Abstract
Background
Dry pleural dissemination (DPD) in non-small cell lung cancer (NSCLC) is defined as having solid pleural metastases without malignant pleural effusion. We aim to identify DPD by applying radiomics, a novel approach to decode the tumor phenotype.
Methods
Preoperative chest computed tomographic images and basic clinical feature were retrospectively evaluated in patients with surgically resected NSCLC between January 1, 2015 and December 31, 2016. Propensity score was applied to match the DPD and non-DPD groups. One thousand and eighty radiomics features were quantitatively extracted by the 3D slicer software and “pyradiomics” package. Least absolute shrinkage and selection operator (LASSO) binary model was applied for feature selection and developing the radiomics signature. The discrimination was evaluated using area under the curve (AUC) and Youden index.
Results
Sixty-four DPD patients and paired 192 non-DPD patients were enrolled. Using the LASSO model, this study developed a radiomics signature including 10 radiomic features. The mean ± standard deviation values of the radiomics signature with DPD status (−2.129±1.444) was significantly higher compared to those with non-DPD disease (0.071±0.829, P<0.001). The ten-feature based signature showed good discrimination between DPD and non-DPD, with an AUC of 0.93 (95% confidence-interval, 0.891–0.958) respectively. The sensitivity and specificity of the radiomics signature was 85.94% and 85.94%, with the optimal cut-off value of −0.696 and Youden index of 0.71.
Conclusions
The signature based on radiomics features can provide potential predictive value to identify DPD in patients with NSCLC.
Keywords: Radiomics, dry pleural dissemination (DPD), predict
Introduction
Malignant pleural dissemination is generally considered as a contraindicative disease stage to surgery (1). In the American Joint Committee on Cancer (AJCC) staging system of non-small cell lung cancer (NSCLC) based on tumor, nodal, and metastatic categories (TNM staging) (2,3), M1a stage was classified having poor clinical outcomes, with a 3-year overall survival less than 23% and a median survival time (MST) of 11.5 months (4).
Patients with M1a disease showed a range of disease diversity, varying from multiple pleural nodules or/with massive malignant pleural effusion to minimal disease with single solid pleural nodule without malignant pleural effusion (5,6). Betweenwhiles, thoracic surgeons may find unexpected pleural malignant nodules in patients who had clinical stage M0 during operation (7,8). Dry pleural dissemination (DPD) in NSCLC is defined as solid pleural metastases without pleural malignant effusion (9). Those clinical dilemmas, which could not be identified preoperatively because of the lack of commonly accompanying malignant pleural effusions, can result in misdiagnose without identifying the presence of DPD (1).
Computed tomographic (CT) findings of DPD present as several small pleural malignant nodules or/with uneven pleural thickening on the basis of existing research (9). It has been proved that the pleural small nodules or/with uneven pleural thickening can generally exhibit scanty fluorine 18 fluorodeoxyglucose (FDG) uptake at positron emission tomography (PET). The accuracy of diagnosing DPD can be improved using CT combining PET/CT. While CT images may strongly suggest the presence of DPD even when PET images showed little FDG uptake, the commonly malignant alerted CT characteristics may prove significance to the diagnosis of DPD. However, by only manually assessing the CT images, doctors lack enough information to detect small pleural nodules or define nodule’s pathological nature (10). To get more detailed information as reference, radiomics, defined as a set of quantitative features from medical images (CT, magnetic resonance imaging, ultrasound, etc.) using data-characterization analyses, may be paly an effective role. Recent researches have reported that radiomic analysis showed huge effect for excellent CT lesion characterization and excellent performance (11,12).
By applying numerous quantitative imaging features, radiomics provides a novel approach to decode the tumor phenotype. Herein, we aim to identify the patients with DPD preoperatively by CT images radiomics analysis.
