TO THE EDITOR: Waldenström macroglobulinemia (WM) is a rare type of low-grade B-cell lymphoma in which overexpression of the IgM monoclonal protein occurs. In most cases of WM, an L265P mutation is present in MYD88 [1], and approximately 30–50% of cases have a chromosomal 6q deletion [2]. Owing to its rarity, reports on therapies from prospective randomized studies for WM are limited. We previously reported the impact of half-dose CHOP therapy [cyclophosphamide (CPA); hydroxy-doxorubicin (ADR); vincristine (VCR); and prednisone (PSL)] combined with the anti-CD20 antibody, rituximab (R) (R-half CHOP), for untreated patients with WM [3], because the standard-dose R-CHOP therapy [4,5] caused severe myelosuppression and peripheral neuropathy (PN). Herein, we have presented the updated outcomes over the median follow-up period of 37.7 months.
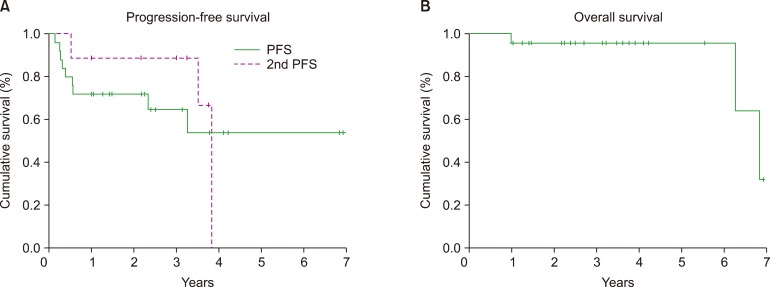
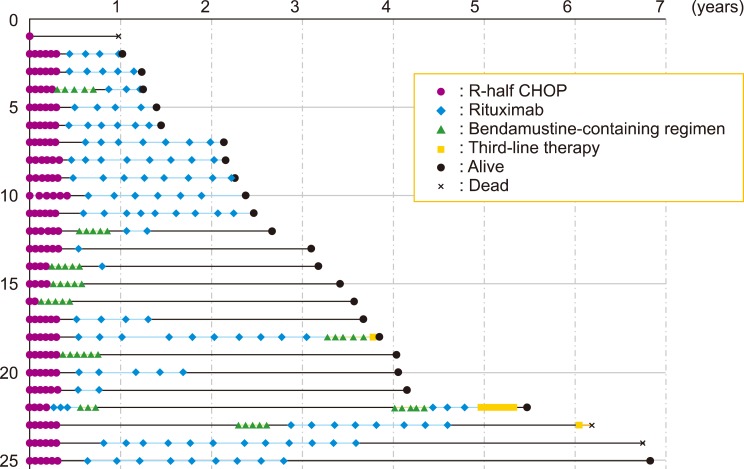
Twenty-five untreated symptomatic patients with WM who received R-half CHOP as the primary therapy at our hospital between April 2011 and April 2017 were analyzed retrospectively. Approval from the Institutional Review Board of our hospital was obtained, and the study was performed according to the Declaration of Helsinki formulated in 1995. R-half CHOP consisted of 6 treatment cycles, with each cycle separated by 3 weeks; however, for 21 patients, vincristine was omitted. Two (8%) patients achieved complete response (CR), 1 (4%) patient achieved very good partial response (VGPR), 12 (48%) patients achieved partial response (PR), and 6 (25%) patients achieved minimal response (MR). The median follow-up of all 25 patients was 37.7 months (range, 12–83.2 mo). The median progression-free survival (PFS) was not reached, although the estimated 2-year and 3-year PFS was 72% and 64%, respectively (Fig. 1A). Nine patients developed refractory disease or progression. All patients received a bendamustine (Benda)-containing regimen as second-line therapy. Subsequently, 2 (22%) patients achieved VGPR and 6 (67%) achieved PR. The estimated 3-year second PFS was 89% (Fig. 1A). The estimated 3-year overall survival (OS) was 96% (Fig. 1B). A swimmer plot of patient responses is presented in Fig. 2. Grade 3/4 leukocytopenia, neutropenia, febrile neutropenia, and Grade 1 peripheral neuropathy (PN) occurred in 33%, 38%, 0%, and 21% of patients, respectively. During the follow-up, 3 patients died: 1 patient experienced traumatic subarachnoid hemorrhage with disease progression, 1 patient had Bing-Neel syndrome, and 1 patient who maintained CR committed suicide (Fig. 2). Furthermore, none of the patients had secondary malignancies. Thus, we confirmed that half-dose R-CHOP was effective and well-tolerated as the primary therapy for untreated WM. In addition, the use of a Benda-containing regimen as second-line therapy had a high response rate and favorable PFS. Therefore, half-dose R-CHOP as first-line therapy and Benda-containing regimen as second-line therapy would be an appropriate treatment strategy for newly diagnosed symptomatic WM.
