Abstract
Alport syndrome (AS) is a hereditary nephropathy characterized by glomerular basement membrane lesions. AS shows a relatively rare entity with autosomal dominant gene mutation (accounts for less than 5% of AS cases) and is widely believed to be a consequence of heterozygous variants in the COL4A3 and COL4A4 genes. Until now, there have been no reports of homozygous variants in genes in AS patients, and it is scarce to detect both homozygous and heterozygous variants in a single AS pedigree. We performed genetic analysis by exome sequencing (exome-seq) in a Chinese family with AS and found four individuals harboring the COL4A4 c.4599T > G variant, a novel COL4A4 nonsense mutation that gains stop codon and results in a truncated protein. The proband and her two siblings were determined to be heterozygous, whereas their mother was homozygous. The proband satisfied the criteria for the diagnosis of AS, which included clinical manifestations of microscopic hematuria and proteinuria, and pathological features of the glomerular basement membrane (GBM), including irregular thickening and splitting. However, the other three individuals who were homozygous or heterozygous for the variant exhibited mild clinical features with isolated microscopic hematuria. In summary, we identified a novel pathogenic variant in either the heterozygous or homozygous state of the COL4A4 gene in a Chinese family with AS. Our results also suggest that the severity of clinical manifestations may not be entirely attributed to by the COL4A4 genetic variant itself in patients.
Keywords: human genetics, collagen type IV, Alport syndrome, COL4A4, genomic variant, hereditary nephropathy
Introduction
Alport syndrome (AS) is a genetic disorder characterized by glomerulonephritis, end-stage kidney disease, ocular abnormalities, and hearing loss. It is caused by pathogenic variants in the COL4A3, COL4A4, and COL4A5 genes, which encode the α3, α4, and α5 chains of collagen type IV (COL4) respectively. The glomerular basement membrane (GBM) contains three layers, including the lamina lucida interna, lamina densa, and lamina lucida externa. COL4 is a major component of the GBM of the kidney. Pathogenic variants involving this gene result in the splitting of the GBM or more severe pathological lesions involving the kidney (Kruegel et al., 2013). More than 1,700 unique variants have been identified in these genes and involve three inheritance patterns: X-linked AS (XLAS), autosomal recessive AS (ARAS), or autosomal dominant AS (ADAS). XLAS is associated with variants in the COL4A5 gene and accounts for 85% of patients with AS, while ARAS is found with variants in the COL4A3 and COL4A4 genes and accounts for 15% of AS patients (Hudson et al., 2003; Nagel et al., 2005; Hertz et al., 2015), and ADAS is seen with variants in the COL4A3 and COL4A4 genes, accounting for 5% of AS patients (Lemmink et al., 1996).
The correlation of genotype and phenotype between COL4 gene mutations and the clinical manifestations of AS have been previously described. XLAS is a highly penetrant disease in hemizygous males, and 70% of these cases rapidly progress to end-stage renal disease (ESRD) before the age of 30. Only a few cases (30%) reach ESRD after 30 years of age (Flinter et al., 1988; Myers et al., 1990; Jais et al., 2000). However, in heterozygous female patients with XLAS, clinical features are variable, ranging from isolated micro-hematuria to ESRD (Rheault, 2012). ARAS, which is a consequence of homozygous variants or compound heterozygous variants in the COL4A3 and COL4A4 genes, usually involves severe early disease in both females and males (Longo et al., 2006; Oka et al., 2014). ADAS has been ascribed to heterozygous variants in the COL4A3 or COL4A4 gene, and clinical manifestations usually include mild and slowly progressing renal disease compared with that in patients with ARAS or males with XLAS(7). Theoretically, the asymptomatic, late-onset, or mildly affected individuals with the autosomal dominant disease may not be diagnosed; consequently, the offspring of two carrier parents may have possibilities to carry two variant alleles and present with a homozygous state. In reality, homozygotes have not yet been described for AS, although they are reported in some pedigrees of autosomal dominant diseases (Chemke et al., 1984; Brandi et al., 1993; Tonini et al., 2004; Arzel-Hezode et al., 2010; Vinciguerra et al., 2014; Conidi et al., 2015; Kosik et al., 2015; Sjouke et al., 2015). In this study, we identified a novel mutation, c.4599T > G (p.Tyr1533Ter), in the COL4A4 gene, which is associated with AS disorder in three generations of a Chinese family. Of particular interest is the coexistence of homozygote and heterozygote carriers in a single pedigree, which, to our knowledge, has not been reported in the literature.
Results
Clinical Characteristics of the Pedigree
Seven members of this family, including four males and three females, did not have high blood pressure, ophthalmic impairment, or audiological abnormalities. The proband (II:3) manifested proteinuria (2.3 g per day), micro-hematuria, and a mildly elevated serum creatinine level (92.5 µmol/L). Her mother and two of her siblings (one brother and one sister) displayed isolated micro-hematuria and normal serum creatinine levels. Her father, her husband, and her son showed no symptomatic glomerulopathy. Consanguinity was denied by the family members. The clinical characteristics of the family are summarized in Table 1 .
Table 1.
