Abstract
Introduction:
Cellular senescence is a stable form of cell-cycle exit. Though they no longer divide, senescent cells remain metabolically active and secrete a plethora of proteins collectively termed the senescence-associated secretory phenotype (SASP). Although senescence-associated cell-cycle exit likely evolved as an anti-tumor mechanism, the SASP contains both anti- and pro-tumorigenic potential.
Areas covered:
In this review, we briefly discuss the discovery of senescent cells and its relationship to cancer and aging. We also describe the initiation and regulation of the SASP upon senescence stimulus onset. We focus on both the pro- and anti-tumorigenic properties of the SASP. Finally, we speculate on the potential benefits of therapy-induced senescence combined with selective SASP inhibition for the treatment of cancer.
Expert opinion:
Further identification and characterization of the SASP factors that are pro-tumorigenic and those that are anti-tumorigenic in specific contexts will be crucial in order to develop personalized therapeutics for the successful treatment of cancer.
Keywords: cancer, cellular senescence, inflammation, SASP, senescence-associated secretory phenotype, tumorigenesis
1. Introduction
While many advances have been made in the diagnosis, prevention, and treatment of cancer, it remains the second-leading cause of death in the United States [1]. Prognosis continues to be exceptionally bleak in certain cancers, such as pancreatic ductal adenocarcinoma. Encouragingly, cancer mortality as a whole is slowly declining, as new therapeutics stem from our increased understanding of disease behavior [1]. As such, there exists a continual need to probe the mechanisms of tumor initiation and progression, as well as discover new targets that can affect tumor cells or its microenvironment.
Cellular senescence is a form of stable cell cycle exit that was initially hypothesized to serve as a barrier to tumorigenesis, as a cell that does not divide cannot give rise to a tumor. Despite their growth arrest, senescent cells remain metabolically active. In addition, they secrete a defined set of cytokines, proteases, and growth factors, collectively referred to as the senescence-associated secretory phenotype (SASP). The SASP may act to reinforce the cell cycle exit elicited by senescent cells. In direct contrast, however, the pro-inflammatory nature of specific SASP factors can favor tumorigenesis. Because of the functional dichotomy of the SASP, great care must be exercised when manipulating senescence for the treatment of cancer.
In this review, we focus on the double-edged sword that is the SASP in the context of cancer. We highlight recent developments that support both pro- and anti-tumorigenic functions of the SASP. We further explore several therapeutic strategies based on these developments and discuss potential consequences of targeting these specific pathways in cancer.
2. Cellular senescence
2.1. Discovery of senescent cells
Senescent cells were first described in 1961 in a seminal paper published by Hayflick and Moorhead [4]. They demonstrated that primary human embryonic cells do not divide indefinitely in culture, but rather cease proliferating after a defined number of population doublings. This proliferation arrest, termed replicative senescence, was later attributed to the gradual shortening of telomeres that occurs with each cell division [5, 6]. Critically short telomeres trigger a DNA damage response (DDR) involving the ATM and p53 pathway, causing cells to stop dividing [7, 8].
Replicative senescence was hypothesized by some to correspond to an in vitro artifact of cell culture. This view was challenged when senescent cells were identified in specific physiological contexts in vivo. Enlarged and flattened endothelial cells were found in atherosclerotic plaques, a similar morphology to senescent fibroblasts in culture [9, 10]. Since then, the usage of the biomarker senescence-associated beta-galactosidase (SA-βgal) allowed for the identification of senescent cells in other tissues, such as human skin, prostate, and breast [11–13]. Importantly, SA-βgal-positive cells harboring dysfunctional telomeres were discovered in the skin of aging baboons, establishing a clear link between senescence and aging [14].
In addition to telomere shortening, other stresses have been described to induce cellular senescence. Constitutively active drivers of mitogenic signals, such as oncogenic Ras, Raf, or Mek, induce many hallmarks of senescence when expressed in primary cells, including cell cycle arrest, SASP activation, and expression of p16INK4A [15–18]. Termed oncogene-induced senescence (OIS), this form of cell cycle exit shares many features of replicative senescence, such as the activation of a DDR, resulting in this case from uncontrolled hyper-proliferation and subsequent fork collapse [19, 20]. Other agents that initiate DDR, including chemotherapeutic agents, irradiation, and histone deacetylase inhibitors can also induce cellular senescence [21–23]. In addition, stimuli that do not trigger DDR, such as oxidative stress and ectopic expression of cyclin-dependent kinase inhibitors (CDKis), have been reported to induce senescence, although the molecular mechanisms underlying these processes remain unclear [24].
Numerous studies support the role of senescence as a barrier against tumorigenesis. Senescent cells have been found in human pre-neoplastic lesions such as melanocytic nevi, but not in their corresponding malignant tissue [17, 25, 26]. Moreover, inactivating the senescence machinery in mouse models resulted in the acceleration of tumor development, while restoration of senescence in growing tumors caused their regression [27–29]. By contrast, senescence may also be responsible for age-associated disorders. Elimination of p16INK4A-positive senescent cells in a progeroid mouse model delayed onset of aging phenotypes, such as cataracts and muscle and fat loss [30]. Moreover, elimination of p16INK4A-positive senescent cells in aged mice resulted in prolonged healthspan and lifespan [31]. The demonstration that senescence can prevent cancer progression while promoting age-associated diseases is consistent with the notion of antagonistic pleiotropy, whereby senescent cells may be beneficial early in life to prevent cancer, but may be detrimental later in life and contribute to aging [32].
2.2. The senescence-associated secretory phenotype
As cells cease dividing and enter senescence, they secrete a plethora of inflammatory agents, growth factors, and proteases known as the SASP. Also coined the senescence-messaging secretome (SMS) [33], the most robust and universally secreted factors include the cytokines interleukin (IL)-1, IL-6 and IL-8 [2]. Other SASP factors include chemokines such as specific CXCLs, growth factors such as EGF and IGFBP, receptors such as ICAMs, and proteases such as MMPs and PAI-1 [34]. However, the nature of the SASP can also vary depending on cell type and senescence stimulus. For example, senescence induced by oncogenic Ras results in much higher levels of specific SASP factors, including IL-6 and IL-1β, compared to senescence induced by X-irradiation or replicative exhaustion [35]. Conversely, senescence induced by p16INK4A over-expression does not induce a SASP [36]. Finally, transcriptome analysis of senescent cells revealed that tissue origin accounted for the most variation in SASP expression [37].
The components of the senescence secretome can also vary over time following senescence induction. The conventional SASP is not induced immediately after cell cycle exit; rather it is expressed days after stimulus onset [38]. Recently, Hoare and colleagues demonstrated that oncogene-induced senescent cells produce two distinct secretomes temporally regulated by Notch1 activity: an early TGF-β secretome, and a late secretome containing the more conventional inflammatory factors [39]. Other studies showed that the secretome dampens down after the cells become fully senescent, consistent with the idea that senescence is a dynamic process even after the initial growth arrest [40, 41].
