA were used for cDNA preparation using Superscript III (Invitrogen), and changes in expression of genes of interest were evaluated by real-time PCR using gene-specific primers and in-house SYBR green mix as previously described [30]. Primers (from 5`to 3’) used in the study are CNR1 (NM_016083.4): GTATGCTCTGCCTGCTGAA and CTGTGAACACTGGCTGCATT; CNR2 (NM_001841.2): GGTCTTGGCACTCTCTTCTT and GTCCCAACACTCATCAGCAA; PRL (NM_000948.5): CCTGCAACGATCACGAACA and CTGGCACAGGAGCAGGTT; IGFBP-1 (NM_000596.4): ATTCCATCCTTTGGGACGCC and GAGTTCTATTCGGCAGGGCT; 18sRNA: GTGGAGCGATTTGTCTGGTT and GAACGCCACTTGTCCCTCT; MMP-2 (NM_004530.5): GCTCAGATCCGTGGTGAGAT and GCCTCGTATACCGCATCAAT; and MMP-9 (NM_004994.2): CGAACTTTGACAGCGACAA and CCCTCAGTGAAGCGGTACAT.
ELISA:
The levels of soluble prolactin (PRL) secreted in media from undecidualized and decidualized THESCs (with and without CB treatments) were quantified using a PRL-ELISA kit (Invitrogen #EHIAPRL) following the manufacturer’s instructions. Media collected were centrifuged at 10,000 rpm at 4°C and stored at −20°C until use. The media was diluted 50-fold prior to adding to the ELISA plates, and colorimetric quantification was then carried out with a microplate reader at 450 nm.
Spheroid assay:
Migration and expansion of individual BeWo cells from BeWo spheroids when placed on a monolayer of decidualized ESCs were used to demonstrate the role of decidualization in inducing trophoblast cell invasion [31]. We used a modified version of this assay to evaluate the effects of THESC decidualization on inducing the invasive behavior in trophoblast cells and to evaluate if CBs can compromise this aspect. Enhanced green fluorescent protein (EGFP)-expressing THESCs (G-THESCs) and mCherry-expressing BeWo (R-BeWo) cells were generated by infecting the cells with lentivirus containing the pLJM1-EGFP (Addgene #19319) or pLV-mCherry (Addgene #36084) plasmid, respectively. G-THESCs were plated and decidualized (with and without CB treatments) in 12-well plates for 7 days, after which the cells were maintained in hormone-free OPTI-MEM for 24 h to allow accumulation of secretory factors from decidualized G-THESCs. R-BeWo cell spheroids, which mimic the trophoectoderm of the blastocyst, were prepared by suspending 2 × 104
cells/ml in 30 μl of “hanging drops” as previously described [32]. After 48 h of culturing, around 3–4 R-BeWo spheroids were placed in each well of decidualized G-THESC cells. Trophoblast cell migration from individual spheroids was assessed after 6 h using an inverted fluorescence microscope (Nikon Eclipse Ti). The number of spheroids which showed some degree of spreading were counted and graphed.
Transwell migration assay/Boyden chamber assay:
This assay was performed using 24-well transwell plates (0.8 μm) (Corning #3422) as follows. Briefly, THESCs were plated in the lower chambers and either left undecidualized or decidualized (with and without CB treatments) for 7 days. The cells were then maintained for 24 h in normal OPTI-MEM devoid of MPA, E2 and FK as well as CB to allow accumulation of secretory factors from decidualized THESCs. On day 8, Martigel (0.2 mg/ml) (Invitrogen #354263) coated transwells were inserted and seeded with 1 × 105 of HTR8-SV/neo (HTR8) cells suspended in serum-free F12-K. After 24 h of incubation, the transwells were placed in 4% ice-cold formaldehyde for 5 minutes to fix the cells. Cotton buds were used to gently clear the martigel. Invading HTR8 trophoblast cells were stained with crystal violet (0.5% w/v in methanol) for one minute and repeatedly washed in PBS to remove excess staining. Individual membranes were gently cut and mounted on positively charged glass slides using ClearMount (ThermoFisher #00–8110). The numbers of invading HTR8 cells in each treatment group were counted and graphed from four random images from inverted microscope (Nikon Eclipse Ti).
Data analysis:
GraphPad Prism 7 software (La Jolla, CA) was used for data analysis. Data shown represent means ± SEM of 3–4 independent experiments. Two-way ANOVA followed by the Bonferroni correction was used to analyze differences among treatment groups. Differences with p value of < 0.05 were considered statistically significant.
Results
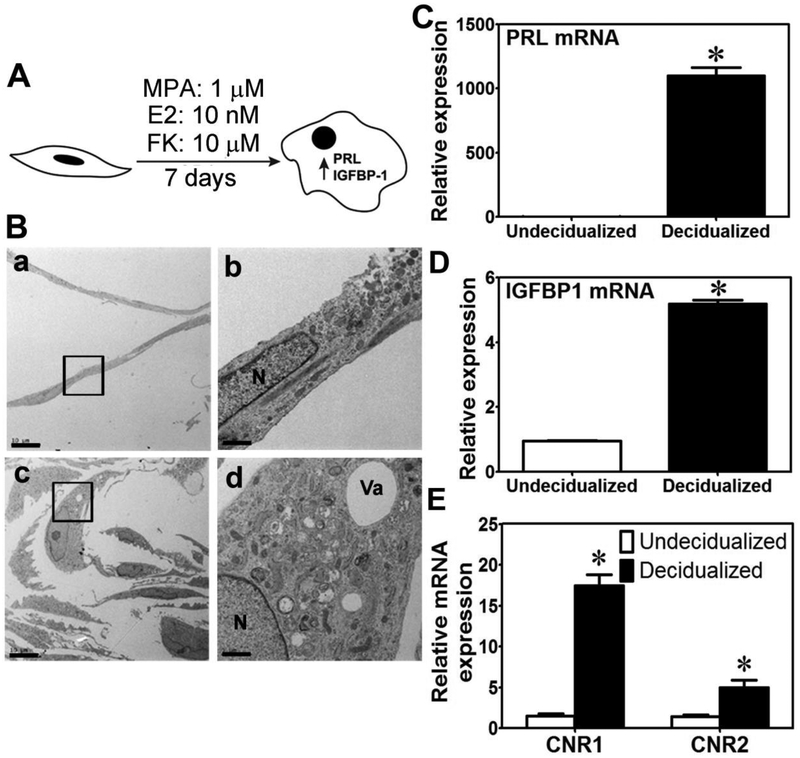
Earlier studies have demonstrated that THESC is a very suitable human cell model to study cellular and biochemical changes associated with ESC decidualization [29, 33, 34]. In this study, we decidualized THESC by culturing them in OPTI-MEM supplemented with 1 μM MPA, 10 nM E2, and 10 μM FK for 7 days (termed MEF treatment) (Fig. 1A). Compared to the control undecidualized cells, MEF treatment resulted in an increase in cell volume, size of the nucleus (Fig. 1B, a vs c), the endoplasmic reticulum (ER) and Golgi content (Fig. 1B, b vs d), along with an increase in mRNA expression of prolactin (PRL) (Fig. 1C) and IGF binding protein1 (IGFBP1) (Fig. 1D) as well as secreted PRL protein levels (see below). These cellular and biochemical changes are in agreement with previous reports [29, 33, 34], suggesting that MEF treatment successfully induced THESC decidualization and hence was used in all subsequent experiments to study the effects of CB. We observed that the mRNA levels of CNR1 and CNR2 in decidualized THESCs were significantly induced ~17-fold and ~6-fold, respectively, compared to undecidualized cells (Fig. 1E).
