Abstract
Microbial pattern recognition critically contributes to innate response, both at extracellular and intracellular cytosolic surveillance pathway (CSP) interface. However, the role of pattern recognition by host innate receptors in CSP is poorly understood in Leishmania donovani infection. Here, we have demonstrated that cytosolic targeting of L.donovani DNA (Ld-DNA) inhibits macrophage responsiveness to IFNɣ, through decreased MHC-II expression and lowered pSTAT1 (Y701) levels, involving host three-prime repair exonuclease-1 (TREX-1). The Ld-DNA potently induced type-1 IFNs, i.e. significant over-production of IFNβ through activation of the IRF pathway. Interestingly, knockdown of TRIF or MyD88 expression in macrophages had no effect on cytosolic Ld-DNA transfection-mediated IFN-β production, indicating involvement of a TLR independent pathway. Contrastingly, Ld-DNA failed to induce IFNβ in both TBK-1 and IRF3KO knockout macrophages. Although IFNβ was not induced by Ld-DNA in STING- knockout macrophages, STING alone was not enough for the induction. Evidently, besides STING, Ld-DNA recognition for induction of IFNβ critically required cytosolic cyclic GMP-AMP synthase (cGAS). Furthermore, the cGAS dependent targeting of Ld-DNA induced IFNβ over-production that contributed to antimony resistance in L.donovani infection. We provide the first evidence that enhanced cytosolic sensing of Ld-DNA in infection by antimony resistant (SBR-LD), but not antimony sensitive L.donovani strains (SBS-LD), was critically regulated by host MDRs, multi drug resistant associated protein 1 (MRP 1) and permeability glycoprotein (P-gp) in macrophages. Collectively, our results disclose Ld-DNA as a vital pathogen associated molecular pattern (PAMP) driving host Type-I IFN responses and antimony resistance. The findings may help in future development of policies for novel anti-leishmanial therapeutics.
Subject terms: Immunology, Pathogenesis
Introduction
Visceral leishmaniasis (VL), also known as “Kala Azar,” is a symptomatic intracellular infection of the liver, spleen, and bone marrow and is caused by L. donovani1. The therapeutic module for VL is beleaguered with several limitations because the currently used drugs are either toxic or only parentally effective or requires extended periods of administration. The Mϕs are the major host effector cells and the key parasite refuge in VL2. The struggle for survival between Leishmania and the host is vital and both have evolved multiple subversion strategies to antagonize each other. Of note, it has shown that the altered responsiveness of macrophage receptors is a crucial host defence subversion point for intracellular Leishmania survival3,4. In this regard, IFN-ɣ is crucial for regulating macrophage responsiveness to support its leishmanicidal Th1-biased activity through IFN-ɣR (IFN-ɣ receptor; IFNGR) mediated pathway involving kinases JAK1/JAK2 and STAT-15.
The innate immune system of the infected host plays a vital role in recognition of microbial patterns for inducing microbicidal responses6,7. Recognition of microbial products by a range of intracellular pattern recognition receptors (PRRs) stimulate a cytosolic surveillance pathway (CSP), leading to induction of type-I IFN production8,9. Type-I IFNs, best defined as IFNα/β, have crucial and context dependent diverse effects on innate and adaptive immune responses during infections. Interestingly, the IFN-cross-priming is very crucial; where type-II IFN (IFNɣ) is regulated by type-I IFN (IFNβ)10–12. As IFNβ is mostly initiated by intracellular receptors, its role in CSP during intracellular infections is prominent13, representing the CSP-mediated large transcriptional response14,15.
Besides intracellular Toll-like receptors (TLRs), the nuclear oligomerization domain (NOD)-like receptors (NLRs), RNA sensors and DNA sensors are critical units for innate recognition of conserved microbial structures in the cytosol16,17. PRRs activate signalling pathways leading to activation of transcription factors such as NF-κB and/or IFN regulatory factor 3 (IRF3) which induce production of pro-inflammatory molecules such as TNFα, IL-8 and pro-IL-1β, or IFNβ, respectively. IFNβ is triggered by type-I IFN receptors (IFNAR; namely IFNAR1 and IFNAR2) and related downstream signalling pathway. Of note, DNA sensors are recently been reported as essential units of CSP and inducers of IFN-β expression18. Intracellular DNA sensing needs activation of the interferon-stimulatory DNA (ISD) pathway. Cytosolic DNA mediated production of type-I IFNs requires the transcription factor IRF3 (IFN regulatory factor 3), TBK1 (TANK-binding-kinase-1) and the transmembrane protein STING (stimulator of IFN genes)19. STING ligands trigger type-I IFN production and the induction of interferon stimulated genes (ISG) through interferon regulatory factors (IRFs). Notably, DNA recognition by cytosolic host DNA sensor cGMP-AMP synthase (cGAS) generates the second-messenger, cyclic GMP–AMP (cGAMP) which binds to STING to stimulate IFN-β production20. In turn, IFNβ activates phosphorylation of transcription factors STAT1 and STAT2 leading to promotion of several gene expression via the IFN-stimulated responsive elements13. Interestingly, protozoan parasites are also reported to induce IFNα/β production13,21–23.
Despite the arsenal of defence mechanisms, Leishmania have evolved strategies to survive within the host Mϕs. Induction of an innate cytosolic surveillance pathway (CSP) has been recently reported to play vital role in bacterial and viral pathogenesis24,25, however has been poorly reported in Leishmaniasis. It has been shown that microbial DNA induces immune modulatory effects in the host26–29. Recently, it was demonstrated that L. donovani DNA contains Ld-CpG-rich motifs, which are potent in modulating the macrophage lifespan for progression of VL through a TLR9-dependent mechanism29. However, the innate sensing of cytosolic Leishmania DNA or the molecular mechanism involved for induction of type-I IFNs are still unclear in VL. In the present study, for the first time, we report the pattern recognition of L. donovani genomic DNA (Ld-DNA) in the macrophage cytosol by cGAS and the effect/s of this phenomenon in pathogenesis in VL. We also show that cytosolic delivery, recognition and targeting of L. donovani DNA inhibits macrophage responsiveness to IFNɣ and TLR-independent induction of IFN-β. Furthermore, we decode the molecular pathway of Leishmania DNA sensing and insinuate a CSP-mediated host immune subversion mechanism by the antimony-resistant L.donovani strains to overproduce IFNβ and enables the upregulation of host multidrug resistance proteins.
Results
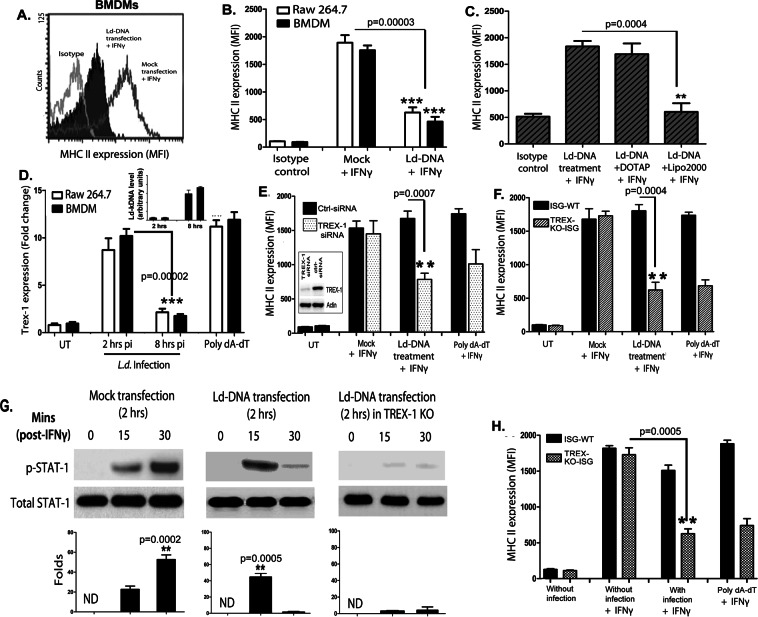
Cytosolic delivery of Leishmania donovani DNA inhibits macrophage responsiveness to IFNɣ
It was previously shown modulation of Mϕ apoptosis by L. donovani gDNA (Ld-DNA)29. To determine the role of Ld-DNA on macrophage responsiveness, mouse bone marrow derived Mϕs (BMDMs) and/or RAW264.7 Mϕs were transfected with Ld-DNA (2.5 µg/ml) in Lipofectamine 2000, 2 hrs before IFNɣ-treatment. Transfected cells were harvested and MHCII surface expressions were analyzed by flowcytometry. IFNɣ treatment for 30 mins induced higher MHCII expression in mock transfected Mϕs than in mouse BMDM cells transfected with Ld-DNA (Fig. 1A). In contrast, Ld-DNA transfection significantly (P < 0.0003) blocked about 96% of IFNɣ -treatment induced MHCII increase on BMDMs and RAW264.7 cells (Fig. 1B). However, Mϕs incubated with Ld-DNA, in the absence of Lipofectamine 2000, failed to downregulate IFNɣ-treatment induced MHCII increase on Mϕs (Fig. 1C). We also repeated the same experiment by transfecting BMDMs and RAW264.7 cells with Ld-DNA in DOTAP, 2 hrs before the IFNɣ treatment for 30 mins. Results suggested that transfection of Ld-DNA with DOTAP failed to block IFNɣ-treatment induced MHCII increase on Mϕs (Fig. 1C). Lipofectamine 2000 transfers the DNA to the host cell cytosol and DOTAP targets the DNA to the endosomes. Therefore, the data clearly verifies that cytosolic transfer of Ld-DNA is crucial for the inhibition of IFNɣ -induced Mϕ responsiveness during infection.
Figure 1.
Ld-DNA suppresses IFN-γ induced macrophage responses. (A–C) Surface expression of MHCII on live gated RAW264.7 (B,C) or BALB/c mouse BMDM (A) that were either mock transfected or Ld-DNA transfected; 2 hrs prior to the addition of fresh media containing no (0) or 100 U/ml IFN-γ treatment [for 30 mins]. Mean intensity is presented (B,C). (D) RAW264.7 or BALB/c mouse BMDM cells were infected with L. donovani parasites (parasite:macrophage = 10: 1) or transfected with 1 µg/ml double-stranded (ds) DNA Poly(dA-dT) for 18 hrs as positive control for indicated time periods. Cells were harvested and relative transcript levels of TREX-1 was measured by qRT-PCR. Leishmania-specific kDNA levels were quantified from both sets by RT-PCR and are shown in inset. Normalized levels for GAPDH are presented. (E,F) In two parallel sets of experiment, either mouse RAW264.7 cells were pre-transfected with 100 nM of targeted TREX-1 siRNA or non-targeted (NT) control siRNA (E) for 48 hrs or RAW264.7-ISG reporter cell derived TREX-1 ablated [TREX-1 KO] cells with control cells [ISG-WT] were employed (F). In both sets, cells were either mock transfected or Ld-DNA transfected [with DOTAP or Lipofectamine 2000] or transfected with dsDNA control Poly(dA-dT); 2 hrs prior to the addition of fresh media containing no (0 = UT) or 100 U/ml IFN-γ treatment. Mean intensity is presented. (G) Immunoblot results of phospho-STAT1 (pY701; p-STAT1) levels after mock transfection or Ld-DNA transfection of RAW264.7-ISG reporter cells or Ld-DNA transfection of RAW264.7-ISG TEX-1 KO cells. After 2 hrs post-transfection, cells were treated with 100 U/ml IFN-γ and then lysed at 15 or 30 mins. (H) RAW264.7-ISG reporter cell derived TREX-1 ablated [TREX-1 KO] cells and control cells [ISG-WT] were employed and pre-treated with 100 U/ml IFN-γ for 30 mins. Then the cells were infected with L.donovani parasites (parasite: macrophage = 10: 1). Surface expression of MHCII on live gated cells was investigated by flowcytometry. The experiment was repeated at least three times yielding similar results (n = 4 for A–C; and n = 3 for D–H) and mean values are presented or one representative result is shown (as in G). Statistically significant results are marked as **p < 0.001 and ***p < 0.0001. ND = not detected.
