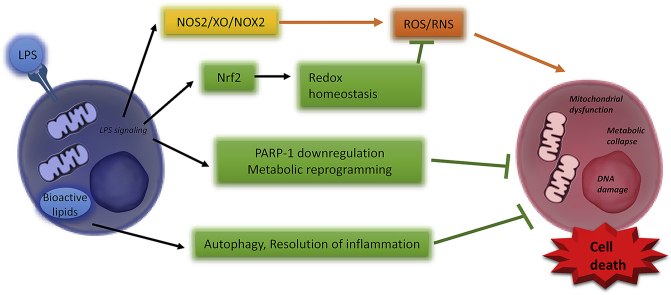
Abstract
Activated macrophages play a central role in both the development and resolution of inflammation. These immune cells need to be functional in harmful conditions with high levels of reactive oxygen and nitrogen species that can damage their basic cell components, which may alter their metabolism. An excessive accumulation of these cell alterations drives macrophages inexorably to cell death, which has been associated to the development of several inflammatory diseases and even with aging in a process termed as “immunosenescence”. Macrophages, however, exhibit a prolonged survival in this hostile environment because they equip themselves with a complex network of protective mechanisms. Here we provide an overview of these self-defense mechanisms with special attention being paid to bioactive lipid mediators, NRF2 signaling and metabolic reprogramming.
Keywords: Inflammation, Immunity, Macrophage, Oxidative burst, Metabolism, Apoptosis, Parthanatos, PARP1, NRF2, HO-1, FOXO, Antioxidant, Eicosanoids, Specialized pro-resolving mediators, Senescence, Aging
Graphical abstract
1. The dual role of macrophages during inflammation and resolution of inflammation: role of reactive oxygen and nitrogen species
Macrophages (ΜΦs) are part of the innate immune system and can differentiate into several subtypes with opposite functions in the course of the immune and inflammatory response [[1], [2], [3]]. Their activation plays a central role in both innate and acquired immunity, which suggests a tightly regulated switch in response to environmental conditions that promote ΜΦ recruitment via tissue infiltration. The high plasticity of ΜΦs enables these cells to respond and adapt to the specific requirements of the inflamed area [1,4,5]. The functions of ΜΦs include host defense against pathogens, phagocytosis and pathogen killing, bone dynamics, antigen presentation, local inflammatory reactions, wound healing, blood lipid homeostasis and tissue remodeling [[6], [7], [8]].
In the course of activation by pathogens and other pathophysiological triggers, ΜΦs express specific sets of genes (e.g. pro-inflammatory cytokines, chemokines and matrix metalloproteinases) that support the initiation of the host defense against these challenges. This initial action is followed by the sequential release of mediators that govern the progression from the early cell-aggressive steps to tissue repair, deactivation of the initial pro-inflammatory response and resolution of the aggression process [[9], [10], [11], [12]]. ΜΦs are very active in synthesizing, processing, and releasing a plethora of bioactive molecules, including prostaglandins, leukotrienes, and other classic eicosanoids that exhibit pro-inflammatory functions; bioactive lipids with anti-inflammatory or pro-resolution activities; and matrix metalloproteinases that accomplish tissue remodeling [[13], [14], [15]]. Reactive oxygen and nitrogen species (ROS and RNS, respectively) are also prototypical ΜΦ mediators that play a central role in effector functions (Fig. 1). ΜΦs increase ROS and RNS production after exposure to a number of different signals including pathogen-derived or damage associated molecular patterns (PAMPs, such as lipopolysaccharide or DAMPs, such as high-mobility box 1 protein, nucleotides, and DNA, respectively), cytokines (e.g. TNFα, IFNγ), metabolic stress (e.g. hyperglycemia, advanced glycation endproducts, oxidized lipoproteins), endoplasmic reticulum stress, unfolded protein accumulation (unfolded protein response; UPR), and various nanoparticles. This exacerbated release of ROS and RNS constitutes the oxidative burst: a defense mechanism initiated by ΜΦs to destroy pathogens thanks to the bactericidal activity of ROS and RNS.
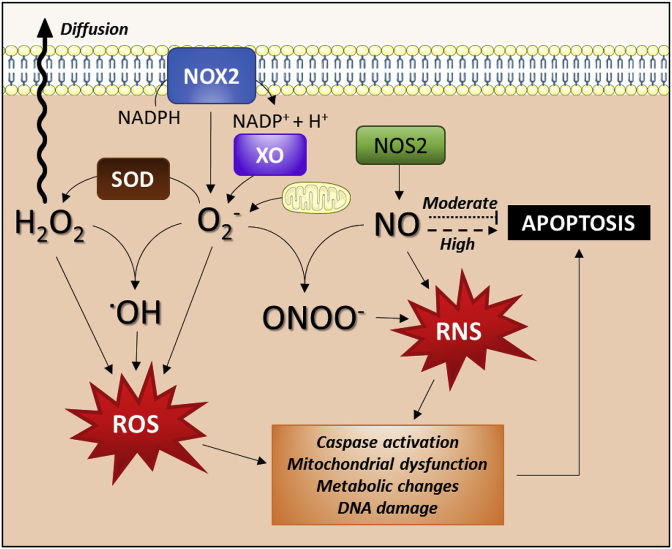
Fig. 1.
Mechanisms of reactive oxygen and nitrogen species generation in macrophages. ROS are generated via enzymatic reactions mediated by NADPH oxidase 2 (NOX2) and xanthine oxidase (XO) and/or as a result of mitochondrial respiratory dysfunction. RNS production is mainly driven by NOS2, whose main product, NO, has either pro- or anti-apoptotic and survival functions, depending on the generation rate. Both ROS and RNS dramatically affect cell metabolism, DNA integrity, and macrophage viability.
The most important sources of ROS in activated ΜΦs are NADPH oxidase 2 (NOX2), xanthine oxidase (XO), and the mitochondrial electron transport chain. Superoxide (O2●-), and hydrogen peroxide (H2O2) generated by these systems play signaling roles, but may also contribute to cell dysfunction and death [16,17]). Superoxide is formed by one-electron reduction of molecular oxygen [18] as catalyzed by NOX or XO enzymes or by the mitochondrial electron transport system. Normally, electrons are transferred through mitochondrial electron transport chain to reduce dioxygen by four electrons, but approximately 1–3% of all electrons leak from the system and produce superoxide [18,19]. Superoxide is converted to H2O2 by the action of superoxide dismutases (SODs). Peroxisomal reactions mediated by oxidant enzymes also represent a source of H2O2 [20]. In the presence of Fe2+ ions, hydrogen peroxide can be broken down to hydroxyl radicals (●OH) in a complex process known as Fenton reaction. Hydroxyl radical is the most reactive ROS and can damage proteins, lipids, carbohydrates and DNA contributing to cell dysfunction and death [21].
In addition to direct DNA damage, ROS can influence the “methylome” through the formation of oxidized DNA lesions with structural similarities to epigenetic signals. Furthermore, they can indirectly modulate the activity of the epigenetic machinery, indicating that epigenetic changes are tightly linked to cellular metabolism and energy levels. All these alterations have been strongly associated with aging and longevity leading to subclinical accumulation of immunosenescence factors. This fact has been suggested to contribute to various diseases in the elderly [[22], [23], [24]].
Nitric oxide (NO) represents the most predominant form of RNS synthesized by ΜΦs and is a major signaling radical capable of modulating various functions as well as viability of ΜΦs. Elevated NO synthesis in activated pro-inflammatory ΜΦs is one of the main cytostatic, cytotoxic, and pro-apoptotic mechanisms participating in the innate immune response, at least in non-primate species [25]. ΜΦs express all three NO synthase (NOS) isoforms. Transcriptionally regulated NOS2 (inducible NOS; NOS2) can produce large amounts of NO for prolonged periods of time while the activity-regulated constitutive isoforms NOS1 (neuronal NOS) and NOS3 (endothelial NOS) respond rapidly to calcium signals [26]. While the role of NOS2 in the anti-microbial activity of ΜΦs is well known [26], NOS1 is required for ΜΦ uptake of LDL during foam cell formation [27] and NOS3 may contribute to the initiation of the immune response [28]. In humans, special mention is required for the expression of NOS2 by activated pro-inflammatory ΜΦs. Despite the high homology of the Nos2/NOS2 gene across mammalian species, the promoter region varies substantially in primates, which explains the poor inducibility of the gene in humans [4,25,[29], [30], [31]]. This situation is perhaps responsible for the development of survival strategies by several pathogens, such as trypanosomes that cannot be efficiently cleared from infected human ΜΦs (Fig. 2). Absence of NO synthesis as it happens in higher mammals restricts the oxidative burst of ΜΦs to superoxide and hydrogen peroxide production only.