Figure 1. Decidualization of THESCs using FK.
A. A schematic representation of THESC decidualization using the MEF treatment. MPA, E2 and FK represent medroxyprogesterone acetate, β-estradiol and forskolin, respectively. B. TEM images a and b show that undecidualized THESCs are narrow with a small nucleus. TEM images c and d indicate that decidualized THESCs have larger cytoplasmic and nuclear volume and that the cytoplasm is enriched with mitochondria, endoplasmic reticulum, vacuoles and vesicles. Boxs in images a and c are magnified in b and d, respectively. The scale bars in images a and c represent 10 μm. The scale bars in images b and d represent 2 μm. N and Va in image d represent nucleus and vacuole, respectively. C and D show that MEF treatments induced THSEC decidualization which is associated with a robust increase in mRNA expression of PRL and IGFBP-1, respectively. *indicates a p value of ≤ 0.05 between undecidualized and decidualized THESCs. E shows that decidualizing THESCs for 7 day leads to a significant increase in mRNA expression of both CNR1 and CNR2. *indicates a p value of ≤ 0.05 between decidualized and undecidualized cells.
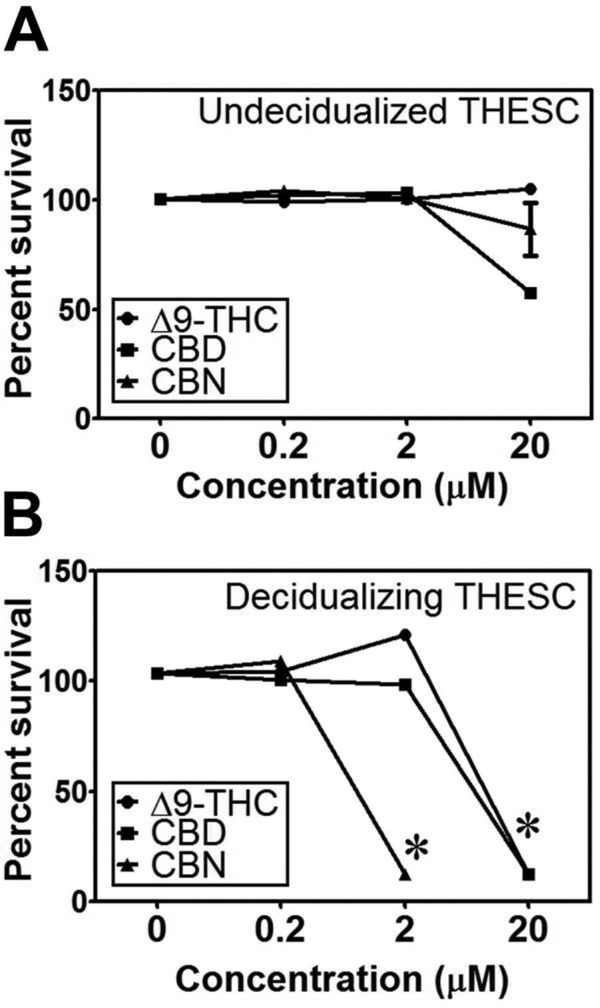
To examine the cytotoxic effects of CBs, we treated THESCs with 0.2, 2 and 20 μM of THC, CBD or CBN for 7 days simultaneously with or without MEF. None of the CBs at concentrations up to 2 μM were cytotoxic to normal undecidualized cells (without MEF treatment) (Fig. 2A); however, THC and CBD at 20 μM and CBN at 2 μM were significantly cytotoxic to decidualizing THESCs (with MEF treatment) (Fig. 2B). Based on these observations, we used 0.5 μM THC, CBD or CBN in all subsequent experiments as none of the CBs showed any apparent cytotoxicity towards THESCs at this concentration. Further, this CB concentration is clinically relevant as it is within the range of the plasma THC concentrations (0.09–0.73 μM) observed in marijuana users [35, 36].
Figure 2. Cytotoxicity of CBs to undecidualized and decidualizing THESCs.
The control undecidualized (A) (without MEF) and decidualizing (B) (with MEF) THESCs were treated with or without MEF for 7 days simultaneously with 0.2, 2 and 20 μM of THC, CBD or CBN. While none of the CBs at up to 2 μM had any cytotoxic effects on undecidualized THESCs, THC and CBD at 20 μM and CBN at 2 μM showed significant cytotoxicity to decidualizing THESCs. *indicates a p value of ≤ 0.05 compared to no treatments with THC, CBD or CBN.