Reportedly, TREX-1 represents host exonucleases that cleave intracellular cytosolic DNA and is an essential negative regulator of the ISD pathway30. Notably, we found that L.donovani infection of RAW264.7 cells or BMDMs significantly increased, but later rapidly decreased TREX-1 transcript levels at 8 hpi compared to untreated controls (Fig. 1D). To further verify the role of TREX-1 in inhibition of Mϕ responsiveness, we employed siRNA-mediated knockdown of TREX-1 in mouse RAW264.7 Mϕs and then transfected with Ld-DNA, 2 hrs before IFNɣ treatment. Flowcytometric data revealed that Ld-DNA or poly(dA-dT) transfection led to further reduction of IFNɣ pre-treatment induced MHCII expression in Mϕs with TREX-1-siRNA (Fig. 1E) and in TREX-1 KO cells (Fig. 1F). Similar patterns of unresponsiveness of Mϕs to IFNɣ, in terms of MHCII surface expression, was also noted with Ld-DNA transfection in THP-1 human monocyte cells with/without TREX-1-siRNA (Supplementary Fig. 1).
IFNɣ potentiates Mϕ activation typically by triggering a signal transducer and activators of transcription (STAT1)-dependent pathway31. To determine the role of STAT1 in Ld-DNA induced Mϕ unresponsiveness to IFNɣ, we aimed to evaluate pSTAT1 (Y701) levels in the experiments. For this, RAW264.7 cells were transfected with Ld-DNA, 2 hrs prior to IFNɣ treatment. Mϕs were harvested at different time points (0 min, 10 mins, 30 mins) after IFNɣ treatment and pSTAT1 levels were assessed by immunoblotting. We found that pSTAT1 was significantly downregulated in Mϕs with Ld-DNA transfection (Fig. 1G). Moreover, the Ld-DNA also significantly lowered pSTAT1 levels compared to the mock-transfected Mϕs (P < 0.001; two to three folds) (Fig. 1G). Interestingly, results also demonstrated almost no induction of pSTAT1 levels with Ld-DNA transfection for 2 hrs in TREX-1KO cells (Fig. 1G). We also tested the effect of TREX-1 with L.donovani infection of Mϕs. We found that L.donovani infection of TREX-1KO-ISG Mϕs rendered significant reduction (p < 0.001) of IFNɣ pre-treatment induced MHCII expression compared to infection in ISG-WT cells (Fig. 1H).
Cytosolic delivery of Ld-DNA induces overproduction of IFNβ
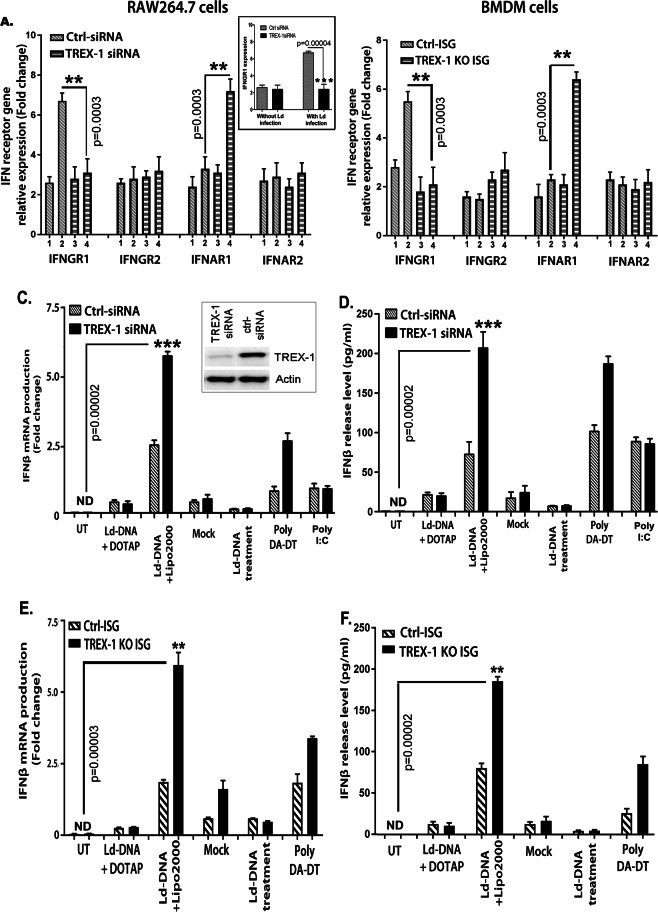
The type I interferon (IFN)2 receptor (IFNAR) is comprised of multiple components, designated as IFNAR1 and IFNAR2. The IFNɣ receptor (IFNGR) is composed of IFNGR1 and IFNGR232. The ability of a cell to respond to IFN is fully dependent on the presence and regulation of its cognate receptor on target cell surface, viz. IFNGR1, IFNGR2, IFNAR1 and IFNAR2; thereby altering the cytokine-specific responsiveness32. To identify the inducers of macrophage unresponsiveness to IFNɣ with Ld-DNA transfection, we first set out to identify changes in transcript levels in IFN receptor genes. In brief, BMDMs with TREX-1-siRNA or control siRNA were transfected with Ld-DNA in Lipofectamine 2000. Total RNA was extracted from both sets of Mϕs after 8 hr and used for transcript analysis of IFNGR1, IFNGR2, IFNAR1 and IFNAR2 levels. Real-time PCR analysis revealed significant downregulation of IFNGR1 (P < 0.001) by Ld-DNA in Mϕs with TREX-1-siRNA compared to Mϕs with control-siRNA (Fig. 2A). Contrastingly, IFNAR1 expression was increased (P < 0.001) by Ld-DNA in Mϕs with TREX-1-siRNA compared with Mϕs with control siRNA (Fig. 2A). However, IFNGR2 and IFNAR2 expressions were not significantly changed in these cells (Fig. 2A). For validation of the results in parasite infection model, we also tested the effect of TREX-1 on regulation of IFNGR1 gene expression with L.donovani infection of siRNA modified Mϕs. We found that L.donovani infection of cells with TREX-1 siRNA induced significant reduction of IFNGR1 expression compared to infection in cells with control siRNA (Fig. 2A inset). For validation of the results, similar experiments were repeated with RAW264.7 ISG reporter Mϕs and the results of BMDM cells for Ld-DNA-mediated changes in IFNGR and IFNAR expression levels were successfully reproduced (Fig. 2B). However, only Ld-DNA treatment or DOTAP-mediated transfection of Ld-DNA of RAW264.7 Mϕs, had no significant effect on IFN receptor gene levels (Not shown). These results clearly indicated the role of intracellular cytosolic targeting of Ld-DNA in reduction of Mϕ responsiveness through down modulation of IFNGR1 and increased level of IFNAR1.
Figure 2.
Cytosolic delivery of Ld-DNA induces TREX-1-dependent IFN-β. (A–D) In two parallel sets of experiment, either mouse RAW264.7 (A,C) or BALB/c mouse BMDM (B,D) cells were pre-transfected with targeted TREX-1 siRNA or non-targeted (NT) control siRNA. These cells were either mock transfected or Ld-DNA transfected [2.5 ug/ml with DOTAP or Lipofectamine 2000] or left untreated. Poly I:C (1 µg/ml for 18 hrs) was used as control (C,D). Total RNA were extracted from all sets of cells and relative transcript levels of IFN receptors [IFNGR1, IFNGR2, IFNAR1, IFNAR2] were analyzed by qRT-PCR. In another set of experiment, TREX-1 siRNA modified mouse RAW264.7 cells were infected with L.donovani parasites (parasite: macrophage = 10: 1) and relative transcript levels of IFNGR1 was evaluated. Mean of relative expression to GAPDH expression (fold change) from three sets of experiments were determined (A,B). The cells from both set of experiments were harvested, 8 hrs post-transfection of mock or Ld-DNA [2.5 ug/ml] and the culture supernatant was analyzed for released IFN-β by ELISA. (E,F) Mouse RAW264.7 reporter derived TREX-1 ablated [TREX-1 KO] cells and control cells [ISG-WT] were mock transfected or Ld-DNA transfected [2.5 ug/ml with DOTAP or Lipofectamine 2000] or transfected with dsDNA control Poly(dA-dT). Production of IFN-β was estimated at the relative transcript level (to GAPDH expression levels) by qRT-PCR (E) and at the release level in the culture supernatant by ELISA. All the experiments were repeated at least three times (n = 4 for A,B; and n = 3 for C–F) and the mean values are presented. Statistically significant results are marked as **p < 0.01 and ***p < 0.0001. [Legend for A and B: 1 = Ctrl siRNA+untreated; 2 = Ctrl siRNA +Ld-DNA; 3 = TREX-1 siRNA +untreated; 4 = TREX-1 siRNA ++Ld-DNA]
As IFNARs are responsible for production in type-I IFNs, we evaluated induction of IFNβ levels after Ld-DNA transfection. For this, the induction of IFNβ production by Ld-DNA was validated at the transcript and release level in RAW264.7 Mϕs (Fig. 2C,D). As shown in Fig. 2C,D, transfection of Ld-DNA induced significant amounts of IFNβ mRNA (2–3 fold; P < 0.0001; Fig. 2C) and IFNβ release (3–4 fold; P < 0.0001; Fig. 2D) from the cells with TREX-1 siRNA compared to the cells containing control siRNA (Fig. 2C,D). In parallel experiments, we transfected Ld-DNA into RAW264.7 ISG reporter cells and the transcript plus release levels of IFNβ were measured. As expected, RAW264.7 ISG TREX-1 ablated reporter cells produced more IFNβ in response to cytosolic transfer of Ld-DNA compared to the wild type cells (Fig. 2E,F). However, RAW264.7 ISG cells incubated with Ld-DNA or with DOTAP, in the absence of Lipofectamine, failed to produce IFNβ (Fig. 2E,F). These data suggests that only cytosolic access of Ld-DNA can induce IFNβ production. Moreover, we found that Ld-DNA transfection of Mϕ induced significant amounts of IFNβ expression with decrease in TREX-1 transcript levels in a time-dependent manner (Supplementary Fig. 2). Simultaneously, we also evaluated release levels of IFN response gene CXCL-10 by ELISA as it denotes measure of biological activity of IFNβ. The levels of CXCL-10 were readily induced by Ld-DNA transfection of RAW264.7 Mϕs and BMDMs (Supplementary Fig. 3). Furthermore, Ld-DNA transfection also induced IFN-α in RAW264.7 cells (data not shown). Overall, the results strongly suggested the induction of biologically active IFNβ production by cytosolic recognition of Ld-DNA in Mϕs.