Clinical and genetic data of the family with Alport syndrome (AS).
| Subject | I:1 | I:2 | II:1 | II:2 | II:3 | III:1 |
| Sex | M | F | F | M | F | M |
| Age (years) | 83 | 80 | 59 | 52 | 46 | 23 |
| Blood pressure | Normal | Normal | Normal | Normal | Normal | Normal |
| Hematuria | No | 3+ | 2+ | 2+ | 3+ | No |
| Proteinuria | No | No | No | No | 3+ | No |
| BUN (mmol/L) | 6 | 7.5 | Normal | Normal | 6.13 | Normal |
| Scr (µmol/L) | 81.5 | 91.3 | Normal | Normal | 92.5 | Normal |
| Audiological examination | Normal | Normal | Normal | Normal | Normal | Normal |
| Ophthalmic examination | Normal | Normal | Normal | Normal | Normal | Normal |
| Genotype | − | Hom | Het | Het | Het | − |
BUN, blood urea nitrogen; Scr, serum creatinine values; Hom, homozygous; Het, heterozygous.
Renal Pathology Manifestation
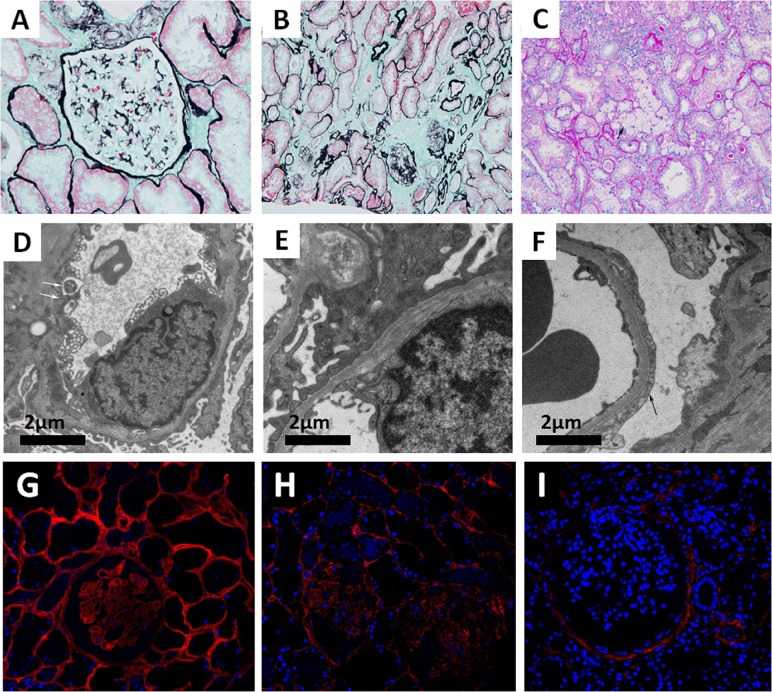
Seventeen glomeruli were available and assessed under a light microscope. Glomerular sclerosis was observed in two of the glomeruli. Among the remaining 15 glomeruli, the glomerular capillary tuft was normal by light microscopy. The tubules showed dispersed granular degeneration and cast formation. The tubular casts included blood cell casts and proteins casts. The areas of the brush border in the kidney tubules were significantly smaller. Interstitial inflammatory cell infiltration and foam cells were observed focally. The intima of the arterioles showed thickening with occasional hyaline changes ( Figure 1A–C ). Immunofluorescence staining showed granular IgM deposition in the mesangium, whereas staining for IgG, IgA, C3, C1q, and C4 was all negative. The ultramicroscopic evaluation revealed that most segments of the GBM were normal, whereas some exhibited irregular thinning (<200 nm) and thickening or splitting. There was localized effacement of podocyte foot processes. No distinct dense deposits in the glomerular basement membrane or in the mesangium were detected ( Figure 1D–F ). For Col4 expression profiling, Col4 immunofluorescence staining was performed on the renal tissues of a patient with minimal change disease (MCD), the proband (II:3), and a male patient with XLAS. The results indicated that although Col4 was positively expressed in the glomeruli of the proband (II:3), it was weaker than that of the MCD patient. As a negative control, no Col4 was observed in the glomeruli of the male patient with XLAS ( Figure 1G–I ).
Figure 1.
(A–C) Images of renal pathology (II:3) under light microscopy by periodic acid-silver methe-namine (PASM) (A and B) and periodic acid-schiff (PAS) staining (C). A non-uniform glomerular basement membrane (GBM) (arrow), 200×. (B) Tubular atrophy and interstitial fibrosis, 200×. (C) The infiltration of foam cells (arrow) in renal interstitium. (D–F) Representative electron micrograph of renal tissue. (D) Some segments of GBM were absent (arrow). (E) Irregular GBM thinning, thickening, and splitting. (F) Effacement of podocyte foot processes (arrow). (G–I) Immunofluorescence staining of Col4A3 (Ab111742, Abcam, USA) in renal tissue from a patient with minimal change disease (MCD) (G, left panel), the proband (II:3) (H, middle panel), and a male patient with XLAS (I, right panel), 400×.
Genomic DNA Extraction and Exome Sequencing
The total DNA concentration of the sample from the proband (II:3) was 68.2 ng/μl, and the ratio of the absorbance at wavelengths of 260 and 280 nm (A260/280) was 1.83, indicating that the isolated DNA was pure. Exome sequencing was also performed to identify sequence variants. A total of 293,903 amplicons were generated by the polymerase chain reaction (PCR) method. Approximately 57,742,646 base pairs were identified in the targeted regions. The average base coverage depth was 114.6×. The uniformity of coverage was 93.24%. These results are summarized in Table 2 .