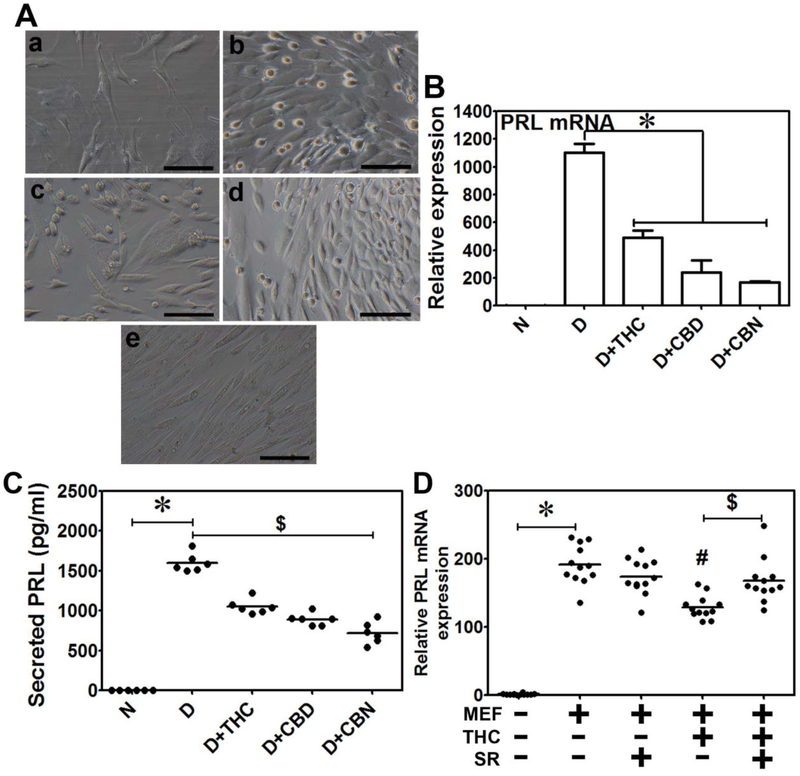
Next, we examined the effects of CB on THESC decidualization. Morphologically, undecidualized THESCs were narrow and fibroblast-like (Fig. 3A, a); while decidualized THESCs (with MEF treatment alone) transformed into secretory cells with larger cytoplasmic volume (Fig. 3A, b). We observed that all the CB at 0.5 μM attenuated THESC decidualization associated morphological changes (Fig. 3A, b vs c-e) and significantly decreased the decidualization-induced PRL mRNA expression (Fig. 3B) and secreted PRL levels (Fig. 3C). To evaluate if the observed changes are mediated via a CNR, we included 10 μM SR141716 (SR), a potent CNR1 inhibitor [37] during decidualization. SR rescued the THC-associated decrease in PRL mRNA, but had no significant effect on MEF-induced PRL mRNA expression (Fig. 3D), suggesting that the effects of THC on THESC decidualization are likely mediated by CNR1. Given the ambiguity as to which CNRs might interact with CBD and CBN, we did not conduct the rescue experiments with CBD and CBN.
Figure 3. THC, CBD and CBN inhibit THESC decidualization.
A. Shown are phase contrasts (a-e) that were recorded to monitor morphological changes during THSEC decidualization. Image a, shows undecidualized THESCs that were narrow and fibroblast-like. Image b, depicts decidualized THESCs (with the MEF treatment alone) that were transformed into secretory cells with large cytoplasmic volume. Images c, d and e illustrate morphologies of decidualizing THESCs in the presence of 0.5 μM THC, CBD and CBN, respectively. The CB treatments compromised the decidualization-associated morphological changes. The CBN treatment led to heavy cellular vacuolation (e) compared to treatments with THC (c) or CBD (d). The scale bars in images a-e represent 250 μm. B. THESC decidualization (with MEF treatment alone) was associated with a significant increase in PRL mRNA expression that was significantly reduced when THESCs were decidualized in the presence of 0.5 μM THC, CBD or CBN. *indicates a p value of ≤ 0.05 compared to decidualized cells with no THC, CBD or CBN treatment. C. THESC decidualization (with MEF treatment alone) was associated with a significant increase in secreted PRL protein levels in media and this increase was significantly reduced when THSECs were decidualized in the presence of 0.5 μM THC, CBD or CBN. *indicates a p value of ≤ 0.05 compared to undecidualized cells, and $ indicates a p value of ≤ 0.05 compared to decidualized cells with no THC, CBD or CBN treatment. N and D in panels B and C represent undecidualized and decidualized THESCs, respectively. Data shown are means ± SEM of three independent experiments performed in duplicate in each experiment. D. The decrease in PRL mRNA by THC treatment was rescued when decidualized THSECs were co-treated with 10 μM SR141716 (SR); however, SR had no effects on PRL mRNA when THSECs were decidualized with no THC treatment. *indicates significant increase (p ≤ 0.05) of PRL mRNA in decidualized THESCs (with MEF treatment alone) compared to undecidualized cells. #indicates significant decrease (p ≤ 0.05) of PRL mRNA in decidualized cells with THC treatment compared to decidualized cells with no THC treatment (MEF alone); $indicates significant increase (p ≤ 0.05) of PRL mRNA in decidualized cells with SR and THC treatment compared to THC treatment alone. Data shown are means ± SEM of three independent experiments performed with 4 replications in each experiment. These results indicate that THC, CBD and CBN can delay or compromise the process of uterine endometrial stromal cell decidualization.