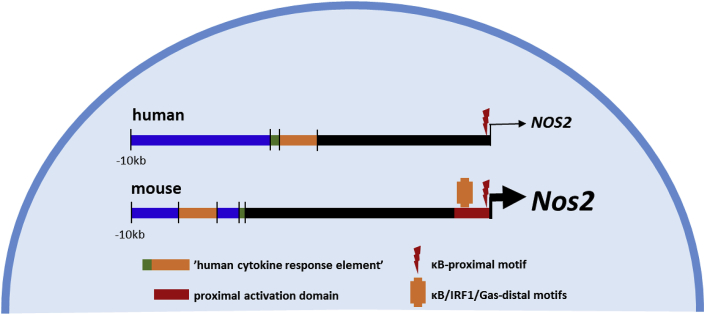
Fig. 2.
Differences in the regulatory elements of the NOS2 gene promoter among mammals. The decrease in pro-inflammatory-triggered NO production in primates lies in the lack of several motifs in the NOS2 gene promoter, which confers a restrictive transcriptional regulation of the gene and, therefore, limits the capacity of cells to accomplish high-output synthesis of NO.
When NO is present in mitochondria either by mitochondrial NOS action or by diffusion from vicinal compartments, it can react with superoxide to yield peroxynitrite (ONOO−) in situ [32].This is a rapid reaction (in fact, one of the fastest reactions in nature, not catalyzed by enzymes) that produces a potent oxidant, more reactive than its precursors. In addition to the nitration (mainly in tyrosine residues) of a large number of proteins, peroxynitrite can also oxidize amino acid residues essential for the function of many proteins, including transcription factors and enzymes [33]. In DNA, ONOO− can induce strand breaks and base modifications (e.g. 8-nitroguanine formation) [34].
Pro-inflammatory ΜΦs can survive for several days under the stressful environment of highly reactive molecules due to their high metabolic plasticity [1]. Uniquely, NO synthesis by ΜΦs leads to a hyperpolarization of the mitochondria, which preserves mitochondrial activity for a while, preventing the release of cytochrome c and other mitochondrial-dependent pro-apoptotic mediators [31]. In addition, mediators such as p53 are highly over-expressed to prevent DNA damage and extend the functional activity of ΜΦs [35]. Moreover, most physiological ΜΦ functions, such as phagocytosis, require preservation of cell integrity, mainly through attenuation of the auto-apoptotic response. Indeed, NO plays a dual role, favoring cell viability or inducing apoptotic/necrotic death depending on the cell type and the amount of NO produced [36,37]. Thus, moderate NO synthesis can inhibit apoptosis in several cell types including B lymphocytes and hepatocytes [36,38]. Several targets have been identified in this protective mechanism mediated by NO, including caspase regulation and the expression of antiapoptotic genes from the inhibitor of apoptosis protein (IAP) family [39]. Caspase processing and activation are inhibited due to the formation of NO-adducts with the cysteine residues present in the catalytic domain of these enzymes via transient S-nitrosylation reactions [40]. Indeed, S-nitrosylation of cysteine residues (for example, in GSH) yielding nitrosothiols is one of the mechanisms that prolong the bioactivity of NO since nitric oxide can be released from nitrosothiols for longer periods of time in a subcellular compartment specific manner. Interestingly, in vitro treatment of ΜΦs with chemical NO donors (which rapidly decompose and release high amounts of NO into cells) promote apoptosis, as confirmed by sequential activation of caspase 9 and 3, loss of plasma membrane asymmetry and DNA fragmentation. However, the kinetics of this process is very dependent on the rate of NO generation. A sustained, moderate release of NO resembling the in vivo response (e.g. that generated by DETA-NONOate; half-life ca. 10 h), induces a significantly reduced apoptotic response [41,42]. The presence of modest levels of NO reduces apoptosis by maintaining higher levels of anti-apoptotic proteins and inhibiting the activity of caspases.
2. The role of functional state of macrophages in determining sensitivity to ROS and RNS
ΜΦs are highly plastic cells with a rapidly changing phenotype in response to altered microenvironment. In a very simplistic way, two principal polarization states have been described, M1 or classically activated macrophages, and M2 or alternatively activated macrophages. M1 ΜΦs exhibit a pro-inflammatory response inducing a robust anti‐microbial and anti‐tumoral activity and M2 ΜΦs promote tissue repair and wound healing. Exposure to cytokines, chemokines, toxins, PAMPs and DAMPscan dramatically shape the functional state and oxidative stress sensitivity of these cells. Despite their high resistance to ROS, ΜΦs are still not fully resistant to ROS-induced death. In fact, when oxidative stress is maintained for long period of time, ΜΦs accumulate massive amounts of oxidized proteins and lipids, leading to metabolic dysfunction. In more advanced stages, the loss of DNA integrity leads to an inevitable cell death. Recently Regdon et al. [43] investigated the mechanisms whereby ΜΦs develop a high degree of resistance against oxidative stress. In their experiments, an exogenous ROS (H2O2) was used to simulate the oxidative microenvironment in order to elucidate the cell death modality of these H2O2-treated ΜΦs. In this model, ΜΦs were treated with high concentrations of H2O2 to elucidate the cell death modality in response to ROS. ΜΦs exposed to high concentrations of H2O2 underwent regulated necrotic cell death similar to parthanatos. Parthanatos is a form of programmed necrotic cell death [44] triggered by severe DNA damage and mediated by the activation of the DNA damage response enzyme, poly(ADP-ribose) polymerase-1 (PARP1) [108].
Interestingly, the functional state of ΜΦs had been related to differential susceptibility of M1 or M2 to cell death. For example, induction of mature ΜΦs from BMDM (bone marrow-derived ΜΦs) by M-CSF treatment caused increased H2O2 resistance. Subsequent exposure to LPS, an inflammatory polarization signal (M1 stimulus), conferred maximal protection to ΜΦs while IL-4 (M2 challenge) had no effect [43]. An important question is how M1 polarization is linked to the development of oxidative stress resistance. The mechanism underlying H2O2 resistance of M1 ΜΦs appears to be based on at least three pillars: inhibition of cell death signaling, upregulation of antioxidant defense and metabolic reprogramming [43]. Conversely, a recent report suggests that in other cell contexts as lipotoxicity, the M2 phenotype can confer protection to adipose-tissue resident ΜΦs enhancing their tolerance to lipid-mediated stress [45].
Inflammatory (e.g. LPS-treated) ΜΦs display a dramatically altered gene expression landscape (Fig. 3). Part of the genetic reprogramming in inflammatory ΜΦs involves cell death genes, such as Parp1. LPS-induced downregulation of PARP1 expression in ΜΦs appears to be a key factor rendering ΜΦs resistant to oxidative stress. The expression of PARP1 from a viral promoter, which is unresponsive to LPS-induced suppressive signals, restores H2O2 sensitivity of ΜΦs, demonstrating the importance of PARP1 suppression [43]. Moreover, the expression of various antioxidant enzymes and proteins vary greatly in LPS-stimulated cells. Interestingly, catalase and SOD1 expression decreased significantly after treatment with LPS, while glutathione reductase, thioredoxin reductase, and peroxiredoxin 1 expression increased [43]. These latter changes may play a role in oxidative stress resistance; however, the most significant change could be observed in SOD2 expression, which increased approximately tenfold in LPS-treated cells. Cells undergoing parthanatos have been shown to display secondary mitochondrial superoxide production [46], and SOD2 silencing was found to abolish the protective effects of LPS against H2O2 cytotoxicity [47]. These results highlight the importance of SOD2 induction in the development of oxidative stress resistance of M1 ΜΦs. Interestingly, the roles of PARP1 and SOD2 appear to be interconnected as PARP1 participates in the regulation of Sod2 gene expression [47]. This regulation is complex and has been suggested to involve a functional interplay between PARP1 and the lysine-specific histone demethylase 1A (LSD1) (for details see Ref. [47]).