Table 2.
Comparison of secondary structure between wild-type COL4A4 and the mutant protein.
| Wild type | Mutant | |||
|---|---|---|---|---|
| Number | Percentage (%) | Number | Percentage (%) | |
| Alpha helix | 58 | 3.43 | 15 | 0.98 |
| 310 helix | 0 | 0 | 0 | 0 |
| Pi helix | 0 | 0 | 0 | 0 |
| Beta bridge | 0 | 0 | 0 | 0 |
| Extended strand | 185 | 10.95 | 142 | 9.27 |
| Beta turn | 138 | 8.17 | 124 | 8.09 |
| Bend region | 0 | 0 | 0 | 0 |
| Random coil | 1,309 | 77.46 | 1,251 | 81.66 |
| Ambiguous states | 0 | 0 | 0 | 0 |
Variant Analysis and COL4A4 Variant Screening
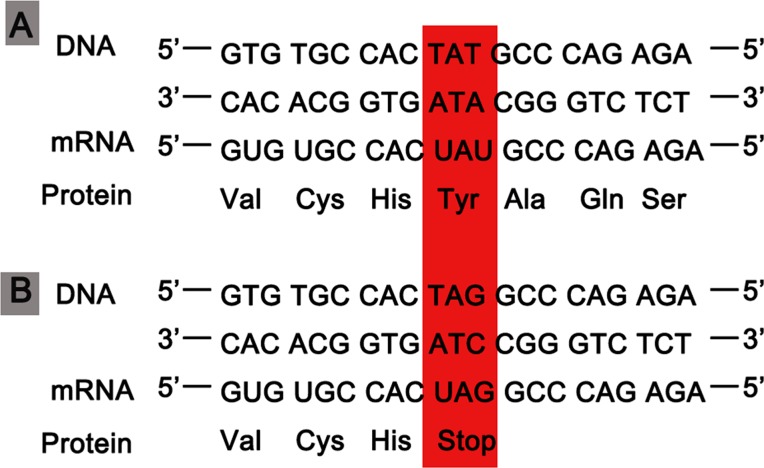
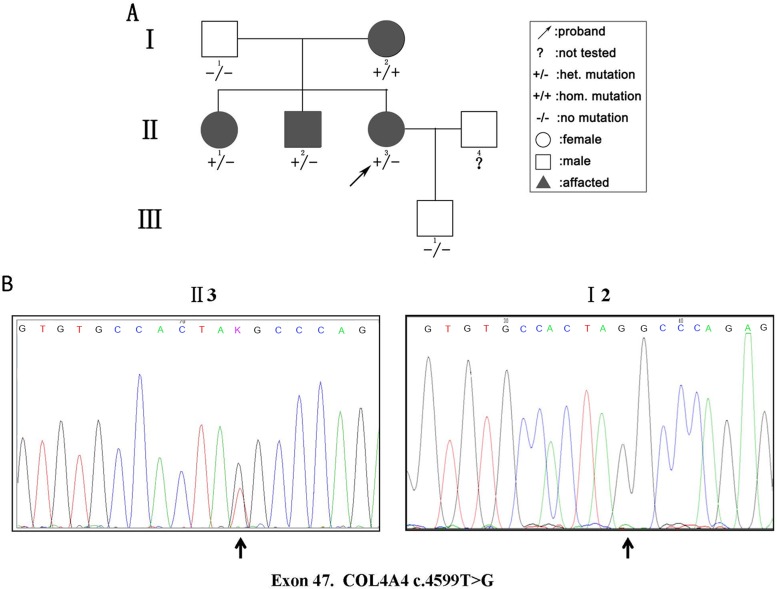
We detected 583 variants in the proband (II:3) and filtered out synonymous and intronic variants using public databases. Variants in genes related to proteinuria were all analyzed in terms of pathogenicity. A novel heterozygous variant c.4599T > G in exon 47 of the COL4A4 gene was classified as pathogenic. The c.4599T > G variant was predicted to result in a premature translation termination codon (TAT > TAG) at amino acid position 1,533 (p.Tyr1533Ter), and the remaining codons of the mRNA were not translated into amino proteins ( Figure 2 ). The variant was confirmed by Sanger sequencing and her family members (I:1, I:2, II:1, II:2, and III:1; Figure 3A ) were subsequently assessed. The c.4599T > G (p.Tyr1533Ter) COL4A4 variant was not detected in her father or her son, whereas her mother was homozygous ( Figure 3B ), and her sister and brother were heterozygous. The results are shown in Table 1 .
Figure 2.
(A) The process of gene expression in the normal wild-type COL4A4. (B) A variant codon in the DNA sequence (TAG) leading to a nonsense change from tyrosine to a stop codon (UGA).
Figure 3.
(A) Pedigree of a three-generation family with AS. Arrow indicates the proband. The proband, her brother, and her sister were heterozygous (het). For the novel pathogenic variant, whereas the mother was homozygous (hom). The shapes filled with black indicate patients affected with hematuria. (B) Sequencing results of the c.4599T > G (p.Tyr1533Ter) variant at the 47th exon of the COL4A4 gene of the proband (II:3) (left panel) and her mother (I:2) (right panel).