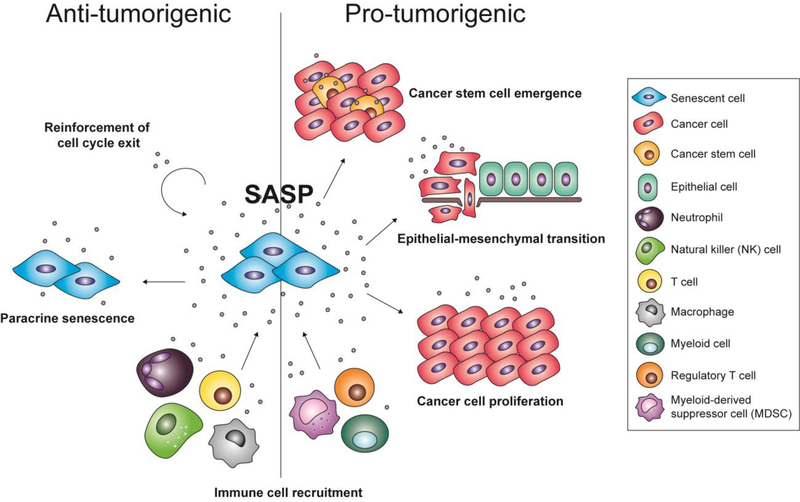
It has also been argued that “priming” of senescent cells constitute another factor that can affect the SASP. Conditioned media from senescent mesenchymal stromal cells (MSCs) was able to trigger apoptosis and senescence in myeloma cells; however, MSCs that came into contact with myeloma cells beforehand exhibited an altered transcriptome, and conditioned media from these cells was unable to affect cancer cell growth [42]. Ultimately, the SASP can alter its microenvironment and has been shown to harbor both pro- and anti-tumorigenic properties depending on cell type and context, which will be described in detail below (Fig. 1).
Figure 1.
The pro- and anti-tumorigenic properties of the senescence-associated secretory phenotype (SASP). The SASP can act to reinforce cell cycle exit in a cell-autonomous manner, induce neighboring cells to exit the cell cycle (termed paracrine senescence), and recruit immune cells that will clear away senescent and potentially cancerous cells. Conversely, the SASP can also promote the proliferation of neoplastic cells, induce epithelial-to-mesenchymal transition in malignant cells, promote the emergence of cancer stem cells, and create an immunosuppressive or inflammatory environment that further drives tumorigenesis.
2.3. Upstream regulators of the SASP
Several transcription factors have been demonstrated to contribute to SASP activation. For instance, NF-κB inhibition or C/EBPβ inhibition results in SASP abrogation [2, 43]. More recent studies revealed a role for the transcription factor GATA4, which activates NF-κB activity and controls SASP expression in response to DDR in human foreskin fibroblasts [44]. Moreover, the kinase p38MAPK was demonstrated to induce the SASP by upregulating NF-κB transcriptional activity in a DDR-independent manner [45]. The JAK/STAT pathway, important for controlling cytokine production, also modulates SASP production, as JAK inhibition suppress the SASP in senescent preadipocytes and human umbilical vein endothelial cells (HUVECs) [46]. Overall, different cell types and modes of senescence induction are likely to contribute to the activation of specific signaling pathways and networks to produce the SASP, which reflects the varied results from different studies.
Studies probing into the mechanism of SASP initiation have shown that the IL-1 pathway functions upstream of NF-κB to elicit SASP production [47]. Indeed, depletion of RelA, the DNA binding subunit of NF-κB, results in the suppression of the GATA4-induced SASP, with the exception of IL-1α [44]. IL-1α is responsible for the secretion of senescence-induced IL-6 and IL-8 [48]. Surprisingly, the related IL-1β cytokine, which engages the same IL-1 receptor, does not share this property with IL-1α. It was then demonstrated that the kinase mTOR also plays a role in SASP induction, by promoting both IL-1α and MAPKAPK2 translation [49, 50]. These factors converge mainly upon the NF-κB-IL-1 signaling axis, suggesting that this pathway serves as the master regulator of the SASP.
Consistent with the notion that the SASP is regulated at the transcriptional level, many epigenetic factors can affect the SASP. Inhibition of the methyltransferase MLL1 prevents the activation of genes involved in DNA damage, thus disabling the SASP [51]. More directly, the chromatin reader BRD4 was found to be recruited to super-enhancers adjacent to SASP genes, and its inhibition can also abolish the SASP [52]. Finally, the histone protein HMGB2 was demonstrated to localize to SASP gene loci in senescent cells, which prevents their heterochromatinization and subsequent transcriptional repression [53].
As aforementioned, Hoare and colleagues demonstrated that Notch1 mediates a switch between an early and late secretome, the latter consisting of the IL-1-dependent SASP. Notch1 levels increased steadily in OIS, but the Notch1 intracellular domain (N1ICD) and its canonical target HES1 returned to basal levels at full senescence. Ectopic N1ICD expression induced a distinct early secretome containing many TGF-β ligands and suppressed the IL-1-dependent SASP. The expression of IL-1-dependent SASP correlated with the return of N1ICD and HES1 to basal levels [39].
While the mechanisms linking NF-κB and IL-1 activation to SASP production have been partially elucidated, how senescence triggers IL-1 engagement in the first place remain largely elusive. However, the recent discovery of DNA fragments outside of the nucleus of senescent cells prompted some to hypothesize that cytoplasmic chromatin fragments (CCF) contribute to the initiation of the SASP [54]. CCFs are recognized by the innate immune system; specifically the cytosolic DNA-sensing machinery cGAS-STING [55]. While some groups have argued that activation of the cGAS-STING pathway is required for senescence initiation, others demonstrated that this pathway only affects the SASP [55–57]. Nevertheless, activation of the cGAS-STING pathway triggers the expression of many inflammatory genes, and has now been described to occur in senescent cells [55, 57]. Whether cGAS-STING drives SASP production through engagement of the IL-1-NF-κB pathway remains to be investigated.
3. Anti-tumorigenic functions of the SASP
3.1. Reinforcement of cell-cycle exit
Early studies argued that expression of specific SASP factors was required for stable cell-cycle arrest. Specifically, inactivation of individual SASP components or upstream regulators of the SASP was shown to alter senescence-associated cell cycle exit. For example, knockdown of plasminogen activator-inhibitor 1 (PAI-1) allowed late passage mouse embryonic fibroblasts and human BJ fibroblasts to sustain proliferation over time, thus bypassing replicative senescence. Conversely, PAI-1 over-expression induced cell cycle exit even in the absence of p53 [58]. Depletion of the SASP factor IL-6 resulted in a bypass of oncogene-induced cell cycle arrest, indicating that IL-6 is required in a cell-autonomous manner for OIS [2]. In another study, many SASP factors were found to bind to the chemokine receptor CXCR2, including Gro-α, Gro-β, and IL-8 [59]. Inhibition of CXCR2 was sufficient to bypass both replicative and OIS, while its ectopic expression induced premature senescence. Furthermore, depletion of C/EBPβ caused human diploid fibroblasts (HDF) to escape oncogenic Braf-induced OIS [2]. In addition, NF-κB was also demonstrated to cooperate with p53 to promote senescence, as knockdown of both factors resulted in senescence bypass in oncogenic Ras-induced IMR90 human fibroblasts [43]. Moreover, as mentioned previously, the cGAS-STING pathway can activate the inflammatory SASP, and disrupting this pathway can result in SASP abrogation, and with it, cell cycle arrest [56, 57].