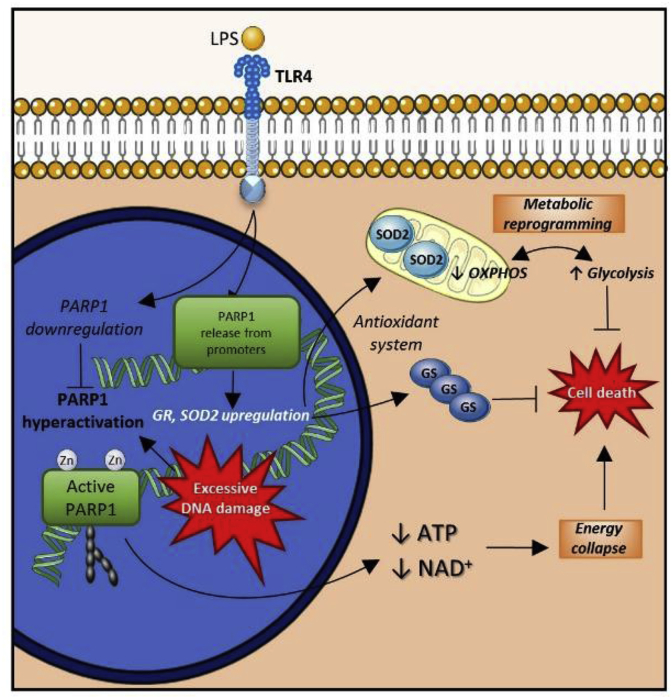
Fig. 3.
Oxidative stress resistance in LPS-treated (M1/pro-inflammatory) macrophages. Severe oxidative DNA damage induces activation of the nuclear DNA nick sensor enzyme, PARP1. PARP1 is the central mediator of the PARylation-dependent cell death route, termed parthanatos. LPS induces M1 differentiation in macrophages via TLR4. Additionally, this stimulation triggers downregulation of PARP1, induction of SOD2 and other antioxidant proteins, and promotion of metabolic reprogramming favoring glycolysis. As a result, cell death pathways, especially parthanatos, become highly inhibited.
The role of metabolic regulation in various cellular functions, including the sensitivity of ΜΦs to toxic stimuli, has been increasingly recognized. The high plasticity of ΜΦs involves dynamic metabolic reprogramming to adjust their metabolism to their functional state. M1 ΜΦs operate a glycolytic metabolism similar to the Warburg phenotype (aerobic glycolysis) of cancer cells [5,43,[48], [49], [50]]. While the rationale for this metabolic shift in cancer cells has been a subject of heated debates, its contribution to a resistant phenotype is widely acknowledged. Parthanatos resistance in LPS-stimulated ΜΦs is associated with the Warburg phenotype [47] through the following possible mechanisms: a) a shift from oxidative to glycolytic metabolism, which prepares cells for the consequences of oxidant-induced damage to mitochondrial respiration; b) downregulation of mitochondrial activity, which limits mitochondrial ROS production; c) rapidly inducible glycolytic ATP production, which may be beneficial in ATP depletion scenarios (e.g. PARP1 over-activation) [10,51]; and d) a glycolytic switch, which may impact epigenetic reprogramming to influence the regulation of cell death and antioxidant enzymes [10]. One must be cautious, however, when translating murine data to human settings as murine and human ΜΦs show fundamental differences in LPS-induced metabolic reprogramming and RNS synthesis [12,25].
3. Molecular mechanisms of self-protection in macrophages
The high amount of ROS and RNS produced inside ΜΦs to defend from pathogens requires the existence of protective mechanisms against this oxidative burst to ensure ΜΦ survival during the inflammatory process. Although the specific mechanisms for ROS sensing and scavenging within ΜΦs remain elusive [52], recent reports inicate that these cytoprotective mechanisms may be acquired during differentiation from monocytes [53]. In fact, the lack of antioxidant mechanisms in monocytes is thought to establish a negative feedback loop where ROS produced by ΜΦs kill monocytes to avoid an exaggerated inflammatory response [53].
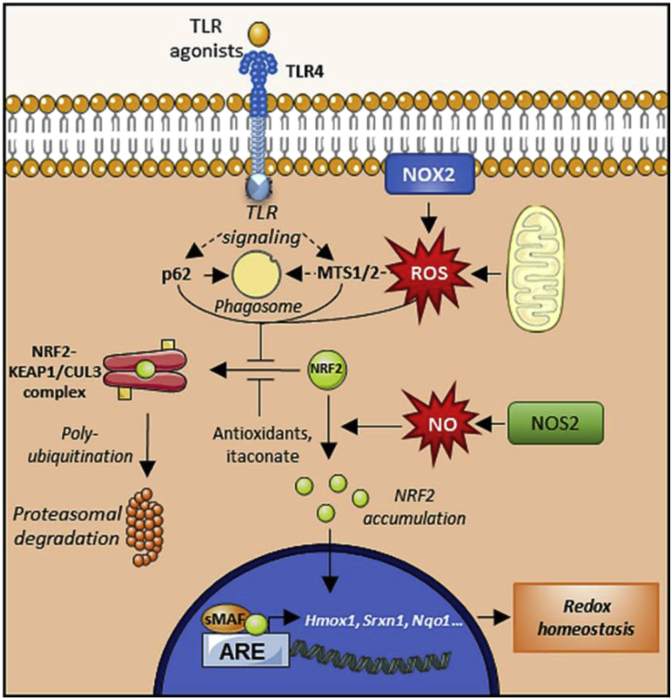
The nuclear factor erythroid 2-related factor 2 (NRF2) pathway represents the main strategy to alleviate oxidative stress in ΜΦs [[54], [55], [56]] (Fig. 4). NRF2 belongs to the cap ‘n’ collar (CNC) subfamily of basic-region leucine zipper (bZIP) transcription factors. When stable, NRF2 translocates to the nucleus and binds to antioxidant response elements (AREs) to upregulate genes implicated in metabolism, proteostasis and redox balance [55,57]. NRF2 target genes include heme-oxygenase 1 (Hmox1) and ferritin complex (Ftl and Fth), which prevent hydroxyl radical production by releasing Fe2+ from heme molecules and oxidizing Fe2+ to Fe3+, respectively. NRF2 also targets the expression of both subunits of the glutamate-cysteine ligase complex (Gclc and Gclm), which participate in glutathione synthesis [57]. Other targets of NFR2 include glutathione peroxidase 2 (Gpx2), glutathione reductase 1 (Gsr1), and several glutathione-S-transferases (Gsta1-3, Gsta5, Gstm1-3 and Gstp1), which are involved in glutathione redox cycle. NRF2 also regulates the expression of thioredoxin reductase 1 (Txnrd1) and sulfiredoxin 1 (Srxn1), which reduce protein thiols [57]. Finally, NRF2 also targets NAD(P)H:quinone oxidoreductase 1 (Nqo1), which reduces reactive quinones to less toxic hydroquinones [58].
Fig. 4.
Antioxidant mechanism of the NRF2 pathway. Under basal conditions, the NRF2 transcription factor is sequestered and polyubiquitinated by the KEAP1/CUL3 complex, ensuring its degradation. ROS are able to inhibit this association, leading to NRF2 stabilization and accumulation. NRF is then translocated into the nucleus where it upregulates a series of genes involved in the antioxidant response. The NRF2-KEAP1/CUL3 complex can also be destabilized by antioxidant molecules, metabolites (e.g. itaconate), and even by autophagic mediators, whereas NO production may induce the NRF2 pathway as an adaptive response.
To appropriately bind to AREs, NRF2 first needs to form a stable complex with members of the small masculoaponeurotic fibrosarcoma (sMAF) family of proteins (MAFF, MAFG and MAFK) [57,59]. In fact, disruption of the NRF2-sMAF complex by deletion of both MAFF and MAFG was shown to inhibit NRF2 signaling [57,59]. In addition to the induction of the antioxidant response, NRF2 signaling actively inhibits the pro-inflammatory response independently of ROS and ARE in ΜΦs, supporting the cytoprotective role of the NRF2 pathway [60].
To ensure the appropriate control of the antioxidant and anti-inflammatory responses, a tightly regulated protein network maintains low levels of NRF2 under unstressed conditions [59]. The main NRF2 regulator is Kelch-like ECH-associated protein 1 (KEAP1), a protein constitutively expressed in cells. KEAP-1 homodimerizes and binds to cullin 3 (CUL3) to form the KEAP1-CUL3 ubiquitin ligase complex. This complex catalyzes the polyubiquitination of NRF2 to induce its proteasomal degradation [59]. Most inducers of the NRF2 pathway inhibit KEAP1 function by preventing its dimerization through targeting of cysteine thiol residues (sulforaphane, bardoxolone), by inhibiting its association with NRF2 (tert-butylhydroquinone) or by promoting the dissociation of NRF2 from the ubiquitin ligase complex (As3+, Cd2+, and Cr6+) [59]. In addition to KEAP1-associated polyubiquitination, NRF2 can be regulated through other mechanisms that promote its nuclear import/export or degradation, including phosphorylation/dephosphorylation or acetylation/deacetylation. In addition, several microRNAs target Nrf2 and Keap1 mRNA to repress or induce the NRF2 pathway, respectively [59].