Bioinformatics Analysis of the Novel COL4A4 Variant
The tyrosine at position 1,533 (p.Tyr1533) is conserved across primates from humans (Homo sapiens) to Nomascus leucogenys (data not shown). Such a scenario indicates that a sequence variant at this position may negatively affect protein structure and function. Secondary structure analysis predicted that the wild-type COL4A4 contains 58 alpha helices and 1,309 random coils, whereas the variant sequence only consists of 15 alpha helices and 1,251 random coils ( Table 2 ).
Discussion
Collagen IV is the main component of the GBM lamina densa (Laurie et al., 1984) and is encoded by six genes (COL4A1–COL4A6) (Harvey et al., 2006). Variants in the COL4A3/A4/A5 genes result in type IV collagen nephropathies, which include AS, thin basement membrane nephropathy (TBMN) (Pierides et al., 2013; Gross et al., 2017; Imafuku et al., 2017; Daga et al., 2019; Lee et al., 2019), and focal segmental glomerulosclerosis (FSGS) (Voskarides et al., 2008; Wu et al., 2016). Here, we report of a Chinese family with AS that involves a novel variant, COL4A4 4599T > G, which occurred in either the homozygous or heterozygous state in affected individuals with mild kidney involvement.
Previous studies have described a causal relationship between heterozygous COL4A3/COL4A4 variants and FSGS (Voskarides et al., 2007; Pierides et al., 2009). Due to this finding, some patients with ADAS may have been diagnosed as familial FSGS (Malone et al., 2014; Xie et al., 2014; Gast et al., 2016). FSGS is one of the major causes of renal failure and ESRD (Korbet, 2012). Large proteinuria (>3 g per day) with hypertension, microscopic hematuria, and kidney failure are common in patients with FSGS. Segmental sclerosis and podocyte lesions are the prominent histological changes in FSGS. These features, either clinical or pathological, were not detected in this family, thus leading to the exclusion of a diagnosis of familiar FSGS.
Inherited glomerular hematuria affects at least 1% of the population and is mainly caused by TBMN and less often is due to AS (Savige et al., 2003; Tryggvason and Patrakka, 2006). Unlike the clinical feature of TBMN, which usually manifests with isolated hematuria, AS is typically characterized by hematuria, proteinuria, renal function impairment, and sensorineural deafness and ocular abnormalities. Besides the difference in clinical appearances, TBMN and AS also have different genetic natures. As a hereditary disease, AS is caused by pathogenic variants in the COL4A3, COL4A4, and COL4A5 genes. COL4A5 pathogenic variants usually cause X-linked AS, whereas those involving the COL4A3 and COL4A4 genes generally lead to autosomal dominant or autosomal recessive types (Lemmink et al., 1996; Hudson et al., 2003; Nagel et al., 2005; Hertz et al., 2015). Hereditary TBMN is a disorder caused by pathogenic variants in the COL4A3 and COL4A4 genes, often presenting as an autosomal dominant genetic disease (Savige et al., 2003), although some biopsy-proven TBMN individuals were found to be carriers of autosomal recessive AS (Lemmink et al., 1996; Boye et al., 1998; Netzer et al., 1999; Buzza et al., 2001). In this report, we describe a three-generational family with autosomal dominant AS and not autosomal TBMN because the proband in this family clinically manifested with proteinuria and hematuria. In addition, the renal pathological changes in the proband displayed GBM irregular thinning and thickening or splitting but no uniform thinning.
ADAS is generally a mild and slowly progressing disease. Its clinical manifestations, especially in younger patients, seem to be significantly milder than those in men with XLAS or patients with ARAS (Jais et al., 2000; AegeanSoftware. NoteExpress, 2005), and its extra-renal manifestations are relatively rare (Jais et al., 2000; Oka et al., 2014). No identifiable phenotype–genotype correlation has been observed in patients with ADAS because their phenotype varies regardless of genetic background. In the pedigree described in this study, most of the individuals carrying the novel pathogenic variant exhibited isolated hematuria without the extra-renal phenotype, except for the proband who presented with hematuria and proteinuria. Particularly, an 80-year old woman (I:2) carrying the homozygous COL4A4 pathogenic mutation also only has the isolated hematuria. This indicates that the COL4A4 c.4599T > G variant does lead to hematuria, but by itself, it is not enough to cause AS, whether in homozygous or heterozygous individuals. Previous studies have shown that functional haplo-insufficiency for COL4A3 or COL4A4 results in hematuria but does not always lead to AS (Lemmink et al., 1997; Boye et al., 1998). There are two possible explanations for this complex ADAS phenotype. First, the nature of the variant causes defects in the position and function of amino acids, thereby resulting in phenotypic manifestations or penetration. With respect to the pedigree, the c.4599T > G variant, which is a nonsense mutation and located in the NC1 domain of the COL4A4 gene, leads to a truncated NC1 α chain. We presume that this shortened α chain may not influence the assembly of the collagen IV protein but just affects the integrity of the collagen IV network, thus not resulting in a severe renal phenotype. Second, except for the alteration in the structure and function of collagen IV that is caused by the mutation itself, the complex phenotype of ADAS could also be attributed to other factors, such as environmental or genetic factors. For example, hypertension and obesity have been identified as contributory factors, and several genetic modifiers were also reported as epistatic factors that regulate the phenotype (Pereira et al., 2004; Kottgen et al., 2008; Deltas et al., 2013; Pierides et al., 2013; Stefanou et al., 2015; Kamiyoshi et al., 2016; Gross et al., 2017; Daga et al., 2019; Lee et al., 2019).
