Abstract
Static magnetic fields (SMFs) are known to alter neural activity, but evidence of their ability to modify learning-related neuroplasticity is lacking. The present study tested the hypothesis that application of static magnetic stimulation (SMS), an SMF applied transcranially via a neodymium magnet, over the primary motor cortex (M1) would alter learning of a serial reaction time task (SRTT). Thirty-nine participants took part in two experimental sessions separated by 24 h where they had to learn the SRTT with their right hand. During the first session, two groups received SMS either over contralateral (i.e., left) or ipsilateral (i.e., right) M1 while a third group received sham stimulation. SMS was not applied during the second session. Results of the first session showed that application of SMS over contralateral M1 impaired online learning as compared to both ipsilateral and sham groups, which did not differ. Results further revealed that application of SMS did not impair offline learning or relearning. Overall, these results are in line with those obtained using other neuromodulatory techniques believed to reduce cortical excitability in the context of motor learning and suggest that the ability of SMS to alter learning-related neuroplasticity is temporally circumscribed to the duration of its application.
Subject terms: Psychophysics, Consolidation, Consolidation, Human behaviour, Human behaviour
Introduction
Exposure to strong static magnetic fields (SMFs) is known to bear an influence on biological systems1,2. At the brain level, exposure to SMF in the context of magnetic resonance imaging (MRI) has been shown to transiently modulate cortical excitability in humans3,4. The evidence that SMF can alter normal brain function has led to the development of a new non-invasive neuromodulatory technique called static magnetic stimulation (SMS)5,6. SMS relies on the application of a strong neodynium magnet positioned directly onto the scalp; this simple approach has been shown to have effects comparable to those initially observed with MRI, inducing a reduction in cortical excitability3,6–12, and an increase in GABAA-mediated intracortical inhibition3,8,9. While the exact mechanisms behind the inhibitory effects of SMS remain to be fully established, they could result from the induction of a rotation in cells’ membrane that causes conformational changes in transmembraneous ions channels, altering the normal passage of ions13–15.
Initial studies suggested that the effects of SMS on the brain were relatively short-lived (i.e., ~10 minutes)3,6,16, but recent transcranial magnetic stimulation (TMS) evidence shows that a 30-minute exposure to SMS over the primary motor cortex (M1) could induce enduring neurophysiological changes consistent with long-term depression (LTD)-like effects8. Whether these lasting LTD-like changes are strong enough to modify behaviour after the stimulation period (i.e. offline effects), or whether the behavioural effects of SMS are temporally circumscribed to the duration of its application, remains to be clarified. Similarly, it is currently unknown if the effects of SMS are spatially circumscribed to the area of the cortex directly under the magnet, or if it also impacts the functioning of other interconnected regions. Answering these questions is important to better understand the limits and capabilities of SMS, and design proper protocols for human research.
The serial reaction time task (SRTT) is a well-established task that is commonly used to assess the effect of neuromodulatory interventions on learning-related neuroplasticity17–23. It consists of consecutive key presses prompted by visual cues. Unbeknownst to the participant, a sequence of key presses is repeated throughout the task, which induces a gradual reduction in response time that is specific for the repeated sequence, indicative of learning. Typically, while neuromodulatory interventions applied prior to an SRTT session on the M1 contralateral to the hand performing the task influences online learning24–27, other evidence further suggests that similar interventions applied to the ipsilateral M1 tend to improve learning, presumably through the release of interhemispheric inhibition20,21,28. Moreover, low-frequency repetitive TMS and continuous theta-burst TMS applied to the contralateral M1, which are believed to induce LTD-like plasticity, appear to interfere with the consolidation processes involved in offline learning of SRTT29–31. The SRTT thus appears well suited to differentiate between the online and offline effects of SMS and clarify its potential distal neuromodulatory influence over the non-stimulated M1. Considering that online SMS is presumed to decrease cortical excitability3,6,9,12, we hypothesized that SMS applied to the contralateral M1 would impair online learning as compared to sham, while SMS over the ipsilateral M1 would improve it. In the eventuality that SMS induced lasting LTD-like effects8, we would expect contralateral SMS to decrease offline learning as compared to the other stimulation conditions.