Methods
Patients
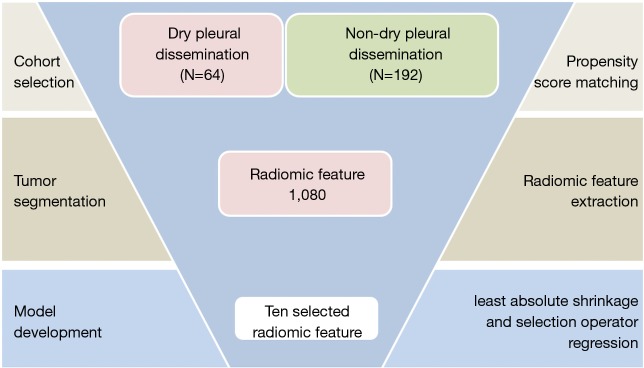
The institutional review board approved this retrospective study with informed consent (IRB No. K18-105). This retrospective study included consecutive patients with histologically confirmed NSCLC of the primary lung cancer from Jan 2015 to Dec 2016. All included cases fulfilled the following inclusion criteria: (I) All patients underwent examinations to exclude remote metastasis. Whole body bone scan, brain magnetic resonance imaging, and upper abdominal ultrasonic examinations confirmed the only locally advanced nature of the tumors preoperatively (II) qualified clinical characteristics, including smoking history, age, sex, Location, histological subtype and TNM stage; (III) Complete thin-section CT images stored in the Picture Archiving and Communication System (PACS); (IV) patient did not received neoadjuvant treatment (including target therapy). Patients were excluded if they reported M1a with malignant pleural effusion postoperatively. Eligible patients were divided into the non-DPD metastases cohort and DPD metastases cohort (Figure 1).
Figure 1.
Overview of radiomics model development.
Propensity scores matching
The propensity scores analysis, which convert the observational (nonrandomized) study to simulate a randomized controlled trial by mimic baseline characteristics, including the following variables: age, sex, smoking history, histological subtype, T stage and N stage represent the probability of being assigned to either non-DPD metastases group and DPD group. After the matching procedure, the non-DPD metastases group and DPD group had balanced distributions of propensity scores, minimizing the distinction in variables between two cohorts. In this study, it was settled as propensity scores one by three using nearest neighbor algorithm, with 0.1 caliper width and no replacement. After propensity score matching, the clinical characteristics comparation were listed, respectively. All clinicopathological and demographic data were presented as median (range) or number (percentage).
CT scanning protocol
All CT scans were obtained by using a Somatom Definition AS (Siemens Medical Systems, Germany). All images were reconstructed at 1.0 mm slice thickness, with 0.7 mm increment, 512 mm × 512 mm and a standard soft kernel (Siemens B31 filter, Siemens Medical Solutions, Forchheim, Germany). All CT scans were obtained in the full inspiratory phase.
Tumor segmentation and radiomic feature extraction
The open-source software 3D-slicer (www.slicer.org) were used in this study as the analysis platform to achieve nodule segmentation and radiomic feature extraction (13). Nodules were delineated on the CT images using a semi-automatic GrowCut segmentation algorithm, which is settled to have best accuracy and speed for the 3D nodule segmentation with an interactive region growing method and allows more robust radiomic feature extraction (14,15). Primary tumor segmentation was confirmed by the radiologist attending (J Shi and X Sun), both who had twenty years of experience in pulmonary radiogram. After tumor segmentation, 1,080 radiomic features (16) were extracted by the “pyradiomics” package with the software (JetBrains PyCharm Community Edition 2017.2.4; https://www.jetbrains.com/pycharm/).
Statistical analysis
Pearson test was used to compare the categorical data, and an independent sample t-test was used to compare the numerical data between two groups. The least absolute shrinkage and selection operator (LASSO) binary regression model were applied to select the most predictive features in order to develop the radiomic signature. The discrimination of the signature was calculated by the area under the curve (AUC). We used the “glmnet” package of R software (version 3.2.2) to run the LASSO analysis. All other statistical analysis was calculated with SPSS, version 23.0. A two-sided P value <0.05 was considered statistically significant.
Results
Clinical characteristics
Finally, matched 192 patients from non-DPD metastases group and 64 patients from DPD group were included in the analysis. Clinical characteristics in both DPD and non-DPD were listed in Table 1. The majority of patients had peripheral lesions (93%). The median age was 58 years. The majority of tumors were T2 stage disease (67%) and adenocarcinoma (95%). After matching, there were no significant differences in age, sex, smoking status, histological subtype, T stage and N stage between the non-DPD and DPD group.