The SASP may also reinforce senescence-associated cell cycle exit in vivo. Mice lacking IL-6 harbored decreased SA-βal-positive vertebrae bone cells after injection of carcinogenic doses of a calcium radioisotope (45Ca) compared to wild-type mice [60]. In another model, oncogenic K-Ras expression in the pancreas leads to the formation of low-grade lesions harboring senescent acinar cells [61]. Inactivation of RelA in the pancreas resulted in the abrogation of K-Ras-induced senescence and accelerated carcinogenesis [62]. The authors further showed that, in this model, the SASP factor CXCL1 promotes senescence-associated cell cycle exit through activation of the CXCR2 receptor.
How the SASP promotes cell cycle exit remains completely unknown. One hypothesis is that the SASP serves to reinforce the irreversibility of senescence-associated cell cycle exit, rather than initiating it. This possibility remains to be investigated, but the emergence of novel resources to trigger senescence in an inducible and reversible manner should allow such studies. It is important to note, however, that several studies have shown that targeting the SASP fails to impair senescence-associated cell cycle arrest. In human foreskin fibroblasts, inhibition of mTOR activity with rapamycin treatment suppresses the SASP, but does not affect the ability to exit the cell cycle following ionizing radiation [49]. Similarly, knockdown of HMGB2 in IMR90 fibroblasts did not affect Ras-induced cell cycle exit, even though it blunted SASP expression [53]. Inactivation of cGAS or STING in IMR90 fibroblasts failed to revert Ras- or etoposide-induced senescence phenotypes such as SA-βgal positivity and p16INK4a induction [55]. These discrepancies may be due to nuances involving different cell types and different strategies of senescence induction in each study. For example, other groups that demonstrated the requirement for the cGAS-STING pathway in cell cycle exit performed their experiments in mouse embryonic fibroblasts that senesced via serial passaging [56, 57].
3.2. Induction of paracrine senescence
Senescent cells have been hypothesized to induce cell cycle arrest in neighboring cells, allowing the propagation of the anti-tumorigenic response, and therefore ensuring that cells residing in a detrimental environment cease division. Senescent cells achieve this effect through secreted proteins, as conditioned media derived from senescent human fibroblasts was sufficient to induce senescence in normal growing fibroblasts [47]. Moreover, it was argued that paracrine senescence occurs in vivo, as fibroblasts and immune cells neighboring oncogenic Braf-positive senescent colon adenomas were also positive for the cell-cycle exit marker p21CIP1 and negative for the proliferation marker Ki67 [47]. Furthermore, paracrine senescence was detected in a mouse model involving cholangiocyte-specific deletion of Mdm2, resulting in biliary senescence. In this model, a subset of hepatocytes was also shown to express the senescence markers 53BP1, γH2A.X, and Dcr2 while retaining Mdm2 expression, suggesting that senescent cholangiocytes can induce neighboring hepatocytes to senesce [63]. This study further demonstrates that the SASP factors IL-1α and TGF-β, as well as NF-κB, are expressed at high levels by cholangiocytes, leading the authors to postulate that the SASP drives paracrine senescence in this context. However, the evidence for paracrine senescence in vivo remains largely correlative at this point, mainly for technical reasons.
3.3. Recruitment of immune cells
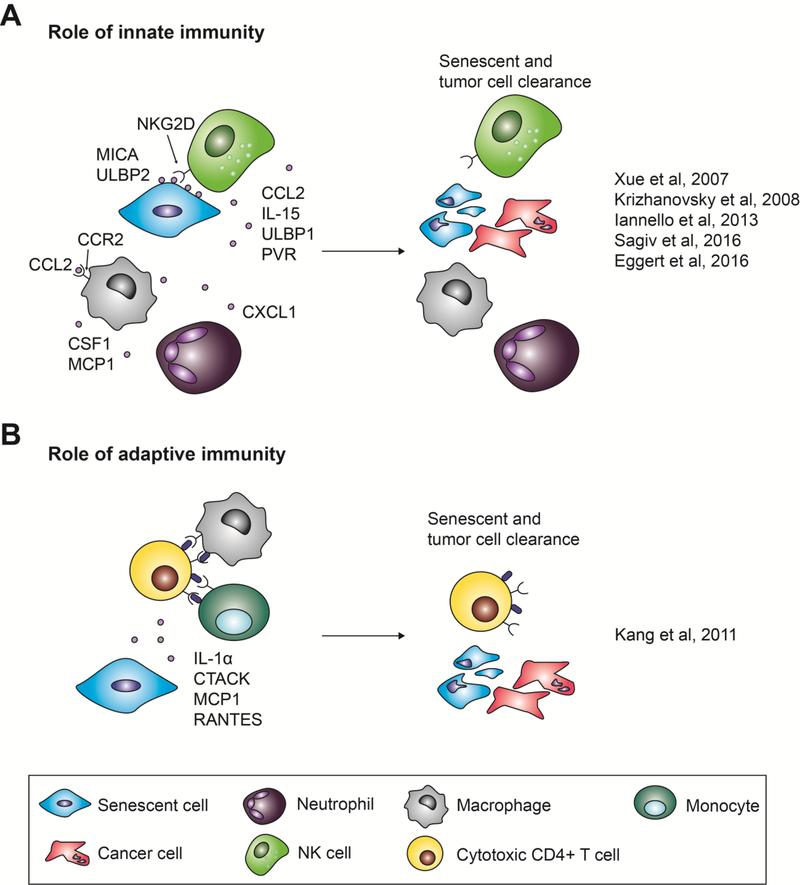
In addition to its ability to promote cell cycle exit in neighboring cells, the SASP has been postulated to recruit immune cells to the site of tissue injury. Once recruited to the site of SASP production, immune cells are able to clear away senescent and potentially pre-cancerous cells, providing yet another mechanism to prevent tumorigenesis. Among the different in vivo evidence for this immune cell recruitment, Krizhanovsky and colleagues demonstrated that, in a mouse model of carbon tetrachloride-induced liver fibrosis, senescence is induced in activated hepatic stellate cells [64]. Abrogating the cells’ ability to senesce by genetically inactivating both p53 and p16INK4A delayed liver fibrosis resolution due to the absence of immune cell-mediated clearance. Interestingly, natural killer (NK) cell receptor (NGK2D) ligands and other NK cell chemokines (i.e. CCL2) were upregulated in both senescent human fibroblasts and hepatic stellate cells [64–66]. Moreover, p53 re-activation in a mouse model of liver carcinoma resulted in tumor regression, correlating with the secretion of NK-cell chemoattractants by senescent tumor cells and their subsequent removal. Finally, antibody neutralization or genetic ablation of NK cells delayed tumor regression after p53 reactivation in the tumors [29, 65]. Together, these studies reveal a role for NK cells in the clearance of senescent cells (Fig. 2A). In addition, inhibition of neutrophils also delayed tumor regression following p53 activation, suggesting that these cells play a role in senescent cell clearance as well (Fig. 2A) [29]. However, re-activation of p53 in Rag2-deleted mice, which lack B and T cells, resulted in tumor regression comparable to wild-type mice, suggesting that the adaptive immunity is dispensable when clearing senescent tumor cells [29, 65].