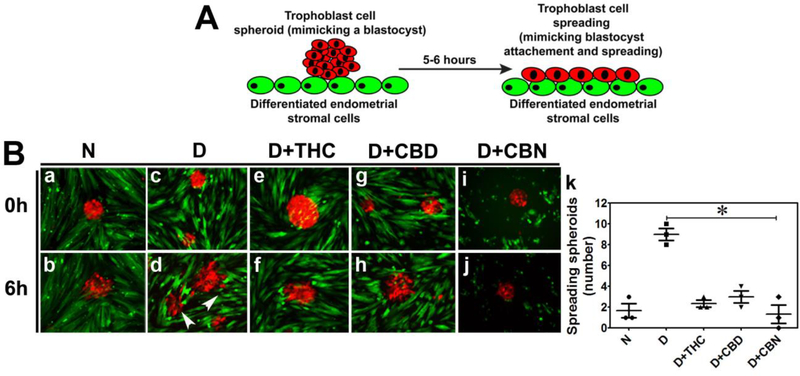
Reciprocal paracrine communication between decidualized endometrial stromal cells and the trophoblast (TB) cells regulates embryo attachment and trophoblast invasion [24, 38, 39]. We used the spheroid attachment assay to investigate the effects of CBs on these processes. TB spheroids mimicking the blastocyst trophoectoderm [40–42] were prepared using R-BeWo cells and placed on a monolayer of decidualized G-THESC (Fig. 4A). Attachment and expansion of R-BeWo spheroids were then visually assessed after 6 h using an inverted microscope (Fig. 4B). Compared to undecidualized THESC, R-BeWo spheroids placed on decidualized G-THESC attached sooner and cells started migrating away from spheroids by 6 h (Fig. 4B, a vs b and c vs d). Treatment with THC, CBD or CBN delayed these processes (Fig. 4B, e vs f, g vs h, and i vs j). Consistent with the qualitative analysis, the number of spreading R-BeWo spheroids placed on decidualized G-THESC was significantly decreased by the treatments with THC, CBD or CBN (Fig. 4B, k).
Figure 4. THC, CBD and CBN compromise trophoblast-endometrium interaction.
A. A schematic representation of the spheroid attachment assay. TB spheroids made from BeWo cells expressing mCherry that mimic trophoectoderm of the blastocyst were placed on a monolayer of undecidualized control or decidualized THESCs with or without THC, CBD or CBN treatment. Spheroid attachment and expansion were then assessed visually after 6 h of incubation. B. Shown are fluorescence images. TB spheroids prepared from mCherry-expressing BeWo cells (R-BeWo shown in red) were placed onto a monolayer of undecidualized control or decidualized EGFP-expressing THSECs (G-THSECs shown in green). Images a and b show that R-BeWo spheroids placed on undecidualized G-THSECs weakly attached and TB cells never migrated from the spheroids after 6 h. Images c and d show that R-BeWo spheroids placed on decidualized THESCs strongly attached and TB cells started migrating away from the spheroids after 6 h. Images e-j show that R-BeWo spheroids placed on decidualized THESCs in the presence of 0.5 μM THC (e and f), CBD (g and h) or CBN (i and j) had decreased attachment and delayed expansion after 6 h. N and D in panel B represent undecidualized and decidualized THESCs, respectively. k shows a significant decrease in the number of spreading R-BeWo spheroids placed on G-THESC decidualized in the presence of THC, CBD or CBN compared to control decidualized THESCs with no CB treatments. *indicates a p value of ≤ 0.05 between with and without CB treatments of decidualized THESCs.
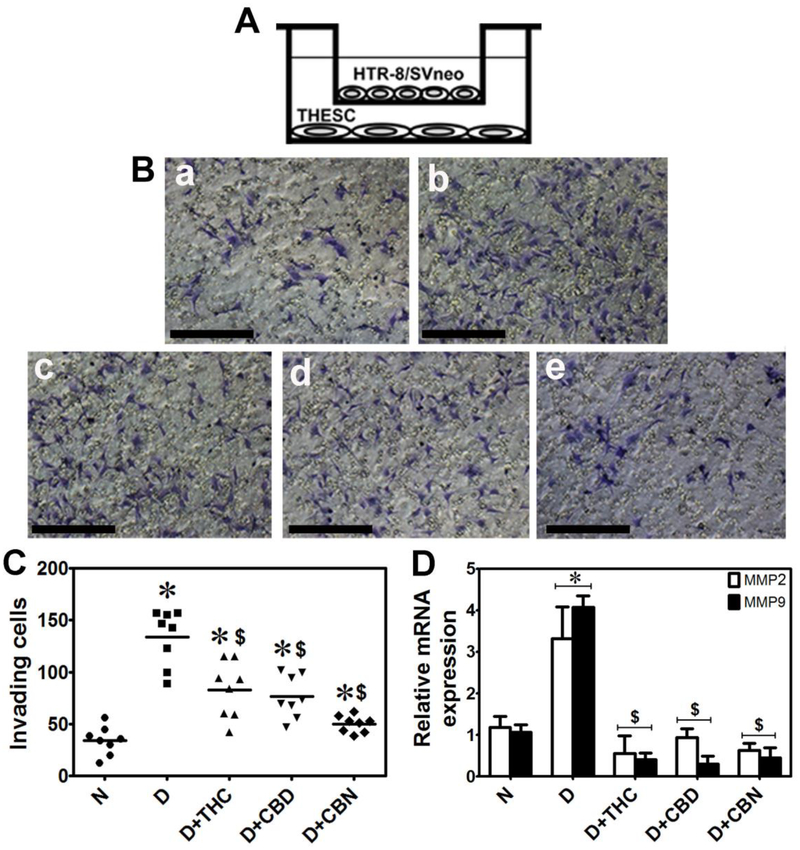
Finally, we used the Boyden chamber assay to investigate if the CBs affected the capacity of decidualized THESC to induce TB invasion (Fig. 5A). THESC were plated and decidualized with or without CB for 7 days and then cultured in plain OPTI-MEM for 24 h, after which Martigel (0.2 μg/ml) coated inserts layered with 1 × 105 of HTR8 trophoblast cells were placed into the wells and HTR8 invasion was quantified 12 h later as previously described [43]. THESC decidualization induced significant HTR8 invasion across the martigel compared to undecidualized cells (Fig. 5B, a vs b; Fig. 5C). The presence of 0.5 μM THC, CBD or CBN during decidualization significantly decreased the number of invading HTR8 cells (Fig. 5B, b vs c-e; Fig. 5C). TB invasion is known to be associated with increase in matrix metalloproteinase (MMP) gene expression, in particular MMP2 and MMP9 [44]. As expected, culturing HTR8 cells in conditioned media from decidualized THESCs for 24 h caused a 3–4-fold increase in MMP2 and MMP9 mRNA, which was significantly decreased to the basal levels (like those cultured in conditioned media from undecidualized THESCs) when HTR8 cells were cultured in conditioned media from THC, CBD or CBN-treated decidualized THESCs (Fig. 5D).