Results
First session
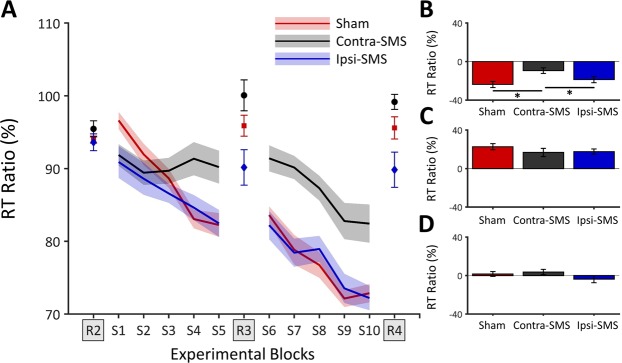
Concerning RT data, results of the MANOVA revealed an effect of Group (F(6,70) = 2.530, p = 0.028, = 0.178), which was broken down using the Roy-Bargmann Stepdown Procedure32. Concerning global learning, a univariate ANOVA revealed a main effect of Group (F(2,36) = 5.516, p = 0.011, = 0.223), where the Contra-SMS showed lower global learning than both the Ipsi-SMS (p = 0.049; Cohen’s d = 0.806) and Sham groups (p = 0.003; Cohen’s d = 1.324). The Ipsi-SMS and Sham groups did not differ (p = 0.231; Cohen’s d = 0.435). Concerning unspecific learning, an ANCOVA, using the RT data from the global learning comparison as a covariate, revealed no effect of Group (F(2,35) = 1.975, p = 0.154, = 0.101). Concerning specific learning, an ANCOVA, using the RT data from the unspecific learning comparison as a covariate, also revealed no effect of Group (F(2,35) = 1.203, p = 0.313, = 0.064). Overall, this suggests that application of SMS selectively over contralateral M1 impaired online learning, but not global motor performance. RT data of the first session are presented in Fig. 1.
Figure 1.
Results of the first SRTT session. (A) Mean RT ratio data for each group as a function of experimental block during the first session. The R1 block was used to normalize RT data into ratio. (B) Global learning (S10-S1) RT ratio data. The Contra-SMS group showed impaired global learning as compared to the two other groups, which did not differ. (C) Specific learning (R4-S10) RT ratio data. (D) Unspecific learning (R4-R2) RT ratio data. Error bars represent SEM. Asterisks (*) denote significant differences (p < 0.05).
Concerning Error data, results from the MANOVAs revealed no effect of Group (F(6,68) = 0.496, p = 0.809, = 0.042), suggesting that SMS did not influence the number of errors committed during the first session among groups.
Offline learning
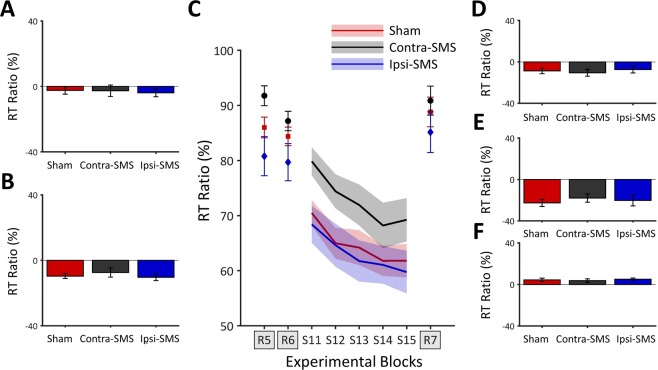
Concerning the occurrence of offline learning in RT data, results revealed that specific offline learning (S11-S10) did not occur in any groups (all p > 0.414; all Cohen’s dz < 0.459), but revealed that unspecific offline learning (R5-R4) occurred in all three groups (all p < 0.022; all Cohen’s dz > 0.756). Similar to results from Meier and Cock (2014)33, this suggests that offline learning of RT data occurred selectively in the unspecific comparison.
Concerning the influence of SMS on group RT data, results of the MANOVA revealed no effect of Group (F(4,70) = 0.295, p = 0.880, = 0.017), suggesting that SMS did not influence offline learning among groups in RT data. Offline learning RT data are presented in Fig. 2.
Figure 2.
Results of the second SRTT session. (A) Specific offline learning (S11-S10) RT ratio data. (B) Unspecific offline learning (R5-R4) RT ratio data. (C) Mean RT ratio data for each group as a function of experimental blocks during the second session. The R1 block from the first session was used to normalize RT data of the second session into ratio. (D) Global learning (S15-S11) RT ratio data. (E) Specific learning (S15-R7) RT ratio data. (F) Unspecific learning (R7-R6) RT ratio data. Error bars represent SEM.
Concerning the occurrence of offline learning in Error data, results revealed that specific (S11-S10) offline learning occurred in both the Sham (p = 0.015; Cohen’s dz = 0.946) and Ipsi-SMS groups (p = 0.009; Cohen’s dz = 1.051), but not in the Contra-SMS group (p = 0.239; Cohen’s dz = 0.423). Results further indicated that unspecific (R5-R4) offline learning occurred in all three groups (all p < 0.009; all Cohen’s dz > 0.647). These results suggest that specific and unspecific offline learning occurred in overall Error data.
Concerning the influence of SMS on group Error data, results of the MANOVA revealed no effect of Group (F(4,72) = 1.037, p = 0.394, = 0.054), suggesting that SMS did not influence offline learning among groups in Error data.