The exacerbation of oxidative damage in ΜΦs and the aggravation of inflammatory diseases upon NRF2 deficiency have been widely described in the literature [54,56]. Interestingly, the role that NRF2 plays in foam cells during atherogenesis remains unclear as conflicting data in the literature report both protective and deleterious functions [60]. In contrast, NO is capable of triggering an adaptive response to oxidative stress in pro-inflammatory ΜΦs by inducing the NRF2 pathway [61]. Recently, the work of Wang et al. revealed that MTS1 and MTS2, two kinases implicated in cell growth, polarity, migration and apoptosis, induce the NRF2-associated antioxidant response by phosphorylating and inactivating KEAP1 in BMDM [52]. Interestingly, MTS1/2 have been previously implicated in ROS trafficking from mitochondria to phagosomes as part of the TLR signaling cascade [62]. Along the same lines, heme oxygenase (HO) has been found to indirectly enhance phagocytic activity in ΜΦs by diminishing intracellular heme levels as part of the antioxidant response, strengthening the bactericidal function [63]. Altogether, these results indicate that NRF2 pathway plays a critical role in ΜΦ self-protection against oxidative damage by finely regulating both ROS generation and detoxification. Thus, these important pathways should be further investigated.
Another canonical antioxidant pathway is the FOXO pathway, which is comprised of four different transcription factors: FOXO1, FOXO3, FOXO4, and FOXO6. The FOXO transcription factors bind to specific DNA motifs (DBEs, DAF-16 family member-binding elements) to induce the expression of target genes, including MnSOD and catalase [64]. Apart from defending against ROS, FOXO pathway is also implicated in cell cycle arrest, apoptosis, differentiation and DNA repair [65]. In the immune system and particularly in ΜΦs, the role of the FOXO pathway remains controversial. Some reports indicate that FOXO3 is predominantly expressed in myeloid cells, including ΜΦs, whereas others did not detect any expression of either FOXO1 or FOXO3 [52,65]. Interestingly, in ΜΦs FOXO1 has been found to stimulate both pro- and anti-inflammatory pathways by upregulating the Tlr4 gene and the IL-10 promoter, respectively [66,67]. The simultaneous deletion of FOXO1, 3 and 4 induces monocytosis and increased NOS2 expression and oxidative stress in mice [68]. These data highlight the versatility of functions and the complexity of the FOXO interaction network, which has not been completely elucidated yet in the context of ΜΦ oxidative stress and inflammation.
Supporting these two major antioxidant pathways, additional cytoprotective mechanisms such as autophagy [69], UPR [70], and the hypoxia-inducible factor 1 (HIF1) pathway [71], also orchestrate the elimination of ROS and RNS to alleviate oxidative damage. Autophagy includes several processes that drive the degradation of different dysfunctional cytoplasmic components inside autophagosomes in order to recycle nutrients upon starvation or other stressful conditions affecting cellular homeostasis, such as oxidative stress [69]. During infections, ΜΦs engulf pathogens to form phagosomes that are subsequently targeted by autophagic proteins (e.g. p62 and NDP52) to form autophagosomes, where oxidative burst takes place [72]. This process has been found to be dependent on ROS production by NOX2, since the inhibition or deletion of this enzyme impairs the recruitment of autophagic components and hence autophagosome formation [73]. Interestingly, p62 is also able to induce NRF2 pathway by degrading KEAP1 via autophagy (Fig. 4) [74]. Therefore, autophagy provides a compartment that confines ROS production and prevents pathogen escape while stimulating antioxidant pathways to counterbalance this oxidative burst [69].
UPR is induced to alleviating endoplasmic reticulum stress generated by aberrant protein accumulation elicited in oxidative stress situations [70]. Specific mediators of the UPR (XBP1 and IRE1) are activated in ΜΦs upon TLR signaling. This activation is dependent on ROS production by NOX2 [74]. As a result, a non-canonical UPR signaling pathway is triggered contributing to enhance the inflammatory response by activating both Il6 and Tnf genes and reducing endoplasmic reticulum stress within an oxidative burst context [70,74]. There is a crosstalk between UPR and the aforementioned NRF2 and FOXO antioxidant pathways, which provides yet another defense mechanism to fight against ROS and RNS overproduction under ER stress conditions [75,76].
The HIF1 pathway is initiated by the HIF1α subunit. When stimulated, the HIF1α subunit becomes stable and associates with the HIF1β constitutive subunit to form a HIF1 transcription factor complex. The HIF1 transcription factor complex translocates to the nucleus and upregulates a series of genes involved in hypoxia resistance and metabolic switch [71]. Oxidative stress is a well-known inductor of HIF1 pathway in pro-inflammatory ΜΦs, ROS induction of the HIF pathway is responsible for the metabolic change towards glycolysis [71]. As a result, mitochondrial respiration is attenuated and ROS production dramatically decreases [77]. Interestingly, this metabolic switch also causes the accumulation of citrate produced in Krebs cycle, which increases NOX2 and NOS2 activity [77]. Altogether, these data suggest that upon stimulation, HIF1 pathway efficiently diminishes oxidative stress generated by mitochondria while promoting inherent ROS and RNS production mechanisms of the inflammatory response in ΜΦs.
The natural resistance-associated macrophage proteins (NRAMPs), a group of secondary carrier proteins that are involved in metal ion transport (i.e. Mn2+/Fe2+ and H+ antiporters) [78] may also contribute to ΜΦ self defense. These proteins are highly conserved across evolution and their loss of function not only reduces the inflammatory response to infection, but also promotes iron recycling by macrophages. Iron is used by cells to support electron exchanges in core biological processes, such as ATP production and mitochondrial electron transport chain, and to transport oxygen and other gases by hemoglobin [79]. Release of high amounts of heme upon red blood cell (RBC) senescence or damage increases iron cytotoxicity, eventually leading to a specific form of programmed cell death termed ferroptosis [80]. A specific macrophage subset is capable of phagocytosing those RBCs [81], metabolizing heme groups and recycling the iron back to erythroid progenitors [81]. NRAMPs can increase HO-1 levels [82], which in turn can also modulate NOX2 expression and activity [83] and generates CO that is cytoprotective [84], suggesting that heme catabolism by HO-1 can also protect macrophages from oxidative stress by modulating NOX2.
Along with antioxidant and cytoprotective pathways that help reducing the oxidative burden, ΜΦs can also make use of ascorbate (vitamin C) and α-tocopherol (vitamin E). Concentrations of these two ROS scavenger molecules depend on dietary intake [85]. Ascorbate is able to scavenge a broad spectrum of free radicals, including superoxide, hydroxyl, aqueous peroxyl and alkoxyl radicals. Ascorbate also neutralizes protein hydroperoxides [85]. In ΜΦs, treatment with ascorbate was shown to alleviate oxidative stress and to improve phagocytic activity whereas ablation of the ascorbate transporter increased ROS susceptibility and apoptosis [86]. α-Tocopherol efficiently neutralizes lipid peroxyl radicals [87], however, the antioxidant potential of α-tocopherol is still under debate. Low levels of α-tocopherol are also able to elicit lipid peroxidation reactions, which may explain the negative results obtained in clinical trials evaluating the effects of α-tocopherol in myocardial infarction patients [88]. Lastly, the antioxidant function of 7,8-dihydroneopterin should also be noted. 7,8-Dihydroneopterin is an intermediate of 5,6,7,8-tetrahydrobiopterin synthesis, the cofactor for NOS2, from GTP [87]. This synthesis is activated mainly by interferons α/γ and LPS; however, in primate ΜΦs these pro-inflammatory stimuli do not increase the expression of 6-pyruvoyltetrahydropterin synthase, the intermediary enzyme that metabolizes 7,8-dihydroneopterin-triphosphate, leading to 7,8-dihydroneopterin accumulation [87]. 7,8-Dihydroneopterin is able to scavenge free radicals such as hydroxyl and peroxyl radicals, HOCl and oxLDL thus reducing cellular oxidative stress, restoring thiol levels and inhibiting cell death [87]. Nonetheless, 7,8-dihydroneopterin levels in activated ΜΦs have not been completely determined nor have any strategies to increase them been designed [87], demanding greater research in this field.
It should be emphasized that the importance of the mechanisms listed above do not solely depend on their antioxidant potential, but also on their interplay with pro-inflammatory and pro-resolving pathways, which is crucial for the appropriate balance between ΜΦ protection and inflammatory function [41,89].