Table 1. Clinical and pathological characteristics in the cohort after propensity scores matching.
| Characteristics | Non-DPD (n=192) | DPD (n=64) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 61.474±9.871 | 58.390±9.694 | 0.943 |
| Gender | 0.718 | ||
| Male | 97 [51] | 34 [53] | |
| Female | 95 [49] | 30 [47] | |
| Tumor size (cm), mean ± SD | 3.267±1.146 | 3.225±1.077 | 0.363 |
| Location | 0.869 | ||
| Peripheral | 183 [95] | 60 [94] | |
| Central | 9 [5] | 4 [6] | |
| Histology subtype | 1 | ||
| Adenocarcinoma | 185 [96] | 61 [95] | |
| Squamous cell carcinoma | 7 [4] | 3 [5] | |
| T status | 0.734 | ||
| T1 | 57 [29] | 19 [30] | |
| T2 | 132 [69] | 43 [67] | |
| T3 | 3 [2] | 2 [3] | |
| Node status | 0.133 | ||
| N0/1 | 71 [37] | 18 [28] | |
| N2 | 95 [49] | 31 [48] | |
| Nx | 26 [14] | 15 [24] | |
| Radiomics score | −2.129±1.444 | 0.071±0.829 | <0.001 |
Data are presented as median ± SD or n [%], DPD, dry pleural dissemination
Feature selection of the radiomic signature
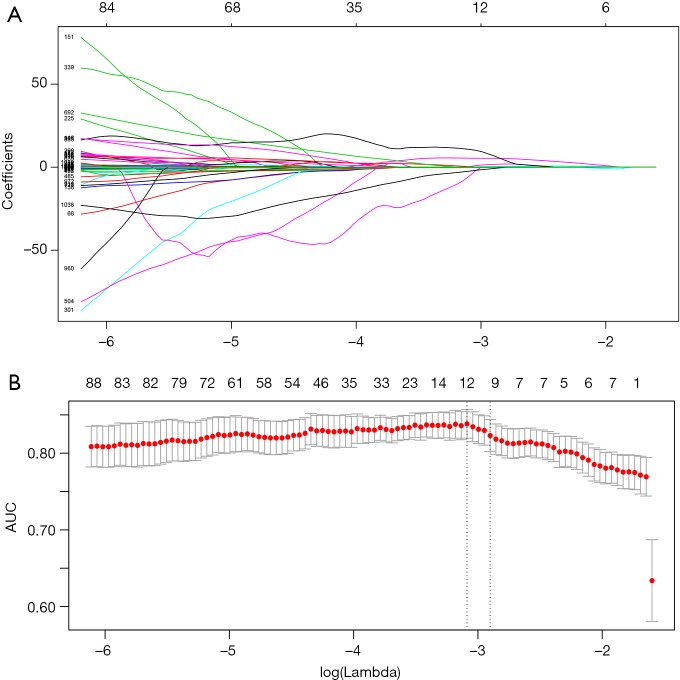
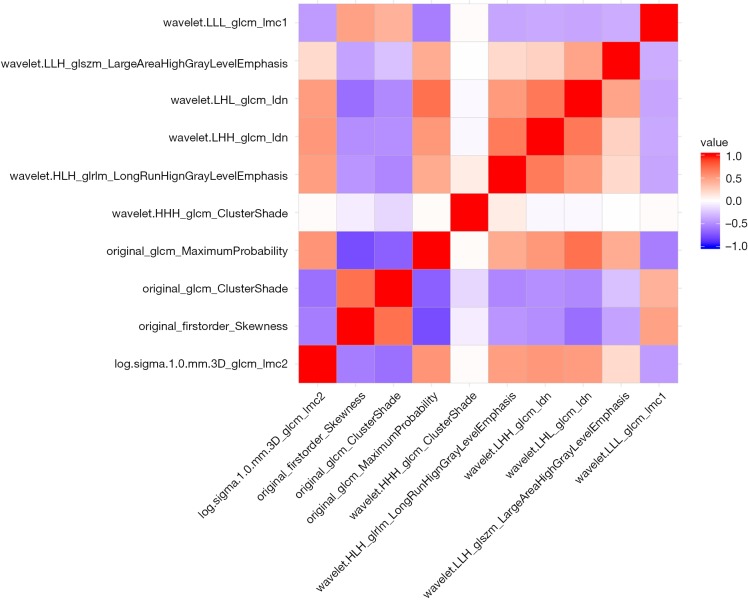
We used 10-fold cross-validation via minimum criteria as tuning parameter (λ) selection in the LASSO model and settled the λ value of 0.018 with log(λ) –3.97 (one standard error of the minimum criteria) for optimal value (Figure 2). Under this procedure, 1,080 radiomic features were reduced to 10 effective predictors with nonzero coefficients in the LASSO model in the cohort. The Figure S1 showed the 10 robust and non-redundant radiomic features (Log.sigma.1.0.mm.3D_glcm_Imc2, Original_firstorder_Skewness, Original_glcm_ClusterShade, Original_glcm_MaximumProbability, Wavelet.HHH_glcm_ClusterShade, Wavelet.HLH_glrlm_LongRunHighGrayLevelEmphasis, Wavelet.LHH_glcm_Idn, Wavelet.LHL_glcm_Idn, Wavelet.LLH_glszm_LargeAreaHighGrayLevelEmphasis, Wavelet.LLL_glcm_Imc1) with Pearson’s correlation matrix.