IRF3 and TBK-1 are required for Ld-DNA mediated IFNβ induction in Mϕs
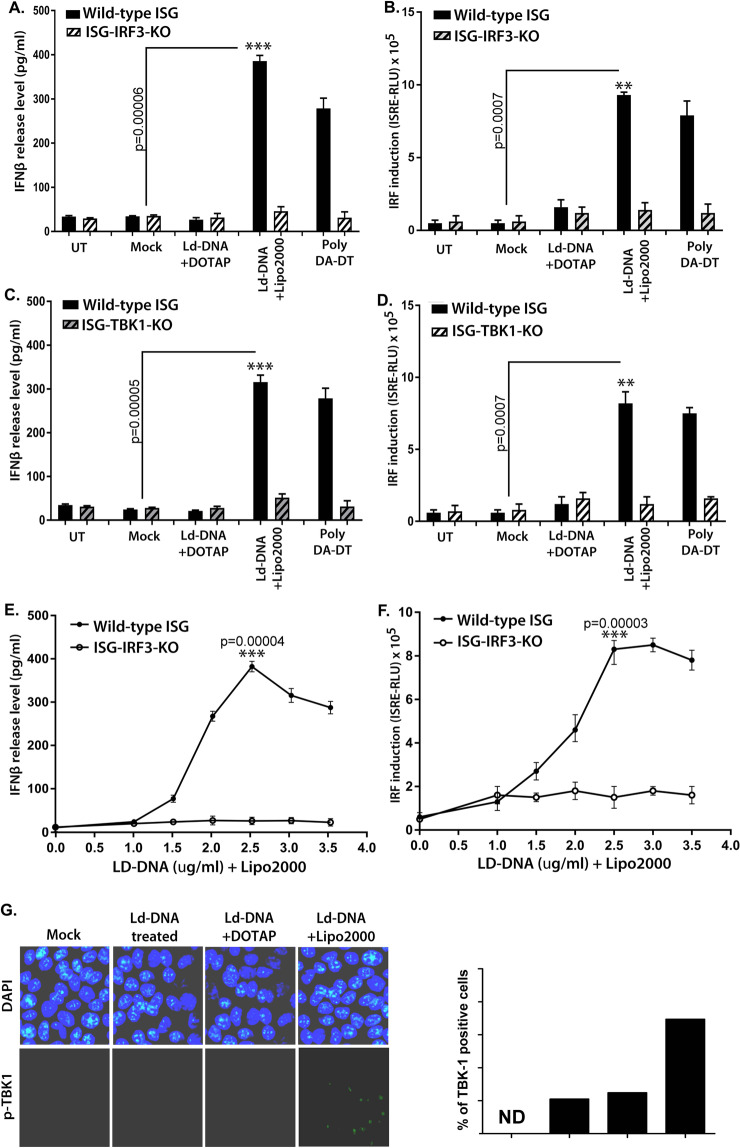
Interferon regulatory factor 3 (IRF3) is a key transcription regulator of type-I interferon (IFN)-dependent innate immunity33. To ascertain the role of IRF3 in IFNβ induction by Ld-DNA, we transfected Ld-DNA into RAW 264.7 ISG reporter cells with IRF3 gene knockout (ISG-KO-IRF3). RAW 264.7 ISG reporter cells (Invivogen) are derived from the murine RAW 264.7 macrophage cell line. Notably, the ISG-KO-IRF3 cells have no ability to respond to cytosolic nucleotides. Results denoted that Ld-DNA failed to induce IFNβ production in ISG-KO-IRF3 cells compared to wild-type cells RAW 264.7 ISG reporter cells (Fig. 3A), indicating the specificity and importance of IRF3 in this process. However, direct transfection of Ld-DNA readily activated IRF pathway in wild-type RAW 264.7 ISG reporter cells, but not in ISG-KO-IRF3 cells (Fig. 3B). Ld-DNA treatment (without transfection) or with DOTAP-mediated transfection did not activate IRF pathway or induce IFNβ production in wild-type RAW 264.7 ISG reporter cells (Fig. 3A,B). On the other hand, Ld-DNA induced IRF and IFN-β in a dose-dependent manner in control ISG cells but not in ISG-KO-IRF3 cells (Fig. 3C,D). This indicates that cytosolic transfer of Ld-DNA is crucial for activation of IRF pathway and stimulation of IFNβ production. To validate the results further, we transfected Ld-DNA into BMDMs with IRF3-siRNA or ctrl-siRNA. The results showed that induction of IFNβ was only possible in BMDM cells with ctrl-IRF3 siRNA, but not in BMDMs with IRF3-siRNA (Supplementary Fig. 4A). Similar pattern of IRF pathway activation was also found in THP-1 human monocyte cells with/without IRF3-siRNA (Supplementary Fig. 4B).
Figure 3.
Contribution of IRF3 and TBK-1 in Ld-DNA induced activation of IFN-β. (A–D) Mouse RAW264.7 ISG reporter derived IRF3 ablated [IRF3 KO] cells (A,B) or TBK-1 ablated [TBK-1 KO] cells (C,D) and control cells [ISG-WT] were mock transfected or Ld-DNA transfected [2.5 ug/ml with DOTAP or Lipofectamine 2000] or transfected with dsDNA control Poly(dA-dT) or left untreated. Production of IFN-β was estimated at the release level in the culture supernatant by ELISA (A,C) and IRF pathway activation was measured by luciferase reporter assay (B,D). (E,F) Mouse RAW264.7 reporter derived IRF3 ablated [IRF3 KO] cells and control cells [ISG-WT] were transfected with different concentrations of Ld-DNA [with Lipofectamine 2000]. Production of IFN-β was estimated at the release level in the culture supernatant by ELISA (E) and IRF pathway activation was measured by luciferase reporter assay (F). (G) Fluorescence microscopy derived images of mouse RAW264.7 cells, fixed after 8 hrs post mock transfection or Ld-DNA transfection [2.5 ug/ml with DOTAP or Lipofectamine 2000] or Ld-DNA treatment [2.5 ug/ml]. The cells were stained with anti-pTBK-1 (green) and counterstained with DAPI (blue) post fixation with PFA. Quantitative analysis of percentage of p-TBK-1 positive cells is shown as inset. All the experiments were repeated at least three times (n = 4 for A–D; and n = 3 for E–G) and the mean values are presented. Statistically significant results are marked as *p < 0.05 and ***p < 0.001.
Simultaneously, we employed RAW 264.7 ISG reporter TBK-1 knockout cells (ISG-KO-TBK-1) to verify if the Ld-DNA induced IFNβ involved activation of TBK-1. We found that Ld-DNA was unable to induce significant amounts of IFNβ production (Fig. 3E) or IRF pathway activation (Fig. 3F) in ISG-KO-TBK-1 cells compared to wild-type cells. These data confirmed the involvement of the IRF3-TBK1 pathway in Ld-DNA mediated IFNβ induction process. Next, TBK1-mediated type-I IFN production was also validated when Ld-DNA transfection induced higher percentage of pTBK-1 positive cells compared with the mock-transfected cells (Fig. 3G). Collectively, the data suggested that down regulation of TREX-1 during L. donovani infection in Mϕs enables sensing of Ld-DNA in the cytosol leading to induction of type-I IFN (IFNβ) through a TBK1-IRF3-dependent pathway.
Ld-DNA utilizes a TLR-independent pathway for induction of IFNβ in Mϕs
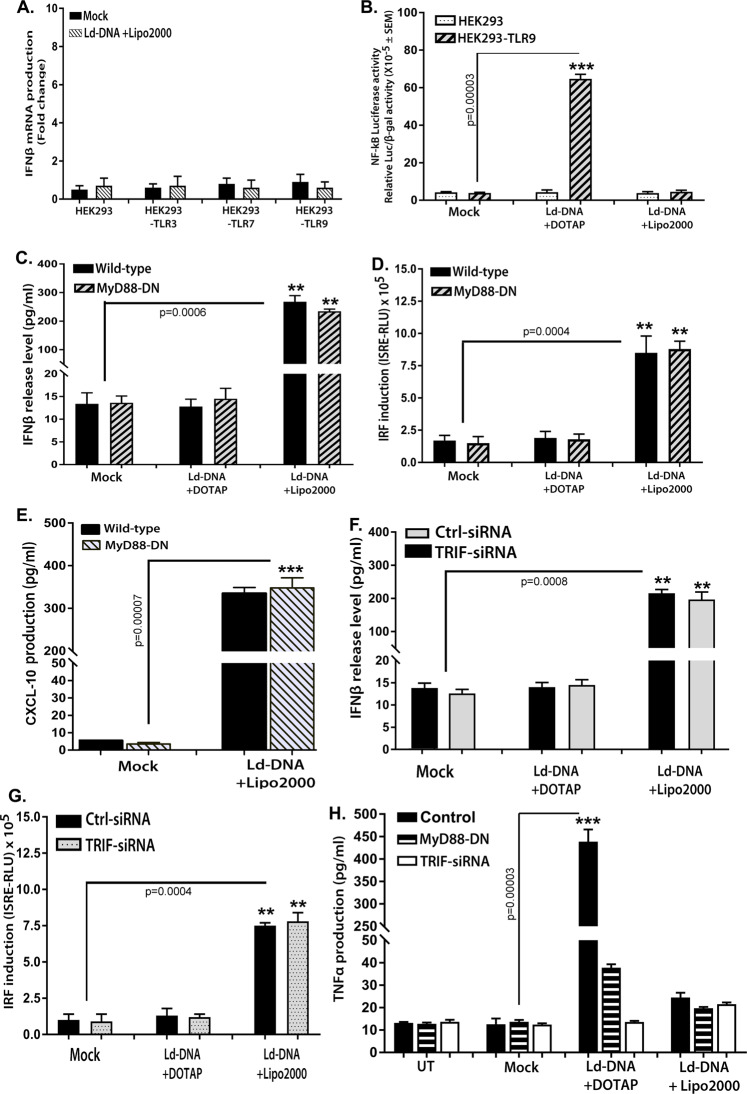
TLR9-dependent IFNβ production in viral infections has been extensively studied34. As Ld-DNA transfection to the cytosol potently induced IFNβ production in Mϕs, we were incited to investigate the potential of TLRs in this activity. Recently, it was demonstrated that CpG-motifs in the Ld-DNA induces a TLR9 dependent delay in macrophage apoptosis in VL29. It was shown that LdDNA transfection with DOTAP activates the TLR9-dependent NF-kB pathway as it promoted expression and activity of luciferase gene under the control of NF-kB elements in HEK-TLR9 cells but not in HEK293-TLR7 cells29. Importantly, HEK293 cells lack TLRs yet retain the downstream components used for TLR signalling. HEK293 cells transfected to express specific TLRs can be used to assess TLR recognition of potential ligands; these cells secrete cytokines in response to TLR signalling and consequent activation of NF-kB. To investigate the role of intracellular TLRs in Ld-DNA-mediated responses, we transfected HEK293, HEK293-TLR9, HEK293-TLR7 and HEK293-TLR3 cells with Ld-DNA in Lipofectamine 2000 and measured the level of IFNβ production by RT-PCR. Surprisingly, Ld-DNA failed to induce IFNβ production in all HEK293 cells (Fig. 4A). Our results were consistent with report of T.cruzi infection failing to induce IFNβ response in HEK293 cells35. Furthermore, it was found that tansfection of Ld-DNA in HEK293-TLR9 with DOTAP activated NF-kB but not with Lipofectamine 2000 mediated transfection (Fig. 4B). Earlier results of this study also suggested that transfection of Ld-DNA with DOTAP failed to block IFNɣ -treatment induced MHCII increase on Mϕs (Fig. 1C). One possibility was that Lipofectamine 2000 transfers the DNA to the host cell cytosol and DOTAP targets the DNA to the endosomes, it was clearly indicated that NF-kB activation required Ld-DNA to be transferred to the endosomes and recognized by TLR929. Therefore, the data clearly indicates that induction of IFNβ required Ld-DNA to be transferred to the cytosol for TLR-independent activation of a cytosolic surveillance pathway (CSP).
Figure 4.
Ld-DNA utilize a TLR-independent pathway for IFN-β activation. (A) HEK293 cells alone or stably expressing specific TLRs [HEK293-TLR3, HEK293-TLR7 and HEK293-TLR9] were mock transfected or Ld-DNA transfected [2.5 ug/ml with Lipofectamine 2000] and production of IFN-β was estimated at the relative transcript level by qRT-PCR. (B) HEK293 cells alone or stably expressing TLR9 [HEK293-TLR9] were transfected with NF-kB reporter luciferase plasmid or empty vector. Later, these cells were mock transfected or Ld-DNA transfected [2.5 ug/ml with DOTAP or Lipofectamine 2000]. Result represent the ratio of luciferase (Luc) to β-galactosidase (β-gal). (C–E) Mouse RAW264.7 reporter ISG derived wild type cells (WT) were either left untreated or were transfected with MyD88-DN plasmid. Later, these cells were mock transfected or Ld-DNA transfected [2.5 ug/ml with DOTAP or Lipofectamine 2000]. Production of IFN-β and CXCL-10 were estimated at the release level in the culture supernatant by ELISA (C,D) and IRF pathway activation was measured by luciferase reporter assay (E). (F–H) Mouse RAW264.7 reporter ISG derived cells were pre-transfected with targeted TRIF siRNA or non-targeted (NT) control siRNA (F–H) or transfected only with MyD88-DN plasmid (H). These cells were later mock transfected or Ld-DNA transfected [2.5 ug/ml with DOTAP or Lipofectamine 2000] or left untreated (UT). Production of IFN-β (F) and TNF-α (H) were estimated at the release level in the culture supernatant by ELISA (F,H) and IRF pathway activation was measured by luciferase reporter assay (G). Data represent mean ± SEM of at least three independent experiments (n = 4 for A–D and H; and n = 3 for E–G). Statistically significant results are marked as *p < 0.05 and ***p < 0.001.