Second session
Concerning RT data, results of the MANOVA revealed no effect of Group (F(6,66) = 0.812, p = 0.564, = 0.069), indicating that relearning did not differ between groups. RT data of the second session are presented in Fig. 2.
Concerning Error data, results of the MANOVA also revealed no effect of Group (F(6,66) = 1.496, p = 0.193, = 0.120), indicating that relearning did not differ between groups.
Discussion
This study provides evidence that SMS influences learning-related neuroplasticity in humans in a manner consistent with its documented transient inhibitory activity. On one hand, results showed that SMS applied selectively over the contralateral M1 impaired online learning without affecting offline learning or relearning measured 24 h later. On the other hand, ipsilateral SMS did not significantly facilitate, nor interfere with, online learning.
SMS over contralateral M1 impaired online learning
The present results show that application of SMS over the contralateral M1 impaired global learning as compared to both Sham and application of SMS over the ipsilateral M1, suggesting that SMS over contralateral M1 disrupted online learning-related neuroplasticity. On one hand, these results fall in line with what is seen following putatively inhibitory rTMS, applied to the contralateral M1 prior to the learning phase27, and opposite to what is typically seen following excitatory neuromodulatory interventions applied to the same hemisphere19,34,35. Importantly, the observed effect on online learning cannot be attributed to a general slowing of motor response, as groups did not differ on RT for unspecific online learning (i.e., random sequences).
On the other hand, the apparent inhibitory nature of SMS observed here is in accordance with previous behavioural results reported in humans16,36,37 and animals38 globally showing that SMS can impair the neuronal activity mediating behavioral performance. In this light, given that SMS over M1 is known to decrease cortical excitability during and for a short period of time after its application3,6–8,12, SMS-induced transient disruption of M1 excitability could putatively mediate the present impairment in online learning19,39,40. However, since recent lines of evidence indicate that changes in M1 excitability during learning are not related to retention41,42, event when assessed 48 h later43, transient inhibition of M1 excitability during online learning may not necessarily interfere with time-dependent processes related to offline learning.
Contralateral SMS did not influence offline learning and relearning measured 24 h later
The presents results revealed that SMS did not impair offline learning or relearning between groups measured 24 h later, suggesting that the effects of SMS were transient in nature and did not alter the neuroplastic processes within M1 that are associated with consolidation shortly after practice31,44. On one hand, these results contrast with what is observed with inhibitory rTMS, as perturbation of M1 before and during motor learning with rTMS can negatively impact time-dependent processes such as consolidation and offline learning29–31,45–48. On the other hand, these results are partly consistent with studies using anodal transcranial direct current stimulation (tDCS) over M1, a neuromodulatory intervention believed to increase cortical excitability49, and showing that modification of performance during online learning does not necessarily influence offline learning (for a review, see50). Hence, one possibility is that transient perturbations of M1 excitability occurring online do not necessarily translate into perturbations of offline learning45.
In stark opposition to the present findings, a recent report showed that application of SMS over contralateral M1 did not impair online learning during SRTT, but rather facilitated offline learning as compared to sham stimulation51. With respect to online learning, one possibility that could account for this discrepancy is the differing application duration and timing of SMS over M1 during the acquisition session of the present design as compared to Nojima and colleagues51. Namely, whereas SMS was applied 10 min before and during the initial SRTT session (i.e., total of ~30 min) in the present design, Nojima and colleagues (2018) applied SMS for ~10 min selectively during acquisition. Given that SMS has been shown to require at least 10 min to induce LTD-like effects on M1 excitability6, one likely possibility is that SMS’s impairing influence on behaviors may discerningly emerge when it has been applied for at least 10 min before learning. With respect to offline learning, although the present results argue for a null effect of SMS on offline learning, how the inhibitory influence of SMS hinges on the complex set of neural processes engaged by offline learning remains unknown. For instance, given that homeostatic and non-homeostatic interactions are known to occur between neuromodulatory interventions and motor learning sessions52,53, application of an inhibitory neuromodulatory intervention during learning M1 could leave unimpaired45, inhibit48 or even facilitate51 processes engaged by offline learning. The extent to which SMS can be used to alter time-dependent processes, such as offline learning, remains a query for future studies. Alternatively, if the short-lasting nature of SMS was to be confirmed, it would constitute a useful tool to investigate temporally-defined mechanisms involved in complex cognitive phenomena such as learning.