4. Bioactive lipids, self-defense mechanisms and macrophage resolution of inflammation
Lipids are not just the major constituents of cell membranes and efficient sources of energy, but also can be key pathophysiological mediators of several intercellular and intracellular processes. Indeed, endogenous lipids are one of the most important players in all phases of inflammation, including the resolution phase [1,3,14,90,91]. This is the reason why lipids are often called “bioactive lipids”. Lipids can be divided into several families according to their biochemical functions: a) classical eicosanoids with pro-inflammatory functions (e.g. prostaglandins, prostacyclins and thromboxanes); b) specialized pro-resolving mediators (SPMs), which are considered pro-resolving lipids due to their anti-inflammatory and pro-resolution functions (e.g. lipoxins, resolvins, protectins and maresins); c) lysoglycerophospholipids/sphingolipidis, structural membrane components that participate in several signaling pathways; and d) endocannabinoids, which regulate numerous cellular processes upstream of mechanisms that control cell and tissue adaptation to inflammatory events. All lipid families are generated from essential ω-6 or ω-3 polyunsaturated fatty acid (PUFA) precursors that are esterified into membrane lipids. Bioactive lipids act by binding to and activating specific G protein coupled receptors (GPCRs), among other targets [92]. Since both pro-inflammatory and anti-inflammatory mediators are derived from the same precursors, the balance between pro- and anti-inflammatory products is crucial to maintain homeostasis and to prevent inappropriate inflammation.
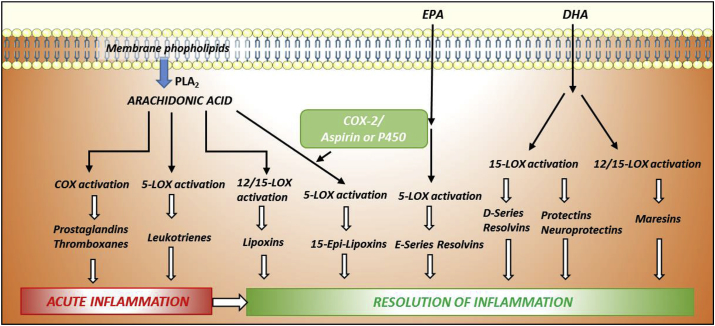
In the course of inflammation, the innate immune cells rapidly generate classical eicosanoids that are highly pro-inflammatory with the aim of removing adverse stimuli. Their main role is the recruitment of immune cells to the damaged area to initiate and amplify the inflammatory response. However, this process needs to be self-limiting and, once the noxious stimulus has been controlled, the cellular scenario changes to the “resolution of inflammation phase”, where the same innate immune cells undergo a temporal lipid mediator class switch and start producing bioactive ‘repair’ lipids: the SPMs. All of these mediators originate from the same PUFAs (Fig. 5). Cis-linoleic acid (LA) is metabolized to arachidonic acid (AA), whereas α-linolenic acid (ALA) is converted to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
Fig. 5.
Metabolic pathways of bioactive lipids production. While all pro-inflammatory classical eicosanoids (prostaglandins, thromboxanes, and leukotrienes) derive from arachidonic acid metabolism, SPMs can either be synthesized from arachidonic acid (lipoxins) or from polyunsaturated fatty acids like EPA (E-series resolvins) or DHA (D-series resolvins, protectins and maresins). The main enzymes implicated in these pathways are lipoxygenases and cyclooxygenases.
To date, more than 20 different SPMs have been identified via sophisticated lipidomic approaches, although LXA4 and RvD1 are the most studied SPMs so far [93]. These mediators are subdivided into six main classes: AA-derived LXs (LXA4 and LXB4), EPA-derived E-series resolvins (RvE1–3), DHA-derived D-series resolvins (RvD1–6), protectins, neuroprotectins (PD1/NPD1 and PDX), and maresins (MaR1 and MaR2). The clupanodonic (DPA)-derived 13-series resolvins (RvT1–4) were also recently identified. SPMs actively terminate inflammation by reducing leukocyte infiltration and the recruitment and stimulation of nonphlogistic mononuclear cells. This situation promotes killing and clearance of pathogens via ΜΦ-mediated phagocytosis of apoptotic granulocytes (efferocytosis) and cellular debris. At the same time, the synthesis of pro-inflammatory cytokines is repressed, while the accumulation of anti-inflammatory mediators shortens the time of resolution and activation of endogenous resolution programs. Overall, the result is the promotion of tissue regeneration and healing to restore full tissue homeostasis. When inflammation is not properly extinguished, due to defective resolution, chronic inflammation develops, which contributes to the development of a majority of prevalent pathologies. Therefore, SPMs are considered key molecules with potential therapeutic uses as STOP-inflammation signals.
During efferocytosis of apoptotic neutrophils, ΜΦs normally overcome oxidative stress-induced apoptosis. ΜΦ death impairs the resolution process inducing a chronic inflammatory scenario. Thus, these immune cells have to modulate their redox metabolism to favor their own survival. In this sense, many groups have described how SPMs can modulate the balance of the main antioxidant enzymes in several pathological models reducing ROS levels in cells. Thus, it is known that NRF2 and NOX2 levels are strictly balanced in ΜΦs and both can be modulated by SPMs. Indeed, LXA4 reduces apoptosis and activates autophagy in ΜΦs mainly via AKT and ERK. In addition, NRF2-dependent transcriptional regulation is an auxiliary anti-oxidant mechanism [15,91]. In fact, autophagy has been identified as part of the self-defense mechanisms of myeloid cells [94]. Moreover, LXA4 improves the phagocytic capacity of these immune cells, favoring the resolution of inflammation. Lee et al. showed that RvD1 reduced ΜΦ apoptosis by upregulating the levels of several anti-apoptotic proteins of the Bcl-2 family and inhibiting the activation of the pro-oxidant enzyme NOX2 [95]. Thus, SPMs in ΜΦs have a protective role, which prolongs their viability and favor an antioxidant balance modulating both NRF2 and NOX2 pathways. In the same context but in a different cell type, SPMs are very effective in upregulating antioxidant defense mechanisms in animal models. For example, an LXA4 derivative blocks ROS generation in endothelial cells by suppressing NOX activity [96]. Furthermore, in a murine model of cigarette smoke-induced emphysema, RvD1 is capable of reducing inflammatory and oxidative markers in lungs, resulting in improved pulmonary functions. These effects are associated with an RvD1-mediated increase in SOD activity [97]. RvD1 also protects against oxidative stress by decreasing LPS-induced synthesis of lipid peroxides and malondialdehyde, and protects against glutathionyl-4-hydroxy-trans-2-nonenal (GS-HNE)-initiated inflammation by inhibiting leukocyte infiltration [98]. RvD1 can also reduce UV-induced skin oxidative stress by preventing UVB-elicited NRF2 downregulation and catalase inactivation, which lead to decreased hydroperoxides and superoxide and maintained GSH levels [99]. Importantly, all SPM levels in humans plasma, tissues or another biological fluids are in the pg to ng/ml range [[100], [101], [102]]. These endogenous concentrations are equivalent to the doses used in the broad majority of the studies where their effects are described. This fact indicates that endogenous SPMs can be active in the antioxidant scenario in a similar manner as exogenous analogs.
ROS and RNS can interact with several enzymes to modify their functionality, which affects SPMs synthesis. In fact, ROS can activate TLR4 receptors to promote the biosynthesis of RvD1 through the upregulation of lipoxygenase enzymes, particularly 12-LOX. Increased RvD1 may also inhibit the nuclear translocation of 5-LOX in an autocrine manner. This creates a positive feedback loop for RvR1 synthesis via the cytosolic interaction of 5-LOX with 12-LOX, which induces the conversion of DHA into RvD1 [103]. In this sense, the rise in ROS may induce the production of SPMs as a self-protective mechanism. In summary, SPMs can modulate the activity of several oxidative enzymes in cells, reducing oxidative stress and even controlling their own synthesis.
5. Ageing, immunosenescence and control of ROS biosynthesis
Immunosenescence refers to the gradual deterioration of the immune system brought on by natural age advancement. This process is associated with chronic ROS production, mainly in phagocytic immune cells, as senescent cells exhibit a decreased capacity to regulate their own redox balance and autophagic capacity [94]. ΜΦs of aging mice accumulate a large number of lipofuscin granules that appear to promote higher ROS accumulation making cells more vulnerable to oxidative stress. Moreover, these senescent ΜΦs show impaired phagocytic and digestive capacity, increased expression and activity of XO and lower levels of antioxidant enzymes, such as catalase and GSH that can be responsible for oxidative stress [104]. Increased XO activity can activate NLRP3, triggering the release of several pro-inflammatory cytokines. Although the mechanisms by which XO is linked to the inflammation process are not entirely clear yet, some authors propose that XO can activate certain TLRs in phagocytes [105]. This harmful inflammation may be perpetuated not only by the synthesis and release of pro-inflammatory metabolites, but also by the reduction of SPM levels. In the elderly, there is a shift in arachidonic acid metabolism towards the formation of pro-inflammatory eicosanoids, decreasing the production of anti-inflammatory derivatives. Along this same line, LXA4 levels are profoundly decreased with ageing [106]. In this context, several factors collaborate to induce the senescent damage: alteration in cell detoxifying metabolism, increases in pro-inflammatory mediator production, and reduction of SPMs. All of these mechanisms drive the senescent cell inexorably to cell death.