Figure 5. THC, CBD and CBN compromise trophoblast invasion and MMP gene expression.
A. A schematic illustration of the Boyden chamber assay that was used to evaluate the effects of THC, CBD or CBN on trophoblast-THESC interaction. First, THESCs were plated and decidualized for 7 days with or without 0.5 μM THC, CBD or CBN. Then, martigel-coated transwells layered with HTR8 cells were placed to the chamber as shown. Trophoblast invasion was assessed 12 hours later following staining with crystal voilet. B. Undecidualized TESECs induced lesser HTR8 cell migration (as indicated in violet color) (image a) compared to decidualized TESECs (MEF alone) (image b). The presence of THC (image c), CBD (image d) or CBN (image e) during decidualization reduced decidualization-induced HTR8 cell migration. The scale bars in images represent 600 μm. C. Invading HTR8 cells were counted after staining with crystal violet. The increase in invading HTR8 cells associated with THESC decidualization was significantly reduced by the presence of THC, CBD or CBN. D. HTR8 cells were cultured in conditioned media collected from undecidualized control and decidualized THESCs with or without THC, CBD or CBN treatment. The significant increase in mRNA levels of MMP2 and MMP9 in HTR8 cells associated with THSEC decidualization was significantly reduced by the presence of THC, CBD or CBN in conditioned media, suggesting that decidualization creates a microenvironment conducive for TB invasion and that CBs can compromise the formation of this microenvironment. *indicates significant increase (p ≤ 0.05) in invading HTR8 cells in the presence of decidualized THSECs compared to undecidualized THSECs or significant increase (p ≤ 0.05) in mRNA levels of MMP2 or MMP9 in HTR8 cells cultured in conditioned media from decidualized THSECs compared to undecidualized THSECs. $indicates significant decrease (p ≤ 0.05) in invading HTR8 cells in the presence of decidualized THSECs treated with THC, CBD or CBN versus no CB treatment or significant decrease (p ≤ 0.05) in mRNA levels of MMP2 or MMP9 in HTR8 cells cultured in conditioned media from decidualized THESCs treated with THC, CBD or CBN versus no CB treatment. Data shown are means ± SEM of four independent experiments performed in duplicate in each experiment. N and D in panels C and D represent undecidualized and decidualized THESCs, respectively.
Discussion
Uterine preparedness, associated with epithelial maturation and proper decidualization of endometrial stromal cells is key for a successful pregnancy [45–47]. The goal of this study was to evaluate the effects of marijuana-derived CBs on cellular aspects associated with endometrial stromal cell decidualization. THESCs were developed by stably transfecting human telomerase coding gene into primary human endometrial stromal cells from healthy females, and utilization of this cell model addresses the limited tissue availability of human ESC samples [29]. THESC can be decidualized by culturing them for 6–7 days in low serum media supplemented with progesterone, estrogen and stable cAMP analogues [29, 33, 34]. The gene expression pattern between these in vitro decidualized THESCs and primary human stromal cells from the late secretor phase is very similar and hence THESCs have been widely used as a cell model system to study diverse biochemical and cellular aspects associated with ESC decidualization [27, 31, 48]. We observed that decidualizing THESCs in MEF-supplemented OPTI-MEM for 7 days (Fig. 1) induced similar cellular and biochemical changes as reported earlier [29, 33, 34], thus establishing a functional in vitro decidualization model which would be used in subsequent experiments to evaluate the effects of marijuana-derived CBs on endometrial stromal cell decidualization.
We used 0.5 μM THC, CBD or CBN in our studies as at this concentration, none of the tested CB (THC, CBD or CBN) showed any apparent cytotoxicity towards decidualizing THESCs. CBN at 2 μM appeared to be more cytotoxic to decidualizing THESCs than THC or CBD (Fig. 2B); however, we estimated that CBN had an IC50 value of ~1.5 μM which is about 3 times the concentration (0.5 μM) used in this study. In addition, this CB concentration is within the range of clinically recorded plasma THC concentrations (0.09–0.73 μM) observed in marijuana users [35, 36]. Therefore, our studies are clinically relevant with respect to CB concentrations used.
In human liver, THC is hydroxylated to 11-hydroxy-tetrahydrocannabinol (11-OH-THC) by cytochrome P450 (CYP) enzymes CYP2C9 and CYP2C19, and is further oxidized to 11-nor-9-carboxy-tetrahydrocannabinol (COOH-THC) by CYP2C9 and CYP3A4 [49–52]. Additionally, hepatic UDP-glucuronosyltransferase (UGT) enzymes, UGT1A9 and UGT1A10, further glucuronidate 11-OH-THC [53]. Among these THC metabolites, 11-OH-THC is the primary and psychoactive metabolite. Up to date, little is known about extrahepatic THC metabolism as well as overall metabolism of CBD and CBN. Apart from CYP3A4 and CYP3A7, no other CYP or UGT enzymes have been identified in the endometrium [54]; hence, the endometrium may not be a significant contributor to or site of CB metabolism. Nevertheless, it is of importance to examine whether 11-OH-THC could exert the same or similar effects on THESC decidualization as THC in future studies as it is the major pharmacologically active metabolite of THC.
Embryo implantation is a critical early pregnancy event and endometrial stromal cell decidualization plays a critical role in temporal regulation of this event. Endometrial stromal cell decidualization prepares a microenvironment conducive for embryo implantation and trophoblast invasion [23, 38, 55, 56]. In line with earlier reports [31], we observed that THESC decidualization induced rapid attachment and expansion of TB spheroids; however, the presence of CB significantly suppressed these cellular events (Fig. 4B). The results of the Boyden chamber assay (Fig. 5) further indicate that the microenvironment created by THESC decidualization can activate the invasive behavior of TBs, which is compromised by the continued presence of CBs. These results suggest that decidualized THESCs create a microenvironment conducive for embryonic TB attachment and invasion and the CBs can negatively affect these critical cellular processes.