Ipsilateral SMS did not impair online or offline learning
The existence of interhemispheric interactions between bilateral motor cortices is well established and appears to be mostly inhibitory in nature54,55. There is evidence that inhibiting one motor cortex suppresses transcallosal inhibition and leads to an increase in cortical excitability in the opposite M1 as probed with TMS20. In the context of SRTT, this release from inhibition induced by low-frequency rTMS has been shown to facilitate online learning in the unstimulated M120,21,28. Moreover, anodal tDCS impairs online learning during SRTT when applied to the ipsilateral M126, further supporting the notion that interhemispheric inhibition regulates the learning-related neuroplasticity during motor sequence learning. In the present study, SMS applied to the ipsilateral M1 did not significantly improve online learning or offline learning as one could have expected from the interhemispheric rivalry hypothesis. The precise reasons for this remain to be clarified, but one possibility is that SMS modulated different intracortical circuits than those responsible for interhemispheric inhibition (IHI). Indeed, while SMS modulates short intracortical inhibition3,8, these intracortical circuits have been documented to be distinct from those involved in IHI56, which would limit SMS capabilities to modulate IHI. Future studies combining SMS and dual coil TMS could provide additional information regarding the precise neurophysiological effect of SMS on IHI.
Conclusion
The present results provide evidence that transcranial application of SMS over contralateral M1 impairs online learning-related neuroplasticity. The detrimental effects of SMS on motor sequence learning appeared both temporally and spatially circumscribed to the duration and brain area of application, respectively. An important step for future research would be to combine SMS with whole brain neuroimaging techniques to determine the mechanisms by which SMS influences learning-related neuroplasticity, an important step to determine SMS’ capabilities for human research.
Materials and Methods
Participants
Thirty-nine right-handed healthy adults with no history of neurological or psychiatric disorder participated in the study (21 females; mean age 23.28 ± 0.47 years; Mean ± SEM). Importantly, participants were carefully randomly assigned to one of the three experimental groups in order to randomize the influence of confounding biological factors on the outcome of a neurostimulation session, such as anatomy of neuronal circuits and state of M1 excitability prior to learning (for a review, see57). Since this work is among the first to assess the influence of SMS on learning-related neuroplasticity, the expected effect size to be achieved which could then be used to power an a priori analysis was unknown. Nonetheless, based on previous SRTT studies using other neuromodulatory interventions that had groups of four to six31, eight27 or eight to thirteen participants29, we made the reasonable assumption that fourteen participants per group should yield acceptable statistical power to detect a meaningful effect of SMS on SRTT. Initially, forty-two participants were collected, but three participants were excluded due to computer malfunction (n = 2) or because they did not show up for the second session (n = 1). The study was approved by the research board of the Centre intégré universitaire de santé et de services sociaux de l’Estrie – Centre hospitalier universitaire de Sherbrooke, participants gave written informed consent, and all procedures were in accordance with the 1964 Declaration of Helsinki.
General procedure
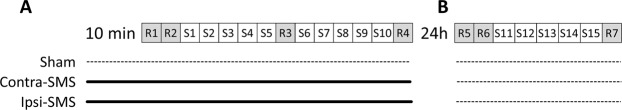
Participants came to the laboratory for two sessions separated by a 24 h interval to perform the SRTT. Sham or active SMS (contralateral, ipsilateral) was applied during the first session only. Offline learning and relearning were assessed upon the second visit. Participants were blind to group membership, and the experimenter collecting data was unaware of the expected effects of SMS application. While double-blinding would have been preferable, we could not ensure efficient blinding of the experimenter due to the obvious interaction of the magnet with ferromagnetic objects.
Static magnetic stimulation (SMS)
Participants were randomly assigned to one of the three following groups: sham SMS (Sham group; n = 12), contralateral SMS (Contra-SMS group; n = 13), or ipsilateral SMS (Ipsi-SMS group; n = 14). For the active SMS groups, a nickel-plated neodymium disc magnet (N52, 50 mm diameter, 50 mm thickness, axially magnetized, 139Kg pull force) was positioned over C3 or C4 sites of the 10–20 EEG system, corresponding to the left and right M1. The SMS was held in place with a custom-made helmet, resulting in approximately 180 mT reaching the cortex58. A non-ferromagnetic stainless steel cylinder of identical size was placed over the non-stimulated hemisphere to counterbalance the weight. For the sham group, stainless steel cylinders were placed on both hemispheres. To ensure that SMS would M1 excitability, application of SMS began 10 minutes before the onset of the SRTT6 and was maintained throughout the task (i.e., ~30 minutes total).
Serial reaction time task (SRTT)
In the SRTT, a visual cue appeared alternately at one of four possible positions within a horizontal array on a screen, each position corresponding to a specific key on a keyboard. In the first session, the SRTT involved 14 blocks of 12-key sequence each, which were performed with the right hand; 10 of these blocks (sequence blocks, S1 to S10) consisted of a repeating sequence (sequence: 4-2-3-1-1-3-2-1-3-4-2-4), while the order of stimuli presentation was randomized in 4 blocks (random blocks, R1 to R4). Two of the random blocks were presented at the beginning of the task (R1, R2), and after the fifth (R3) and tenth (R4) sequence blocks (Fig. 3 for details). The second part of the task, conducted 24 h later and aiming to measure offline learning and relearning, consisted in the presentation of the same first eight blocks of the first session in the same order (i.e., hereafter referred to as R5 to R7 and S11 to S15). Reaction time (RT), corresponding to the time between visual cue onset and the key press response (i.e., hereafter referred to as RT data), and errors, corresponding to the number of incorrect responses during the task (i.e., hereafter referred to as Error data), were recorded.