Though the data indicated involvement of a TLR-independent pathway, we attempted to verify if MyD88 contributed to Ld-DNA mediated IFNβ production in Mϕs. Our experiments showed that transient transfection of RAW-Lucia™ ISG cells (Invivogen) with the dominant negative MyD88 construct (MyD88DN- encoding a dominant negative MyD88 with a deletion of its death domain to evaluate roles of MyD88) had no effect on Ld-DNA mediated IFNβ production (Fig. 4C) or IRF pathway induction (Fig. 4D). It was also found that equal amount of Ld-DNA transfection triggered similar significant amounts CXCL-10 in both RAW-ISG-MyD88DN cells and RAW-ISG wild-type cells (Fig. 4E). Nextly, it was demonstrated that siRNA-mediated knockdown of TRIF expression in RAW 264.7 ISG reporter cells had no effect on production of IFNβ in response or IRF pathway induction to Ld-DNA transfection (Fig. 4F,G). For control, we measured levels of TNF-α mRNA level changes with Ld-DNA transfection in RAW264.7 cells with MyD88DN or TRIF-siRNA. Here, we found that TNF-α mRNA induction was significantly tampered in cells containing TRIF-1 siRNA or MyD88DN with Ld-DNA transfection with DOTAP (Fig. 4H). Therefore, the findings confirmed that MyD88 and TRIF-1 are not essential molecules for induction of IFNβ in response to Ld-DNA transfection and delivery to the cytosol. Taken together, it was indicated that the induction of IFNβ in Mϕs by cytosolic access of Ld-DNA is evidently TLR-independent, but requires a pathway that typically congregates on IRF3 and TBK1.
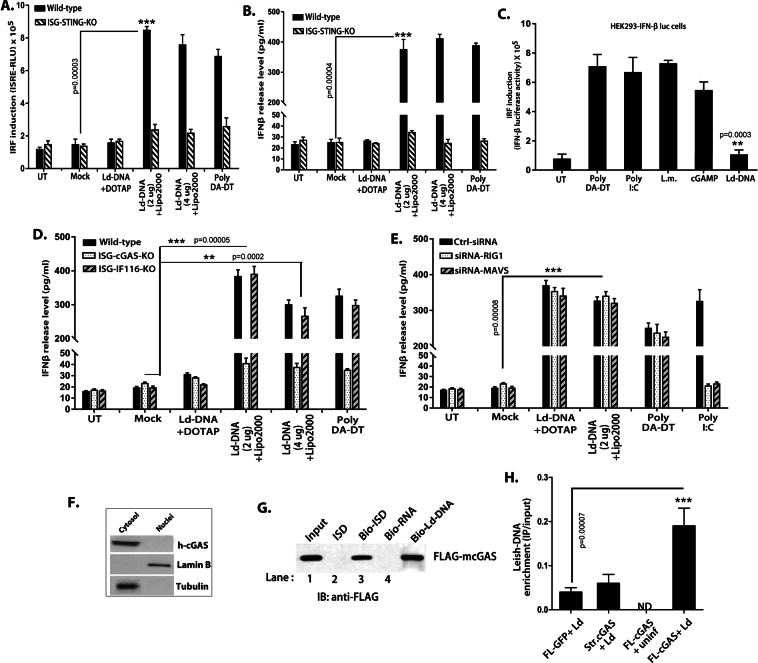
Cytosolic Ld-DNA is targeted by cyclic GMP-AMP synthase (cGAS) for induction of IFNβ
In this study, results denoted that Ld-DNA induced type-I IFN and activated the cytosolic surveillance pathway in Mϕs. Therefore, involvement of cytosolic DNA sensors in host Mϕs was indicated. We first investigated the contribution of STING to the Ld-DNA induced IFNβ response. Though STING is not a DNA sensor, it can indirectly identify cytosolic DNA to induce IFN- β during infection19. STING ligands trigger IFNβ response and the induction of ISGs through IRFs. Therefore, we took advantage of RAW264.7 macrophage ISG reporter cells with STING knockout (ISG-KO-STING) for Ld-DNA transfection. Notably, Ld-DNA transfection was unable to induce significant IFNβ response or activate IRF pathway in ISG-KO-STING cells (Fig. 5A,B). We found significant IFNβ response and activation IRF pathway in wild-type RAW264.7 macrophage ISG reporter cells with Ld-DNA transfection (Fig. 5A,B). However, LPS could induce significant IFNβ production in ISG-KO-STING cells (Not shown). Simultaneously, Ld-DNA transfection failed to stimulate significant IFNβ response in THP-1 reporter cells with stable knockdown of STING (THP-1 DualTM STING-KO) that was reversed in wild-type THP-1 cells (Supplementary Fig. 5). Though LPS-mediated IFNβ induction is STING-independent, our findings indicated that Ld-DNA mediated induction of IFNβ is STING-dependent.
Figure 5.
Cytosolic DNA sensor cGAS is targeted by Ld-DNA for for IFN-β activation. (A,B) Mouse RAW264.7 reporter derived STING ablated [STING KO] cells and control cells [ISG-WT] were mock transfected or with Ld-DNA [2.5 ug/ml with DOTAP or Lipofectamine 2000]. IRF pathway activation was measured by luciferase reporter assay (A) and production of IFN-β was estimated at the release level in the culture supernatant by ELISA (B). (C) HEK293- IFN-β-luciferase reporter cells were transfected with poly(DA-DT) or poly(I:C) or Ld-DNA or treated with cGAMP or infected with L. monocytogenes. After overnight stimulations, IRF induction was measured through luciferase activity. (D,E) Mouse RAW264.7 reporter derived cGAS ablated [cGAS KO] cells or IFI16 ablated [IFI16 KO] and control cells [ISG-WT] (D) or pre-transfected with with targeted RIG-1-siRNA or MAVS-siRNA or non-targeted (NT) control siRNA (E) were employed. These cells were mock transfected or with Ld-DNA [2.5 ug/ml with DOTAP or Lipofectamine 2000] or poly (DA-DT). Production of IFN-β was estimated at the release level in the culture supernatant by ELISA (D,E). (F) THP-1 cells were transfected with Ld-DNA [2.5 ug/ml with Lipofectamine 2000]. The cytosolic and nuclear fractions were prepared after 8 hrs post transfection. The fractions were immunoblotted with the indicated antibodies. (G) FLAG-m-cGAS was expressed and purified from E.coli. Subsequently, the FLAG-tagged cGAS was incubated with ISD or biotinylated-ISD or biotinylated-RNA in the presence streptavidin beads [See method section]. The bound proteins were then eluted in SDS sample buffer for immunoblotting with an anti-FLAG antibody. (H) HEK293 cells stably expressing FLAG-tagged constructs of mouse cGAS [HEK293-FLAG-m-cGAS and HEK293-FLAG-m-strep-cGAS] were infected with L.donovani parasites (parasite: macrophage = 10: 1) and crosslinked with 4% PFA. The m-cGAS was then precipitated with anti-FLAG antibody. The DNA in the precipitate was then subjected to qRT-PCR of L.donovani specific kDNA sequence. Quartiles were normalized to the inputs. Data represent mean ± SEM of at least three independent experiments (n = 4 for A–C and G; and n = 3 for D–F and H). Statistically significant results are marked as **p < 0.001 and ***p < 0.0001.
Interestingly, HEK293 cells lack TLRs, but possess STING20, yet Ld-DNA failed to induce IFNβ in HEK293 cells (Fig. 4A,B). Further, we found that though poly(dA-dT) or poly(I:C) or cGAMP transfection or Listeria monocytogenes infection could, Ld-DNA transfection failed to induce significant IFNβ-luciferase reporter activation in HEK-293-IFNβ-luc reporter cells (Fig. 5C). Therefore, it was concluded that STING alone was not responsible IFNβ induction by Ld-DNA. Our results were also supported by findings in T cruzi infection and therefore, involvement of a novel pathway was proposed35.
Recent research has identified cyclic GMP-AMP synthase (cGAS) as the central cytoplasmic DNA sensor upstream of STING to play major role for IFNβ induction upon DNA transfection and in viral infections20,36,37. To ascertain the probable contribution of cGAS in targeting cytosolic Ld-DNA for induction of IFNβ, if any, we transfected Ld-DNA to RAW 264.7 macrophage ISG reporter cells with either cGAS knockout (ISG-KO-cGAS) or IFI16 knockout (ISG-KO-IFI16). Interestingly, results demonstrated that Ld-DNA transfection failed to induce significant amounts of IFNβ in ISG-KO-cGAS cells compared with wild-type control reporter cells (Fig. 5D). Notably, stable knockout IFI16 in ISG-KO-IFI16 did not alter Ld-DNA-mediated IFNβ production in RAW 264.7 ISG reporter Mϕs (Fig. 5D).
Additionally the RIG-1-MAVS pathway has been shown to play vital role in type-I IFN production38. In a parallel sets, we transfected Ld-DNA to RAW 264.7 cells with RIG-1 siRNA or with MAVS siRNA or control siRNA. Ld-DNA transfection in RAW264.7 cells with RIG-1 siRNA or MAVS siRNA demonstrated no significant alterations in IFNβ production compared to cells with control siRNA (Fig. 5E). Results demonstrated that RIG-1 or MAVS has no effect on the ability of Ld-DNA to induce IFNβ (Fig. 5E). Simultaneously, siRNA silencing of RIG-1 had no effect on significant IFNβ response in THP-1 DualTM reporter cells with Ld-DNA transfection, this was reversed in wild-type THP-1 cells (Supplementary Fig. 6).
Next, we transfected THP-1 cells with Ld-DNA in Lipofectamine 2000 and after 6 hr, the cytosolic and nuclear fractions were separated by high speed centrifugation. Immunoblot analysis of human cGAS protein proved that Ld-DNA transfection significantly enhanced the cGAS protein expression only in the cytosol, compared to the mock transfected sets (Fig. 5F). The results confirm Ld-DNA mediated activation of cGAS in the cytosol. Thus, we were prompted to verify the in vivo physical interaction of Ld-DNA with cGAS as a mechanism for Ld-DNA-mediated IFNβ induction in host cells. As also reported earlier20,39, FLAG fused to mouse-cGAS (FLAG–m-cGAS) was precipitated by streptavidin pull-down assay with biotinylated ISD, but not with biotinylated RNA (Fig. 5G). To identify the physical binding of cGAS with unlabelled Ld-DNA, we followed a previously reported chromatin-IP protocol in live HEK293T cells40. Interestingly, it has been reported that though HEK293T cells possess STING, these cells lack the cGAS gene20. HEK293T cells stably expressing m-cGAS (HEK293-FLAG-m-cGAS) were infected with L.donovani parasites, crosslinked with formaldehyde and cGAS was immunoprepitated with anti-FLAG antibodies. In the resultant cGAS precipitate, abundance of L.donovani specific kDNA sequence frequency was measured by qPCR. As expected, L.donovani specific kDNA sequences were significantly intensified in FLAG-m-cGAS precipitates compared to precipitate sets with FLAG-GFP or cGAS with a different epitope tag (Strep-cGAS) controls (Fig. 5G). Collectively, the data indicated that STING and cGAS are both crucial for cytosolic sensing of Ld-DNA in Mϕs for activation of IFNβ response.