Figure 2.
Radiomic feature selection using the least absolute shrinkage and selection operator (LASSO) binary logistic regression model.
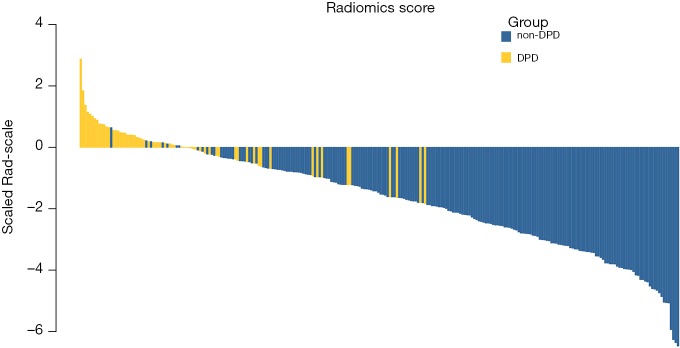
The radiomic signature, which was based on selected 10 radiomic features, was calculated for each case (http://fp.amegroups.cn/cms/atm.2019.05.20-1.pdf). The score of radiomics signature calculated for each case was shown in a waterfall plot (Figure 3) and demonstrated by heatmap (Figure S1). The values were significantly different between non-DPD and DPD status for all 10 selected radiomics features (P<0.001). The mean values of the radiomics signature with DPD status (−2.129±1.444) was significantly higher compared to those without DPD disease (0.071±0.829; P<0.001; Table 1).
Figure 3.
Rad-score for all patients in the cohort. Classification of dry pleural dissemination status was marked with different colors.
Performance of the radiomics signature
The radiomic signature that we developed showed a significant capability to distinguish DPD status from non-DPD status in the cohort (AUC: 0.93, 95% CI, 0.891 to 0.958; Figure 4). The sensitivity and specificity of the radiomics signature was 85.94% and 85.94%, with the optimal cut-off value of −0.696 and Youden index of 0.71.
Figure 4.
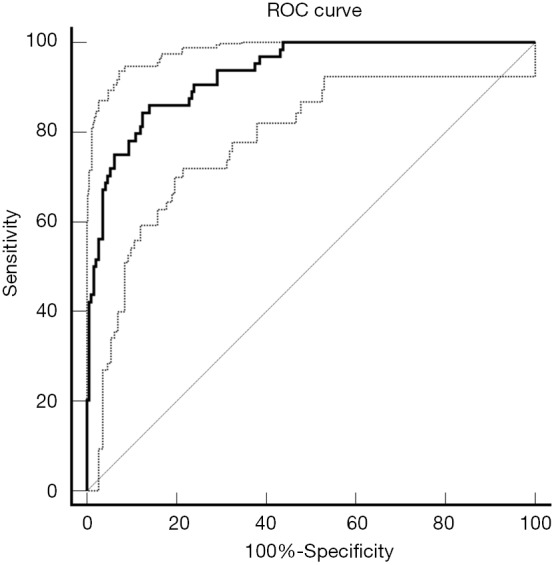
Area under the curve (AUC) of the radiomic signature
Discussion
Radiomic assessment of the image phenotype can be used with non-invasive CT scan that are used routinely in clinical prevention procedure for lung cancer (17,18). Our innovative point was using quantitative radiomic measurements, which should more individually reflect tumor malignancy. In this study, by using a propensity scores matching screening dataset of image, we demonstrate that radiomic model cam distinguish DPD and non-DPD status with sensitivity 0.859 and specificity 0.859. This approach, after being valid in clinical trials, could improve the management of screen-identified primary lung cancer and potentially minimize health care costs and reduce unnecessary surgery associated with the clinical routine for the evaluation and management of undetected DPD status before surgery.