Figure 3.
Overview of the SRTT protocol. (A) First session. Sham or SMS stimulation was applied 10 minutes before the onset of the SRTT protocol. SMS stimulation ceased upon first session termination. (B) Second session occurring 24 h later. No SMS or Sham stimulation was applied. “S” and “R” denote sequence and random blocks, respectively.
Data processing and analyses
For each participant, aberrant trials were identified within each block individually (±2.5 SD, <3% of trials) and excluded to compute representative mean RT (ms)59. Performance in the two sessions was normalized using the first block of the first session (R1) to compute a percentage ratio (%) for RT data. Because some participants made no error in the R1 block, Error data were not normalized.
The first analysis sought to determine whether SMS influenced global, specific or unspecific learning during the SRTT60–62. For that purpose, multivariate analysis of variance (MANOVAs), with Group (Sham, Contra-SMS, Ipsi-SMS) as the fixed factor were used63. The MANOVAs had three dependent variables, which were the RT ratio difference between S10-S1, R4-S10, and R4-R2 to assess global, specific, and unspecific learning, respectively.
To determine if offline learning occurred and differed between groups, specific learning and unspecific learning were calculated from the RT ratio difference between S11-S10 and between R5-R4, respectively. First, to determine whether specific or unspecific offline learning occurred, RT ratio difference data of each group were compared to the value 0 using one-sample t-tests, which were corrected for multiple comparisons (see below). Significant differences from the value 0 would indicate that offline learning occurred.
Second, to assess the impact of SMS on specific and unspecific offline learning among groups, the RT ratio difference data were submitted to a MANOVA using Group (Sham, Contra-SMS, Ipsi-SMS) as the fixed effect. The MANOVA had two dependent variables, which were the RT data of specific (S11-S10) and unspecific (R5-R4) offline learning. Finally, to assess the potential lingering effect of SMS on relearning during the second session, a similar MANOVA was also conducted. The MANOVA had three dependent variables, which were the RT ratio difference used to assess global learning (S15-S11), specific learning (R7-S15) and unspecific learning (R7-R6).
Moreover, to determine if the number of committed errors was influenced by the application of SMS, the same analyses were conducted on the same comparisons as with RT data but on Error data.
If a MANOVA returned a significant effect of Group, the Roy-Bargmann Stepdown Procedure was used as a follow-up analysis to determine the contribution of each dependent variable to the detected effect32. Briefly, the Roy-Bargmann procedure first requires the dependent variables to be ordered in descending order according to their level of theoretical importance. Then, a univariate ANOVA is conducted on the variable of highest interest. Subsequently, ANCOVAs that use the data of the immediately higher level of importance as covariates are conducted in a descending stepwise fashion to evaluate the influence of single variables on the MANOVA results32. In the present context, assessment of global learning was deemed to be the response variable of highest interest it was deemed to best represent learning-related performance improvements. Assessments of unspecific and specific learning were deemed to be of second and third level of theoretical importance, respectively.
For all statistical analyses, normality of distribution and homogeneity of variance were verified using Shapiro-Wilk’s64 and Levene’s tests (Box’s M for multivariate analyses)65, respectively. For post-hoc comparisons, independent t-tests or Mann-Whitney U tests were used, depending on data normality and homogeneity of variance. To control for inflated type 1 errors upon multiple comparisons66, the Benjamini-Hochberg procedure was used67,68.
Acknowledgements
This work was supported by grants from the Fonds de la Recherche du Québec- Santé [FRQS, Grant 33140] and the Natural Sciences and Engineering Research Council of Canada [NSERC; Grant RGPIN-2017-05510] awarded to J.F.L. L.P.B. received an undergraduate student research award from the NSERC; A.L. is supported by a graduate scholarship award from the FRQS; R.H. is supported by a graduate scholarship award from the NSERC; L.D.B. is supported by a Junior 1 Salary Award from the FRQS. We thank Eric Pelletier and Usinage Maska for skillful technical help.
Author Contributions
J.F.L. designed the experiment. A.L. and L.P.B. collected the data and redacted an initial version of the manuscript, under the supervision of J.F.L. R.H. and P.M.B. thoroughly revised all sections of the manuscript, performed the statistical analyses, and prepared the figures. J.F.L., P.M.B. and L.D.B. reviewed and provided constructive comments.
Data Availability
Data is available by contacting the corresponding author.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Angélina Lacroix, Léa Proulx-Bégin and Raphaël Hamel contributed equally.