6. Concluding remarks
Survival of ΜΦs and other myeloid cells represents a relevant issue in the execution of their physiological and homeostatic functions. The presence of high amounts of ROS and RNS in the inflammatory scenario has a dual role in the survival/death balance depending on the immune cell subset. In fact, the weak functionality of aged ΜΦs has been related to a reduced capacity to retain viability in the last steps of the activation process resulting in impaired autophagic function in aged individuals and a lower pro-resolutive capacity of immune cells [94,107]. Therefore, unraveling the mechanisms of functional MΦ deterioration and developing drugs to restore the full homeostatic and tissue repair capacity of ΜΦs based on the retention of their self-defense pathways may result in new therapeutic interventions to treat diseases in which these characteristic properties have been attenuated or even lost.
Acknowledgements
LV received funding from the National Research Development and Innovation Office grants GINOP-2.3.2-15-2016-00048-STAYALIVE, GINOP-2.3.2-15-2016-00020 TUMORDNS", OTKA K112336 and from the Faculty of Medicine of University of Debrecen. LB and PP were supported by the following grants: SAF2017-82436R from Ministerio de Economía, Industria y competitividad (MINECO), S2017/BMD-3686 and S2010/BMD-2423 from Gobierno de España, Comunidad de Madrid (Spain), CIVP18A3864 from Ramón Areces Foundation and CIBERCV (funded by the Instituto de Salud Carlos III) and Fondos FEDER. The authors are grateful for the careful English language editing by Dr. Karen Uray.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101261.
Contributor Information
László Virág, Email: lvirag@med.unideb.hu.
Rafael I. Jaén, Email: rafaelinigo@iib.uam.es.
Zsolt Regdon, Email: regdon.zsolt@med.unideb.hu.
Lisardo Boscá, Email: lbosca@iib.uam.es.
Patricia Prieto, Email: pprietoc@iib.uam.es.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bosca L., Gonzalez-Ramos S., Prieto P., Fernandez-Velasco M., Mojena M., Martin-Sanz P., Alemany S. Metabolic signatures linked to macrophage polarization: from glucose metabolism to oxidative phosphorylation. Biochem. Soc. Trans. 2015;43:740–744. doi: 10.1042/BST20150107. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A., Garlanda C., Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler. Thromb. Vasc. Biol. 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 3.Martinez F.O., Sica A., Mantovani A., Locati M. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 4.Hortelano S., Zeini M., Bosca L. Nitric oxide and resolution of inflammation. Methods Enzymol. 2002;359:459–465. doi: 10.1016/s0076-6879(02)59208-9. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Prados J.C., Traves P.G., Cuenca J., Rico D., Aragones J., Martin-Sanz P., Cascante M., Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 8.Spann N.J., Garmire L.X., McDonald J.G., Myers D.S., Milne S.B., Shibata N., Reichart D., Fox J.N., Shaked I., Heudobler D., Raetz C.R., Wang E.W., Kelly S.L., Sullards M.C., Murphy R.C., Merrill A.H., Jr., Brown H.A., Dennis E.A., Li A.C., Ley K., Tsimikas S., Fahy E., Subramaniam S., Quehenberger O., Russell D.W., Glass C.K. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltzschig H.K., Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly B., O'Neill L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swirski F.K., Weissleder R., Pittet M.J. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009;29:1424–1432. doi: 10.1161/ATVBAHA.108.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijayan V., Pradhan P., Braud L., Fuchs H.R., Gueler F., Motterlini R., Foresti R., Immenschuh S. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide - a divergent role for glycolysis. Redox Biol. 2019;22:101147. doi: 10.1016/j.redox.2019.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae Y.S., Oh H., Rhee S.G., Yoo Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hortelano S., Castrillo A., Alvarez A.M., Bosca L. Contribution of cyclopentenone prostaglandins to the resolution of inflammation through the potentiation of apoptosis in activated macrophages. J. Immunol. 2000;165:6525–6531. doi: 10.4049/jimmunol.165.11.6525. [DOI] [PubMed] [Google Scholar]
- 15.Prieto P., Cuenca J., Traves P.G., Fernandez-Velasco M., Martin-Sanz P., Bosca L. Lipoxin A4 impairment of apoptotic signaling in macrophages: implication of the PI3K/Akt and the ERK/Nrf-2 defense pathways. Cell Death Differ. 2010;17:1179–1188. doi: 10.1038/cdd.2009.220. [DOI] [PubMed] [Google Scholar]
- 16.Weigert A., von Knethen A., Fuhrmann D., Dehne N N., Brüne B. Redox-signals and macrophage biology. Mol. Asp. Med. 2018;63:70–87. doi: 10.1016/j.mam.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drose S., Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 19.Afonso V., Champy R., Mitrovic D., Collin P., Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Jt. Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 20.De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles) Physiol. Rev. 1966;46:323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- 21.Puppo A., Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Is haemoglobin a biological Fenton reagent? Biochem. J. 1988;249:185–190. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cencioni C., Spallotta F., Martelli F., Valente S., Mai A., Zeiher A.M., Gaetano C. Oxidative stress and epigenetic regulation in ageing and age-related diseases. Int. J. Mol. Sci. 2013;14:17643–17663. doi: 10.3390/ijms140917643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sen P., Shah P.P., Nativio R., Berger S.L. Epigenetic mechanisms of longevity and aging. Cell. 2016;166:822–839. doi: 10.1016/j.cell.2016.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Avraham D., Muzumdar R.H., Atzmon G. Epigenetic genome-wide association methylation in aging and longevity. Epigenomics. 2012;4:503–509. doi: 10.2217/epi.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rico D., Vaquerizas J.M., Dopazo H., Bosca L. Identification of conserved domains in the promoter regions of nitric oxide synthase 2: implications for the species-specific transcription and evolutionary differences. BMC Genomics. 2007;8:271. doi: 10.1186/1471-2164-8-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Roy A., Banerjee S., Saqib U., Baig M.S. NOS1-derived nitric oxide facilitates macrophage uptake of low-density lipoprotein. J. Cell. Biochem. 2019;120(7):11593–11603. doi: 10.1002/jcb.28439. [DOI] [PubMed] [Google Scholar]
- 28.Connelly L., Jacobs A.T., Palacios-Callender M., Moncada S., Hobbs A.J. Macrophage endothelial nitric-oxide synthase autoregulates cellular activation and pro-inflammatory protein expression. J. Biol. Chem. 2003;278:26480–26487. doi: 10.1074/jbc.M302238200. [DOI] [PubMed] [Google Scholar]
- 29.Bosca L., Hortelano S. Mechanisms of nitric oxide-dependent apoptosis: involvement of mitochondrial mediators. Cell. Signal. 1999;11:239–244. doi: 10.1016/s0898-6568(98)00064-3. [DOI] [PubMed] [Google Scholar]
- 30.Bosca L., Zeini M., Traves P.G., Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005;208:249–258. doi: 10.1016/j.tox.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 31.Hortelano S., Alvarez A.M., Bosca L. Nitric oxide induces tyrosine nitration and release of cytochrome c preceding an increase of mitochondrial transmembrane potential in macrophages. FASEB J. 1999;13:2311–2317. doi: 10.1096/fasebj.13.15.2311. [DOI] [PubMed] [Google Scholar]
- 32.Valez V., Cassina A., Batinic-Haberle I., Kalyanaraman B., Ferrer-Sueta G., Radi R. Peroxynitrite formation in nitric oxide-exposed submitochondrial particles: detection, oxidative damage and catalytic removal by Mn-porphyrins. Arch. Biochem. Biophys. 2013;529:45–54. doi: 10.1016/j.abb.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartesaghi S., Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018;14:618–625. doi: 10.1016/j.redox.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yermilov V., Rubio J., Becchi M., Friesen M.D., Pignatelli B., Ohshima H. Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis. 1995;16:2045–2050. doi: 10.1093/carcin/16.9.2045. [DOI] [PubMed] [Google Scholar]
- 35.Brune B., Courtial N., Dehne N., Syed S.N., Weigert A. Macrophage NOS2 in tumor leukocytes. Antioxidants Redox Signal. 2017;26:1023–1043. doi: 10.1089/ars.2016.6811. [DOI] [PubMed] [Google Scholar]