Of interest is our finding that decidualizing THESCs for 7 days led to a significant increase in mRNA expression of both CNR1 and CNR2 compared to undecidualized cells (Fig. 1E). Similar observation was reported earlier by Moghadam et al., where decidualizing endometrial stromal cells treated with synthetic cannabinoid, WIN-55,212–2, were shown to have higher CNR expression [57]. Keeping this in perspective, it could be argued that CBs are more effective in exerting their cellular effects on decidualized THESCs than on undecidualized cells. In other words, endometrial stromal cell decidualization could enhance and sustain the effects of CBs by increasing CNR expression. This argument is consistent with earlier studies which showed that sustained activation of CNR by synthetic cannabinoids or endocannabinoids compromised ESC survival and decidualization [57, 58].
It has been well documented that THC can inhibit placental trophoblast proliferation and differentiation [59–61]. These previous findings and the data of this study suggest that CBs can adversely affect both ESC and placental TB proliferation and differentiation, meaning that pregnant women could be vulnerable to the detrimental effects of CBs, especially during early gestation when embryo implantation occurs and placenta begins to develop.
Conclusions
Our study for the first time demonstrates that exposure to marijuana-derived CBs can have adverse effects on endometrial stromal cell decidualization and potentially interfere with the trophoblast-endometrium cross-talk. Consequently, marijuana (cannabis) use during early gestation could pose a risk to critical pre-pregnancy and early pregnancy events. We recognize that the results of this study were obtained using a transformed human ESC cell model, therefore further studies using primary human ESCs and in vivo animal models are necessary to verify if the CBs exert similar effects and elucidate their mechanisms of action.
Highlights.
THC, CBD and CBN at a clinically relevant concentration had no cytotoxicity
THC, CBD and CBN inhibited endometrial stromal cell decidualization
THC, CBD and CBN adversely affected trophoblast-endometrium cross-talk
Funding:
This work was supported by the National Institute on Drug Abuse [grant number DA032507] to QM and a pilot grant from the University of Washington National Institute on Drug Abuse-sponsored Alcohol and Drug Abuse Institute to NNK.
Abbreviations:
- CB
cannabinoid
- THESC
immortalized human endometrial stromal cells
- TB
trophoblast
- PRL
prolactin
- THC
Δ9-tetrahydrocannabinol
- CBD
cannabidiol
- CBN
cannabinol
- MPA
medroxyprogesterone acetate
- E2
β-estradiol
- FK
forskolin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: The authors have no conflict of interest.
Conflict of interest
The authors have no conflict of interest.
References
- [1].Huestis MA, Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol, Handbook of experimental pharmacology (168) (2005) 657–90. [DOI] [PubMed] [Google Scholar]
- [2].Huestis MA, Human cannabinoid pharmacokinetics, Chemistry & biodiversity 4(8) (2007) 1770–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Striegel H, Rossner D, Simon P, Niess AM, The World Anti-Doping Code 2003--consequences for physicians associated with elite athletes, International journal of sports medicine 26(3) (2005) 238–43. [DOI] [PubMed] [Google Scholar]
- [4].Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS, Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002–2014, JAMA 317(2) (2017) 207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Forman-Hoffman VL, Glasheen C, Batts KR, Marijuana Use, Recent Marijuana Initiation, and Progression to Marijuana Use Disorder Among Young Male and Female Adolescents Aged 12–14 Living in US Households, Substance abuse : research and treatment 11 (2017) 1178221817711159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].E.a.M. National Academies of Sciences, The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research, National Academies of Sciences, Engineering and Medicine, 2017. [PubMed] [Google Scholar]
- [7].A.C.o.O.a. Gynecologists, Marijuana use during pregnancy and lactation, American College of Obstetricians and Gynecologists, Obstet Gynecol, 2015, pp. 234–238. [Google Scholar]
- [8].Volkow ND, Compton WM, Wargo EM, The Risks of Marijuana Use During Pregnancy, JAMA 317(2) (2017) 129–130. [DOI] [PubMed] [Google Scholar]
- [9].Hayatbakhsh MR, Flenady VJ, Gibbons KS, Kingsbury AM, Hurrion E, Mamun AA, Najman JM, Birth outcomes associated with cannabis use before and during pregnancy, Pediatric research 71(2) (2012) 215–9. [DOI] [PubMed] [Google Scholar]
- [10].Gunn JK, Rosales CB, Center KE, Nunez A, Gibson SJ, Christ C, Ehiri JE, Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis, BMJ open 6(4) (2016) e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kaminski NE, Koh WS, Yang KH, Lee M, Kessler FK, Suppression of the humoral immune response by cannabinoids is partially mediated through inhibition of adenylate cyclase by a pertussis toxin-sensitive G-protein coupled mechanism, Biochemical pharmacology 48(10) (1994) 1899–908. [DOI] [PubMed] [Google Scholar]
- [12].Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI, Structure of a cannabinoid receptor and functional expression of the cloned cDNA, Nature 346(6284) (1990) 561–4. [DOI] [PubMed] [Google Scholar]
- [13].Munro S, Thomas KL, Abu-Shaar M, Molecular characterization of a peripheral receptor for cannabinoids, Nature 365(6441) (1993) 61–5. [DOI] [PubMed] [Google Scholar]