Enhancement of IFNβ production contributes to antimony resistance in L.donovani
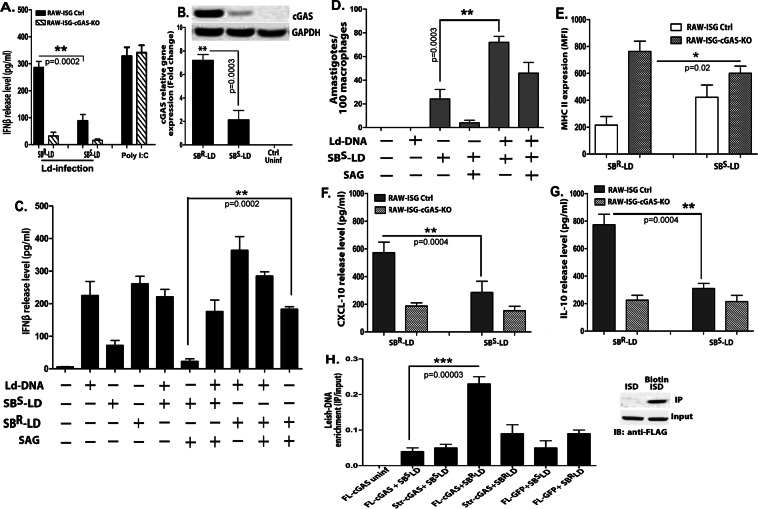
The results confirmed that cytosolic delivery of Ld-DNA was necessary for activation of IFNβ response during L. donovani infection of Mϕs. It is well established that type-I IFNs negatively regulate IL-12 and IFNɣ production in Mϕs10. The induction of these latter pro-inflammatory cytokines negatively impact survival of intracellular L.donovani parasites in Mϕs. Thus, we speculated that over-production of IFNβ may help in enhanced intracellular survival of the parasite and could additionally support in developing drug resistance in L.donovani infection. As antimony (Sodium Stibo-gluconate; SSG) resistance is mostly widespread in VL, we sought to investigate the relation between cytosolic delivery of Ld-DNA in IFNβ overproduction and this phenomenon, if any. Firstly, we infected antimony-resistant (SBR-LD) and antimony-sensitive (SB-SLD) L.donovani strains to RAW 264.7 ISG reporter cells or RAW 264.7 ISG reporter cells with cGAS knockout and resultant IFNβ production was evaluated. Interestingly, significantly higher production of IFNβ (P < 0.001 three to four folds) was detected in ISG reporter cells infected with SBR-LD parasites compared to SBS-LD infection after 18 hpi (Fig. 6A). Simultaneously, we found that infection with both SBR-LD and SBS-LD strains failed to induce any significant IFNβ production in ISG-KO-cGAS cells compared to wild-type control reporter cells (Fig. 6A). Therefore, the production of IFNβ by infection with SBR-LD or SBS-LD strain was dependent on the availability of cGAS. We also noted significant rise in cGAS expression (over 3 fold; P < 0.001) in ISG reporter cells infected with SBR-LD strains compared to infection with SBS-LD parasites (Fig. 6B). However, it was verified that cytosolic transfection of equal amounts of separate Ld-DNA preparations from SBR-LD or SBS-LD parasites in RAW 264.7 ISG reporter cells induced similar amounts of IFNβ production with same activation of the IRF pathway and ROS generation (Supplementary Fig. 7A,B,D). It was thus confirmed that difference in induction of IFNβ by SBS-LD and SBR-LD strains was not due to any difference in their DNA. Hence, the overproduction of IFNβ might be due to the differential delivery for enhanced cytosolic access and cGAS-mediated recognition of Ld-DNA from the SBR-LD strains in the Mϕs. Simultaneously, we found that infection with SBR-LD strain resulted in accumulation of more cytosolic Ld-DNA than in infection with SBS-LD strain. This was identified by quantifying L. donovani specific kDNA expression separately in the nuclear and cytosolic fractions in the infected RAW 264.7 ISG reporter cells (Supplementary Fig. 7C).
Figure 6.
Contribution of Ld-DNA for IFN-β activation in antimony resistance. (A,B) Mouse RAW264.7 reporter derived cGAS ablated [cGAS KO] and control [ISG-WT] cells were infected with antimony resistant (SBR-LD) or antimony sensitive (SBS-LD) L.donovani parasites (parasite: macrophage = 10: 1). Poly I:C was used as control. Production of IFN-β was estimated at the release level in the culture supernatant by ELISA (A) and relative transcript level of m-cGAS IRF was measured by qRT-PCR (B). (C) Mouse RAW264.7 cells were mock transfected or with Ld-DNA [2.5 ug/ml with Lipofectamine 2000]. The pre-transfected cells were either left uninfected or infected with antimony resistant (SBR-LD) or antimony sensitive (SBS-LD) L.donovani parasites (parasite: macrophage = 10: 1) and later treated/untreated with SAG (60 ug/ml). Production of IFN-β was estimated at the release level in the culture supernatant by ELISA. (D) Mouse RAW264.7 cells were mock transfected or with Ld-DNA [2.5 ug/ml with Lipofectamine 2000]. The pre-transfected cells were either left uninfected or infected with antimony sensitive (SBS-LD) L.donovani parasites (parasite: macrophage = 10: 1) and later treated/untreated with SAG (60 ug/ml). Number of intracellular amastigotes per 100 macrophages on infection was microscopically evaluated on stained coverslip preparations from each experiment. (E–G) Mouse RAW264.7 reporter derived cGAS ablated [cGAS KO] and control [ISG-WT] cells were either left uninfected or infected with antimony resistant (SBR-LD) or antimony sensitive (SBS-LD) L.donovani parasites (parasite: macrophage = 10: 1). Surface expression of MHCII on live gated RAW264.7 cells were determined by flowcytometry and mean intensity is presented (E). Production of CXCL-10 (F) and IL-10 (G) was estimated at the release level in the culture supernatant by ELISA. (H) HEK293 cells stably expressing FLAG-tagged constructs of mouse cGAS [HEK293-FLAG-m-cGAS and HEK293-FLAG-m-strep-cGAS] were either left uninfected or infected with antimony resistant (SBR-LD) or antimony sensitive (SBS-LD) L.donovani parasites (parasite: macrophage = 10: 1) and crosslinked with 4% PFA. The m-cGAS was then precipitated with anti-FLAG antibody. The DNA in the precipitate was then subjected to qRT-PCR of L.donovani specific kDNA sequence. Quartiles were normalized to the inputs. Data represent mean ± SEM of at least three independent experiments (n = 4 for A–D and H; and n = 3 for E–G). Statistically significant results are marked as *p < 0.05, **p < 0.001 and ***p < 0.0001.
Interestingly, in separate experiments, SBR-LD or SBS-LD infection in RAW264.7 cells with/without Ld-DNA pre-transfection, confirmed the crucial role of Ld-DNA in IFNβ production in antimony resistance (Fig. 6C). It was seen that Ld-DNA pre-transfection enhanced the capacity of the SBR-LD or SBS-LD parasite to induce more IFNβ production after infection (Fig. 6C). However, this enhancement of IFNβ production by Ld-DNA pre-transfection could not be controlled by optimal SSG treatment post-infection (Fig. 6C). To further verify this, in separate experiments, the effect of Ld-DNA transfection before SBS-LD infection and intracellular parasite number was determined in the presence or absence of SSG in ISG reporter cells. Notably, Ld-DNA pre-transfection resulted in significantly (p < 0.001; three folds) more parasite load in Mϕs compared to cells not pre-transfected with Ld-DNA before SBS-LD infection (Fig. 6D). As expected, in the presence of SSG, we noted significantly more reduction (P < 0.001; about four fold) in intracellular parasite number in Mϕs infected with SBS-LD without Ld-DNA pre-transfection compared to the Mϕs infected with SBS-LD with Ld-DNA pre-transfection (Fig. 6D). Therefore, cytosolic delivery of Ld-DNA during infection could amount for significant SSG resistance in the host Mϕs.
To further check the contribution of IFNβ in antimony resistance phenomenon, we evaluated macrophage responsiveness to IFNɣ in RAW 264.7 ISG reporter cells and RAW 264.7 ISG reporter cells with cGAS knockout, infected with either SBR-LD or SBS-LD L.donovani strains. Flowcytometric data ascertained significant decrease in MHCII expression by IFNɣ treatment in cells with SBR-LD infection compared with SBR-LD infection in wild-type RAW 264.7 ISG reporter cells but not in ISG-KO-cGAS cells, at 18 hpi (3 fold; P < 0.0001) (Fig. 6E). Notably, besides induction of IFNβ production, the SBR-LD infection mediated decrease in MHCII expression corresponded with increase in CXCL-10 expression (Fig. 6F) and IL-10 expression (Fig. 6G). In parallel experiments, we also used human THP-1 cGAS-KO reporter cells that validated the results obtained with RAW 264.7 cells (Supplementary Fig. 8A,B). Notably, there was no change in infection rates in control wild type and cGAS-KO cells by both SBR-LD and SBS-LD L.donovani strains (Supplementary Fig. 8C).
We desired to identify the differential binding of Ld-DNA with cGAS in SBR-LD and SBS-LD infection. For that, HEK293T cells stably expressing m-cGAS (HEK293-FLAG-m-cGAS) were infected with either SBR-LD or SBS-LD parasites, crosslinked with formaldehyde and cGAS was immune-precipitated with anti-FLAG antibodies. In the resultant cGAS precipitate from both the infection sets, abundance of L.donovani specific kDNA sequence frequency was measured by qPCR. Interestingly, L.donovani specific DNA sequences were significantly intensified in FLAG-m-cGAS precipitates from the cells infected with SBR-LD compared to precipitate sets with SBS-LD infection (Fig. 6H). However, no differences were observed in the abundance of host DNA (mouse actin) in the precipitates that could be due to low non-specific binding of host-DNA with cGAS in normal conditions (Fig. 6H). Collectively, these data confirms enhanced access of Ld-DNA to the cytosol during SBR-LD infection for increased induction of IFNβ.
Overproduction of IFNβ upregulates host MDRs and facilitates antimony resistance
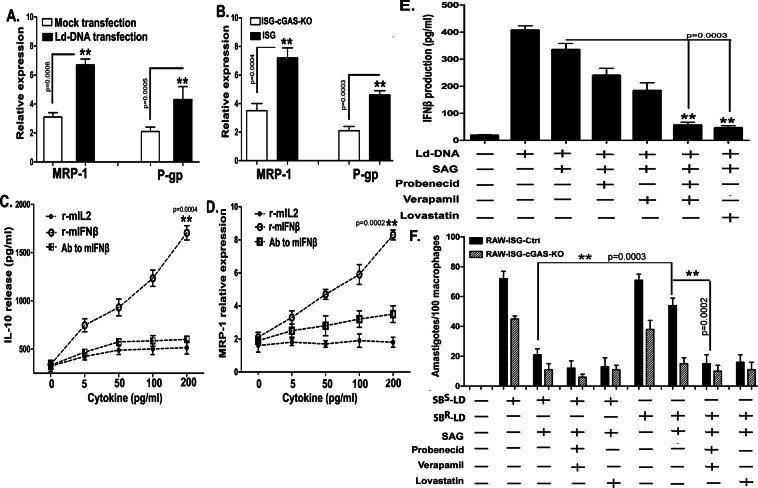
Reportedly, L. donovani induces the upregulation of host MDRs, multidrug resistance-associated protein-1 (MRP1) and permeability glycoprotein (P-gp) in host Mϕs and are well known for playing crucial role in antimony resistance in VL41. We hypothesized a cross-relation between Ld-DNA-mediated IFNβ production and regulation of host MDR1 transporter proteins in antimony resistance. To verify the relation of Ld-DNA and host MDRs, if any, we evaluated expression status of host cell MRP1 and P-gp in infected RAW 264.7 ISG reporter cells after Ld-DNA transfection. Results confirmed that cells transfected with Ld-DNA demonstrated significant upregulation of MRP1 (P < 0.001) and P-gp (P < 0.001) compared to their levels in non-transfected cells (Fig. 7A). Notably, in parallel experiments, we found that Ld-DNA transfection failed to induce MRP1 and P-gp expression in ISG-KO-cGAS cells compared to wild-type control reporter cells (Fig. 7B). Therefore, cytosolic recognition of Ld-DNA by cGAS directly/indirectly influenced MRP1 expression. Notably, IL-10 is known to induce MDR1 expression in a dose-dependent manner42. We show that different concentrations of mouse recombinant IFNβ (rm-IFNβ) treatment resulted in significant (P < 0.001; two to three folds) dose-dependent enhancement in IL-10 production (Fig. 7C) and simultaneous increase in MRP1 expression (0.0001; four to five folds) in Mϕs (Fig. 7D). However, the surge of IL-10 or MRP-1 was inhibited by antibodies to rm-IFNβ (Fig. 7D inset). We and others have also shown that SBR-LD infection induced significant surge of IL-10 expression (Fig. 6G). Therefore, the findings confirm that overproduction of IFNβ directly influences upregulation of IL-10 that in turn helps in enhancement of host MDRs.
Figure 7.