Figure 2.
Senescence-associated secretory phenotype (SASP)-mediated immune cell recruitment aiding in tumor clearance. Both the innate and adaptive immune systems have been described to aid in SASP-mediated tumor clearance. (A) Various SASP factors recruit natural killer (NK) cells, macrophages, and neutrophils to the site of tissue damage, resulting in the clearance of both senescent and cancerous cells. (B) Various SASP factors recruit macrophages and monocytes, as well as cytotoxic CD4+ T cells. Macrophages and monocytes can activate these T cells, which then kill senescent and cancer cells.
In contrast, other studies have pointed to a contribution of adaptive immunity in the clearance of senescent cells. SASP-mediated recruitment of helper T cells was postulated to limit liver cancer development in a different mouse model of hepatocellular carcinoma, where hydrodynamic injection of oncogenic N-Ras resulted in senescence induction in hepatocytes [67]. These senescent hepatocytes secreted the SASP factors IL-1α, MCP1, and RANTES, and were eventually cleared from the liver. Strikingly, in mice lacking CD4+ T cells, N-Ras-positive cells remained present and formed liver tumors (Fig. 2B). The discrepancy regarding immune cell requirement for senescent cell clearance in these two models is likely explained by the different genetic tools used to induce liver cancer. These differences also suggest that additional studies will be required to extrapolate any conclusions to human cancers.
To further complicate the matter, macrophages have also been implicated in senescence surveillance. Using the same system of hydrodynamic delivery of N-Ras to induce senescence in hepatocytes, Eggert and colleagues demonstrated that CCL2 secreted by senescent hepatocytes led to the recruitment of immature myeloid cells and macrophages (Fig. 2A) [68]. Genetic inactivation of the CCL2 receptor (CCR2) in senescent cells resulted in reduced macrophage infiltration and increased HCC development in mice. This evidence suggests that SASP-induced recruitment of macrophages also play a role in limiting tumorigenesis.
4. Pro-tumorigenic functions of the SASP
4.1. Enhancement of cancer cell proliferation
One of the earliest described functions of the SASP was its ability to directly promote cancer cell proliferation, contrasting with the original proposed anti-tumorigenic role of senescent cells. Indeed, Krtolica and colleagues observed that conditioned media derived from senescent human fibroblasts was able to stimulate the proliferation of pre-neoplastic, but not normal, epithelial cells [69]. This effect was confirmed for multiple forms of senescence induction, including replicative, oncogene-induced, and H2O2 treatment-induced senescence. Moreover, senescent fibroblasts, but not normal fibroblasts, were able to stimulate tumorigenesis when co-injected with pre-neoplastic epithelial cells in immunocompromised mice. More specifically, IL-6 secretion from senescent human umbilical cord mesenchymal stem cells enhanced proliferation of breast cancer cells in vitro and in xenograft transplants [70]. Similarly, matrix metalloproteases secreted from bleomycin-induced senescent human fibroblasts increased the proliferative capacity of MDA-MB-231 breast cancer cells in nude mice xenograft transplants [71]. Therefore, while the SASP appears to induce senescence in normal benign cells, it can promote proliferation in pre-neoplastic cells. This dichotomy of the SASP is interestingly reminiscent of the effect of oncogenic Ras, whose activation induces senescence in primary cells, but promotes transformation in pre-neoplastic cells [15].
4.2. Induction of epithelial-mesenchymal transition
Other studies reveal that the SASP can enhance tumorigenic properties, like the epithelial-to-mesenchymal transition (EMT). Conditioned media from replicative or irradiated senescent fibroblasts enhanced invasion of non-aggressive breast cancer cells [35]. These cells exhibited increased expression of vimentin and decreased expression of β-catenin and E-cadherin, hallmarks of EMT. Breast cancer cells exposed to the SASP also exhibited epithelial cell scattering and reduced cell-cell adhesions, another mesenchymal phenotype [35]. These EMT phenotypes may be mediated by IL-6 and IL-8, as exposure to recombinant IL-6 or IL-8 promoted migration and invasion in MCF7 breast cancer cells [72]. In addition, the SASP factors MMP-1 and MMP-2 secreted by senescent human dermal fibroblasts enhanced the migratory ability of human epidermal keratinocytes [73]. The SASP may even enhance tumorigenic properties of cells that have either failed to senesce or have escaped from senescence. For example, treating malignant pleural mesothelioma cells (MPM) with the chemotherapeutic drug pemetrexed induced only a subset of cells to senesce. Culturing the MPM cells that failed to senesce with conditioned media from senescent MPM cells resulted in the emergence of an elongated fibroblast-like morphology and increased levels of vimentin and fibronectin, all features of EMT [74].
4.3. Induction of cancer cell stemness
In addition to stimulating EMT, the SASP has been suggested to promote stemness in both cancer and non-cancer settings. For example, multiple myeloma (MM) cells exposed to senescence-inducing irradiation or doxorubicin exhibited decreased CD138 levels and increased CD45 and CD20 levels, considered stemness markers in MM [75]. This increase in stemness markers was correlated with the upregulation of specific components of the SASP, particularly RANTES. Consistent with increased stemness potential, injection of MM cells following irradiation resulted in increased tumorigenesis. Whether these effects are directly related to SASP or correspond to a senescence-independent by-product of irradiation remains to be investigated.
Recent studies underlined a more direct contribution of the SASP in the promotion of cancer cell stemness. Indeed, MCF7 breast cancer cells treated with conditioned medium from senescent fibroblasts acquired stem cell surface markers and were able to form mammospheres, a hallmark of stemness in breast cancer cells [72]. Recombinant IL-6 and IL-8 exposure also induced mammosphere formation, suggesting that these cytokines can also mediate senescence-induced cancer stemness. Similarly, explanted cells from tumors that arise from the co-injection of human embryonic kidney (HEK) cells and senescent fibroblasts were able to form spheres to a greater extent when compared to parental HEK cells that had not been injected [76]. These explanted cells were also able to differentiate into chondrocytes, osteocytes, and adipocytes more efficiently than parental cells. Finally, sphere formation was promoted by culturing HEK cells with conditioned media derived from senescent cells, a direct demonstration that the SASP drives stem cell emergence in cancer cell populations.