- [14].Paria BC, Das SK, Dey SK, The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling, Proceedings of the National Academy of Sciences of the United States of America 92(21) (1995) 9460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pertwee RG, The diverse CB(1) and CB(2) receptor pharmacology of three plant cannabinoids: Δ(9)-tetrahydrocannabinol, cannabidiol and Δ(9)-tetrahydrocannabivarin, British journal of pharmacology 153(2) (2008) 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BAJ, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Brosens JJ, Macklon NS, Natural Selection of Human Embryos: Decidualizing Endometrial Stromal Cells Serve as Sensors of Embryo Quality upon Implantation, PloS one 5(4) (2010) e10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dunn CL, Kelly RW, Critchley HO, Decidualization of the human endometrial stromal cell: an enigmatic transformation, Reprod Biomed Online 7(2) (2003) 151–61. [DOI] [PubMed] [Google Scholar]
- [18].Garrido-Gomez T, Quinonero A, Antunez O, Diaz-Gimeno P, Bellver J, Simon C, Dominguez F, Deciphering the proteomic signature of human endometrial receptivity, Human reproduction (Oxford, England) 29(9) (2014) 1957–67. [DOI] [PubMed] [Google Scholar]
- [19].Haouzi D, Dechaud H, Assou S, De Vos J, Hamamah S, Insights into human endometrial receptivity from transcriptomic and proteomic data, Reprod Biomed Online 24(1) (2012) 23–34. [DOI] [PubMed] [Google Scholar]
- [20].Hu S, Yao G, Wang Y, Xu H, Ji X, He Y, Zhu Q, Chen Z, Sun Y, Transcriptomic changes during the pre-receptive to receptive transition in human endometrium detected by RNA-Seq, The Journal of clinical endocrinology and metabolism 99(12) (2014) E2744–53. [DOI] [PubMed] [Google Scholar]
- [21].Krjutskov K, Katayama S, Saare M, Vera-Rodriguez M, Lubenets D, Samuel K, Laisk-Podar T, Teder H, Einarsdottir E, Salumets A, Kere J, Single-cell transcriptome analysis of endometrial tissue, Human reproduction (Oxford, England) 31(4) (2016) 844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lockwood CJ, Krikun G, Schatz F, Decidual cell-expressed tissue factor maintains hemostasis in human endometrium, Ann N Y Acad Sci 943 (2001) 77–88. [DOI] [PubMed] [Google Scholar]
- [23].Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC, Global gene profiling in human endometrium during the window of implantation, Endocrinology 143(6) (2002) 2119–38. [DOI] [PubMed] [Google Scholar]
- [24].Pellicer A, Dominguez F, Remohi J, Simón C, Molecular basis of implantation, Reproductive biomedicine online 5 (2002) 44–51. [DOI] [PubMed] [Google Scholar]
- [25].Simon C, Martin JC, Pellicer A, Paracrine regulators of implantation, Bailliere’s best practice & research. Clinical obstetrics & gynaecology 14(5) (2000) 815–26. [DOI] [PubMed] [Google Scholar]
- [26].Salamonsen LA, Nie G, Hannan NJ, Dimitriadis E, Society for Reproductive Biology Founders’ Lecture 2009. Preparing fertile soil: the importance of endometrial receptivity, Reproduction, fertility, and development 21(7) (2009) 923–34. [DOI] [PubMed] [Google Scholar]
- [27].Classen-Linke I, Alfer J, Hey S, Krusche CA, Kusche M, Beier HM, Marker molecules of human endometrial differentiation can be hormonally regulated under in-vitro conditions as in-vivo, Hum Reprod Update 4(5) (1998) 539–49. [DOI] [PubMed] [Google Scholar]
- [28].Berlanga O, Bradshaw HB, Vilella-Mitjana F, Garrido-Gomez T, Simon C, How endometrial secretomics can help in predicting implantation, Placenta 32 Suppl 3 (2011) S271–5. [DOI] [PubMed] [Google Scholar]
- [29].Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ, A novel immortalized human endometrial stromal cell line with normal progestational response, Endocrinology 145(5) (2004) 2291–6. [DOI] [PubMed] [Google Scholar]
- [30].Neradugomma NK, Drafton K, O’Day DR, Liao MZ, Han LW, Glass IA, Mao Q, Marijuana use differentially affects cannabinoid receptor expression in early gestational human endometrium and placenta, Placenta 66 (2018) 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gonzalez M, Neufeld J, Reimann K, Wittmann S, Samalecos A, Wolf A, Bamberger AM, Gellersen B, Expansion of human trophoblastic spheroids is promoted by decidualized endometrial stromal cells and enhanced by heparin-binding epidermal growth factor-like growth factor and interleukin-1 beta, Molecular human reproduction 17(7) (2011) 421–33. [DOI] [PubMed] [Google Scholar]
- [32].Spelke DP, Ortmann D, Khademhosseini A, Ferreira L, Karp JM, Methods for embryoid body formation: the microwell approach, Methods in molecular biology 690 (2011) 151–62. [DOI] [PubMed] [Google Scholar]
- [33].Graham A, Holbert J, Nothnick WB, miR-181b-5p Modulates Cell Migratory Proteins, Tissue Inhibitor of Metalloproteinase 3, and Annexin A2 During In Vitro Decidualization in a Human Endometrial Stromal Cell Line, Reproductive sciences (Thousand Oaks, Calif.) 24(9) (2017) 1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gellersen B, Brosens J, Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair, The Journal of endocrinology 178(3) (2003) 357–72. [DOI] [PubMed] [Google Scholar]
- [35].Andrenyak DM, Moody DE, Slawson MH, O’Leary DS, Haney M, Determination of −9-Tetrahydrocannabinol (THC), 11-hydroxy-THC, 11-nor-9-carboxy-THC and Cannabidiol in Human Plasma using Gas Chromatography-Tandem Mass Spectrometry, Journal of analytical toxicology 41(4) (2017) 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Huestis MA, Henningfield JE, Cone EJ, Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH), Journal of analytical toxicology 16(5) (1992) 283–90. [DOI] [PubMed] [Google Scholar]
- [37].Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, et al. , SR141716A, a potent and selective antagonist of the brain cannabinoid receptor, FEBS letters 350(2–3) (1994) 240–4. [DOI] [PubMed] [Google Scholar]
- [38].Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM, Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals, Human reproduction (Oxford, England) 25(4) (2010) 862–73. [DOI] [PubMed] [Google Scholar]