Host MDR driven overproduction of IFN-β facilitates antimony resistance. (A,B) Mouse RAW264.7 wild type reporter cells were either mock transfected or with Ld-DNA [2.5 ug/ml with Lipofectamine 2000] (A) or mouse RAW264.7 reporter derived cGAS ablated [cGAS KO] and control [ISG-WT] cells were transfected with Ld-DNA [2.5 ug/ml with Lipofectamine 2000] (B). Cells were harvested and relative transcript levels of MRP-1 and P-gp were measured by qRT-PCR. Normalized levels for GAPDH are presented. (C,D) Mouse RAW264.7 cells were either treated with r-mIL-2 or r-mIFN-β or antibody (Ab) to mIFN-β. Production of IL-10 was estimated at the release level in the culture supernatant by ELISA (C) and relative transcript level of host MRP1 was measured by qRT-PCR (D). (E) Mouse RAW264.7 wild type reporter cells were either pre-treated with probenecid (200 uM) and/or verapamil (2 uM) or lovastatin (10 uM). Then these pre-treated cells were either mock transfected or transfected with Ld-DNA [2.5 ug/ml with Lipofectamine 2000]. Production of IFN-β was estimated at the release level in the culture supernatant by ELISA. (F) Mouse RAW264.7 reporter derived cGAS ablated [cGAS KO] and control [ISG-WT] cells were cells were either pre-treated with probenecid (200 uM) and/or verapamil (2 uM) or lovastatin (10 uM). Then these pre-treated cells were either left uninfected or infected with antimony sensitive (SBS-LD) or antimony resistant (SBR-LD) L.donovani parasites (parasite: macrophage = 10: 1) and later treated/untreated with SAG (60 ug/ml). Number of intracellular amastigotes per 100 macrophages on infection was microscopically evaluated on stained coverslip preparations from each experiment. Data represent mean ± SEM of at least three independent experiments (n = 4 for A–C and F; and n = 3 for D,E). Statistically significant results are marked as **p < 0.001.
To further investigate the relation of host MDRs and IFNβ production in antimony resistance of L.donovani infection, if any, we took advantage of well-defined pharmacological inhibitors of MRP-1 and P-gp. Treatment with a combination of probenecid and verapamil or with lovastatin alone, prior to cytosolic transfection of Ld-DNA in wild-type RAW 264.7 ISG reporter cells, demonstrated significant reduction in IFNβ production compared to cells without the specified treatments (Fig. 7E). Moreover, the effect of pharmacological inhibition of the MRP-1 and P-gp was also checked on parasite clearance in RAW264.7-ISG cells [wild-type and cGAS-KO cells] infected with SBS-LD or SBR-LD parasite strains. Results showed that inhibition of MRP-1 and P-gp in macrophages helped SAG to efficiently clear both SBS-LD and SBR-LD intracellular parasites (Fig. 7F). Therefore, the necessity of MDRP1 and Pgp in the delivery of SBR-LD Ld-DNA to the cytosol for induction of IFNβ and subsequent resistance to antimony treatment was confirmed.
Discussion
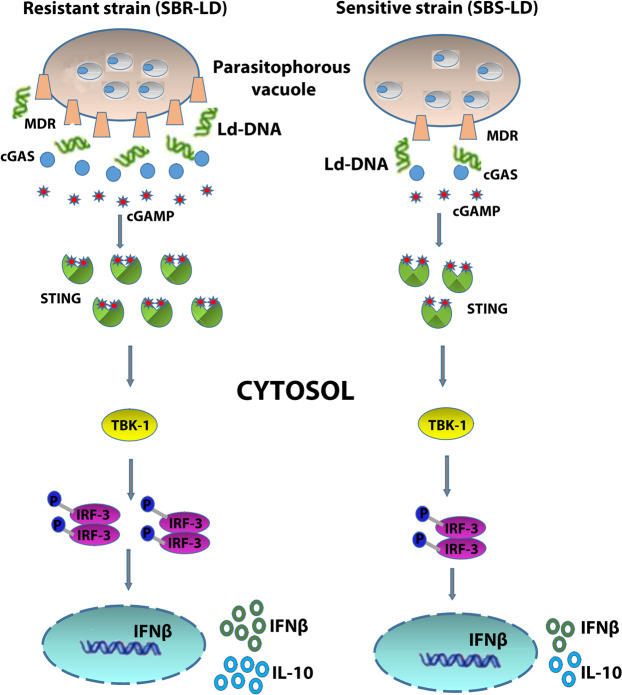
Sensing of intracellular pathogens in the host cell cytosol is essential for activation of the innate immune response and subsequent pathogen clearance. Since the first report from bacterial pathogen Listeria monocytogenes43, very few studies have focused on the sensing of pathogen-specific DNA structures in the host cell cytosol44. In a previous study, they identified the immunomodulatory role of Leishmania donovani DNA (Ld-DNA) in inducing TLR9-dependent macrophage apoptosis during VL infection29. DNA sensors in the cytosol are newly identified weapons of the host to mount an efficient innate immune response to combat the pathogen. Type I IFN, mainly IFNβ has several crucial roles on the immune system during any infection13. In most cell types, IFNβ production is mainly induced by activation of the operative cytosolic receptors by xenogenic or autologous nucleic acids45. Therefore, the identification of DNA sensor- cGAS, instigated us to examine the role of Ld-DNA in inducing IFNβ production during VL infection and its implications in disease pathogenesis. The data of this study support the existence of a model where the cytosolic targeting of cGAS by Ld-DNA activates the production of IFNβ leading to induction of macrophage unresponsiveness during VL infection (results are summarized in Fig. 8). Subsequent delivery of Ld-DNA to the cytosol targets the cellular events through IRF3-TBK1 activation leading to antimony resistance through physical and functional upregulation of host MDRs.
Figure 8.
Graphical abstract.
First, we focused on the effect of cytosolic sensing of Ld-DNA on macrophage responsiveness. Macrophage responsiveness to IFNγ is a crucial event in deciding the outcome of VL infection. Here, we provide direct evidence on the inhibition of IFNγ–induced macrophage responsiveness by Ld-DNA (Fig. 1B,C). IFN receptor genes are crucial regulators of IFN-mediated responses in the cell and their expression level decides the degree of binding to their specific ligands32. To identify the key players of macrophage unresponsiveness to IFNɣ after Ld-DNA transfection, we set out to identify changes in transcript levels in IFN receptor genes. The results clearly demonstrate that down modulation of IFNGR1 and increased level of IFNAR1 by cytosolic targeting of Ld-DNA activates macrophage unresponsiveness. The data also reveals that the effects of macrophage unresponsiveness could only be achieved by cytosolic transfer, but not by endosomal transfer, of Ld-DNA.
The host cytosolic 3′ repair exonuclease-1 (TREX-1) efficiently degrades excess intracellular cytosolic DNA30 and negate the production of IFNβ46. Therefore, it was not surprising to find that absence of TREX-1 boosts effects of cytosolic Ld-DNA on cellular responses. Our results confirmed the role of TREX-1 in Ld-DNA-mediated induction of macrophage unresponsiveness by using si-RNA mediated knockdown mechanisms (Fig. 1E,F). However, it is important to note that modified (oxidized/UV damaged) DNA are less susceptible to TREX-1 mediated degradation compared to unmodified DNA in the cytosol47. Thus, it is also possible that antimicrobial oxidative burst mediated modifications of Ld-DNA may reduce its susceptibility to cytosolic cleavage by TREX-1 and gain the opportunity to bind to and activate cytosolic sensors. The mechanism of the modifications of Ld-DNA by antimicrobial oxidative burst needs further investigation.
Second, we focused on the molecular cascade of events in identification of Ld-DNA in the host cell cytosol, subsequent IRF3 activation and IFNβ production. TBK-1 is the essential molecule to phosphorylate IRF3 leading to production of IFNs33. We were motivated towards finding the role of IRF3-TBK1 in cytosolic recognition of Ld-DNA in VL infection. Our data showed that Ld-DNA failed to induce IFNβ production in ISG-KO-IRF3 cells compared to wild-type cells RAW 264.7 ISG reporter cells (Fig. 3A), indicating the specificity and importance of IRF3 in this process. Further experiments of the current study suggest that downregulation of TREX-1 during L. donovani infection enables sensing of Ld-DNA in the cytosol leading to induction of IFNβ production through a TBK1-IRF3-dependent pathway.
The cytosolic sensing of Listeria monocytogenes DNA is generally described as TLR-independent, but dependent on IRF325. However, endosomal TLR9 is well known for identification of CpG motifs in the bacterial DNA48. Recently, it was demonstrated that CpG-motifs in the Ld-DNA induced a TLR9 dependent delay in macrophage apoptosis in VL29. It was shown that LdDNA transfection with DOTAP activates the TLR-dependent NF-kB pathway as it promoted expression and activity of luciferase gene under the control of NF-kB elements in HEK-TLR9 cells, but not in HEK293-TLR7 cells. TLR9-dependent IFNβ production in viral infections has been reported34. Therefore, it was tempting to speculate the involvement of the TLRs in sensing Ld-DNA. Our data showed that Ld-DNA transfection failed to induce IFNβ production in the HEK293 cells (Fig. 4A). Our results were consistent with report of T.cruzi infection failing to induce IFNβ response in HEK293 cells35. The transfection of Ld-DNA in HEK293-TLR9 with DOTAP activated NF-kB but not with Lipofectamine 2000 mediated transfection (Fig. 4B). The confusion was cleared as Lipofectamine 2000 transfers the DNA to the host cell cytosol and DOTAP targets the DNA to the endosomes, it was clearly indicated that NF-kB activation required Ld-DNA to be transferred to the endosomes and recognized by TLR9. Therefore, induction of IFNβ required Ld-DNA to be transferred to the cytosol involving activation of a CSP through a TLR-independent pathway, especially no contribution of TLR9 was observed.
The observation that the presence of STING alone in HEK293T cells could not compensate the deficiency of this cell-line in sensing of cytosolic DNA, unveiled the crucial role of cGAS in this process20. cGAS can directly interact with DNA and can also stimulate STING through an enzymatic function for IFNβ production. Studies have shown that ISD-induced IFNβ production in several cell types depends directly upon expression level of cGAS20. Our data analysis of human cGAS protein immunoblot demonstrated that Ld-DNA transfection significantly enhanced the cGAS protein expression, only in the cytosol (Fig. 5E). However, the data also confirms that STING and cGAS are both crucial for cytosolic sensing of Ld-DNA for activation of IFNβ responses in VL.
Third, we aimed to find out the possible mechanism that enables the cytosolic transfer of Ld-DNA from the parasitophorous vacuoles where the amastigotes reside and the significance of such transfer. Previous studies on Listeria monocytogenes suggest the role of multidrug resistance transporters (MDRs) in regulation of CSP and activation of IFNβ responses49. Overexpression of ABC transporters, like P-gp or MRP1, results in multiple drug resistance in different diseases [Ref] and are well known for playing crucial role in conferring antimony resistance in VL41,42. It is quite relevant to recall that the organic pentavalent antimony compound Sb(V) has been considered as the first line of drug for treatment of VL. However, antimony resistance is widely observed in VL-endemic zones50. In this study, we found that infection with both antimony resistant strain (SBR-LD) and antimony sensitive strain (SBS-LD) failed to induce any significant IFNβ production in ISG-KO-cGAS cells compared to wild-type control reporter cells (Fig. 6A). Moreover, the comparative overproduction of IFNβ by infection with SBR-LD, than by SBS-LD strain, was dependent on the availability of cGAS. Thus, the comparatively higher binding of SBR-LD DNA to cGAS might be facilitated by enhanced access of this DNA to the cytosol by the MDRs. Therefore, it was quite possible that sensing of Ld-DNA by cGAS could have role in antimonial drug resistance.
However, the relation between targeting of cytosolic DNA sensors (like cGAS) and access of Ld-DNA to the macrophage cytosol by ABC transporters and the role of type-I IFNs in antimony resistance development is unknown in VL infection. We observed a direct relation of IFNβ production with enhanced survival of SBR-LD parasites. Our studies with specific pharmacological inhibitors confirmed the necessity of MDRP1 and Pgp in the delivery of SBR-LD Ld-DNA to the cytosol for induction of IFNβ and subsequent resistance to antimony treatment. Therefore, it is tempting to speculate the role of ABC transporters like P-gp or MRP1 in transporting Ld-DNA to the cytosol. The pharmacological manipulation of ABC transporters rendered surge in IL-10 and IFNβ production by Ld-DNA sensing to achieve antimony resistance.