4.4. Inflammation and immunosuppression
It is well known that the tumor microenvironment in many different cancers largely consists of immune cells, which can affect the proliferation, migration, and survival of malignant cells [77]. As discussed above, in addition to affecting cancer cells directly, the SASP modulates immune cell recruitment (see section 3.3). These immune cells can be immunosuppressive, thereby preventing immune clearance of tumor cells. Alternatively, SASP-mediated recruitment of immune cells can create an inflammatory environment that further drives tumorigenesis. For example, in a mouse model of pancreatic cancer, a well characterized site of tumorigenesis fueled by inflammation, abrogating the senescence machinery by genetically inactivating the chromatin scaffolding protein Sin3B resulted in the delayed development of neoplastic lesions. In this model, the delay in cancer progression correlated with decreased expression of specific SASP markers, including IL-1 and IL-6 [3].
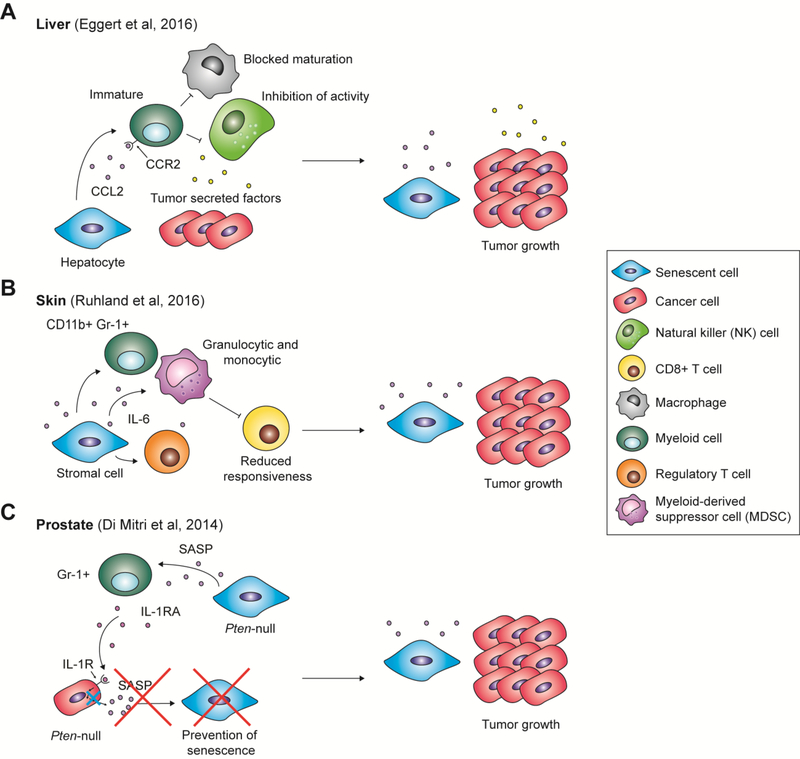
As mentioned in section 3.3, the SASP factor CCL2 recruited macrophages to N-Ras induced senescent hepatocytes, resulting in their clearance and the prevention of liver tumorigenesis. However, hydrodynamic injection of N-Ras in the liver prior to transplantation of transformed hepatocytes significantly accelerated HCC development [68]. This effect is attributed to CCL2-mediated recruitment of immature myeloid cells in addition to macrophages, which in turn inhibit NK cell activity (Fig. 3A). This study therefore implies that while the SASP suppresses tumor initiation in a mouse model of HCC, it promotes cancer progression after HCC is fully established.
Figure 3.
Senescence-associated secretory phenotype (SASP)-mediated immune cell recruitment that favors tumor progression. The SASP has been described in various contexts to create an immunosuppressive environment that drives tumor progression. (A) In the liver, the SASP factor CCL2 acts to recruit immature myeloid cells. However, combined with tumor-secreted factors, CCL2 drives myeloid cells to block the maturation of macrophages and inhibit natural killer (NK) cell activity, resulting in uninhibited tumor growth. (B) In skin, the SASP factor IL-6 recruits Gr-1+ myeloid cells, regulatory T cells, and granulocytic and monocytic myeloid-derived suppressor cells (MDSCs), which reduce CD8+ T cell responsiveness. This immunosuppressive environment allows tumor cell proliferation. (C) In the prostate, the SASP recruits Gr-1+ myeloid cells, which secrete IL-1 receptor antagonist (IL-1RA). IL-1RA binds IL-1R, blocking IL-1-mediated SASP production necessary for the induction of paracrine senescence, thus allowing tumor growth.
Moreover, senescent skin stroma has been shown to promote tumorigenesis through the recruitment of immunosuppressive myeloid cells by IL-6 [78]. In this study, senescence and SASP expression were achieved in the skin of mice via p27Kip1 activation specifically in cells of mesenchymal origin. CD11b+ Gr-1+ myeloid cells and regulatory T cells were found enriched in mouse skin containing senescent cells compared to skin with no senescent cells (Fig. 3B). Consistently, co-injection of tumor cells with senescent fibroblasts into mice resulted in increased tumor growth compared to injection with growing fibroblasts. Surprisingly, this difference was abrogated when cells were injected into nude mice, suggesting that immune cells drive tumorigenesis acceleration in this context.
In another example, Di Mitri and colleagues utilized a mouse model where genetic inactivation of the tumor suppressor Pten in the prostate resulted in the formation of benign and senescent lesions that progressed to aggressive tumors after a long latency. In this model, expression of the SASP correlated with the recruitment of Gr-1+ myeloid cells, which in turn prevented senescence by secreting IL-1 receptor antagonist (IL-1RA) and interfering with IL-1 signaling in a subset of prostate cells (Fig. 3C) [79]. The authors hypothesized that abrogation of senescence in these specific cells made them capable of initiating tumorigenesis. In conclusion, the SASP can affect the tumor microenvironment in a multitude of ways, and assessing the therapeutic window offered by SASP targeting will be crucial in developing cancer treatments aimed at modulating the senescence program.
