Abstract
Oral Submucous Fibrosis (OSMF) is an insidious, chronic, complex, crippling, debilitating, irreversible, progressive, scarring, potentially malignant and collagen metabolic disorder, induced by a known carcinogen areca nut; wherein the oral mucosa, and occasionally the pharynx and esophagus is subjected to various pathological changes with significant clinical manifestations at different stages of progression, leading to functional morbidity; and with a risk of malignant transformation in the overlying epithelium. Although the condition is mainly diagnosed based on classic clinical manifestations, the commonly used existing definition for oral submucous fibrosis is primarily based on histological features. The authors have conducted extensive clinical research studies on OSMF and intends to propose a new clinical definition as ‘a debilitating, progressive, irreversible collagen metabolic disorder induced by chronic chewing of areca nut and its commercial preparations; affecting the oral mucosa and occasionally the pharynx and esophagus; leading to mucosal stiffness and functional morbidity; and has a potential risk of malignant transformation.’ Thus, a new clinical definition is put forward so as to assist the academicians, researchers and clinicians in terming and grouping this disease according to its clinical and biological behaviour for its subsequent management.
Keywords: Areca nut, Definition, Collagen metabolic disorder, Fibrous bands, Malignant transformation, Oral potentially malignant disorder, Oral submucous fibrosis
1. Introduction
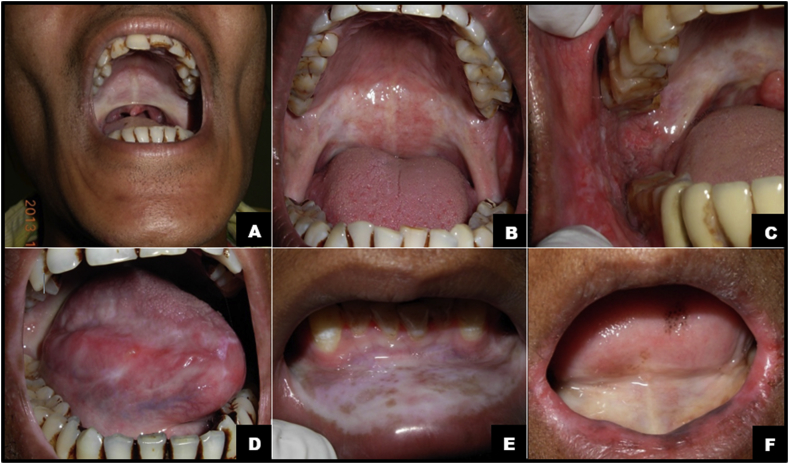
A disease is often construed as an abnormal medical condition that negatively affects the body mechanism or functions of part or complete body and is associated with specific signs and symptoms. It is broadly referred to any condition that causes pain, weakness, impairment, social problems, grief or death to the affected person. The disease may include infection, disorder, syndrome, injury, disability etc., which may cause secluded symptoms, unusual deviation in structure & function. Oral Submucous Fibrosis (OSMF) is one such disease, which is an insidious, chronic, complex, crippling, debilitating, irreversible, persistent progressive, scarring, potentially malignant and collagen metabolic disorder; primarily affecting the oral cavity and occasionally the pharynx and esophagus; wherein the oral mucosa undergoes various changes like inflammation, ulcerations, pigmentation, loss of resilience and pliability, to a significant blanched and fibrosed appearance leading to rigidity and progressive inability to open mouth (Fig. 1). In severe stage; hypomobility of soft palate & tongue, xerostomia, loss of gustatory sensations, fibrosis of pharyngeal & esophageal mucosa, hearing impairment, sunken cheeks, muscular dystrophy, hoarseness of voice, nasal twang and significant functional morbidity is noticed.1, 2, 3, 4
Fig. 1.
(A) sunken cheeks, restricted mouth opening, severely blanched palatal mucosa and altered shape (inverted) of uvula. (B) Significant blanching or marble-like appearance of the soft palate and faucial pillars. (C) Note the severe erythematous right buccal mucosa, stiff buccal mucosa and blanched soft palate & faucial pillar. (D) Blanched and fibrosed lateral border of tongue with multiple erythematous areas. (E) blanched and severely fibrosed lower labial mucosa. Note multiple brownish pigmented patches. (F) Note significant blanching of ventral surface of tongue and floor of mouth, thinning of lips, microstomia and angular chelitis.