In this family, we found that the proband, who was heterozygous for the pathogenic variant, exhibited more severe renal symptoms than her mother, who was homozygous for this variant. Autosomal dominant hereditary diseases are generally exhibited in heterozygous individuals carrying a pathogenic variant; homozygous individuals are rarely observed and often result from assortative mating or consanguinity. A few cases of homozygotes in the disease pedigree have been previously reported (Chemke et al., 1984; Brandi et al., 1993; Zlotogora, 1997; Tonini et al., 2004; Arzel-Hezode et al., 2010; Vinciguerra et al., 2014; Conidi et al., 2015; Kosik et al., 2015; Sjouke et al., 2015), and their clinical phenotype is usually more severe than that of the heterozygous individuals (Chemke et al., 1984; Arzel-Hezode et al., 2010; Vinciguerra et al., 2014; Conidi et al., 2015; Kosik et al., 2015). However, exceptions to homozygotes displaying milder phenotypes have also been reported (Zlotogora, 1997; Tonini et al., 2004; Sjouke et al., 2015), and these individuals not only develop clinical symptoms at a later age but also exhibit slower disease progression than do heterozygotes. An important reason seen, as mentioned above, is that in addition to the alteration in the structure and function of the protein caused by the mutation itself, other factors, such as environmental or genetic factors, also play important roles in the manifestation severity of ADAS (Pereira et al., 2004; Kottgen et al., 2008; Deltas et al., 2013; Stefanou et al., 2015; Kamiyoshi et al., 2016).
To our knowledge, homozygous AS has not been reported in the literature. In this AS pedigree, one homozygote was found and manifested with a milder renal phenotype than the heterozygous proband. This is similar to previously reported cases of other diseases and indicates that the phenotype of kidney disease in patients with AS is determined by multiple factors in addition to the genetic background of a single gene. We will continue to follow up the family and do whole genome sequencing to further identify other possible reasons in the future. The molecular mechanism underlying the variations in phenotypic consequences remains elusive and thus needs further investigation using cellular and/or animal models.
In summary, ADAS has long been considered as a consequence of heterozygous pathogenic variants in the COL4A3 and COL4A4 genes, and homozygous carriers have never been reported in an ADAS family. We have described one Chinese pedigree with ADAS that involves a novel pathogenic variant, COL4A4 c.4599T > G, which is predicted to result in ADAS, yet its pedigree includes both homozygous and heterozygous carriers. Our results show that the proband with the heterozygous variant state had severe clinical manifestations than had others with either homozygous or heterozygous variant. This suggests that the severity of clinical manifestations may be attributed to not only the genetic variants but also other unknown factors.
Materials and Methods
Family History
This study has been approved by the Ethics Committee of Renmin Hospital of Wuhan University (Wuhan City, Hubei Province of China), and written informed consent was obtained from the parents or guardians of the participant for the publication of this case report. Three generations of a Chinese family consisting of seven members were included in this research. Urinalysis and renal function evaluation were performed on all included family members. Peripheral blood samples were also obtained for next-generation sequencing, including four affected members (I:2, II:1, II:2, and II:3; Figure 3A ) and two unaffected individuals (I:1 and III:1; Figure 3A ). The whole family also underwent routine examination for renal function, vision, and hearing. Renal biopsy was performed on the proband of the family (II:3).
Renal Biopsy and Renal Pathology Examination
A renal biopsy of the proband (II:3) was collected on the condition that this procedure had no contraindications. With the assistance of sonography, a nephrologist inserted a biopsy needle into the kidney under local anesthesia and successfully obtained a tissue sample. The pathologists then evaluated the tissue by light, electron, and fluorescence microscopy.
Genomic DNA Extraction (gDNA) and Exome Sequencing
gDNA was isolated from peripheral blood of six members (I:1, I:2, II:1, II:2, II:3, and III:1). Exome sequencing of gDNA was performed using the Life Technologies Proton sequencer by the laboratory medicine department of Renmin Hospital, Wuhan, China. A DNA library was constructed using the Ion AmpliSeq™ Exome Enrichment Kit (Life Technologies, USA), and templates were prepared using Ion PGM™ Enrichment Beads (Life Technologies, USA) according to the manufacturer’s recommendation. Then, sequencing was performed using an Ion PI™ Chip Kit v2 (Life Technologies, USA), and data analysis was performed by comparing the results to the sequences of the AS-associated genes (COL4A3–COL4A6) using the Ion Reporter™ software (Life Technologies, USA).
Variant Analysis
All synonymous and intronic variants were filtered out using the data from public databases, including the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/), the UCSC database (https://genome.ucsc.edu/), the single nucleotide polymorphism database (dbSNP, http://www.ncbi.nlm.nih.gov/projects/SNP/snp_summary.cgi), and 1000 Genomes Project (http://www.1000genomes.org/). After the benign variants were excluded, the remaining variants were designated as novel. We performed Sanger sequencing for variant confirmation and searched the HGMD database (http://www.hgmd.cf.ac.uk/ac/index.php) and PubMed database (http://www.ncbi.nlm.gov/pubmed) to predict the potential gene dysfunction.