5. Therapeutic strategies
5.1. Induction of senescence in cancer cells paired with specific targeting of the SASP
One of the many proposed strategies to combat cancer is therapy-induced senescence, whereby chemotherapeutics are used to induce senescence in cancer cells and therefore limit their proliferation. As discussed in section 3.3, restoration of p53 in cancerous liver cells induced senescence and tumor regression through SASP-mediated immune cell clearance [29]. However, therapy-induced senescence could be detrimental as well, due to the pro-tumorigenic effects of the SASP as outlined in section 4. Therefore, pairing therapy-induced senescence with SASP abrogation may yield more effective results. In particular, in Pten-deficient mouse prostate tumors, induction of senescence via docetaxel paired with a CXCR2 antagonist, which blocks the recruitment of Gr-1+ myeloid cells, resulted in increased senescence coupled with a marked reduction of tumor cell size [79]. Additionally, inhibition of the JAK/STAT pathway in a mouse model of prostate cancer reduced the levels of immunosuppressive chemokines in the SASP, while maintaining high levels of chemokines that attract B, T and NK cells [80]. Moreover, induction of senescence with docetaxel combined with inhibition of the JAK/STAT pathway resulted in a marked reduction in prostate tumor size.
Transcription factors and global regulators have been found to control the SASP without affecting cell-cycle exit, such as p38/MAPK, NF-κB, and mTOR [35, 45, 49, 50]. Inhibition of these factors has been shown to reduce SASP expression. However, targeting such ubiquitous factors may affect many other physiological processes. Therefore, therapeutics that target regulators directly upstream of the SASP may be more beneficial. As IL-1α has been demonstrated to be an upstream regulator of the inflammatory SASP, drugs that target the IL-1 receptor, such as anakinra, may prove to be a more useful therapeutic [48]. More studies will need to be conducted to find additional suitable targets in the context of cancer.
5.2. Ablation of senescent cells
While therapy-induced senescence may have promising anti-tumorigenic effects, accumulation of senescent cells may favor the emergence of age-associated diseases, including, paradoxically, cancer [30]. Therefore, therapies that eliminate senescent cells may represent an additional and promising strategy to mitigate cancer progression. Harnessing the immune system could be one approach to eliminate senescent cells, as outlined in section 5.1. Other studies have used biological approaches to ablate senescent cells, but their application in the clinic currently remains improbable [30, 81].
A promising and more realistic approach may be the use of chemicals that specifically kill senescent cells, termed senolytics. The first senolytics were apoptosis-inducing drugs, as senescent cells were known to be resistant to apoptosis [82]. The Bcl-2 inhibitor navitoclax (ABT-263) was demonstrated to selectively target senescent IMR90 fibroblasts and HUVECs, as well as senescent hematopoietic stem cells (HSCs), resulting in the rejuvenation of the HSC population in aged mice [83, 84]. The combination of the tyrosine kinase inhibitor dasatinib and the flavonoid quercetin, both apoptosis-inducing agents, reduced senescent cell abundance in human adipose tissue and extended both healthspan and lifespan in mice [85]. More recent senolytics include drug delivery systems that selectively target senescent cells, such as GalNP [86]. These are sugar-coated beads that are efficiently endocytosed and eventually exocytosed. However, β-galactosidase, present at high levels in senescent cells, can digest the sugar coating of the beads, resulting in the release of the encapsulated drug. With these systems, more potent and ubiquitous cytotoxic drugs can be used to selectively eliminate senescent cells.
In addition to treating age-associated disorders, senolytics may be exploited as an adjuvant for cancer therapy, whereby malignant cells are first induced to senesce, and then are subsequently eliminated. For example, Wang and colleagues demonstrated that aurora kinase inhibitors induce senescence in cultured melanoma cells, while treatment with ABT-263 subsequently induced their apoptosis [87]. The CDK4/6 inhibitor palbociclib has been shown to induce tumor cell senescence in multiple different tumor types, such as melanoma, HCC, and glioblastoma [88–90]. Therapy-induced senescence may result in immune cell clearance of these tumors, or may be paired with senolytics to effectively ablate senescent cancer cells. Extensive research is currently being conducted to apply our understanding of senescence to translational approaches, and the development of senolytics in conjunction with therapy-induced senescence may have vast implications in cancer therapy.
6. Conclusion
In this review, we discussed the regulation of the senescence-associated secretory phenotype, as well as its pros and cons in the context of cancer. Due to the dual nature of the SASP, there may be detrimental effects of either inducing or ablating senescent cells as cancer therapy. While abrogation of senescent cells may reverse aging phenotypes, it could also give full reign for existing tumor cells to divide without inhibition. Conversely, induction of senescence in cancer cells will prevent them from dividing, but the accompanying SASP may induce non-senescent cancer cells to divide, or prevent the immune system from detecting and killing cancer cells. Designing apt therapies will likely depend on cell and tissue context and therefore more studies will need to be completed to address the complexity of senescence.
7. Expert opinion
Senescent cells were originally hypothesized to halt tumor formation, as stable growth arrest will prevent damaged cells from giving rise to tumors. We now know that senescence results in an array of phenotypes; some beneficial and some detrimental to human health. Among these phenotypes, the SASP itself is heterogeneous in nature and in function. The SASP can drive tumor progression in different ways, including in a non-cell autonomous manner. These seemingly opposite functions of senescence as it relates to tumor progression create challenges in devising therapeutics that modulate senescence, as targeting its pro-tumorigenic properties may also abolish anti-tumorigenic properties as well. Therefore, therapeutics should be developed to target a specific feature of senescent cells. Since the SASP has been shown to harbor both pro- and anti-tumorigenic function, further research should identify which SASP factors exhibit pro-tumorigenic properties and which ones possess anti-tumorigenic properties. This information can aid in the development of therapeutics that only inhibit the pro-tumorigenic portion of the SASP.
Additionally, a context-specific effect of the SASP on cancer progression justifies a more thorough analysis of its effect in a given tumor type. Indeed, our group has demonstrated using mouse models that disabling senescence delays tumorigenesis in the pancreas, but enhances tumor progression in the prostate [3, 91]. The bases for these seemingly opposite effects of senescence include the SASP-mediated recruitment of immune cells. These immune cells can either eliminate potentially dangerous senescent cells, or create an immunosuppressive environment that promotes tumor progression, as described in detail in sections 3.3 and 4.4. A deeper and more systematic knowledge of the composition of the SASP under different senescence inducers and in different tissues is much needed to design strategies to modulate its impact in specific cancers.
A promising therapeutic strategy would be to induce senescence in cancer cells in conjunction with the abrogation of the SASP without affecting cell cycle exit. While inhibition of global factors such as NF-κB and mTOR impairs SASP production, it is also likely to affect other physiological and beneficial functions in the body. Inhibition of a more specific regulator of the SASP, such as the IL-1 pathway, could prove to be a potent and narrow therapeutic approach. As IL-1 inhibitors already exist in the clinic, they may be repurposed to blunt the SASP in inflammation-driven cancers. Ideally, abrogation of the pro-tumorigenic SASP will leave a secretome that can still attract cytotoxic immune cells to clear away senescent cancer cells. In the scenario where immune cells and clearance of senescent cells are absent, introduction of senolytics may become useful. As studies have demonstrated that elimination of senescent cells may reverse aging phenotypes, this may have the double benefit of treating cancer while extending human healthspan.