OSMF is a form of pathological fibrosis which is characterized by progressive inability to open the mouth due to loss of elasticity and development of vertical fibrous bands in labial and buccal tissues. The disease is seen in any age group, including children and adolescents, but the prevalence is high in the age group of 18–35 years. The prevalence of OSMF cases have increased from 0.03% to 6.42% in the last four decades, making it a significant public health problem in India. The disease contributes significantly to mortality because of its high malignant transformation rate.3, 4, 5, 6 This condition was initially confined to Indian subcontinent, but is also noticed in South and South-east Asian countries.3,4 Various factors are suggested to trigger the disease process including areca nut chewing, genetic predisposition and immunologic processes. The nutritional deficiency and ingestion of chillies may further contribute to the course of the disease.4, 5, 6
There is compelling evidence to implicate the chewing of areca nut and its commercial preparations with the development of OSMF. Areca nut is recognized as a group I carcinogen.6 It is one of the most widely consumed addictive substance in the world after nicotine, ethanol and caffeine, and is consumed by approximately 10–20% of the world's population in various forms depending on geographical location.4,7, 8, 9 It causes various deleterious effects on oral and general health. Numerous studies have revealed that constituents of areca nut have an important role in the pathogenesis of OSMF.7,10 Primarily, the areca nut constitutes of Alkaloids – arecoline, arecaidine, guvacine and guvacoline; Flavonoids-tannins (areca tannin and gallic acid) and catechins; and Copper. The alkaloids of areca nut stimulate fibroblast proliferation and cause increased collagen formation; whereas the tannins in areca nut reduces the collagen degradation by inhibiting collagenases. The combined effect of alkaloids and tannins in areca nut forms the basis for fibrosis. All these compounds contribute to histologic changes in the oral mucosa.3,6,9,10 It is also hypothesized that the increased collagen in OSMF patients is because of presence of high copper content in areca nut and subsequent upregulation of copper dependant enzyme, lysyl oxidase. An analysis of cultured OSMF fibroblasts demonstrated theoretically an apparently large size cell capable of producing larger amounts of collagen, suggesting phenotypic alteration in cells.3,4,8,11,12
The chewing of areca nut and its commercial preparations (gutkha, mawa, pan masala, flavored supari, etc.) is a regular and rampant practice in asian countries, irrespective of age and sex. In recent years, the prevalence of OSMF is on rapid rise, may be due to an upsurge in the popularity of the commercial preparations of areca nut and its increase use.4,7,8 Several case series are reported on Asian immigrants of UK, USA, and Africa. Recent literatures from European and Western countries have reported sporadic cases of OSMF in non-Asians.4 The literature are replete with the clinico-pathologic presentation of OSMF. Once the process of uncontrolled fibrosis is initiated, the condition is not reversible at any stage of the disease process even after cessation of chewing areca nut or its substitute. Diagnosis and staging thus becomes very important as it affects the treatment. The diseased condition may remain either stationary or become severe, leaving an individual handicapped, both physically and psychologically, affecting the persons perspective on life. The pathological changes in OSMF are similar to those of systemic sclerosis (scleroderma), but are limited to oral tissues.4,9, 10, 11
The presently available treatment modalities have not shown marked effectiveness in the management of OSMF. The most preferred approach is education and counseling the patient, through which the disease can be prevented and controlled from progression into malignancy. The malignant transformation may arise in any oral site affected with OSMF. The clinically normal appearing oral mucosa may have molecular aberrations or dysplasia of varying degree. This observation emphasizes the potential risk or predisposing the oral mucosa for malignancy.11, 12, 13 The malignant transformation rate of OSMF is 3–13% for Oral Squamous Cell Carcinoma. The oral malignancy arising in OSMF is clinically more invasive and exhibits higher metastasis and high risk of recurrence.13,14 OSMF may also be associated with other OPMD's such as Oral Leukoplakia, Oral Lichen planus etc. This association carries higher and earlier risk of malignant transformation.4,15 Currently, OSMF is a major global health problem.13,14,16
2. Discussion
In 600 B.C., Shushrutha, an ancient Indian Physician, in his book ‘Shushrutha Samhita', reported an oro - pharyngeal condition called ‘Vidari’, which mimicked to Oral Submucous Fibrosis. It was first reported and described by J.Schwartz in 1952, among the five Indian migrant women in East Africa - Kenya.4,17,18 Since then, various researchers and clinicians around the world have described the disease and suggested various terminologies or nomenclatures (Table 1) and only one definition till date. In 1966, Jens J. Pindborg and Satyavati M. Sirsat had coined the term Oral Submucous Fibrosis and defined it as ‘an insidious chronic disease affecting any part of the oral cavity and sometimes the pharynx. Although occasionally preceded by and/or associated with vesicle formation, it is always associated with a juxta-epithelial inflammatory reaction followed by a fibro-elastic change of the lamina propria, with epithelial atrophy leading to stiffness’.2 This definition describes the basic histological understanding of the disease and is used most commonly worldwide more than half a century without any modifications, inspite of its drawbacks; even though OSMF is mainly diagnosed clinically, based on the classic clinical manifestations.
Table 1.