Bioinformatics Analysis of the Detected Sequence Variants
We compared the nucleotide sequences with sequence databases, and we aligned multiple sequences among various species using the Basic Local Alignment Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi). In addition, we also searched for protein sequences and functional information in UniProt databases (http://www.uniprot.org). For secondary structure comparison between the wild-type and variant proteins, we used the single nucleotide polymorphism (NPS) server (https://prabi.ibcp.fr/htm/site/web/home). The identified new nonsense mutant, COL4A4 c.4599T > G (p.Tyr1533Ter), was submitted to ClinVar database with a submission ID SUB5664647.
Contribution to the Field
We performed the genetic analyses by exome-seq in a Chinese family with AS and found four individuals harboring the COL4A4 c.4599T > G variant in the family. We identified a novel pathogenic variant in either the heterozygous or homozygous state of the COL4A4 gene in this family. Our results also suggest that the severity of clinical manifestations may not be entirely attributed to the COL4A4 genetic variant itself in patients.
Consent for Publication
All participants signed a consent form for publication.
Data Availability Statement
The datasets supporting the conclusions of this article are available in ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/).
Ethics Statement
This study has been approved by the Ethical Committee of Renmin Hospital of Wuhan University, and written informed consent was obtained from the parents or guardians of the participant for the publication of this case report.
Author Contributions
HW: Manuscript writing and overall instruction.
CY: Manuscript writing and data collection.
YS: Manuscript writing and data collection.
ZC: Fluorescence microscopic examination.
XY: Pathology materials preparation.
XC: Clinical data collection.
GD: Clinical data collection.
YG: Electron microscope examination.
YT: Sequencing.
CS: Manuscript writing and preparation.
MM: Manuscript writing and preparation.
Funding
The National Natural Science Foundation of China (81370800) and National Institute of Hospital Administration (an Explorer Program to Promote the Application of Peritoneal Dialysis in China) supported this study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The results presented in this paper have not been published previously in whole or in part, except for an abstract.
Acknowledgments
The authors thank all patients who participated in this article. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
- AegeanSoftware NoteExpress (ed 2.0); 2005: NoteExpress is a perfect assistant and information manager for researchers, scholars, students, and librarians. NoteExpress is designed to help you organize research notes and bibliographic references, generate bibliographies automatically, search and capture bibliographic data from Internet with efficiency and ease. NoteExpress is well integrated with Microsoft Word. It can format bibliographies in many popular styles.
- Arzel-Hezode M., Sternberg D., Tabti N., Vicart S., Goizet C., Eymard B., et al. (2010). Homozygosity for dominant mutations increases the severity of muscle channelopathies. Muscle Nerve 41, 470–477. 10.1002/mus.21520 [DOI] [PubMed] [Google Scholar]
- Boye E., Mollet G., Forestier L., Cohen-Solal L., Heidet L., Cochat P., et al. (1998). Determination of the genomic structure of the COL4A4 gene and of novel mutations causing autosomal recessive Alport syndrome. Am. J. Hum. Genet. 63, 1329–1340. 10.1086/302106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandi M. L., Weber G., Svensson A., Falchetti A., Tonelli F., Castello R., et al. (1993). Homozygotes for the autosomal dominant neoplasia syndrome (MEN1). Am. J. Hum. Genet. 53, 1167–1172. [PMC free article] [PubMed] [Google Scholar]
- Buzza M., Wilson D., Savige J. (2001). Segregation of hematuria in thin basement membrane disease with haplotypes at the loci for Alport syndrome. Kidney Int. 59, 1670–1676. 10.1046/j.1523-1755.2001.0590051670.x [DOI] [PubMed] [Google Scholar]
- Chemke J., Nisani R., Feigl A., Garty R., Cooper M., Bårash Y., et al. (1984). Homozygosity for autosomal dominant Marfan syndrome. J. Med. Genet. 21, 173–177. 10.1136/jmg.21.3.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conidi M. E., Bernardi L., Puccio G., Smirne N., Muraca M. G., Curcio S. A., et al. (2015). Homozygous carriers of APP A713T mutation in an autosomal dominant Alzheimer disease family. Neurology 84, 2266–2273. 10.1212/wnl.0000000000001648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daga S., Fallerini C., Furini S., Pecoraro C., Scolari F., Ariani F., et al. (2019). Non-collagen genes role in digenic Alport syndrome. BMC Nephr. 20, 70–79. 10.1186/s12882-019-1258-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltas C., Pierides A., Voskarides K. (2013). Molecular genetics of familial hematuric diseases. Nephrol. Dial. Transplant. 28, 2946–2960. 10.1093/ndt/gft253 [DOI] [PubMed] [Google Scholar]