Fig. 1. Survival curve. (A) Progression-free survival (PFS). The median PFS of half-dose R-CHOP therapy was not reached, and the estimated 2-year PFS was 72% and 3-year PFS was 64%. The estimated 3-year second PFS by a bendamustine-containing regimen was 89%. (B) Overall survival (OS). The estimated 3-year OS was 96%.
Fig. 2. Swimmer plot for 25 patients who received half-dose R-CHOP therapy. Nine patients developed refractory disease or progression and 3 patients received third-line therapy.
Buske et al. [6] previously reported treatment and outcome patterns for 454 patients with WM outside of clinical trials between 2000 and 2014 in 10 European countries. One hundred and ninety-three (43%) patients received monotherapy including chlorambucil (Chl, 27%), and R (6%); 164 patients (36%) received R and alkylating agents (chemo-immunotherapy), such as R-CHOP (11%) [4,5], and dexamethasone, R, and cyclophosphamide (DRC) therapy (6%) [7]. Olszewski et al. [8] reported patterns in treatment regimens associated with survival for patients of ≥65 years of age with WM for whom first-line rituximab-based therapy was initiated in 2008–2014 by using the Surveillance, Epidemiology, and End Results registry in the US. Of the 681 patients, 58% received R alone, 22% received chemo-immunotherapy, 11% received a bortezomib (Bor)-containing regimen, and 9% received a Benda-containing regimen. They also found no significant difference in OS between immune-chemotherapy combinations with classical agents and those with Bor-containing or Benda-containing regimens, although the proportion of Benda- or Bor-containing regimens increased significantly between 2008 and 2014. In studies from Asia, Lee et al. [9] reported the prevalence of Chl alone (35.2%) followed by an alkylating regimen (±R, 28.2%) in a Korean study. This was similar in Japan, with Saito et al. reporting the prevalence of oral alkylating agent therapy alone (46.5%) and CHOP-like regimens (25.4%) [10]. Therefore, the regimens of R alone, oral alkylating agents alone, and R+alkylating agent regimens, including R-CHOP therapy, are popular, according to clinical treatment data.
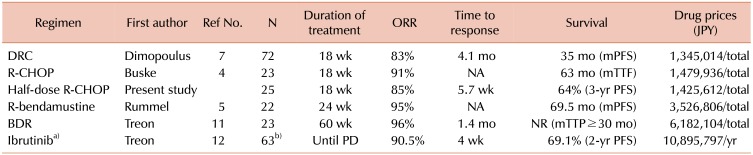
In a consideration of the costs of WM therapy, Olszewski et al. [8] reported no apparent survival benefit and higher costs of treatment for Bor- or Benda-containing regimens. Thus, their value compared with classical regimens should be reconsidered in US practice. We also calculated the drug prices of each regimen in Japanese yen, as shown in Table 1. The prices were calculated for a patient with a body-surface area (BSA) of 1.74 m2, which was the average BSA of Japanese men between 65 and 69 years of age in 2017. The costs of Benda-containing regimens and Bor-containing regimens [11] were more than 2 and 4 times higher than those for R-alkylating regimens, respectively.
Table 1. Summary of responses to each regimen, survival, and drug prices.
a)Ibrutinib is not approved in Japan for WM. b)Data relapsed/refractory WM.
Abbreviations: R, rituximab; DRC, dexamethasone, R, cyclophosphamide; R-CHOP, R, cyclophosphamide, hydroxyl-doxorubicin, vincristine, prednisone; BDR, bortezomib, dexamethasone, R; ORR, overall response rate; JPY, japanese yen; PFS, progression-free survival; TTF, time to treatment failure; TTP, time to progression; PD, progressive disease; NA, not applicable; NR, not reached.