Various nomenclatures for Oral Submucous Fibrosis (OSMF).
| Years | Details |
|---|---|
| 600 B.C. | Shushrutha, an ancient Indian Physician, was the chief practitioner of ancient medicine, described a oro -pharyngeal condition called ‘Vidari’ in his book ‘Shushrutha Samhita', which mimicked to Oral Submucous Fibrosis. Shushrutha Samhita is the ancient treatise which addresses all aspects of general medicine and is considered a foundational text of Ayurveda. |
| 1952 | J. Schwartz first described the similar condition in five Indian migrant women, in East Africa- Kenya, under the term Atropica idiopathica (tropica) Mucosae Oris. |
| 1953 | S. G. Joshi from Bombay – India, broadly described the condition and termed it as Submucous Fibrosis of palate and pillars. |
| 1953 | D Lal described the pathology as Diffuse oral submucous fibrosis. |
| 1954 | Su I. P. from Taiwan described similar condition, which he called it as Idiopathic Scleroderma of mouth. |
| 1957 | J. V. Desa termed the condition as Submucous fibrosis of palate and cheek. |
| 1958 | A. T. George described the condition as Submucous fibrosis of palate and mucosa membrane. |
| 1962 | A. B. N. Rao termed the condition as Idiopathic palatal fibrosis. |
| 1962 | P. N. Behl described the condition as Sclerosing Stomatitis. |
| 1962 | S. M. Sirsat and V. R. Khanolkar designated the condition as Submucous fibrosis of the palate. |
| 1964 | Jens J. Pindborg and Satyavati M. Sirsat described the condition as Juxta epithelial fibrosis. |
| 1965 | Jens J. Pindborg and J. Zachariah emphasized the precancerous nature of OSMF. |
| 1966 | Jens. J. Pindborg and Satyavati. M. Sirsat termed the condition as Oral Submucous Fibrosis and put forth the definition of OSMF. |
| 1970 | Goleria described the condition as Sub-epithelial fibrosis. |
| 1970 | B. M. Abrol and S. Krishnamoorthy labelled the condition as Idiopathic Fibrosis. |
| 1975 | K. Ramanathan and S. K. Dharmalingam described OSMF as an oral precancerous condition. |
| 1981 | K. Ramanathan described the condition as Asian Sideropenic Dysphagia. |
| 2005 | S. Warnakulasuriya, Newell. W. Johnson, I. Van der Waal Categorized Oral Submucous Fibrosis as Oral Potentially Malignant Disorder. |
| 2009 | Pankaj Chaturvedi proposed a new name to OSMF as Gutkha syndrome or Areca nut chewer's syndrome. |
| 2018 | Chandramani More broadly described Oral Submucous Fibrosis as Areca nut induced Oral Fibrosis and a Collagen metabolic disorder. |
| 2019 | Chandramani More and Naman Rao, Proposed Clinical Definition for Oral Submucous Fibrosis. |
Since 1952, numerous research studies have been conducted worldwide especially on clinical manifestations, diagnostic and therapeutic interventions. The outcome of these studies has changed the concept of the existing definition. The authors of this paper have extensively studied numerous patients affected with OSMF. Because of the expertise on OSMF, the authors are proposing a new definition so as to assist the academicians, researchers and clinicians in terming and grouping this disease according to its clinical and biological behavior and for its subsequent management.
2.1. Proposed definition
The authors would like to propose a precise, definite and distinct New Clinical Definition which depicts the exact nature of this devastating disorder. Oral Submucous Fibrosis is defined as 'a debilitating, progressive, irreversible collagen metabolic disorder induced by chronic chewing of areca nut and its commercial preparations; affecting the oral mucosa and occasionally the pharynx and esophagus; leading to mucosal stiffness and functional morbidity; and has a potential risk of malignant transformation.' This definition describes the characterization, biological behavior, etiology and prognosis of the disease. It gives more clarity and is consistent with the current disease trend (Table 2).
Table 2.
Definitions for oral submucous fibrosis –
| Year | Details |
|---|---|
| 1966 | Jens J. Pindborg and Satyavati M. Sirsat defined Oral Submucous Fibrosis as ‘an insidious chronic disease affecting any part of the oral cavity and sometimes the pharynx. Although occasionally preceded by and/or associated with vesicle formation, it is always associated with a juxta-epithelial inflammatory reaction followed by a fibro-elastic change of the lamina propria, with epithelial atrophy leading to stiffness’. |
| 2019 | Chandramani More and Naman Rao defined Oral Submucous Fibrosis as ‘a debilitating, progressive, irreversible collagen metabolic disorder induced by chronic chewing of areca nut and its commercial preparations; affecting the oral mucosa and occasionally the pharynx and oesophagus; leading to mucosal stiffness and functional morbidity; and has a potential risk of malignant transformation.’ |
3. Conclusion
The best definition would be one which emphasizes the nature of disease, including etio-pathogenesis and clinico-histopathological manifestations. The proposed definition for OSMF in this article is precise, comprehensive and reflects the progressive disease array. This will strengthen the understanding of the disease in a better way. Based on the expertise, the authors have brought a new definition, which will meet the requirement of the clinicians, researchers and academicians worldwide, in the proper diagnosis and management of OSMF. The drawbacks of the previous definitions are evolved up to certain extent. In near future, as the new knowledge accumulates, there shall be a scope for revision.