- 36.Genaro A.M., Hortelano S., Alvarez A., Martinez C., Bosca L. Splenic B lymphocyte programmed cell death is prevented by nitric oxide release through mechanisms involving sustained Bcl-2 levels. J. Clin. Investig. 1995;95:1884–1890. doi: 10.1172/JCI117869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hortelano S., Zeini M., Castrillo A., Alvarez A.M., Bosca L. Induction of apoptosis by nitric oxide in macrophages is independent of apoptotic volume decrease. Cell Death Differ. 2002;9:643–650. doi: 10.1038/sj.cdd.4401017. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Sanz P., Diaz-Guerra M.J., Casado M., Bosca L. Bacterial lipopolysaccharide antagonizes transforming growth factor beta 1-induced apoptosis in primary cultures of hepatocytes. Hepatology. 1996;23:1200–1207. doi: 10.1002/hep.510230539. [DOI] [PubMed] [Google Scholar]
- 39.Liu L., Stamler J.S. NO: an inhibitor of cell death. Cell Death Differ. 1999;6:937–942. doi: 10.1038/sj.cdd.4400578. [DOI] [PubMed] [Google Scholar]
- 40.Dimmeler S., Haendeler J., Nehls M., Zeiher A.M. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J. Exp. Med. 1997;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hortelano S., Traves P.G., Zeini M., Alvarez A.M., Bosca L. Sustained nitric oxide delivery delays nitric oxide-dependent apoptosis in macrophages: contribution to the physiological function of activated macrophages. J. Immunol. 2003;171:6059–6064. doi: 10.4049/jimmunol.171.11.6059. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y.M., Talanian R.V., Billiar T.R. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J. Biol. Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 43.Regdon Z., Robaszkiewicz A., Kovacs K., Rygielska Z., Hegedus C., Bodoor K., Szabo E., Virag L. LPS protects macrophages from AIF-independent parthanatos by downregulation of PARP1 expression, induction of SOD2 expression, and a metabolic shift to aerobic glycolysis. Free Radic. Biol. Med. 2019;131:184–196. doi: 10.1016/j.freeradbiomed.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 44.Vanden Berghe T., Linkermann A., Jouan-Lanhouet S., Walczak H., Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 45.Dai L., Bhargava P., Stanya K.J., Alexander R.K., Liou Y.H., Jacobi D., Knudsen N.H., Hyde A., Gangl M.R., Liu S., Lee C.H. Macrophage alternative activation confers protection against lipotoxicity-induced cell death. Mol Metab. 2017;6:1186–1197. doi: 10.1016/j.molmet.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virag L., Salzman A.L., Szabo C. Poly(ADP-ribose) synthetase activation mediates mitochondrial injury during oxidant-induced cell death. J. Immunol. 1998;161:3753–3759. [PubMed] [Google Scholar]
- 47.Tokarz P., Ploszaj T., Regdon Z., Virag L., Robaszkiewicz A. PARP1-LSD1 functional interplay controls transcription of SOD2 that protects human pro-inflammatory macrophages from death under an oxidative condition. Free Radic. Biol. Med. 2019;131:218–224. doi: 10.1016/j.freeradbiomed.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Hard G.C. Some biochemical aspects of the immune macrophage. Br. J. Exp. Pathol. 1970;51:97–105. [PMC free article] [PubMed] [Google Scholar]
- 49.Newsholme P., Curi R., Gordon S., Newsholme E.A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem. J. 1986;239:121–125. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newsholme P., Gordon S., Newsholme E.A. Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem. J. 1987;242:631–636. doi: 10.1042/bj2420631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Icard P., Shulman S., Farhat D., Steyaert J.M., Alifano M., Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist. Updates. 2018;38:1–11. doi: 10.1016/j.drup.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Wang P., Geng J., Gao J., Zhao H., Li J., Shi Y., Yang B., Xiao C., Linghu Y., Sun X., Chen X., Hong L., Qin F., Li X., Yu J.S., You H., Yuan Z., Zhou D., Johnson R.L., Chen L. Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 2019;10:755. doi: 10.1038/s41467-019-08680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponath V., Kaina B. Death of monocytes through oxidative burst of macrophages and neutrophils: killing in trans. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Itoh K., Mochizuki M., Ishii Y., Ishii T., Shibata T., Kawamoto Y., Kelly V., Sekizawa K., Uchida K., Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2) Mol. Cell. Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li N., Alam J., Venkatesan M.I., Eiguren-Fernandez A., Schmitz D., Di Stefano E., Slaughter N., Killeen E., Wang X., Huang A., Wang M., Miguel A.H., Cho A., Sioutas C., Nel A.E. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J. Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- 56.Luo J.F., Shen X.Y., Lio C.K., Dai Y., Cheng C.S., Liu J.X., Yao Y.D., Yu Y., Xie Y., Luo P., Yao X.S., Liu Z.Q., Zhou H. Activation of Nrf2/HO-1 pathway by nardochinoid C inhibits inflammation and oxidative stress in lipopolysaccharide-stimulated macrophages. Front. Pharmacol. 2018;9:911. doi: 10.3389/fphar.2018.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional regulation by Nrf2. Antioxidants Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siegel D., Gustafson D.L., Dehn D.L., Han J.Y., Boonchoong P., Berliner L.J., Ross D. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol. Pharmacol. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 59.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ooi B.K., Goh B.H., Yap W.H. Oxidative stress in cardiovascular diseases: involvement of Nrf2 antioxidant redox signaling in macrophage foam cells formation. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18112336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbas K., Breton J., Planson A.G., Bouton C., Bignon J., Seguin C., Riquier S., Toledano M.B., Drapier J.C. Nitric oxide activates an Nrf2/sulfiredoxin antioxidant pathway in macrophages. Free Radic. Biol. Med. 2011;51:107–114. doi: 10.1016/j.freeradbiomed.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 62.Geng J., Sun X., Wang P., Zhang S., Wang X., Wu H., Hong L., Xie C., Li X., Zhao H., Liu Q., Jiang M., Chen Q., Zhang J., Li Y., Song S., Wang H.R., Zhou R., Johnson R.L., Chien K.Y., Lin S.C., Han J., Avruch J., Chen L., Zhou D. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat. Immunol. 2015;16:1142–1152. doi: 10.1038/ni.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mullebner A., Dorighello G.G., Kozlov A.V., Duvigneau J.C. Interaction between mitochondrial reactive oxygen species, heme oxygenase, and nitric oxide synthase stimulates phagocytosis in macrophages. Front. Med. 2017;4:252. doi: 10.3389/fmed.2017.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonora M., Wieckowsk M.R., Chinopoulos C., Kepp O., Kroemer G., Galluzzi L., Pinton P. Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene. 2015;34:1608. doi: 10.1038/onc.2014.462. [DOI] [PubMed] [Google Scholar]
- 65.Dejean A.S., Hedrick S.M., Kerdiles Y.M. Highly specialized role of Forkhead box O transcription factors in the immune system. Antioxidants Redox Signal. 2011;14:663–674. doi: 10.1089/ars.2010.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung S., Ranjan R., Lee Y.G., Park G.Y., Karpurapu M., Deng J., Xiao L., Kim J.Y., Unterman T.G., J.W Christman Distinct role of FoxO1 in M-CSF- and GM-CSF-differentiated macrophages contributes LPS-mediated IL-10: implication in hyperglycemia. J. Leukoc. Biol. 2015;97:327–339. doi: 10.1189/jlb.3A0514-251R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan W., Morinaga H., Kim J.J., Bae E., Spann N.J., Heinz S., Glass C.K., J.M Olefsky FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J. 2010;29:4223–4236. doi: 10.1038/emboj.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsuchiya K., Westerterp M., Murphy A.J., Subramanian V., Ferrante A.W., Jr., Tall A.R., Accili D. Expanded granulocyte/monocyte compartment in myeloid-specific triple FoxO knockout increases oxidative stress and accelerates atherosclerosis in mice. Circ. Res. 2013;112:992–1003. doi: 10.1161/CIRCRESAHA.112.300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Filomeni G., De Zio D., Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grootjans J., Kaser A., Kaufman R.J., Blumberg R.S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 2016;16:469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corcoran S.E., O'Neill L.A. HIF1alpha and metabolic reprogramming in inflammation. J. Clin. Investig. 2016;126:3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bah A., Vergne I. Macrophage autophagy and bacterial infections. Front. Immunol. 2017;8:1483. doi: 10.3389/fimmu.2017.01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang J., Brumell J.H. NADPH oxidases contribute to autophagy regulation. Autophagy. 2009;5:887–889. doi: 10.4161/auto.9125. [DOI] [PubMed] [Google Scholar]