References
- 1.McLean MJ, et al. Effects of a static magnetic field on audiogenic seizures in black Swiss mice. Epilepsy Res. 2008;80:119–131. doi: 10.1016/j.eplepsyres.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Ward BK, Roberts DC, Della Santina CC, Carey JP, Zee DS. Vestibular stimulation by magnetic fields. Ann. N. Y. Acad. Sci. 2015;1343:69–79. doi: 10.1111/nyas.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nojima I, Koganemaru S, Fukuyama H, Mima T. Static magnetic field can transiently alter the human intracortical inhibitory system. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015;126:2314–2319. doi: 10.1016/j.clinph.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Schlamann M, et al. Short term effects of magnetic resonance imaging on excitability of the motor cortex at 1.5T and 7T. Acad. Radiol. 2010;17:277–281. doi: 10.1016/j.acra.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Oliviero A, et al. Safety Study of Transcranial Static Magnetic Field Stimulation (tSMS) of the Human Cortex. Brain Stimulat. 2015;8:481–485. doi: 10.1016/j.brs.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Oliviero A, et al. Transcranial static magnetic field stimulation of the human motor cortex. J. Physiol. 2011;589:4949–4958. doi: 10.1113/jphysiol.2011.211953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arias P, Adán-Arcay L, Puerta-Catoira B, Madrid A, Cudeiro J. Transcranial static magnetic field stimulation of M1 reduces corticospinal excitability without distorting sensorimotor integration in humans. Brain Stimulat. 2017;10:340–342. doi: 10.1016/j.brs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Dileone M, Mordillo-Mateos L, Oliviero A, Foffani G. Long-lasting effects of transcranial static magnetic field stimulation on motor cortex excitability. Brain Stimulat. 2018;11:676–688. doi: 10.1016/j.brs.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Kirimoto H, et al. Effect of transcranial static magnetic field stimulation over the sensorimotor cortex on somatosensory evoked potentials in humans. Brain Stimulat. 2014;7:836–840. doi: 10.1016/j.brs.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Kufner M, Brückner S, Kammer T. No modulatory effects by transcranial static magnetic field stimulation of human motor and somatosensory cortex. Brain Stimulat. 2017;10:703–710. doi: 10.1016/j.brs.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Nojima I, Koganemaru S, Mima T. Combination of Static Magnetic Fields and Peripheral Nerve Stimulation Can Alter Focal Cortical Excitability. Front. Hum. Neurosci. 2016;10:598. doi: 10.3389/fnhum.2016.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silbert BI, Pevcic DD, Patterson HI, Windnagel KA, Thickbroom GW. Inverse correlation between resting motor threshold and corticomotor excitability after static magnetic stimulation of human motor cortex. Brain Stimulat. 2013;6:817–820. doi: 10.1016/j.brs.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Albuquerque WWC, Costa RMPB, Fernandes TdeSE, Porto ALF. Evidences of the static magnetic field influence on cellular systems. Prog. Biophys. Mol. Biol. 2016;121:16–28. doi: 10.1016/j.pbiomolbio.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Hashemi S, Abdolali A. Three-dimensional analysis, modeling, and simulation of the effect of static magnetic fields on neurons. Bioelectromagnetics. 2017;38:128–136. doi: 10.1002/bem.22019. [DOI] [PubMed] [Google Scholar]
- 15.Rosen AD. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem. Biophys. 2003;39:163–173. doi: 10.1385/CBB:39:2:163. [DOI] [PubMed] [Google Scholar]
- 16.Lozano-Soto E, et al. Transcranial static magnetic field stimulation (tSMS) of the visual cortex decreases experimental photophobia. Cephalalgia Int. J. Headache. 2018;38:1493–1497. doi: 10.1177/0333102417736899. [DOI] [PubMed] [Google Scholar]
- 17.Buch ER, et al. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2017;128:589–603. doi: 10.1016/j.clinph.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Censor N, Cohen LG. Using repetitive transcranial magnetic stimulation to study the underlying neural mechanisms of human motor learning and memory. J. Physiol. 2011;589:21–28. doi: 10.1113/jphysiol.2010.198077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashemirad F, Zoghi M, Fitzgerald PB, Jaberzadeh S. The effect of anodal transcranial direct current stimulation on motor sequence learning in healthy individuals: A systematic review and meta-analysis. Brain Cogn. 2016;102:1–12. doi: 10.1016/j.bandc.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi M, Hutchinson S, Théoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91–98. doi: 10.1212/WNL.62.1.91. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M, Théoret H, Pascual-Leone A. Suppression of ipsilateral motor cortex facilitates motor skill learning. Eur. J. Neurosci. 2009;29:833–836. doi: 10.1111/j.1460-9568.2009.06628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollok B, Boysen A-C, Krause V. The effect of transcranial alternating current stimulation (tACS) at alpha and beta frequency on motor learning. Behav. Brain Res. 2015;293:234–240. doi: 10.1016/j.bbr.2015.07.049. [DOI] [PubMed] [Google Scholar]