In conclusion, we believe that an improved understanding of context-dependent functions of the SASP and regulators specific to the SASP will enable the generation of novel therapeutic strategies for the treatment of cancer. Specifically, we propose that induction of senescence coupled with SASP inhibition may be useful for targeting growing tumor cells without creating an immunosuppressive environment. Strategies that inhibit the SASP selectively such that cytotoxic immune cell activity is maintained could be a promising treatment in specific cancers. Moreover, developing therapeutics that will specifically target the SASP without affecting global cell function will be a difficult but necessary endeavor. Moving forward, studies should focus on how to better target specific portions of the SASP so that the anti-tumorigenic aspect can be specifically and therapeutically enhanced.
Article highlights box.
Senescent cells are hypothesized to act as a barrier to tumorigenesis, but may contribute to aging and therefore be detrimental later in life, a concept termed antagonistic pleiotropy.
Senescent cells produce a SASP that is likely regulated by the IL-1-NF-κB signaling axis.
Anti-tumorigenic properties of the SASP include induction of senescence and recruitment of immune cells.
The SASP can act to promote tumorigenesis by enhancing cancer cell properties and by creating an immunosuppressive environment that favors cancer cell growth.
Abrogation of senescence disrupts the SASP but may allow uninhibited tumor proliferation, while therapy-induced senescence may induce a SASP that is conducive to tumorigenesis.
Therapy-induced senescence combined with selective SASP inhibition represents a promising strategy for the treatment of cancer; more studies must be completed to elucidate the context-specific impact of the SASP on tumor promotion or regression.
Acknowledgments
Funding
This work was funded by the National Institute of Health (R01CA148639 and R21CA155736).
Footnotes
Declaration of Interests
G David has received support from The Samuel Waxman Cancer Research Foundation and a Feinberg NYU individual grant. L. Lau was supported by a predoctoral NIH/NCI NRSA (F31 CA206387). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: Cancer J Clin 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2**.Kuilman T, Michaloglou C, Vredeveld LC, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008;133(6):1019–31. This is one of the first articles describing SASP composition and function. [DOI] [PubMed] [Google Scholar]
- 3.Rielland M, Cantor DJ, Graveline R, et al. Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression. J Clin Invest 2014;124(5):2125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayflick L, and Moorhead PS The serial cultivation of human diploid cell strains. Exp Cell Res 1961;25:585–621. [DOI] [PubMed] [Google Scholar]
- 5.Lundblad V, Szostak JW. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 1989;57(4):633–43. [DOI] [PubMed] [Google Scholar]
- 6.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990;345(6274):458–60. [DOI] [PubMed] [Google Scholar]
- 7.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003;426(6963):194–8. [DOI] [PubMed] [Google Scholar]
- 8.Herbig U, Jobling WA, Chen BP, et al. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 2004;14(4):501–13. [DOI] [PubMed] [Google Scholar]
- 9.Rosen EM, Mueller SN, Noveral JP, et al. Proliferative characteristics of clonal endothelial cell strains. J Cell Physiol 1981;107(1):123–37. [DOI] [PubMed] [Google Scholar]
- 10.Burrig KF. The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler Thromb 1991;11(6):1678–89. [PubMed] [Google Scholar]
- 11.Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 1995;92(20):9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi J, Shendrik I, Peacocke M, et al. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology 2000;56(1):160–6. [DOI] [PubMed] [Google Scholar]
- 13.te Poele RH, Okorokov AL, Jardine L, et al. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 2002;62(6):1876–83. [PubMed] [Google Scholar]
- 14*.Herbig U, Ferreira M, Condel L, et al. Cellular senescence in aging primates. Science 2006;311(5765):1257 This article links the presence of senescent cells to aging. [DOI] [PubMed] [Google Scholar]
- 15**.Serrano M, Lin AW, McCurrach ME, et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997;88(5):593–602. This article shows that constitutively active oncogenes serves as another method to induce cellular senescence. [DOI] [PubMed] [Google Scholar]
- 16.Lin AW, Barradas M, Stone JC, et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev 1998;12(19):3008–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michaloglou C, Vredeveld LC, Soengas MS, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005;436(7051):720–4. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Woods D, McMahon M, et al. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev 1998;12(19):2997–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartkova J, Rezaei N, Liontos M, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 2006;444(7119):633–7. [DOI] [PubMed] [Google Scholar]
- 20*.Di Micco R, Fumagalli M, Cicalese A, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 2006;444(7119):638–42. This article demonstrates that DNA damage triggers the SASP. [DOI] [PubMed] [Google Scholar]
- 21.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007;8(9):729–40. [DOI] [PubMed] [Google Scholar]
- 22.Di Leonardo A, Linke SP, Clarkin K, et al. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev 1994;8(21):2540–51. [DOI] [PubMed] [Google Scholar]
- 23.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003;421(6922):499–506. [DOI] [PubMed] [Google Scholar]
- 24.McConnell BB, Starborg M, Brookes S, et al. Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol 1998;8(6):351–4. [DOI] [PubMed] [Google Scholar]
- 25.Collado M, Gil J, Efeyan A, et al. Tumour biology: senescence in premalignant tumours. Nature 2005;436(7051):642. [DOI] [PubMed] [Google Scholar]
- 26.Braig M, Lee S, Loddenkemper C, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 2005;436(7051):660–5. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005;436(7051):725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lujambio A, Akkari L, Simon J, et al. Non-cell-autonomous tumor suppression by p53. Cell 2013;153(2):449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007;445(7128):656–60. This article demonstrates p53 restoration in tumors can induce their senescence and result in tumor regression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011;479(7372):232–6. This paper provides the first evidence that abrogating senescent cells can delay aging phenotypes, suggesting that senescence is a causal effect of aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016;530(7589):184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campisi J Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005;120(4):513–22. [DOI] [PubMed] [Google Scholar]
- 33.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 2009;9(2):81–94. [DOI] [PubMed] [Google Scholar]
- 34.Coppe JP, Desprez PY, Krtolica A, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008;6(12):2853–68. This article describes the components that make up the SASP and demonstrates that the SASP can induce EMT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coppe JP, Rodier F, Patil CK, et al. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem 2011;286(42):36396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernandez-Segura A, de Jong TV, Melov S, et al. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr Biol 2017;27(17):2652–60 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodier F, Coppe JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 2009;11(8):973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoare M, Ito Y, Kang TW, et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol 2016;18(9):979–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhaumik D, Scott GK, Schokrpur S, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging 2009;1(4):402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Studencka M, Schaber J. Senoptosis: non-lethal DNA cleavage as a route to deep senescence. Oncotarget 2017;8(19):30656–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozcan S, Alessio N, Acar MB, et al. Myeloma cells can corrupt senescent mesenchymal stromal cells and impair their anti-tumor activity. Oncotarget 2015;6(37):39482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chien Y, Scuoppo C, Wang X, et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev 2011;25(20):2125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang C, Xu Q, Martin TD, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 2015;349(6255):aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO 2011;30(8):1536–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu M, Tchkonia T, Ding H, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA 2015;112(46):E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Acosta JC, Banito A, Wuestefeld T, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 2013;15(8):978–90. This article shows that the SASP can induce paracrine senescence and is controlled by the IL-1 signaling pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Orjalo AV, Bhaumik D, Gengler BK, et al. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci 2009;106(40):17031–6. This paper demonstrates that IL-1α, but not IL-1β, may act as an upstream regulator of the SASP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laberge RM, Sun Y, Orjalo AV, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 2015;17(8):1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herranz N, Gallage S, Mellone M, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat cell Biol 2015;17(9):1205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capell BC, Drake AM, Zhu J, et al. MLL1 is essential for the senescence-associated secretory phenotype. Genes Dev 2016;30(3):321–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tasdemir N, Banito A, Roe JS, et al. BRD4 Connects Enhancer Remodeling to Senescence Immune Surveillance. Cancer Discov 2016;6(6):612–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aird KM, Iwasaki O, Kossenkov AV, et al. HMGB2 orchestrates the chromatin landscape of senescence-associated secretory phenotype gene loci. J Cell Biol 2016;215(3):325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivanov A, Pawlikowski J, Manoharan I, et al. Lysosome-mediated processing of chromatin in senescence. J Cell Biol 2013;202(1):129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Dou Z, Ghosh K, Vizioli MG, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 2017;550(7676):402–06. This article links the presence of CCFs to the induction of the SASP through the cGAS-STING pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang H, Wang H, Ren J, et al. cGAS is essential for cellular senescence. Proc Natl Acad Sci USA 2017;114(23):E4612–E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gluck S, Guey B, Gulen MF, et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol 2017;19(9):1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 2006;8(8):877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Acosta JC, O’Loghlen A, Banito A, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008;133(6):1006–18. [DOI] [PubMed] [Google Scholar]
- 60.Kansara M, Leong HS, Lin DM, et al. Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J Clin Invest 2013;123(12):5351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4(6):437–50. [DOI] [PubMed] [Google Scholar]
- 62.Lesina M, Wormann SM, Morton J, et al. RelA regulates CXCL1/CXCR2-dependent oncogene-induced senescence in murine Kras-driven pancreatic carcinogenesis. J Clin Invest 2016;126(8):2919–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreira-Gonzalez S, Lu WY, Raven A, et al. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat Comm 2018;9(1):1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64**.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell 2008;134(4):657–67. This article demonstrates that senescent cells can recruit immune cells to the site of tissue injury, thereby limiting fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iannello A, Thompson TW, Ardolino M, et al. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 2013;210(10):2057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sagiv A, Burton DG, Moshayev Z, et al. NKG2D ligands mediate immunosurveillance of senescent cells. Aging 2016;8(2):328–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67**.Kang TW, Yevsa T, Woller N, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011;479(7374):547–51. This article also demonstrates that senescent cells can recruit immune cells to potentially cancerous cells in order to facilitate their clearance. [DOI] [PubMed] [Google Scholar]
- 68*.Eggert T, Wolter K, Ji J, et al. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell 2016;30(4):533–47. This article nicely highlights both the pro- and anti-tumorigenic effect of the SASP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Krtolica A, Parrinello S, Lockett S, et al. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA 2001;98(21):12072–7. This article provides the first evidence that the SASP can enhance tumor proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di GH, Liu Y, Lu Y, et al. IL-6 secreted from senescent mesenchymal stem cells promotes proliferation and migration of breast cancer cells. PloS One 2014;9(11):e113572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res 2007;67(7):3117–26. [DOI] [PubMed] [Google Scholar]
- 72.Ortiz-Montero P, Londono-Vallejo A, Vernot JP. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal 2017;15(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malaquin N, Vercamer C, Bouali F, et al. Senescent fibroblasts enhance early skin carcinogenic events via a paracrine MMP-PAR-1 axis. PloS One 2013;8(5):e63607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Canino C, Mori F, Cambria A, et al. SASP mediates chemoresistance and tumor-initiating-activity of mesothelioma cells. Oncogene 2012;31(26):3148–63. [DOI] [PubMed] [Google Scholar]
- 75.Cahu J, Bustany S, Sola B. Senescence-associated secretory phenotype favors the emergence of cancer stem-like cells. Cell Death Dis 2012;3:e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Castro-Vega LJ, Jouravleva K, Ortiz-Montero P, et al. The senescent microenvironment promotes the emergence of heterogeneous cancer stem-like cells. Carcinogenesis 2015;36(10):1180–92. [DOI] [PubMed] [Google Scholar]
- 77.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420(6917):860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78*.Ruhland MK, Loza AJ, Capietto AH, et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat Comm 2016;7:11762 This paper demonstrates that IL-6 can recruit MDSCs and regulatory T cells, creating an immunosuppressive environment that favors tumor progression in the skin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Di Mitri D, Toso A, Chen JJ, et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 2014;515(7525):134–7. This article desmonstrates that the SASP recruits myeloid cells that secrete IL-1RA, which prevents SASP activation and senescence and promotes tumor progression. [DOI] [PubMed] [Google Scholar]
- 80*. Toso A, Revandkar A, Di Mitri D, et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep 2014;9(1):75–89. This article demonsrates that therapy-induced senescence coupled with SASP inhibition may be a promising therapy strategy for the treatment of cancer. [DOI] [PubMed] [Google Scholar]
- 81.Yosef R, Pilpel N, Tokarsky-Amiel R, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Comm 2016;7:11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017;21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016;15(3):428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84*. Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 2016;22(1):78–83. This article introduces the concept of senolytics as a potential treatment to counter aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med 2018;24(8):1246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Munoz-Espin D, Rovira M, Galiana I, et al. A versatile drug delivery system targeting senescent cells. EMBO Mol Med 2018;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Leite de Oliveira R, Wang C, et al. High-Throughput Functional Genetic and Compound Screens Identify Targets for Senescence Induction in Cancer. Cell Rep 2017;21(3):773–83. [DOI] [PubMed] [Google Scholar]
- 88.Yoshida A, Lee EK, Diehl JA. Induction of Therapeutic Senescence in Vemurafenib-Resistant Melanoma by Extended Inhibition of CDK4/6. Cancer Res 2016;76(10):2990–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bollard J, Miguela V, Ruiz de Galarreta M, et al. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut 2017;66(7):1286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Michaud K, Solomon DA, Oermann E, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res 2010;70(8):3228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bainor AJ, Deng FM, Wang Y, et al. Chromatin-Associated Protein SIN3B Prevents Prostate Cancer Progression by Inducing Senescence. Cancer Res 2017;77(19):5339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]