Furthermore, novel agents, such as ibrutinib (Ibr), have been developed for WM. Ibr is an orally administered inhibitor of Bruton's tyrosine kinase (BTK). In 2015, it was approved by the US Food and Drug Administration and the European Medicine Agency for adults with relapsed/refractory WM or for previously untreated patients with WM for whom treatment with chemo-immunotherapy is not suitable [12,13]. Ibr monotherapy was highly active, associated with sustainable responses, and safe [12], and the use of Ibr with R resulted in a significantly higher PFS than the use of R alone [13]. However, as the cost of Ibr treatment is very high, Olszewski et al. [14] examined the cost-effectiveness of Ibr use compared with chemo-immunotherapy. Italian medical and economical experts analyzed the cost-effectiveness of single-agent Ibr compared with the Italian current therapeutic pathways (CTP) for relapse/refractory WM by using an incremental cost-effectiveness ratio [15]. They concluded that Ibr increased the Life Years Gained and costs were comparable with CTP. These reports have confirmed the need for additional analyses of the cost-effectiveness of each drug and regimen, including Ibr.
In conclusion, numerous drugs are available currently; however, in addition to the drug approval status, the age of patients to be treated, presence of severe symptoms such as hyperviscosity syndrome, treatment period, response rate, long-term survival rate, secondary malignancies, costs, and any other relevant factors, should be comprehensively examined to allow making an informed decision on the treatment regimen.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström's macroglobulinemia. N Engl J Med. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 2.Sekiguchi N, Nomoto J, Nagata A, et al. Gene expression profile signature of aggressive Waldenström macroglobulinemia with chromosome 6q deletion. Biomed Res Int. 2018;2018:6728128. doi: 10.1155/2018/6728128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekiguchi N, Hamano A, Kitagawa T, et al. Impact of rituximab and half-dose CHOP as primary therapy for untreated symptomatic Waldenström Macroglobulinemia: review of a combined regimen of rituximab with an alkylating agent. Blood Res. 2018;53:117–122. doi: 10.5045/br.2018.53.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buske C, Hoster E, Dreyling M, et al. The addition of rituximab to front-line therapy with CHOP (R-CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: results of a randomized trial of the German Low-Grade Lymphoma Study Group (GLSG) Leukemia. 2009;23:153–161. doi: 10.1038/leu.2008.261. [DOI] [PubMed] [Google Scholar]
- 5.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 6.Buske C, Sadullah S, Kastritis E, et al. Treatment and outcome patterns in European patients with Waldenström's macroglobulinaemia: a large, observational, retrospective chart review. Lancet Haematol. 2018;5:e299–e309. doi: 10.1016/S2352-3026(18)30087-5. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007;25:3344–3349. doi: 10.1200/JCO.2007.10.9926. [DOI] [PubMed] [Google Scholar]
- 8.Olszewski AJ, Treon SP, Castillo JJ. Application and outcomes of bendamustine- or bortezomib-based therapy for Waldenstrom's macroglobulinemia. Blood (ASH Annual Meeting Abstracts) 2017;130(Suppl):abst 348. [Google Scholar]
- 9.Lee HS, Kim K, Yoon DH, et al. Clinical factors associated with response or survival after chemotherapy in patients with Waldenström macroglobulinemia in Korea. Biomed Res Int. 2014;2014:253243. doi: 10.1155/2014/253243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito A, Isoda A, Kojima M, et al. Retrospective analysis of prognostic factors for Waldenström macroglobulinemia: a multicenter cooperative study in Japan. Int J Hematol. 2017;106:681–690. doi: 10.1007/s12185-017-2297-y. [DOI] [PubMed] [Google Scholar]
- 11.Treon SP, Ioakimidis L, Soumerai JD, et al. Primary therapy of Waldenström macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J Clin Oncol. 2009;27:3830–3835. doi: 10.1200/JCO.2008.20.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström's macroglobulinemia. N Engl J Med. 2015;372:1430–1440. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström's macroglobulinemia. N Engl J Med. 2018;378:2399–2410. doi: 10.1056/NEJMoa1802917. [DOI] [PubMed] [Google Scholar]
- 14.Olszewski AJ, Castillo JJ. Ibrutinib and rituximab in Waldenström's macroglobulinemia. N Engl J Med. 2018;379:1973–1974. doi: 10.1056/NEJMc1809505. [DOI] [PubMed] [Google Scholar]
- 15.Aiello A, D'Ausilio A, Lo Muto R, Randon F, Laurenti L. Cost-effectiveness analysis of ibrutinib in patients with Waldenström macroglobulinemia in Italy. J Mark Access Health Policy. 2017;5:1393308. doi: 10.1080/20016689.2017.1393308. [DOI] [PMC free article] [PubMed] [Google Scholar]