- Flinter F. A., Cameron J. S., Chantler C., Houston I., Bobrow M. (1988). Genetics of classic Alport’s syndrome. Lancet. 2, 1005–1007. 10.1016/s0140-6736(88)90753-2 [DOI] [PubMed] [Google Scholar]
- Gast C., Pengelly R. J., Lyon M., Bunyan D. J., Seaby E. G., Graham N., et al. (2016). Collagen (COL4A) mutations are the most frequent mutations underlying adult focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 31, 961–970. 10.1093/ndt/gfv325 [DOI] [PubMed] [Google Scholar]
- Gross O., Kashtan C. E., Rheault M. N., Flinter F., Savige J., Miner J. H., et al. (2017). Advances and unmet needs in genetic, basic and clinical science in Alport syndrome: report from the 2015 International workshop on Alport syndrome. Nephrol. Dial. Transplant. 32, 916–924. 10.1093/ndt/gfw095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S. J., Perry J., Zheng K., Chen D., Sado Y., Jefferson B., et al. (2006). Sequential expression of type IV collagen networks: testis as a model and relevance to spermatogenesis. Am. J. Pathol. 168, 1587–1597. 10.2353/ajpath.2006.050816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz J. M., Thomassen M., Storey H., Flinter F. (2015). Clinical utility gene card for: Alport syndrome—update 2014. Eur. Heart J. 23, 1–4. 10.1038/ejhg.2014.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B. G., Tryggvason K., Sundaramoorthy M., Neilson E. G. (2003). Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N. Engl. J. Med. 348, 2543–2556. 10.1056/nejmra022296 [DOI] [PubMed] [Google Scholar]
- Imafuku A., Nozu K., Sawa N., Hasegawa E., Hiramatsu R., Kawada M., et al. (2017). Autosomal dominant form of type IV collagen nephropathy exists among patients with hereditary nephritis difficult to diagnose clinicopathologically. Nephrology (Carlton) 23 (10), 940–947. 10.1111/nep.13115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jais J. P., Knebelmann B., Giatras I., De Marchi M., Rizzoni G., Renieri A., et al. (2000). X-linked Alport syndrome: natural history in 195 families and genotype–phenotype correlations in males. J. Am. Soc. Nephrol. 11, 649–657. [DOI] [PubMed] [Google Scholar]
- Kamiyoshi N., Nozu K., Fu X. J., Morisada N., Nozu Y., Ye M. J., et al. (2016). Genetic, clinical, and pathologic backgrounds of patients with autosomal dominant Alport syndrome. Clin. J. Am. Soc. Nephrol. 11, 1441–1449. 10.2215/cjn.01000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbet S. M. (2012). Treatment of primary FSGS in adults. J. Am. Soc. Nephrol. 23, 1769–1776. 10.1681/ASN.2012040389 [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Munoz C., Lopez L., Arcila M. L., García G., Madrigal L., et al. (2015). Homozygosity of the autosomal dominant Alzheimer disease presenilin 1 E280A mutation. Neurology 84, 206–208. 10.1212/wnl.0000000000001130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottgen A., Hsu C. C., Coresh J., Shuldiner A. R., Berthier-Schaad Y., Gambhir T. R., et al. (2008). The association of podocin R229Q polymorphism with increased albuminuria or reduced estimated GFR in a large population-based sample of US adults. Am. J. Kidney Dis. 52, 868–875. 10.1053/j.ajkd.2008.02.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel J., Rubel D., Gross O. (2013). Alport syndrome—insights from basic and clinical research. Nat. Rev. Nephrol. 9, 170–178. 10.1038/nrneph.2012.259 [DOI] [PubMed] [Google Scholar]
- Laurie G. W., Leblond C. P., Inoue S., Martin G. R., Chung A. (1984). Fine structure of the glomerular basement membrane and immunolocalization of five basement membrane components to the lamina densa (basal lamina) and its extensions in both glomeruli and tubules of the rat kidney. Am. J. Anat. 169, 463–481. 10.1002/aja.1001690408 [DOI] [PubMed] [Google Scholar]
- Lee J. H., Nozu K., Choi D. E., Kang H. G., Ha I. S., Cheong H. I., et al. (2019). Features of autosomeal recessive Alport syndrome: a systematic review. J. Clin. Med. 8, 178–195. 10.3390/jcm8020178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmink H. H., Nillesen W. N., Mochizuki T., Schroder C. H., Brunner H. G., van Oost B. A., et al. (1996). Benign familial hematuria due to mutation of the type IV collagen alpha4 gene. J. Clin. Invest. 98, 1114–1118. 10.1172/jci118893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmink H. H., Schroder C. H., Monnens L. A., Smeets H. J. (1997). The clinical spectrum of type IV collagen mutations. Hum. Mutat. 9, 477–499. [DOI] [PubMed] [Google Scholar]
- Longo I., Scala E., Mari F., Caselli R., Pescucci C., Mencarelli M. A., et al. (2006). Autosomal recessive Alport syndrome: an in-depth clinical and molecular analysis of five families. Nephrol. Dial. Transplant. 21, 665–671. 10.1093/ndt/gfi312 [DOI] [PubMed] [Google Scholar]