- 74.Martinon F., Chen X., Lee A.H., Glimcher L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cullinan S.B., Zhang D., Hannink M., Arvisais E., Kaufman R.J., Diehl J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pajares M., Cuadrado A., Rojo A.I. Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox Biol. 2017;11:543–553. doi: 10.1016/j.redox.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao T., Zhu Y., Morinibu A., Kobayashi M., Shinomiya K., Itasaka S., Yoshimura M., Guo G., Hiraoka M., Harada H. HIF-1-mediated metabolic reprogramming reduces ROS levels and facilitates the metastatic colonization of cancers in lungs. Sci. Rep. 2014;4:3793. doi: 10.1038/srep03793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nevo Y., Nelson N. The NRAMP family of metal-ion transporters. Biochim. Biophys. Acta. 2006;1763:609–620. doi: 10.1016/j.bbamcr.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Kosman D.J. Redox cycling in iron uptake, efflux, and trafficking. J. Biol. Chem. 2010;285:26729–26735. doi: 10.1074/jbc.R110.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., 3rd, Stockwell Ferroptosis B.R. An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Korolnek T., Hamza I. Macrophages and iron trafficking at the birth and death of red cells. Blood. 2015;125:2893–2897. doi: 10.1182/blood-2014-12-567776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soe-Lin S., Sheftel A.D., Wasyluk B., Ponka P. Nramp1 equips macrophages for efficient iron recycling. Exp. Hematol. 2008;36:929–937. doi: 10.1016/j.exphem.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 83.Taille C., El-Benna J., Lanone S., Dang M.C., Ogier-Denis E., Aubier M., Boczkowski J. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J. Biol. Chem. 2004;279:28681–28688. doi: 10.1074/jbc.M310661200. [DOI] [PubMed] [Google Scholar]
- 84.Brouard S., Otterbein L.E., Anrather J., Tobiasch E., Bach F.H., Choi A.M., Soares M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gieseg S.P., Leake D.S., Flavall E.M., Amit Z., Reid L., Yang Y.T. Macrophage antioxidant protection within atherosclerotic plaques. Front. Biosci. 2009;14:1230–1246. doi: 10.2741/3305. [DOI] [PubMed] [Google Scholar]
- 86.Babaev V.R., Whitesell R.R., Li L., Linton M.F., Fazio S., May J.M. Selective macrophage ascorbate deficiency suppresses early atherosclerosis. Free Radic. Biol. Med. 2011;50:27–36. doi: 10.1016/j.freeradbiomed.2010.10.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gieseg S.P., Baxter-Parker G., Lindsay Neopterin A. Inflammation, and oxidative stress: what could we Be missing? Antioxidants. 2018;7 doi: 10.3390/antiox7070080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rode S., Rubic T., Lorenz R.L. Alpha-Tocopherol disturbs macrophage LXRalpha regulation of ABCA1/G1 and cholesterol handling. Biochem. Biophys. Res. Commun. 2008;369:868–872. doi: 10.1016/j.bbrc.2008.02.132. [DOI] [PubMed] [Google Scholar]
- 89.Yin S., Cao W. Toll-like receptor signaling induces Nrf2 pathway activation through p62-triggered Keap1 degradation. Mol. Cell. Biol. 2015;35:2673–2683. doi: 10.1128/MCB.00105-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 91.Prieto P., Rosales-Mendoza C.E., Terron V., Toledano V., Cuadrado A., Lopez-Collazo E., Bannenberg G., Martin-Sanz P., Fernandez-Velasco M., Bosca L. Activation of autophagy in macrophages by pro-resolving lipid mediators. Autophagy. 2015;11:1729–1744. doi: 10.1080/15548627.2015.1078958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chiurchiu V., Leuti A., M Maccarrone bioactive lipids and chronic inflammation: managing the fire within. Front. Immunol. 2018;9:38. doi: 10.3389/fimmu.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Serhan C.N., Levy B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018;128:2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clarke A.J., Simon A.K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 2019;19:170–183. doi: 10.1038/s41577-018-0095-2. [DOI] [PubMed] [Google Scholar]
- 95.Lee H.N., Surh Y.J. Resolvin D1-mediated NOX2 inactivation rescues macrophages undertaking efferocytosis from oxidative stress-induced apoptosis. Biochem. Pharmacol. 2013;86:759–769. doi: 10.1016/j.bcp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 96.Nascimento-Silva V., Arruda M.A., Barja-Fidalgo C., Fierro I.M. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb. Haemostasis. 2007;97:88–98. [PubMed] [Google Scholar]
- 97.Posso S.V., Quesnot N., Moraes J.A., Brito-Gitirana L., Kennedy-Feitosa E., Barroso M.V., Porto L.C., Lanzetti M., Valenca S.S. AT-RVD1 repairs mouse lung after cigarette smoke-induced emphysema via downregulation of oxidative stress by NRF2/KEAP1 pathway. Int. Immunopharmacol. 2018;56:330–338. doi: 10.1016/j.intimp.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 98.Spite M., Summers L., Porter T.F., Srivastava S., Bhatnagar A., Serhan C.N. Resolvin D1 controls inflammation initiated by glutathione-lipid conjugates formed during oxidative stress. Br. J. Pharmacol. 2009;158:1062–1073. doi: 10.1111/j.1476-5381.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saito P., Melo C.P.B., Martinez R.M., Fattori V., Cezar T.L.C., Pinto I.C., Bussmann A.J.C., Vignoli J.A., Georgetti S.R., Baracat M.M., Verri W.A., Jr., Casagrande R. The lipid mediator resolvin D1 reduces the skin inflammation and oxidative stress induced by UV irradiation in hairless mice. Front. Pharmacol. 2018;9:1242. doi: 10.3389/fphar.2018.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fedirko V., McKeown-Eyssen G., Serhan C.N., Barry E.L., Sandler R.S., Figueiredo J.C., Ahnen D.J., Bresalier R.S., Robertson D.J., Anderson C.W., Baron J.A. Plasma lipoxin A4 and resolvin D1 are not associated with reduced adenoma risk in a randomized trial of aspirin to prevent colon adenomas. Mol. Carcinog. 2017;56:1977–1983. doi: 10.1002/mc.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hashimoto A., Hayashi I., Murakami Y., Sato Y., Kitasato H., Matsushita R., Iizuka N., Urabe K., Itoman M., Hirohata S., Endo H. Antiinflammatory mediator lipoxin A4 and its receptor in synovitis of patients with rheumatoid arthritis. J. Rheumatol. 2007;34:2144–2153. [PubMed] [Google Scholar]
- 102.Kirkby N.S., Chan M.V., Lundberg M.H., Massey K.A., Edmands W.M., MacKenzie L.S., Holmes E., Nicolaou A., Warner T.D., Mitchell J.A. Aspirin-triggered 15-epi-lipoxin A4 predicts cyclooxygenase-2 in the lungs of LPS-treated mice but not in the circulation: implications for a clinical test. FASEB J. 2013;27:3938–3946. doi: 10.1096/fj.12-215533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y., Igwe O.J. Role of Toll-like receptor 4/oxidant-coupled activation in regulating the biosynthesis of omega-3 polyunsaturated fatty acid derivative resolvin D1 in primary murine peritoneal macrophage. Biochem. Pharmacol. 2018;158:73–83. doi: 10.1016/j.bcp.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 104.Vida C., de Toda I.M., Cruces J., Garrido A., Gonzalez-Sanchez M., De la Fuente M. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol. 2017;12:423–437. doi: 10.1016/j.redox.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abooali M., Lall G.S., Coughlan K., Lall H.S., Gibbs B.F., Sumbayev V.V. Crucial involvement of xanthine oxidase in the intracellular signalling networks associated with human myeloid cell function. Sci. Rep. 2014;4:6307. doi: 10.1038/srep06307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gangemi S., Pescara L., D'Urbano E., Basile G., Nicita-Mauro V., Davi G., Romano M. Aging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levels. Exp. Gerontol. 2005;40:612–614. doi: 10.1016/j.exger.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Franz K.M., Kagan J.C. Innate immune receptors as competitive determinants of cell fate. Mol. Cell. 2017;66:750–760. doi: 10.1016/j.molcel.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robinson N., Ganesan R., Hegedűs C., Kovács K., Kufer T., A., Virág L. Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biology. 2019;26:101239. doi: 10.1016/j.redox.2019.101239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.