From the beginning of seventh TNM staging for NSCLC, pleural dissemination was transferred to the M1a category, which including pleural metastasis and/or contralateral metastatic nodules (19). For now, DPD has not been clearly classified, but the surgeons suspected that DPD can be defined as a type of intrathoracic tumor spread. One important aim of promoting the application of CT features in patients with lung cancer is to more accurately diagnose M1a patients before surgery. Previous studies (20,21) have revealed that DPD can more frequently appeared when the primary lesion is adjacent to the pleura or the fissure. Kim et al. (9) reported that NSCLCs with lower T and N status can invade into the thorax breakthrough pleura when the lesions are adjacent to the fissure or the pleura.
CT image is widely used in oncological practice for lung cancer detection. Usually, we diagnose the lung nodule rely on the manual assessment of physicians; there are tiny features, which can not be detected manually and require computer-aided techniques. Many published researches have revealed that the use of CT-based radiomics texture analysis in prediction of driver mutations in NSCLC (13,22,23). To the best of our knowledge, only a few studies have focused on the association between CT radiomics features and DPD status. Here, this study presented a sophisticated radiomic feature analysis using semi-automatic segmentation in 192 patients from non-DPD metastases group and 64 patients from DPD metastases group. Radiomics features were extracted to evaluate the potential to predict DPD status preoperatively. To avoid the overfitting because of vast sum of radiomic features in the classification model, LASSO binary model and 10-fold validation was used to develop the radiomics signature. After above procedure, ten radiomic features were set up from two different feature categories, which showed significantly association with DPD status.
There are main differences between histological and radiological based exam. Medical image diagnoses can collect the malignant features at a macroscopic level (CT high pixel), while histology relies on a microscopic level and provides a more detailed analysis of underlying pathological processes. However, Imaging outweigh histology because it can capture the complete tumor burden in a single scan. Imaging-based features could potentially be extracted in clinical situations as adding clinical information ahead of bio-histology results. More validation trial should focus on comparing radiomics to liquid biopsy (such as ctDNA or cfDNA), since both of these approaches show dominant advance in reflecting the overall tumor burden as opposed to the histological exam (24).
There were some limitations of our study: (I) this was a retrospective study from a single institution from China, and there may have been selection bias in the study cohort. (II) Our analysis did not analysis the perifissural nodules, while Mets et al. indicated that incidental perifissural nodules do not represent lung cancer in a routine care, heterogeneous population (25). (III) In our study, radiomics features were applied for only the main lung primary lesion of each case, which could influence sensitivity and specificity of the model. (IV) positron emission tomography CT (PET-CT) test information were not included in this study. However, in spite of these limitations potentially adding noise to the data, this study using quantitative radiomic measurements to identify a strong signal predictive of DPD preoperatively. Further innovation test and validation of imaging phenotype is an important aspect for the introduction of imaging-based features.
Conclusions
In conclusion, this research revealed a strong association between the imaging phenotype captured from radiomic features and DPD status. The signature based on radiomics features can provide added predictive value to identify DPD.
Figure S1.
Heatmap of ten selected radiomic signature.
Acknowledgments
Funding: Supported by the projects from technical research and development project of technology benefiting for people of Ningbo, Zhejiang Province, China [grant number 2015C50032], Shanghai Hospital Development Center (16CR3116B), National Key R&D Program of China (grant number 2016YFC0905402)].