Conflicts of interest
None declared.
Financial Support
Nil.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jobcr.2019.06.016.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Warnakulasuriya S., Johnson N., van der Waal I. Nomenclature and Classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36(10):575–580. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 2.Rajalalitha P., Vali S. Molecular Pathogenesis of oral submucous fibrosis – a collagen metabolic disorder. J Oral Pathol Med. 2005;34:321–328. doi: 10.1111/j.1600-0714.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 3.Pindborg J.J., Sirsat S.M. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1966 Dec;22(6):764–779. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 4.More C.B., Das S., Patel H., Adalja C., Kamatchi V., Venkatesh R. Proposed clinical classification for oral submucous fibrosis. Oral Oncol. 2012;48:200–202. doi: 10.1016/j.oraloncology.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev P.K., Jeanne Freeland-Graves J., Beretvas S., Sanjeevi N. Zinc, copper, and iron in oral submucous fibrosis: a meta-analysis. Int J Dent. 2018;(4):1–14. doi: 10.1155/2018/3472087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.More C.B., Patel H. Trace elements in potentially oral malignant disorders and oral malignant lesion - a biochemical study. Int J Oral Maxillofac Dis. 2016;1(2):1–7. [Google Scholar]
- 7.Gupta P.C. Areca nut use in India. Ind J Med Sci. 2007;61:317–319. [PubMed] [Google Scholar]
- 8.Arakeri G., Brennan P.A. Oral Submucous Fibrosis: an overview of the aetiology, pathogenesis, classification, and principles of management. Br J Oral Maxillofac Surg. 2013 Oct;51(7):587–593. doi: 10.1016/j.bjoms.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Hastak K., Lubri N., Jakhi S.D. Effect of turmeric oil and turmeric oleoresin on cytogenetic damage in patients suffering from oral submucous fibrosis. Cancer Lett. 1997;116(2):265–269. doi: 10.1016/s0304-3835(97)00205-x. [DOI] [PubMed] [Google Scholar]
- 10.Gupta P.C., Warnakulasuriya S. Global epidemiology of areca nut usage. Addict Biol. 2002;7:77–83. doi: 10.1080/13556210020091437. [DOI] [PubMed] [Google Scholar]
- 11.Murti P.R., Bhonsle R.B., Gupta P.C., Daftary D.K., Pindborg J.J., Mehta F.S. Etiology of oral submucous fibrosis with special reference to the role of areca nut chewing. J Oral Pathol Med. 1995;24:145–152. doi: 10.1111/j.1600-0714.1995.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 12.More C., Shilu K., Gavli N., Rao N.R. Etio-pathogenesis and clinical manifestations of oral submucous fibrosis, a potentially malignant disorder: an update. Int J Cur Res. 2018;10(7):71816–71820. [Google Scholar]
- 13.More C., Gupta S., Joshi J., Varma S. Classification system of oral submucous fibrosis. J Ind Acad Oral Med Radiol. 2012;24(1):24–29. [Google Scholar]
- 14.VanWyk C.W. Oral submucous fibrosis: the south African experience. Ind J Dent Res. 1997;8(2):39–45. [PubMed] [Google Scholar]
- 15.Arakeri G., Patil S.G., Aljabab A.S. Oral submucous fibrosis: an update on pathophysiology of malignant transformation. J Oral Pathol Med. 2017;46(6):413–417. doi: 10.1111/jop.12582. [DOI] [PubMed] [Google Scholar]
- 16.Paymaster J.C. Cancer of the buccal mucosa; a clinical study of 650 cases in Indian patients. Cancer. 1956;9:431–435. doi: 10.1002/1097-0142(195605/06)9:3<431::aid-cncr2820090302>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.More C., Peter R., Nishma G., Chen Y., Rao N. Association of Candida species with oral submucous fibrosis and oral Leukoplakia: a case control study. Ann Clin Lab Res. 2018;6(3):248. [Google Scholar]
- 18.More C.B., Gavli N., Chen Y., Rao N.R. A novel clinical protocol for therapeutic intervention in oral submucous fibrosis: an evidence based approach. J Oral Maxillofac Pathol. 2018;22:382–391. doi: 10.4103/jomfp.JOMFP_223_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.