- 23.Reis J, et al. Consensus: Can transcranial direct current stimulation and transcranial magnetic stimulation enhance motor learning and memory formation? Brain Stimulat. 2008;1:363–369. doi: 10.1016/j.brs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Amadi U, Allman C, Johansen-Berg H, Stagg CJ. The Homeostatic Interaction Between Anodal Transcranial Direct Current Stimulation and Motor Learning in Humans is Related to GABAA Activity. Brain Stimulat. 2015;8:898–905. doi: 10.1016/j.brs.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantak SS, Mummidisetty CK, Stinear JW. Primary motor and premotor cortex in implicit sequence learning–evidence for competition between implicit and explicit human motor memory systems. Eur. J. Neurosci. 2012;36:2710–2715. doi: 10.1111/j.1460-9568.2012.08175.x. [DOI] [PubMed] [Google Scholar]
- 26.Keitel A, Øfsteng H, Krause V, Pollok B. Anodal Transcranial Direct Current Stimulation (tDCS) Over the Right Primary Motor Cortex (M1) Impairs Implicit Motor Sequence Learning of the Ipsilateral Hand. Front. Hum. Neurosci. 2018;12:289. doi: 10.3389/fnhum.2018.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson L, Teo JT, Obeso I, Rothwell JC, Jahanshahi M. The contribution of primary motor cortex is essential for probabilistic implicit sequence learning: evidence from theta burst magnetic stimulation. J. Cogn. Neurosci. 2010;22:427–436. doi: 10.1162/jocn.2009.21208. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi M. Effect of slow repetitive TMS of the motor cortex on ipsilateral sequential simple finger movements and motor skill learning. Restor. Neurol. Neurosci. 2010;28:437–448. doi: 10.3233/RNN-2010-0562. [DOI] [PubMed] [Google Scholar]
- 29.Breton, J. & Robertson, E. M. Dual enhancement mechanisms for overnight motor memory consolidation. Nat. Hum. Behav. 1 (2017). [DOI] [PMC free article] [PubMed]
- 30.Hotermans C, Peigneux P, de Noordhout AM, Moonen G, Maquet P. Repetitive transcranial magnetic stimulation over the primary motor cortex disrupts early boost but not delayed gains in performance in motor sequence learning. Eur. J. Neurosci. 2008;28:1216–1221. doi: 10.1111/j.1460-9568.2008.06421.x. [DOI] [PubMed] [Google Scholar]
- 31.Robertson EM, Press DZ, Pascual-Leone A. Off-line learning and the primary motor cortex. J. Neurosci. Off. J. Soc. Neurosci. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finch, W. H. Performance of the Roy-Bargmann Stepdown Procedure as a Follow Up to a Significant MANOVA.
- 33.Meier B, Cock J. Offline consolidation in implicit sequence learning. Cortex J. Devoted Study Nerv. Syst. Behav. 2014;57:156–166. doi: 10.1016/j.cortex.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Bütefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J. Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- 35.Narayana S, et al. Concurrent TMS to the primary motor cortex augments slow motor learning. NeuroImage. 2014;85(Pt 3):971–984. doi: 10.1016/j.neuroimage.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrasco-López C, et al. Static Magnetic Field Stimulation over Parietal Cortex Enhances Somatosensory Detection in Humans. J. Neurosci. Off. J. Soc. Neurosci. 2017;37:3840–3847. doi: 10.1523/JNEUROSCI.2123-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Rosa JJ, et al. Static Magnetic Field Stimulation over the Visual Cortex Increases Alpha Oscillations and Slows Visual Search in Humans. J. Neurosci. Off. J. Soc. Neurosci. 2015;35:9182–9193. doi: 10.1523/JNEUROSCI.4232-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguila J, Cudeiro J, Rivadulla C. Effects of Static Magnetic Fields on the Visual Cortex: reversible Visual Deficits and Reduction of Neuronal Activity. Cereb. Cortex N. Y. N 1991. 2016;26:628–638. doi: 10.1093/cercor/bhu228. [DOI] [PubMed] [Google Scholar]
- 39.Hirano M, et al. Acquisition of skilled finger movements is accompanied by reorganization of the corticospinal system. J. Neurophysiol. 2018;119:573–584. doi: 10.1152/jn.00667.2017. [DOI] [PubMed] [Google Scholar]
- 40.Hirano M, Kubota S, Koizume Y, Tanaka S, Funase K. Different Effects of Implicit and Explicit Motor Sequence Learning on Latency of Motor Evoked Potential Evoked by Transcranial Magnetic Stimulation on the Primary Motor Cortex. Front. Hum. Neurosci. 2016;10:671. doi: 10.3389/fnhum.2016.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bologna M, et al. Reversal of Practice-related Effects on Corticospinal Excitability has no Immediate Effect on Behavioral Outcome. Brain Stimulat. 2015;8:603–612. doi: 10.1016/j.brs.2015.01.405. [DOI] [PubMed] [Google Scholar]