- [39].Salamonsen LA, Evans J, Nguyen HP, Edgell TA, The Microenvironment of Human Implantation: Determinant of Reproductive Success, American journal of reproductive immunology (New York, N.Y. : 1989) 75(3) (2016) 218–25. [DOI] [PubMed] [Google Scholar]
- [40].Campbell S, Rowe J, Jackson CJ, Gallery ED, In vitro migration of cytotrophoblasts through a decidual endothelial cell monolayer: the role of matrix metalloproteinases, Placenta 24(4) (2003) 306–15. [DOI] [PubMed] [Google Scholar]
- [41].Carver J, Martin K, Spyropoulou I, Barlow D, Sargent I, Mardon H, An in‐vitro model for stromal invasion during implantation of the human blastocyst, Human Reproduction 18(2) (2003) 283–290. [DOI] [PubMed] [Google Scholar]
- [42].Cohen M, Bischof P, Coculture of Decidua and Trophoblast to Study Proliferation and Invasion, in: Vaillancourt C, Lafond J (Eds.), Human Embryogenesis: Methods and Protocols, Humana Press, Totowa, NJ, 2009, pp. 63–72. [DOI] [PubMed] [Google Scholar]
- [43].Liu L, Wang Y, Shen C, He J, Liu X, Ding Y, Gao R, Chen X, Benzo(a)pyrene inhibits migration and invasion of extravillous trophoblast HTR-8/SVneo cells via activation of the ERK and JNK pathway, Journal of applied toxicology : JAT 36(7) (2016) 946–55. [DOI] [PubMed] [Google Scholar]
- [44].Jovanovic M, Stefanoska I, Radojcic L, Vicovac L, Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1, Reproduction (Cambridge, England) 139(4) (2010) 789–98. [DOI] [PubMed] [Google Scholar]
- [45].Hohn HP, Linke M, Denker HW, Adhesion of trophoblast to uterine epithelium as related to the state of trophoblast differentiation: in vitro studies using cell lines, Molecular reproduction and development 57(2) (2000) 135–45. [DOI] [PubMed] [Google Scholar]
- [46].Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Brosens JJ, Macklon NS, Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation, PloS one 5(4) (2010) e10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhu H, Hou CC, Luo LF, Hu YJ, Yang WX, Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions, Gene 551(1) (2014) 1–14. [DOI] [PubMed] [Google Scholar]
- [48].Graham A, Holbert J, Nothnick WB, miR-181b-5p Modulates Cell Migratory Proteins, Tissue Inhibitor of Metalloproteinase 3, and Annexin A2 During In Vitro Decidualization in a Human Endometrial Stromal Cell Line, Reproductive Sciences 24(9) (2016) 1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bland TM, Haining RL, Tracy TS, Callery PS, CYP2C-catalyzed delta9-tetrahydrocannabinol metabolism: kinetics, pharmacogenetics and interaction with phenytoin, Biochemical pharmacology 70(7) (2005) 1096–103. [DOI] [PubMed] [Google Scholar]
- [50].Patilea-Vrana GI, Anoshchenko O, Unadkat JD, Hepatic Enzymes Relevant to the Disposition of (−)-(9)-Tetrahydrocannabinol (THC) and Its Psychoactive Metabolite, 11-OH-THC, Drug metabolism and disposition: the biological fate of chemicals 47(3) (2019) 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sachse-Seeboth C, Pfeil J, Sehrt D, Meineke I, Tzvetkov M, Bruns E, Poser W, Vormfelde SV, Brockmoller J, Interindividual variation in the pharmacokinetics of Delta9-tetrahydrocannabinol as related to genetic polymorphisms in CYP2C9, Clinical pharmacology and therapeutics 85(3) (2009) 273–6. [DOI] [PubMed] [Google Scholar]
- [52].Stott C, White L, Wright S, Wilbraham D, Guy G, A Phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of Rifampicin, Ketoconazole, and Omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers, SpringerPlus 2(1) (2013) 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mazur A, Lichti CF, Prather PL, Zielinska AK, Bratton SM, Gallus-Zawada A, Finel M, Miller GP, Radominska-Pandya A, Moran JH, Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids, Drug metabolism and disposition: the biological fate of chemicals 37(7) (2009) 1496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sarkar MA, Vadlamuri V, Ghosh S, Glover DD, Expression and cyclic variability of CYP3A4 and CYP3A7 isoforms in human endometrium and cervix during the menstrual cycle, Drug metabolism and disposition: the biological fate of chemicals 31(1) (2003) 1–6. [DOI] [PubMed] [Google Scholar]
- [55].Gellersen B, Wolf A, Kruse M, Schwenke M, Bamberger AM, Human endometrial stromal cell-trophoblast interactions: mutual stimulation of chemotactic migration and promigratory roles of cell surface molecules CD82 and CEACAM1, Biology of reproduction 88(3) (2013) 80. [DOI] [PubMed] [Google Scholar]
- [56].Grummer R, Hohn HP, Mareel MM, Denker HW, Adhesion and invasion of three human choriocarcinoma cell lines into human endometrium in a three-dimensional organ culture system, Placenta 15(4) (1994) 411–29. [DOI] [PubMed] [Google Scholar]
- [57].Moghadam KK, Kessler CA, Schroeder JK, Buckley AR, Brar AK, Handwerger S, Cannabinoid receptor I activation markedly inhibits human decidualization, Molecular and cellular endocrinology 229(1–2) (2005) 65–74. [DOI] [PubMed] [Google Scholar]
- [58].Sun X, Deng W, Li Y, Tang S, Leishman E, Bradshaw HB, Dey SK, Sustained Endocannabinoid Signaling Compromises Decidual Function and Promotes Inflammation-induced Preterm Birth, J Biol Chem 291(15) (2016) 8231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chang X, Bian Y, He Q, Yao J, Zhu J, Wu J, Wang K, Duan T, Suppression of STAT3 Signaling by Delta9-Tetrahydrocannabinol (THC) Induces Trophoblast Dysfunction, Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 42(2) (2017) 537–550. [DOI] [PubMed] [Google Scholar]
- [60].Costa MA, Fonseca BM, Keating E, Teixeira NA, Correia-da-Silva G, 2-arachidonoylglycerol effects in cytotrophoblasts: metabolic enzymes expression and apoptosis in BeWo cells, Reproduction (Cambridge, England) 147(3) (2014) 301–11. [DOI] [PubMed] [Google Scholar]
- [61].Khare M, Taylor AH, Konje JC, Bell SC, Delta9-tetrahydrocannabinol inhibits cytotrophoblast cell proliferation and modulates gene transcription, Molecular human reproduction 12(5) (2006) 321–33. [DOI] [PubMed] [Google Scholar]