Ethical Statement: The Shanghai Pulmonary Hospital institutional ethics committee approved this retrospective study (IRB No. K18-105).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Iida T, Shiba M, Yoshino I, et al. Surgical Intervention for Non-Small-Cell Lung Cancer Patients with Pleural Carcinomatosis: Results From the Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2015;10:1076-82. 10.1097/JTO.0000000000000554 [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. Journal of Thoracic Oncology 2016;11:39-51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 3.Tendler S, Grozman V, Lewensohn R, et al. Validation of the 8th TNM classification for small-cell lung cancer in a retrospective material from Sweden. Lung Cancer 2018;120:75-81. 10.1016/j.lungcan.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 4.Chikaishi Y, Shinohara S, Kuwata T, et al. Complete resection of the primary lesion improves survival of certain patients with stage IV non-small cell lung cancer. J Thorac Dis 2017;9:5278-87. 10.21037/jtd.2017.11.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez de Cos Escuin J, Abal Arca J, Melchor Iniguez R, et al. Tumor, node and metastasis classification of lung cancer--M1a versus M1b--analysis of M descriptors and other prognostic factors. Lung Cancer 2014;84:182-9. 10.1016/j.lungcan.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 6.Zhong WZ, Li W, Yang XN, et al. Accidental invisible intrathoracic disseminated pT4-M1a: a distinct lung cancer with favorable prognosis. J Thorac Dis 2015;7:1205-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren Y, Dai C, Shen J, et al. The prognosis after contraindicated surgery of NSCLC patients with malignant pleural effusion (M1a) may be better than expected. Oncotarget 2016;7:26856-65. 10.18632/oncotarget.8566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorelli A, Santini M. In lung cancer patients where a malignant pleural effusion is found at operation could resection ever still be justified? Interact Cardiovasc Thorac Surg 2013;17:407-12. 10.1093/icvts/ivt153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YK, Lee HY, Lee KS, et al. Dry pleural dissemination in non-small cell lung cancer: prognostic and diagnostic implications. Radiology 2011;260:568-74. 10.1148/radiol.11110053 [DOI] [PubMed] [Google Scholar]
- 10.Hallifax RJ, Haris M, Corcoran JP, et al. Role of CT in assessing pleural malignancy prior to thoracoscopy. Thorax 2015;70:192-3. 10.1136/thoraxjnl-2014-206054 [DOI] [PubMed] [Google Scholar]
- 11.Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014;5:4006. 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chae HD, Park CM, Park SJ, et al. Computerized texture analysis of persistent part-solid ground-glass nodules: differentiation of preinvasive lesions from invasive pulmonary adenocarcinomas. Radiology 2014;273:285-93. 10.1148/radiol.14132187 [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, She Y, Xie D, et al. A Texture Analysis-Based Prediction Model for Lymph Node Metastasis in Stage IA Lung Adenocarcinoma. Ann Thorac Surg 2018;106:214-20. 10.1016/j.athoracsur.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 14.Parmar C, Rios Velazquez E, Leijenaar R, et al. Robust Radiomics feature quantification using semiautomatic volumetric segmentation. PLoS One 2014;9:e102107. 10.1371/journal.pone.0102107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velazquez ER, Parmar C, Jermoumi M, et al. Volumetric CT-based segmentation of NSCLC using 3D-Slicer. Sci Rep 2013;3:3529. 10.1038/srep03529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beig N, Khorrami M, Alilou M, et al. Perinodular and Intranodular Radiomic Features on Lung CT Images Distinguish Adenocarcinomas from Granulomas. Radiology 2019;290:783-92. 10.1148/radiol.2018180910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma V, Simone CB, 2nd, Krishnan S, et al. The Rise of Radiomics and Implications for Oncologic Management. J Natl Cancer Inst 2017;109. doi: . 10.1093/jnci/djx055 [DOI] [PubMed] [Google Scholar]
- 18.Rizzo S, Petrella F, Buscarino V, et al. CT Radiogenomic Characterization of EGFR, K-RAS, and ALK Mutations in Non-Small Cell Lung Cancer. Eur Radiol 2016;26:32-42. 10.1007/s00330-015-3814-0 [DOI] [PubMed] [Google Scholar]
- 19.Detterbeck FC, Boffa DJ, Tanoue LT. The New Lung Cancer Staging System. Chest 2009;136:260-71. 10.1378/chest.08-0978 [DOI] [PubMed] [Google Scholar]
- 20.Murayama S, Murakami J, Yoshimitsu K, et al. CT diagnosis of pleural dissemination without pleural effusion in primary lung cancer. Radiat Med 1996;14:117-9. [PubMed] [Google Scholar]
- 21.Leung AN, Muller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol 1990;154:487-92. 10.2214/ajr.154.3.2106209 [DOI] [PubMed] [Google Scholar]
- 22.Rios Velazquez E, Parmar C, Liu Y, et al. Somatic Mutations Drive Distinct Imaging Phenotypes in Lung Cancer. Cancer Res 2017;77:3922-30. 10.1158/0008-5472.CAN-17-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou JY, Zheng J, Yu ZF, et al. Comparative analysis of clinicoradiologic characteristics of lung adenocarcinomas with ALK rearrangements or EGFR mutations. Eur Radiol 2015;25:1257-66. 10.1007/s00330-014-3516-z [DOI] [PubMed] [Google Scholar]
- 24.Song C, Liu Y, Fontana R, et al. Elimination of unaltered DNA in mixed clinical samples via nuclease-assisted minor-allele enrichment. Nucleic Acids Res 2016;44:e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mets OM, Chung K, Scholten ET, et al. Incidental perifissural nodules on routine chest computed tomography: lung cancer or not? Eur Radiol 2018;28:1095-101. 10.1007/s00330-017-5055-x [DOI] [PMC free article] [PubMed] [Google Scholar]