- 42.López-Alonso V, Cheeran B, Fernández-del-Olmo M. Relationship Between Non-invasive Brain Stimulation-induced Plasticity and Capacity for Motor Learning. Brain Stimulat. 2015;8:1209–1219. doi: 10.1016/j.brs.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 43.Hannah Ricci, Iacovou Anna, Rothwell John C. Direction of TDCS current flow in human sensorimotor cortex influences behavioural learning. Brain Stimulation. 2019;12(3):684–692. doi: 10.1016/j.brs.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muellbacher W, et al. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- 45.Iezzi E, et al. Theta-burst stimulation over primary motor cortex degrades early motor learning. Eur. J. Neurosci. 2010;31:585–592. doi: 10.1111/j.1460-9568.2010.07090.x. [DOI] [PubMed] [Google Scholar]
- 46.Richardson AG, et al. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J. Neurosci. Off. J. Soc. Neurosci. 2006;26:12466–12470. doi: 10.1523/JNEUROSCI.1139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riek S, Hinder MR, Carson RG. Primary motor cortex involvement in initial learning during visuomotor adaptation. Neuropsychologia. 2012;50:2515–2523. doi: 10.1016/j.neuropsychologia.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 48.Stöckel T, Summers JJ, Hinder MR. Reversed Effects of Intermittent Theta Burst Stimulation following Motor Training That Vary as a Function of Training-Induced Changes in Corticospinal Excitability. Neural Plast. 2015;2015:578620. doi: 10.1155/2015/578620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bastani A, Jaberzadeh S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: a systematic review and meta-analysis. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2012;123:644–657. doi: 10.1016/j.clinph.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 50.Savic B, Meier B. How Transcranial Direct Current Stimulation Can Modulate Implicit Motor Sequence Learning and Consolidation: A Brief Review. Front. Hum. Neurosci. 2016;10:26. doi: 10.3389/fnhum.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nojima I, et al. Transcranial static magnetic stimulation over the primary motor cortex alters sequential implicit motor learning. Neurosci. Lett. 2018;696:33–37. doi: 10.1016/j.neulet.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Karabanov A, et al. Consensus Paper: Probing Homeostatic Plasticity of Human Cortex With Non-invasive Transcranial Brain Stimulation. Brain Stimulat. 2015;8:442–454. doi: 10.1016/j.brs.2015.01.404. [DOI] [PubMed] [Google Scholar]
- 53.Müller-Dahlhaus F, Ziemann U. Metaplasticity in human cortex. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry. 2015;21:185–202. doi: 10.1177/1073858414526645. [DOI] [PubMed] [Google Scholar]
- 54.Chen R, Yung D, Li J-Y. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J. Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- 55.Ferbert A, et al. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J. Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Y-Z, et al. Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2017;128:2318–2329. doi: 10.1016/j.clinph.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Tharayil JJ, Goetz SM, Bernabei JM, Peterchev AV. Field Distribution of Transcranial Static Magnetic Stimulation in Realistic Human Head Model. Neuromodulation J. Int. Neuromodulation Soc. 2018;21:340–347. doi: 10.1111/ner.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller J. A warning about median reaction time. J. Exp. Psychol. Hum. Percept. Perform. 1988;14:539–543. doi: 10.1037/0096-1523.14.3.539. [DOI] [PubMed] [Google Scholar]
- 60.Lévesque J, Théoret H, Champoux F. Reduced procedural motor learning in deaf individuals. Front. Hum. Neurosci. 2014;8:343. doi: 10.3389/fnhum.2014.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morin-Parent F, de Beaumont L, Théoret H, Lepage J-F. Superior non-specific motor learning in the blind. Sci. Rep. 2017;7:6003. doi: 10.1038/s41598-017-04831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez MA, Wise SP, Willingham DT, Cohen LG. Neurophysiological mechanisms involved in transfer of procedural knowledge. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:1045–1053. doi: 10.1523/JNEUROSCI.4128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tabachnick, B. G. & Fidell, L. S. Using Multivariate Statistics. (Pearson Education, 2013).
- 64.Razali, N. M. & Wah, Y. B. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. In (2011).
- 65.Field, A. Discovering Statistics Using IBM SPSS Statistics. (SAGE, 2013).
- 66.Ludbrook J. Multiple comparison procedures updated. Clin. Exp. Pharmacol. Physiol. 1998;25:1032–1037. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 67.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple. Testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- 68.Verhoeven KJF, Simonsen KL, McIntyre LM. Implementing false discovery rate control: increasing your power. Oikos. 2005;108:643–647. doi: 10.1111/j.0030-1299.2005.13727.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available by contacting the corresponding author.