- Malone A. F., Phelan P. J., Hall G., Cetincelik U., Homstad A., Alonso A. S., et al. (2014). Rare hereditary COL4A3/COL4A4 variants may be mistaken for familial focal segmental glomerulosclerosis. Kidney Int. 86, 1253–1259. 10.1038/ki.2014.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Jones T. A., Pohjolainen E. R., Kadri A. S., Goddard A. D., Sheer D., et al. (1990). Molecular cloning of alpha 5(IV) collagen and assignment of the gene to the region of the X chromosome containing the Alport syndrome locus. Am. J. Hum. Genet. 46, 1024–1033. [PMC free article] [PubMed] [Google Scholar]
- Nagel M., Nagorka S., Gross O. (2005). Novel COL4A5, COL4A4, and COL4A3 mutations in Alport syndrome. Hum. Mutat. 26, 60. 10.1002/humu.9349 [DOI] [PubMed] [Google Scholar]
- Netzer K. O., Seibold S., Weber M. (1999). Thin basement membrane—do we have a window for understanding the molecular pathogenesis? Nephrol. Dial. Transplant. 14, 1060–1061. 10.1093/ndt/14.5.1060 [DOI] [PubMed] [Google Scholar]
- Oka M., Nozu K., Kaito H., Fu X. J., Nakanishi K., Hashimura Y., et al. (2014). Natural history of genetically proven autosomal recessive Alport syndrome. Pediatr. Nephrol. 29, 1535–1544. 10.1007/s00467-014-2797-4 [DOI] [PubMed] [Google Scholar]
- Pereira A. C., Pereira A. B., Mota G. F., Cunha R. S., Herkenhoff F. L., Pollak M. R., et al. (2004). NPHS2 R229Q functional variant is associated with microalbuminuria in the general population. Kidney Int. 65, 1026–1030. 10.1111/j.1523-1755.2004.00479.x [DOI] [PubMed] [Google Scholar]
- Pierides A., Voskarides K., Athanasiou Y., Ioannou K., Damianou L., Arsali M., et al. (2009). Clinico-pathological correlations in 127 patients in 11 large pedigrees, segregating one of three heterozygous mutations in the COL4A3/COL4A4 genes associated with familial haematuria and significant late progression to proteinuria and chronic kidney disease from focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 24, 2721–2729. 10.1093/ndt/gfp158 [DOI] [PubMed] [Google Scholar]
- Pierides A., Voskarides K., Kkolou M., Hadjigavriel M., Deltas C., et al. (2013). X-linked, COL4A5 hypomorphic Alport mutations such as G624D and P628L may only exhibit thin basement membrane nephropathy with microhematuria and late onset kidney failure. Hippokratia 17, 207–213. [PMC free article] [PubMed] [Google Scholar]
- Rheault M. N. (2012). Women and Alport syndrome. Pediatr. Nephrol. 27, 41–46. 10.1007/s00467-011-1836-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savige J., Rana K., Tonna S., Buzza M., Dagher H., Wang Y. Y. (2003). Thin basement membrane nephropathy. Kidney Int. 64, 1169–1178. 10.1046/j.1523-1755.2003.00234.x [DOI] [PubMed] [Google Scholar]
- Sjouke B., Kusters D. M., Kindt I., Besseling J., Defesche J. C., Sijbrands E. J., et al. (2015). Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur. Heart J. 36, 560–565. 10.1093/eurheartj/ehu058 [DOI] [PubMed] [Google Scholar]
- Stefanou C., Pieri M., Savva I., Georgiou G., Pierides A., Voskarides K., et al. (2015). Co-inheritance of functional podocin variants with heterozygous collagen IV mutations predisposes to renal failure. Nephron 130, 200–212. 10.1159/000432406 [DOI] [PubMed] [Google Scholar]
- Tonini M. M., Pavanello R. C., Gurgel-Giannetti J., Lemmers R. J., van der Maarel S. M., Frants R. R., et al. (2004). Homozygosity for autosomal dominant facioscapulohumeral muscular dystrophy (FSHD) does not result in a more severe phenotype. J. Med. Genet. 41, e17. 10.1136/jmg.2003.010637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryggvason K., Patrakka J. (2006). Thin basement membrane nephropathy. J. Am. Soc. Nephrol. 17, 813–822. 10.1681/asn.2005070737 [DOI] [PubMed] [Google Scholar]
- Vinciguerra C., Rufa A., Bianchi S., Sperduto A., De Santis M., Malandrini A., et al. (2014). Homozygosity and severity of phenotypic presentation in a CADASIL family. Neurol. Sci. 35, 91–93. 10.1007/s10072-013-1580-9 [DOI] [PubMed] [Google Scholar]
- Voskarides K., Damianou L., Neocleous V., Zouvani I., Christodoulidou S., Hadjiconstantinou V., et al. (2007). COL4A3/COL4A4 mutations producing focal segmental glomerulosclerosis and renal failure in thin basement membrane nephropathy. J. Am. Soc. Nephrol. 18, 3004–3016. 10.1681/asn.2007040444 [DOI] [PubMed] [Google Scholar]
- Voskarides K., Patsias C., Pierides A., Deltas C. (2008). COL4A3 founder mutations in Greek-Cypriot families with thin basement membrane nephropathy and focal segmental glomerulosclerosis dating from around the 18th century. Genet. Test. 12, 273–278. 10.1089/gte.2007.0110 [DOI] [PubMed] [Google Scholar]
- Wu Y., Hu P., Xu H., Yuan J., Yuan L., Xiong W., et al. (2016). A novel heterozygous COL4A4 missense mutation in a Chinese family with focal segmental glomerulosclerosis. J. Cell. Mol. Med. 20, 2328–2332. 10.1111/jcmm.12924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Wu X., Ren H., Wang W., Wang Z., Pan X., et al. (2014). COL4A3 mutations cause focal segmental glomerulosclerosis. J. Mol. Cell. Biol. 6, 498–505. 10.1093/jmcb/mju040 [DOI] [PubMed] [Google Scholar]
- Zlotogora J. (1997). Dominance and homozygosity. Am. J. Med. Genet. 68, 412–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are available in ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/).