Type-I IFNs have additional role in activation of haematopoietic stem cells (HSCs) which has a prominent effect on recruitment of immune cells at the site of infection51. Of note, IFN-I receptor knockout (Ifnar1-/-) mice develop significant defects in the infiltration of Ly6Chi monocytes and neutrophils after influenza infection in the lung52. The Ly6Chi inflammatory monocytes are also reported to promote susceptibility to L. donovani infection in the liver and spleen53. Additionally, IFN-I plays a critical role in regulating neutrophil functions to Leishmania parasites54. Thus, surge in IFN-I by Ld-DNA sensing probably induces modulation of macrophage and neutrophil infiltration to achieve antimony resistance in L. donovani infection. Previous studies have strongly recommended the role of selective impairment of IFN-γ signalling in macrophages for reduced control of Leishmania sp parasites55. Our current study demonstrated the role of Ld-DNA in reduction of IFN-γ responsiveness in macrophages. It is well known that Leishmania infection has a suppressive effect on responsiveness to IFN-γ56. Thus unresponsiveness to IFN-γ and lowering of MHCII expression may lead to compromised activation of T cells that would lead to enhanced parasite survival and ultimately mediate drug resistance phenomenon.
Recent studies have also suggested the role of Leishmania secreted vesicles in pathogenesis57. Leishmania-secreted exosomes were found to be predominantly immunosuppressive and regulated both innate and adaptive immune responses58. Notably, exosomes were found to attenuate IFN-γ-induced pro-inflammatory TNF-α by Leishmania-infected monocytes while conversely enhancing production of the anti-inflammatory IL-10. Furthermore, the tumour-derived exosomes has been reported to contain dsDNA and are currently being used as biomarkers for cancer detection59,60. Thus, it would not be surprising if exosomal cargo of macrophages has Ld-DNA and also found responsible for transferring it to the cell cytosol. Therefore, the access of Ld-DNA to the cell cytosol and its impact on the disease outcome is a new area of importance and is unlikely to be simple in vivo. Instead, it is part of a constellation of regulatory mechanisms which requires further investigation.
Materials and Methods
Ethics statement
All studies or protocols were approved by the Institutional Review Boards and Ethical Committees of All-India Institute of Medical Sciences, Patna and ICMR-Rajendra Memorial Research Institute of Medical Sciences, Patna. All experiments were performed in accordance with relevant guidelines and regulations.
Reagents
All chemicals were purchased from Sigma unless otherwise indicated. The ISD was prepared by mixing equimolar amounts of sense and antisense DNA oligonucleotides [Sense strand 5′-TACAGATCTACTAGTGATCTATGACTGATCTGTACATGATCTACA-3′]. The oligos were heated at 95 °C (5 mins) and then cooled to RT. Poly(I:C), Poly(DA-DT) and cGAMP was purchased from Invivogen. Puromycin, blasticidin, zeocin, Quanti-Luc (InvivoGen), kanamycin (Sigma-Aldrich) and hygromycin (Roche).
Parasite strains
Three strains of L. donovani, SAG-sensitive (SBS-LD) MHOM/IN/1983/AG83 (AG83) and two SAG-resistant (SBR-LD) strains, RMRI-108/2014 and K39 (both isolated from a SAG-unresponsive patient and characterized in the laboratory), were used. Amastigotes obtained from the spleens of infected hamsters were cultured axenically to obtain promastigotes as described previously29. Briefly, promastigotes were cultured at 22 °C in endotoxin-free RPMI-1640 medium (pH 7.4; Gibco-BRL, USA), supplemented with 10% FBS (Gibco-BRL), 25 mM HEPES (Sigma-Aldrich), 100 U/ml penicillin G-sodium (Sigma-Aldrich), and 100 mg/ml streptomycin sulfate (Sigma-Aldrich). The axenic cultures of the promastigote stage were maintained at 22 °C and subcultured at every 72 h when cultures reached confluence of 1–2 × 106 cells/ml. Cells were counted, and viability was tested by trypan blue exclusion technique (=95%). Stationary-phase promastigotes were collected from in vitro cultures in complete RPMI-1640 medium.
Parasite genomic DNA preparation
Genomic DNA (Ld-DNA) was purified from stationary-phase promastigotes by GFX genomic DNA purification kit (GE Healthcare, USA). The DNA was treated with 20 mg/ml RNase B and 20 mg/ml proteinase K and extracted further with ethanol. Purity and concentrations of DNA samples were determined before use in experiments. The purified DNA was also tested for the presence of glucans and endotoxin by use of LAL (BioWhittaker, USA); endotoxin levels were ≤0.02 endotoxin units/mg DNA.
Culture of primary cells and cell lines
For primary BMDM culture, cells were flushed from both femurs of young BALB/c mice and cultured for 6 days in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL, Germany) supplemented with 1% sodium pyruvate, 1% L-glutamine, 2-mercaptoethanol, 1% penicillin-streptomycin (Gibco BRL), 10% fetal bovine serum (FBS; HyClone) and 10% L-cell conditioned media. At day 3, fresh media was added and BMMs were used for the experiments on day 7.
The variants of RAW264.7 and THP-1 cells used in this study were cultured as per the suppliers protocol (InvivoGen) in DMEM and RPMI-GlutaMAX, respectively (Life Technologies) with 10% heat-inactivated FBS. Murine RAW264.7 derived wild-type and knockout (KO) macrophages such as RAW-LuciaTMISG, RAW-LuciaTMISG-KO-STING, RAW-LuciaTMISG-KO-cGAS, RAW-LuciaTMISG-KO-TREX-1, RAW-LuciaTMISG-KO-IRF3, RAW-LuciaTMISG-KO-TBK1, RAW-LuciaTMISG-KO-IFI16, RAW-LuciaTMISG-KO-RIG1 and human monocyte THP1-Dual™, THP1-Dual™ ISG-KO-STING cells were all commercially purchased from Invivogen and used for in vitro experiments. Plated macrophages were incubated overnight at 37 °C to adhere at 5% CO2, and then stimulated accordingly. The viability of cells was >98%, as assessed by trypan blue dye exclusion technique.
Flow cytometry
DNA treated, transfected or mock transfected RAW256.7 cells or mouse BMDM cells were subjected to flowcytometric detection of MHCII expression. To detect MHCII expression, cells were stained with PE-Cyanine5 MHC II antibody (I-A/I-E; clone M5/114.15.2; eBioscience). Stained cells were run on BD FACS Aria II and the results were analysed using BD FACS DIVA software. To compare the effects of these treatments on MHCII surface expression levels, the mean channel fluorescence intensities (MFIs) for each of the treated/transfected samples per group were normalized to mean control MFI of mock samples. All graphs represent mean values with SD.
Ld-DNA transfection
Mouse primary cells and cell lines, human THP-1 or HEK293 cells were transfected with Ld-DNA (2.5 µg/ml) in Lipofectamine 2000 or DOTAP or were mock transfected. In IFN-ɣ stimulation experiments, transfection was done 2 hrs prior to IFN-ɣ (100 U/ml) treatment. After transfection, the cells received fresh culture media with no (0) or 100 U/ml of IFN-ɣ addition. Cells were also treated/transfected with RNA analog poly(I:C) in parallel for negative control. The cells were harvested 6–8 hr after the treatment or Ld-DNA transfection.
RNA-mediated interference
Preconfirmed TREX-1, TRIF, IRF-3, RIG-1 and MAVS-specific mouse small interfering RNA (siRNA) and non-targeting (NT) control siRNA were purchased from Dharmacon. The target macrophages were plated at 105 cells/well in 24 well plates for 18–24 hrs before transfection. The siRNA were transfected into the target macrophages with SMARTPool siGENOME siRNA buffer and DharmaFECT transfection reagents, according to the manufacturer’s protocol. The media were then replaced with fresh, antibiotic-free complete RPMI 1640 [containing 10% heat inactivated human AB serum] after 48 hr of the first siRNA transfection. The same process of transfection was repeated with half volume of siRNA of the first transfection to achieve maximum knockdown in expression levels (checked by immunoblotting with specific antibodies as described below). After 18 hrs of the second siRNA transfection, the cells were treated with indicated stimulations for a desired period of time or were transfected with Ld-DNA (as described above) for different time periods. Cells were also transfected with either ISD (for positive control) or with RNA analog poly I:C (for negative control) in parallel experiments.
NF-kB luciferase reporter assay in HEK293 cell line
HEK293 cells, stably transfected with TLR3 (293-TLR3), TLR7 (293-TLR7) and TLR9 (293-TLR9) were purchased from InvivoGen. These cells were mock-transfected or transfected with Ld-DNA as described above and the cell culture supernatant was collected for ELISA. In another experiment, HEK293 or HEK293-TLR9 cells were seeded at 5 × 105 cell/well and transfected overnight in 6-well plates by a CaCl2 precipitation method with 1 mg pNF-kB-Luc (Stratagene, USA) or control plasmid, and were used for testing the activity of LdDNA. To correct for differences in transfection efficiency, each group of cells was transfected with 80 ng pSV40/LACZ and co-transfected with 3 mg control vector and incubated overnight. The ratio of luciferase activity to β-galactosidase activity in each sample served as a measure of luciferase activity, normalized to control transfection results. HEK293 with pNFKB-Luc cells were mock-transfected or transfected with Ld-DNA or ISD (for positive control) or RNA analog poly I:C (for negative control) in parallel experiments. The cell extracts were prepared for determination of luciferase activity by enhanced luciferase assay reagents (Analytical Luminescence Laboratory, USA), according to the manufacturer’s instructions on a luminometer (Analytical Luminescence Laboratory).
Immunofluorescence
Ld-DNA transfected or mock-transfected macrophages were fixed after 8 hrs and stained with pTBK-1 antibody (green; Ser172, D52C2; Cell Signalling Technologies); and/or Nuclei-blue [DAPI; Life Technologies] as per manufacturers’ protocol. Merged (Blue + green) channel images of macrophages were captured by fluorescence microscope (Olympus) and percentage of pTBK-1 cells were counted per case.
IRF induction estimation by luciferase reporter assay
The RAW-LuciaTMISG or RAW-LuciaTM ISG-KO cells were derived from the murine RAW 264.7 macrophage cell line by stable integration of an interferon regulatory factor (IRF)-inducible luciferase reporter constructs, under the control of the I-ISG54 promoter, which is comprised of the IFN-inducible ISG54 promoter enhanced by a multimeric ISRE. After required treatment in fresh DMEM, supernatants were collected for estimation of IRF induction by Luminescence assay using QUANTI-Luc (InvivoGen). THP1-Dual™ were derived from the human THP-1 monocyte cell line by stable integration of an interferon regulatory factor (IRF)-inducible SEAP reporter construct and maintained as per supplier instructions. After required treatment in fresh DMEM, supernatants were collected for estimation of IRF induction by SEAP colorimetric assay using QUANTI-Luc reagent (InvivoGen).
Isolation of RNA and RT-PCR
Total RNA was isolated from 2 × 106 cells of differentially stimulated/transfected macrophages by RNAqueous kit (Ambion, Life Technologies, USA), according to the manufacturer’s protocol. The extracted RNA was then treated with 2 U RNase-free DNase (Ambion, Life Technologies), followed by deactivation of DNase. One mg RNA was converted into complementary DNA using platinum quantitative RT-PCR Thermoscript One-Step System kit (Invitrogen). To determine the differential transcription levels of IFN-β, TREX-1, TNF-α, IFNAR-1, IFNAR-2, IFNGR-1 and IFNGR-2 in macrophages, semiquantitative PCR was performed. One PCR cycle consisted of denaturation at 95 °C for 60 s, annealing at 58–60 °C for 60 s, and extension at 72 °C for 90 s. PCR reactions were performed for 25 cycles for IFN-β or TREX-1, GAPDH and TNF-α, and for 27 cycles for all IFN receptors. The final extension was carried out at 72 °C for 7 mins.
Next, 10 ml PCR products were electrophoresed in 1.2% agarose gel containing 0.5 mg/ml ethidium bromide. DNA size markers (1 Kb Plus DNA Ladder; Invitrogen, Life Technologies) were run in parallel. The resultant bands were densitometrically scanned (Quantity One software; Bio-Rad Laboratories, Hercules, CA, USA) and normalized to the expression of GAPDH. The primers (sequences of human origin obtained from the GenBank/EMBL database), designed by Primer 3 software, are presented in Supplementary Table 1.
Immunoblotting
Control or mock transfected or transfected RAW264.7-ISG or RAW264.7-TREX-1-KO cells were treated with 100 U/ml of IFN-ɣ at 2 hpi. Cells were rinsed in PBS and lysed in SDS buffer P (62.5 mM Tris-Hcl, pH 6.8; 2% SDS; 10% glycerol, 50 mM DTT and 0.01% bromophenol blue) supplemented with phosphatase and protease inhibitor cocktails (Roche, USA). Lysates were separated by loading equal amounts of protein (30–50 mg) from each sample onto a 12% SDS-PAGE gel. Proteins were size fractionated, transferred to a Hybond-P membrane (GE Healthcare), blocked with 5% skimmed milk, and immunoblotted with anti-pSTAT-1, total STAT-1 or β-actin using commercially purchased antibodies (Cell signalling technologies, USA). The blots were developed by an ECL Western blotting detection system (GE Healthcare), and different exposition times were performed for each blot with a charged coupling device camera in a luminescent image analyzer (Molecular Imager; Bio-Rad Laboratories) to ensure the linearity of the band intensities. Values of densitometry were determined by use of Quantity One software (Bio-Rad Laboratories). To insure equal loading, the blots were then stripped and reprobed with mouse β-actin antibody.
Experimental infection of cultured macrophages with L. donovani parasites
A total of 106 macrophages (as indicated in different experiments) was coincubated at 37 °C under a humidified atmosphere containing 5% CO2, with L. donovani promastigotes at a parasite:macrophage ratio of 10:1 in 1 ml of complete RPMI-1640 medium (with 10% heat-inactivated autologous serum). After incubating cultures overnight at 37 °C and 5% CO2 in complete RPMI-1640 medium, non-ingested promastigotes were washed off. Then, the number of infected cells was determined by microscopic evaluation after May-Gruenwald-Giemsa staining of the coverslips containing the infected adherent macrophages.
Preparation of cellular fractions
Following infection/stimulations, the cells were fractionated into the cytosolic and the nuclear fractions by use of a commercially available kit [NE-PER Nuclear and Cytoplasmic Extraction Reagents, Thermo Scientific, USA] as per manufacturer’s protocol. The suspended collection of cells were harvested and washed by centrifuging at 500 × g for 5 minutes in PBS. Cells were then separated in different tubes for separate extraction of cytoplasmic and nuclear fractions as per the kit content and protocol.
Assay for DNA binding
The coding sequences for mouse cGAS were subcloned into pcDNA3 in frame with an N-terminal FLAG tag. Indicated FLAG fusion proteins were expressed and purified from E.coli. Recombinant FLAG-tagged m-cGAS protein was incubated with streptavidin plus Ultralink resin beads (Pierce) in the presence of ISD, biotinylated-ISD, biolytinated Ld-DNA in lysis buffer [50 nM Tris-Hcl (pH 7.4), 100 nM NaCl, 10% glycerol, 0.5% NP40, 0.5 nM EDTA, 0.5 mM EGTA]. Biotin RNA sequence (ACGGAAAGACCCCGU) from 23 S rRNA of DH5α was used as negative control. Biotinylation of ISD and Ld-DNA was performed using Pierce Biotin 3′-End DNA labelling kit (Thermo Fischer Scientific).
In vitro cGAS pull down assay
Mouse cGAS (m-cGAS) expressing HEK293T was infected with Ld parasites and crosslinked with 4% PFA after 4 hrs. cGAS was immunoprecipitated with M2 FLAg magnetic beads (Sigma) and then eluted with FLAG peptide (Bioneer). The IP efficiency was confirmed by immunoblot analysis. The precipitate was later analysed by qPCR for abundance of Leishmania donovani kinetoplastid DNA (Ld-kDNA) using specific primers.
Estimation of cytokine production by ELISA
Macrophages (5 3 106/ml) were pretreated with different blockers and then transfected with LdDNA [of SBS-LD or SBR-LD parasites] for 18 h at 37 °C with 5% CO2 in 24-well tissue-culture plates (Costar, USA) in RPMI-1640 medium. Supernatants were stored at 270 °C for no longer than 15 d before assay. The release level of cytokines (IFN-β, CXCL-10, TNF-α and IL-10) was detected simultaneously in supernatants by use of the BD mouse cytokine kit (BD Biosciences, USA). The absorption was measured at 450 nm, and wavelength correction was performed at 570 nm.
Statistical analysis
Each experiment was repeated 3–4 times in separate sets, and the mean values 6 SEM are presented in the paper. All in vitro experiments were performed in triplicates, and representative data from each set of these experiments were presented with the mean values. All statistical analysis was performed by use of GraphPad Prism software, version 5.0 (GraphPad Software, La Jolla, CA, USA). Comparisons were based on Mann-Whitney U-test. The values which were considered to be significantly different were represented by an asterisk.
Supplementary information
Author Contributions
S.D. provided the concept and design of the study. S.D., A.K.2., A.M., S.V. and K.A. performed the experiments. S.D. A.K.1 and P.D. analyzed the data and interpretation, P.D. and S.D. secured the availability of equipment, chemicals, reagents, etc. S.D. wrote the main manuscript and P.D. prepared all the figures.
Competing Interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41598-022-09098-9
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/22/2022
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-022-09098-9
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-45800-0.
References
- 1.Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22(12):552–7. doi: 10.1016/j.pt.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 2002;3(11):1041–7. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 3.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin. Microbiol. Rev. 2005;18(2):293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das S, et al. TGF-β1 re-programs TLR4 signaling in L. donovani infection: enhancement of SHP-1 and ubiquitin-editing enzyme A20. Immunol. Cell Biol. 2012;90(6):640–54. doi: 10.1038/icb.2011.80. [DOI] [PubMed] [Google Scholar]
- 5.Igarashi K, et al. Interferon-gamma induces tyrosine phosphorylation of interferon-gamma receptor and regulated association of protein tyrosine kinases, Jak1 and Jak2, with its receptor. J. Biol. Chem. 1994;269(20):14333–6. [PubMed] [Google Scholar]
- 6.Medzhitov R, Janeway C., Jr. The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8(10):452–6. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 7.Rosadini CV, Kagan JC. Microbial strategies for antagonizing Toll-like-receptor signal transduction. Curr. Opin. Immunol. 2015;32:61–70. doi: 10.1016/j.coi.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immunesystem. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015;15(2):87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cousens LP, Orange JS, Su HC, Biron CA. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc. Natl. Acad. Sci. USA. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautier G, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 2005;201(9):1435–46. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 13.Beiting DP. Protozoan parasites and type I interferons: a cold case reopened. Trends Parasitol. 2014;30(10):491–8. doi: 10.1016/j.pt.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 2007;204(5):987–94. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaffrey RL, et al. A specific gene expression program triggered by Gram-positive bacteria in the cytosol. Proc. Natl. Acad. Sci. USA. 2004;101(31):11386–91. doi: 10.1073/pnas.0403215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corr SC, O’Neill LA. Listeria monocytogenes infection in the face of innate immunity. Cell. Microbiol. 2009;11(5):703–9. doi: 10.1111/j.1462-5822.2009.01294.x. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaussabel D, et al. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102(2):672–81. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 22.Diefenbach A, et al. Type 1 interferon (IFN alpha/beta) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8(1):77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 23.Mattner J, et al. Regulation of type 2 nitric oxide synthase by type 1 interferons in macrophages infected with Leishmania major. Eur. J. Immunol. 2000;30(8):2257–67. doi: 10.1002/1521-4141(2000)30:8<2257::AID-IMMU2257>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 24.Dey B, et al. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat. Med. 2015;21:401–406. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc. Natl. Acad. Sci. USA. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38:870–880. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez-Ortiz ZG, et al. Toll-like receptor 9-dependent immune activation by unmethylatedCpG motifs in Aspergillus fumigatus DNA. Infect. Immunity. 2008;76(5):2123–9. doi: 10.1128/IAI.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parroche P, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Nat. Acad. Sci. USA. 2007;104(6):1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das S, et al. Unmethylated CpG motifs in the L. donovani DNA regulate TLR9-dependent delay of programmed cell death in macrophages. J. Leukoc. Biol. 2015;97(2):363–78. doi: 10.1189/jlb.4A0713-378RR. [DOI] [PubMed] [Google Scholar]
- 30.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines and their receptors. Immunol. Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 2012;5:ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Versteeg GA, et al. Species-specific antagonism of host ISGylation by the influenza B virus NS1 protein. J. Virol. 2010;84(10):5423–30. doi: 10.1128/JVI.02395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chessler AD, Ferreira LR, Chang TH, Fitzgerald KA, Burleigh BA. A Novel IFN Regulatory Factor 3-Dependent Pathway Activated by Trypanosomes Triggers IFN-β in Macrophages and Fibroblasts. J. Immunol. 2008;181(11):7917–7924. doi: 10.4049/jimmunol.181.11.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao D, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341(6148):903–6. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, et al. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity. 2013;39(6):1019–31. doi: 10.1016/j.immuni.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ablasser A, et al. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J. Immunol. 2014;192:5993–5997. doi: 10.4049/jimmunol.1400737. [DOI] [PubMed] [Google Scholar]
- 39.Liang Q, et al. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe. 2014;15(2):228–38. doi: 10.1016/j.chom.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson RO, et al. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe. 2015;17(6):811–819. doi: 10.1016/j.chom.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mookerjee Basu J, et al. Inhibition of ABC Transporters abolishes Antimony Resistance in Leishmania Infection. Antimicrob. Agents. Chemother. 2008;52(3):1080–1093. doi: 10.1128/AAC.01196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukherjee B, et al. Antimony-resistant but not antimony-sensitive Leishmania donovani up-regulates host IL-10 to overexpress multidrug-resistant protein 1. Proc. Natl. Acad. Sci USA. 2013;110(7):E575–82. doi: 10.1073/pnas.1213839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Sharma S, et al. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity. 2011;26(35(2)):194–207. doi: 10.1016/j.immuni.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trinchieri G. Type I interferon: friend or foe? J. Exp. Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. The cytosolic exonuclease TREX1 inhibits the innate immune response to HIV-1. Nat. Immunol. 2010;11(11):1005–1013. doi: 10.1038/ni.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gehrke N, et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39(3):482–95. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad-Nejad P, et al. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 49.Crimmins GT, et al. Listeria monocytogenes multidrug resistance transporters activate a cytosolic surveillance pathway of innate immunity. Proc. Natl. Acad. Sci. USA. 2008;105(29):10191–6. doi: 10.1073/pnas.0804170105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakravarty J, Sundar S. Drug resistance in leishmaniasis. J. Glob. Infect. Dis. 2010;2(2):167–176. doi: 10.4103/0974-777X.62887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Essers MA, et al. IFN alpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904–8. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 52.Seo SU, et al. Type I interferon signaling regulates Ly6C(hi) monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog. 2011;7(2):e1001304. doi: 10.1371/journal.ppat.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terrazas C, et al. Ly6Chi inflammatory monocytes promote susceptibility to Leishmania donovani infection. Sci. Rep. 2017;7(1):14693. doi: 10.1038/s41598-017-14935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xin L, et al. Type I IFN Receptor Regulates Neutrophil Functions and Innate Immunity to Leishmania Parasites. J. Immunol. 2010;184(12):7047–7056. doi: 10.4049/jimmunol.0903273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lykens JE, et al. Mice with a selective impairment of IFN-gamma signalling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J. Immunol. 2010;184:877–885. doi: 10.4049/jimmunol.0902346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kima PE, Soong L. Interferon gamma in Leishmaniasis. Front. Immunol. 2013;4:156. doi: 10.3389/fimmu.2013.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atayde VD, et al. Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cell. Immunol. 2016;309:7–18. doi: 10.1016/j.cellimm.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Silverman. JM, et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 2010;185(9):5011–22. doi: 10.4049/jimmunol.1000541. [DOI] [PubMed] [Google Scholar]
- 59.Kahlert C, Kalluri R. Exosomes in Tumor Microenvironment Influence Cancer Progression and Metastasis. J. Mol Med. 2013;91(4):431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thakur BK, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell. Res. 2014;24(6):766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.